ABSTRACT
Mycobacterium abscessus causes acute and chronic bronchopulmonary infection in patients with chronic lung damage, of which cystic fibrosis (CF) patients are particularly vulnerable. The major threat posed by this organism is its high intrinsic antibiotic resistance. A typical treatment regimen involves a 6- to 12-month-long combination therapy of clarithromycin and amikacin, with cure rates below 50% and multiple side effects, especially due to amikacin. In the present work, we show that M. abscessus whiB7, a homologue of Mycobacterium tuberculosis and Mycobacterium smegmatis whiB7 with previously demonstrated effects on intrinsic antibiotic resistance, is strongly induced when exposed to clinically relevant antibiotics that target the ribosome: erythromycin, clarithromycin, amikacin, tetracycline, and spectinomycin. The deletion of M. abscessus whiB7 results in sensitivity to all of the above-mentioned antibiotics. Further, we have defined and compared the whiB7 regulon of M. abscessus with the closely related nontuberculous mycobacterium (NTM) M. smegmatis to demonstrate the induction of a species-specific repertoire of genes. Finally, we show that one such gene, eis2, is specifically induced in M. abscessus by whiB7 and contributes to its higher levels of intrinsic amikacin resistance. This species-specific pattern of gene induction might account for the differences in drug susceptibilities to other antibiotics and between different mycobacterial species.
KEYWORDS: Mycobacterium, abscessus, antibiotic resistance, intrinsic, whiB7
INTRODUCTION
The Mycobacterium abscessus group is a rapid-growing, nontuberculous species of mycobacteria (NTM/RGM) comprised of three subspecies, M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. abscessus massiliense, and has emerged as an important human pathogen over the last 10 years (1–8). M. abscessus is one of the most prominent causes of bronchopulmonary infections in patients with underlying lung damage, such as bronchiectasis, prior tuberculosis, and cystic fibrosis (CF), and leads to either acute lung failure or chronic disease with a progressive decline in lung function. M. abscessus infections comprise 80% of all RGM-associated pulmonary infections, with an incidence of ∼1 per 100,000 of the general population and a prevalence of 6 to 13% in CF patients (4, 9–13). In addition, M. abscessus causes skin and soft tissue infections (SSTI) postsurgery and posttrauma (14, 15). In rare cases, M. abscessus can also cross the blood-brain barrier to cause meningitis and cerebral abscesses in immunocompetent individuals (16, 17).
Mycobacteria are intrinsically drug resistant; nontuberculous mycobacteria (NTM) are particularly drug resistant, of which M. abscessus stands out as one of the most resistant bacterial species known. M. abscessus is resistant to most antimicrobial agents, including antituberculosis drugs (rifampin, isoniazid, ethambutol, and pyrazinamide), and it presents a challenge in public health settings (18, 19). Only a few antibiotics, clarithromycin (0 to 38%), cefoxitin (15%), and amikacin (7.7%), exhibit activity against M. abscessus (with low rates of acquired resistance shown in parenthesis) (20, 21). A particularly vexing aspect of M. abscessus therapy is that there is poor correlation between in vitro antibiotic susceptibility and in vivo efficacy; a large part of macrolide resistance is attributed to inducible erm(41) expression (20, 22–25). The current regimen of treatment typically involves an extended (12-month) combination therapy of a macrolide (clarithromycin or azithromycin) and intravenous amikacin and cefoxitin/imipenem. Despite such aggressive treatments, the average rate of eradication is only 45% (26).
Intrinsic resistance in mycobacteria is thought to be a synergistic action of a waxy and impermeable cell envelope and internal defense mechanisms (27). Diffusion of antibiotics into the bacterial cytosol, albeit at a reduced rate, induces a massive transcriptional reprogramming that results in changes in growth rate, metabolism, and induction of genes facilitating drug resistance, such as those encoding efflux pumps and enzymes that modify either the antibiotic or its target. The existence of ∼190 transcription regulators which include two-component systems, protein kinases, as well as >100 transcription activators and repressors in the mycobacterial genome suggests an exceptionally intricate and flexible system of gene regulation (28); however, the hierarchy and topology of molecular networks in the antibiotic-induced global reprogramming of gene expression are poorly understood. One of the best studied regulators of this reprogramming circuit is WhiB7, a transcriptional activator that belongs to the WhiB family of transcriptional regulators conserved in actinomycetes (29, 30). WhiB7 proteins have a variable N terminus and a conserved core sequence characterized by four iron-binding conserved cysteine residues, a G/Y-rich motif, and a positively charged AT-hook that binds an AT-rich region in DNA (31). whiB7 is induced in the presence of several structurally unrelated antibiotics, such as tetracycline, macrolides, and aminoglycosides, as well as compounds that perturb respiration, redox balance, and iron starvation (30–34). A deletion of whiB7 in Mycobacterium smegmatis and Mycobacterium tuberculosis results in multidrug sensitivity (34). Whole-genome sequencing of M. abscessus subsp. abscessus ATCC 19977 (referred to as M. abscessus in the current work) reveals the presence of a transcription factor, MAB_3508c, which is 75% identical to M. smegmatis and M. tuberculosis whiB7.
In the present study, we have investigated the effect of MAB_3508c deletion on the sensitivity of M. abscessus to six unrelated antibiotics: erythromycin, clarithromycin, streptomycin, spectinomycin, amikacin, and tetracycline. We establish that MAB_3508c is the M. abscessus whiB7 and is required for intrinsic resistance of M. abscessus to all antibiotics tested; this effect is specific, and a deletion of MAB_3508c (here referred to as MabwhiB7) does not influence rifampin or isoniazid resistance. Further, we show that although whiB7 is required for multidrug resistance in both M. abscessus and M. smegmatis, the whiB7 regulon shows minimal overlap between the two species. The species-specific repertoire of whiB7 gene induction between different mycobacterial species might account for the observed differences in their drug susceptibilities. This is supported by the whiB7-dependent induction of eis2 exclusively in M. abscessus and a direct role of eis2 in high levels of amikacin resistance.
RESULTS
MAB_3508c is highly induced by ribosome-targeting antibiotics.
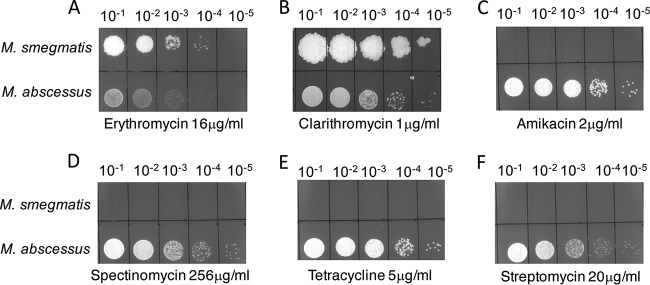
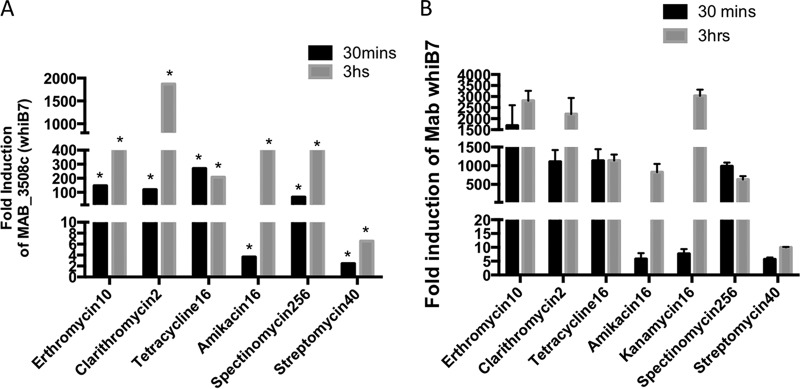
M. abscessus and M. smegmatis are two fast-growing NTMs with a high conservation in their genes and genetic organization. However, M. abscessus and M. smegmatis have strikingly distinct susceptibilities to amikacin, tetracycline, spectinomycin, and streptomycin (Table 1 and Fig. 1) while showing comparable tolerance to the macrolides erythromycin and clarithromycin. In an ongoing RNA sequencing (RNA-Seq) study of antibiotic-induced genes in M. abscessus (data not shown), we found that MAB_3508c is the earliest and the most highly induced gene upon exposure to sublethal concentrations of all ribosome-targeting antibiotics tested (Fig. 2A and S1). These results were subsequently confirmed using quantitative PCR (Fig. 2B). The extent of induction of MAB_3508c is a function of the time of exposure as well as the antibiotic used. As seen in Fig. 2A and B, sublethal doses of erythromycin, clarithromycin, tetracycline, and spectinomycin were the strongest inducers of MAB_3508c (induced >100-fold within 30 min of exposure). The induction of MAB_3508c by amikacin is much weaker than that by the above-mentioned antibiotics. We tested a range of amikacin concentrations and found comparable levels of induction using 16 μg/ml; even at this concentration, the induction of MAB_3508c was low at early times of exposure but increased upon extension of the exposure time (Fig. 2 and S2). However, the related aminoglycoside kanamycin is a stronger inducer of MAB_3508c. Finally, streptomycin was the weakest inducer of MAB_3508c.
TABLE 1.
Survival of wild-type M. abscessus ATCC 19977 and M. smegmatis mc2155 in a 2-fold dilution series of antibiotics in Middlebrook 7H9 medium
| Antibiotic | MIC99 (μg/ml)a |
|
|---|---|---|
| M. abscessus WT | M. smegmatis WT | |
| Erythromycin | 0.5–1 | 2.5 |
| Clarithromycin | 0.25–0.5 | 0.25–0.5 |
| Amikacin | 8 | 0.625 |
| Spectinomycin | >256 | 50 |
| Tetracycline | 16 | 0.2 |
| Streptomycin | 16 | 0.3 |
MIC99, the minimum concentration of antibiotic required to inhibit 99% of growth.
FIG 1.
Differences in antibiotic sensitivities between M. abscessus ATCC 19977 and M. smegmatis mc2155 (A to F). Ten-fold serial dilutions of M. abscessus ATCC 19977 and M. smegmatis mc2155 grown to A600 of 0.7 and spotted on Middlebrook 7H10 containing indicated concentrations of antibiotics. M. abscessus is more resistant to amikacin, tetracycline, streptomycin, and spectinomycin than M. smegmatis but the sensitivities to clarithromycin and erythromycin are comparable.
FIG 2.
Time course of induction of whiB7 upon antibiotic exposure. (A) The fold induction of transcript of MAB_3508c in wild-type M. abscessus ATCC 19977 exposed to amikacin (16 μg/ml), clarithromycin (2 μg/ml), erythromycin (10 μg/ml), tetracycline (16 μg/ml), streptomycin (40 μg/ml), and spectinomycin (256 μg/ml) over unexposed samples was determined in previous RNA-Seq studies. The asterisks (*) indicate q values of <0.001 (B) The results were verified by quantitative PCR (qPCR) and expressed as a fold overexpression over unexposed samples. Data represent the mean ± standard deviation (SD), n = 3. sigA was used as an endogenous control.
Deletion of MAB_3508c causes varied levels of susceptibility to ribosome-targeting antibiotics.
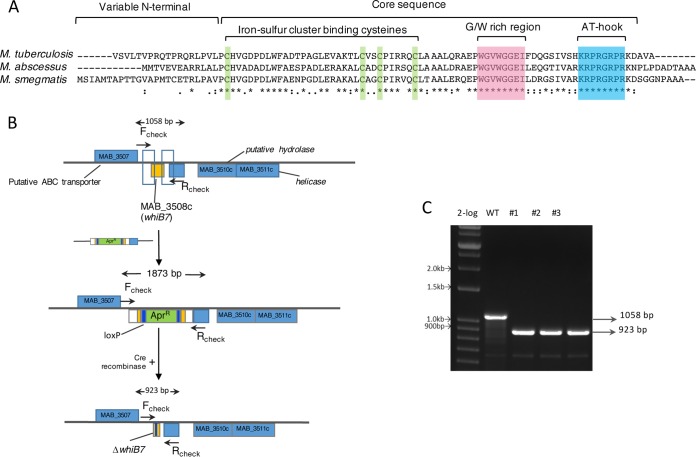
MAB_3508c is 75% identical to M. smegmatis and M. tuberculosis whiB7, suggesting a conserved role of MAB_3508c in M. abscessus drug resistance (Fig. 3A). We constructed an isogenic deletion of the gene in M. abscessus ATCC 19977 using phage recombineering (Fig. 3B) (35). Following transformation with the knockout construct in the recombineering strain, we obtained 130 apramycin-resistant (Aprr) colonies, of which 20 were screened using PCR using the flanking Fcheck and Rcheck primers. Three colonies were found to contain the expected fragment size corresponding to a double-crossover event (∼15%). The frequency correlated with previously reported recombineering efficiencies (36). The insertional mutant was unmarked by recombination between the loxP sites mediated by the Cre recombinase, as shown in Fig. 3B and C. The unmarked deletion mutant was also confirmed using PCR and sequencing. A complemented strain was constructed by transforming the deletion strain with pMH94-MAB_3508c, a phage L5-based integration vector, in which MAB_3508c is expressed from the constitutive promoter Phsp60.
FIG 3.
Deletion of M. abscessus whiB7. (A) Multiple-sequence alignment of WhiB7 from M. abscessus, M. smegmatis, and M. tuberculosis showing identical residues (*) and conserved motifs. (B) Schematic representation of creating a deletion of MAB_3508c (whiB7) using phage recombineering and unmarking using the Cre-lox system. (C) Three clones were selected (no. 1 to 3), and the whiB7 gene was amplified using the Fcheck and Rcheck primers, followed by confirmation by Sanger sequencing.
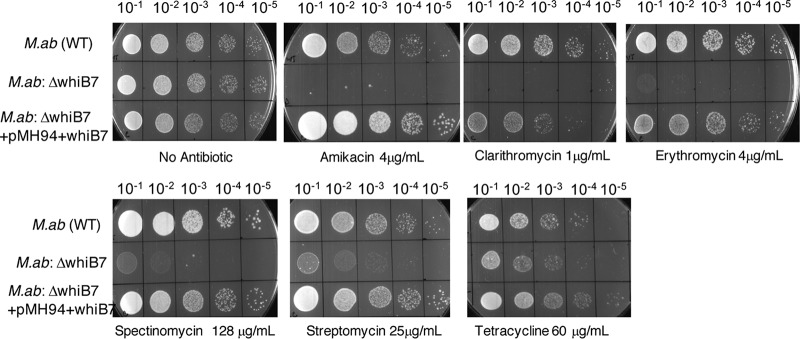
Wild-type M. abscessus, the ΔMAB_3508c mutant, and the complemented strain were tested for their susceptibilities to erythromycin, clarithromycin, tetracycline, amikacin, streptomycin, and spectinomycin by spotting a 10-fold serial dilution of each strain on Middlebrook 7H10 containing oleic acid-albumin-dextrose-catalase (OADC) and the indicated concentrations of antibiotics (Fig. 4). MICs were also determined in liquid media as described in Materials and Methods and Table 2. The deletion of MAB_3508c resulted in sensitivity to all the antibiotics tested and is reminiscent of the behavior of M. tuberculosis and M. smegmatis ΔwhiB7 mutants. Moreover, this effect was specific, and a deletion of MAB_3508c did not influence resistance to rifampin or isoniazid (data not shown). We here refer to MAB_3508c as MabwhiB7. The deletion of MabwhiB7 resulted in a spectrum of effects on antibiotic resistance that were not entirely predictable from the induction profile. The ΔMabwhiB7 mutant is hypersensitive to erythromycin, clarithromycin, and spectinomycin compared to the wild-type parent strain and correlates with the high levels of induction of MabwhiB7 upon exposure to these drugs. The sensitivity of the mutant is restored in the complemented strain (Fig. 4). Streptomycin is an ineffective inducer of MabwhiB7, and the ΔMabwhiB7 mutant shows a modest defect in streptomycin sensitivity (Fig. 4). However, although amikacin is a weak inducer, the ΔMabwhiB7 mutant is highly sensitive to amikacin. In contrast, tetracycline is a potent inducer of MabwhiB7, but the deletion mutant is only mildly more sensitive to tetracycline than the wild type (Fig. 4). Together, these findings suggest that the role of MabwhiB7 is complex and that it plays a varied role in the intrinsic resistance to different antibiotics. Moreover, there is little correlation between the level of induction of MabwhiB7 and the extent of resistance conferred. While MabwhiB7 is perhaps the primary regulator of intrinsic resistance toward erythromycin, clarithromycin, and spectinomycin, additional WhiB7-independent determinants may be required to confer resistance to tetracycline and streptomycin.
FIG 4.
Deletion of MAB_3508c (whiB7) renders M. abscessus hypersensitive to multiple antibiotics. Ten-fold dilutions of the ΔwhiB7 mutant cells were spotted on Middlebrook 7H10/OADC containing indicated concentrations of antibiotics. The mutant is hypersensitive to clarithromycin, erythromycin, amikacin, streptomycin, and spectinomycin but marginally more sensitive to tetracycline than the wild-type parent strain. A complementing strain containing an integrated copy of whiB7 expressed from a constitutive promoter Phsp60 restores antibiotic resistance. M.ab, M. abscessus.
TABLE 2.
Survival of wild-type M. abscessus ATCC 19977 and ΔMAB_3508c in a 2-fold dilution series of antibiotics in Middlebrook 7H9 medium
| Antibiotic | MIC99 (μg/ml)a |
|
|---|---|---|
| M. abscessus WT (μg/ml) | M. abscessus ΔwhiB7 mutant (μg/ml) | |
| Amikacin | 8 | 2 |
| Clarithromycin | 0.25–0.5 | 0.0625 |
| Erythromycin | 0.5–1 | 0.0625 |
| Spectinomycin | >256 | 32–64 |
| Tetracycline | 16 | 8 |
| Streptomycin | 16 | 8 |
MIC99, the minimum concentration of antibiotic required to inhibit 99% of growth after 96 h.
WhiB7 regulates species-specific repertoire of genes in M. smegmatis and M. abscessus.
Despite a central contribution of WhiB7 in drug resistance of M. abscessus and M. smegmatis, the level of resistance between the two species differs by 20- to 200-fold. We speculated that the whiB7 regulon of M. abscessus may be different from that of M. smegmatis and includes genes that confer increased levels of intrinsic drug resistance. We used RNA-Seq to determine the whiB7 regulon of M. abscessus and M. smegmatis using the ΔwhiB7 mutant strains of the two species complemented with respective whiB7 genes expressed from a constitutive promoter, Phsp60. (see RNA-Seq in Data Set S1 in the supplemental material). We identified 128 and 96 genes comprising the whiB7 regulon of M. abscessus and M. smegmatis, respectively, using the criteria of >4-fold induction in the whiB7-overexpressing strain compared to the ΔwhiB7 mutant and q values of <0.01. Exceptions for statistical significance were made for highly induced genes. Surprisingly, we found an overlap of only 16 genes in the whiB7 regulons of the two species (Fig. 5, Table 3, and RNA-Seq in Data Set S1). Of the remaining 111 genes in the M. abscessus WhiB7 regulon, 56 genes were unique to M. abscessus (Fig. 5, shaded in green). Notably, 55 genes, despite having clear orthologues in M. smegmatis, did not comprise the M. smegmatis WhiB7 regulon (Fig. 5, shaded in yellow).
FIG 5.
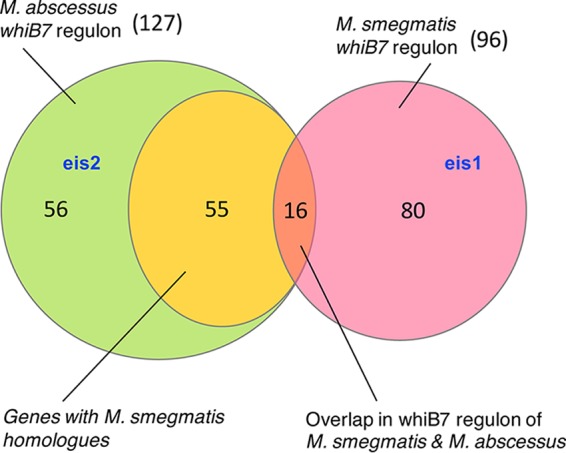
Species-specific regulons of WhiB7 in M. smegmatis and M. abscessus. M. smegmatis and M. abscessus whiB7 were expressed from constitutive promoters in respective ΔwhiB7 mutant strains. RNA-Seq analysis was performed to determine the regulon of WhiB7 in each species, and the overlap in the regulon is represented. eis2 is WhiB7 regulated exclusively in M. abscessus, whereas eis1 is exclusively induced in M. smegmatis by WhiB7. Numbers refer to the number of genes in each category.
TABLE 3.
Overlapping genes in the whiB7 regulons of M. smegmatis and M. abscessus
| M. abscessus gene | M. smegmatis homologue | Producta |
|---|---|---|
| MAB_1341 | MSMEG_5087 | Hypothetical protein |
| MAB_1342 | MSMEG_5086 | Probable fatty-acid-CoA ligase FadD |
| MAB_1395 | MSMEG_5047 | Multidrug resistance transporter, Bcr/CflA family |
| MAB_1846 | MSMEG_5102 | ABC transporter ATP-binding protein |
| MAB_2273 | MSMEG_2795 | Putative MFS transporter |
| MAB_2355c | MSMEG_3140 | ABC transporter ATP-binding protein |
| MAB_2396 | MSMEG_2306 | Acetyltransferase |
| MAB_2595 | MSMEG_6576 | Pyridoxamine 5′-phosphate oxidase |
| MAB_2736c | MSMEG_3140 | ABC transporter |
| MAB_2780c | MSMEG_5187 | TetV efflux pump |
| MAB_3042c | MSMEG_2736 | GTP-binding protein |
| MAB_3078 | MSMEG_0091 | TetR transcriptional regulator |
| MAB_3467c | MSMEG_5611 | 18-kDa antigen (HSP 16.7) |
| MAB_3508c | MSMEG_1953 | whiB7 |
| MAB_3762 | MSMEG_1530 | Hypothetical protein |
| MAB_4294 | MSMEG_0688 | Aspartate aminotransferase |
CoA, coenzyme A; MFS, major facilitator superfamily.
MAB_4532c confers amikacin resistance in M. abscessus.
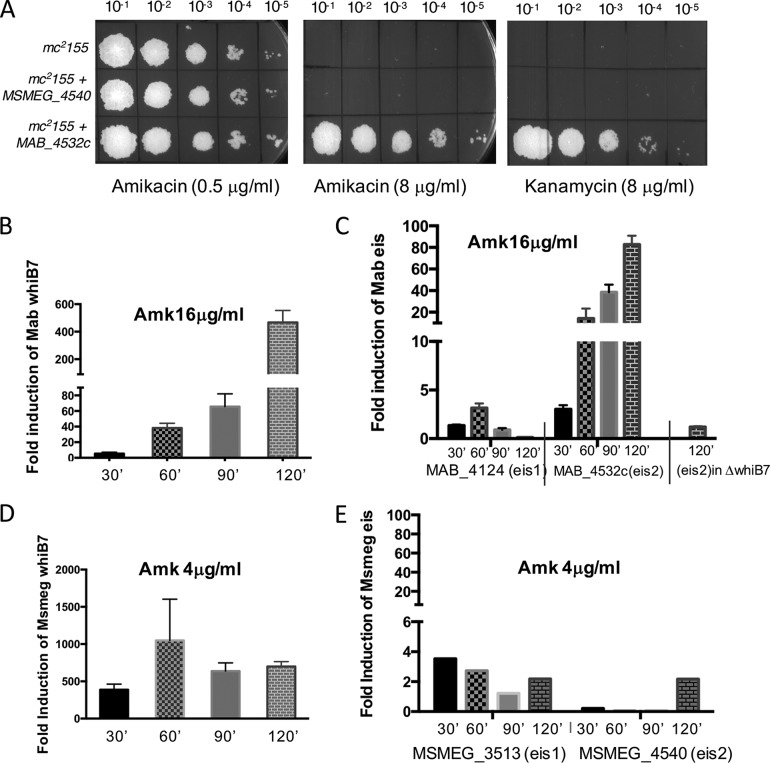
Amikacin is the front-line drug used in combination with clarithromycin against M. abscessus infections. However, intrinsic resistance to amikacin is ∼10-fold higher in M. abscessus than in M. smegmatis, despite the fact that whiB7 is upregulated in both species (more strongly in M. smegmatis) upon amikacin exposure (Fig. 6B and D). This could be due to one of two possibilities: (i) the M. abscessus-specific whiB7 regulon contains genes uniquely induced in M. abscessus but not in M. smegmatis which contribute to the enhanced amikacin resistance in M. abscessus, or (ii) amikacin induces genes outside the whiB7 regulon in M. abscessus that contribute to its increased tolerance to amikacin. In order to identify such determinants of M. abscessus that contribute to the 10-fold greater resistance to amikacin, we generated and introduced a partial M. abscessus genomic DNA library into M. smegmatis and screened for the appearance of growth on Middlebrook 7H10/OADC containing 8 μg/ml amikacin, a concentration that is lethal to M. smegmatis. We obtained 500 amikacin-resistant colonies (compared to none when using a control vector), of which 40 were amplified by PCR using junction primers and sequenced. All sequenced amikacin-resistant clones were found to contain MAB_4532c. MAB_4532c was recloned into an integrating vector and was confirmed to increase the amikacin resistance of wild-type M. smegmatis from 0.4 μg/ml to 8 μg/ml, as well as kanamycin resistance from 1 μg/ml to 8 μg/ml (Fig. 6A). However, resistance to streptomycin was unaffected (data not shown). This is in agreement with observations of Rominski et al., who recently showed that a deletion of MAB_4532c results in amikacin and kanamycin sensitivity (37).
FIG 6.
MAB_4532c is induced exclusively in M. abscessus as part of the whiB7 regulon and confers amikacin (Amk) resistance. (A) Heterologous expression of MAB_4532c (eis2) from a constitutive promoter integrated at the L5 attB site increases amikacin and kanamycin resistance of M. smegmatis from 0.4 μg/ml to 8 μg/ml. Similar overexpression of MSMEG_4540 (eis2) is insufficient to enhance amikacin/kanamycin resistance in M. smegmatis. (B to E) Wild-type M. smegmatis and M. abscessus and the ΔMabwhiB7 mutant were grown to an A600 of 0.7, exposed to sublethal concentrations of amikacin, as indicated, and the amount of whiB7, eis1, and eis2 transcripts were determined by qPCR and plotted as fold induction over an unexposed control. Data represent the mean ± SD, n = 3. Although whiB7 is induced in both bacteria, eis2 is induced only in M. abscessus in a whiB7-dependent manner and confers species-specific amikacin sensitivity.
Kanamycin resistance of M. tuberculosis has been previously attributed to a member encoding GNAT family acetyltransferases, eis1 (38). Sequence analysis suggests that MAB_4532c belongs to the GNAT family of acetyltransferases with 29% homology to M. abscessus eis1 (MAB_4124) and is referred to as eis2 (37). Interestingly, both paralogs of eis, eis1 (MSMEG_3513) and eis2 (MSMEG_4540), are found in M. smegmatis. MAB_4532c (eis2) is induced ∼100-fold upon amikacin exposure in M. abscessus in a whiB7-dependent manner (RNA-Seq in Data Set S1 and Fig. 6C), whereas MAB_4124 (eis1) is not amikacin inducible (Fig. 6C and S2). In contrast, while MSMEG_3513 (eis1) comprises the M. smegmatis WhiB7 regulon and is induced to low levels by amikacin, MSMEG_4540 (eis2) remains largely unresponsive and is also not a part of the M. smegmatis WhiB7 regulon (Fig. 6E and RNA-Seq in Data Set S1). Overexpression of MSMEG_4540 (eis2) from a constitutive promoter also does not increase amikacin or kanamycin resistance of M. smegmatis to the levels displayed by eis2 of M. abscessus, suggesting that although eis2 from the two species share ∼30% identity at the level of protein sequence, they are not functionally equivalent (Fig. 6A).
DISCUSSION
The influx of antibiotics into the mycobacterial cytosol induces a massive transcriptional reprogramming that results in changes in growth rate, metabolism, and induction of drug resistance genes. One of the best studied regulators of this reprogramming circuit is M. tuberculosis WhiB7, a transcriptional activator that regulates the expression of several genes involved in resistance to tetracycline, macrolides, and aminoglycosides (30, 31, 33, 34). Consistent with previous findings, our study here demonstrates an important role of WhiB7 in the intrinsic resistance of M. abscessus to several ribosome-targeting antibiotics. Similar to M. tuberculosis, whiB7 is the earliest gene induced in M. abscessus and M. smegmatis in response to ribosomal antibiotics and plays a critical role in the expression of downstream drug resistance effectors. Previous work by Ramón-García et al. demonstrates that the disruption of whiB7 from three different actinomycetes (M. smegmatis, Streptomyces lividans, and Rhodococcus jostii) results in different resistance profiles despite WhiB7 being functionally equivalent in these bacteria and is presumably due to the regulation of distinct sets of genes (31). In the present study, we have defined the whiB7 regulons of M. abscessus and M. smegmatis, which reveals a minimal overlap of 16 genes. This suggests that although WhiB7 is the master regulator in both species, the set of genes regulated by WhiB7 is specific to a given mycobacterial species. We hypothesize that the existence of species-specific repertoires of genes likely forms the basis of varying antibiotic susceptibilities within mycobacterial species. The extreme antibiotic resistance of M. abscessus can therefore be attributed to two classes of genes within the whiB7 regulon: (i) genes that lack orthologues in M. smegmatis, and (ii) genes that have orthologues in M. smegmatis but are not whiB7 inducible in M. smegmatis. This idea is further supported by the involvement of eis2 in amikacin and kanamycin resistance of M. abscessus. Amikacin and kanamycin induce eis2, but not eis1, via the whiB7 pathway in M. abscessus. M. smegmatis eis2 is not a part of the whiB7 regulon, even though amikacin is a fairly strong inducer of whiB7 in M. smegmatis. The differential amikacin sensitivity between the two species therefore occurs due to the species-specific inclusion of a gene with unique enzymatic properties, MAB_4532c (eis2), in the whiB7 regulon of M. abscessus. An in-depth study of species-specific patterns of gene induction could additionally account for the differences in drug susceptibilities to other antibiotics and between different mycobacterial species.
Our results further demonstrate that WhiB7 contributes differentially to resistance to different antibiotics. For example, the macrolides clarithromycin and erythromycin are potent inducers and cause near-saturating levels of induction of whiB7 within the first 30 min of exposure. Consistent with this rapid induction, a whiB7 mutant loses the distinctive resistance to these antibiotics and is suggestive of macrolide resistance being determined entirely by genes within the whiB7 regulon. In contrast, the deletion of MabwhiB7 results in a moderate reduction in streptomycin and tetracycline resistance, suggesting that genes outside the whiB7 regulon also play a role in the resistance to these antibiotics. Furthermore, induction of whiB7 by the synthetic aminoglycosides amikacin and kanamycin is gradual and only achieves high levels of expression upon extending the exposure time and concentration. This delayed induction of whiB7 and its downstream drug resistance determinants possibly overestimates the amikacin sensitivity of M. abscessus in in vitro drug susceptibility assays. The efficacy of amikacin in vivo therefore may not reflect in vitro conclusions. Moreover, our data suggest that the timing and rate of induction of whiB7 by clinically relevant antibiotics can affect the efficacy of the treatment regimen. The use of macrolides early in treatment would cause a rapid induction of whiB7 and compromise the outcome of amikacin therapy for M. abscessus infections. However, an initial amikacin treatment that is slow to induce MabwhiB7, followed up by macrolide treatment, is likely to enhance the effectiveness of this combined regimen and concomitantly reduce the duration of therapy.
MATERIALS AND METHODS
Media and strains.
Mycobacterium smegmatis was grown at 37°C in Middlebrook 7H9 (Difco) supplemented with 10% ADC and 0.05% Tween 20. Mycobacterium abscessus was grown at 37°C in Middlebrook 7H9 (Difco) supplemented with 10% OADC and 0.05% Tween 20 (Table 4). Antibiotics were added as required to the indicated amounts. Gene replacement mutants were constructed using recombineering, as described previously (35). The recombineering construct was generated by cloning in the multiple-cloning sites flanking the apramycin cassette of pYUB854. The left arm and right arms were generated using the primer pairs 5′-GTGCCTTTGTCGTCTTAAGCCGATCGC-3′/5′-GTGGAGGCCTCTAGATCT-3′ and 5′-AAGCTTCCCACTGCCCGA-3′/5′-GGCCAAAGCGGTCTGACTAGTAATCCATCACCTG-3′, respectively. Mutant clones were checked using Fcheck (5′-CGGAGACACCTTGTGGCGTGATGCC-3′) and Rcheck (CCCGGACAGCTGAACGTCCGG).
TABLE 4.
Strains and plasmids used in the present study
| Lab IDa | Strain | Strain description |
|---|---|---|
| M. abscessus | M. abscessus subsp. abscessus ATCC 19977 | Wild-type ATCC strain |
| MABPG1 | M. abscessus subsp. abscessus ATCC 19977 ΔMAB_3508c | Isogenic deletion in type strain |
| MABPG2 | M. abscessus subsp. abscessus ATCC 19977 ΔMAB_3508c/pMH95hspMab3508c | MABPG1 overexpressing MAB_3508c from Phsp60 integrated at L5 locus |
| MSPG1 | M. smegmatis ΔMSMEG_1953 | Isogenic deletion of MSMEG_1953 in type strain |
| MSPG2 | M. smegmatis ΔMSMEG_1953/pSJ25hspMSMEG_1953 | MSPG1 overexpressing MSMEG_1953 from Phsp60 integrated at Bxb1 locus |
ID, identification.
Antibiotic sensitivity assays.
Wild-type and whiB7 mutant strains of M. smegmatis and M. abscessus were grown to an A600 of 0.6 to 0.7. Cells were tested for their susceptibility to various antibiotics by spotting a 10-fold serial dilution initially on Middlebrook 7H10 (Difco) plates containing a range of each drug: tetracycline, 0.05 to 120 μg/ml; clarithromycin, 0.01 to 1.0 μg/ml; erythromycin, 4 to 40 μg/ml; spectinomycin, 1 to 256 μg/ml; streptomycin, 0.2 to 20 μg/ml; amikacin, 0.05 to 8 μg/ml; and kanamycin, 0.05 to 8 μg/ml. The concentration of antibiotic showing the best dynamic range in each case was then used in subsequent experiments. Antibiotic susceptibility in liquid media was assayed by inoculating the desired strain in a 2-fold dilution series of each antibiotic at an initial A600 of 0.0004. The cultures were incubated at 37°C, and the A600 was measured after 48 h for M. smegmatis and after 96 h for M. abscessus.
RNA preparation, quantitative PCR, and RNA-Seq analysis.
Total RNA was prepared from wild-type M. abscessus ATCC 19977, wild-type M. smegmatis mc2155, and the corresponding ΔwhiB7 mutant strains containing a chromosomally integrated copy of whiB7 expressed from a constitutive promoter and grown to exponential phase in Middlebrook 7H9-ADC/OADC using the Qiagen RNA preparation kit, followed by DNase I treatment. Approximately 5 μg of total RNA samples was treated with the Ribo-Zero rRNA removal procedure (Illumina) to enrich for mRNA. Approximately 500 ng of RNA was used for library preparation using the ScriptSeq version 2 RNA-Seq kit and high-throughput sequencing on the Illumina NextSeq platform. The sequence data were analyzed using Rockhopper, in which the data are normalized by upper-quartile normalization and transcript abundance is reported as reads per kilobase per million (RPKM). Differential gene expression is tested for each transcript, and q values are then reported that control the false-discovery rate (39, 40).
Wild-type M. abscessus, M. abscessus ΔwhiB7 mutant, and wild-type M. smegmatis were exposed to different concentrations of antibiotics for either 30 min or 3 h. Total RNA was prepared using the Qiagen RNA preparation kit, followed by DNase I treatment. Primers for quantitative reverse transcription-PCR (qRT-PCR) were generated using Primer Quest software (IDT). cDNA was generated using random hexamers and Maxima reverse transcriptase (Fisher Scientific), and qRT-PCR was performed using the Maxima SYBR green qPCR master mix (Fisher Scientific) using the following primer pairs: for MAB_3508c, 5′-CCTGTGGTTCGCGGAAA-3′/5′-CCCTGCTCAAGAATCTCACC-3′; for MAB_4532c, 5′-GAGCTTCATGTGCAAGAGGT-3′/GCGCCGTGATACTTGATCTT; for MAB_4124, 5′-CCCGTCAAGCCTTATGTAGTG-3′/5′-CAAGATCGTCAACGGATATGGT-3′; for MSMEG_4540, 5′-GCGACGATCGAAGTGGATG-3′/5′-GCCGGTTGTTGGTGTAGAT-3′; and for MSMEG_3513, 5′-GAACCCGAACAGACACAGG-3′/5′-GCTGCATGTCCAGGTACAG-3′. The Applied Biosystems 7300 real-time PCR system was used with cycling conditions of 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min.
Supplementary Material
ACKNOWLEDGMENTS
We thank The Wadsworth Center Genomics Core facility for sequencing of RNA-Seq libraries, The Bioinformatics Core for data analysis, and the Media Core for preparation of media and buffers. We also thank Keith Derbyshire and Anil Ojha for critical reading of the manuscript.
P.G. was supported by a New York Trust Community Grant and the Wadsworth Center.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/AAC.01353-17.
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01347-17.
REFERENCES
- 1.Leung JM, Olivier KN. 2013. Nontuberculous mycobacteria: the changing epidemiology and treatment challenges in cystic fibrosis. Curr Opin Pulm Med 19:662–669. doi: 10.1097/MCP.0b013e328365ab33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN. 2010. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qvist T, Gilljam M, Jonsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, Svahn A, Kotz K, Hansson L, Hollsing A, Hansen CR, Finstad PL, Pressler T, Hoiby N, Katzenstein TL, Scandinavian Cystic Fibrosis Study Consortium (SCFSC). 2015. Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros 14:46–52. doi: 10.1016/j.jcf.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, Edwards LJ, Chakraborti S, Knowles MR, Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. 2003. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 167:828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 5.Brown-Elliott BA, Wallace RJ Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benwill JL, Wallace RJ Jr. 2014. Mycobacterium abscessus: challenges in diagnosis and treatment. Curr Opin Infect Dis 27:506–510. doi: 10.1097/QCO.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 7.Cho YJ, Yi H, Chun J, Cho SN, Daley CL, Koh WJ, Shin SJ. 2013. The genome sequence of ‘Mycobacterium massiliense’ strain CIP 108297 suggests the independent taxonomic status of the Mycobacterium abscessus complex at the subspecies level. PLoS One 8:e81560. doi: 10.1371/journal.pone.0081560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sassi M, Drancourt M. 2014. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics 15:359. doi: 10.1186/1471-2164-15-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. 2012. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esther CR Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux AL, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, Bellis G, Vibet MA, Le Roux E, Lemonnier L, Gutierrez C, Vincent V, Fauroux B, Rottman M, Guillemot D, Gaillard JL, Jean-Louis Herrmann for the OMA Group. 2009. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol 47:4124–4128. doi: 10.1128/JCM.01257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodman DM, Polis JM, Heltshe SL, Sontag MK, Chacon C, Rodman RV, Brayshaw SJ, Huitt GA, Iseman MD, Saavedra MT, Taussig LM, Wagener JS, Accurso FJ, Nick JA. 2005. Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med 171:621–626. doi: 10.1164/rccm.200403-404OC. [DOI] [PubMed] [Google Scholar]
- 13.Catherinot E, Roux AL, Vibet MA, Bellis G, Ravilly S, Lemonnier L, Le Roux E, Bernede-Bauduin C, Le Bourgeois M, Herrmann JL, Guillemot D, Gaillard JL, OMA Group. 2013. Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J Cyst Fibros 12:74–80. doi: 10.1016/j.jcf.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Kothavade RJ, Dhurat RS, Mishra SN, Kothavade UR. 2013. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis 32:161–188. doi: 10.1007/s10096-012-1766-8. [DOI] [PubMed] [Google Scholar]
- 15.Nakanaga K, Hoshino Y, Era Y, Matsumoto K, Kanazawa Y, Tomita A, Furuta M, Washizu M, Makino M, Ishii N. 2011. Multiple cases of cutaneous Mycobacterium massiliense infection in a “hot spa” in Japan. J Clin Microbiol 49:613–617. doi: 10.1128/JCM.00817-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talati NJ, Rouphael N, Kuppalli K, Franco-Paredes C. 2008. Spectrum of CNS disease caused by rapidly growing mycobacteria. Lancet Infect Dis 8:390–398. doi: 10.1016/S1473-3099(08)70127-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee MR, Cheng A, Lee YC, Yang CY, Lai CC, Huang YT, Ho CC, Wang HC, Yu CJ, Hsueh PR. 2012. CNS infections caused by Mycobacterium abscessus complex: clinical features and antimicrobial susceptibilities of isolates. J Antimicrob Chemother 67:222–225. doi: 10.1093/jac/dkr420. [DOI] [PubMed] [Google Scholar]
- 18.Brown-Elliott BA, Nash KA, Wallace RJ Jr. 2012. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 25:545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Kim S, Park EM, Kim H, Kwon OJ, Chang CL, Lew WJ, Park YK, Koh WJ. 2008. In vitro antimicrobial susceptibility of Mycobacterium abscessus in Korea. J Korean Med Sci 23:49–52. doi: 10.3346/jkms.2008.23.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 21.Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. 2015. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 21:1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown-Elliott BA, Hanson K, Vasireddy S, Iakhiaeva E, Nash KA, Vasireddy R, Parodi N, Smith T, Gee M, Strong A, Barker A, Cohen S, Muir H, Slechta ES, Wallace RJ Jr. 2015. Absence of a functional erm gene in isolates of Mycobacterium immunogenum and the Mycobacterium mucogenicum group, based on in vitro clarithromycin susceptibility. J Clin Microbiol 53:875–878. doi: 10.1128/JCM.02936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash KA, Andini N, Zhang Y, Brown-Elliott BA, Wallace RJ Jr. 2006. Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrob Agents Chemother 50:3476–3478. doi: 10.1128/AAC.00402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash KA, Brown-Elliott BA, Wallace RJ Jr. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash KA, Zhang Y, Brown-Elliott BA, Wallace RJ Jr. 2005. Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum. J Antimicrob Chemother 55:170–177. doi: 10.1093/jac/dkh523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martiniano SL, Nick JA. 2015. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med 36:101–115. doi: 10.1016/j.ccm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen L, Thompson CJ. 2006. Foundations of antibiotic resistance in bacterial physiology: the mycobacterial paradigm. Trends Microbiol 14:304–312. doi: 10.1016/j.tim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 29.Soliveri JA, Gomez J, Bishai WR, Chater KF. 2000. Multiple paralogous genes related to the Streptomyces coelicolor developmental regulatory gene whiB are present in Streptomyces and other actinomycetes. Microbiology 146:333–343. [DOI] [PubMed] [Google Scholar]
- 30.Burian J, Ramon-Garcia S, Howes CG, Thompson CJ. 2012. WhiB7, a transcriptional activator that coordinates physiology with intrinsic drug resistance in Mycobacterium tuberculosis. Expert Rev Anti Infect Ther 10:1037–1047. doi: 10.1586/eri.12.90. [DOI] [PubMed] [Google Scholar]
- 31.Ramón-García S, Ng C, Jensen PR, Dosanjh M, Burian J, Morris RP, Folcher M, Eltis LD, Grzesiek S, Nguyen L, Thompson CJ. 2013. WhiB7, an Fe-S-dependent transcription factor that activates species-specific repertoires of drug resistance determinants in actinobacteria. J Biol Chem 288:34514–34528. doi: 10.1074/jbc.M113.516385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geiman DE, Raghunand TR, Agarwal N, Bishai WR. 2006. Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob Agents Chemother 50:2836–2841. doi: 10.1128/AAC.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burian J, Yim G, Hsing M, Axerio-Cilies P, Cherkasov A, Spiegelman GB, Thompson CJ. 2013. The mycobacterial antibiotic resistance determinant WhiB7 acts as a transcriptional activator by binding the primary sigma factor SigA (RpoV). Nucleic Acids Res 41:10062–10076. doi: 10.1093/nar/gkt751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris RP, Nguyen L, Gatfield J, Visconti K, Nguyen K, Schnappinger D, Ehrt S, Liu Y, Heifets L, Pieters J, Schoolnik G, Thompson CJ. 2005. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 102:12200–12205. doi: 10.1073/pnas.0505446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Kessel JC, Hatfull GF. 2007. Recombineering in Mycobacterium tuberculosis. Nat Methods 4:147–152. doi: 10.1038/nmeth996. [DOI] [PubMed] [Google Scholar]
- 36.Medjahed H, Reyrat JM. 2009. Construction of Mycobacterium abscessus defined glycopeptidolipid mutants: comparison of genetic tools. Appl Environ Microbiol 75:1331–1338. doi: 10.1128/AEM.01914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rominski A, Selchow P, Becker K, Brulle JK, Dal Molin M, Sander P. 2017. Elucidation of Mycobacterium abscessus aminoglycoside and capreomycin resistance by targeted deletion of three putative resistance genes. J Antimicrob Chemother 72:2191–2200. doi: 10.1093/jac/dkw466. [DOI] [PubMed] [Google Scholar]
- 38.Zaunbrecher MA, Sikes RD Jr, Metchock B, Shinnick TM, Posey JE. 2009. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 106:20004–20009. doi: 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tjaden B. 2015. De novo assembly of bacterial transcriptomes from RNA-seq data. Genome Biol 16:1. doi: 10.1186/s13059-014-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B. 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41:e140. doi: 10.1093/nar/gkt444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.