ABSTRACT
Spinal cord injury (SCI) often leads to substantial disability due to loss of motor function and sensation below the lesion. Neural stem cells (NSCs) are a promising strategy for SCI repair. However, NSCs rarely differentiate into neurons; they mostly differentiate into astrocytes because of the adverse microenvironment present after SCI. We have shown that myelin-associated inhibitors (MAIs) inhibited neuronal differentiation of NSCs. Given that MAIs activate epidermal growth factor receptor (EGFR) signaling, we used a collagen scaffold-tethered anti-EGFR antibody to attenuate the inhibitory effects of MAIs and create a neuronal differentiation microenvironment for SCI repair. The collagen scaffold modified with anti-EGFR antibody prevented the inhibition of NSC neuronal differentiation by myelin. After transplantation into completely transected SCI animals, the scaffold-linked antibodies induced production of nascent neurons from endogenous and transplanted NSCs, which rebuilt the neuronal relay by forming connections with each other or host neurons to transmit electrophysiological signals and promote functional recovery. Thus, a scaffold-based strategy for rebuilding the neuronal differentiation microenvironment could be useful for SCI repair.
KEYWORDS: biomaterials, EGFR, microenvironment, myelin associated inhibitors, neural stem cell, neuronal differentiation, spinal cord injury
INTRODUCTION
Spinal cord injury (SCI) is a devastating neurologic disorder that often leads to loss of voluntary motor function and sensation below the injury level. SCI initiates a cascade of biochemical reactions that cause continued and pervasive cell death and tissue damage.1 Inducing neural regeneration and reconstructing neurologic function after SCI remain global challenges. Neural stem cells (NSCs) are a type of multipotent cell that can self-renew and differentiate into neurons and glial cells. NSCs have been identified in and isolated from the brain, and some reports have demonstrated that NSCs also exist in the spinal cord.2,3 After SCI, quiescent NSCs are activated and migrate toward the injury site.4-6 Neuronal differentiation of NSCs residing in the spinal cord is regarded as a promising strategy for replenishing lost neurons after SCI. However, endogenous and exogenous transplanted NSCs rarely differentiate into neurons; they primarily differentiate into astrocytes and thus contribute to formation of a glial scar in the injury site, which greatly affects the therapeutic efficacy of NSCs for SCI repair.7-9 Rebuilding a regenerative microenvironment to promote neuronal differentiation of endogenous or exogenous NSCs is pivotal for the recovery of neurologic function after SCI. Our studies have revealed that a collagen scaffold modified with an anti-epidermal growth factor receptor (EGFR) antibody was able to rebuild a regenerative microenvironment that promoted neuronal differentiation of NSCs; the nascent neurons formed neuronal relays for SCI repair.6,10-12 Here, we summarize related research to highlight the importance of the neuronal differentiation microenvironment for SCI repair.
The adverse microenvironment present after SCI inhibits neuronal differentiation of NSCs
Although NSCs have been regarded as promising source cells for SCI repair, early clinical trials treating patients with SCI with embryonic stem cells or neural stem cells have not achieved the desired therapeutic effects. For example, the California biotech firm Geron began the first trial of embryonic stem cell therapy for patients with SCI in 2010, but shut down the program in 2011. In 2016, the company Stemcells announced the termination of a phase II clinical study of neural stem cell transplantation for SCI. Many previous animal studies have demonstrated that endogenous and exogenous transplanted NSCs rarely differentiate into neurons, but rather primarily differentiate into astrocytes at the injury site,13,14 which greatly impairs the therapeutic efficacy of NSCs for SCI repair.
The inhibitive pathophysiological microenvironment present after SCI may be a key factor affecting the therapeutic effects of stem cells in SCI repair. SCI leads to formation of an adverse microenvironment that inhibits axonal regeneration. Myelin-associated inhibitors (MAIs) including Nogo, oligodendrocyte myelin glycoprotein (OMgp) and myelin-associated glycoprotein (MAG) have been identified as the major components of this adverse microenvironment.15 Moreover, our previous study found that in addition to inhibiting axonal regeneration, MAIs also inhibited neuronal differentiation of NSCs.16 Nogo-A is a potent myelin-associated protein expressed mainly in oligodendrocytes, which has been identified as an inhibitor of neurite outgrowth in the central nervous system. We discovered that an active fragment of Nogo-A (Nogo-66) expressed on the cell surface inhibited neuronal differentiation of NSCs and promoted differentiation of NSCs into astrocytes. Nogo-66 receptor (NgR) is a glycosyl phosphatidyl inositol (GPI)-linked axonal surface protein that is also expressed in NSCs, which binds Nogo-66 with high affinity to inhibit axon growth. Using NEP1–40, a competitive antagonist of NgR, we demonstrated that NgR activation is involved in the inhibitory effect of Nogo-66 on neuronal differentiation of NSCs.
Small GTPases are critical signaling mediators involved in the inhibition of axonal regeneration by MAIs. MAIs activate the small GTPase RhoA and RhoA-associated kinase (ROCK) to mediate reorganization of cytoskeletal structures, thereby inhibiting neuronal axon growth. However, the RhoA–ROCK pathway is not involved in the inhibitory effect of Nogo-66 on neuronal differentiation of NSCs, as demonstrated by a study showing that a synthetic inhibitor of ROCK, Y27632, did not prevent Nogo-66-induced inhibition of NSC differentiation. We found that Nogo-66 inhibited neuronal differentiation by activating mammalian target of rapamycin (mTOR) and STAT316. These data provided the first evidence that MAIs inhibit neuronal differentiation of NSCs, and suggest that MAI antagonism might be a potential strategy for promoting neuronal differentiation after SCI.
Collagen scaffolds modified with anti-EGFR antibody prevent inhibition of neuronal differentiation of NSCs by myelin
EGFR is a transmembrane protein expressed in many cells. It is commonly activated by binding of its specific ligands. Upon activation, EGFR dimerization stimulates intracellular protein tyrosine kinase activity, which leads to activation of the MAPK/Akt pathway and thus modulates cell migration, adhesion and proliferation.17 Previous studies showed that MAIs promoted intracellular calcium influx and activated EGFR signaling.18,19 Activation of EGFR signaling by myelin is indispensable for inhibition of neurite outgrowth, and attenuating EGFR signaling with the small molecule inhibitor PD168393 improved locomotor and sensory function in a rat model of SCI.20
Because EGFR plays a critical role in mediating MAI signaling, we hypothesized that inhibiting EGFR function might prevent inhibition of neuronal differentiation of NSCs by MAIs after SCI. We have investigated the use of biomaterials combined with an anti-EGFR antibody to rebuild an NSC neuronal differentiation microenvironment (Table 1).6,10-12 A linearly ordered collagen scaffold with excellent biocompatibility and biodegradability, termed NeuroRegen scaffold, was developed to deliver functional biomolecules and stem cells and thus construct a favorable regenerative microenvironment at the site of SCI. The scaffold promoted axonal growth along its collagen fibers and inhibited scar tissue formation.21 The anti-EGFR antibody interfered with the binding of EGFR to its ligand and thus inhibited EGFR signaling. To avoid rapid diffusion of the anti-EGFR antibody from the collagen scaffold, the antibody was covalently conjugated to the scaffold. Traut's reagent was used to introduce sulfhydryl groups to the collagen scaffold, and then the heterobifunctional crosslinker Sulfo-SMCC was applied to conjugate the amine group of the anti-EGFR antibody to the sulfhydryl group of the collagen scaffold. The collagen scaffold-conjugated anti-EGFR antibody prevented the inhibition of neuronal differentiation of NSCs by myelin.12
TABLE 1.
Summary of collagen scaffolds modified with anti-EGFR antibodies for spinal cord injury repair.
| Scaffolds |
Molecules |
Cells |
Function |
Reference |
| NeuroRegen Scaffold | Chemical conjugated 151IgG, CBD-BDNF | None | Axon regeneration and electrophysiological recovery | Han et al. 201011 |
| Collagen scaffold | Chemical conjuagted Cetuximab | NSCs | Neuronal differentiation, and neuronal relay formation and locomotion function recovery | Li et al. 201312 |
| NeuroRegen Scaffold | CBD-EGFR-Fab | None | Endogenous neuronal differentiation, synaptic formation, myelination, electrophysiological and motor functional recovery | Fan et al. 20176 |
| NeuroRegen Scaffold | CBD-EGFR-Fab | NSCs | NSCs Retainment in SCI sites, neuronal differentiation, synapse formation, electrophysiological and motor functional recovery | Xu et al. 201710 |
Although chemical conjugation is a convenient method for achieving high levels of anti-EGFR antibody binding to a scaffold, it may affect the biologic activity of EGFR because of the formation of unexpected and excessive intermolecular covalent crosslinking. Our previous studies demonstrated that when multiple recombinant growth factors were tethered to a collagen scaffold via a collagen-binding domain (CBD) introduced by genetic engineering technology, the scaffold exhibited controlled release of the growth factors, and thus promoted repair of different tissue injuries.22-31 The binding between the CBD and the collagen scaffold imitated the interaction between collagen and collagenase, and the binding force between CBD and collagen was comparable to that between the ligand and receptor.32 Thus, this approach to achieving controlled release of functional proteins from a collagen scaffold has obvious advantages over chemical crosslinking. We therefore prepared a recombinant CBD–EGFR-Fab protein by fusing the CBD with the fragment antigen-binding (Fab) region of anti-EGFR antibody. The Fab molecule is a 50-kDa fragment of the anti-EGFR antibody that consists of light chain variable and constant domains and a truncated heavy chain, which retains the EGFR-binding ability of its parent antibody but penetrates tissues more quickly because of its lower molecular weight.33 A functional biomaterial composed of CBD–EGFR-Fab and NeuroRegen scaffold was prepared to promote neuronal differentiation of NSCs. We found that CBD–EGFR-Fab had higher binding affinity for the collagen scaffold than EGFR-Fab without CBD (NAT–EGFR-Fab), and achieved controlled release from the collagen scaffold. CBD–EGFR-Fab inhibited the phosphorylation of EGFR by myelin, which suggests that it blocked myelin-induced EGFR activation in NSCs. The NeuroRegen scaffold combined with CBD–EGFR-Fab prevented the inhibition of neuronal differentiation of cultured NSCs by myelin.6,10
Collagen scaffolds modified with anti-EGFR antibody promote neuronal differentiation in animal models of SCI
To evaluate the therapeutic effects of the collagen scaffold modified with anti-EGFR antibody in SCI, we established rat and canine models of complete spinal cord transection. Unlike previous models of SCI that used contusion, compression, dislocation or partial transection, our SCI model had a gap in the spinal cord—the spinal cord was completely separated and no spinal cord tissue was left in the lesion site. This produced a more uniform model that could be used to precisely evaluate the efficacy of biomaterial transplantation.
151IgG is an EGFR-neutralizing antibody that inhibits EGFR signaling activity. 151IgG conjugated to NeuroRegen scaffold using Traut's reagent and Sulfo-SMCC was combined with collagen-binding brain-derived neurotrophic factor (BDNF) (CBD–BDNF) to repair complete spinal cord transection in rats. When transplanted into rats with 6-mm complete spinal cord transection, this scaffold promoted axon regeneration and recovery of electrophysiological function.11 To explore the neuronal differentiation effects of anti-EGFR antibody in our animal model of SCI, we conjugated a clinical anti-EGFR antibody drug, Cetuximab, to the collagen scaffold. When implanted with exogenous NSCs into rats with 4-mm complete spinal cord transection, this scaffold increased neuronal differentiation and decreased astrocytic differentiation of the transplanted NSCs, and promoted neuronal relay formation and locomotor function recovery. Additionally, we created a canine model of SCI with 5-mm complete transection to examine the therapeutic effects of the NeuroRegen scaffold with Cetuximab. The NeuroRegen scaffold combined with Cetuximab improved locomotion recovery in this canine model of SCI (unpublished), which lays the foundation for future clinical studies.
In our recent studies, we evaluated the therapeutic outcome of NeuroRegen scaffold combined with CBD–EGFR-Fab in rats with 4-mm complete spinal cord transection. Given that massive activation of endogenous NSCs is observed in SCI sites, we implanted the NeuroRegen scaffold combined with CBD–EGFR-Fab to evaluate its potential for promoting neuronal differentiation of endogenous NSCs. Transplantation of the NeuroRegen scaffold combined with CBD–EGFR-Fab inhibited EGFR phosphorylation in endogenous NSCs in the SCI site and promoted their neuronal differentiation, and ultimately improved synapse formation, myelination, and recovery of electrophysiological and motor function in rats with SCI.6 The NeuroRegen scaffold combined with CBD–EGFR-Fab also promoted differentiation of exogenous NSCs into neurons in the SCI site. We found that CBD–EGFR-Fab bound to EGFR in NSCs and retained exogenous NSCs at the site of SCI. Moreover, it also promoted differentiation of implanted NSCs into multiple functional neurons and induced synapse formation between grafted and host neurons, which is necessary for recovery of electrophysiological and motor functional recovery after SCI.10
CONCLUSIONS AND PERSPECTIVES
SCI repair is challenging because of the inadequate regenerative ability of central nervous system axons and the inhibitive pathophysiological microenvironment present after SCI. The activation and neuronal differentiation of endogenous NSCs is critical for SCI repair. Our studies provide the first evidence that creating a neuronal differentiation microenvironment with biomaterials that attenuate MAI activitypromotes NSC differentiation for SCI repair. The nascent neurons derived from NSCs could form a neuronal relay, which could overcome the bottleneck problem caused by the inadequate axon regrowth ability of long tracts after SCI. Implantation of the NeuroRegen scaffold could inhibit scar tissue formation and guide the orientated growth of axons along the collagen fibers of the scaffold, which could also help nascent neurons to form a neuronal relay and reconnect with the transected host long tracts at the caudal and rostral segments of the spinal cord.
We propose that functional biomaterials composed of a collagen scaffold and CBD–EGFR-Fab could be used to create a regenerative microenvironment for SCI repair. The specific binding between collagen scaffold and CBD–EGFR-Fab could achieve prolonged and controlled release of CBD–EGFR-Fab. The scaffold may create a comparatively stable microenvironment to help attenuate the inhibitory effects of MAIs on neuronal differentiation. Additionally, a NeuroRegen scaffold composed of longitudinally arranged collagen fibers was used to inhibit scar tissue formation after SCI in our studies. The data demonstrated that the NeuroRegen scaffold inhibited deposition of chondroitin sulfate proteoglycans (CSPGs) in the SCI site. Because CSPGs are one of the main inhibitory molecules expressed by reactive cells after SCI, inhibition of CSPGs by the scaffold provide may allow axon regeneration. This would ultimately contribute to formation of neuronal relays between nascent and host neurons and promote recovery of neurologic function after SCI.
The corticospinal tract (CST) is an important motor nerve of the central nervous system that starts at the cortex and terminates at motor neurons, and controls the movement of the limbs and trunk. It is commonly thought that regeneration of CST fibers is critical for recovery of motor function after SCI. Many efforts have been made to induce long descending tract regeneration, but little evidence has been produced to demonstrate long-distance regeneration of CST fibers crossing the damaged area after SCI. The CST fibers did not regenerate across the lesion in our SCI model in our preliminary work. Thus, instead of CST regeneration, we propose that neuronal relay formation by differentiated functional neurons in the injured long tract axon might be the main mechanism for biomaterial-based SCI repair in our studies. A model of SCI repair was proposed in Fig. 1, endogenous NSCs proliferate and migrate toward the injury site immediately after SCI, but NSCs rarely differentiate into neurons because of the existence of MAIs around the lesion site. After transplantation with a functional scaffold composed of NeuroRegen scaffold and CBD–EGFR-Fab, NSCs differentiated into mature neurons that rebuilt the neuronal relay by forming connections with each other or with host neurons, and thus transmitted electrophysiological signals and promoted functional recovery of animals with SCI.
Figure 1.
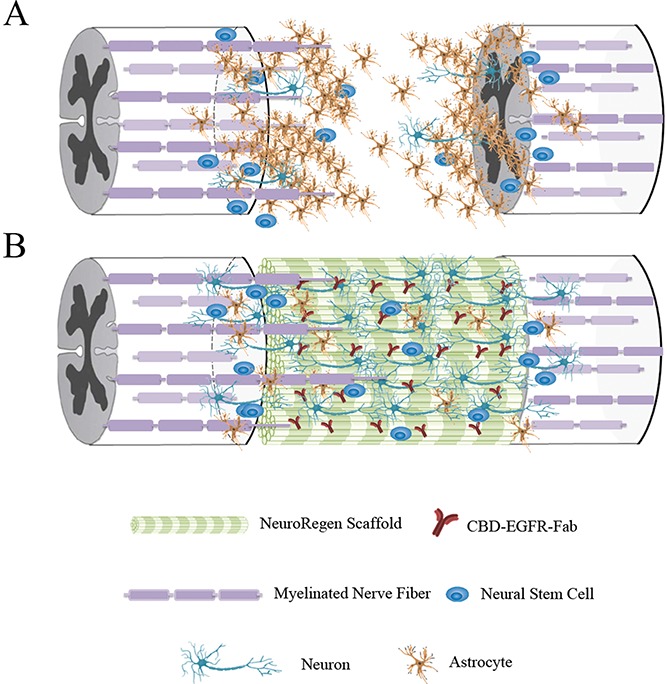
A model of SCI repair. NeuroRegen scaffold modified with CBD–EGFR-Fab promotes neuronal differentiation of neural stem cells (NSCs) for spinal cord injury (SCI) repair. (A) The adverse microenvironment at the site of SCI induces NSCs to differentiate into astrocytes rather than neurons. (B) Transplantation of NeuroRegen scaffold combined with CBD–EGFR-Fab promotes NSC differentiation into mature neurons that form a neuronal relay to bridge the injury gap and thus promote functional recovery in animals with SCI.
An in-depth understanding of the spinal cord microenvironment and the neuronal differentiation mechanism of NSCs would allow biomaterial-based strategies for creating a regenerative microenvironment to be optimized. Scaffold-based, precise, spatiotemporally controlled release of functional molecules to promote neuronal differentiation is promising for SCI repair.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- CBD
collagen-binding domain
- CSPGs
chondroitin sulfate proteoglycans
- CST
corticospinal tract
- EGFR
Epidermal growth factor receptor
- GPI
glycosyl phosphatidyl inositol
- MAG
myelin-associated glycoprotein
- MAIs
myelin associated inhibitors
- mTOR
mammalian target of rapamycin
- NgR
Nogo-66 receptor
- NSCs
neural stem cells
- Omgp
oligodendrocyte myelin glycoprotein
- ROCK
RhoA-associated kinase
- SCI
spinal cord injury
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This work was supported by a grant from the Key Research Program (Grant No. ZDRW-ZS-2016–2) and Youth Innovation Promotion Association (Grant No. 2016096) of the Chinese Academy of Sciences.
REFERENCES
- [1].Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 2014; 114:25-57; PMID:24269804; https://doi.org/ 10.1016/j.pneurobio.2013.11.002 [DOI] [PubMed] [Google Scholar]
- [2].Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992; 255:1707-10; PMID:1553558; https://doi.org/ 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- [3].Shihabuddin LS. Adult rodent spinal cord derived neural stem cells. Isolation and characterization. Methods Mol Biol 2002; 198:67-77 [DOI] [PubMed] [Google Scholar]
- [4].Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 2005; 131:177-87; PMID:15680701; https://doi.org/ 10.1016/j.neuroscience.2004.10.011 [DOI] [PubMed] [Google Scholar]
- [5].Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisén J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 2008; 6:e182; PMID:18651793; https://doi.org/ 10.1371/journal.pbio.0060182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fan C, Li X, Xiao Z, Zhao Y, Liang H, Wang B, Han S, Li X, Xu B, Wang N, et al.. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater 2017; 51:304-16; PMID:28069497; https://doi.org/ 10.1016/j.actbio.2017.01.009 [DOI] [PubMed] [Google Scholar]
- [7].Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010; 7:470-82; PMID:20887953; https://doi.org/ 10.1016/j.stem.2010.07.014 [DOI] [PubMed] [Google Scholar]
- [8].Sabelstrom H, Stenudd M, Reu P, Dias DO, Elfineh M, Zdunek S, Damberg P, Göritz C, Frisén J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science 2013; 342:637-40; PMID:24179227; https://doi.org/ 10.1126/science.1242576 [DOI] [PubMed] [Google Scholar]
- [9].Liu Y, Tan B, Wang L, Long Z, Li Y, Liao W, Wu Y. Endogenous neural stem cells in central canal of adult rats acquired limited ability to differentiate into neurons following mild spinal cord injury. Int J Clin Exp Pathol 2015; 8:3835-42; PMID:26097566 [PMC free article] [PubMed] [Google Scholar]
- [10].Xu B, Zhao Y, Xiao Z, Wang B, Liang H, Li X, Fang Y, Han S, Li X, Fan C, et al.. A dual functional scaffold tethered with EGFR antibody promotes neural stem cell retention and neuronal differentiation for spinal cord injury repair. Adv Healthc Mater 2017; 6(9): https://doi.org/ 10.1002/adhm.201601279 [DOI] [PubMed] [Google Scholar]
- [11].Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W, Chen L, Zhao Y, et al.. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials 2010; 31:9212-20; PMID:20869112; https://doi.org/ 10.1016/j.biomaterials.2010.08.040 [DOI] [PubMed] [Google Scholar]
- [12].Li X, Xiao Z, Han J, Chen L, Xiao H, Ma F, Hou X, Li X, Sun J, Ding W, et al.. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials 2013; 34:5107-16; PMID:23591390; https://doi.org/ 10.1016/j.biomaterials.2013.03.062 [DOI] [PubMed] [Google Scholar]
- [13].Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol 2001; 167:48-58; PMID:11161592; https://doi.org/ 10.1006/exnr.2000.7536 [DOI] [PubMed] [Google Scholar]
- [14].Yang H, Lu P, McKay HM, Bernot T, Keirstead H, Steward O, Gage FH, Edgerton VR, Tuszynski MH. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci 2006; 26:2157-66; PMID:16495442; https://doi.org/ 10.1523/JNEUROSCI.4070-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci 2003; 4:703-13; PMID:12951563; https://doi.org/ 10.1038/nrn1195 [DOI] [PubMed] [Google Scholar]
- [16].Wang B, Xiao Z, Chen B, Han J, Gao Y, Zhang J, Zhao W, Wang X, Dai J. Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS One 2008; 3:e1856; PMID:18365011; https://doi.org/ 10.1371/journal.pone.0001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004; 59:21-6; PMID:15142631; https://doi.org/ 10.1016/j.ijrobp.2003.11.041 [DOI] [PubMed] [Google Scholar]
- [18].Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, et al.. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 2005; 310:106-10; PMID:16210539; https://doi.org/ 10.1126/science.1115462 [DOI] [PubMed] [Google Scholar]
- [19].Giger RJ, Venkatesh K, Chivatakarn O, Raiker SJ, Robak L, Hofer T, Lee H, Rader C. Mechanisms of CNS myelin inhibition: evidence for distinct and neuronal cell type specific receptor systems. Restor Neurol Neurosci 2008; 26:97-115; PMID:18820405 [PMC free article] [PubMed] [Google Scholar]
- [20].Erschbamer M, Pernold K, Olson L. Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J Neurosci 2007; 27:6428-35; PMID:17567803; https://doi.org/ 10.1523/JNEUROSCI.1037-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin H, Chen B, Wang B, Zhao Y, Sun W, Dai J. Novel nerve guidance material prepared from bovine aponeurosis. J Biomed Mater Res A 2006; 79:591-8; PMID:16817216; https://doi.org/ 10.1002/jbm.a.30862 [DOI] [PubMed] [Google Scholar]
- [22].Shi J, Sun J, Zhang W, Liang H, Shi Q, Li X, Chen Y, Zhuang Y, Dai J. Demineralized bone matrix scaffolds modified by CBD-SDF-1alpha promote bone regeneration via recruiting endogenous stem cells. ACS Appl Mater Interfaces 2016; 8:27511-27522; https://doi.org/ 10.1021/acsami.6b08685 [DOI] [PubMed] [Google Scholar]
- [23].Jia W, Tang H, Wu J, Hou X, Chen B, Chen W, Zhao Y, Shi C, Zhou F, Yu W, et al.. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015; 69:45-55; PMID:26280949; https://doi.org/ 10.1016/j.biomaterials.2015.08.009 [DOI] [PubMed] [Google Scholar]
- [24].Cao J, Xiao Z, Jin W, Chen B, Meng D, Ding W, Han S, Hou X, Zhu T, Yuan B, et al.. Induction of rat facial nerve regeneration by functional collagen scaffolds. Biomaterials 2013; 34:1302-10; PMID:23122676; https://doi.org/ 10.1016/j.biomaterials.2012.10.031 [DOI] [PubMed] [Google Scholar]
- [25].Shi C, Chen W, Zhao Y, Chen B, Xiao Z, Wei Z, Hou X, Tang J, Wang Z, Dai J. Regeneration of full-thickness abdominal wall defects in rats using collagen scaffolds loaded with collagen-binding basic fibroblast growth factor. Biomaterials 2011; 32:753-9; PMID:20937527; https://doi.org/ 10.1016/j.biomaterials.2010.09.038 [DOI] [PubMed] [Google Scholar]
- [26].Liang W, Han Q, Jin W, Xiao Z, Huang J, Ni H, Chen B, Kong J, Wu J, Dai J. The promotion of neurological recovery in the rat spinal cord crushed injury model by collagen-binding BDNF. Biomaterials 2010; 31:8634-41; PMID:20716462; https://doi.org/ 10.1016/j.biomaterials.2010.07.084 [DOI] [PubMed] [Google Scholar]
- [27].Fan J, Xiao Z, Zhang H, Chen B, Tang G, Hou X, Ding W, Wang B, Zhang P, Dai J, et al.. Linear ordered collagen scaffolds loaded with collagen-binding neurotrophin-3 promote axonal regeneration and partial functional recovery after complete spinal cord transection. J Neurotrauma 2010; 27:1671-83; PMID:20597688; https://doi.org/ 10.1089/neu.2010.1281 [DOI] [PubMed] [Google Scholar]
- [28].Chen W, Shi C, Yi S, Chen B, Zhang W, Fang Z, Wei Z, Jiang S, Sun X, Hou X, et al.. Bladder regeneration by collagen scaffolds with collagen binding human basic fibroblast growth factor. J Urol 2010; 183:2432-9; PMID:20403614; https://doi.org/ 10.1016/j.juro.2010.02.042 [DOI] [PubMed] [Google Scholar]
- [29].Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H, Chen B, Xiao Z, Dai J. The effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials 2009; 30:4649-56; PMID:19573907; https://doi.org/ 10.1016/j.biomaterials.2009.05.037 [DOI] [PubMed] [Google Scholar]
- [30].Sun W, Lin H, Xie H, Chen B, Zhao W, Han Q, Zhao Y, Xiao Z, Dai J. Collagen membranes loaded with collagen-binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth Factors 2007; 25:309-18; PMID:18236209; https://doi.org/ 10.1080/08977190701803885 [DOI] [PubMed] [Google Scholar]
- [31].Chen B, Lin H, Wang J, Zhao Y, Wang B, Zhao W, Sun W, Dai J. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials 2007; 28:1027-35; PMID:17095085; https://doi.org/ 10.1016/j.biomaterials.2006.10.013 [DOI] [PubMed] [Google Scholar]
- [32].Huang X, Li X, Wang Q, Dai J, Hou J, Chen L. Single-molecule level binding force between collagen and collagen binding domain-growth factor conjugates. Biomaterials 2013; 34:6139-46; PMID:23706541; https://doi.org/ 10.1016/j.biomaterials.2013.04.057 [DOI] [PubMed] [Google Scholar]
- [33].Liang H, Li X, Wang B, Chen B, Zhao Y, Sun J, Zhuang Y, Shi J, Shen H, Zhang Z, et al.. A collagen-binding EGFR antibody fragment targeting tumors with a collagen-rich extracellular matrix. Sci Rep 2016; 6:18205; PMID:26883295; https://doi.org/ 10.1038/srep18205 [DOI] [PMC free article] [PubMed] [Google Scholar]


