Abstract
In synthetic biology, researchers assemble biological components in new ways to produce systems with practical applications. One of these practical applications is control of the flow of genetic information (from nucleic acid to protein), a.k.a. gene regulation. Regulation is critical for optimizing protein (and therefore activity) levels and the subsequent levels of metabolites and other cellular properties. The central dogma of molecular biology posits that information flow commences with transcription, and accordingly, regulatory tools targeting transcription have received the most attention in synthetic biology. In this mini-review, we highlight many past successes and summarize the lessons learned in developing tools for controlling transcription. In particular, we focus on engineering studies where promoters and transcription terminators (cis-factors) were directly engineered and/or isolated from DNA libraries. We also review several well-characterized transcription regulators (trans-factors), giving examples of how cis- and trans-acting factors have been combined to create digital and analogue switches for regulating transcription in response to various signals. Last, we provide examples of how engineered transcription control systems have been used in metabolic engineering and more complicated genetic circuits. While most of our mini-review focuses on the well-characterized bacterium Escherichia coli, we also provide several examples of the use of transcription control engineering in non-model organisms. Similar approaches have been applied outside the bacterial kingdom indicating that the lessons learned from bacterial studies may be generalized for other organisms.
Keywords: Promoter, Transcription, Synthetic biology, Bacteria, Termination, Metabolic engineering
1. Introduction
A central tenet of synthetic biology is the ability to assemble a practical system from biological components. In most cases, the components are proteins synthesized from genetic information encoded in DNA. One of the challenges faced by synthetic biologists is designing the appropriate genetic sequence to control protein expression at the level that optimizes system function. The central dogma of molecular biology (Fig. 1) describes the flow of genetic information from nucleic acid polymers (DNA and RNA) to amino acid polymers (proteins) [1]. Transcription is the process during which information encoded in DNA is converted to a transient carrier of information, mRNA. Translation is the process in which the mRNA code is converted to an amino acid polymer by ribosomes, themselves RNA catalysts (ribozymes) [2], [3], [4], [5]. The flow of genetic information terminates with the production of a protein, but often post-translational modifications and processes (see below) are needed to produce the final functional component. To optimize the abundance and other properties of the protein and the performance of the ultimate application, the rate and timing of each of these processes can be controlled by both cis-acting (within or adjacent to the protein coding sequence) and trans-acting (separate from the protein coding sequence) components.
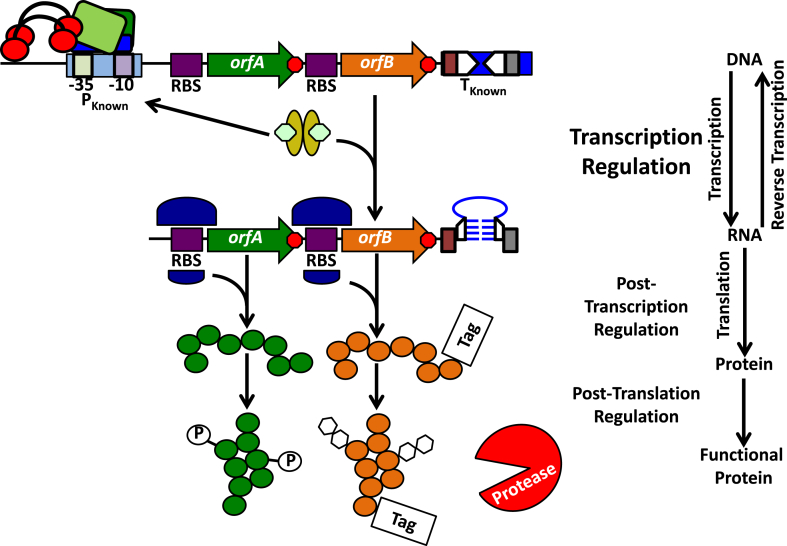
Fig. 1.
Overview of the central dogma of molecular biology. An operon encoding orfA and orfB is under the control of the indicated promoter (PKnown), which is engaged by the RNA polymerase holoenzyme (green and blue boxes, red circles, and black arcs). Sequences that will be transcribed into the ribosome binding sites (RBSs) for orfA and orfB (purple boxes) and the transcription terminator (TKnown; two inverted repeats [inverted arrowheads] flanked by a poly A-tract [red box] and poly U-tract [grey box]) are indicated. Red octagons represent translation stop codons. At the level of transcription regulation, alterations to the promoter and terminator sequences, the presence of transcription activators and repressors (depicted as golden ovals with green hexagon ligands bound), and nucleoid/supercoiling states may all influence orfA and orfB transcription (not shown). Through the process of transcription itself, the associated orfA and orfB mRNA is produced, which may in turn be bound by ribosomes (depicted as two variable size purple half circles) at the RBS. To terminate transcription, the depicted terminator sequence forms a stem-loop (blue), mediated by base pairing/stacking with the complementary sequences of the former inverted repeats (half arrowheads), and mRNA synthesis ceases at this site (poly A- and ploy U-tracts are indicated as above). Modifying RBS strength, RNA riboswitches, aptazymes (aptamer ribozymes), and RNA stability may each contribute to post-transcription regulation (not shown). Folding of a translated protein (green and orange circles) with the assistance of folding chaperones (not shown), enzymes that promote post-translation modifications such as phosphorylation (P in a white circle) or glycosylation (white hexagons), the presence of proteolytic enzymes (indicated in the figure and potentially targeted to a protein through a specific motif [Tag]) represent methods of post-translation regulation.
Transcriptional control is a prevalent mode of regulating protein production because it is the first step in the process. Accordingly, it has received the most attention in terms of tool development and engineering applications. Transcriptional regulation allows a cell to allocate its valuable resources towards production of proteins when these proteins would increase fitness in a given environment. Furthermore, transcriptional regulation also provides a method for the cell to control the levels/stoichiometry of enzymes to avoid futile cycles, underutilized enzymes, build-up of undesired/toxic metabolites, and assembly of macromolecular structures. Over the last several decades, researchers have assembled methodologies for creating tools that enable fine-tuning of gene expression without the evolution time required to optimize natural systems. These tools have been applied to many organisms and heterologous systems. Indeed, fine tuning the levels of enzymes and other proteins has proven to be essential to many applications in metabolic engineering [6], [7], [8], [9], [10], [11], [12], [13], [14], [15] and common synthetic biology systems such as toggle switches and Boolean logic circuits [16], [17], [18], [19], [20], [21], [22], [23], cellular memory and calculation elements [24], [25], [26], [27], and regulatory oscillators [13], [20], [28]. Promoter and terminator libraries [12], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40] have enabled many of these applications.
This mini-review will describe elements of transcriptional control and applications for engineered systems, but many control topics are beyond its scope. Although we will focus primarily on well-characterized examples of transcriptional control in model bacteria (e.g., Escherichia coli), we acknowledge that these transcriptional control methods are being increasingly extended to non-model organisms and eukaryotes. We are confident that the approaches developed for E. coli and other simple systems will lead to the creation of transcription tools for these organisms and find use in both metabolic engineering and synthetic biology applications. While this review will focus on transcription control, there are additional layers of control over protein expression at the post-transcriptional (e.g., riboswitches/toehold RNA switches [41], [42], [43], [44], RNA splicing/ribozymes [9], [45], [46], [47], and at the level of modifying ribosome binding site [RBS] strength [48], [49]) and post-translational levels (e.g., proteolytic activation/inactivation [50], [51], [52], [53], [54], [55], [56], glycosylation [57], [58], [59], [60], phosphopantetheine addition [61], [62], [63], phosphorylation [64], [65], [66], [67], [68], quaternary structure formation [69], [70], [71], [72], [73], [74], protease tags [75], and folding chaperones [76]). However, these are methods beyond the intended scope of this mini-review. Likewise, most genes in bacteria are arranged in operons [77], [78], [79], [80], [81]. In addition to understanding promoter strength and transcription termination (discussed here, but in a different context), engineering synthetic operons requires careful RBS design and positioning [48], [49]; an understanding of gene positioning, order, and orientation [78], [79], [80], [81]; an understanding of RNA stability and structure [82], [83], [84]; and an understanding of intergenic DNA and RNA sequences (related to the other operon engineering considerations) [29], [30], [85]. These too are all beyond the scope of this mini-review. Please note that transcription and translation are coupled in prokaryotes (i.e., simultaneously occurring in the same location). Intriguingly, once these processes are decoupled, transcripts become rapidly inactivated through such mechanisms as alterations to the RNA structure, RNA degradation, and forming RNA-DNA R-Loops [86], [87], [88], [89], [90], [91]. Furthermore, the bacterial nucleoid structure and DNA supercoiling surely play an overwhelming [92], [93], [94], [95], [96], [97], [98], but still poorly understood, function during transcription regulation. Nevertheless, transcription-translation decoupling, DNA structure, and supercoiling are all beyond the intentional scope of this mini-review.
2. Prokaryotic promoters, transcription initiation, termination, and tools
A logical point to begin any discussion of transcription regulation is at the level of the machinery and DNA/RNA sequence elements that facilitate mRNA transcript production. In particular, the machinery referred to here includes bacterial RNA polymerase (RNAP) holoenzymes. The DNA/RNA sequence elements referred to above include promoters and terminators. Remarkably, the components of the RNAP holoenzyme, α2ββ′ωσ, are conserved among bacteria [74], [99], [100] and have similar characteristics to eukaryotic and viral RNA polymerases [70], [73], [99], [100]. Additionally, RNAP holoenzymes have different activities toward different promoters and terminators [16], [35], [36], [101], [102], [103], [104], [105], [106], [107], [108], which motivates promoter and terminator library development.
2.1. Elements of promoter recognition by RNA polymerases
Among the well-characterized prokaryotic promoters are those promoters recognized by σ70 (sigma-70) and other group 1 sigma factors. Indeed, group 1 sigma factors represent those factors that drive the expression of genes necessary for cell viability and other housekeeping activities [109], [110], [111]. In E. coli, σ70 is the principal sigma factor (σA is the principal sigma factor in other prokaryotes) [109], [110], [111]. Although there are many additional sigma factors that serve important functions under a variety of different cellular conditions [109], [110], [111], [112], [113], we will primarily focus on σ70- or σA-regulated promoters.
σ70, in complex with RNAP, recognizes key promoter sequences during early events of transcription initiation (for additional descriptions of transcription initiation and the anatomy of RNAP holoenzyme-promoter interactions please see Fig. 2A, Fig. 2B, and references [70], [71], [72], [110], [111], [114], [115], [116], [117], [118], [119], [120], [121]). This sigma factor is further sub-divided into four regions; elements of sub-region 4 (sub-region 4.2) and 2 (sub-regions 2.3 and 2.4) are involved in recognizing the −35 and −10 regions of a typical σ70 promoter, respectively [70], [71], [72], [109], [110], [111], [114], [115], [120], [121]. The −35 sequence has the consensus sequence TTGACA, while the −10 sequence has the consensus sequence (TATAAT) [121], [122], [123]. An additional TGN sequence upstream of the −10 sequence (the extended −10 sequence) is present in some bacterial promoters and is recognized by the 3.0 sub-region of σ70 [70], [71], [72], [111], [114], [115], [120], [121]. The optimal spacing between the end of the −35 sequences and the beginning of the −10 sequences is 17-bp (base pairs), which is consistent with sub-regions 2, 3, and 4 positioning when σ70 is docked into the RNAP holoenzyme [70], [71], [72], [109], [110], [111], [114], [120], [121], [122], [123]. In addition, a 6- to 8-bp variable region (recognized by the 1.2 sub-region of σ70) immediately downstream of the −10 sequence (the discriminator) also serves a function during early transcription initiation [71], [72], [110], [111], [114], [117], [118], [119], [120], [121].
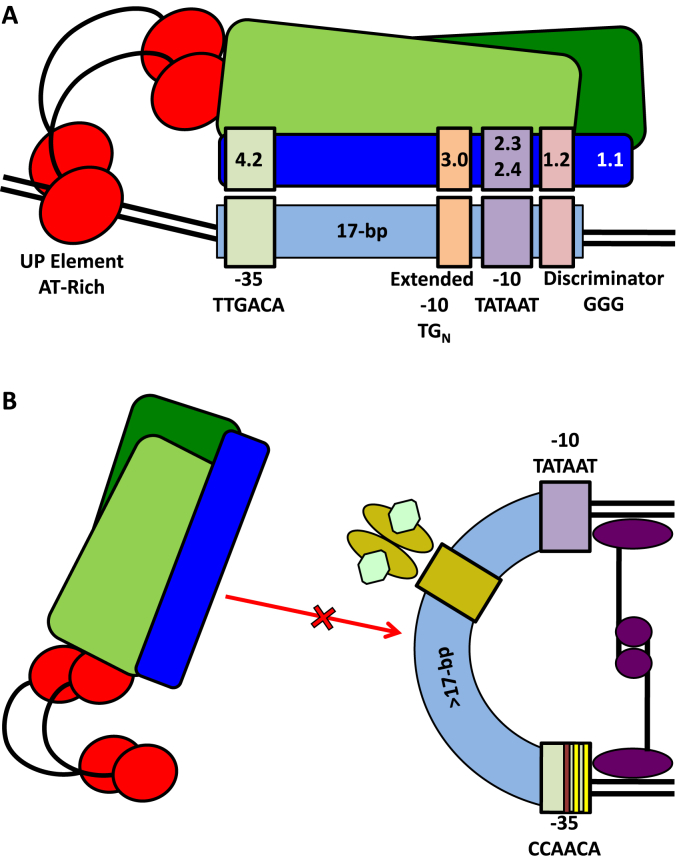
Fig. 2.
A representation of RNA polymerase holoenzyme-promoter interactions. (A) RNAP β and β′ subunits are indicated by green boxes. The RNAP α subunits (red circles and black arcs) are in contact with the bent upstream UP element. σ70 (dark blue box) sub-region 4.2 (pale green), 3.0 (pale orange), 2.3/2.4 (pale purple), 1.2 (pale pink), and 1.1 are associated with the indicated −35 sequence (pale green), extended −10 sequence (pale orange), −10 sequence (pale purple), discriminator (pale pink), and double stranded DNA, respectively. In this case, promoter elements are separated by 17-bp. (B) RNAP holoenzyme promoter binding is obstructed (red arrow and a red cross) by DNA bending mediated by nucleoid structuring factors or transcription factors (purple ovals, circles, and lines), other transcription regulators (golden ovals with green hexagon ligands bound) bound to operator sequences (gold box), spacing greater than 17-bp between the −35 and −10 sequences, and a −35 sequence deviating from the canonical −35 sequence (yellow and red bars). These figures are modified from the work by Browning and Busby [121].
Optimal spacing and function of promoter sequences reflects the position of individual components in the RNAP holoenzyme both during promoter recognition and subsequent transcription initiation events. Briefly, during the early stages of promoter recognition, the RNAP holoenzyme α2 subunits CTDs (carboxyl-terminal domains) bind to the promoter UP elements (an AT-rich sequence [114], [121], [124], [125]), the σ70 sub-region 4.2 (proximal to the α2 subunits CTDs) associates with the promoter −35 sequence, and sub-regions 2.3 and 2.4 associate with the −10 sequence [70], [71], [72], [109], [110], [111], [114], [115], [120], [121]. A linker region between σ70 sub-region 4.2 and sub-regions 2.3, 2.4, and 3.0 accounts for the spacing between the −35 and −10 sequences (i.e., the linker is in an extended conformation when σ70 is docked into the RNAP holoenzyme) [70], [71], [72], [109], [110], [111], [114], [120], [121]. The clamp and jaw formed by the β and β′ subunits of the RNAP holoenzyme, respectively, are in an open conformation, while promoter DNA at this point is still in a closed duplex (referred to as the closed complex) [71], [72], [114], [126]. After initial contact with a promoter, DNA is bent around the RNAP holoenzyme; first the upstream portion of DNA proximal to the α2 subunits CTDs is bent, followed by bending of the region immediately upstream of the −10 sequence through the −10 sequence itself [70], [71], [72], [114]. During this bending, σ70 sub-region 1.2 is in contact with the discriminator, base stacks in duplex DNA are disrupted in the AT-rich −10 sequence, the base at position −11 (usually an A) is flipped into σ70 sub-region 2.3, and the −11/-10 nucleotide through the transcription initiation site (+1) (usually initiated with a purine [122], [127]) and adjacent bases (+2/+3) of duplex DNA are eventually dehybridized (i.e., this is step is kinetically limiting) forming the open complex [70], [71], [72], [111], [114], [115], [117], [120], [121], [128]. A separation between the clamp and jaw of RNAP, where central Mg2+ ions are coordinated by amino acids found in the β′ subunit and water molecules coordinated by amino acids found in both the β′ and β subunits [70], [71], [72], [74], [114], forms the active site cleft. The single stranded transcriptional start site (on the template strand), typically seven- or eight-nucleotides downstream of the −10 sequence [70], [71], [72], [110], [114], [120], is positioned into the active site cleft, and isomerization events result in a tightening (closing) of the RNAP jaw and clamp [71], [72], [114], [126]. Intriguingly, recent work shows that the RNAP jaw and clamp may transiently close during initial promoter recognition and reopen before the final tightening above [126]. Discriminator sequence-RNAP holoenzyme contacts presumably assist in positioning the template strand (i.e., open DNA strands may be scrunched into this cleft with different DNA-RNAP holoenzyme interactions leading to transcription initiation sites different from the typical seven- or eight- nucleotides downstream of the −10 sequence noted above) [71], [72], [114], [116], [119], [120].
The discriminator also serves a function in mediating open complex stability and may influence length of the initially transcribed RNA polymer at the time of promoter escape. As noted above, there is no consensus sequence for the discriminator region [116], [118]. Therefore, it instead seems that interactions between open DNA and σ70 sub-region 1.2, the presence of G/(G/C) bases at positions −6/-5, interactions between DNA and σ70 sub-region 1.1, continued interactions between σ70 and the aforementioned promoter elements above, spacing of these promoter elements, and other interactions between open- and closed-form DNA and RNAP holoenzyme subunits all likely contribute to recognition of the discriminator sequence [70], [71], [72], [110], [111], [114], [115], [116], [117], [118], [119], [120], [121]. It has recently been demonstrated that open complexes at certain promoters have discriminator regions that mediate stable open complexes and increased length of abortive, and presumably initial escape transcripts, while other promoters with discriminators that mediate unstable open complexes have shorter length abortive and initial escape transcripts [116]. The reason for these differences is likely accounted for by weaker discriminator sequence interactions with the RNAP holoenzyme along with needing to prescrunch these sequences into the RNAP active site cleft to make any productive interactions [70], [116], [118], [119]. Thus, relieving stress buildup resulting from scrunching open complex DNA into the active site cleft may be a significant driver of RNAP promoter escape [116], [119], [129], [130].
2.2. Promoter libraries
RNAP holoenzyme activity can vary over a wide range depending on the sequence of a series of promoters [16], [36], [101], [102], [103], [104], [105]. This correlation has been used to tune control of gene expression at the level of promoter recognition and transcription initiation. Constitutive promoters under the control of σ70, in contrast to non-constitutive inducible and repressible promoters (see the Transcription Factors section below), usually direct comparable levels of gene expression independent of environmental and regulator context. Although libraries of characterized non-constitutive promoters exist [33], [34], [38], [39], [40] (http://parts.igem.org/Promoters/Catalog/Ecoli/Multiple, http://parts.igem.org/Promoters/Catalog/Ecoli/Repressible, http://parts.igem.org/Promoters/Catalog/Ecoli/Positive), this mini-review will primarily focus on constitutive promoter libraries (e.g., http://parts.igem.org/Promoters/Catalog/Ecoli/Constitutive). These constitutive promoters are often characterized as being weak (yielding low levels of reporter gene expression) or strong (yielding high levels of reporter gene expression). Although weak promoters often deviate from the −35 and −10 consensus sequences and typical element spacing (strong promoters closely match these consensus sequences and typical element spacing), considerable variability may still exist between promoters with identical or nearly identical −35 and −10 elements [32], [37], [101], [102], [105], [131]. In addition, interactions between the 5′ UTRs (untranslated regions) and identical promoters contribute to gene expression variability, suggesting that promoter context is as important as the −35 and −10 sequences [29], [30].
Promoter libraries are usually generated either through synthesizing putative constitutive promoter sequences [29], [33], [37], randomly mutating the promoter sequence [12], [34], or generating libraries of putative promoter inserts [29], [30], [36]. For instance, Jensen et al. [37] demonstrated that a synthetic promoter sequence library originally engineered for Lactococcus lactis (see below) and inserted upstream of lacZ (encoding β-galactosidase), could be used in E. coli to titrate lacZ expression over four different orders of magnitude. This library was synthesized with changes primarily to regions flanking the −35 and −10 sequences, with a few −35 and −10 sequence variants. As one may expect, the strongest promoters in this collection have the −35 and −10 consensus sequence, along with optimal spacing. However many of the weaker promoters in this collection also match these elements and some strong promoters do not. The reasons for these deviations remain unclear, but may result from complex promoter-RNAP-holenzyme interactions, DNA supercoiling, and 3D DNA architecture noted in previous sections above. Indeed, Mutalik et al. [30] developed an analysis of variance (ANOVA) tool to quantify transcription variability arising from different promoter and 5′ UTR couplings. In other work by Mutalik et al. [29], these researchers subsequently leveraged known and random promoters, coupled to 5′ UTR, monocistron, and bicistron variants, to obtain transcription and translation initiation constructs that promote reliable reporter gene expression over a 1000-fold dynamic range. In the work by Alper et al. [12] the PLTet-O1 promoter was randomly mutated, inserted upstream of a gfp (encoding green fluorescent protein) reporter gene, and a library of 22 promoter mutants were selected for further characterization based on high reporter expression across the entire population of E. coli harboring these constructs. This promoter library had a 196-fold range in GFP production among member constructs. The cat (chloramphenicol acetyltransferase) gene was also swapped for gfp in this promoter library, the minimum inhibitory concentrations (MICs) of chloramphenicol were measured, and these MICs were correlated with promoter strength. This swap not only demonstrates that gene expression from these library promoters was constitutive, but that these promoters were modular. Leveraging this modularity, Alper et al. inserted library promoters upstream of ppc (encoding phosphoenolpyruvate carboxylase) and dxs (encoding deoxy-xylulose-P synthase). Biomass yields and lycopene production were to varying degrees associated with promoter strength. The Anderson promoter library (http://parts.igem.org/Promoters/Catalog/Anderson) is a tool composed of 20 promoters for titrating gene expression over three different orders of magnitude. This library is composed primarily of −35 and −10 sequence variants. Finally, the Weizmann Institute of Science promoter library [36] features nearly 2000 unique members for titratable and dynamic expression of a gfp reporter. This library was engineered using random inserts of genomic DNA cloned upstream of a previously promoterless reporter gene, a technique often followed when promoter sequences or regulation profiles are unknown [36], [132], [133], [134], [135], [136], [137]. Analyzing transcriptome data for putative transcription start sites or conserved upstream sequences are additional methods to identify promoter elements [127], [138], [139], [140], [141].
Promoter libraries have been developed and implemented in non-model organisms. As alluded to above, Jensen et al. [37] synthesized an Lc. lactis library using the known consensus sequences of the −35 and −10 regions along with variable sequence adjacent motifs. This library yielded titratable lacZ expression over four different orders of magnitude in this organism. Unlike E. coli above, in terms of lacZ reporter expression, Lc. lactis is less tolerant to changes in the −35 and −10 sequences. However, as was the case with E. coli, the spacer region and additional promoter elements also contribute to titratable lacZ expression in Lc. lactis. As was the case for Lc. Lactis [37], to engineer a Corynebacterium glutamicum library Rytter et al. [33] took consensus sequences for the C. glutamicum −10 region (TGNGNTAYAATGG), the E. coli −35 region with and without an AT-rich region upstream, and primarily changed the spacer between these regions to titrate lacZ expression over three different orders of magnitude. Finally, Markley et al. [34] used mutagenesis of a truncated version of the well-characterized Synechococcus elongatus strain PCC 7002 promoter PCpcB to engineer a library of 29 unique members to control expression of a yfp (encoding yellow fluorescent protein) reporter gene. This library yielded titratable expression of yfp over three orders of magnitude, with a mutation changing the original PCpcB −10 sequence (TATAAA) to the E. coli −10 consensus being responsible for much of the increased yfp expression of the top performing library member. Fig. 3 provides a roadmap for engineering promoter libraries, which may be a useful guide for further extending promoter libraries in other non-model organisms.
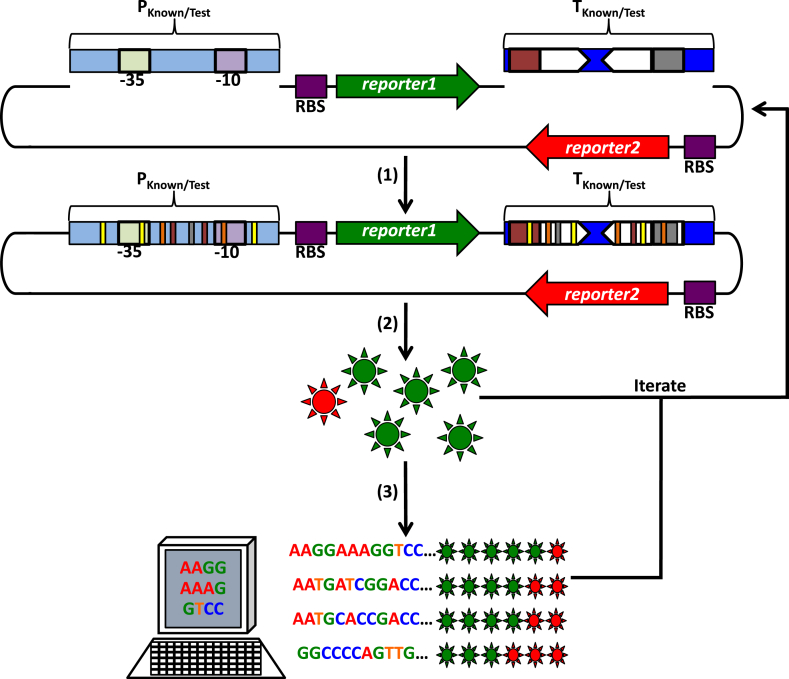
Fig. 3.
A procedure to generate promoter and terminator libraries. (1) To test putative promoters as sites of transcription initiation, known promoters (PKnown) or random sequences (PTest) may be inserted upstream of a previously promoterless ribosome binding site (RBS)-reporter1 gene translational fusion (purple box with a green arrow). These promoters may be constitutive (shown) or subject to transcription regulation by activators and repressors (not shown). Pale green and pale purple boxes indicate the −35 and −10 promoter sequences, respectively. To test putative transcription terminators, known terminators (TKnown) or random sequences (TTest) may be inserted upstream of a promoterless RBS-reporter2 translational fusion (purple box with a red arrow) and downstream of the PKnown/Test-reporter1 transcription fusion above. Additional known or random terminators may be inserted in tandem, and a known terminator may be inserted downstream of reporter2 to ensure termination of transcription after RNAP reads-through this gene (not shown). Inverted white arrows represent the sequence forming a terminator stem-loop (inverted repeats), and red and grey boxes represent poly A- and poly U-tracts, respectively. (2) To introduce variability in promoter and terminator libraries (thin yellow, orange, red, grey, and white bars) these cis-regulatory elements may be derived from randomly sheared DNA, synthesized on demand with desired sequences, subjected to mutagenic PCR (polymerase chain reaction), or other similar methods. These cis-regulatory element constructs may be introduced into an organism of interest, reporter gene expression may be assessed for each library member (green and red suns), and this procedure may be iterated (starting with new random/mutated sequences or using library member sequences as inputs for further sequence variation generation) to achieve a desired result or a wider expression variability profile. (3) To identify sequence characteristics that may be responsible for reporter gene expression level differences (rows of green [gene expression from promoters] and red [gene expression from transcription read-through] suns) among library member sequences (indicated), these constructs should be sequenced and compared with other members or known promoter sequences (iterated as desired).
2.3. Transcription terminators
In E. coli, transcription primarily terminates either with the assistance of Rho helicase (Rho-dependent termination) or through innate elements of the RNA transcript itself (Rho-independent termination) [142], [143], [144]. Engineering termination elements becomes especially important for many transcriptional control systems to avoid polar effects associated with transcription. Many of these polar effects may include transcription unit desegmentation (i.e., circumventing transcription regulation of downstream genes) [35], [145], [146], [147], [148], [149], [150], [151], [152], increased translation of upstream genes by prolonging transcription-translation coupling along with other alterations to translation mechanics (i.e., translational coupling) [91], [144], [150], [153], [154], [155], [156], potentially arresting downstream transcription through R-Loop formation [144], [157], [158], [159], altering DNA supercoiling [160], and influencing plasmid copy number [161]. Multiple terminators present in a single cell must be designed such that homologous recombination will not delete or invert intervening sequences between these constructs, underscoring the need to have a broad understanding of a variety of different terminators. To further characterize transcriptional termination [142], [143], [144]. As a hexamer complex, Rho associates with RNA, recognizes a C-rich and G-poor RNA rut (Rho utilization) sequence, allows RNA to threaded through an open region in the Rho complex, and subsequently closes around the associated RNA [142], [143], [144]. Signals that are still poorly understood pause the procession of RNAP-mediated RNA polymer elongation or promote RNAP backtracking (albeit, nucleoid structure may serve a function perturbing RNAP elongation dynamics along with promoting RNAP backtracking) [162], [163], and Rho, coupled to ATP hydrolysis, proceeds wrapping RNA around the hexamer complex [142], [143] and catches up to this previously paused RNAP (i.e., resuming transcript elongation may be a requirement for Rho-mediated termination [143], [164]). Subsequently, Rho disrupts the RNA-DNA duplex inside of RNAP and transcription terminates after dissociation of the RNA-DNA duplex and rehybridization of the DNA-DNA duplex [142], [143]. In contrast to Rho-dependent termination, Rho-independent termination events usually occur when an RNA stem-loop forms in the RNAP exit channel and a poly U-tract downstream of this stem loop is dehybridized from the associated DNA poly A-tract [35], [142], [144], [165]. In particular, pausing at this poly U-tract allows the nucleation of a GC-rich stem [35], [142], [144]. An additional poly A-tract is sometimes present upstream of some terminator stem-loops [35], [142], [144], but the precise function for this sequences remains unclear (e.g., this sequence may be the poly U-tract for transcription directed from the opposite strand of DNA, promote extension of the terminator stem into the poly U-tract, etc). Well known Rho-independent transcription terminators include the TRrnB1 and TRrnB2 terminators [166], [167], [168], the TTE T7 phage terminator [167], [168], and the λ phage TO terminator [169], [170], [171]. To further characterize transcriptional termination and solve the issue of integrating multiple terminators in tandem without promoting homologous recombination, additional libraries of known and tested Rho-independent terminators have been developed (http://parts.igem.org/Terminators/Catalog) [35]. As was the case for promoter libraries above, RNAP also has a wide range of activities toward intrinsic terminators [35], [106], [107], [108].
2.4. Terminator libraries
In the work by Chen et al. [35], 582 natural and synthetic Rho-independent terminators were characterized and a biophysical model of terminator strength was developed. This group used an experimental setup where a single promoter, PBAD (see below), drove expression of a gfp reporter gene. Downstream of gfp, putative terminators were positioned to terminate expression 5′ of a promoterless rfp (red fluorescent protein) reporter gene (i.e., rfp will only be expressed if RNAP reads through these tested terminators). Terminator strengths were determined by taking the ratio of gfp to rfp expression levels, normalized to the same ratio for a terminator-less RNA molecule, and varied over three different orders of magnitude. As was previously known for intrinsic terminators, the presence of a poly A-tract upstream (5′) of a GC-rich terminator stem, a longer terminator stem (approximately 8-bp), a stable unpaired loop region, and a poly U-tract downstream (3′) of the stem were all characteristics associated with strong terminators studied by this group. Free energy changes associated with the formation of all of these features, thus, went into the design of a biophysical model for predicting terminator strength. In addition, through identifying a variety of strong terminators in their library and model, Chen et al. were able to predictably terminate transcription with reduced homologous recombination-mediated changes to these terminator constructs. Fig. 3, in addition to describing promoter library generation, describes building terminator libraries.
3. Transcription control through transcription factor tools
As described above, promoters can often be classified as weak or strong. However, trans-acting factors, referred to as transcription factors, have the potential to invert these promoter strength metrics (i.e., a weak promoter could be transformed into a strong promoter, and a strong promoter into a weak one) (Fig. 4A). Factors that transform a weak promoter into a strong one are referred to as transcription activators; the factors that transform a strong promoter into a weak one are referred to as transcription repressors. Promoters with titratable (linear/dynamic) activity toward these transcription factors are generally used for analogue-type computation (Fig. 4A and B) [25]. Promoters that strongly respond to transcription activators or repressors (often with feedforward and feedback loops), resulting in a step-change in promoter strength, are generally used for digital-type gene circuits [16], [18], [19], [25] (Fig. 4A and C). DNA binding by many of these transcription factors is affected by environmental conditions, such as the presence of small metabolites and other ligands. In this way, small molecules can be used as switches of transcriptional control. Of particular note, in terms of digital transcription control design, Nielson et al. [23] have developed the automated system Cello (http://www.cellocad.org/). The Cello system uses the Verilog design language, which can be converted into DNA sequences by this system, and reliable and modular gene circuit components to automate gene circuit engineering. In the following sections, we highlight some of the more commonly used transcription factors and how they have been used to control gene expression by synthetic biologists.
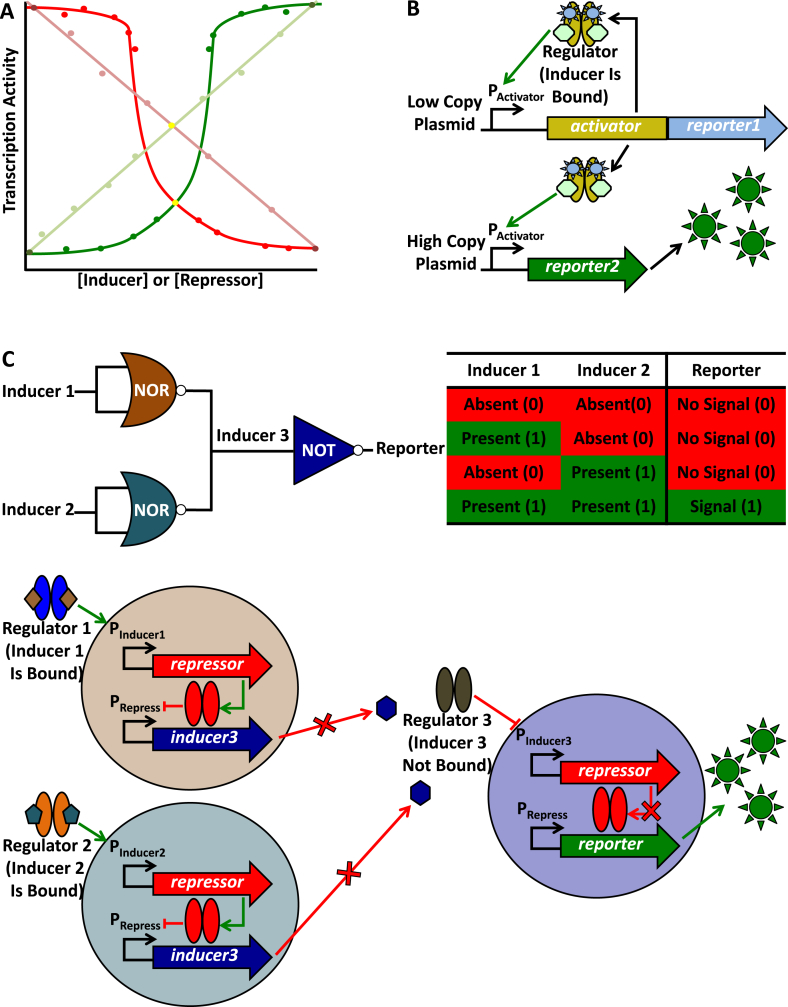
Fig. 4.
An overview of transcription regulation following analogue and digital expression dynamics. (A) A hypothetical transcription activity dynamics plot is depicted. Faint green and red lines represent transcription activity (activation and repression, respectively) subject to linear/analogue dynamics. Dark green and red curves represent activation and repression subject to step-change/digital dynamics, respectively. Individual points represent one hypothetical measurement of transcription activity. Concentrations of hypothetical activator or repressor ligands increase from left to right on the horizontal axis, and transcription activity increases from the bottom to the top of the vertical axis. (B) An example of a system subject to linear/analogue dynamics. This model is modified from the work of Daniel et al. [25]. Activator (gold ovals) fused to Reporter 1 (blue suns) is produced from a gene expressed from a low copy plasmid and subject to a positive feedback (auto-activation) loop at PActivator (green arrow). Activator (bound to inducer) also binds target sites on a high copy number plasmid activating reporter2, again at PActivator, and simultaneously titrates Activator from the low copy plasmid above. Based on inducer concentration, this model allows for log-linear titration of Reporter 2 (green suns) levels [25]. (C) A digital AND logic gate (engineered from two NOR gates and a NOT gate) is symbolically represented along with the associated truth table. The depicted model is modified from the work of Tamsir et al. [18]. This circuit is distributed among three co-cultured cells (pale brown, blue, and purple circles), and the scenario depicted represents the ON output state, when Reporter (green suns) is produced (1 and a green box in the truth table). Both inducer 1 (brown diamond) and inducer 2 (blue pentagon) are present (1 and a green box in the truth table) and bound to Regulators 1 and 2, respectively. These regulators bind PInducer1 and PInducer2, respectively, activating repressor (green arrow). Repressor is subsequently produced and represses inducer3 (red crossbar), the gene encoding the producer of inducer 3 (purple hexagon), at PRepress. Inducer 3 is not secreted (red arrow with a cross), and Regulator 3 (without inducer 3 bound) binds PInducer3 repressing repressor. Subsequently, Repressor does not repress reporter, at PRepress, leading to reporter expression and Reporter production. In the absence of either or both inducers 1 and 2 (0 and red boxes in the truth table) inducer3 is not repressed, which leads to inducer 3 secretion. Regulator 3 (with inducer 3 bound) activates repressor, at PRepress. Repressor subsequently represses reporter, blocking Reporter production (0 and a red box in the truth table).
3.1. CI and Cro
Together, CI and Cro are transcription factors that naturally function as a toggle switch and have been utilized in a variety of synthetic regulatory systems. In nature, Cro binds PRM and represses cI, and CI binds PR and represses cro [172], [173], [174], [175], [176]. This toggle switch controls the fate of a cell infected with bacteriophage λ. If the Cro binding state is predominate a lytic infection with this phage occurs, but if the CI state predominates the phage may enter into the lysogenic cycle [172], [173], [174], [175], [176]. The Cro and CI binding sites have the same consensus sequence (TATCACCGGCGGTGATA), but each repressor has different affinities for variants of this sequence, explaining the switch like behavior [172], [173], [174], [175], [176], [177], [178]. The Cro and CI binding sites overlap the respective promoters of each repressor, which suggests that each blocks transcription by sterically interfering with RNAP holoenzyme promoter binding (potentially through DNA looping) [115], [121], [173], [174], [175], [179]. In synthetic biology, CI was one of three repressors tested in early toggle switches development by Gardner et al. [16]. Gardner et al. concluded that the CI857 temperature-sensitive variant (functional as a repressor at 30 °C, but not at 42 °C [180]) is an extremely efficient repressor at PLS1con. Kotula et al. [24] constructed a Cro and CI memory element based on the natural PR and PRM promoters. This group used a stable CI-ON state (resulting in lacZ reporter gene repression) coupled to TetR (see below) repression of cro. Adding aTc (anhydrotetracycline) derepressed cro, which in turn repressed cI and generated a stable Cro-ON state (resulting in lacZ reporter gene expression). Using a mouse model, where mice were treated with a strain of E. coli harboring this memory element and with or without aTc, Kotula et al. also demonstrated that this memory element is a useful in vivo reporter system. Finally, Tamsir et al. [18] used CI, CAP (catabolite activator protein), AraC, and TetR (see below) during their construction of all 16 two-input Boolean logic gates.
3.2. CAP (catabolite activator protein)
CAP serves a key regulatory function in catabolite repression and has been leveraged in synthetic systems to control gene expression. When CAP binds cAMP (cyclic AMP) it has a higher affinity for its cognate binding sites (AATGTGATCTAGATCACATTT), which are often upstream of operons related to carbohydrate metabolism [181], [182], [183], [184]. Indeed, the αCTD subunits of the RNAP holoenzyme make contacts with CAP, which improves promoter recognition by the holoenzyme [115], [181], [185], [186]. In terms of catabolite repression, adenylyl cyclase activity and cAMP synthesis is modulated by the availability of glucose in the extracellular milieu [184]. When glucose levels are high, adenylyl cyclase activity is low, and when glucose levels are low, adenylyl cyclase activity is high [184]. Synthetic systems based on promoters that are subject to catabolite repression (e.g., PBAD [found on vector pBAD and others], PLacUV5 expression systems, etc) are often used when strong negative regulation of genes is desired (i.e., for auto-induction systems that will only produce desired proteins when glucose is depleted from the extracellular milieu) [187], [188]. Daniel et al. [25], additionally, used PBAD to engineer transcription systems capable of performing analogue computation. Other components of these transcription control systems include LacI, AraC, and LuxR (see below).
3.3. LacI
Describing LacI-mediated repression of the E. coli lac operon represents one of the earliest models of gene regulation [77], [189]. Naturally, LacI dimers bind the operator sequence (TGGAATTGTGAGCGGATAACAATT) in the absence of the small molecule allolactose (or isopropyl β-d-1-thiogalactopyranoside [IPTG]) [77], [189], [190], [191], [192], [193], [194]. Occupancy at these operator sequences obstructs RNAP holoenzyme binding to PLac (a weak promoter without additional CAP-mediated activation) [121], [189], [192], [195], [196], [197]. In addition, LacI-mediated DNA looping (potentially assisted by nucleoid structuring factors and DNA supercoiling) may obstruct RNAP holoenzyme-PLac association [115], [121], [179], [189], [195], [196], [198]. When present, allolactose or IPTG occupy ligand-binding pockets on LacI, which results in a conformational change of this regulator and reduces its affinity for operator binding sequences [77], [189], [191], [193], [194]. Numerous regulation tools (noted above and below) have been based off of LacI-mediated repression.
LacI-mediated repression is a tool used for engineering a variety of genetic circuits. To explore intrinsic and extrinsic noise in gene expression, Elowitz et al. [199] used LacI-mediated repression of reporter genes yfp and cfp (encoding the cyan fluorescent protein) placed nearly equidistant from the origin of replication on opposite sides of the E. coli chromosome. This group identified noisy reporter gene expression when LacI repressed these reporter genes. However, they found that varying levels of IPTG affected intrinsic and extrinsic reporter gene noise differently. Increasing IPTG levels decreased intrinsic noise, and extrinsic noise initially increased then decreased. LacI was used in addition to CI (see above) and TetR (see below) repressors, to construct toggle switches used by Gardner et al. [16]. In this work, LacI repressed cI or tetR expression in addition to a gfp reporter gene expressed from PTrc-2 (a strong synthetic promoter generated by fusing PTrp and PLac—containing a single LacI operator sequence). This approximates a step-change in reporter expression, where derepression by addition of IPTG is supplemented by cI expression and subsequent CI-mediated lacI repression at PLS1con. Earlier work has established that LacI-mediated regulation is subject to the all or nothing phenomenon (a step-change as opposed to a titration in gene expression) [200], [201]. Indeed, suboptimal induction with IPTG leads to a heterogeneous response at the single cell level (i.e., some cells express high levels of lacZ while others do not), but at the cell population level may appear as though the cells have uniform or expression levels similar to a fully induced system [201], [202]. Nevertheless, other groups have used systems where it was possible to titrate IPTG-mediated derepression [25], [200], [202]. For example, titratable expression is also sometimes achieved when lacY (the lactose and IPTG importer) is deleted from the background of the E. coli host strain [200], [202].
3.4. AraC
AraC-mediated regulation of the ara operon is another well-characterized system of transcriptional control. In nature, AraC is both a repressor and activator depending on l-arabinose-mediated isomerizations of this regulator [203], [204]. Indeed, when l-arabinose is absent in the extracellular milieu, AraC dimers bind promoter proximal and upstream distal sites in an outstretched configuration, which bends/loops DNA and obstructs RNAP holoenzyme promoter binding [115], [121], [204]. AraC recognizes an AT-rich sequence (usually 6-bp long) flanked by the sequence TRTGGAC on the upstream side and GCTA on the downstream side [203]. In the presence of l-arabinose, AraC binds this metabolite and isomerizes to a collapsed configuration [204]; AraC and CAP (PAra is a weak promoter without CAP activation) occupy binding sites proximal to PAra and PAraC and induce RNAP holoenzyme promoter binding and open complex formation [204]. This may be achieved by locally bending DNA such that the αCTDs of the RNA holoenzyme can still contact CAP despite the presence of AraC dimers between these two elements [115], [204].
AraC has been mobilized to engineer a variety of systems under the transcriptional control of this regulator. Many of these engineered systems promote both digital/step-change dynamics [18], [23] and analogue/linear dynamics [25], [205] It is intriguing to note, however, that for AraC-mediated induction at subsaturating levels of l-arabinose (intermediate induction) a dichotomy exists at the single cell level [205], [206]. Thus, a subset of cells in the population express a gene of interest from PBAD, while the remaining cells do not express this gene of interest. This gives the false impression that all cells in the population express the gene of interest at the same intermediate level [205], [206]. As was the case for LacI above, however, systems can be engineered where AraC-mediated regulation can be titratable. For example, titratable expression may be achieved when arabinose importers (e.g. araE) are constitutively expressed (natively, expression is linked to the presence of arabinose) from the E. coli chromosome [205].
3.5. TetR
TetR-mediated negative regulation of PTet is another model of tight E. coli gene repression. In its natural context, TetR binds as a dimer to the operator sequence (TCTATCATTGATAGG), which in turn obstructs RNAP holoenzyme-promoter interactions [115], [207], [208], [209]. In the presence of tetracycline (or the analogue aTc) this small molecule binds a tetracycline binding pocket in each dimer, which in turn causes a conformational change in this regulator and reduces its affinity for the above operator sequence [103], [207], [208], [209], [210]. Regulation by TetR at cognate promoters is incredibly tight, with as high as a 5000-fold difference between aTc induced and uninduced expression from some promoters [103].
TetR-mediated regulation has been leveraged to engineer synthetic regulatory elements. When constructing all two-input Boolean logic gates using site-specific recombinases to invert promoters or terminators controlling reporter gene expression, Siuti et al. [19] used TetR- and aTc-mediated regulation to control one of two site-specific recombinases (the other recombinase being controlled by a small regulatory RNA and LuxR- and AHL (acyl-homoserine lactone)-mediated regulation at PLux [see below]). TetR, CAP, LacI, and AraC (discussed above) are also useful components for automated logic gate assembly by the Cello design system [23]. Kotula et al. [24], as noted above, used TetR- and aTc-mediated regulation as a trigger element to regulate cro expression. Elowitz and Leibler [28] used TetR, along with LacI and CI (see above), to construct a synthetic repressilator. As the name of this construct suggests, Elowitz and Leibler described the synchronization and subsequent innate oscillatory character of reporter and regulator protein production and turnover.
3.6. LuxR
Although best understood for its functions during the quorum sensing phenomenon [211], [212], [213], [214], AHL-mediated regulation of LuxR is also associated with a variety of engineered transcription control systems. In nature, LuxI synthesizes the auto-inducer AHL, which in turn binds LuxR once sufficiently high levels of AHL are present in the cellular milieu (i.e., AHL binds reversibly and weakly to LuxR at low AHL levels) [211], [212], [213], [214], [215]. AHL-bound LuxR dimers recognize a LuxR box (ACCTGTAGGATCGTACAGGT) upstream of the extended −10 sequence of PLux [211], [212], [213], [214], [215], [216], [217]. In the case of PLux, LuxR dimers make contact with the αCTD subunits of the RNAP holoenzyme, which in turn facilitates promoter recognition (of the weak PLux) [216]. In synthetic biology, leveraging the natural functions of AHL and LuxR in quorum sensing allowed Basu et al. [218] to engineer a system where cells could be induced (aTc-mediated derepression of PLtet-O1) to produce AHL (sender cells) that in turn binds LuxR in receiver cells leading to a pulse of gfp expression (under control of PLux). Due to limited diffusion of AHL in solid media, follow-up work [219] extended LuxR-mediated regulation to demonstrate that band patterns of fluorescent reporter production could form when undifferentiated mixtures of receiver cells (harboring a complex mixture of plasmids with varying copy numbers encoding CI, LuxR variants, LacI variants, promoters responsive to these regulators, and gfp or dsred reporters) were plated with AHL-sender cells. Thus, LuxR- and AHL-mediated regulation could be useful for engineering auto-induction systems [220], [221] to simplify protein production and assist in cross-strain and cross-species communication in microbial consortia [222], [223], [224], [225], [226].
3.7. TALEs (transcription activator-like effectors)
Unlike the previous regulators above, which recognize specific DNA sequences, TALEs (transcription activator-like effectors) have a modular structure and may be naturally or artificially engineered to recognize nearly any specific DNA sequence. Thus, these regulators are especially useful tools for engineering transcription control regulation at any sequence on demand (i.e., where no known regulators are able to bind to desired target sequences or known regulators have been used elsewhere in the same construct) [227]. Although originally described as plant transcriptional activators secreted by prokaryotic plant pathogens [228], [229], [230], [231], [232], TALEs have been well-studied as modular DNA binding proteins. TALEs are large (>120 kDa) DNA binding proteins with N- (amino-) and C-termini containing secretion and nuclear localization signals, respectively, and a highly repetitive central helical region [228], [229], [231], [232], [233], [234]. The internal helical region typically contains 15.5–19.5 near perfect repeats of 34 amino acids, which accounts for most of the size of these regulators and imparts the overwhelming majority of DNA sequence-specific binding activity [228], [231], [232], [233], [234], [235], [236], [237], [238]. Indeed, Boch et al. [235] and Moscou and Bogdanove [236] cracked the TALE DNA binding specificity code, where residues 12/13 of the 34 amino acid repeat impart this nucleotide binding specificity (H/D=C, N/G=T, N/I=A, N/S=N, N/N=R, N/—=Y, H/G=T, H/A=V, N/D=C, N/K=G, H/—=T, H/I=C, H/N=G, N/A=G, and I/G=T). Thus, shuffling different repeat regions imparts TALEs with new DNA binding sequence specificities [21], [237], [239], [240], [241], [242], [243], [244], [245], a testament to the TALE modularity. The region immediately N-terminal to the central repeat region is required for initial nonspecific DNA binding [231], [233], [246], [247], [248]. In this initial event, the TALE associates loosely with DNA in an extended conformation and begins a search for its cognate binding sequence through 1D diffusion [246]. Upon recognition of its cognate binding sequence, the extended TALE conformation contracts, and the helical repeats tightly wrap around the major groove of this binding sequence [246]. In addition, the C-terminal domain of natural TALEs may contain regions that interact with transcription factors or may be fused to other regulatory proteins [21], [228], [230], [232], [240], [243], [244], [245].
TALEs have been mobilized as tools to control gene expression in synthetic biology. For the most part, TALE tools have been implemented in eukaryotes [21], [237], [239], [240], [243], [244], [245]. However, Politz et al. [242] demonstrated that a TALE recognizing the LacI operator sequence could effectively block transcription initiation and elongation. Expanding on this work and to demonstrate that TALE-mediated repression in E. coli could be reversed with the addition of small molecule ligands, Copeland et al. [241] used TALEs with TEV protease [249], [250] cleavage sequences and a tev gene expression construct under the control of PTet (aTc inducible). Upon TetR depression of tev (by the addition of aTc) and subsequent TEV-mediated TALE cleavage, a formerly TALE-repressed gfp or mcherry reporter could be subsequently expressed. This response to a small ligand (aTc) had similar dynamics to IPTG-mediated LacI derepression of a mcherry reporter gene, despite being an indirect response. In addition, by changing the binding specificity of the repeat region inside of the TALE scaffold used above, Copeland et al. demonstrated multiplexed gene repression at two E. coli chromosomal loci, the lysA and sucA promoters.
3.8. CRISPRi (clustered regularly interspaced short palindromic repeats interference)
The CRISPR (clustered regularly interspaced short palindromic repeats) system is believed to have evolved as a defensive system against foreign DNA [251], [252], [253], [254], [255]. Spacers intervening between inverted repeats (form stem-loops in RNA transcripts) contain nucleic acid sequences of bacteriophages and other mobile genetic elements [251], [252], [253], [254], [255]. In the case of Type II CRISPR systems, the spacer locus is transcribed as a single RNA transcript, a tracrRNA binds adjacent to the spacers (in the stem-loop), and RNase III-mediated processing generates mature guide crRNAs (containing the spacer nucleic acid sequences above) and tracrRNAs [256], [257]. These RNAs are subsequently loaded into Cas9 forming a mature endonuclease complex that will cleave DNAs in a sequence specific manner (directed by the guide crRNAs above) [257], [258], [259], [260], [261], [262], [263]. An additional sequence motif, referred to as the PAM (protospacer adjacent motif), also serves functions during target sequence recognition and subsequent DNA-RNA hybridization [257], [258], [259], [260], [261], [262], [263]. In the case of the commonly used Cas9 variant from Streptococcus pyogenes, NGG (the reverse complement of which occurs upstream of the DNA sequence hybridized to the RNA spacer sequence) is the PAM utilized by this enzyme [261], [262]. Although the catalytically active Cas9 has been extensively used for recombination-mediated genetic engineering [261], [262], [264], [265], [266], catalytically inactive Cas9 (dCas9) also has important regulatory functions [6], [7], [8], [267], [268], [269], [270], [271], [272], [273].
Modifications to the CRISPR system promote sequence-specific transcriptional regulation, CRISPRi (CRISPR interference). Indeed, CRISPR spacer sequences and dCas9 can be directed to nearly any DNA sequence, a similar phenomenon to what was described for TALEs above. Thus, as was the case for TALEs, CRISPRi is an especially useful tool for targeting regulatory proteins (e.g., dCas9) to any DNA sequence on demand [227]. For example, Qi et al. [268] used a dCas9 variant to block transcription initiation and elongation using sgRNAs (single guide RNAs) to various targets in the promoters and coding sequences of variant rfp and gfp reporter genes. Blocking transcription elongation was the most efficient method to decrease expression of the tested reporter genes. To demonstrate that CRISPRi systems could be used to probe a complex regulatory network, Qi et al. also used sgRNAs directed against genes in the lac operon and involved in catabolite repression (see above) to knockdown expression of tested genes. Bikard et al. [269] used a dCas9 variant to block transcription initiation and elongation by targeting the gfp reporter open reading frame, the reporter gfp promoter, and the gfp reporter ribosome binding site using crRNA guides directed against these sequences. This same group also fused the ω subunit of the RNAP core to dCas9 and demonstrated that transcription activation could be promoted by crRNA guides directed at sequences 43 to 59 base pairs upstream of the −35 sequence of a weak promoter. To successfully minimize acetate production in E. coli, Fernandez-Rodriguez et al. [14] used dCas9, a series of light-responsive two-component systems (see below), and sgRNAs directed against poxB, pta, and ackA (all involved in acetate production [274]). This demonstrates that dCas9 is also useful for controlling transcription of genes that encode factors controlling flux through a metabolic pathway. Through fusing activators and repressors to dCas9, others have also shown (for the most part in eukaryotes) that these hybrid proteins can mediate transcription activation and repression [270], [271], [272], [273].
In non-model organisms, CRISPRi can also be used to regulate gene expression. Indeed, Yao et al. [6] used CRISPRi to first regulate gfp reporter gene expression in the cyanobacterium Synechcocystis sp. PCC 6803. This group then used CRISPRi to repress expression of phaE (involved in polyhydroxybutyrate synthesis [275]), glgC (involved in generating glycogen storage molecules [276]), and a variety of aldehyde dehydrogenases and reductases (involved in depleting aldehyde pools available for alkane production [277]). Gordon et al. [8] used CRISPRi in S. elongatus strain PCC 7002 to tune expression of the cpc operon (involved in phycocyanin antennae synthesis) by using sgRNAs directed against cpcB. They also used sgRNAs directed against glnA (depletes α-ketoglutarate pools by converting this metabolite to glutamine, which promotes ammonium [preferred nitrogen source of cyanobacteria] assimilation and is involved in signaling carbon/nitrogen balance [278]) to moderately increase α-ketoglutarate levels, activate NtcA (a catabolic gene regulator activated by reduced ammonium assimilation and the modest increase in α-ketoglutarate levels [278], [279], [280]), and subsequently increase lactate levels (driven by NtcA-mediated enhanced flux to pyruvate from fixed carbon [279]). In the same pursuit, to redirect carbon flow toward succinate accumulation, Huang et al. [7] targeted glgC, sdhA, and sdhB (blocks production of fumarate from succinate [281], [282]) with sgRNAs directed against these genes. Thus, CRISPRi is a useful technology for rationally routing metabolites for the purposes of metabolic engineering.
3.9. Two-component regulatory systems
Unlike the one-component systems described above, two-component systems perturb gene expression in response to signals that are often located outside of the cytoplasm. In general, a sensor kinase binds a ligand (or in some cases low ligand concentration shifts the receptor-ligand complex equilibrium toward releasing a ligand). In turn, this ligand binding (or disengagement) stimulates autokinase activity (often at specific histidine residues) of the sensor kinase [64], [65], [66]. The specific response regulator for the activated sensor kinase (crosstalk is generally avoided in prokaryotic systems [64], [283], [284], [285]) is subsequently phosphorylated (usually at specific aspartate residues), undergoes conformational changes, and subsequently mediates differential gene regulation (direct or indirect activation or repression) at regulator-specific promoters [64], [65], [66].
Two-component systems have been used for regulation of synthetic transcription systems. Anderson et al. [22] used the PhoPQ system (PhoQ kinase is inactive under high Mg2+ concentrations [64], [65]), the cognate PMgrB, PLux and PSal (positively regulated by NahR in the presence of salicylate [286]), and gfp and inv (Invasin) [287] reporters to develop an AND digital logic gate. Intriguingly, this logic gate can be used to program bacterial cell invasion of mammalian cells of interest. Using a chimeric receptor (composed of a Cph1 photoreceptor [288], [289], [290] and the EnvZ histidine kinase domain [291]), Levskaya et al. [292] developed a sensor that would be inactivated in the presence of red light (i.e., OmpR would not be phosphorylated by EnvZ [291], [293] to drive expression of a lacZ reporter from POmpC). In an additional impressive feat, this same chimeric light receptor above, LuxR, LuxI, CI, and a lacZ reporter were used by Tabor et al. [294] to build a system that could recognize the edge of projected shapes. Recently, to engineer E. coli capable of distinguishing between red, green, and blue light, Fernandez-Rodriguez et al. [14] used the above chimeric receptor (switched on by infrared light and off by red light, switching the OmpR response regulator off or on, respectively); the CcaS receptor (switched on by green light and off by far-red light, switching the CcaR response regulator on or off, respectively [295], [296]); the YF1 receptor (switched off by blue light, which switches off response regulator FixJ [297]); CI and PhlF regulators; a T7 RNAP core enzyme; supplemental sigma factors for this T7 RNAP core; promoters recognized by each regulator or T7 RNAP holoenzyme; and bFMO, lacZ, and gusA reporter genes. These complex circuits allowed this group to replicate several colored images projected onto E. coli cultures harboring these constructs.
4. Conclusion
In this mini-review we have discussed several features of the natural biology of transcriptional regulation and several systems that have been used to control transcriptional regulation in model bacteria. Engineering control at this level is especially valuable for titrating protein (and therefore activity) levels, which leads to titratability of metabolites of interest, and engineering a plethora of gene circuits. Indeed, as outlined by the central dogma of molecular biology, transcriptional regulation represents the first level that the flow of genetic information from nucleic acid polymers (DNA and RNA) to amino acid polymers (proteins) can be regulated. Transcriptional regulation, as discussed in detail above, is mediated by controlling promoter strength, positioning and strength of terminators, and by transcriptional regulators. These methods of engineering transcription control can be readily extrapolated to controlling metabolic flux and control engineering in non-model organisms and eukaryotes. Nevertheless, there are still additional layers of post-transcriptional and post-translational control not covered by this mini-review. Furthermore, translation is reduced once transcription of an mRNA ceases. However, this phenomenon and the global effects of nucleoid structure and DNA supercoiling on gene transcription and translation (presumably at the apex of genetic information flow) remain poorly understood. Thus, along with combining the well-characterized regulatory systems discussed above in novel and interesting ways (potentially for practical applications or improved predictability of engineered constructs), exploring the use of novel regulatory systems and expanding control engineering to non-model organisms and eukaryotes remain areas of active research.
Acknowledgements
MDE was supported by an NHGRI training grant to the Genomic Sciences Training Program 5T32-HG002760. BFP was supported by a grant from the National Science Foundation (EFRI-1240268).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Crick F. Central dogma of molecular biology. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 2.Cech T.R. Structural biology. The ribosome is a ribozyme. Science. 2000;289:878–879. doi: 10.1126/science.289.5481.878. [DOI] [PubMed] [Google Scholar]
- 3.Ban N., Nissen P., Hansen J., Moore P.B., Steitz T.A. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 4.Nissen P., Hansen J., Ban N., Moore P.B., Steitz T.A. The structural basis of ribosome activity in peptide bond synthesis. Science. 2000;289:920–930. doi: 10.1126/science.289.5481.920. [DOI] [PubMed] [Google Scholar]
- 5.Muth G.W., Ortoleva-Donnelly L., Strobel S.A. A single adenosine with a neutral pKa in the ribosomal peptidyl transferase center. Science. 2000;289:947–950. doi: 10.1126/science.289.5481.947. [DOI] [PubMed] [Google Scholar]
- 6.Yao L., Cengic I., Anfelt J., Hudson E.P. Multiple gene repression in cyanobacteria using CRISPRi. ACS Synth Biol. 2016;5:207–212. doi: 10.1021/acssynbio.5b00264. [DOI] [PubMed] [Google Scholar]
- 7.Huang C.H., Shen C.R., Li H., Sung L.Y., Wu M.Y., Hu Y.C. CRISPR interference (CRISPRi) for gene regulation and succinate production in cyanobacterium S. elongatus PCC 7942. Microb Cell Fact. 2016;15:196. doi: 10.1186/s12934-016-0595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon G.C., Korosh T.C., Cameron J.C., Markley A.L., Begemann M.B., Pfleger B.F. CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metab Eng. 2016;38:170–179. doi: 10.1016/j.ymben.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens J.T., Carothers J.M. Designing RNA-based genetic control systems for efficient production from engineered metabolic pathways. ACS Synth Biol. 2015;4:107–115. doi: 10.1021/sb400201u. [DOI] [PubMed] [Google Scholar]
- 10.Farmer W.R., Liao J.C. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat Biotechnol. 2000;18:533–537. doi: 10.1038/75398. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F., Carothers J.M., Keasling J.D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol. 2012;30:354–359. doi: 10.1038/nbt.2149. [DOI] [PubMed] [Google Scholar]
- 12.Alper H., Fischer C., Nevoigt E., Stephanopoulos G. Tuning genetic control through promoter engineering. Proc Natl Acad Sci U. S. A. 2005;102:12678–12683. doi: 10.1073/pnas.0504604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung E., Wong W.W., Suen J.K., Bulter T., Lee S.G., Liao J.C. A synthetic gene-metabolic oscillator. Nature. 2005;435:118–122. doi: 10.1038/nature03508. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Rodriguez J., Moser F., Song M., Voigt C.A. Engineering RGB color vision into Escherichia coli. Nat Chem Biol. 2017;13:706–708. doi: 10.1038/nchembio.2390. [DOI] [PubMed] [Google Scholar]
- 15.Atsumi S., Cann A.F., Connor M.R., Shen C.R., Smith K.M., Brynildsen M.P. Metabolic engineering of Escherichia coli for 1-butanol production. Metab Eng. 2008;10:305–311. doi: 10.1016/j.ymben.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Gardner T.S., Cantor C.R., Collins J.J. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 17.Purcell O., Lu T.K. Synthetic analog and digital circuits for cellular computation and memory. Curr Opin Biotechnol. 2014;29:146–155. doi: 10.1016/j.copbio.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamsir A., Tabor J.J., Voigt C.A. Robust multicellular computing using genetically encoded NOR gates and chemical 'wires'. Nature. 2011;469:212–215. doi: 10.1038/nature09565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siuti P., Yazbek J., Lu T.K. Synthetic circuits integrating logic and memory in living cells. Nat Biotechnol. 2013;31:448–452. doi: 10.1038/nbt.2510. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson M.R., Savageau M.A., Myers J.T., Ninfa A.J. Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell. 2003;113:597–607. doi: 10.1016/s0092-8674(03)00346-5. [DOI] [PubMed] [Google Scholar]
- 21.Gaber R., Lebar T., Majerle A., Šter B., Dobnikar A., Benčina M. Designable DNA-binding domains enable construction of logic circuits in mammalian cells. Nat Chem Biol. 2014;10:203–208. doi: 10.1038/nchembio.1433. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J.C., Voigt C.A., Arkin A.P. Environmental signal integration by a modular AND gate. Mol Syst Biol. 2007;3:133. doi: 10.1038/msb4100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen A.A., Der B.S., Shin J., Vaidyanathan P., Paralanov V., Strychalski E.A. Genetic circuit design automation. Science. 2016;352 doi: 10.1126/science.aac7341. aac7341. [DOI] [PubMed] [Google Scholar]
- 24.Kotula J.W., Kerns S.J., Shaket L.A., Siraj L., Collins J.J., Way J.C. Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc Natl Acad Sci U. S. A. 2014;111:4838–4843. doi: 10.1073/pnas.1321321111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel R., Rubens J.R., Sarpeshkar R., Lu T.K. Synthetic analog computation in living cells. Nature. 2013;497:619–623. doi: 10.1038/nature12148. [DOI] [PubMed] [Google Scholar]
- 26.Friedland A.E., Lu T.K., Wang X., Shi D., Church G., Collins J.J. Synthetic gene networks that count. Science. 2009;324:1199–1202. doi: 10.1126/science.1172005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farzadfard F., Lu T.K. Synthetic biology. Genomically encoded analog memory with precise in vivo DNA writing in living cell populations. Science. 2014;346:1256272. doi: 10.1126/science.1256272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elowitz M.B., Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 29.Mutalik V.K., Guimaraes J.C., Cambray G., Lam C., Christoffersen M.J., Mai Q.A. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat Methods. 2013;10:354–360. doi: 10.1038/nmeth.2404. [DOI] [PubMed] [Google Scholar]
- 30.Mutalik V.K., Guimaraes J.C., Cambray G., Mai Q.A., Christoffersen M.J., Martin L. Quantitative estimation of activity and quality for collections of functional genetic elements. Nat Methods. 2013;10:347–353. doi: 10.1038/nmeth.2403. [DOI] [PubMed] [Google Scholar]
- 31.Hammer K., Mijakovic I., Jensen P.R. Synthetic promoter libraries–tuning of gene expression. Trends Biotechnol. 2006;24:53–55. doi: 10.1016/j.tibtech.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Jensen P.R., Hammer K. Artificial promoters for metabolic optimization. Biotechnol Bioeng. 1998;58:191–195. [PubMed] [Google Scholar]
- 33.Rytter J.V., Helmark S., Chen J., Lezyk M.J., Solem C., Jensen P.R. Synthetic promoter libraries for Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2014;98:2617–2623. doi: 10.1007/s00253-013-5481-x. [DOI] [PubMed] [Google Scholar]
- 34.Markley A.L., Begemann M.B., Clarke R.E., Gordon G.C., Pfleger B.F. Synthetic biology toolbox for controlling gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. ACS Synth Biol. 2015;4:595–603. doi: 10.1021/sb500260k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y.J., Liu P., Nielsen A.A., Brophy J.A., Clancy K., Peterson T. Characterization of 582 natural and synthetic terminators and quantification of their design constraints. Nat Methods. 2013;10:659–664. doi: 10.1038/nmeth.2515. [DOI] [PubMed] [Google Scholar]
- 36.Zaslaver A., Bren A., Ronen M., Itzkovitz S., Kikoin I., Shavit S. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat Methods. 2006;3:623–628. doi: 10.1038/nmeth895. [DOI] [PubMed] [Google Scholar]
- 37.Jensen P.R., Hammer K. The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl Environ Microbiol. 1998;64:82–87. doi: 10.1128/aem.64.1.82-87.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H.H., Lindblad P. Wide-dynamic-range promoters engineered for cyanobacteria. J Biol Eng. 2013;7:10. doi: 10.1186/1754-1611-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camsund D., Heidorn T., Lindblad P. Design and analysis of LacI-repressed promoters and DNA-looping in a cyanobacterium. J Biol Eng. 2014;8:4. doi: 10.1186/1754-1611-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zucca S., Pasotti L., Politi N., Casanova M., Mazzini G., Cusella De Angelis M.G. Multi-faceted characterization of a novel LuxR-repressible promoter library for Escherichia coli. PLoS One. 2015;10:e0126264. doi: 10.1371/journal.pone.0126264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breaker R.R. Riboswitches and the RNA world. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green A.A., Silver P.A., Collins J.J., Yin P. Toehold switches: de-novo-designed regulators of gene expression. Cell. 2014;159:925–939. doi: 10.1016/j.cell.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler W.C., Breaker R.R. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 44.Sudarsan N., Hammond M.C., Block K.F., Welz R., Barrick J.E., Roth A. Tandem riboswitch architectures exhibit complex gene control functions. Science. 2006;314:300–304. doi: 10.1126/science.1130716. [DOI] [PubMed] [Google Scholar]
- 45.Perriman R., Ares M., Jr. Circular mRNA can direct translation of extremely long repeating-sequence proteins in vivo. RNA. 1998;4:1047–1054. doi: 10.1017/s135583829898061x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belfort M., Pedersen-Lane J., West D., Ehrenman K., Maley G., Chu F. Processing of the intron-containing thymidylate synthase (td) gene of phage T4 is at the RNA level. Cell. 1985;41:375–382. doi: 10.1016/s0092-8674(85)80010-6. [DOI] [PubMed] [Google Scholar]
- 47.Kruger K., Grabowski P.J., Zaug A.J., Sands J., Gottschling D.E., Cech T.R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 48.Salis H.M., Mirsky E.A., Voigt C.A. Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol. 2009;27:946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Espah Borujeni A., Salis H.M. Translation initiation is controlled by RNA folding kinetics via a ribosome drafting mechanism. J Am Chem Soc. 2016;138:7016–7023. doi: 10.1021/jacs.6b01453. [DOI] [PubMed] [Google Scholar]
- 50.Alba B.M., Leeds J.A., Onufryk C., Lu C.Z., Gross C.A. DegS and YaeL participate sequentially in the cleavage of RseA to activate the σE-dependent extracytoplasmic stress response. Genes Dev. 2002;16:2156–2168. doi: 10.1101/gad.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanehara K., Ito K., Akiyama Y. YaeL (EcfE) activates the σE pathway of stress response through a site-2 cleavage of anti-σE, RseA. Genes Dev. 2002;16:2147–2155. doi: 10.1101/gad.1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown M.S., Ye J., Rawson R.B., Goldstein J.L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 53.Stragier P., Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 54.LaBell T.L., Trempy J.E., Haldenwang W.G. Sporulation-specific σ factor σ29 of Bacillus subtilis is synthesized from a precursor protein, P31. Proc Natl Acad Sci U. S. A. 1987;84:1784–1788. doi: 10.1073/pnas.84.7.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christensen S.K., Maenhaut-Michel G., Mine N., Gottesman S., Gerdes K., Van Melderen L. Overproduction of the Lon protease triggers inhibition of translation in Escherichia coli: involvement of the yefM-yoeB toxin-antitoxin system. Mol Microbiol. 2004;51:1705–1717. doi: 10.1046/j.1365-2958.2003.03941.x. [DOI] [PubMed] [Google Scholar]
- 56.Gottesman S. Regulation by proteolysis: developmental switches. Curr Opin Microbiol. 1999;2:142–147. doi: 10.1016/S1369-5274(99)80025-3. [DOI] [PubMed] [Google Scholar]
- 57.Nothaft H., Szymanski C.M. Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol. 2010;8:765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- 58.Mescher M.F., Strominger J.L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976;251:2005–2014. [PubMed] [Google Scholar]
- 59.Sleytr U.B. Heterologous reattachment of regular arrays of glycoproteins on bacterial surfaces. Nature. 1975;257:400–402. doi: 10.1038/257400a0. [DOI] [PubMed] [Google Scholar]
- 60.Sleytr U.B., Thorne K.J. Chemical characterization of the regularly arranged surface layers of Clostridium thermosaccharolyticum and Clostridium thermohydrosulfuricum. J Bacteriol. 1976;126:377–383. doi: 10.1128/jb.126.1.377-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y.M., Frank M.W., Virga K.G., Lee R.E., Rock C.O., Jackowski S. Acyl carrier protein is a cellular target for the antibacterial action of the pantothenamide class of pantothenate antimetabolites. J Biol Chem. 2004;279:50969–50975. doi: 10.1074/jbc.M409607200. [DOI] [PubMed] [Google Scholar]
- 62.Clifton G., Bryant S.R., Skinner C.G. N1-(substituted) pantothenamides, antimetabolites of pantothenic acid. Arch Biochem Biophys. 1970;137:523–528. doi: 10.1016/0003-9861(70)90470-4. [DOI] [PubMed] [Google Scholar]
- 63.Magnuson K., Jackowski S., Rock C.O., Cronan J.E., Jr. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993;57:522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitrophanov A.Y., Groisman E.A. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008;22:2601–2611. doi: 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Groisman E.A. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stock A.M., Robinson V.L., Goudreau P.N. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 67.Stock J.B., Ninfa A.J., Stock A.M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garnak M., Reeves H.C. Phosphorylation of isocitrate dehydrogenase of Escherichia coli. Science. 1979;203(4385):1111–1112. doi: 10.1126/science.34215. [DOI] [PubMed] [Google Scholar]
- 69.Klotz I.M., Langerman N.R., Darnall D.W. Quaternary structure of proteins. Annu Rev Biochem. 1970;39:25–62. doi: 10.1146/annurev.bi.39.070170.000325. [DOI] [PubMed] [Google Scholar]
- 70.Murakami K.S., Darst S.A. Bacterial RNA polymerases: the whole story. Curr Opin Struct Biol. 2003;13:31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 71.Saecker R.M., Record M.T., Jr., deHaseth P.L. Mechanism of bacterial transcription initiation: RNA polymerase - promoter binding, isomerization to initiation-competent open complexes, and initiation of RNA synthesis. J Mol Biol. 2011;412:754–771. doi: 10.1016/j.jmb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haugen S.P., Ross W., Gourse R.L. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat Rev Microbiol. 2008;6:507–519. doi: 10.1038/nrmicro1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ebright R.H. RNA polymerase: structural similarities between bacterial RNA polymerase and eukaryotic RNA polymerase II. J Mol Biol. 2000;304:687–698. doi: 10.1006/jmbi.2000.4309. [DOI] [PubMed] [Google Scholar]
- 74.Vassylyev D.G., Sekine S., Laptenko O., Lee J., Vassylyeva M.N., Borukhov S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 75.Farrell C.M., Grossman A.D., Sauer R.T. Cytoplasmic degradation of ssrA-tagged proteins. Mol Microbiol. 2005;57(6):1750–1761. doi: 10.1111/j.1365-2958.2005.04798.x. [DOI] [PubMed] [Google Scholar]
- 76.Kim Y.E., Hipp M.S., Bracher A., Hayer-Hartl M., Hartl F.U. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 77.Jacob F., Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- 78.Price M.N., Arkin A.P., Alm E.J. The life-cycle of operons. PLoS Genet. 2006;2:e96. doi: 10.1371/journal.pgen.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf Y.I., Rogozin I.B., Kondrashov A.S., Koonin E.V. Genome alignment, evolution of prokaryotic genome organization, and prediction of gene function using genomic context. Genome Res. 2001;11:356–372. doi: 10.1101/gr.gr-1619r. [DOI] [PubMed] [Google Scholar]
- 80.Ermolaeva M.D., White O., Salzberg S.L. Prediction of operons in microbial genomes. Nucleic Acids Res. 2001;29:1216–1221. doi: 10.1093/nar/29.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Price M.N., Huang K.H., Alm E.J., Arkin A.P. A novel method for accurate operon predictions in all sequenced prokaryotes. Nucleic Acids Res. 2005;33:880–892. doi: 10.1093/nar/gki232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Selinger D.W., Saxena R.M., Cheung K.J., Church G.M., Rosenow C. Global RNA half-life analysis in Escherichia coli reveals positional patterns of transcript degradation. Genome Res. 2003;13:216–223. doi: 10.1101/gr.912603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emory S.A., Bouvet P., Belasco J.G. A 5'-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- 84.Watters K.E., Abbott T.R., Lucks J.B. Simultaneous characterization of cellular RNA structure and function with in-cell SHAPE-Seq. Nucleic Acids Res. 2016;44:e12. doi: 10.1093/nar/gkv879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfleger B.F., Pitera D.J., Smolke C.D., Keasling J.D. Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat Biotechnol. 2006;24:1027–1032. doi: 10.1038/nbt1226. [DOI] [PubMed] [Google Scholar]
- 86.Hansen M.M., Ventosa Rosquelles M., Yelleswarapu M., Maas R.J., van Vugt-Jonker A.J., Heus H.A. Protein synthesis in coupled and uncoupled cell-free prokaryotic gene expression systems. ACS Synth Biol. 2016;5:1433–1440. doi: 10.1021/acssynbio.6b00010. [DOI] [PubMed] [Google Scholar]
- 87.Iost I., Dreyfus M. The stability of Escherichia coli lacZ mRNA depends upon the simultaneity of its synthesis and translation. EMBO J. 1995;14:3252–3261. doi: 10.1002/j.1460-2075.1995.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewicki B.T., Margus T., Remme J., Nierhaus K.H. Coupling of rRNA transcription and ribosomal assembly in vivo. Formation of active ribosomal subunits in Escherichia coli requires transcription of rRNA genes by host RNA polymerase which cannot be replaced by bacteriophage T7 RNA polymerase. J Mol Biol. 1993;231:581–593. doi: 10.1006/jmbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- 89.Massé E., Drolet M. Escherichia coli DNA topoisomerase I inhibits R-loop formation by relaxing transcription-induced negative supercoiling. J Biol Chem. 1999;274:16659–16664. doi: 10.1074/jbc.274.23.16659. [DOI] [PubMed] [Google Scholar]
- 90.Iskakova M.B., Szaflarski W., Dreyfus M., Remme J., Nierhaus K.H. Troubleshooting coupled in vitro transcription-translation system derived from Escherichia coli cells: synthesis of high-yield fully active proteins. Nucleic Acids Res. 2006;34:e135. doi: 10.1093/nar/gkl462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim H.N., Lee Y., Hussein R. Fundamental relationship between operon organization and gene expression. Proc Natl Acad Sci U. S. A. 2011;108:10626–10631. doi: 10.1073/pnas.1105692108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolfe A.J. The acetate switch. Microbiol Mol Biol Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mogil L.S., Becker N.A., Maher L.J., 3rd Supercoiling effects on short-range DNA looping in E. coli. PLoS One. 2016;11:e0165306. doi: 10.1371/journal.pone.0165306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q., Wu J., Friedberg D., Plakto J., Calvo J.M. Regulation of the Escherichia coli lrp gene. J Bacteriol. 1994;176:1831–1839. doi: 10.1128/jb.176.7.1831-1839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Travers A., Muskhelishvili G. DNA supercoiling - a global transcriptional regulator for enterobacterial growth? Nat Rev Microbiol. 2005;3:157–169. doi: 10.1038/nrmicro1088. [DOI] [PubMed] [Google Scholar]
- 96.Kelly A., Goldberg M.D., Carroll R.K., Danino V., Hinton J.C., Dorman C.J. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology. 2004;150:2037–2053. doi: 10.1099/mic.0.27209-0. [DOI] [PubMed] [Google Scholar]
- 97.Hommais F., Krin E., Laurent-Winter C., Soutourina O., Malpertuy A., Le Caer J.P. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol Microbiol. 2001;40(1):20–36. doi: 10.1046/j.1365-2958.2001.02358.x. [DOI] [PubMed] [Google Scholar]
- 98.Higgins C.F., Dorman C.J., Stirling D.A., Waddell L., Booth I.R., May G. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 99.Werner F., Grohmann D. Evolution of multisubunit RNA polymerases in the three domains of life. Nat Rev Microbiol. 2011;9:85–98. doi: 10.1038/nrmicro2507. [DOI] [PubMed] [Google Scholar]
- 100.Werner F. Structural evolution of multisubunit RNA polymerases. Trends Microbiol. 2008;16:247–250. doi: 10.1016/j.tim.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 101.Deuschle U., Kammerer W., Gentz R., Bujard H. Promoters of Escherichia coli: a hierarchy of in vivo strength indicates alternate structures. EMBO J. 1986;5:2987–2994. doi: 10.1002/j.1460-2075.1986.tb04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mulligan M.E., Hawley D.K., Entriken R., McClure W.R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984;12:789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lutz R., Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997;25(6):1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ross W., Aiyar S.E., Salomon J., Gourse R.L. Escherichia coli promoters with UP elements of different strengths: modular structure of bacterial promoters. J Bacteriol. 1998;180:5375–5383. doi: 10.1128/jb.180.20.5375-5383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Boer H.A., Comstock L.J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U. S. A. 1983;80:21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.d'Aubenton Carafa Y., Brody E., Thermes C. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990;216:835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]
- 107.Yager T.D., von Hippel P.H. A thermodynamic analysis of RNA transcript elongation and termination in Escherichia coli. Biochemistry. 1991;30:1097–1118. doi: 10.1021/bi00218a032. [DOI] [PubMed] [Google Scholar]
- 108.von Hippel P.H., Yager T.D. Transcript elongation and termination are competitive kinetic processes. Proc Natl Acad Sci U. S. A. 1991;88:2307–2311. doi: 10.1073/pnas.88.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paget M.S., Helmann J.D. The σ70 family of sigma factors. Genome Biol. 2003;4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feklístov A., Sharon B.D., Darst S.A., Gross C.A. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu Rev Microbiol. 2014;68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 111.Österberg S., del Peso-Santos T., Shingler V. Regulation of alternative sigma factor use. Annu Rev Microbiol. 2011;65:37–55. doi: 10.1146/annurev.micro.112408.134219. [DOI] [PubMed] [Google Scholar]
- 112.Kazmierczak M.J., Wiedmann M., Boor K.J. Alternative sigma factors and their roles in bacterial virulence. Microbiol Mol Biol Rev. 2005;69:527–543. doi: 10.1128/MMBR.69.4.527-543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruff E.F., Record M.T., Jr., Artsimovitch I. Initial events in bacterial transcription initiation. Biomolecules. 2015;5:1035–1062. doi: 10.3390/biom5021035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Browning D.F., Busby S.J. The regulation of bacterial transcription initiation. Nat Rev Microbiol. 2004;2:57–65. doi: 10.1038/nrmicro787. [DOI] [PubMed] [Google Scholar]
- 116.Henderson K.L., Felth L.C., Molzahn C.M., Shkel I., Wang S., Chhabra M. Mechanism of transcription initiation and promoter escape by E. coli RNA polymerase. Proc Natl Acad Sci U. S. A. 2017;114:E3032–E3040. doi: 10.1073/pnas.1618675114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruff E.F., Drennan A.C., Capp M.W., Poulos M.A., Artsimovitch I., Record M.T., Jr. E. coli RNA polymerase determinants of open complex lifetime and structure. J Mol Biol. 2015;427:2435–2450. doi: 10.1016/j.jmb.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haugen S.P., Berkmen M.B., Ross W., Gaal T., Ward C., Gourse R.L. rRNA promoter regulation by nonoptimal binding of σ region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 119.Winkelman J.T., Chandrangsu P., Ross W., Gourse R.L. Open complex scrunching before nucleotide addition accounts for the unusual transcription start site of E. coli ribosomal RNA promoters. Proc Natl Acad Sci U. S. A. 2016;113:E1787–E1795. doi: 10.1073/pnas.1522159113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bae B., Feklistov A., Lass-Napiorkowska A., Landick R., Darst S.A. Structure of a bacterial RNA polymerase holoenzyme open promoter complex. Elife. 2015:4. doi: 10.7554/eLife.08504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Browning D.F., Busby S.J. Local and global regulation of transcription initiation in bacteria. Nat Rev Microbiol. 2016;14(10):638–650. doi: 10.1038/nrmicro.2016.103. [DOI] [PubMed] [Google Scholar]
- 122.Hawley D.K., McClure W.R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harley C.B., Reynolds R.P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987;15:2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aiyar S.E., Gourse R.L., Ross W. Upstream A-tracts increase bacterial promoter activity through interactions with the RNA polymerase α subunit. Proc Natl Acad Sci U. S. A. 1998;95:14652–14657. doi: 10.1073/pnas.95.25.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sivaraman K., Seshasayee A.S., Swaminathan K., Muthukumaran G., Pennathur G. Promoter addresses: revelations from oligonucleotide profiling applied to the Escherichia coli genome. Theor Biol Med Model. 2005;2:20. doi: 10.1186/1742-4682-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feklistov A., Bae B., Hauver J., Lass-Napiorkowska A., Kalesse M., Glaus F. RNA polymerase motions during promoter melting. Science. 2017;356:863–866. doi: 10.1126/science.aam7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mendoza-Vargas A., Olvera L., Olvera M., Grande R., Vega-Alvarado L., Taboada B. Genome-wide identification of transcription start sites, promoters and transcription factor binding sites in E. coli. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007526. e7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heyduk E., Kuznedelov K., Severinov K., Heyduk T. A consensus adenine at position -11 of the nontemplate strand of bacterial promoter is important for nucleation of promoter melting. J Biol Chem. 2006;281:12362–12369. doi: 10.1074/jbc.M601364200. [DOI] [PubMed] [Google Scholar]
- 129.Revyakin A., Liu C., Ebright R.H., Strick T.R. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science. 2006;314:1139–1143. doi: 10.1126/science.1131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kapanidis A.N., Margeat E., Ho S.O., Kortkhonjia E., Weiss S., Ebright R.H. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science. 2006;314:1144–1147. doi: 10.1126/science.1131399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Mey M., Maertens J., Lequeux G.J., Soetaert W.K., Vandamme E.J. Construction and model-based analysis of a promoter library for E. coli: an indispensable tool for metabolic engineering. BMC Biotechnol. 2007;7:34. doi: 10.1186/1472-6750-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kawano M., Storz G., Rao B.S., Rosner J.L., Martin R.G. Detection of low-level promoter activity within open reading frame sequences of Escherichia coli. Nucleic Acids Res. 2005;33:6268–6276. doi: 10.1093/nar/gki928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bron P.A., Hoffer S.M., Van S., II, De Vos W.M., Kleerebezem M. Selection and characterization of conditionally active promoters in Lactobacillus plantarum, using alanine racemase as a promoter probe. Appl Environ Microbiol. 2004;70:310–317. doi: 10.1128/AEM.70.1.310-317.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stout V. Identification of the promoter region for the colanic acid polysaccharide biosynthetic genes in Escherichia coli K-12. J Bacteriol. 1996;178:4273–4280. doi: 10.1128/jb.178.14.4273-4280.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Slauch J.M., Camilli A. IVET and RIVET: use of gene fusions to identify bacterial virulence factors specifically induced in host tissues. Methods Enzymol. 2000;326:73–96. doi: 10.1016/s0076-6879(00)26047-3. [DOI] [PubMed] [Google Scholar]
- 136.Mahan M.J., Slauch J.M., Mekalanos J.J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 137.Valdivia R.H., Falkow S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science. 1997;277:2007–2011. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]
- 138.Thomason M.K., Bischler T., Eisenbart S.K., Förstner K.U., Zhang A., Herbig A. Global transcriptional start site mapping using differential RNA sequencing reveals novel antisense RNAs in Escherichia coli. J Bacteriol. 2015;197:18–28. doi: 10.1128/JB.02096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yan B., Núñez C., Ueki T., Esteve-Núñez A., Puljic M., Adkins R.M. Computational prediction of RpoS and RpoD regulatory sites in Geobacter sulfurreducens using sequence and gene expression information. Gene. 2006;384:73–95. doi: 10.1016/j.gene.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 140.Weber H., Polen T., Heuveling J., Wendisch V.F., Hengge R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J Bacteriol. 2005;187:1591–1603. doi: 10.1128/JB.187.5.1591-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kröger C., Dillon S.C., Cameron A.D., Papenfort K., Sivasankaran S.K., Hokamp K. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U. S. A. 2012;109:E1277–E1286. doi: 10.1073/pnas.1201061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ray-Soni A., Bellecourt M.J., Landick R. Mechanisms of bacterial transcription termination: all good things must end. Annu Rev Biochem. 2016;85:319–347. doi: 10.1146/annurev-biochem-060815-014844. [DOI] [PubMed] [Google Scholar]
- 143.Banerjee S., Chalissery J., Bandey I., Sen R. Rho-dependent transcription termination: more questions than answers. J Microbiol. 2006;44:11–22. [PMC free article] [PubMed] [Google Scholar]
- 144.Peters J.M., Vangeloff A.D., Landick R. Bacterial transcription terminators: the RNA 3'-end chronicles. J Mol Biol. 2011;412:793–813. doi: 10.1016/j.jmb.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Conway T., Creecy J.P., Maddox S.M., Grissom J.E., Conkle T.L., Shadid T.M. Unprecedented high-resolution view of bacterial operon architecture revealed by RNA sequencing. MBio. 2014;5 doi: 10.1128/mBio.01442-14. e01442-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Epshtein V., Toulmé F., Rahmouni A.R., Borukhov S., Nudler E. Transcription through the roadblocks: the role of RNA polymerase cooperation. EMBO J. 2003;22:4719–4727. doi: 10.1093/emboj/cdg452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U. S. A. 1977;74:4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gottesman M.E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980;140:57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- 149.Yang X.J., Hart C.M., Grayhack E.J., Roberts J.W. Transcription antitermination by phage λ gene Q protein requires a DNA segment spanning the RNA start site. Genes Dev. 1987;1:217–226. doi: 10.1101/gad.1.3.217. [DOI] [PubMed] [Google Scholar]
- 150.Olson E.R., Flamm E.L., Friedman D.I. Analysis of nutR: a region of phage lambda required for antitermination of transcription. Cell. 1982;31:61–70. doi: 10.1016/0092-8674(82)90405-6. [DOI] [PubMed] [Google Scholar]
- 151.Grayhack E.J., Roberts J.W. The phage λ Q gene product: activity of a transcription antiterminator in vitro. Cell. 1982;30:637–648. doi: 10.1016/0092-8674(82)90260-4. [DOI] [PubMed] [Google Scholar]
- 152.Friedman D.I., Court D.L. Transcription antitermination: the λ paradigm updated. Mol Microbiol. 1995;18:191–200. doi: 10.1111/j.1365-2958.1995.mmi_18020191.x. [DOI] [PubMed] [Google Scholar]
- 153.Turnbough C.L., Jr., Switzer R.L. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol Mol Biol Rev. 2008;72:266–300. doi: 10.1128/MMBR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Richardson J.P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U. S. A. 1975;72:1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- 156.Oppenheim D.S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980;95:785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harinarayanan R., Gowrishankar J. Host factor titration by chromosomal R-loops as a mechanism for runaway plasmid replication in transcription termination-defective mutants of Escherichia coli. J Mol Biol. 2003;332:31–46. doi: 10.1016/s0022-2836(03)00753-8. [DOI] [PubMed] [Google Scholar]
- 158.Leela J.K., Syeda A.H., Anupama K., Gowrishankar J. Rho-dependent transcription termination is essential to prevent excessive genome-wide R-loops in Escherichia coli. Proc Natl Acad Sci U. S. A. 2013;110:258–263. doi: 10.1073/pnas.1213123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aguilera A., García-Muse T. R loops: from transcription byproducts to threats to genome stability. Mol Cell. 2012;46:115–124. doi: 10.1016/j.molcel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 160.Liu L.F., Wang J.C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U. S. A. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stueber D., Bujard H. Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J. 1982;1:1399–1404. doi: 10.1002/j.1460-2075.1982.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kotlajich M.V., Hron D.R., Boudreau B.A., Sun Z., Lyubchenko Y.L., Landick R. Bridged filaments of histone-like nucleoid structuring protein pause RNA polymerase and aid termination in bacteria. Elife. 2015:4. doi: 10.7554/eLife.04970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Landick R. Transcriptional pausing without backtracking. Proc Natl Acad Sci U. S. A. 2009;106:8797–8798. doi: 10.1073/pnas.0904373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dutta D., Chalissery J., Sen R. Transcription termination factor rho prefers catalytically active elongation complexes for releasing RNA. J Biol Chem. 2008;283:20243–20251. doi: 10.1074/jbc.M801926200. [DOI] [PubMed] [Google Scholar]
- 165.Hein P.P., Kolb K.E., Windgassen T., Bellecourt M.J., Darst S.A., Mooney R.A. RNA polymerase pausing and nascent-RNA structure formation are linked through clamp-domain movement. Nat Struct Mol Biol. 2014;21:794–802. doi: 10.1038/nsmb.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orosz A., Boros I., Venetianer P. Analysis of the complex transcription termination region of the Escherichia coli rrnB gene. Eur J Biochem. 1991;201:653–659. doi: 10.1111/j.1432-1033.1991.tb16326.x. [DOI] [PubMed] [Google Scholar]
- 167.Uptain S.M., Chamberlin M.J. Escherichia coli RNA polymerase terminates transcription efficiently at rho-independent terminators on single-stranded DNA templates. Proc Natl Acad Sci U. S. A. 1997;94:13548–13553. doi: 10.1073/pnas.94.25.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Telesnitsky A.P., Chamberlin M.J. Sequences linked to prokaryotic promoters can affect the efficiency of downstream termination sites. J Mol Biol. 1989;205:315–330. doi: 10.1016/0022-2836(89)90343-4. [DOI] [PubMed] [Google Scholar]
- 169.Scholtissek S., Grosse F. A cloning cartridge of λ t(o) terminator. Nucleic Acids Res. 1987;15:3185. doi: 10.1093/nar/15.7.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McDowell J.C., Roberts J.W., Jin D.J., Gross C. Determination of intrinsic transcription termination efficiency by RNA polymerase elongation rate. Science. 1994;266:822–825. doi: 10.1126/science.7526463. [DOI] [PubMed] [Google Scholar]
- 171.Hajimorad M., Gray P.R., Keasling J.D. A framework and model system to investigate linear system behavior in Escherichia coli. J Biol Eng. 2011;5:3. doi: 10.1186/1754-1611-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in λ. Proc Natl Acad Sci U. S. A. 1970;66:855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oppenheim A.B., Kobiler O., Stavans J., Court D.L., Adhya S. Switches in bacteriophage lambda development. Annu Rev Genet. 2005;39:409–429. doi: 10.1146/annurev.genet.39.073003.113656. [DOI] [PubMed] [Google Scholar]
- 174.Schubert R.A., Dodd I.B., Egan J.B., Shearwin K.E. Cro's role in the CI Cro bistable switch is critical for λ's transition from lysogeny to lytic development. Genes Dev. 2007;21:2461–2472. doi: 10.1101/gad.1584907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Johnson A., Meyer B.J., Ptashne M. Mechanism of action of the cro protein of bacteriophage λ. Proc Natl Acad Sci U. S. A. 1978;75:1783–1787. doi: 10.1073/pnas.75.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Herskowitz I., Hagen D. The lysis-lysogeny decision of phage λ: explicit programming and responsiveness. Annu Rev Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- 177.Benson N., Youderian P. Phage λ Cro protein and cI repressor use two different patterns of specific protein-DNA interactions to achieve sequence specificity in vivo. Genetics. 1989;121:5–12. doi: 10.1093/genetics/121.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Humayun Z., Jeffrey A., Ptashne M. Completed DNA sequences and organization of repressor-binding sites in the operators of phage lambda. J Mol Biol. 1977;112:265–277. doi: 10.1016/s0022-2836(77)80143-5. [DOI] [PubMed] [Google Scholar]
- 179.Vilar J.M., Saiz L. DNA looping in gene regulation: from the assembly of macromolecular complexes to the control of transcriptional noise. Curr Opin Genet Dev. 2005;15:136–144. doi: 10.1016/j.gde.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 180.Lieb M. Studies of heat-inducible lambda bacteriophage. I. Order of genetic sites and properties of mutant prophages. J Mol Biol. 1966;16:149–163. doi: 10.1016/s0022-2836(66)80269-3. [DOI] [PubMed] [Google Scholar]
- 181.Lawson C.L., Swigon D., Murakami K.S., Darst S.A., Berman H.M., Ebright R.H. Catabolite activator protein: DNA binding and transcription activation. Curr Opin Struct Biol. 2004;14:10–20. doi: 10.1016/j.sbi.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ebright R.H., Ebright Y.W., Gunasekera A. Consensus DNA site for the Escherichia coli catabolite gene activator protein (CAP): CAP exhibits a 450-fold higher affinity for the consensus DNA site than for the E. coli lac DNA site. Nucleic Acids Res. 1989;17:10295–10305. doi: 10.1093/nar/17.24.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zheng D., Constantinidou C., Hobman J.L., Minchin S.D. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 2004;32:5874–5893. doi: 10.1093/nar/gkh908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Görke B., Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 185.Igarashi K., Ishihama A. Bipartite functional map of the E. coli RNA polymerase α subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991;65:1015–1022. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- 186.Niu W., Kim Y., Tau G., Heyduk T., Ebright R.H. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guzman L.M., Belin D., Carson M.J., Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Blommel P.G., Becker K.J., Duvnjak P., Fox B.G. Enhanced bacterial protein expression during auto-induction obtained by alteration of lac repressor dosage and medium composition. Biotechnol Prog. 2007;23:585–598. doi: 10.1021/bp070011x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lewis M. The lac repressor. C R Biol. 2005;328:521–548. doi: 10.1016/j.crvi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 190.Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U. S. A. 1973;70:3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lewis M., Chang G., Horton N.C., Kercher M.A., Pace H.C., Schumacher M.A. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271:1247–1254. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- 192.Dickson R.C., Abelson J., Barnes W.M., Reznikoff W.S. Genetic regulation: the Lac control region. Science. 1975;187:27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- 193.Daber R., Stayrook S., Rosenberg A., Lewis M. Structural analysis of Lac repressor bound to allosteric effectors. J Mol Biol. 2007;370:609–619. doi: 10.1016/j.jmb.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jobe A., Bourgeois S. Lac Repressor-operator interaction. VI. The natural inducer of the lac operon. J Mol Biol. 1972;69:397–408. doi: 10.1016/0022-2836(72)90253-7. [DOI] [PubMed] [Google Scholar]
- 195.Oehler S., Eismann E.R., Krämer H., Müller-Hill B. The three operators of the lac operon cooperate in repression. EMBO J. 1990;9:973–979. doi: 10.1002/j.1460-2075.1990.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Swigon D., Coleman B.D., Olson W.K. Modeling the Lac repressor-operator assembly: the influence of DNA looping on Lac repressor conformation. Proc Natl Acad Sci U. S. A. 2006;103:9879–9884. doi: 10.1073/pnas.0603557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schlax P.J., Capp M.W., Record M.T., Jr. Inhibition of transcription initiation by lac repressor. J Mol Biol. 1995;245:331–350. doi: 10.1006/jmbi.1994.0028. [DOI] [PubMed] [Google Scholar]
- 198.Becker N.A., Kahn J.D., Maher L.J., 3rd Bacterial repression loops require enhanced DNA flexibility. J Mol Biol. 2005;349:716–730. doi: 10.1016/j.jmb.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 199.Elowitz M.B., Levine A.J., Siggia E.D., Swain P.S. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 200.Jensen P.R., Westerhoff H.V., Michelsen O. The use of lac-type promoters in control analysis. Eur J Biochem. 1993;211:181–191. doi: 10.1111/j.1432-1033.1993.tb19885.x. [DOI] [PubMed] [Google Scholar]
- 201.Novick A., Weiner M. Enzyme induction as an all-or-none phenomenon. Proc Natl Acad Sci U. S. A. 1957;43:553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maloney P.C., Rotman B. Distribution of suboptimally induces β-D-galactosidase in Escherichia coli: the enzyme content of individual cells. J Mol Biol. 1973;73:77–91. doi: 10.1016/0022-2836(73)90160-5. [DOI] [PubMed] [Google Scholar]
- 203.Lee N., Francklyn C., Hamilton E.P. Arabinose-induced binding of AraC protein to araI2 activates the araBAD operon promoter. Proc Natl Acad Sci U. S. A. 1987;84:8814–8818. doi: 10.1073/pnas.84.24.8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schleif R. Regulation of the L-arabinose operon of Escherichia coli. Trends Genet. 2000;16:559–565. doi: 10.1016/s0168-9525(00)02153-3. [DOI] [PubMed] [Google Scholar]
- 205.Khlebnikov A., Risa Ø., Skaug T., Carrier T.A., Keasling J.D. Regulatable arabinose-inducible gene expression system with consistent control in all cells of a culture. J Bacteriol. 2000;182:7029–7034. doi: 10.1128/jb.182.24.7029-7034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Siegele D.A., Hu J.C. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc Natl Acad Sci U. S. A. 1997;94:8168–8172. doi: 10.1073/pnas.94.15.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ramos J.L., Martinez-Bueno M., Molina-Henares A.J., Terán W., Watanabe K., Zhang X. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Orth P., Schnappinger D., Hillen W., Saenger W., Hinrichs W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat Struct Biol. 2000;7:215–219. doi: 10.1038/73324. [DOI] [PubMed] [Google Scholar]
- 209.Hillen W., Schollmeier K., Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J Mol Biol. 1984;172:185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- 210.Hinrichs W., Kisker C., Düvel M., Müller A., Tovar K., Hillen W. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science. 1994;264:418–420. doi: 10.1126/science.8153629. [DOI] [PubMed] [Google Scholar]
- 211.Miller M.B., Bassler B.L. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 212.Fuqua W.C., Winans S.C., Greenberg E.P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fuqua C., Greenberg E.P. Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 214.Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 215.Urbanowski M.L., Lostroh C.P., Greenberg E.P. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J Bacteriol. 2004;186:631–637. doi: 10.1128/JB.186.3.631-637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Finney A.H., Blick R.J., Murakami K., Ishihama A., Stevens A.M. Role of the C-terminal domain of the alpha subunit of RNA polymerase in LuxR-dependent transcriptional activation of the lux operon during quorum sensing. J Bacteriol. 2002;184:4520–4528. doi: 10.1128/JB.184.16.4520-4528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Devine J.H., Shadel G.S., Baldwin T.O. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proc Natl Acad Sci U. S. A. 1989;86:5688–5692. doi: 10.1073/pnas.86.15.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Basu S., Mehreja R., Thiberge S., Chen M.T., Weiss R. Spatiotemporal control of gene expression with pulse-generating networks. Proc Natl Acad Sci U. S. A. 2004;101:6355–6360. doi: 10.1073/pnas.0307571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Basu S., Gerchman Y., Collins C.H., Arnold F.H., Weiss R. A synthetic multicellular system for programmed pattern formation. Nature. 2005;434:1130–1134. doi: 10.1038/nature03461. [DOI] [PubMed] [Google Scholar]
- 220.Nocadello S., Swennen E.F. The new pLAI (lux regulon based auto-inducible) expression system for recombinant protein production in Escherichia coli. Microb Cell Fact. 2012;11:3. doi: 10.1186/1475-2859-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pai A., Tanouchi Y., You L. Optimality and robustness in quorum sensing (QS)-mediated regulation of a costly public good enzyme. Proc Natl Acad Sci U. S. A. 2012;109:19810–19815. doi: 10.1073/pnas.1211072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Federle M.J., Bassler B.L. Interspecies communication in bacteria. J Clin Invest. 2003;112:1291–1299. doi: 10.1172/JCI20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Song H., Ding M.Z., Jia X.Q., Ma Q., Yuan Y.J. Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev. 2014;43:6954–6981. doi: 10.1039/c4cs00114a. [DOI] [PubMed] [Google Scholar]
- 224.You L., Cox R.S., III, Weiss R., Arnold F.H. Programmed population control by cell-cell communication and regulated killing. Nature. 2004;428:868–871. doi: 10.1038/nature02491. [DOI] [PubMed] [Google Scholar]
- 225.Saeidi N., Wong C.K., Lo T.M., Nguyen H.X., Ling H., Leong S.S. Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol. 2011;7:521. doi: 10.1038/msb.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Balagaddé F.K., You L., Hansen C.L., Arnold F.H., Quake S.R. Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science. 2005;309:137–140. doi: 10.1126/science.1109173. [DOI] [PubMed] [Google Scholar]
- 227.Copeland M.F., Politz M.C., Pfleger B.F. Application of TALEs, CRISPR/Cas and sRNAs as trans-acting regulators in prokaryotes. Curr Opin Biotechnol. 2014;29:46–54. doi: 10.1016/j.copbio.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bogdanove A.J., Schornack S., Lahaye T. TAL effectors: finding plant genes for disease and defense. Curr Opin Plant Biol. 2010;13:394–401. doi: 10.1016/j.pbi.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 229.Bonas U., Stall R.E., Staskawicz B. Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria. Mol Gen Genet. 1989;218:127–136. doi: 10.1007/BF00330575. [DOI] [PubMed] [Google Scholar]
- 230.Römer P., Hahn S., Jordan T., Strauss T., Bonas U., Lahaye T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318:645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- 231.Mak A.N., Bradley P., Bogdanove A.J., Stoddard B.L. TAL effectors: function, structure, engineering and applications. Curr Opin Struct Biol. 2013;23:93–99. doi: 10.1016/j.sbi.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Boch J., Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol. 2010;48:419–436. doi: 10.1146/annurev-phyto-080508-081936. [DOI] [PubMed] [Google Scholar]
- 233.Mak A.N., Bradley P., Cernadas R.A., Bogdanove A.J., Stoddard B.L. The crystal structure of TAL effector PthXo1 bound to its DNA target. Science. 2012;335:716–719. doi: 10.1126/science.1216211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Deng D., Yan C., Pan X., Mahfouz M., Wang J., Zhu J.K. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science. 2012;335:720–723. doi: 10.1126/science.1215670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 236.Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 237.Streubel J., Blücher C., Landgraf A., Boch J. TAL effector RVD specificities and efficiencies. Nat Biotechnol. 2012;30(7):593–595. doi: 10.1038/nbt.2304. [DOI] [PubMed] [Google Scholar]
- 238.Rinaldi F.C., Doyle L.A., Stoddard B.L., Bogdanove A.J. The effect of increasing numbers of repeats on TAL effector DNA binding specificity. Nucleic Acids Res. 2017;45:6960–6970. doi: 10.1093/nar/gkx342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mahfouz M.M., Li L., Shamimuzzaman M., Wibowo A., Fang X., Zhu J.K. De novo-engineered transcription activator-like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double-strand breaks. Proc Natl Acad Sci U. S. A. 2011;108:2623–2628. doi: 10.1073/pnas.1019533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Polstein L.R., Perez-Pinera P., Kocak D.D., Vockley C.M., Bledsoe P., Song L. Genome-wide specificity of DNA binding, gene regulation, and chromatin remodeling by TALE- and CRISPR/Cas9-based transcriptional activators. Genome Res. 2015;25:1158–1169. doi: 10.1101/gr.179044.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Copeland M.F., Politz M.C., Johnson C.B., Markley A.L., Pfleger B.F. A transcription activator-like effector (TALE) induction system mediated by proteolysis. Nat Chem Biol. 2016;12:254–260. doi: 10.1038/nchembio.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Politz M.C., Copeland M.F., Pfleger B.F. Artificial repressors for controlling gene expression in bacteria. Chem Commun (Camb) 2013;49:4325–4327. doi: 10.1039/c2cc37107c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lienert F., Torella J.P., Chen J.H., Norsworthy M., Richardson R.R., Silver P.A. Two- and three-input TALE-based AND logic computation in embryonic stem cells. Nucleic Acids Res. 2013;41:9967–9975. doi: 10.1093/nar/gkt758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cong L., Zhou R., Kuo Y.C., Cunniff M., Zhang F. Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. Nat Commun. 2012;3:968. doi: 10.1038/ncomms1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mercer A.C., Gaj T., Sirk S.J., Lamb B.M., Barbas C.F., III Regulation of endogenous human gene expression by ligand-inducible TALE transcription factors. ACS Synth Biol. 2014;3:723–730. doi: 10.1021/sb400114p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cuculis L., Abil Z., Zhao H., Schroeder C.M. Direct observation of TALE protein dynamics reveals a two-state search mechanism. Nat Commun. 2015;6:7277. doi: 10.1038/ncomms8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Joung J.K., Sander J.D. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gao H., Wu X., Chai J., Han Z. Crystal structure of a TALE protein reveals an extended N-terminal DNA binding region. Cell Res. 2012;22:1716–1720. doi: 10.1038/cr.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lucast L.J., Batey R.T., Doudna J.A. Large-scale purification of a stable form of recombinant tobacco etch virus protease. Biotechniques. 2001;30:544–546. doi: 10.2144/01303st06. [DOI] [PubMed] [Google Scholar]
- 250.Kapust R.B., Waugh D.S. Controlled intracellular processing of fusion proteins by TEV protease. Protein Expr Purif. 2000;19:312–318. doi: 10.1006/prep.2000.1251. [DOI] [PubMed] [Google Scholar]
- 251.Sorek R., Lawrence C.M., Wiedenheft B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu Rev Biochem. 2013;82:237–266. doi: 10.1146/annurev-biochem-072911-172315. [DOI] [PubMed] [Google Scholar]
- 252.Horvath P., Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 253.Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 254.Marraffini L.A., Sontheimer E.J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322:1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wiedenheft B., Sternberg S.H., Doudna J.A. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 256.Deltcheva E., Chylinski K., Sharma C.M., Gonzales K., Chao Y., Pirzada Z.A. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Makarova K.S., Haft D.H., Barrangou R., Brouns S.J., Charpentier E., Horvath P. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nishimasu H., Ran F.A., Hsu P.D., Konermann S., Shehata S.I., Dohmae N. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156:935–949. doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jiang F., Taylor D.W., Chen J.S., Kornfeld J.E., Zhou K., Thompson A.J. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science. 2016;351:867–871. doi: 10.1126/science.aad8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Anders C., Niewoehner O., Duerst A., Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014;513:569–573. doi: 10.1038/nature13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mekler V., Minakhin L., Severinov K. Mechanism of duplex DNA destabilization by RNA-guided Cas9 nuclease during target interrogation. Proc Natl Acad Sci U. S. A. 2017;114:5443–5448. doi: 10.1073/pnas.1619926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 266.Jinek M., East A., Cheng A., Lin S., Ma E., Doudna J. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Marraffini L.A., Sontheimer E.J. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet. 2010;11:181–190. doi: 10.1038/nrg2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bikard D., Jiang W., Samai P., Hochschild A., Zhang F., Marraffini L.A. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41:7429–7437. doi: 10.1093/nar/gkt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gilbert L.A., Horlbeck M.A., Adamson B., Villalta J.E., Chen Y., Whitehead E.H. Genome-Scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chavez A., Tuttle M., Pruitt B.W., Ewen-Campen B., Chari R., Ter-Ovanesyan D. Comparison of Cas9 activators in multiple species. Nat Methods. 2016;13:563–567. doi: 10.1038/nmeth.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zalatan J.G., Lee M.E., Almeida R., Gilbert L.A., Whitehead E.H., La Russa M. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160:339–350. doi: 10.1016/j.cell.2014.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.De Mey M., De Maeseneire S., Soetaert W., Vandamme E. Minimizing acetate formation in E. coli fermentations. J Ind Microbiol Biotechnol. 2007;34:689–700. doi: 10.1007/s10295-007-0244-2. [DOI] [PubMed] [Google Scholar]
- 275.Hein S., Tran H., Steinbüchel A. Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch Microbiol. 1998;170:162–170. doi: 10.1007/s002030050629. [DOI] [PubMed] [Google Scholar]
- 276.Gründel M., Scheunemann R., Lockau W., Zilliges Y. Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology. 2012;158:3032–3043. doi: 10.1099/mic.0.062950-0. [DOI] [PubMed] [Google Scholar]
- 277.Rodriguez G.M., Atsumi S. Toward aldehyde and alkane production by removing aldehyde reductase activity in Escherichia coli. Metab Eng. 2014;25:227–237. doi: 10.1016/j.ymben.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Muro-Pastor M.I., Reyes J.C., Florencio F.J. Ammonium assimilation in cyanobacteria. Photosynth Res. 2005;83:135–150. doi: 10.1007/s11120-004-2082-7. [DOI] [PubMed] [Google Scholar]
- 279.Osanai T., Imamura S., Asayama M., Shirai M., Suzuki I., Murata N. Nitrogen induction of sugar catabolic gene expression in Synechocystis sp. PCC 6803. DNA Res. 2006;13:185–195. doi: 10.1093/dnares/dsl010. [DOI] [PubMed] [Google Scholar]
- 280.Herrero A., Muro-Pastor A.M., Flores E. Nitrogen control in cyanobacteria. J Bacteriol. 2001;183:411–425. doi: 10.1128/JB.183.2.411-425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhang S., Bryant D.A. The tricarboxylic acid cycle in cyanobacteria. Science. 2011;334:1551–1553. doi: 10.1126/science.1210858. [DOI] [PubMed] [Google Scholar]
- 282.Steinhauser D., Fernie A.R., Araújo W.L. Unusual cyanobacterial TCA cycles: not broken just different. Trends Plant Sci. 2012;17:503–509. doi: 10.1016/j.tplants.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 283.Laub M.T., Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- 284.Rowland M.A., Deeds E.J. Crosstalk and the evolution of specificity in two-component signaling. Proc Natl Acad Sci U. S. A. 2014;111:5550–5555. doi: 10.1073/pnas.1317178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Capra E.J., Perchuk B.S., Skerker J.M., Laub M.T. Adaptive mutations that prevent crosstalk enable the expansion of paralogous signaling protein families. Cell. 2012;150:222–232. doi: 10.1016/j.cell.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schell M.A. Transcriptional control of the nah and sal hydrocarbon-degradation operons by the nahR gene product. Gene. 1985;36:301–309. doi: 10.1016/0378-1119(85)90185-4. [DOI] [PubMed] [Google Scholar]
- 287.Isberg R.R., Voorhis D.L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 288.Hughes J., Lamparter T., Mittmann F., Hartmann E., Gärtner W., Wilde A. A prokaryotic phytochrome. Nature. 1997;386:663. doi: 10.1038/386663a0. [DOI] [PubMed] [Google Scholar]
- 289.Lamparter T., Mittmann F., Gärtner W., Börner T., Hartmann E., Hughes J. Characterization of recombinant phytochrome from the cyanobacterium Synechocystis. Proc Natl Acad Sci U. S. A. 1997;94:11792–11797. doi: 10.1073/pnas.94.22.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yeh K.C., Wu S.H., Murphy J.T., Lagarias J.C. A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277:1505–1508. doi: 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]
- 291.Forst S., Delgado J., Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U. S. A. 1989;86:6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Levskaya A., Weiner O.D., Lim W.A., Voigt C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mascher T., Helmann J.D., Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev. 2006;70:910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tabor J.J., Salis H.M., Simpson Z.B., Chevalier A.A., Levskaya A., Marcotte E.M. A synthetic genetic edge detection program. Cell. 2009;137:1272–1281. doi: 10.1016/j.cell.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hirose Y., Shimada T., Narikawa R., Katayama M., Ikeuchi M. Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proc Natl Acad Sci U. S. A. 2008;105:9528–9533. doi: 10.1073/pnas.0801826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tabor J.J., Levskaya A., Voigt C.A. Multichromatic control of gene expression in Escherichia coli. J Mol Biol. 2011;405:315–324. doi: 10.1016/j.jmb.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Möglich A., Ayers R.A., Moffat K. Design and signaling mechanism of light-regulated histidine kinases. J Mol Biol. 2009;385:1433–1444. doi: 10.1016/j.jmb.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]