Abstract
Although the cytochrome P450 CYP27B1 plays a critical role in vitamin D biology, the molecular mechanisms involved in regulation of CYP27B1 have remained undefined. A new study has identified a kidney-specific control module distal to the Cyp27b1 gene that mediates the basal activity and hormonal regulation of Cyp27b1. This work provides a novel mechanism indicating differential regulation of Cyp27b1 in renal and non-renal cells and has implications for vitamin D biology in multiple sclerosis and perhaps other autoimmune diseases as well.
Introduction
Vitamin D is critical for calcium homeostasis and is required for the development and maintenance of skeletal integrity. In addition, it has been suggested to have roles in extraskeletal health including cancer, immunity, and autoimmune diseases. Vitamin D undergoes two enzymatic steps to form the active compound 1,25-dihydroxyvitamin D3 (1,25(OH)2D3).2 CYP27B1 is a cytochrome P450 enzyme that performs the second step in this process, metabolizing 25-hydroxyvitamin D3 to 1,25(OH)2D3 (1, 2), and thus controls the biological activity of vitamin D. Inactivating mutations in the gene encoding CYP27B1 cause vitamin D-dependent rickets type 1 (VDDRI) (also known as pseudovitamin D deficiency rickets) despite normal intake of vitamin D (3), indicating the importance of this enzyme for skeletal integrity. CYP27B1 is present predominantly in the proximal straight tubule of the kidney and is also expressed in low levels in other tissues including placenta, immune cells, and malignant epithelia (1). However, whether CYP27B1 serves a function at sites other than the kidney and placenta under normal physiological conditions is a matter of debate (1) that has been difficult to resolve. This has been due at least in part to the fact that the detailed regulatory mechanisms controlling this enzyme are not clear and tools to establish these mechanisms, particularly in a location-sensitive manner, are lacking. A new report by Meyer et al. (4) addresses these gaps using ChIP-seq analysis as well as newly generated genetic mouse models to identify a kidney-specific regulatory module that controls basal and hormone-regulated expression of Cyp27b1.
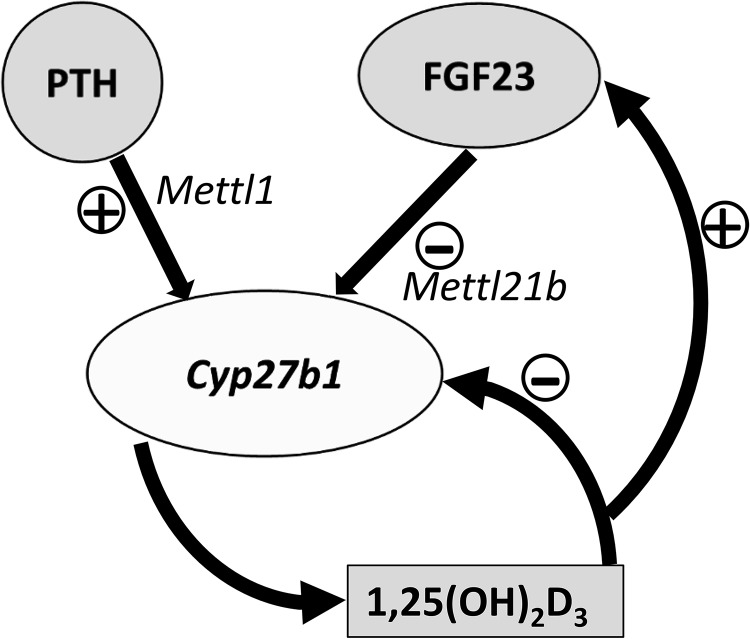
CYP27B1 is tightly regulated. A primary signal in mediating induction of 1,25(OH)2D3 in the kidney is elevated parathyroid hormone (PTH). This was demonstrated in early animal studies in which thyroparathyroidectomy resulted in reduced production of 1,25(OH)2D3, whereas administration of parathyroid extract restored 1,25(OH)2D3 production almost to control levels (5). 1,25(OH)2D3 is known to regulate its own production by inhibiting CYP27B1. In addition to 1,25(OH)2D3, the phosphaturic factor fibroblast growth factor 23 (FGF23), which acts as an endocrine factor, also suppresses expression of renal CYP27B1 (1, 2) (Fig. 1). But, what are the molecular mechanisms connecting these hormones to CYP27B1 and each other? Some initial hints have emerged. Early studies showed that 1,25(OH)2D3 treatment could suppress Cyp27b1 expression in both thyroparathyroidectomy and sham-operated rats, suggesting that activation by PTH and suppression by 1,25(OH)2D3 are two distinct events (6). It was suggested that 1,25(OH)2D3-mediated suppression may not be based on direct binding of the vitamin D receptor to a consensus vitamin D response element in the Cyp27b1 gene but rather may be indirect (7).
Figure 1.
Regulation of renal CYP27B1. Although PTH, FGF23, and 1,25(OH)2D3 play key roles in the regulation of renal CYP27B1, the mechanisms involved have remained undefined. Meyer et al. (4) describe for the first time a kidney-specific control module distal to Cyp27b1 that controls both basal and hormone-regulated expression of Cyp27b1 in vivo. The authors found that regions that mediate PTH induction and FGF23 and 1,25(OH)2D3 suppression of renal Cyp27b1 are located in selected introns of Mettl1 and Mettl21b genes, respectively. Suppression of Cyp27b1 that includes induction of FGF23 by 1,25(OH)2D3 (or increased Pi) is also suggested by the findings of Meyer et al. (4).
Meyer et al. (4) identified, through ChIP-seq analysis, a genomic region distal to Cyp27b1 as a potential control module to explain the regulation observed. The authors used in vivo studies with mice generated via CRISPR/Cas9 genome–editing methods to directly evaluate the functional contribution of this potential regulatory region to control Cyp27b1 expression. The authors found regions of DNA that mediate PTH induction and 1,25(OH)2D3 and FGF23 suppression of renal Cyp27b1 expression located in selected introns of the nearby Mettl1 and Mettl21b genes (genes that produce methyltransferase-like proteins). Dissection of these regions pointed to distinct sections responsible for basal expression, PTH induction, and 1,25(OH)2D3- and FGF23-mediated suppression. In mice in which the intronic enhancer located at Mettl1 (M1-IKO) had been deleted, basal Cyp27b1 expression was strikingly reduced, and sensitivity to PTH induction (but not to FGF23 and 1,25(OH)2D3 suppression) was lost. Deletion of the Mettl21b enhancer (M21-IKO) resulted in mice with a more limited decrease in basal Cyp27b1 expression that were still sensitive to PTH but insensitive to FGF23 and 1,25(OH)2D3 treatment. Altered vitamin D metabolism and a debilitating skeletal phenotype similar to the Cyp27b1 KO mouse were observed in M1-IKO but not in the M21-IKO mice. The striking phenotype specifically of M1-IKO mice emphasizes the essential role of PTH in the control of bone mineralization and calcium homeostasis.
In additional experiments, Meyer et al. (4) explored the localization and hierarchy of this newly discovered circuit. The authors noted that the ability of another known regulator, LPS, to induce Cyp27b1 in non-renal target cells was unaffected by the enhancer deletion, suggesting a separate mechanism for modulation of locally produced 1,25(OH)2D3. Moreover, because 1,25(OH)2D3 induces FGF23 in bone, the authors tested whether 1,25(OH)2D3 might suppress Cyp27b1 by inducing FGF23. 1,25(OH)2D3 was indeed found to suppress Cyp27b1 in kidney within the same time frame as its effect on induction of FGF23 in bone (calcium and Pi were also induced by 1,25(OH)2D3). Although these findings are consistent with suppression of Cyp27b1 that involves induction of FGF23 by 1,25(OH)2D3 (or by increased Pi), further mechanistic studies are needed.
Finally, Meyer et al. (4) noted the relevance of their findings to genome-wide association studies (GWAS) that have correlated single-nucleotide polymorphisms to the prevalence of multiple sclerosis. MS-associated single-nucleotide polymorphisms were identified in a region that contains the CYP27B1 gene as well as METTL1 and METTL21B (8). High expression of CYP27B1 was also reported to be associated with the MS protective genotype (rs10877013-T allele) (9). The new data from Meyer et al. (4) indicating that the regulatory module of renal Cyp27b1 is located in selected introns of Mettl1 and Mettl21b suggest that multiple sclerosis is associated with renal production of 1,25(OH)2D3. Although further studies examining the mechanisms of regulation of the human CYP27B1 gene are needed, these findings have implications for vitamin D biology in MS and perhaps other autoimmune diseases.
The identification of a kidney-specific multicomponent control module distal to Cyp27b1 that mediates basal activity and hormonal activation and suppression represents a novel mechanism for the control of Cyp27b1. This is an important advance in the vitamin D field that has provided insight for the first time using a genome-wide perspective on the mechanisms that control Cyp27b1 expression. These findings provide evidence for distinct regulation of Cyp27b1 for the control of mineral metabolism and for the control of other pleiotropic actions of vitamin D. These pioneering studies, including novel mouse models, will enable future studies related to the identification of transcription factors and exact sites of hormonal regulation of Cyp27b1. In addition, the impact of locally produced 1,25(OH)2D3 at sites other than the kidney under normal physiological conditions and in disease (including autoimmune disease and cancer), which has been a matter of debate (1), can now be determined.
The author declares that she has no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- 1,25(OH)2D3
- 1α,25-dihydroxyvitamin D3
- PTH
- parathyroid hormone
- MS
- multiple sclerosis.
References
- 1. Jones G., Prosser D. E., and Kaufmann M. (2014) Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 55, 13–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christakos S., Dhawan P., Verstuyf A., Verlinden L., and Carmeliet G. (2016) Vitamin D: Metabolism, molecular mechanism of action and pleiotropic effects. Physiol. Rev. 96, 365–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kitanaka S., Takeyama K., Murayama A., Sato T., Okumura K., Nogami M., Hasegawa Y., Niimi H., Yanagisawa J., Tanaka T., and Kato S. (1998) Inactivating mutations in the 25-hydroxyvitamin D3 1α-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N. Engl. J. Med. 338, 653–661 [DOI] [PubMed] [Google Scholar]
- 4. Meyer M. B., Benkusky N. A., Kaufmann M., Lee S. M., Onal M., Jones G., and Pike J. W. (2017) A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J. Biol. Chem. 292, 17541–17558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garabedian M., Holick M. F., Deluca H. F., and Boyle I. T. (1972) Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc. Natl. Acad. Sci. U.S.A. 69, 1673–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenza H. L., and DeLuca H. F. (2000) Regulation of 25-hydroxyvitamin D3 1α-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch. Biochem. Biophys. 381, 143–152 [DOI] [PubMed] [Google Scholar]
- 7. Brenza H. L., Kimmel-Jehan C., Jehan F., Shinki T., Wakino S., Anazawa H., Suda T., and DeLuca H. F. (1998) Parathyroid hormone activation of the 25-hydroxyvitamin D3-1α-hydroxylase gene promoter. Proc. Natl. Acad. Sci. U.S.A. 95, 1387–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alcina A., Fedetz M., Fernández O., Saiz A., Izquierdo G., Lucas M., Leyva L., García-León J. A., Abad-Grau Mdel M., Alloza I., Antigüedad A., Garcia-Barcina M. J., Vandenbroeck K., Varadé J., de la Hera B., Arroyo R., Comabella M., Montalban X., Petit-Marty N., Navarro A., Otaegui D., Olascoaga J., Blanco Y., Urcelay E., and Matesanz F. (2013) Identification of a functional variant in the KIF5A-CYP27B1-METTL1-FAM119B locus associated with multiple sclerosis. J. Med. Genet. 50, 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karaky M., Alcina A., Fedetz M., Barrionuevo C., Potenciano V., Delgado C., Izquierdo G., and Matesanz F. (2016) The multiple sclerosis-associated regulatory variant rs 10877013 affects expression of CYP27B1 and VDR under inflammatory or vitamin D stimuli. Mult. Scler. 22, 999–1006 [DOI] [PubMed] [Google Scholar]