Abstract
Endocrine therapy (ET) of hormone receptor (HR)-positive and human epidermal growth factor receptor 2-(HER2)-negative metastatic breast cancer (MBC) historically focused on estrogen deprivation and antagonism. The identification of several intracellular pathways promoting resistance to antiestrogen therapy led to the introduction of novel endocrine drug combinations that reformed treatment schema and expanded therapeutic options. There is no doubt that efforts to overcome or delay resistance to ET are fruiting, particularly with the introduction of cyclin-dependent kinase 4/6 inhibitors such as palbociclib and ribociclib, and mechanistic target of rapamycin inhibitors such as everolimus. Although still considered incurable by currently available treatment modalities, many patients with MBC nowadays enjoy several years of good quality life coupled with decent tumor control. The diversity of therapies and unusual pattern of side effects can be quite perplexing to the treating physician. The sequence of variable agents and management of side effects, in addition to the timing of initiation of cytotoxic chemotherapy, is among the challenges faced by oncologists. In this review, we shed a spotlight on mechanisms of resistance to ET, and provide a review of landmark studies that have recently reshaped the landscape of treatment options for patients with metastatic HR-positive, HER2-negative MBC. A suggested treatment strategy for newly diagnosed patients is also discussed herein.
Key words: Breast cancer, endocrine therapy, metastatic
INTRODUCTION
Breast cancer is considered, in many cases, a chronically relapsing form of cancer. This is even more evident in the hormone receptor (HR)-positive operable disease as some patients remain at the risk of relapse even beyond 20 years from diagnosis. Although the annual risk of relapse is highest in the first 5 years from the diagnosis, it can persist well beyond 5 years. A post hoc analysis of the annual hazard rate of recurrence for 4104 patients who participated in the International Breast Cancer Study Group trials between 1978 and 1985 found that patients with a node-negative disease have a recurrence risk of 2.0%, 2.1%, and 1.1% annually for years 10–15, 15–20, and 20–25 from diagnosis, respectively. For patients with node-positive disease (N1, 1–3 involved nodes), the recurrence risk was found to be at 3.0%, 3.5%, and 1.5%, for the same time intervals, respectively.[1] Approximately 5%–10% of patients with Stage I breast cancer, 15%–20% of patients with Stage II, and ~50% of patients with stage III will recur distally and are likely to die from their disease.[2,3] On top of that, about 5%–10% of newly diagnosed patients with metastatic breast cancer (MBC) have de novo disease.[4,5] Historically, median survival of patients with MBC that is hormone-receptor positive, human epidermal growth factor receptor 2 (HER2)-negative who were diagnosed and treated decades ago has been reported to range between 16 and 26 months.[6,7] In comparison, the introduction of cyclin-dependent kinase (CDK) inhibitors and mechanistic target of rapamycin (mTOR) inhibitors, combinations with available endocrine therapies recently led to significantly improved progression-free survival (PFS) and overall survival (OS) that surpassed previously reported outcomes.[6,8,9,10,11,12] Hence, the aforementioned advancements mandate that treatment algorithms and guidelines be updated accordingly so that patients may be offered most effective and least toxic therapeutic options based on breakthrough clinical trials data. The introduction of numerous novel agents can make treatment choices somewhat perplexing. The purpose of this review is to provide a simplified comprehensive and evidence-based approach on how to treat newly diagnosed patients with HR-positive, HER2-negative MBC.
We have used the term “hormone receptor” to denote “estrogen receptor and/or progesterone receptor.” Treatment of metastatic triple-negative or HER2-positive breast cancer follows a different path and is not within the scope of this review.
SAMPLING OF METASTATIC DISEASE
It is strongly recommended that, whenever feasible, a biopsy be attempted from a distant metastatic site before treatment initiation for suspected MBC. This is important for several reasons; first, there is a need to examine the pathology of metastatic focus to aid in confirming breast cancer pathology as a source of primary. Other benign etiologies can mimic metastatic disease on imaging, such as in reactive lymph node enlargement and some benign bone lesions. Second, it is strongly recommended that repeat testing for estrogen receptor (ER), progesterone receptor (PR), and HER2 expression be performed on metastatic tissue since up to 40% may have a different HR status on relapse. HER2 status may also change from negative to positive or vice versa in 15%–33% of the case.[13,14] Several studies demonstrated that ER, PR, and HER2 statuses are unstable throughout tumor progression, which can lead to discordant results. Although not all trials required fresh biopsy, treatment of metastatic disease should be based on most recently reported receptors status in the metastatic disease, if known. Caution should be exercised when interpreting tumor marker (CA27.29 and CA15-3) values as they can be elevated in several other unrelated diseases, including benign conditions, such as benign breast disease, liver diseases, and systemic lupus, in addition to several types of other malignancies.[15]
TREATMENT SEQUENCE
In most cases and in the absence of a visceral crisis, two to four lines of endocrine therapy (ET) may be attempted before resorting to cytotoxic chemotherapy, unless visceral crises from metastatic disease are suspected. There is no uniformly agreed-upon definition for visceral crisis; however, in general, a significant threat to an organ function by burden or location of metastases can be considered a crisis. Examples include diffuse metastatic disease of the liver, lymphangitic carcinomatosis of the lungs or peritoneum, and leptomeningeal disease.
Once HR and HER2 statuses are confirmed, treatment may proceed. Special attention should be paid to sites requiring urgent local therapy, such as in cases of central nervous system (CNS) involvement or vertebral involvement leading to spinal canal stenosis or cord compromise.
PREMENOPAUSAL WOMEN
Patients who are premenopausal on diagnosis may have significantly less endocrine therapeutic options unless they undergo some sort of ovarian ablation. Aromatase inhibitors (AIs) do not suppress estrogen production in premenopausal women since the main source of estrogen is ovarian production rather than peripheral conversion.
Surgical oophorectomy is one of the oldest endocrine therapies known to cancer medicine and was based on observations that the undertaking of oophorectomy in premenopausal women with MBC led to the regression of disease in some cases. Introducing menopause in premenopausal women, surgically or medically, is still considered an important therapeutic approach. Nevertheless, well-designed, large randomized trials to prove its efficacy are lacking. Most trials conducted and published in the last few decades of 20th century are flawed due to several reasons, such as inclusion of ER-negative or ER-unknown tumors, lack of a balanced randomization, noncomparative single-arm patient population, small sample size, etc.[16] An Eastern Cooperative Oncology Group study published in 1995 randomized 147 patients with MRC to receive either chemotherapy alone (cyclophosphamide, doxorubicin, 5-fluorouracil regimen) or chemotherapy plus surgical oophorectomy. The study found that patients treated with oophorectomy had longer than expected median survival time.[17] The study, however, had some flaws, as it included patients with ER-negative tumors that were assigned, rather than randomized, to the chemotherapy-alone arm. Flawed randomization, along with small sample size, would significantly affect interpretation of results, knowing that patients with ER-negative tumors have inherently worse prognosis, a fact that probably was not routinely acknowledged at the time of study conduction.
It is fair to believe now that chemical ovarian ablation with gonadotropin-releasing hormone (GnRH) analogs is as effective as surgical oophorectomy. Most studies comparing the efficacy of the two approaches found no significant differences. We performed PubMed searches using keywords “oophorectomy” with “goserelin”/“leuprolide,” and found two underpowered trials that potentially looked at metastatic disease.[18,19] Results are summarized in Table 1.
Table 1.
Trials comparing gonadotropin-releasing hormone agonists with oophorectomy in metastatic breast cancer

Premenopausal women with metastatic ER-positive breast cancer are recommended to undergo ovarian ablation; this may be accomplished either chemically using a GnRH agonist, such as monthly goserelin, or surgically through oophorectomy. It is important to mention that ovarian ablation/suppression should be utilized along with other antiestrogen therapies such as AIs or tamoxifen. A meta-analysis of four trials comparing GnRH agonist alone or in combination with tamoxifen in 506 patients concluded that combination therapy leads to improved response rate (RR), PFS, and OS.[20,21]
Likewise, the use of single-agent tamoxifen without ovarian ablation as the sole treatment for premenopausal patients has been historically utilized.[22] Nevertheless, accumulating evidence suggests that ovarian ablation is associated with improved RR and improved survival in premenopausal women with HR-positive MBC and, hence, we discourage the use of tamoxifen without ovarian suppression as such.[23]
POSTMENOPAUSAL WOMEN
For treatment purposes and based on clinical trial data, postmenopausal women diagnosed with HR-positive HER2-negative MBC can be subcategorized into two major subgroups. The first group includes either patient diagnosed with de novo metastatic disease or those who have not received any AI therapy within the preceding 12 months before their relapse date. These patients should be offered treatment with the first-line combination of letrozole and a CDK4/6 inhibitor, either palbociclib or ribociclib. The benefit of CDK4/6 inhibitors in combination with AIs as an effective first-line option was first demonstrated in Phase II clinical trial, PALOMA-1 and was later confirmed in the Phase III trial PALOMA-2.[9,24] The latter study randomized a total of 666 postmenopausal women in a 2:1 fashion to receive either palbociclib plus letrozole or placebo plus letrozole. Patients were included in the PALOMA-2 trial if they were treatment-naïve for metastatic disease and have not received any AI within the preceding year, i.e., in the adjuvant setting. A statistically significant ~10-month median PFS improvement was demonstrated in the palbociclib/letrozole group compared to the placebo/letrozole group (24.8 vs. 14.5 months, respectively). OS data are yet to be reported. Common adverse events in the palbociclib group included neutropenia (79.5%), fatigue (37.4%), nausea (35.1%), arthralgia (33.3%), and alopecia (32.9%). Grade 3 neutropenia occurred in 56.1% of patients who received palbociclib and letrozole, but only 1.8% developed febrile neutropenia. Although neutropenia was very common, complicated and febrile neutropenia rates were very low as above. The low rate of complications related to neutropenia is likely due to the fact that CDK4/6 inhibitory effect on the bone marrow is associated with minimal apoptotic consequences at clinically relevant concentrations as demonstrated by Hu et al. in a preclinical in vitro and in vivo models.[25] By inhibiting cell-cycle progression from G1 to S, this class of drugs appears to cause irreversible senescence in tumor cells. However, in bone marrow progenitor cells, it appears to lead to a nonapoptotic cell-cycle arrest.
Palbociclib has recently been joined by another CDK4/6 inhibitor, ribociclib, which was Food and Drug Administration-approved in March 2017 for use in the first-line setting in combination with letrozole, based on the findings of the MONALEESA-2 trial. Compared to placebo, the addition of ribociclib to letrozole significantly improved PFS in the first-line metastatic settings, in a fashion similar to that of palbociclib.[26] Ribociclib requires a regular monitoring of hepatic transaminases and electrocardiogram as it may be associated with hepatotoxicity and QTc prolongation, respectively. It is also important to note that there is currently insufficient evidence to suggest using a different CDK4/6 inhibitor in a subsequent line of therapy, in case of disease progression on previously used agent from the same class. Likewise, continuing a CDK4/6 inhibitor beyond progression is not recommended for the same reason.
Patients diagnosed with metastatic recurrence while taking an AI in the adjuvant setting, or who completed their adjuvant AI therapy within the preceding 12 months, should not be offered letrozole plus palbociclib. Instead, two alternative options can be considered with equal level of evidence. For such patients, the first-treatment option to offer is a combination of the steroidal AI, exemestane, with the mTOR inhibitor, everolimus, given that they have not progressed on, or shortly after, taking exemestane before. This recommendation is based on findings of the BOLERO-2 trial which randomized 724 pretreated postmenopausal women with HR-positive, HER2-negative MBC to receive treatment with either exemestane plus everolimus or exemestane plus placebo. Eligibility criteria included patients who had progression or recurrence after having received a nonsteroidal AI. Patients were allowed to have had a single line of chemotherapy in the metastatic setting before enrollment. Median PFS by central assessment was 10.4 months in the everolimus group compared to 4.1 months in the placebo group. The most prominent grade 3 or 4 events in the everolimus group were stomatitis (8%), anemia (6%), fatigue (4%), dyspnea (4%), hyperglycemia (4%), and pneumonitis (3%).[27] OS did not reach statistical significance although trended toward favoring the everolimus group (median OS 31 months in the everolimus groups vs. 26.6 months in the placebo group, P = 0.14);[10] however, the study was not powered to detect a 4–5 months OS difference. This combination is generally well tolerated, albeit some side effects of everolimus can be serious such as pneumonitis, or may have negative impact on the quality of life, such as fatigue and stomatitis. We suggest the routine prophylactic use of dexamethasone mouthwash to reduce the incidence of stomatitis. The benefit of swish for 2 min and spit of a 10 mg dexamethasone solution four times daily was demonstrated in the SWISH trial. The incidence of all-grade stomatitis was reduced from 67% as previously seen in the BOLERO-2 trial to 19.8% in the SWISH trial. No Grade 3 stomatitis was seen in patients treated prophylactically with dexamethasone oral solution.[28] Everolimus-induced pneumonitis is increasingly being recognized. It should be suspected in patients presenting with progressive shortness of breath or cough. Hypoxia or fever may also be evident in the more severe cases. Everolimus should be held and computed tomographic of the chest should be performed. The presence of bilateral opacities is a typical characteristic finding. Differential diagnoses include infectious etiologies, especially in setting of immunosuppression from everolimus, and lymphangitic carcinomatosis of the lungs.[29]
Antiestrogen therapy, especially AIs, can cause several musculoskeletal symptoms, most notably arthralgia. They can be effectively managed with physical activity and exercise as demonstrated in a randomized trial conducted in 121 breast cancer patients.[30] Pharmacologic treatment, such as duloxetine, is another option for selected patients.[31]
It is noteworthy that everolimus has also been combined with tamoxifen, nonsteroidal AIs, or fulvestrant in various Phase II trials. Such alternatives may be considered in patients who previously progressed after or were unable to tolerate exemestane.[32,33,34]
A second option that could be discussed with patients is a combination of fulvestrant and palbociclib in patients who are naïve to both agents. It is an appropriate option for patients who are diagnosed with recurrent metastatic disease while on AI therapy or within 12 months of completion of such therapy. The PALOMA-3 trial randomized 521 women with HR-positive, HER2-negative disease to receive either fulvestrant plus palbociclib (347 patients) or fulvestrant plus placebo (174 patients). Patients were eligible if they had disease relapse while on adjuvant ET or within 12 months of completion of such therapy. Patients were also allowed to have had prior ET, other than fulvestrant, or one line of chemotherapy in the metastatic setting. Patients could be of any menopausal status; however, pre- and peri-menopausal women were required to receive goserelin for ovarian suppression. Median PFS was 9.5 months in the palbociclib group compared to 4.6 months in the placebo group. Common side effects included neutropenia, fatigue, nausea, headache, diarrhea, constipation, anemia, leukopenia, and thrombocytopenia. Grade 3 or 4 side effects in the palbociclib arm included neutropenia (65%), anemia (3%), leukopenia (28%), and thrombocytopenia (3%). OS data are yet to be reported.[11]
Patients who previously received palbociclib are also candidates for single-agent fulvestrant,[6,35,36] a unique type of ER antagonist. Not only does it block ERs but also it leads to their degradation; hence, the term selective ER downregulator/degrader.[37]
The combination of fulvestrant and AIs in the first-line metastatic setting was considered the standard of care by many experts before the introduction palbociclib. However, it is currently discouraged due to the emergence of CDK4/6 inhibitors-ET combinations as discussed above. The combination of AI and fulvestrant in the front-line setting renders patients to fall outside of eligibility criteria of clinical trials that demonstrated added benefit of CDK4/6 inhibition. For example, once these patients have disease progression, they are no longer considered first line (PALOMA-2 and MONALEESA-2) and no longer fulvestrant-naïve (PALOMA-3). It may still be a good choice for patients who are thought to be ineligible for CDK4/6 inhibitors due to poor performance status or significant baseline neutropenia.[38,39,40] It is also of some concern that the use of fulvestrant in combination with anastrozole significantly decreases serum concentrations of anastrozole as demonstrated in Southwest Oncology Group trial (SWOG S0226).[21,41]
Likewise, the use of single-agent AIs may be considered for selected patients that have not previously received an AI and who are not able to take a CDK4/6 inhibitor, due to poor performance status, or baseline neutropenia. Single-agent fulvestrant is also an appropriate option for such patients, as per the FALCON study which showed significant improvement in PFS on using upfront fulvestrant compared to anastrozole.[35,42]
For tamoxifen-naïve patients, it is very reasonable to consider a treatment trial with this agent at some point in the disease course. A Phase III trial comparing tamoxifen versus exemestane in the treatment of patients who have received no more than one line of chemotherapy and no prior ET, found no difference in OS although RR and PFS were better in the exemestane group. No information provided on how often patients crossed over to the other arm.[43] Another Phase III study showed that the nonsteroidal AI, letrozole, is superior to tamoxifen in the time to progression end-point in 907 patients with advanced breast cancer. The study allowed one previous line of chemotherapy and no previous ET in the metastatic settings. OS was numerically, but not statistically, better in the letrozole group. However, it is noteworthy that the cross-over rate between treatment arms after progression was 49%–54%.[44]
Patients whose tumor progresses after 2–3 lines of ET may be considered for further endocrine-targeting agents based on treatment history and tumor burden. Patients maintaining an indolent course of disease, especially those who have bone-only disease or have minimal visceral involvement that does not threaten organ function, may be treated with another line of ET. Some prohormonal agents have been used in small-scale trials with variable degree of benefit, including progestins, such as megestrol acetate, and estrogens. The mechanism of action of this paradoxical effect is not well understood; however, one theory presume that chronically estrogen-deprived tumor cells may undergo apoptosis on reexposure to estrogen.[45,46]
A suggested treatment algorithm is summarized in Figure 1.
Figure 1.
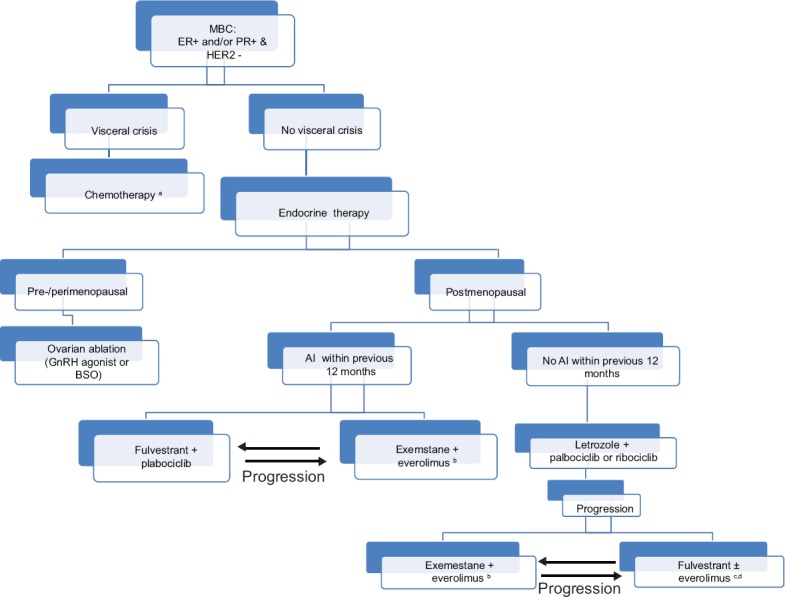
A suggested treatment algorithm for newly diagnosed MBC patients. a: Switching to endocrine therapy after a period of chemotherapy may be considered in this scenario. b: In patients who previously received exemestane in the metastatic settings or within 12 months before relapse, consider other endocrine therapy combinations with everolimus such as either fulvestrant or tamoxifen. c: Consider this combination option, especially in everolimus-naïve patients who previously received exemestane. d: There is insufficient evidence to support the continued use of CDK4/6 inhibitors in patients who progressed while taking an agent from the same class. *Clinical trial participation is encouraged in the first line of therapy or at the time of progression, whenever feasible. MBC: Metastatic breast cancer, AI: Aromatase inhibitor, GnRH: Gonadotropin-releasing hormone, BSO: Bilateral salpingo-oophorectomy, CDK: Cyclin-dependent kinase
METASTATIC HORMONE POSITIVE MALE BREAST CANCER
Male breast cancer is uncommon, comprising approximately 1% of all breast cancers.[47] Endocrine manipulation remains the recommended first-line therapy and tamoxifen remains the mainstay of such therapy in men with MBC who are not in visceral crisis.[48] AIs seem to be less effective, probably due to their inability to inhibit synthesis of estrogen in the testes. Fulvestrant remains of unproven benefit but may be used after failure of tamoxifen.[49] There is no doubt that a big gap in knowledge exists in the literature of male MBC, likely due to rarity of the disease and lack of large randomized trials. Men were excluded from participation in many large randomized trials such the FALCON, PALOMA-2, PALOMA-3, and BOLERO-2 trials.
MECHANISMS OF RESISTANCE TO ANTIESTROGEN THERAPY
A significant proportion of patients still develop tumor resistance to ET, especially in the metastatic settings. Understanding mechanisms of resistance has been the cornerstone step leading to the development of abovementioned treatment strategies utilizing endocrine-novel drug combinations.
ER gene (ESR1) mutations represent a frequent alteration reported in MBC. While the incidence of ESR1 mutations is reported to occur in as few as 2% of primary and treatment-naïve tumors, the incidence in recurrent and metastatic tumors has been reported in as many as 25%–30%. ESR1 mutation usually occurs in the ligand-binding domain of the receptor and can potentially lead to ligand-independent constituent activation of ER. Resistance to AIs and, to a lesser degree, selective ER modulators and degraders ensues as a result of this mutation.[50,51,52]
Another frequently identified alteration in MBC is the upregulation of the growth and survival pathway, phosphoinositide 3-kinase (PI3K)/Akt/mTOR, and is believed to represent a very important mechanism of acquired resistance to ET. The frequency of this mutation in ER-positive MBC is around 30%–40%, most of which are an activating or gain-of-function mutation.[53,54]
Amplification of the HER2 gene is noted in 10% of recurrent HR-positive MBC and is a well-known mechanism of resistance to ET. Bidirectional crosstalk between ER and HER2 renders tumors less sensitive to estrogen blockade and grants them proliferative properties. The presence of hyperactive growth factor pathways increases ER transcriptional activities in a ligand-independent pattern, i.e., even in the presence of estrogen antagonism.[55,56]
Oncogenic somatic mutations of the HER2 gene are thought to represent a driver mutation for a subset of recurrent and metastatic invasive breast carcinomas. One interesting study of 22 relapsed invasive lobular carcinoma samples reported that a gene alteration in HER2 is present in 6 patients (27%), including 4 with gene mutation, 1 with gene fusion, and 1 with overexpression.[57] Combining HER2 tyrosine kinase inhibitors (TKIs) with antiestrogens in unamplified HER2-mutated MBC is currently in early phases of clinical testing (NCT01670877).
Cyclin D1 expression and the continuous phosphorylation of retinoblastoma gene leads to continuous, uninterrupted cell-cycle progression and cell proliferation even in the absence of estrogen. It is now considered a very important mechanism of resistance to antiestrogen therapy, especially in luminal B tumor subtypes.[58,59]
Fibroblast growth factor receptor (FGFR) amplification is oncogenic in breast cancers and potentially leads to resistance to ET. FGFR1 overexpression activates the mutagen-activated protein kinase (MAPK) and PI3K-Akt pathways and is commonly co-amplified with cyclin D1 gene (CCND1). FGFR1 amplification occurs in 10% of breast cancers and in up to 27% of the luminal B subtype, and it appears that it leads to suppression of PR expression. It received special attention recently as it has been linked to poor prognosis and early relapse. It may eventually have therapeutic implications.[60,61]
The insulin-like growth factor 1 (IGF1) also appears to play a role in mediating resistance to estrogen deprivation through the activation of the PI3K-Akt and MAPK pathways. ER activation targets IGF1 directly which in turn promotes cell growth and survival. This effect is further enhanced through IGF type 1 receptor-epidermal growth factor receptor (EGFR) crosstalk and plays as an escape pathway from hormone dependence in breast cancer.[62,63]
FUTURE ADVANCES
Other CDK4/6 inhibitors are currently in various stages of clinical development. Abemaciclib is notable to have more single-agent activity, even in patients who failed multiple lines of prior therapy, as demonstrated in the MONARCH I trial.[64] Diarrhea is a very common side effect of abemaciclib and is the lead cause for dose reductions. It seems to target CDK4 more than CDK6 and hence believed to cause less marrow suppression and can be dosed continuously. Abemaciclib also seems to have better CNS penetration than other drugs in the same class.[65]
Modifying epigenetic alterations are another mechanism by which resistance to antiestrogen therapy can be reversed or delayed. Entinostat is a histone deacetylase (HDAC) inhibitor currently being tested in a Phase III trial in combination with exemestane. HDAC is a critical enzyme in gene expression regulation, and its inhibition with entinostat has been shown to prolong PFS and restore sensitivity to ET in a cohort of pretreated patients in a pilot Phase II trial.[66]
Buparlisib and alpelisib are PI3K inhibitors aimed at overcoming resistance to ET and are currently in clinical testing.[67] In a Phase III trial, the BELLE-3, 432 pretreated patients were randomized to buparlisib plus fulvestrant versus placebo plus fulvestrant. Patients assigned to the buparlisib arm had 2.1-month improvement in PFS. The presence of PIK3CA mutation predicted significantly greater benefit from buparlisib, a finding that is of particular interest.[68]
Immune checkpoint inhibitors have also been tested in HR-positive MBC. The programmed cell death 1 (PD-1) inhibitor, pembrolizumab, showed some activity in a small cohort of selected patients with ER-positive HER2-negative MBC staining positive for programmed cell death ligand 1 (PD-L1) by immunohistochemistry. In the Phase Ib trial, KEYNOTE-028, out of 248 patients, 19.4% were found to stain positive for PD-L1 (positivity defined as ≥1% membranous staining or any degree of stromal staining). Although RR in the treated assessable cohort was only 14%, it is noteworthy to mention that study included patients who were heavily pretreated; in fact, the vast majority of patients having received 3 or more lines of therapy, including cytotoxic chemotherapy, before enrollment.[69]
Preclinical data suggested CDK4/6 inhibition may sensitize to PI3K inhibitors.[70] Triple therapy using ET in combination with palbociclib and PI3K inhibitors is being tested in early phase trials in PIK3CA-mutant breast cancers (NCT02389842).
The FGFR TKI, dovitinib, also inhibits VEGFR and PDGFR, showed promising activity when combined with fulvestrant in a phase II trial in patients with MBC who failed prior ET.[71]
Neratinib, an irreversible EGFR/HER2 TKI, showed promising activity as single agent for a cohort of patients with HER2-mutated unamplified MBC in a pilot Phase II trial. Out of 14 patients with activating HER2 mutations, 36% achieved clinical benefit. Median PFS was 5 months, considered to be significant in this heavily pretreated cohort. The vast majority of patients (95%) had ER+ disease.[72] The protocol was amended to add fulvestrant to neratinib (NCT01670877).
CONCLUSION
Combining targeted therapy with endocrine modulating drugs is a promising treatment strategy. It has proven effective, safe and have indeed reshaped the landscape of ET. Many patients with HR-positive MBC nowadays enjoy decent tumor control paired with very good quality of life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thürlimann B, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol. 2016;34:927–35. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Based on November 2015 SEER Data Submission, Posted April, 2016. Bethesda, MD: National Cancer Institute; [Last accessed on 2016 Dec 01]. SEER Cancer Statistics Review, 1975-2013. Available from: http://www.seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 3.van de Water W, Markopoulos C, van de Velde CJ, Seynaeve C, Hasenburg A, Rea D, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA. 2012;307:590–7. doi: 10.1001/jama.2012.84. [DOI] [PubMed] [Google Scholar]
- 4.O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–9. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 5.Zeichner SB, Herna S, Mani A, Ambros T, Montero AJ, Mahtani RL, et al. Survival of patients with de-novo metastatic breast cancer: Analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Cancer Res Treat. 2015;153:617–24. doi: 10.1007/s10549-015-3564-3. [DOI] [PubMed] [Google Scholar]
- 6.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Final overall survival: Fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106:djt337. doi: 10.1093/jnci/djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milla-Santos A, Milla L, Portella J, Rallo L, Pons M, Rodes E, et al. Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: A prospective, randomized, phase III study. Am J Clin Oncol. 2003;26:317–22. doi: 10.1097/01.COC.0000047126.10522.F9. [DOI] [PubMed] [Google Scholar]
- 8.Chia SK, Speers CH, D'yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–9. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 10.Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2. Ann Oncol. 2014;25:2357–62. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma S, Bartlett CH, Schnell P, DeMichele AM, Loi S, Ro J, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed Safety Analysis from a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3) Oncologist. 2016;21:1165–75. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yardley DA, Noguchi S, Pritchard KI, Burris HA, 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–84. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarneri V, Giovannelli S, Ficarra G, Bettelli S, Maiorana A, Piacentini F, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: Impact on patient management. Oncologist. 2008;13:838–44. doi: 10.1634/theoncologist.2008-0048. [DOI] [PubMed] [Google Scholar]
- 14.Lindström LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–8. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 15.Fejzic H, Mujagic S, Azabagic S, Burina M. Tumor marker CA 15-3 in breast cancer patients. Acta Med Acad. 2015;44:39–46. doi: 10.5644/ama2006-124.125. [DOI] [PubMed] [Google Scholar]
- 16.Harvey HA, Lipton A, Max DT, Pearlman HG, Diaz-Perches R, de la Garza J. Medical castration produced by the GnRH analogue leuprolide to treat metastatic breast cancer. J Clin Oncol. 1985;3:1068–72. doi: 10.1200/JCO.1985.3.8.1068. [DOI] [PubMed] [Google Scholar]
- 17.Falkson G, Holcroft C, Gelman RS, Tormey DC, Wolter JM, Cummings FJ. Ten-year follow-up study of premenopausal women with metastatic breast cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol. 1995;13:1453–8. doi: 10.1200/JCO.1995.13.6.1453. [DOI] [PubMed] [Google Scholar]
- 18.Boccardo F, Rubagotti A, Perrotta A, Amoroso D, Balestrero M, De Matteis A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: Results of a multicentric Italian study. Ann Oncol. 1994;5:337–42. doi: 10.1093/oxfordjournals.annonc.a058837. [DOI] [PubMed] [Google Scholar]
- 19.Taylor CW, Green S, Dalton WS, Martino S, Rector D, Ingle JN, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: An intergroup study. J Clin Oncol. 1998;16:994–9. doi: 10.1200/JCO.1998.16.3.994. [DOI] [PubMed] [Google Scholar]
- 20.Klijn JG, Blamey RW, Boccardo F, Tominaga T, Duchateau L, Sylvester R. Combined Hormone Agents Trialists' Group and the European Organization for Research and Treatment of Cancer. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: A meta-analysis of four randomized trials. J Clin Oncol. 2001;19:343–53. doi: 10.1200/JCO.2001.19.2.343. [DOI] [PubMed] [Google Scholar]
- 21.Shah PD, Dickler MN. Endocrine therapy for advanced breast cancer. Clin Adv Hematol Oncol. 2014;12:214–23. [PubMed] [Google Scholar]
- 22.Manni A. Tamoxifen therapy of metastatic breast cancer. J Lab Clin Med. 1987;109:290–9. [PubMed] [Google Scholar]
- 23.Klijn JG, Beex LV, Mauriac L, van Zijl JA, Veyret C, Wildiers J, et al. Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: A randomized study. J Natl Cancer Inst. 2000;92:903–11. doi: 10.1093/jnci/92.11.903. [DOI] [PubMed] [Google Scholar]
- 24.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 25.Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res. 2016;22:2000–8. doi: 10.1158/1078-0432.CCR-15-1421. [DOI] [PubMed] [Google Scholar]
- 26.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 27.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rugo H, Seneviratne L, Beck J, Glaspy J, Peguero J, Pluard T, et al. Prevention of everolimus/exemestane (EVE/EXE) stomatitis in postmenopausal (PM) women with hormone receptor-positive (HR+) metastatic breast cancer (MBC) using a dexamethasone-based mouthwash (MW): Results of the SWISH trial. J Clin Oncol. 2016;34 ???. [Suppl; Abstr. 525] [Google Scholar]
- 29.Zhang X, Ran YG, Wang KJ. Risk of mTOR inhibitors induced severe pneumonitis in cancer patients: A meta-analysis of randomized controlled trials. Future Oncol. 2016;12:1529–39. doi: 10.2217/fon-2016-0020. [DOI] [PubMed] [Google Scholar]
- 30.Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104–11. doi: 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry NL, Banerjee M, Wicha M, Van Poznak C, Smerage JB, Schott AF, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer. 2011;117:5469–75. doi: 10.1002/cncr.26230. [DOI] [PubMed] [Google Scholar]
- 32.Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J Clin Oncol. 2012;30:2718–24. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 33.Massarweh S, Romond E, Black EP, Van Meter E, Shelton B, Kadamyan-Melkumian V, et al. A phase II study of combined fulvestrant and everolimus in patients with metastatic estrogen receptor (ER)-positive breast cancer after aromatase inhibitor (AI) failure. Breast Cancer Res Treat. 2014;143:325–32. doi: 10.1007/s10549-013-2810-9. [DOI] [PubMed] [Google Scholar]
- 34.Wheler JJ, Moulder SL, Naing A, Janku F, Piha-Paul SA, Falchook GS, et al. Anastrozole and everolimus in advanced gynecologic and breast malignancies: Activity and molecular alterations in the PI3K/AKT/mTOR pathway. Oncotarget. 2014;5:3029–38. doi: 10.18632/oncotarget.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiówka M, Hewson N, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: Overall survival analysis from the phase II FIRST Study. J Clin Oncol. 2015;33:3781–7. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: Follow-up analysis from the randomized 'FIRST' study. Breast Cancer Res Treat. 2012;136:503–11. doi: 10.1007/s10549-012-2192-4. [DOI] [PubMed] [Google Scholar]
- 37.Yeh WL, Shioda K, Coser KR, Rivizzigno D, McSweeney KR, Shioda T. Fulvestrant-induced cell death and proteasomal degradation of estrogen receptor a protein in MCF-7 cells require the CSK c-Src tyrosine kinase. PLoS One. 2013;8:e60889. doi: 10.1371/journal.pone.0060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergh J, Jönsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattström D, et al. FACT: An open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30:1919–25. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 39.Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): A composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14:989–98. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 40.Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–44. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hertz DL, Barlow WE, Kidwell KM, Albain KS, Vandenberg TA, Dakhil SR, et al. Fulvestrant decreases anastrozole drug concentrations when taken concurrently by patients with metastatic breast cancer treated on SWOG study S0226. Br J Clin Pharmacol. 2016;81:1134–41. doi: 10.1111/bcp.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellis M, Bondarenko I, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. FALCON: A Phase III Randomised Trial of Fulvestrant 500 mg vs Anastrozole for Hormone Receptor-Positive Advanced Breast Cancer. Late Breaking Abstract_LBA14_PR [Oral Presentation]. Presented at the European Society for Medical Oncology (ESMO) Congress; Copenhagen, Denmark. 2016. [Google Scholar]
- 43.Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–90. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mouridsen H, Sun Y, Gershanovich M, Perez-Carrion R, Becquart D, Chaudri-Ross HA, et al. Superiority of letrozole to tamoxifen in the first-line treatment of advanced breast cancer: Evidence from metastatic subgroups and a test of functional ability. Oncologist. 2004;9:489–96. doi: 10.1634/theoncologist.9-5-489. [DOI] [PubMed] [Google Scholar]
- 45.Abrams J, Aisner J, Cirrincione C, Berry DA, Muss HB, Cooper MR, et al. Dose-response trial of megestrol acetate in advanced breast cancer: Cancer and leukemia group B phase III study 8741. J Clin Oncol. 1999;17:64–73. doi: 10.1200/JCO.1999.17.1.64. [DOI] [PubMed] [Google Scholar]
- 46.Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, et al. Lower-dose vs. high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: A phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: A population-based study. Cancer. 2004;101:51–7. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 48.Jaiyesimi IA, Buzdar AU, Sahin AA, Ross MA. Carcinoma of the male breast. Ann Intern Med. 1992;117:771–7. doi: 10.7326/0003-4819-117-9-771. [DOI] [PubMed] [Google Scholar]
- 49.Patten DK, Sharifi LK, Fazel M. New approaches in the management of male breast cancer. Clin Breast Cancer. 2013;13:309–14. doi: 10.1016/j.clbc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Clatot F, Augusto L, Di Fiore F. ESR1 mutations in breast cancer. Aging (Albany NY) 2017;9:3–4. doi: 10.18632/aging.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations – A mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12:573–83. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4:1116–30. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–60. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng CK, Piscuoglio S, Geyer FC, Burke KA, Pareja F, Eberle C, et al. The Landscape of Somatic Genetic Alterations in Metaplastic Breast Carcinomas. Clin Cancer Res. 2017:pii: Clincanres 28572016. doi: 10.1158/1078-0432.CCR-16-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr Relat Cancer. 2001;8:191–5. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- 56.Lousberg L, Collignon J, Jerusalem G. Resistance to therapy in estrogen receptor positive and human epidermal growth factor 2 positive breast cancers: Progress with latest therapeutic strategies. Ther Adv Med Oncol. 2016;8:429–49. doi: 10.1177/1758834016665077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross JS, Wang K, Sheehan CE, Boguniewicz AB, Otto G, Downing SR, et al. Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin Cancer Res. 2013;19:2668–76. doi: 10.1158/1078-0432.CCR-13-0295. [DOI] [PubMed] [Google Scholar]
- 58.Rugo HS, Vidula N, Ma C. Improving response to hormone therapy in breast cancer: New targets, new therapeutic options. Am Soc Clin Oncol Educ Book. 2016;35:e40–54. doi: 10.1200/EDBK_159198. [DOI] [PubMed] [Google Scholar]
- 59.Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, et al. Therapeutically activating RB: Reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–45. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.André F, Bachelot T, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, et al. Targeting FGFR with dovitinib (TKI258): Preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19:3693–702. doi: 10.1158/1078-0432.CCR-13-0190. [DOI] [PubMed] [Google Scholar]
- 61.Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–94. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox EM, Miller TW, Balko JM, Kuba MG, Sánchez V, Smith RA, et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011;71:6773–84. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: A supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–18. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 64.Dickler M, Sara Tolaney, Rugo H, Cortes J, Diéras V, Patt D, et al. MONARCH1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2-breast cancer, after chemotherapy for advanced disease. J Clin Oncol. 2016;34 [Suppl; Abstr 510] [Google Scholar]
- 65.Sánchez-Martínez C, Gelbert LM, Lallena MJ, de Dios A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorg Med Chem Lett. 2015;25:3420–35. doi: 10.1016/j.bmcl.2015.05.100. [DOI] [PubMed] [Google Scholar]
- 66.Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013;31:2128–35. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32:1202–9. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Leo A, Keun S, Ciruelos E, Lønning P, Janni W, O'Regan R, et al. BELLE-3: A Phase III Study of Buparlisib + Fulvestrant in Postmenopausal Women with HR+, HER2-, Aromatase Inhibitor-Treated, Locally Advanced or Metastatic Breast Cancer, Who Progressed on or after mTOR Inhibitor-Based Treatment. San Antonio Breast Cancer Symposium. 2016 [Abstract S4-07] [Google Scholar]
- 69.Rugo H, Delord JP, Im SA, Ott P, Piha-Paul S, Bedard P, et al. Abstract S5-07: Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1-positive, estrogen receptor-positive (ER+)/HER2-negative advanced breast cancer enrolled in KEYNOTE-028. Cancer Res. 2016;76(4 Suppl):S5. [Google Scholar]
- 70.Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–49. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musolino A, Campone M, Neven P, Denduluri N, Barrios CH, Cortes J, et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2– breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017;19:18. doi: 10.1186/s13058-017-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma C, Bose R, Gao F, Freedman R, Pegram M, Blackwell K, et al. Phase II trial of neratinib for HER2 mutated, non-amplified metastatic breast cancer (HER2mut MBC) J Clin Oncol. 2016;34 [Suppl; Abstr 516] [Google Scholar]


