Abstract
New vaccines designed to prevent diseases endemic in low and middle-income countries (LMICs) are now being introduced without prior record of utilization in countries with robust pharmacovigilance systems. To address this deficit, our objective was to demonstrate feasibility of an international hospital-based network for the assessment of potential epidemiological associations between serious and rare adverse events and vaccines in any setting. This was done through a proof-of-concept evaluation of the risk of immune thrombocytopenic purpura (ITP) and aseptic meningitis (AM) following administration of the first dose of measles-mumps-containing vaccines using the self-controlled risk interval method in the primary analysis. The World Health Organization (WHO) selected 26 sentinel sites (49 hospitals) distributed in 16 countries of the six WHO regions. Incidence rate ratios (IRR) of 5.0 (95% CI: 2.5-9.7) for ITP following first dose of measles-containing vaccinations, and of 10.9 (95% CI: 4.2-27.8) for AM following mumps-containing vaccinations were found. The strain-specific analyses showed significantly elevated ITP risk for measles vaccines containing Schwarz (IRR: 20.7; 95% CI: 2.7-157.6), Edmonston-Zagreb (IRR: 11.1; 95% CI: 1.4-90.3), and Enders´Edmonston (IRR: 8.5; 95% CI: 1.9-38.1) strains. A significantly elevated AM risk for vaccines containing the Leningrad-Zagreb mumps strain (IRR: 10.8; 95% CI: 1.3-87.4) was also found. This proof-of-concept study has shown, for the first time, that an international hospital-based network for the investigation of rare vaccine adverse events, using common standardized procedures and with high participation of LMICs, is feasible, can produce reliable results, and has the potential to characterize differences in risk between vaccine strains. The completion of this network by adding large reference hospitals, particularly from tropical countries, and the systematic WHO-led implementation of this approach, should permit the rapid post-marketing evaluation of safety signals for serious and rare adverse events for new and existing vaccines in all settings, including LMICs.
Keywords: Post-marketing surveillance, vaccine safety, Global Vaccine Safety Initiative (GVSI), adverse events following immunization (AEFI)
Introduction
With increasing number of vaccine products available, expansion of vaccine manufacturing capabilities, and availability of new vaccines targeted against diseases highly prevalent in low and middle-income countries (LMICs) (1), there is a need to enhance vaccine pharmacovigilance infrastructures globally (2). Many countries do not have technical capacity and/or large enough populations to permit the evaluation of rare adverse events following immunization (AEFI) (2, 3). Enhancement of vaccine pharmacovigilance capabilities is a key activity for the World Health Organization (WHO) Global Vaccine Safety Initiative (GVSI) (4–6). A previous international pilot study sponsored by WHO and the Food and Drug Administration (FDA), to evaluate the safety of the 2009-10 pandemic influenza vaccine, demonstrated that multinational hospital-based vaccine safety studies were feasible and could provide a useful framework for the evaluation of safety concerns (7). Optimization of operational models, centralization of case adjudication, improvements in data quality control, closer supervision of data abstraction, and demonstration of the feasibility of such international collaborations, with high participation from LMICs, were identified by WHO as issues to be resolved (7). Thus, for a subsequent demonstration project, it was important to reach higher participation from LMICs, select a vaccine widely used, and an AEFI that, at least in severe cases, would require hospitalization (2). It was also essential to select an AEFI known to be associated with some of the vaccine strains being used.
Measles-containing vaccines are live-attenuated, often given in combination with mumps and rubella vaccines. The first dose is usually given at one year of age, although it is administered at nine months of age in countries with ongoing measles transmission (8). The second dose is either given at 15-18 months of age, at 4-6 years of age, or in campaigns. Our objective was to demonstrate feasibility of an international hospital-based network for assessing epidemiological associations between rare adverse events and vaccines in any setting, including LMICs. Two well-established associations were chosen: risk of aseptic meningitis (AM) following first dose of mumps-containing vaccines (9–11), and risk of immune thrombocytopenic purpura (ITP) following first dose of measles-containing vaccines (8, 12–14).
Methods
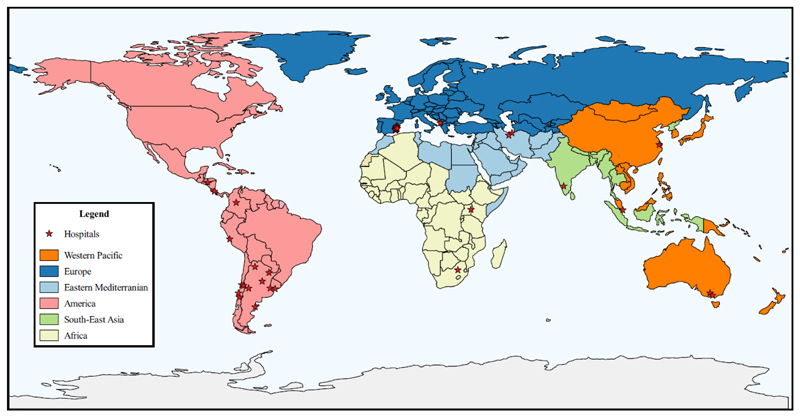
International hospital-based retrospective observational study conducted as proof-of-concept for the investigation of rare AEFI using two analytical case-only methods: self-controlled risk interval (SCRI) and case-crossover (15, 16). For this purpose, WHO selected 26 sentinel sites (49 hospitals) distributed in 16 countries of the six WHO regions (Figure 1). Selection criteria and capability assessments are described elsewhere (Bravo-Alcántara P, Perez-Vilar S, Molina-León HF et al. (accepted for publication in Vaccine)).
Figure 1. Geographical distribution of participating hospitals in the WHO regions.
Disclaimer: Lines on the map represent approximate border lines for which there may not yet be full agreement.
Study population
The study population included children ages 9-23 months admitted to a network-participating hospital during January 2010-March 2014, with a discharge diagnosis of either AM or ITP. Only individuals living in the pre-defined catchment area of the hospital, or, for those hospitals without a pre-specified catchment area, in the same city in which the hospital was located, were eligible.
Case ascertainment and classification
Participating hospitals identified potential cases through hospital discharge databases using pre-specified ICD-9/ICD-10 codes (Supplementary material; Table S-1) whereas hospitals not using a discharge codification system or not having electronic databases used free text. A trained physician or nurse blinded to vaccination status reviewed medical records of potential cases according to established case definitions (Supplementary material; Tables S-2 and S-3). Potential cases for which medical records were not available were excluded. Only first episodes of AM or ITP were considered.
Potential AM cases were excluded if they met criteria for encephalitis (17) (Supplementary material; Table S-4), the medical records showed that a physician ruled out a diagnosis of AM, a meningitis pathogen other than mumps virus was identified in cerebrospinal fluid (CSF), CSF protein concentration (in absence of traumatic lumbar puncture or intracerebral event) was ≥50mg/dL with ≥10 leukocytes/mm3 and glucose ≤40mg/dL in CSF, or if polymorphonuclear leukocytes (PMNs) in the CSF were >1,000/mm3 with glucose ≤40mg/dL (modified from Lussiana et al.) (18).
Potential ITP cases were excluded if classified as chronic (defined as lasting >6 months) (12, 14), with onset of symptoms occurring >42 days prior to hospital admission, or if a physician diagnosis in the medical records ruled out the diagnosis of ITP or thrombocytopenia. ITP cases with medical conditions associated with higher ITP risk (congenital/hereditary thrombocytopenia, aplastic anemia, defibrination syndrome, acquired hemolytic anemia, chronic liver disease, malignancy, or drug-induced thrombocytopenia) were also excluded. For the analyses presented here, patients treated with platelet-depleting medications (amiodarone, heparin, carbamazepine, phenytoin, valproic acid, quinidine, quinine, rifampicin, ethambutol, sulfisoxazole, vancomycin, ampicillin, trimethoprim-sulfamethoxazole, naproxen, or ranitidine) during hospitalization or in the 42 days prior, unless there was evidence that the drug was administered after disease onset date, were also excluded.
All cases were classified as either confirmed (Level 1-3 of diagnosis certainty) or non-confirmed (Supplementary material; Tables S-2 and S-3). Only confirmed cases entered the analyses.
The event date for AM cases was onset date of signs and symptoms suggestive of meningitis, admission date, or date of first physician diagnosis, whichever occurred earlier. The event date for ITP cases was onset date of spontaneous bleeding (19), date of first laboratory result with a platelet count <50,000/μL performed within 42 days prior to hospital admission or during hospitalization, admission date, or date of first physician diagnosis, whichever occurred earlier.
Vaccination status
Vaccination status was retrieved, for confirmed cases only, from vaccine registries, vaccination cards, and medical records. The exposure of interest was first dose of measles/mumps-containing vaccine. Patients were considered as non-vaccinated when any other vaccinations, but not measles-containing vaccines, were registered in the consulted sources. Individuals without any vaccination record were excluded from the study.
Data collection and sharing
Sites collected data using a common protocol, and transferred them into electronic case report forms using the purpose-built Chameleon® system (Erasmus Medical Center (EMC)). Chameleon® classified the cases automatically according to their level of diagnostic certainty. Outcome and exposure-coded datasets containing non-identifiable time interval-only data created by Chameleon® were uploaded to a central remote research environment, located at EMC, through a secure connection.
Quality assurance
In parallel with the study protocol and manual of procedures, a quality assurance plan was developed. It included roles and responsibilities for feasibility assessment, protocol development, data collection/transformation, analysis and reporting. The coordination team trained investigators through on-site and/or virtual meetings and through a simulation exercise using dummy cases, reviewed data submitted using standardized procedures, and sent reports to the sites detailing inconsistencies and missing data found. Following these communications, sites were asked to submit final data for analyses. Detailed information on quality assurance activities implemented and operating procedures followed for data collection, entry, and submission can be found elsewhere (Bravo-Alcántara P, Perez-Vilar S, Molina-León HF et al. (accepted for publication in Vaccine)).
Statistical analyses
The risks of AM following mumps-containing vaccination and ITP following measles-containing vaccination were estimated using self-controlled risk interval (SCRI) analyses (15, 20, 21). The observation period started on the day following first-dose vaccination and ended on day 84 post-vaccination. Days 8-35 were considered the risk period, days 1-7 and 36-42 washout periods, and days 43-84 the non-risk period. Thus, only vaccinated cases for which the event occurred within 84 days following vaccination were included. Poisson regression conditioned on the fact that the event occurred was used to estimate the incidence rate ratio. Differential risk of AM and ITP in the risk and non-risk windows due to circulation of wild viruses linked to the diseases of interest and age were adjusted for in the models as follows: (1) cut-off points for seasonality were March 31, June 30, September 30, and December 31; (2) age was controlled for with periods ending at 365, 457, 549, 641 days, and 732 days of age.
Per protocol, a case-crossover design was chosen as secondary analysis (22). The observation period was 84 days prior to event occurrence (case window: days -1 to -42; control window: days -43 to -84). Thus, cases without at least 84 days of follow-up prior to the event were excluded, regardless of vaccination status. The risk periods were days -8 to -35 for the case window and days -50 to -77 for the control window. The remaining periods were considered washout periods. Crude odds ratios were estimated using conditional logistic regression.
One site did not collect complete vaccination dates for any of the confirmed cases; thus, the day of vaccination was randomly imputed by Chameleon® within the month and year provided. Because of the importance of having exact vaccination dates for case-only methods, analyses with and without cases from this site (Iran-01) were performed. Because the risks for AM and ITP may vary by virus strain, (8–11, 23–25), exploratory analyses were performed by mumps and measles strain received, respectively. The two participating Iranian sites reported that three measles-mumps-rubella (MMR) vaccines, manufactured by Razi Vaccine, Serum Institute of India and Sanofi Pasteur, were used in the country during the study period, but they could not identify which specific product was administered to an individual patient. Thus, a separate analysis for the two Iranian sites was also conducted. Measles/mumps strains included in the vaccine products used by participating countries are shown in Table 1.
Table 1.
Measles and mumps strains included in the vaccine products used by the participating countries during the study period
| Vaccine product | Measles strain | Mumps strain |
|---|---|---|
| Priorix®, GlaxoSmithKline Biologicals | Schwarz | RIT 4385* |
| Priorix Tetra®, GlaxoSmithKline Biologicals | Schwarz | RIT 4385* |
| MMR, Shanghai Institute of Biological Products, Co., Ltd. | Shanghai-191 | S79 |
| Measles, Lanzhou Institute of Biological Products Co., Ltd. | Shanghai-191 | - |
| Measles-Rubella, Beijing Tiantan Biological Products, Co.,Ltd. | Shanghai-191 | - |
| M-M-R-II®, Merck Sharp & Dohme Corp. | Enders´ Edmonston | Jeryl Lynn (Level B) |
| MMR, Razi Vaccine and Serum Research Institute | AIK-C | Hoshino |
| M-M-RVAXPRO®, Sanofi Pasteur-MSD | Enders´Edmonston | Jeryl Lynn (Level B) |
| Trimovax®, Sanofi Pasteur | Schwarz | Urabe Am9 |
| Measles, Serum Institute of India Pvt. Ltd | Edmonston-Zagreb | - |
| Measles-Rubella, Serum Institute of India Pvt. Ltd | Edmonston-Zagreb | - |
| MMR, Serum Institute of India Pvt. Ltd | Edmonston-Zagreb | Leningrad-Zagreb |
| Tresivac®, Serum Institute of India Pvt. Ltd | Edmonston-Zagreb | Leningrad-Zagreb |
| Rouvax®, Sanofi Pasteur | Schwarz | - |
Abbreviations: MMR (measles-mumps-rubella);
Derived from Jeryl Lynn strain
All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC). The WHO Ethics Review Committee and all local Ethics Committees approved the study and provided a waiver of informed consent according to article 32 of the Declaration of Helsinki (26). Given the need for accurate information on vaccination status, a waiver to contact parents or legal representatives in case of lack of vaccination information was also obtained.
Results
A total of 84 confirmed AM cases and 183 confirmed ITP cases were eligible for inclusion in the case-only analyses. Number of confirmed cases successfully linked to vaccination records by site/country, level of diagnosis certainty, and site characteristics, including case ascertainment methods, vaccination data sources, and identifiers used to link exposures and outcomes, are shown in Table 2.
Table 2.
Characteristics of participating sentinel sites
| Site1 | Beds | Case ascertainment | Vaccination status ascertainment | Common outcome-exposure identifier | Confirmed aseptic meningitis cases2 | Confirmed ITP cases2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | ICD codes | Free text | Electronic vaccine registry | Vaccination cards | Medical records | Parents contacted3 | Unique identification number | Clinical history number | National identity card | Level 1 (n) | Level 2 (n) | Level 3 (n) | Level 1 (n) | Level 2 (n) | Level 3 (n) | |
| Albania | 240 | ICD-9 | - | ▯ | ▯ | - | - | - | ▯ | - | 1 | - | - | 5 | - | - |
| Argentina-01 | 330 | ICD-10 | - | ▯ | - | - | ▯ | - | ▯ | ▯ | 1 | - | - | 6 | - | 1 |
| Argentina-02 | 78 | ICD-10 | - | ▯ | - | - | ▯ | - | ▯ | ▯ | - | - | - | 1 | - | - |
| Argentina-03 | 380 | ICD-10 | - | ▯ | - | ▯ | ▯ | - | ▯ | ▯ | - | - | - | 4 | - | - |
| Argentina-04 | 246 | ICD-10 | - | ▯ | - | - | - | - | ▯ | ▯ | - | - | - | - | - | - |
| Argentina-05 | 224 | ICD-10 | - | ▯ | - | - | - | - | ▯ | ▯ | - | - | - | 4 | - | - |
| Argentina-06 | 61 | ICD-10 | - | ▯ | - | - | ▯ | - | ▯ | ▯ | - | - | - | 2 | - | - |
| Australia-01 | 334 | ICD-10 | - | ▯ | - | - | - | ▯ | ▯ | - | 2 | 5 | - | 5 | - | 2 |
| Australia-02 | 184 | ICD-10 | - | ▯ | ▯ | - | - | ▯ | ▯ | - | - | - | 1 | 4 | - | - |
| Chile-01 | 440 | ICD-10 | - | ▯ | - | - | - | - | - | ▯ | 3 | - | - | 2 | - | - |
| Chile-02 | 300 | ICD-10 | - | ▯ | - | - | ▯ | - | - | ▯ | 5 | - | 1 | 4 | - | - |
| Chile-03 | 704 | ICD-10 | - | ▯ | - | - | - | - | - | ▯ | - | - | - | 6 | - | - |
| Chile-04 | 876 | ICD-10 | - | ▯ | - | - | ▯ | - | - | ▯ | - | 1 | - | 5 | - | - |
| China | 500+ | ICD-10 | - | ▯ | - | - | - | - | - | - | - | - | 1 | 7 | - | - |
| Colombia | 340 | ICD-10 | - | ▯ | - | - | - | - | - | ▯ | - | - | - | 2 | - | - |
| Costa Rica | 313 | ICD-10 | - | ▯ | - | - | ▯ | - | - | ▯ | 1 | 2 | 1 | 13 | - | - |
| Honduras | 1,109 | ICD-10 | - | - | - | ▯ | ▯ | - | ▯ | ▯ | - | - | - | 1 | - | - |
| India | 1,200 | ICD-9, ICD-10 | - | - | - | ▯ | ▯ | ▯ | - | - | 3 | 5 | - | 1 | 1 | - |
| Iran-01 | 246 | ICD-10 | - | - | - | ▯ | - | - | - | - | 8 | 16 | 2 | 14 | 3 | - |
| Iran-02 | 340 | ICD-10 | - | - | - | ▯ | ▯ | - | - | - | 9 | 6 | 1 | 20 | - | - |
| Peru | 465 | ICD-10 | - | - | - | ▯ | ▯ | - | - | ▯ | - | - | - | 7 | - | - |
| Singapore | 830 | ICD-9, ICD-10 | - | ▯ | - | ▯ | - | ▯ | ▯ | ▯ | - | - | - | 17 | 1 | 2 |
| South Africa | 3,200 | ICD-10 | - | - | - | ▯ | ▯ | - | - | - | - | 1 | 2 | - | - | - |
| Spain4 | 10,987 | ICD-9 | - | ▯ | - | - | - | ▯ | - | - | 2 | - | 3 | 32 | 2 | 6 |
| Uganda | 254 | - | ▯ | - | ▯ | ▯ | ▯ | - | - | - | - | - | - | - | - | - |
| Uruguay | 245 | ICD-10 | - | ▯ | - | - | - | - | - | ▯ | 1 | - | - | 3 | - | - |
The study period was January 2010 to March 2014, except for Australia, which retrospectively included the first 25 most recent cases that fulfill inclusion criteria for each condition (for both sites combined)
Only the highest level of diagnosis certainty achieved applies. The cases correspond to confirmed cases for which a link to vaccination data was available. Confirmed cases for which vaccination status was unknown were excluded from the study
Parents contacted were asked to provide a copy of the vaccination cards
Spain was designated as one site, but included all public hospitals of the Valencia Region, its hospital beds correspond to the total number of beds from the combined hospitals
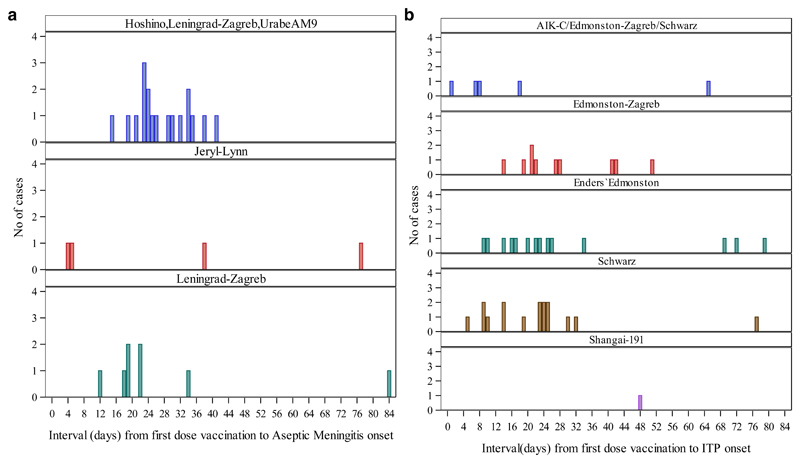
Among 84 AM cases, 80 (95%) received a first dose of mumps-containing vaccines (Table 3). A total of 51 (61%) and a total of 73 (87%) were eligible for inclusion in the SCRI and case-crossover analyses, respectively. The risk of AM following mumps containing vaccines was 10.9 (95% CI 4.2-27.8) with the SCRI analysis. Sensitivity analyses excluding Iran-01 resulted in an IRR estimate of 11.7 (95% CI 3.5-39.3). Intervals between first dose of mumps-containing vaccine and aseptic meningitis onset for cases included in the strain-specific SCRI analyses are shown in Figure 2a. A significantly increased AM risk was found for the Leningrad-Zagreb mumps strain (IRR: 10.8; 95% CI: 1.3-87.4). Risk estimates for S79, UrabeAm9 and RIT 4385/Jeryl-Lynn strains could not be assessed given small numbers. For the vaccine products used in Iran (Hoshino/Leningrad-Zagreb/UrabeAm9), an IRR of 20.3 (95% CI: 4.8-85.2) was identified (Table 4). Case-crossover analysis produced an overall unadjusted OR of 35.0 (95% CI: 4.8-255.5). When cases from Iran-01 were excluded, the OR estimate was 22.0 (95% CI: 3.0-163.2).
Table 3.
Characteristics of children with confirmed aseptic meningitis or immune thrombocytopenic purpura (ITP)
| Characteristic | Confirmed aseptic meningitis cases n=84 | Confirmed ITP cases n=183 |
|---|---|---|
| Male sex (n, %) | 54 (64%) | 98 (54%) |
| Age at onset in months (median; IQR) | 13 (12-15) | 15 (12-19) |
| Mumps-containing first dose vaccination (n, %) | 80 (95%) | - |
| Exact date known (n, %) | 60 (75%) | - |
| Vaccine brand known (n, %) | 41 (51%) | - |
| Age at vaccination in months (median; IQR) | 12 (11-12.5) | - |
| Measles-containing first dose vaccination (n, %) | - | 172 (94%) |
| Exact date known (n, %) | - | 159 (92%) |
| Vaccine brand known (n, %) | - | 125 (73%) |
| Age at vaccination in months (median; IQR) | - | 12 (12-15) |
Two aseptic meningitis cases died during the observation period, one case in Spain 78 days after disease onset date and another case in Australia 608 days following disease onset. None ITP case was known to die during the observation period.
Figure 2.
a. Interval between first dose of mumps-containing vaccines and aseptic meningitis onset by mumps vaccine strain
Only aseptic meningitis cases w ith onset on days 0 to 84 are shown
Cases from Iran01 for which exact vaccination dates were unavailable were excluded
From sites Iran01 and Iran02, strain used was not clear but it was either
Hoshino/UrabeAm9/Leningrad-Zagreb
b. Interval between first dose of measles-containing vaccines and immune thrombocytopenic purpura) ITP onset by measles vaccine strain
Only ITPcases which onset on days 0-84 days are shown
Cases fromIran01 for which vaccination dates were imputed were excluded
Table 4.
Risk of aseptic meningitis following mumps-containing vaccination and risk of immune thrombocytopenic purpura (ITP) following measles-containing vaccination; overall and by vaccine strain
| SCRI analyses | |||||
|---|---|---|---|---|---|
| Mumps vaccine strain1 | Eligible confirmed aseptic meningitis cases2 | Follow-up (days) | Relative incidence (IRR) | ||
| Event in risk period (8-35 days) | Event in non-risk period (43-84 days) | Median (P25-P75) | Unadjusted (95% CI) | Adjusted 95% CI | |
| Overall | 35 | 5 | 85 (85, 85) | 10.9 (4.2- 27.8) | 10.8 (4.0-29.2) |
| Overall3 | 22 | 3 | 85 (85, 85) | 11.7 (3.5-39.3) | 12.4 (3.1-49.1) |
| Hoshino/Leningrad-Zagreb/UrabeAm9 | 27 | 2 | 85 (85, 85) | 20.3 (4.8-85.2) | Non-estimable |
| Hoshino/Leningrad-Zagreb/UrabeAm9* | 14 | 0 | 85 (85, 85) | Non-estimable | Non-estimable |
| Leningrad-Zagreb | 7 | 1 | 85 (85, 85) | 10.8 (1.3-87.4) | 6.4 (0.3-124.4) |
| RIT 4385/Jeryl Lynn (Level B) | 0 | 1 | 85 (85, 85) | Non-estimable | Non-estimable |
| Measles vaccine strain | Eligible confirmed ITP cases1 | Follow-up (days) | Relative incidence (IRR) | ||
| Event in risk period (8-35 days) | Event in non-risk period (43-84 days) | Median (P25-P75) | Unadjusted 95% CI | Adjusted 95% CI | |
| Overall | 36 | 12 | 85 (70, 85) | 5.0 (2.5-9.7) | 5.6 (2.7-11.9) |
| Overall3 | 36 | 8 | 85 (70, 85) | 7.7 (3.5-17.3) | 9.1 (3.7-22.3) |
| AIK-C/ Edmonston-Zagreb /Schwarz | 2 | 5 | 85 (85, 85) | 0.51 (0.10-2.54) | 0.54 (0.08-3.55) |
| Edmonston-Zagreb | 7 | 1 | 85 (67, 85) | 11.1 (1.4-90.3) | 8.4 (0.7-100.3) |
| Enders´ Edmonston | 11 | 3 | 85 (43, 85) | 8.5 (1.9-38.1) | 28.7 (1.9-443.5) |
| Schwarz | 14 | 1 | 85 (76, 85) | 20.7 (2.7-157.6) | Non-estimable |
| Shanghai-191 | 0 | 1 | 85 (85, 85) | Non-estimable | Non-estimable |
There were no cases within days 8-35 or days 43-84 following first dose vaccination with mumps strains S79 or Urabe Am9.
The remaining cases occurred during the washout periods (days 1-7, days 36-42 following vaccination)
Excluding cases from Iran-01 since this site did not provide exact vaccination dates
Among 183 ITP cases, 172 (94%) were vaccinated with first dose of measles-containing vaccines. Of them, 55 (30%) and 152 (83%) were eligible for inclusion in the SCRI and case-crossover analyses, respectively. The risk of ITP following measles vaccination was 5.0 (95% CI: 2.5-9.7); exclusion of cases from Iran-01 resulted in an IRR estimate of 7.7 (95% CI: 3.5-17.3). Intervals between first dose of measles-containing vaccine and ITP onset for cases included in the strain-specific SCRI analyses are shown in Figure 2b. This analysis showed a significantly elevated ITP risk for measles vaccines containing Schwarz (IRR: 20.7; 95% CI: 2.7-157.6), Edmonston-Zagreb (IRR: 11.1; 95% CI: 1.4-90.3), and Enders´Edmonston (IRR: 8.5; 95% CI: 1.9-38.1) strains. Risk estimates for Shanghai-191 could not be assessed because of small numbers. Our estimates for the vaccine product/s used in Iran (AIK-C/ Edmoston-Zagreb/Schwarz) did not show an increased risk of ITP (IRR: 0.51; 95% CI: 0.10-2.54) (Table 4). The case-crossover analysis produced an overall unadjusted OR of 4.7 (95% CI: 2.1-10.7). When cases from Iran-01 were excluded, the OR estimate was 6.6 (95% CI: 2.6-16.9).
Discussion
The success of this proof-of-concept study in obtaining participation and data useful for analysis from sites located in all regions of the world using a common protocol has demonstrated the feasibility of international collaborative hospital-based studies, with high participation of LMICs, for the investigation of serious and rare AEFI. Moreover, the study has confirmed increased risks of AM following first dose of mumps-containing vaccines, and of ITP following first dose of measles-containing vaccines. It has also shown, potential risk differences between vaccine strains for both associations. The elevated risk estimates found for the Leningrad-Zagreb mumps strain are consistent with previous studies (27, 28). Regarding Jeryl-Lynn-derived strain vaccines, although the study did not have enough power to confirm the absence of risk for these strains, our finding of zero cases in the risk window was consistent with the hypothesis of no association (25, 29). The two Iranian sites reported that three vaccine products, containing the mumps strains Hoshino, Leningrad-Zagreb and UrabeAm9 were used during the study period, but they did not differentiate between them. Therefore, we could not assign the high risk of AM identified in Iran to one or other of these three strains (23, 24, 27, 28, 30–32). This would require further investigation in subsequent studies, particularly to determine the risk associated with the Hoshino strain, given the limited literature available on its safety profile (33–36). AM usually occurs within 2-5 weeks following mumps vaccination (9, 11, 31, 32, 37, 38); therefore, our study used a risk window of 8-35 days post-vaccination. Our study found a statistically significant risk when the washout period (days 1-7 and days 36-42 post-vaccination) was compared to the non-risk periods (days 43-84 post-vaccination) for the vaccine/s products used in Iran (IRR: 12.9; 95% CI: 2.8-59.7), which suggests the possibility of an increased risk also for the washout period, that deserves investigation in future studies.
The elevated risk of ITP following measles-containing vaccination is consistent with the literature (12–14). Our strain-specific unadjusted analysis showed a significantly elevated ITP risk for measles vaccines containing the Schwarz, Edmonston-Zagreb, and Enders´Edmonston strains. No risk of ITP was identified in Iran, which reported the concurrent distribution of three vaccine products including the AIK-C, Edmonston-Zagreb and Schwarz strains, without distinguishing between them. Among 172 vaccinees included in this study, at least 155 (90%) received MMR or measles-rubella vaccines. Given the known association between wild rubella infection and ITP (39), and the existence of a few studies showing mostly mild thrombocytopenia following rubella vaccination in some adults (19, 40–42), a potential contribution of the rubella component of the vaccine to our findings may not be excluded.
Case-only methods can be efficient epidemiological designs for use in vaccine safety, particularly for LMICs, given that population denominators or separate controls are not required; moreover, time-fixed confounders are inherently adjusted for (16). Self-controlled case series (SCCS) methods have been successfully implemented in similar international collaborations, such as the hospital-based international collaborative investigation of Guillain-Barre syndrome following the H1N1 2009-2010 pandemic influenza vaccination (7), and the investigation of the association between intussusception and rotavirus in Mexico and Brazil (43). In our study, some of the participating sites could not identify end of the follow-up period independently of the event being investigated, thus, modifying the duration of the observation period in ways that could potentially bias results (44). The SCRI approach simplifies the SCCS design by reducing the length of the control interval (21). The selection of shorter non-risk periods, as done in our study under the assumption that participants were not lost to follow-up during this 84-day period, not only may solve this limitation for LMICs, but may also decrease the effect of time-varying confounders on the risk estimates, because risk variations in such a short period may be negligible (21). Nonetheless, adjustments for age group and seasonality were performed, when possible. For comparison purposes, we used case-crossover as a secondary analysis, given that it does not require follow-up after case occurrence; to decrease the possibility of bias associated with variations in the distributions of exposures over time, only one control window of the same duration as the case window was selected (16). The method requires the same underlying probability of vaccination in all time intervals, which is unlikely to hold true for pediatric vaccines, which are usually administered according to pre-specified schedules (16). However, our case-crossover unadjusted risk estimates for ITP following measles-containing vaccines and for AM following mumps-containing vaccines were comparable to those obtained using the SCRI method, although the latter estimate was less stable due to limited study power.
Case-only methods demand careful determination of event onset and vaccination dates. Therefore, we were particularly thorough in training site investigators. Given that one site could not provide exact vaccination dates (only month/year of vaccination were recorded), we performed analyses both excluding and including this site (using imputed dates for the site). Although these analyses showed differences in point estimates, all results were significant and the confidence intervals overlapped. Since SCRI uses data only from vaccinees, the approach minimizes potential misclassification due to incomplete/absent data on vaccination status, another frequent shortcoming in LMICs. Nonetheless, a possible limitation in the approach used here is that site variability may be a potential source of selection bias as the sites may have differences in access to vaccination records and in patient’s health-seeking behavior. Bias could also be associated with site differences in diagnosis capabilities and quality of medical records. Also, our use of self-controlled analytical methods did not permit estimations of absolute risk (20).
Our results show that collaborative studies for the investigation of different vaccine products by strain and potentially by manufacturer are feasible. The power to do so, and to investigate risk by country/region (Supplementary material; Tables S-5 and S-6) will increase when additional large hospitals with medical specialties for rare and difficult to diagnose events, high quality medical records and easy access to vaccination records are included (2). The inclusion of large referral hospitals with electronic discharge databases should decrease per case investigation costs by reducing efforts associated with data extraction, study coordination, training, data quality assessment, and provide quality medical records and higher reliability in disease codification. The use of large hospitals would also reduce the likelihood of having participating hospitals that do not contribute cases to the analysis, as has occurred in some of our sites. Because easy and unequivocal linkages between hospital and vaccination records and proven access to vaccination information would increase data quality and efficiency, it is important to carefully select the participating sites, particularly in LMICs. Given the current interest on the development of vaccines for diseases such as dengue, malaria, and Zika, prioritization should be given to the addition of sites from tropical/sub-tropical areas in LMICs for future studies.
Conclusions
This collaboration has demonstrated, for the first time, that a multi-country hospital-based network with high participation of LMICs, using a common protocol and standardized procedures, permits the investigation of rare vaccine adverse events, can produce reliable results, and has the potential to characterize risk differences between vaccine strains. The completion of this network with the addition of large referral hospitals, including from tropical/subtropical countries, and the systematic implementation of this hospital-based approach, should permit the rapid and sustainable evaluation of safety signals for serious and rare AEFI for new and existing vaccines in all settings, and the comparison of safety profiles for vaccine products.
Supplementary Material
Acknowledgements
The authors thank Hector S. Izurieta for his valuable contributions to the conception of the project, Mees Mosseveld and Peter Rijnbeek for development of data collection and data sharing tools, Sergio Castillo-Pérez for mapping, and personnel from participating sentinel sites for facilitating the study implementation.
Funding
Center for Biologics Evaluation and Research (CBER)-U.S. Food and Drug Administration (FDA) funded this project. GRiP, Global Research in Pediatrics, European Union Seventh framework Programme (FP7/2007-2013) provided additional funding under grant agreement n° 261060.
Abbreviations
- AEFI
Adverse events following immunization
- AM
Aseptic meningitis
- CI
Confidence Interval
- CSF
Cerebrospinal fluid
- EMC
Erasmus Medical Center
- FDA
Food and Drug Administration
- GVSI
Global Vaccine Safety Initiative
- ITP
Immune thrombocytopenic purpura
- IRR
Incidence Rate Ratio
- LMICs
Low and middle-income countries
- MMR
Measles, mumps, rubella vaccines
- PMNs
Polymorphonuclear leukocytes
- OR
Odds ratio
- SCCS
Self-controlled case series
- SCRI
Self-controlled risk interval
- WHO
World Health Organization
Footnotes
Conflicts of interest
DW has received honoraria from GlaxoSmithKline Biologicals (GSK) for consultancies on malaria vaccine safety studies and implementation unrelated to the content of this manuscript. SB is a consultant for GSK. MS is heading a research group that conducts PASS studies for pharmaceutical companies including GSK.
All other authors confirm that there are no known conflicts of interest associated with this publication.
References
- 1.Gavi. The Vaccine Alliance 2017. [cited 2017 February 27]; Available from: http://www.gavi.org.
- 2.Izurieta HS, Zuber P, Bonhoeffer J, Chen RT, Sankohg O, Laserson KF, et al. Roadmap for the international collaborative epidemiologic monitoring of safety and effectiveness of new high priority vaccines. Vaccine. 2013;31(35):3623–7. doi: 10.1016/j.vaccine.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. [cited 2016 February, 7];Global vaccine safety blueprint. The landscape analysis. WHO/IVB/12.04: Quality, Safety, and Standards unit of the Department of Immunizations, Vaccines and Biologicals. 2012 Available from: http://apps.who.int/iris/bitstream/10665/70854/1/WHO_IVB_12.04_eng.pdf.
- 4.Amarasinghe A, Black S, Bonhoeffer J, Carvalho SM, Dodoo A, Eskola J, et al. Effective vaccine safety systems in all countries: a challenge for more equitable access to immunization. Vaccine. 2013;31(Suppl 2):B108–14. doi: 10.1016/j.vaccine.2012.10.119. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. [cited 2016 February 7];Global vaccine safety blueprint. WHO/IVB/12.07: Quality, Safety, and Standards unit of the Department of Immunization, Vaccines and Biologicals. 2012 Available from: http://extranet.who.int/iris/restricted/bitstream/10665/70919/1/WHO_IVB_12.07_eng.pdf?ua=1.
- 6.Maure CG, Dodoo AN, Bonhoeffer J, Zuber PL. The Global Vaccine Safety Initiative: enhancing vaccine pharmacovigilance capacity at country level. Bull World Health Organ. 2014;92(9):695–6. doi: 10.2471/BLT.14.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, et al. International collaboration to assess the risk of Guillain Barre Syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448–58. doi: 10.1016/j.vaccine.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;84(35):349–60. [PubMed] [Google Scholar]
- 9.Tapiainen T, Prevots R, Izurieta HS, Abramson J, Bilynsky R, Bonhoeffer J, et al. Aseptic meningitis: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007;25(31):5793–802. doi: 10.1016/j.vaccine.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Mumps virus vaccines. Wkly Epidemiol Rec. 2007;82(7):51–60. [PubMed] [Google Scholar]
- 11.Bonnet MC, Dutta A, Weinberger C, Plotkin SA. Mumps vaccine virus strains and aseptic meningitis. Vaccine. 2006;24(49–50):7037–45. [Google Scholar]
- 12.O'Leary ST, Glanz JM, McClure DL, Akhtar A, Daley MF, Nakasato C, et al. The risk of immune thrombocytopenic purpura after vaccination in children and adolescents. Pediatrics. 2012;129(2):248–55. doi: 10.1542/peds.2011-1111. [DOI] [PubMed] [Google Scholar]
- 13.Mantadakis E, Farmaki E, Buchanan GR. Thrombocytopenic purpura after measles-mumps-rubella vaccination: a systematic review of the literature and guidance for management. J Pediatr. 2010;156(4):623–8. doi: 10.1016/j.jpeds.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.France EK, Glanz J, Xu S, Hambidge S, Yamasaki K, Black SB, et al. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics. 2008;121(3):e687–92. doi: 10.1542/peds.2007-1578. [DOI] [PubMed] [Google Scholar]
- 15.Maclure M, Fireman B, Nelson JC, Hua W, Shoaibi A, Paredes A, et al. When should case-only designs be used for safety monitoring of medical products? Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):50–61. doi: 10.1002/pds.2330. [DOI] [PubMed] [Google Scholar]
- 16.Farrington CP. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine. 2004;22(15–16):2064–70. doi: 10.1016/j.vaccine.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Sejvar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5771–92. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Lussiana C, Loa Clemente SV, Pulido Tarquino IA, Paulo I. Predictors of bacterial meningitis in resource-limited contexts: an Angolan case. PLoS One. 2011;6(10):e25706. doi: 10.1371/journal.pone.0025706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wise RP, Bonhoeffer J, Beeler J, Donato H, Downie P, Matthews D, et al. Thrombocytopenia: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5717–24. doi: 10.1016/j.vaccine.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 20.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–97. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 21.Li R, Stewart B, Weintraub E. Evaluating efficiency and statistical power of self-controlled case series and self-controlled risk interval designs in vaccine safety. J Biopharm Stat. 2016;26(4):686–93. doi: 10.1080/10543406.2015.1052819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 23.Ki M, Park T, Yi SG, Oh JK, Choi B. Risk analysis of aseptic meningitis after measles-mumps-rubella vaccination in Korean children by using a case-crossover design. Am J Epidemiol. 2003;157(2):158–65. doi: 10.1093/aje/kwf167. [DOI] [PubMed] [Google Scholar]
- 24.Fujinaga T, Motegi Y, Tamura H, Kuroume T. A prefecture-wide survey of mumps meningitis associated with measles, mumps and rubella vaccine. Pediatr Infect Dis J. 1991;10(3):204–9. doi: 10.1097/00006454-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Black S, Shinefield H, Ray P, Lewis E, Chen R, Glasser J, et al. Risk of hospitalization because of aseptic meningitis after measles-mumps-rubella vaccination in one- to two-year-old children: an analysis of the Vaccine Safety Datalink (VSD) Project. Pediatr Infect Dis J. 1997;16(5):500–3. doi: 10.1097/00006454-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 26.World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.da Cunha SS, Rodrigues LC, Barreto ML, Dourado I. Outbreak of aseptic meningitis and mumps after mass vaccination with MMR vaccine using the Leningrad-Zagreb mumps strain. Vaccine. 2002;20(7–8):1106–12. doi: 10.1016/s0264-410x(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 28.da Silveira CM, Kmetzsch CI, Mohrdieck R, Sperb AF, Prevots DR. The risk of aseptic meningitis associated with the Leningrad-Zagreb mumps vaccine strain following mass vaccination with measles-mumps-rubella vaccine, Rio Grande do Sul, Brazil, 1997. Int J Epidemiol. 2002;31(5):978–82. doi: 10.1093/ije/31.5.978. [DOI] [PubMed] [Google Scholar]
- 29.Makela A, Nuorti JP, Peltola H. Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics. 2002;110(5):957–63. doi: 10.1542/peds.110.5.957. [DOI] [PubMed] [Google Scholar]
- 30.Dourado I, Cunha S, Teixeira MG, Farrington CP, Melo A, Lucena R, et al. Outbreak of aseptic meningitis associated with mass vaccination with a urabe-containing measles-mumps-rubella vaccine: implications for immunization programs. Am J Epidemiol. 2000;151(5):524–30. doi: 10.1093/oxfordjournals.aje.a010239. [DOI] [PubMed] [Google Scholar]
- 31.Sugiura A, Yamada A. Aseptic meningitis as a complication of mumps vaccination. Pediatr Infect Dis J. 1991;10(3):209–13. doi: 10.1097/00006454-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Mumps meningitis and MMR vaccination. Lancet. 1989;2(8670):1015–6. [PubMed] [Google Scholar]
- 33.Dorreh FHM. Adverse events associated with MMR vaccines in Arak. Journal of Iranian Clinical Research. 2015;1:6–10. [Google Scholar]
- 34.Esteghamati A, Keshtkar A, Heshmat R, Gouya MM, Salar Amoli M, Armin S, et al. Adverse reactions following immunization with MMR vaccine in children at selected provinces of Iran. Arch Iran Med. 2011;14(2):91–5. [PubMed] [Google Scholar]
- 35.Ueda K, Miyazaki C, Hidaka Y, Okada K, Kusuhara K, Kadoya R. Aseptic meningitis caused by measles-mumps-rubella vaccine in Japan. Lancet. 1995;346(8976):701–2. doi: 10.1016/s0140-6736(95)92311-x. [DOI] [PubMed] [Google Scholar]
- 36.Sood A, Mitra M, Joshi HA, Nayak US, Siddaiah P, Babu TR, et al. Immunogenicity and safety of a novel MMR vaccine (live, freeze-dried) containing the Edmonston-Zagreb measles strain, the Hoshino mumps strain, and the RA 27/3 rubella strain: Results of a randomized, comparative, active controlled phase III clinical trial. Hum Vaccin Immunother. 2017:1–8. doi: 10.1080/21645515.2017.1302629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura M, Kuno-Sakai H, Yamazaki S, Yamada A, Hishiyama M, Kamiya H, et al. Adverse events associated with MMR vaccines in Japan. Acta Paediatr Jpn. 1996;38(3):205–11. doi: 10.1111/j.1442-200x.1996.tb03471.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller E, Andrews N, Stowe J, Grant A, Waight P, Taylor B. Risks of convulsion and aseptic meningitis following measles-mumps-rubella vaccination in the United Kingdom. Am J Epidemiol. 2007;165(6):704–9. doi: 10.1093/aje/kwk045. [DOI] [PubMed] [Google Scholar]
- 39.Toltzis P. 50 Years Ago in The Journal of Pediatrics: Some Recently Recognized Manifestations of the Rubella Syndrome. J Pediatr. 2015;167(2):441. doi: 10.1016/j.jpeds.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Forrest JM, Honeyman MC, Lovric VA. Rubella vaccination and thrombocytopenia. Aust N Z J Med. 1974;4(4):352–5. doi: 10.1111/j.1445-5994.1974.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 41.Freestone DS, Prydie J, Smith SG, Laurence G. Vaccination of adults with Wistar RA 27/3 rubella vaccine. J Hyg (Lond) 1971;69(3):471–7. doi: 10.1017/s0022172400021720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartos HR. Thrombocytopenia associated with rubella vaccination. N Y State J Med. 1972;72(4):499. [PubMed] [Google Scholar]
- 43.Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283–92. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 44.Weldeselassie YG, Whitaker HJ, Farrington CP. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011;139(12):1805–17. doi: 10.1017/S0950268811001531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.