Abstract
Objective
The decline in insulin sensitivity (SI) associated with puberty increases the difficulty of achieving glycemic control in adolescents with type 1 diabetes (T1D). The aim of this study was to determine whether glutamine supplementation affects blood glucose by enhancing SI in adolescents with T1D.
Methods
Thirteen adolescents with T1D (HbA1C 8.2 ± 0.1%) were admitted to perform afternoon exercise (four 15-min treadmill/5-min rest cycles of exercise) on two occasions within a 4-wk period. They were randomized to receive a drink containing either glutamine (0.25 g/kg) or placebo before exercise, at bedtime, and early morning in a double-blind, crossover design. Blood glucose was monitored overnight, and a hyperinsulinemic-euglycemic clamp was performed the following morning.
Results
Blood glucose concentration dropped comparably during exercise on both days. However, the total number of nocturnal hypoglycemic events (17 versus 7, P = 0.045) and the cumulative probability of overnight hypoglycemia (50% versus 33%, P = 0.02) were higher on the glutamine day than on the placebo day. During clamp, glucose infusion rate was not affected by glutamine supplementation (7.7 ± 1 mg • kg−1 • min−1 versus 7.0 ± 1; glutamine versus placebo; P = 0.4).
Conclusions
Oral glutamine supplementation decreases blood glucose in adolescents with T1D after exercise. Insulin sensitivity, however, was unaltered during the euglycemic clamp. Although the mechanisms involved remain to be elucidated, studies to explore the potential use of glutamine to improve blood glucose control are needed.
Keywords: Amino acids, Exercise, Hypoglycemia, Hyperinsulinemic-euglycemic clamp clamp, Stable isotopes, GLP-1
Introduction
Puberty is associated with a physiological decline in insulin sensitivity [1,2]. This is of particular concern in adolescents with type 1 diabetes (T1D) [3] as insulin resistance can make it more difficult to achieve glycemic control during this stage of life [3]. Glutamine is the most abundant free amino acid in the body and contributes to the regulation of protein and energy homeostasis. Glutamine (GLN) is a major carbon donor for gluconeogenesis [4, 5] and glycogen synthesis [6]. It is thought to inhibit lipolysis in fasting dogs [7] and postabsorptive human volunteers [8], and is a potent stimulus of glucagon-like peptide (GLP)-1 secretion in healthy individuals [9], and adults with type 2 diabetes [10]. It has been suggested that GLN improves glucose tolerance, insulin sensitivity, or both in various settings, including in critically ill patients [11], trauma patients [12], children with cystic fibrosis treated with recombinant growth hormone [13], and experimental animals [14]. GLN also enhanced glycogen storage after exercise in healthy individuals [6]. Recent studies suggest GLP-1 reduces endogenous glucose production through mechanisms independent of insulin [15] and GLP-1 improved insulin sensitivity in rodents [16]. Lower nighttime blood glucose concentrations after afternoon exercise sessions in adolescents with T1D who received oral GLN compared with placebo (PL) have been observed [17]. As adolescents with T1D have no significant endogenous insulin production, we hypothesized that GLN had a role in modulating insulin sensitivity. The present study was designed to determine whether oral GLN increased insulin sensitivity, as assessed by the hyperinsulinemic-euglycemic clamp technique. Whether GLN affects GLP-1 and free fatty acids (FFA) concentrations were assessed as secondary outcomes.
Material and methods
After approval by the Wolfson Children’s Hospital Institutional Review Committee,13 adolescents (8 boys and 5 girls; mean age 15.9 ± 1.6 y) with T1D on insulin pump therapy were recruited among patients followed at the Nemours Children’s Clinic, Jacksonville, Florida after written informed consent and participant’s assent. Inclusion criteria included diabetes duration >1 y, on stable insulin therapy, body mass index (BMI) above the 10th but below the 85th percentile, hemoglobin (Hb) A1C >7.5 but <10%, and Tanner V puberty, as determined by a pediatric endocrinologist based on breast maturity or testicular volume.
Adolescents were excluded if they received any dietary supplement or medication likely to interfere with glucose metabolism. Other medications were continued at constant doses throughout the study. None of the participants had any diabetes-related complications.
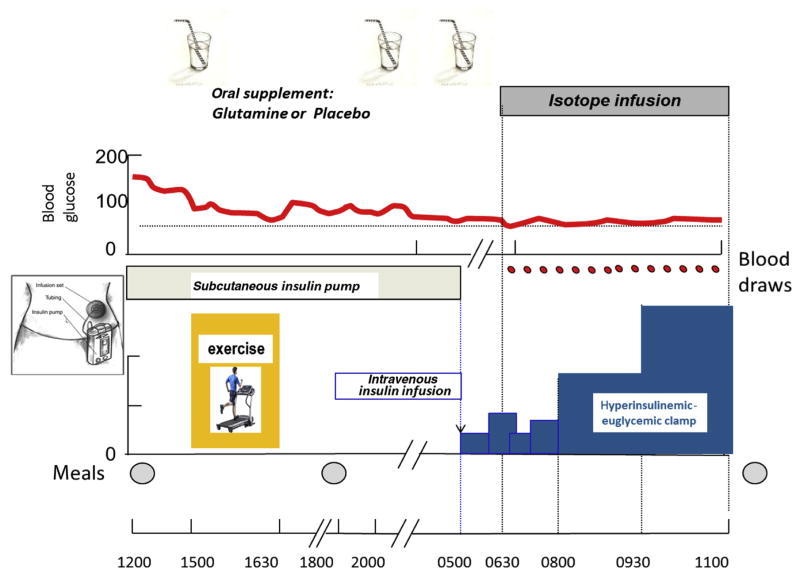
Each adolescent was admitted on two separate occasions to the Clinical Research Center (CRC) at Wolfson Children’s Hospital, once for the GLN study, and the second time for the PL study. Studies were performed within a 4-wk window, and in randomized order. Patients, investigators, and CRC staff were blinded as to randomization. Participants maintained a similar dietary intake and exercise routine as measured by an accelerometer (GTX+3, Actigraph) 3 d before each admission. Diabetes was managed as per their home routine. Patients were admitted at 1200 h. Lunch, dinner, and snacks were provided based on patients’ dietary history. The calorie and protein intake was identical on both CRC admissions. Protocol is depicted in Figure 1. After lunch, an intravenous (IV) line was placed in the antecubital fossa for blood glucose (BG) sampling. BG was titrated (129 ± 37 mg/dL) before exercise at 1500 h. Children were randomized to receive a drink containing either GLN (0.25 g • kg−1 •dose−1) or PL (calorie- and nitrogen-free) before exercise, at bedtime, and at 0530 h the following morning. The dose (0.75 g • kg−1 • 24 h−1) was chosen to match doses used in clinical trials in other settings [11–13] and found to affect BG in our earlier study [17]; such doses are of the same order of magnitude as endogenous GLN production (348 μmol • kg−1 • h−1, i.e., 1.2 g • kg−1 • d−1) [18].
Fig. 1.
Protocol design to assess the effect of glutamine on insulin sensitivity in children with type 1 diabetes.
Exercise and overnight monitoring
The exercise sessions consisted of four consecutive 15-min treadmill cycles with 5-min rest breaks, for a total of 75 min as previously described [17]. BG concentration was checked during each rest period and 30 min after exercise. If BG was <70 mg/dL, patients were not allowed back on the treadmill until BG concentration was restored to >70 mg/dL. Exercise intensity was titrated to achieve a heart rate of ~140 bpm. Basal subcutaneous insulin infusion was discontinued during exercise if adolescents had BG of <80 mg/dL at exercise initiation or after a first episode of hypoglycemia. Plasma GLN concentrations were measured immediately before and at the end of exercise. Patients were not allowed food or liquids after midnight until the end of the euglycemic clamp the following day. BG was monitored hourly overnight from 2100 h to 0500 h. A BG of ≤70 mg/dL prompted treatment with a fast-acting oral carbohydrate (until 2400 h) or 10% dextrose IV (after 2400 h). Treatment was repeated as needed until BG was ≥80 mg/dL.
Stable isotope infusion and hyperinsulinemic-euglycemic clamp
At 0500 h the following morning, the subcutaneous insulin pump was stopped. Another catheter was inserted in a retrograde fashion into a vein of the contralateral arm, heated to arterialize venous blood during sampling. An IV insulin infusion using regular insulin mixed with normal saline (1:1 dilution) was started at 0.05 unit • kg−1 • h−1 and adjusted every 30 min to maintain BG between 80 and 160 mg/dL.
At 0630 h, a 5-h primed, continuous infusion of D-[6,6-2H2] glucose (8 μmol • kg−1 • h−1) (Cambridge Isotope Laboratories, Andover, MA, USA) was started, and continued until completion of the clamp, using a calibrated, volume-controlled syringe. At 0800 h, a 3-h, two-dose hyperinsulinemic-euglycemic clamp was started: Insulin was infused at a low-dose (8 mU • m−2 •min−1) for the first 90 min followed by a high-dose (80 mU •m−2 •min−1) for the subsequent 90 min. BG concentration was kept constant using an IV infusion of 12.5% dextrose, adjusted based on every 5- to 10-min BG sampling using published algorithms [19]. Arterialized venous blood samples were obtained at baseline, midclamp, and at 10-min intervals during plateau (last 30 min of each portion of the clamp) for the determination of substrate and hormone concentrations, and stable isotope enrichments.
After IV infusion was discontinued, patients were fed and discharged home on their usual treatment regimen. They returned to the CRC within 4 wk for a second, identical study. The PL or GLN treatment order was randomized.
Calculations
Glucose infusion rate (GIR) (mg •kg−1•min−1) was calculated using the mean rate of dextrose infused during the last 30 min of each portion of the clamp. Rate of glucose appearance (Ra, mg • kg−1 •min−1) into plasma was calculated using equations for near-steady state conditions with a single pool model as Ra = i × [(Ei/Ep)–1], where i is the tracer infusion rate (mg • kg−1 • min−1), Ei and Ep are isotope enrichments (mol %excess) in the IV infusate and plasma at steady state, respectively. Endogenous glucose production (EGP) (mg • kg−1 • min−1) was calculated by subtracting GIR from Ra (at plateau) (EGP = Ra–GIR), and Ra reduction was determined by subtracting Ra at baseline (i.e., before the start of the clamp) from EGP (Ra reduction = EGP–Ra (baseline)) at the end of the low-dose hyperinsulinemic clamp. Insulin sensitivity index (SI, mL • kg−1 •min−1 per μU/mL) was calculated, using the following equation: SI = M/(BG × ΔI), where M is the glucose disposal rate (assumed to be equal to GIR during the high-dose clamp), BG is the mean steady-state plasma glucose (mg/mL) concentration at plateau, and ΔI is the difference between fasting and steady-state plasma-free insulin concentrations.
Assays
BG concentrations were measured with a FreeStyle Lite meter (Abbott Laboratories, Lake Bluff, IL, USA) and plasma glucose by glucose oxidase method with a YSI 2300 STAT Plus glucose analyzer (YSI, Yellow Springs, OH, USA). Insulin was measured by radioimmunoassay. Bioactive GLP-1 was measured by enzyme-linked immunosorbent assay. Plasma amino acid concentrations were measured by liquid chromatography mass spectrometry. FFAs were quantitated using an enzymatic colorimetric method and [2H2]glucose enrichments and GLN concentrations measured via gas chromatography-mass spectrometry (Agilent 6890 GC interfaced with a 5975 B Mass Spec in EI mode) after derivatization to glucose pentacetate, and N-acetyl propylester-glutamate, respectively, as previously described [5]. Ammonia was measured using an automated chemistry analyzer.
Statistical analysis
Baseline and demographic characteristics are summarized. Sample size was designed to detect a minimum of 20% difference in mean glucose disposal rate between GLN and PL with a power of 90% at the 5% level of significance using a two-sided, paired t test. Quantitative variables are summarized by mean ± SE. A mixed-effects model was used to compare mean glucose homeostasis between GLN and PL days. Treatment (PL or GLN), period, and the interaction of treatment and period were used as fixed effects and participants were used as random effect. We used an AR(1) covariance structure. Estimated marginal means and P values are presented. All tests were two-tailed with a level of significance of 0.05. Correlation between insulin sensitivity and the number of hypoglycemic events was tested using Spearman test. All analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY, USA).
Results
Thirteen adolescents were recruited, 12 of 13 completed the study; 2 did not receive isotope infusions but underwent all other procedures and were included in all analyses. Selected clinical characteristics are listed in Table 1. BMI was in the normal range (69th ± 16th percentile).
Table 1.
Clinical characteristics of study participants (means ± SE)
| Parameter | Males | Females |
|---|---|---|
| Height, cm | 173 ± 2 | 161 ± 4 |
| Weight, kg | 66 ± 11 | 59 ± 2 |
| Body mass index, kg/m2 | 22 ± 0.6 | 23 ± 0.4 |
| Sex ratio, M:F | 8:5 | |
| Duration of diabetes, y | 7.9 ± 1.3 | |
| HbA1C, % | 8.2 ± 0.1 |
Exercise
Participants achieved a mean heart rate of 143 ± 8 bpm. Baseline BG before exercise was comparable between study visits (GLN: 132 ± 11; PL: 125 ± 11 mg/dL; P = 0.66), and decreased comparably postexercise (GLN: −30 ± 12; PL: −20 ± 14 mg/dL; P = 0.58). The mean amount of carbohydrate consumed during exercise to maintain euglycemia was similar on both days (GLN: 21 ± 5; PL: 27 ± 8 g; P = 0.56).
Baseline GLN concentrations were identical on both study days (GLN: 591 ± 33; PL: 597 ± 30 μmol/L; P = 0.9) and increased ~20% after exercise on the GLN day (734 ± 37; P = 0.0012 versus baseline), whereas they remained unaltered on the PL day (616 ± 43; P = 0.1 versus baseline).
Plasma GLP-1 and free fatty acids
GLP-1 concentrations did not differ at baseline (preexercise) between study days (GLN: 4.47 ± 0.9; PL: 4.45 ± 0.9 pmol/L; P = 0.98). GLP-1 concentrations did not differ between regimens either in the immediate postexercise period (GLN: 4.6 ± 0.8; PL: 5.6 ± 0.7 pmol/L; P = 0.37) or in the postabsorptive state the following morning (0600) (GLN: 2.3 ± 0.3; PL: 1.8 ± 0.1 pmol/L; P = 0.2).
Concentration of FFA did not differ between study days either before (P = 0.66) or after exercise (P = 0.78; data not shown).
Overnight glucose control
The number of postexercise, nocturnal hypoglycemic events (BG <70 mg/dL) was higher after GLN administration than PL (17 versus 7, P = 0.045). The cumulative probability of overnight hypoglycemia was increased on the GLN day compared with PL day (50 versus 33% respectively, P = 0.02; Fig. 2).
Fig. 2.
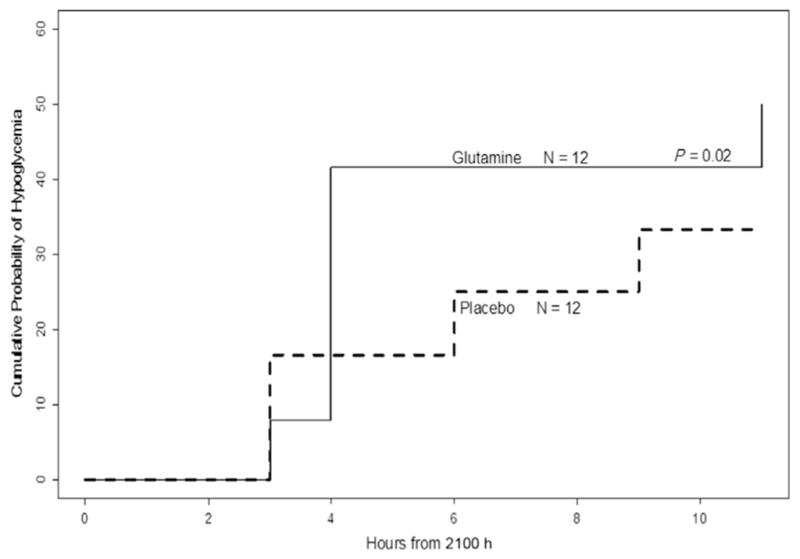
Cumulative probability of nighttime hypoglycemia (BG ≤70 mg/dL) if the same adolescent with type 1 diabetes mellitus took glutamine or placebo before afternoon exercise and at bedtime.
Low-dose, hyperinsulinemic-euglycemic clamp
Basal plasma-free insulin concentrations were not significantly different between study days (GLN: 140 ± 34; PL: 98 ± 19 μU/mL; P = 0.14). Baseline glucose concentrations at the initiation of the clamp were similar on both days (GLN: 103 ± 7; PL: 113 ± 10 mg/dL; P = 0.35) and maintained at plateau with a mean coefficient of variation of <6% (low dose: GLN: 136 ± 11; PL: 145 ± 12 mg/dL; high dose: GLN: 95 ± 4; PL: 92 ± 6 mg/dL; Table 2).
Table 2.
Glucose homeostasis during the hyperinsulinemic-euglycemic clamp in adolescents with type 1 diabetes receiving either glutamine or placebo
| Parameter | Glutamine | Placebo | P value* |
|---|---|---|---|
| Low-dose (8 mU • m−2 • min−1), hyperinsulinemic-euglycemic clamp | |||
| Plasma glutamine at plateau, μmol/L† | 608 ± 26 | 560 ± 25 | 0.15 |
| Plasma glucose at plateau, mg/dL | 136 ± 11 | 145 ± 12 | 0.6 |
| Free insulin at baseline, μU/mL | 140 ± 34 | 98 ± 19 | 0.14 |
| Free insulin at plateau, μU/mL | 72 ± 21 | 83 ± 20 | 0.2 |
| Glucose infusion rate, mg • kg−1 • min−1 | 1.4 ± 0.8 | 0.7 ± 0.3 | 0.4 |
| Baseline glucose Ra, mg • kg−1• min−1 | 6.9 ± 1.0 | 6.0 ± 1.0 | 0.2 |
| Glucose Ra at plateau, mg • kg−1 • min−1 | 6.0 ± 0.9 | 4.8 ± 0.9 | 0.3 |
| Endogenous glucose production at plateau, mg • kg−1 • min−1 | 4.2 ± 0.8 | 4.3 ± 0.6 | 0.96 |
| Reduction in endogenous glucose production, mg • kg−1 • min−1 | −2.7 ± 0.5 | −1.8 ± 0.4 | 0.14 |
| High-dose (80 mU • m−2 • min−1), hyperinsulinemic-euglycemic clamp | |||
| Plasma glutamine at plateau, μmol/L | 516 ± 29 | 488 ± 29 | 0.14 |
| Plasma glucose at plateau, mg/dL | 95 ± 4 | 92 ± 6 | 0.7 |
| Free insulin at plateau, μU/mL | 231 ± 36 | 235 ± 33 | 0.8 |
| Glucose infusion rate, mg • kg−1 • min−1 | 7.8 ± 0.8 | 7.0 ± 0.8 | 0.4 |
| Insulin sensitivity, mL • kg−1 • min−1 per μU/mL | 0.097 ± 0.02 | 0.071 ± 0.02 | 0.19 |
Estimated marginal mixed-effects model for a crossover design.
Last dose of oral glutamine given at 0530 h.
During the low-dose clamp, participants required a larger GIR on the GLN day than on the PL day, but this trend did not reach significance (GLN: 1.6 ± 0.6; PL: 0.6 ± 0.6 mg • kg−1 • min−1; P = 0.25). Endogenous glucose production (EGP) at plateau was nearly identical between days (GLN: 4.24 ± 0.8; PL: 4.32 ± 0.6 mg • kg−1 •min−1; P = 0.9). Reduction in EGP, defined as the change in EGP between baseline and plateau during the low-dose insulin clamp tended to be larger on the GLN day but the trend did not reach significance (GLN: −2.74 ± 0.53; PL: −1.82 ± 0.45; P = 0.14). Similar free insulin concentrations were reached at plateau during the low-dose clamp on both days (GLN: 72 ± 21; PL: 83 ± 20 μU/mL; P = 0.2).
High-dose hyperinsulinemic-euglycemic clamp
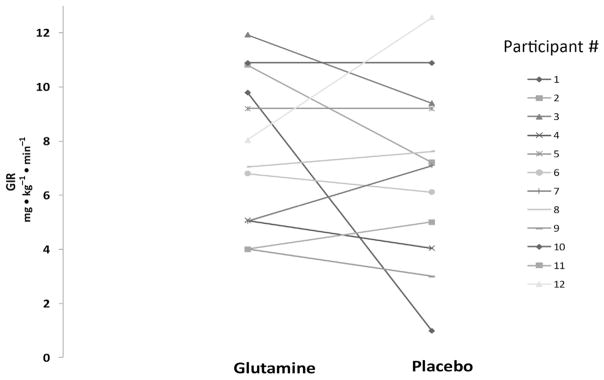
Participants required comparable GIRs on GLN day and PL day (7.7 ± 1 and 7 ± 1 mg • kg−1 •min−1, respectively; P = 0.4) and nearly identical free insulin concentrations were reached at plateau during the high-dose clamp on both days (GLN: 231 ± 36; PL: 235 ± 33 μU/mL; P = 0.82; Fig. 3 and Table 2).
Fig. 3.
GIR during high-dose hyperinsulinemic-euglycemic clamp after participant received glutamine or placebo. Individual changes are depicted by lines joining individual data points. GIR, glucose infusion rate.
Insulin sensitivity
Insulin sensitivity did not differ between the days (GLN: 0.10 ± 0.02; PL: 0.07 ± 0.02 mL • kg−1 • min−1 per μU/mL; P = 0.186; Fig. 3).
Discussion
Consistent with an earlier preliminary report [17], the present study demonstrates that GLN supplementation acutely increases the likelihood of postexercise, overnight hypoglycemia in adolescents with T1D. These data demonstrate that after strenuous exercise, GLN decreases glucose production or increases glucose utilization in T1D. As these participants had long-standing T1D with no residual β-cell function, this effect is unlikely due to any increase in insulin secretion. Yet the exact mechanism remains unclear.
In theory, GLN may affect glucose homeostasis through multiple mechanisms including
Enhanced insulin sensitivity;
Direct, noninsulin-mediated effects of GLN on glucose transport or glycogen synthesis [6,20–23], or lipolysis [7,8];
Enhanced secretion of hormones (other than insulin) affecting glucose metabolism such as GLP-1 [9,10]; and
Insulin-independent mechanisms such as those involving leptin [24], brain-derived neurotrophic factor [25] or fibroblast growth factor 19 [26].
We are aware of a single earlier study that explored the effect of GLN on glucose metabolism in patients with T1D in the literature [27]. That study compared BG recovery after acute hypoglycemia was induced by a gradual, hypoglycemic, hyperinsulinemic clamp in adults with T1D who received an infusion of either alanyl-glutamine dipeptide, or a PL, and found that glucagon secretion was enhanced in the group receiving alanyl-glutamine. That study design and the present study differ in several aspects:
The present study enrolled adolescents compared with adults in the previous study.
In the present study, participants had a session of vigorous exercise the night before the euglycemic clamp, whereas in the previous study, hypoglycemia was induced by a hypoglycemic, hyperinsulinemic clamp;
We administered natural L-glutamine orally, whereas a dipeptide of GLN and alanine was administered intravenously in the earlier study.
Such differences likely explain the discrepant results: We indeed observed an increase in the incidence of nocturnal hypoglycemia with oral GLN administration, whereas the earlier study observed enhanced glucagon secretion with the alanyl-glutamine dipeptide. Additionally, much higher plasma GLN concentrations were achieved in the previous study, most likely due to the IV route of delivery. Although we did not measure plasma glucagon concentrations, the incidence of hypoglycemic events was higher, rather than lower, in our participants on the GLN day, suggesting glucagon secretion likely did not increase on the GLN day. We speculate that the rise in plasma glucagon observed in the previous study may be an effect of alanine because the dipeptide delivered equimolar amounts of alanine and GLN, and alanine is known to increase glucagon secretion [28]; in contrast, we only administered GLN. Moreover, impaired secretion of counterregulatory hormones has been documented in children with T1D even with a relatively short diabetes duration [29,30].
In the literature, GLN was shown to enhance GLP-1 secretion in healthy volunteers [9] or adults with type 2 diabetes [10]; although GLP-1 primarily increases insulin secretion through an incretin effect, GLP-1 also affects glucose metabolism via insulin-independent mechanisms [15]. Yet no rise in GLP-1 was observed on the GLN day in the present study.
In other settings, glutamine was found to inhibit lipolysis [7,8] and therefore could decrease energy substrate availability, potentially leading to postexercise hypoglycemia. Yet FFA concentrations after exercise did not differ in our patients between the 2 days.
Such insulin-independent mechanisms therefore are unlikely. We did not observe a clearcut difference in insulin sensitivity during the hyperinsulinemic-euglycemic clamp after GLN administration. There were, however, nonstatistically significant trends toward a greater reduction in endogenous glucose production, and a greater glucose infusion rate on GLN day. Moreover, the postexercise incidence of nocturnal hypoglycemic events correlated with insulin sensitivity on the GLN day (r = 0.65; P = 0.03), whereas such correlation was not evident on PL day (data not shown). Whether GLN truly improves insulin sensitivity in T1D thus remains to be established.
Limitations in our study could have affected our ability to detect a significant rise in insulin sensitivity after oral GLN supplementation. First, only 13 patients were studied: We may have missed an effect of insulin because of a type II statistical error due to small sample size. Based on the ≈ 10% difference observed in GIR, an unrealistically high number of patients (>80) would need to be enrolled for such difference to reach statistical significance. Second, the hyperinsulinemic-euglycemic clamp was performed 15 h after exercise, by which time the effects of GLN, in combination with exercise, could have dissipated. Additionally, plasma GLN concentrations during clamp were not significantly different between the 2 days, which can be explained by the timing of the last dose of GLN (2 h before clamp initiation). Therefore, we speculate that GLN may only transiently improve insulin sensitivity while circulating GLN concentration is elevated, as it was immediately postexercise. It also is possible that the lower BG concentrations prevailing overnight on the GLN-supplemented day could enhance the secretion of counterregulatory hormone concentrations, which in turn could counteract any insulin-sensitizing effect of GLN during the clamp the following morning. Yet, counterregulatory responses are variable between individuals as blunted overnight counter-regulatory hormone response to hypoglycemia has been observed in toddlers and adolescents with T1D even early after diagnosis [28,29].
Regardless of the mechanism involved, the lower blood sugars induced by GLN is an intriguing finding as it occurred in the absence of residual insulin or glucagon secretion. As a nitrogen-free PL was used in the present study, further studies using a nonessental amino acid PL mix would be needed to ensure the effect is specific for GLN per se. If so, studies with more extended periods of GLN supplementation and larger population samples would be warranted to determine whether GLN supplementation improves insulin sensitivity in patients with T1D. Should such effect of GLN on glucose metabolism be sustained with repeat dietary GLN supplementation, large-scale clinical trials of long-term dietary GLN supplementation in the home setting would be warranted in adolescents with T1D.
Conclusion
The present study confirmed our previous observation that GLN has acute glucose-lowering effects in adolescents with longstanding T1D after exercise. As such patients do not retain endogenous insulin secretion, the effect cannot be due to increased insulin secretion. Although no change in insulin sensitivity was observed the following morning, insulin sensitivity correlated with the number of nocturnal hypoglycemic events. Whether GLN directly stimulates postexercise glucose transport into skeletal muscle or exerts a transient effect on insulin sensitivity after exercise is unclear. Although the current finding suggests the potential role of GLN as a glucose-lowering agent under conditions of insulin deficiency, further studies would clearly be needed to determine whether GLN supplementation has benefit improving glucose control in clinical practice.
Acknowledgments
This work was funded by grants from the Thrasher Research Fund (LTS) and the Nemours Research Programs (DD). The funding source(s) had no involvement in study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication. DD acted as the principal investigator of the study, wrote the grant, and assisted in the preparation of the manuscript. NM was a co-investigator, assisted in grant writing, and reviewed/edited the manuscript. LT-S was a co-investigator, coordinated all aspects of the study, recruited the participants, carried out the experiments, analyzed the data, and wrote the paper. HJ was the principal biostatistician of the study, helped analyze the data, created graphs, and wrote the statistical section. ALW was a co-investigator, served as consultant in exercise physiology, and critiqued the manuscript. All authors have approved the final article.
The authors acknowledge Shawn Sweeten B.S. and Karl Mann B.S., for laboratory assistance, and Katie Black B.A. for research support. The authors also acknowledge Frances Heatherington and Tifanny Deckerhoff, the expert nursing staff of the clinical research center at Wolfson Children’s Hospital for the dedicated care of our patients. The authors also acknowledge all the children with diabetes and their families for their participation and support in these studies.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Caprio S, Cline G, Boulware S, Permanente C, Shulman GI, Sherwin RS, et al. Effects of puberty and diabetes on metabolism of insulin-sensitive fuels. Am J Physiol. 1994;266:E885–91. doi: 10.1152/ajpendo.1994.266.6.E885. [DOI] [PubMed] [Google Scholar]
- 2.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–9. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 3.Arslanian S, Heil BV, Kalhan SC. Hepatic insulin action in adolescents with insulin-dependent diabetes mellitus: Relationship with long-term glycemic control. Metabolism. 1993;42:283–90. doi: 10.1016/0026-0495(93)90075-y. [DOI] [PubMed] [Google Scholar]
- 4.Hankard RG, Haymond MW, Darmaun D. Role of glucose in the regulation of glutamine metabolism in health and in type 1 insulin-dependent diabetes. Am J Physiol Endocrinol Metab. 2000;279:E608–13. doi: 10.1152/ajpendo.2000.279.3.E608. [DOI] [PubMed] [Google Scholar]
- 5.Hankard RG, Haymond MW, Darmaun D. Role of glutamine as a glucose precursor in fasting humans. Diabetes. 1997;46:1535–41. doi: 10.2337/diacare.46.10.1535. [DOI] [PubMed] [Google Scholar]
- 6.Varnier M, Leese GP, Thompson J, Rennie MJ. Stimulatory effect of glutamine on glycogen accumulation in human skeletal muscle. Am J Physiol. 1995;269:E309–15. doi: 10.1152/ajpendo.1995.269.2.E309. [DOI] [PubMed] [Google Scholar]
- 7.Cersosimo E, Williams P, Hoxworth B, Lacy W, Abumrad N. Glutamine blocks lipolysis and ketogenesis of fasting. Am J Physiol. 1986;250:E248–52. doi: 10.1152/ajpendo.1986.250.3.E248. [DOI] [PubMed] [Google Scholar]
- 8.Déchelotte P, Darmaun D, Rongier M, Hecketsweiler B, Rigal O, Desjeux JF. Absorption and metabolic effects of enterally administered glutamine in humans. Am J Physiol. 1991;260:G677–82. doi: 10.1152/ajpgi.1991.260.5.G677. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–13. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samocha-Bonet D, Wong O, Synnott EL, Piyaratna N, Douglas A, Gribble FM, et al. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J Nutr. 2011;141:1233–8. doi: 10.3945/jn.111.139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coeffier M, Hecketsweiler B, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34:598–604. doi: 10.1097/01.CCM.0000201004.30750.D1. [DOI] [PubMed] [Google Scholar]
- 12.Bakalar B, Duska F, Pachl J, Fric M, Otahal M, Pazout J, et al. Parenterally administered dipeptide alanyl-glutamine prevents worsening of insulin sensitivity in multiple-trauma patients. Crit Care Med. 2006;34:381–6. doi: 10.1097/01.ccm.0000196829.30741.d4. [DOI] [PubMed] [Google Scholar]
- 13.Darmaun D, Hayes V, Schaeffer D, Welch S, Mauras N. Effects of glutamine and recombinant human growth hormone on protein metabolism in pre-pubertal children with cystic fibrosis. J Clin Endocrinol Metab. 2004;89:1146–52. doi: 10.1210/jc.2003-031409. [DOI] [PubMed] [Google Scholar]
- 14.Prada PO, Hirabara SM, de Souza CT, Schenka AA, Zecchin HG, Vassallo J, et al. L-glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle of rats with diet-induced obesity. Diabetologia. 2007;50:1949–59. doi: 10.1007/s00125-007-0723-z. [DOI] [PubMed] [Google Scholar]
- 15.Prigeon RL, Quddusi S, Paty B, D’Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285:E701–7. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 16.Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2010;299:E318–24. doi: 10.1152/ajpendo.00191.2010. [DOI] [PubMed] [Google Scholar]
- 17.Mauras N, Xing D, Fox LA, Englert K, Darmaun D. Effects of glutamine on glycemic control during and after exercise in adolescents with type 1 diabetes: A pilot study. Diabetes Care. 2010;33:1951–3. doi: 10.2337/dc10-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol. 1986;251:E117–26. doi: 10.1152/ajpendo.1986.251.1.E117. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 20.Goodyear LJ, King PA, Hirshman MF, Thompson CM, Horton ED, Horton ES. Contractile activity increases plasma membrane glucose transporters in absence of insulin. Am J Physiol. 1990;258:E667–72. doi: 10.1152/ajpendo.1990.258.4.E667. [DOI] [PubMed] [Google Scholar]
- 21.Meijer AJ, Baquet A, Gustafson L, van Woerkom GM, Hue L. Mechanism of activation of liver glycogen synthase by swelling. J Biol Chem. 1992;267:5823–8. [PubMed] [Google Scholar]
- 22.Iwashita S, Williams P, Jabbour K, Ueda T, Kobayashi H, Baier S, et al. Impact of glutamine supplementation on glucose homeostasis during and after exercise. J Appl Physiol. 1985;99:1858–65. doi: 10.1152/japplphysiol.00305.2005. [DOI] [PubMed] [Google Scholar]
- 23.Bowtell JL, Gelly K, Jackman ML, Patel A, Simeoni M, Rennie MJ. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J Appl Physiol. 1999;86:1770–7. doi: 10.1152/jappl.1999.86.6.1770. [DOI] [PubMed] [Google Scholar]
- 24.German JP, Thaler JP, Wisse BE, Oh I, Sarruf DA, Matsen ME, et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152:394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meek TH, Wisse BE, Thaler JP, Guyenet SJ, Matsen ME, Fischer JD, et al. BDNF action in the brain attenuates diabetic hyperglycemia via insulin-independent inhibition of hepatic glucose production. Diabetes. 2013;62:1512–8. doi: 10.2337/db12-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton GJ, Matsen ME, Bracy DP, Meek TH, Nguyen HT, Stefanovski D, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123:4799–808. doi: 10.1172/JCI70710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.M’Bemba J, Cynober L, de Bandt P, Taverna M, Chevalier A, Bardin C, et al. Effects of dipeptide administration on hypoglycaemic counterregulation in type 1 diabetes. Diabetes Metab. 2003;29:412–7. doi: 10.1016/s1262-3636(07)70052-6. [DOI] [PubMed] [Google Scholar]
- 28.Wiethop BV, Cryer PE. Alanine and terbutaline in treatment of hypoglycemia in IDDM. Diabetes Care. 1993;16:1131–6. doi: 10.2337/diacare.16.8.1131. [DOI] [PubMed] [Google Scholar]
- 29.Arbelaez AM, Xing D, Cryer PE, Kollman C, Beck RW, Sherr J, et al. Blunted glucagon but not epinephrine responses to hypoglycemia occurs in youth with less than 1 yr duration of type 1 diabetes mellitus. Pediatr Diabetes. 2014;15:127–34. doi: 10.1111/pedi.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsalikian E, Tamborlane W, Xing D, Becker DM, Mauras N, Fiallo-Scharer R, et al. Blunted counterregulatory hormone responses to hypoglycemia in young children and adolescents with well-controlled type 1 diabetes. Diabetes Care. 2009;32:1954–9. doi: 10.2337/dc08-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]