Abstract
Background
Chromosome 16p11.2 microdeletion is associated with early-onset obesity. Information is limited about the effect of bariatric surgery in patients with genetic obesity.
Objective
To examine effects of bariatric surgery in obese patients with chromosome 16p11.2 microdeletion.
Setting
Academic research institution.
Methods
The Swedish Obese Subjects (SOS) study is a prospective study with 2010 participants receiving bariatric surgery. Deoxyribonucleic acid (DNA) was available in 1843 participants. Multiplex ligation-dependent probe amplification (MLPA) was used to identify 16p11.2 microdeletion carriers. Follow up time was 10 years. In carriers and non-carriers, follow-up rate was 86% and 82% at 10 years, respectively.
Results
Nine carriers of the chromosome 16p11.2 microdeletion (9/1843, 0.49%) were found. At baseline, most risk factors were similar, however carriers had higher body mass index (BMI), insulin levels and systolic blood pressure compared to non-carriers. At the 1-year examination, the percent excess BMI lost (%EBMIL) in carriers and non-carriers was 71.9 and 62.2, respectively; p=0.031 (37.9 and 30.6 kg). This was followed by partial weight regain in both groups, and after 10 years %EBMIL was 25.5 and 41.5 (15.7 and 21.3 kg), respectively (p=0.377). Changes in risk factors were similar in the carriers and non-carriers. Two carriers who had type 2 diabetes at baseline were both in remission at the 2-year follow-up, but had relapsed at the 10-year follow-up. Perceived health status was similar in carriers and non-carriers during follow-up (overall p=0.198).
Conclusions
Despite small sample size, our results indicate that bariatric surgery is a treatment option in obese patients with chromosome 16p11.2 microdeletion.
Keywords: Obesity, bariatric surgery, chromosome 16p11.2 microdeletion
Introduction
Bariatric surgery is the most effective treatment to achieve substantial long-term weight loss and reduced co-morbidities and mortality in severe obesity1. The mechanisms by which bariatric surgery leads to weight loss include enhanced gastrointestinal endocrine signaling of satiety pathways2. Many patients with monogenic or syndromic obesity have hyperphagia, probably caused by disruption of satiety pathways. Consequently, the effectiveness of bariatric surgery may differ dependent on if the specific genetic alteration causes disruption of satiety pathways that are influenced by bariatric surgery.
There is limited and conflicting information about the effectiveness of bariatric surgery in patients with syndromic obesity, such as Prader-Willi (PWS) and Bardet-Biedl (BBS) syndromes3, 4. Another common form of syndromic obesity is caused by a 593kb deletion at the 16p11.2 locus, here referred to as 16p11.2 microdeletion5. Deletions at this region are also associated with autism; the deletion is seen in approximately 1% of individuals with autism compared to a frequency of 0.01% in the general population6. In addition, duplications of the same region have been associated with underweight7. To our knowledge there are no reports on the effects of bariatric surgery in patients with 16p11.2 microdeletion. The aim of this study was to determine the frequency of 16p11.2 microdeletion in the Swedish Obese Subjects (SOS) study and compare the outcomes after bariatric surgery in carriers and non-carriers.
Subjects
The SOS study is a prospective, matched, intervention trial comparing the effects of bariatric surgery (n=2010) and conventional obesity care (n=2037)8–10. The current study comprises 1843 subjects from the surgery group in which deoxyribonucleic acid (DNA) was available for analysis of the 16p11.2 microdeletion. Of these, 1243 (67.4%) underwent vertical banded gastroplasty (VBG), 350 (19.0%) banding and 250 (13.6%) gastric bypass (GBP). Inclusion criteria were age between 37–60 years and body mass index (BMI) >34kg/m2 for males and >38kg/m2 for females. Exclusion criteria10 were minimal and aimed to ensure that the patients were suitable for surgery. Baseline examinations took place 4 weeks before surgery and follow-up examinations were scheduled at 0.5, 1, 2, 3, 4, 6, 8 and 10 years after surgery. Number of individuals available (excluding those who died during follow-up) and seen at each follow-up visit is presented in Supplementary table 1. Biochemical variables were measured at baseline and after 2 and 10 years at the Central Laboratory, Sahlgrenska University Hospital, Gothenburg, Sweden, accredited according to ISO/IEC 15 189. Self-reported energy intake and perceived health status were obtained from SOS questionnaires. Perceived health status was examined using the question: “Please rate your general health on the 7-grade scale below”, where 1 is “I feel great, couldn’t feel better” and 7 is “I feel really bad, couldn’t feel worse”. Seven regional ethics review boards approved the study and informed consent was obtained. The SOS study is registered at ClinicalTrials.gov (identifier: NCT01479452)
Methods
Chromosome 16p11.2 microdeletion screening
To identify carriers of the 16p11.2 microdeletion, 21 single nucleotide polymorphisms (SNPs), of which 14 were within, 3 were upstream and 4 were downstream of the deletion were genotyped using the Sequenom MassARRAY platform (Sequenom Inc., San Diego, California) at the Mutation Analysis Core Facility (MAF), Karolinska Institute, Stockholm, Sweden. The assay was designed using the MassARRAY Assay Design 3.1 software (Sequenom) (primer sequences are available upon request). The presence of the deletion was confirmed using 100ng of DNA and the multiple ligation dependent probe amplification (MLPA) Autism kit (cat no: P343 Holland, Amsterdam, Netherlands) containing 11 probes in the 16p11.2 region, 9 within the deleted region and 2 in the regions flanking the deletion. Labeled MLPA-products were detected using an ABI Prism 3739 Genetic Analyzer (Life Technologies Ltd, Paisley, UK) with GeneScan500 LIZ size standard (Life Technologies) at the KIGene Core Facility, Karolinska Institute, Stockholm, Sweden.
Statistical methods
Descriptive statistics are given as means with standard deviations (SD). Multilevel mixed-effect regression models were fitted to the data to assess longitudinal changes in carriers and non-carriers of the chromosome 16p11.2 microdeletion. For systolic blood pressure and serum insulin, a logarithmic transformation was applied before statistical testing due to a few extreme values. For dichotomous variables during follow-up (complications, mortality), no formal statistical testing was conducted due to low number of persons with the chromosome 16p11.2 microdeletion. Statistical analyses were carried out using the Stata statistical package 12.1 (Stata-Corp. 2011, Stata Statistical Software: Release 12, College Station, TX, USA; StataCorp LP).
Results
Baseline characteristics of carriers and non-carriers of the chromosome 16p11.2 microdeletion
Amongst the 1843 patients we found 9 carriers (4 men, 5 women) of the 16p11.2 microdeletion (0.49%) (Table 1). None of the individuals were previously diagnosed with syndromic obesity. At baseline, most risk factors were similar, however the 16p11.2 microdeletion carriers had higher BMI, fasting insulin and systolic blood pressure compared to the non-carriers (Table 1). Two (22.2%) of the 16p11.2 microdeletion carriers had type 2 diabetes.
Table 1.
Baseline characteristics of carriers and non-carriers of the 16p11.2 microdeletion. Standard deviations are presented in brackets (where applicable).
| Carriers | Non-carriers | p-value | |
|---|---|---|---|
| N | 9 | 1834 | |
| Age, yers | 46.9 (4.4) | 47.2 (6.0) | 0.887 |
| Male gender, % | 44.4 | 29.7 | 0.464 |
| BMI, kg/m2 | 46.8 (6.0) | 42.3 (4.5) | 0.003 |
| Weight, kg | 124.3 (23.2) | 120.8 (16.6) | 0.530 |
| Blood glucose, mmol/L | 5.6 (2.6) | 5.2 (2.0) | 0.557 |
| Insulin, mU/L | 32.4 (21.1) | 21.4 (13.7) | 0.029 |
| Systolic blood pressure, mmHg | 159.8 (16.2) | 144.9 (18.8) | 0.018 |
| Diastolic blood pressure, mmHg | 94.0 (10.9) | 89.8 (11.2) | 0.261 |
| Triglycerides, mmol/L | 1.8 (0.3) | 2.3 (1.6) | 0.359 |
| Total cholesterol, mmol/L | 5.6 (1.2) | 5.9 (1.1) | 0.553 |
| HDL cholesterol, mmol/L | 1.4 (0.3) | 1.4 (0.3) | 0.896 |
| Diabetes, % | 22.2 | 17.5 | 0.662 |
| Hypertension, % | 88.9 | 78.2 | 0.693 |
| Smoking, % | 22.2 | 26.3 | 1.000 |
| Caloric intake/day, kcal | 3017 (989) | 2948 (1266) | 0.871 |
| Perceived health status, score | 3.6 (1.1) | 3.7 (1.3) | 0.751 |
Abbreviations: BMI - body mass index, HDL - high density lipoprotein
Changes in body weight, BMI, energy intake and perceived heath status
Among the nine 16p11.2 deletion carriers, four underwent vertical banded gastroplasty, three banding and two gastric bypass. After surgery, rapid weight loss was seen in both carriers and non-carriers (Figure 1a). At 1 year, carriers and non-carriers had 71.9 and 62.2 percent excess body mass index lost (%EBMIL), respectively; p=0.031 (37.9 and 30.6 kg). After partial weight regain, %EBMIL at 10 years was 25.5 and 41.5 (15.7 and 21.3 kg) in carriers and non-carriers, respectively (p=0.377). The individual BMI trajectories over time varied markedly among the carriers of the 16p11.2 microdeletion (Figure 1b). Detailed follow-up information is presented in Supplementary table 1 and mean weight changes in each surgery subgroup (banding, vertical banded gastroplasty, gastric bypass) in Supplementary table 2.
Figure 1.
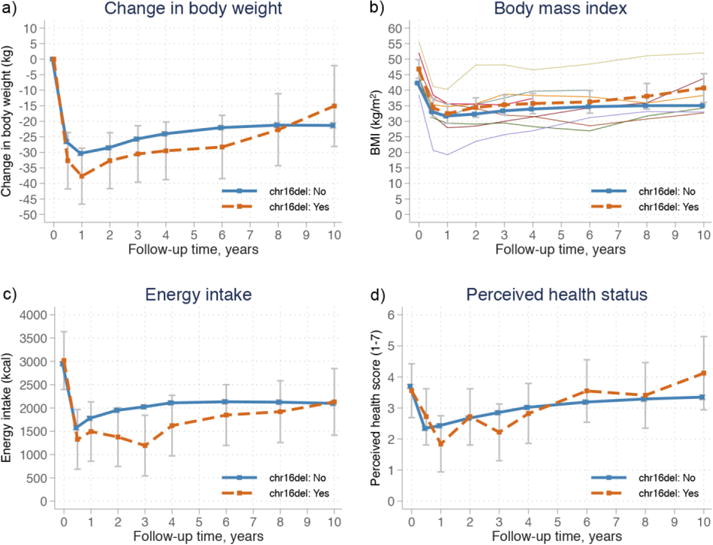
Estimated means from mixed-models with 95% confidence intervals for body weight (a), body mass index (BMI) (b), energy intake (c) and perceived health status (d) in carriers and non-carriers of the 16p11.2 microdeletion followed for 10 years after bariatric surgery in the Swedish Obese Subjects (SOS) study. Orange dashed lines represent carriers of the 16p11.2 microdeletion, while solid blue lines represent non-carriers. b) Individual BMI trajectories for carriers of the 16p11.2 microdeletion are presented with narrow lines.
Compared to baseline, energy intake was significantly reduced 0.5 years after surgery in both carriers and non-carriers of the 16p11.2 microdeletion (p<0.001 for both groups), and remained lower compared to baseline during the 10-year follow-up (Figure 1c), with no statistical difference in overall trend between the groups (p=0.173). Perceived health status was similar in carriers and non-carriers during follow-up (overall p=0.198), with improvement during the first year as compared to baseline in both groups (Figure 1d).
Changes in risk factors and diabetes remission
After surgery, cardiovascular risk factors improved and changes in blood glucose, serum insulin, total cholesterol, high density lipoprotein (HDL) cholesterol, systolic and diastolic blood pressure during follow-up were similar in carriers and non-carriers of the 16p11.2 microdeletion (overall p=0.239, p=0.318, p=0.714, p=0.676 and p=0.974, respectively; Supplementary Figure 1). The two carriers that had type 2 diabetes at baseline were both in remission at the 2-year follow-up, while at the 10-year follow-up both had relapsed.
Post-surgical complications
Three of the nine carriers of the 16p11.2 microdeletion had complications within 90 days after surgery. One patient died 2 days after surgery (gastric bypass), one was re-operated and one had a wound infection.
Discussion
In this study, we demonstrate that the response to bariatric surgery is similar in obese patients with and without chromosome 16p11.2 microdeletion. Bariatric surgery resulted in large long-term weight loss and seemed to have the same positive effects on risk factors in carriers and non-carriers of the microdeletion.
Few studies that have explored the effects of bariatric surgery on monogenic and syndromic forms of obesity, and the results have been inconsistent with some studies reporting large weight losses and improvement of risk factors, while others found no weight changes at all3, 4. Furthermore, there is limited information on safety of bariatric surgery in patients with genetic obesity4. In the current study, there was one postoperative death among the 9 patients with the 16p11.2 microdeletion compared to a 0.25% postoperative mortality rate in whole SOS surgery group9. Due to the limited number of patients with diagnosed genetic obesity who undergo bariatric surgery, safety evaluation is challenging. However, reporting adverse events after bariatric surgery in specific genetic forms of obesity is critical for patient safety4.
It is unknown how the 16p11.2 microdeletion leads to obesity. None of the 27 genes within the deletion stands out as a candidate gene for obesity11, however studies suggest that several factors together may cause the obese phenotype. This includes changes in brain structures associated with the reward system and eating behavior12, 13 and changes in satiety response14, sweet food intake15, and altered expression of obesity associated genes16.
Many rare genetic forms of obesity have in common that they affect eating behavior4, 12. Eating disorders have been associated with less favorable outcome of bariatric surgery and patients with severe eating disorders are not recommended to undergo bariatric surgery. Patients with eating disorders who underwent bariatric surgery initially had the same decrease in BMI as those without disorders, but this was followed by greater weight regain17–19. Some studies indicate that 16p11.2 microdeletion carriers have an altered eating behavior12–15, however our results show that the carriers of the 16p11.2 microdeletion can reduce their energy intake to a similar degree as the non-carriers. In addition, carriers reported similar perceived health score as non-carriers, indicating that there was no difference in subjective health between the groups.
A limitation of this study is the small number of individuals with the 16p11.2 microdeletion. The frequency of the 16p11.2 microdeletion in the surgery group of the SOS study was 0.49%, which is slightly lower than what we5 and others20 have previously found in individuals with morbid obesity. A potential explanation is the fact that the chromosome 16p11.2 microdeletion is associated with cognitive deficits11 and that this may have led to a lower proportion of carriers of the 16p11.2 microdeletion among the candidates for inclusion in the study compared to non-carriers. Also, the exclusion criteria in the SOS study, including psychological problems suspected to result in poor cooperation10, might have disfavored selection of deletion carriers.
Conclusions
We conclude that bariatric surgery has similar effects in carriers and non-carriers of the 16p11.2 microdeletion, with large sustained weight loss and improved risk factors in both groups. This suggests that bariatric surgery is a treatment option for obesity in patients with chromosome 16p11.2 microdeletion.
Supplementary Material
Acknowledgments
We thank the SOS-study participants, the staff members at the SOS secretariat and at the 480 primary health-care centers and 25 surgical departments in Sweden that participated in the study.
Funding: This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK105948 (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health), the Swedish Research Council K2013-99X-22279-01, K2013-54X-11285-19, Sahlgrenska University Hospital ALF research grant, the Swedish Diabetes Foundation, the Åke Wiberg foundation, the Tore Nilson foundation for medical research, the Magnus Bergwall foundation, the IngaBritt and Arne Lundberg Foundation and the Finnish Cultural Foundation (via the Finnish Post Doc Pool).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
LMSC has obtained lecture fees from AstraZeneca, Johnson&Johnson and MSD. BC is employed by AstraZeneca and holds stocks in the same company. No other conflict of interest relevant to this study was reported.
Author contribution
FMK and LMSC designed the study. FMK and JCA-A were responsible for genotyping. NK analyzed energy intake. MP had full access to data and performed statistical analysis. All authors contributed to data interpretation. FMK wrote the first version of the paper. All authors critically reviewed the content and contributed to manuscript revision, approved the final version and agree to be accountable for all aspects of the work.
Contributor Information
Felipe M. Kristensson, Institute of Medicine, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
Johanna C. Andersson-Assarsson, Institute of Medicine, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden.
Noora Kanerva, Institute of Medicine, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, SwedenThe Department of Health, National Institute for Health and Welfare, Helsinki, Finland.
Markku Peltonen, The Department of Health, National Institute for Health and Welfare, Helsinki, Finland.
Björn Carlsson, Institute of Medicine, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, SwedenInnovative Medicines and Early Development Biotech Unit, AstraZeneca Gothenburg, Mölndal, Sweden.
Lena M.S. Carlsson, Institute of Medicine, The Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden.
References
- 1.Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947–56. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 2.Abdeen G, le Roux CW. Mechanism Underlying the Weight Loss and Complications of Roux-en-Y Gastric Bypass. Review Obes Surg. 2016;26(2):410–21. doi: 10.1007/s11695-015-1945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Sayed Moustafa JS, Froguel P. From obesity genetics to the future of personalized obesity therapy. Nature reviews Endocrinology. 2013;9(7):402–13. doi: 10.1038/nrendo.2013.57. [DOI] [PubMed] [Google Scholar]
- 4.Huvenne H, Dubern B, Clement K, Poitou C. Rare Genetic Forms of Obesity: Clinical Approach and Current Treatments in 2016. Obes Facts. 2016;9(3):158–73. doi: 10.1159/000445061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters RG, Jacquemont S, Valsesia A, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463(7281):671–5. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358(7):667–75. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 7.Jacquemont S, Reymond A, Zufferey F, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478(7367):97–102. doi: 10.1038/nature10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 9.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrom L, Larsson B, Backman L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord. 1992;16(6):465–79. [PubMed] [Google Scholar]
- 11.D’Angelo CS, Koiffmann CP. Copy number variants in obesity-related syndromes: review and perspectives on novel molecular approaches. J Obes. 2012;2012:845480. doi: 10.1155/2012/845480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill R, Chen Q, D’Angelo D, Chung WK. Eating in the absence of hunger but not loss of control behaviors are associated with 16p11.2 deletions. Obesity Silver Spring, Md. 2014;22(12):2625–31. doi: 10.1002/oby.20892. [DOI] [PubMed] [Google Scholar]
- 13.Maillard AM, Ruef A, Pizzagalli F, et al. The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol Psychiatry. 2015;20(1):140–7. doi: 10.1038/mp.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillard AM, Hippolyte L, Rodriguez-Herreros B, et al. 16p11.2 Locus modulates response to satiety before the onset of obesity. Int J Obes (Lond) 2016;40(5):870–6. doi: 10.1038/ijo.2015.247. [DOI] [PubMed] [Google Scholar]
- 15.Keskitalo K, Knaapila A, Kallela M, et al. Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr. 2007;86(1):55–63. doi: 10.1093/ajcn/86.1.55. [DOI] [PubMed] [Google Scholar]
- 16.Loviglio MN, Leleu M, Mannik K, et al. Chromosomal contacts connect loci associated with autism, BMI and head circumference phenotypes. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu LK, Benotti PN, Dwyer J, et al. Nonsurgical factors that influence the outcome of bariatric surgery: a review. Psychosom Med. 1998;60(3):338–46. doi: 10.1097/00006842-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Hsu LK, Sullivan SP, Benotti PN. Eating disturbances and outcome of gastric bypass surgery: a pilot study. Int J Eat Disord. 1997;21(4):385–90. doi: 10.1002/(sici)1098-108x(1997)21:4<385::aid-eat12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Kalarchian MA, Marcus MD, Wilson GT, Labouvie EW, Brolin RE, LaMarca LB. Binge eating among gastric bypass patients at long-term follow-up. Obes Surg. 2002;12(2):270–5. doi: 10.1381/096089202762552494. [DOI] [PubMed] [Google Scholar]
- 20.Bochukova EG, Huang N, Keogh J, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463(7281):666–70. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


