Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is associated with increased cardiovascular morbidity and mortality. Cardiac arrhythmias are common in patients with OSA. However, the prevalence and significance of cardiac arrhythmias in Asian patients with OSA are not well studied. The aim of this study is to determine the prevalence of cardiac arrhythmias in patients with OSA in Singapore and to evaluate possible factors that may predispose patients with OSA to arrhythmias.
Methods:
A retrospective study of 2,019 patients was carried out from January 2011 to December 2012 at a sleep center in a tertiary medical center. Of the population, 1,457 patients were found to have OSA and 144 patients were found to have cardiac arrhythmias. Data collected included patient demographics, comorbidities, and polysomnogram parameters.
Results:
The prevalence of cardiac arrhythmias in our OSA population is 8.0%, compared to that of primary snorers at 4.8% (P = .015). The univariate analysis revealed that older age, higher body mass index, comorbidities, and severity of OSA, including apnea-hypopnea index (AHI), lowest oxygen saturation (LSAT) and hypoxic time were correlated with a higher prevalence of cardiac arrhythmias (P < .05). However, the multivariate analysis showed that only age and body mass index were significantly correlated with arrhythmias. AHI, LSAT, and hypoxic time were no longer statistically significant.
Conclusions:
Our study demonstrated that cardiac arrhythmias are common in patients with OSA in Singapore. It also suggests that given the different demographics of our population, ethnicity may play a significant role in the development of cardiovascular disease among patients with OSA.
Commentary:
A commentary on this article appears in this issue on page 1229.
Citation:
Neo WL, Ng AC, Rangabashyam M, Hao Y, Ho KL, Senin SR, Toh ST. Prevalence of cardiac arrhythmias in Asian patients with obstructive sleep apnea: a Singapore sleep center experience. J Clin Sleep Med. 2017;13(11):1265–1271.
Keywords: Asian, cardiac arrhythmias, epidemiology, obesity, obstructive sleep apnea
INTRODUCTION
It is estimated that the prevalence of moderate-to-severe obstructive sleep apnea (OSA) in Singapore is 30%.1 This percentage is markedly higher than the 2% to 4% often quoted for the Western population.2 OSA has been associated with an increased prevalence of cardiovascular morbidity and mortality including hypertension,3 cerebrovascular accidents,4,5 heart failure,6 and coronary artery disease.5,6 The correlation between OSA and cardiac arrhythmias has been increasingly studied, with a prevalence of up to 48%7 of patients with OSA in the Western population.
Various mechanisms have been postulated to cause arrhythmias among patients with OSA. Recurrent episodes of hypoxemia and hypercarbia, with fluctuations in intrathoracic and hence intracardiac pressures, may result in alterations in the sympathetic and parasympathetic nervous system, leading to electrical and structural remodeling. This, in turn, leads to arrhythmogenesis.8
We noted that most studies on the relationship of cardiac arrhythmias and OSA were predominantly done in the Western population. There is a dearth of basic information on the relationship between OSA and cardiac arrhythmias in the Asian population. This, coupled with differences in prevalence and demographics, prompted us to evaluate the relationship between cardiac arrhythmias and OSA. As such, the primary objective of our study is to assess the prevalence of cardiac arrhythmias among patients with OSA in the Singapore context. The secondary objective is to determine possible factors that may predict cardiac arrhythmias among these patients with OSA.
BRIEF SUMMARY
Current Knowledge/Study Rationale: It has been postulated that obstructive sleep apnea (OSA) may affect Asians differently compared with Caucasians. There is a lack of information on the prevalence of cardiac arrhythmias in the Asian population with OSA.
Study Impact: Our study demonstrates that cardiac arrhythmias are more common in patients with OSA in the Singaporean context. In addition, it suggests that ethnicity may play a significant role in the development of cardiovascular disease among patients with OSA.
METHODS
Study Design
This is a retrospective study of patients with suspected OSA who underwent a diagnostic polysomnogram in an accredited sleep center in a tertiary medical center. Polysomnographic and clinical data of patients who presented to our Sleep Disorders Unit from January 2011 to December 2012 were systemically reviewed. All patients were referred to the Sleep Disorders Unit for evaluation of possible OSA with symptoms including daytime somnolence and snoring. Patients with other forms of sleep-disordered breathing such as central sleep apnea (ie, 5 or more central apnea events per hour of sleep9) or Cheyne-Stokes breathing were excluded from this study. A total of 2,019 patients were recruited for this study.
Covariate Data
Characteristics of patients who had OSA were then analyzed, including demographic data including age, sex, body mass index (BMI), neck circumference, and ethnicity. Data on the patient's comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, and previous arrhythmia, were collected.
Polysomnography Data
All the patients recruited in our study underwent an in-hospital polysomnogram with Profusion 3 (Compumedics, Melbourne, Victoria, Australia). Recordings of the electroencephalogram, electrooculogram, electromyogram of the chin, electrocardiogram (ECG), airflow measurement, pulse oximetry, body position, respiratory effort, snoring sound, pulse rate, and limb movement channels were included in the study. Each polysomnogram was reviewed independently by a registered polysomnograph technologist and a physician. The interscorer reliability was more than 80%.
Polysomnogram variables collected included apneahypopnea index (AHI), lowest oxygen saturation (LSAT), and hypoxic time (ie, time spent with oxygen saturation [SpO2] < 90%). LSAT referred to the lowest oxygen saturations detected via pulse oximetry during sleep. Hypoxic time refers to the percentage of time spent with oxygen saturation less than 90%, with reference to total sleep time.
Apneas and hypopneas were scored in accordance with the American Academy of Sleep Medicine 2007 recommended scoring guidelines. Apneas were scored when there was more than 90% drop in airflow for at least 10 seconds from the baseline using a oronasal thermistor.10 Hypopneas were scored when there was more than 30% drop in airflow (for at least 10 seconds) as measured from the nasal pressure transducer with a 4% desaturation. AHI referred to the average number of disordered breathing events (apneas and hypopneas) per hour. Because all patients were symptomatic, patients with an AHI of 5 or more were classified as having OSA.11,12
Arrhythmia Analysis
ECG data were collected for the duration of sleep using a lead II bipolar ECG. All ECG data were manually reviewed by 2 medical doctors, who were blinded to respiratory events. The data were further reviewed by a cardiologist subspecializing in cardiac electrophysiology. The estimated interrater reliability was 90% for arrhythmic events. Arrhythmias were analyzed as dichotomous outcomes (present or absent).
Arrhythmias were then classified as supraventricular arrhythmias, ventricular arrhythmias, mixed arrhythmias, conduction delay arrhythmias, and sinus arrhythmias. Supraventricular arrhythmias included rhythms emanating from the sinus node, atrial tissue, and junctional as well as accessory mediated pathways,13 including premature atrial complexes, atrial fibrillation and junctional arrhythmias. Ventricular arrhythmias included premature ventricular complexes, bigeminy, trigeminy, quadrigeminy, and nonsustained ventricular tachycardia (3 or more consecutive ventricular ectopic beats with a heart rate exceeding 100 bpm). Conduction delay arrhythmias included first-, second-, and third-degree atrioventricular blocks and sinus pauses (3 or more seconds). Mixed arrhythmias referred to patients who had more than one subtype of arrhythmias.
This study was approved by Singhealth Centralised Institutional Review Board, reference number 2009/595/A. Because of the retrospective nature of this study, the need for informed consent was waived.
Statistical Analysis
R 3.0.2 (The R Project for Statistical Computing, Vienna, Austria) was used for statistical analysis. In view of the correlation of arrhythmia and OSA, both univariate and multivariate analysis were carried out. Fisher exact test or chi square test was used for categorical variables, whereas Mann-Whitney U test was used for continuous variables in univariate analysis.
Categorical data was presented as absolute numbers and percentages and continuous data as mean and standard deviation (normal distribution) and mean and interquartile ranges (nonparametric data).
We performed multiple logistics regression models to obtain the adjusted odds ratio of arrhythmia for OSA. All the covariates, which were statistically significant in the univariate analysis, were included in the model. In the multivariate analysis, the association between OSA and each continuous variable are reported as odds ratio (OR) per 1-U increase with the 95% confidence interval. A value of P < .05 was considered statistically significant for all analyses.
RESULTS
Of the 2,019 patients who underwent diagnostic polysomnography, 1,457 (72%) had OSA, whereas 562 did not meet the criteria, and were classified as having primary snoring. The prevalence of arrhythmias was 8.0% in patients with OSA, which was significantly higher than that of those who had primary snoring, 4.8% (P = .015) (Table 1). In addition, the prevalence of arrhythmias increased with the severity of OSA, with only 5.5% of the patients with mild OSA, 7.6% in the moderate group, and 10.2% in the severe group (P = .015) (Table 2).
Table 1.
Prevalence of arrhythmias.

Table 2.
Prevalence of arrhythmias according to severity of OSA.

Patients with OSA were more likely to be male (76.3% versus 62.3%, P < .001), older (48 versus 40 years, P < .001), heavier (BMI = 28.3 versus 24.1 kg/m2, P < .001) and have a larger neck size (41 versus 37 cm, P < .001) (Table 3), compared with the primary snorers. Significantly, only 23 patients (1.1%) of our study population were known to have arrhythmias (Table 3). In addition, patients with OSA were more likely to have pre-existing comorbidities such as hypertension (37.8% versus 14.4%, P < .001), diabetes mellitus (13.5% versus 6.4%, P < .001), hyperlipidemia (25.1% versus 16%, P < .001), and ischemic heart disease (5.7% versus 3.4%, P = .044) (Table 3).
Table 3.
Demographics, comorbidities, and polysomnography characteristics of study population.
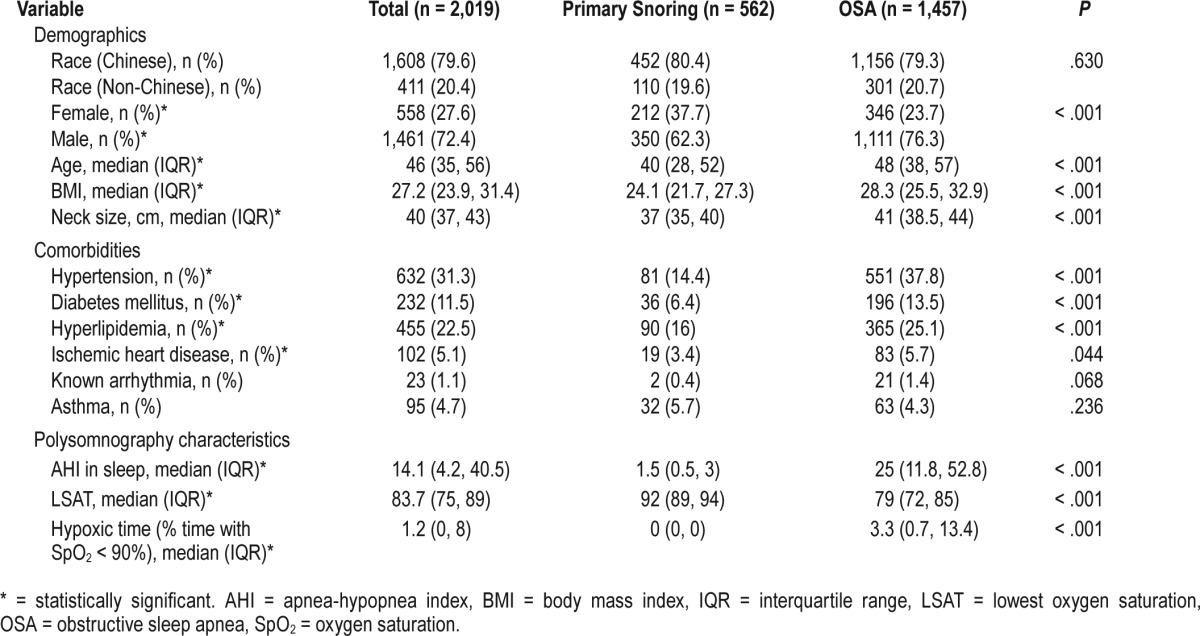
From the univariate analysis, patients with OSA who were older (54.7 versus 47.7 years, P < .001) had higher BMI (32.0 versus 29.7 kg/m2, P < .001) and a larger neck size (42.2 versus 40.9 cm, P < .001) were more likely to have arrhythmias (Table 4). In addition, the mean BMI of patients with OSA and arrhythmias was 32.0 kg/m2, and this is well within the high-risk category for Asian patients.14 Patients with OSA who had arrhythmias also tended to have more severe sleep apnea (AHI = 43.7 versus 34.4 events/h, P < .001), spent a longer time in hypoxia (hypoxic time = 20.7% versus 12.0%, P < .001), and reached lower LSAT (SpO2 = 74.1% versus 77.4%, P = .006) (Table 4). In terms of comorbidities, patients with OSA and arrhythmias were more likely to have pre-existing diabetes mellitus (23.1% versus 12.6%, P = .002), ischemic heart disease (12.0% versus 5.1%, P = .004), and preexisting arrhythmias (6.8% versus 1.0%, P < .001). Of note, there was no significant difference in sex nor race, and patients with OSA who had hypertension or hyperlipidemia were not more likely to have arrhythmias as their counterparts who did not have these comorbidities (Table 4).
Table 4.
Demographics, comorbidities and polysomnography characteristics of the study population with OSA.
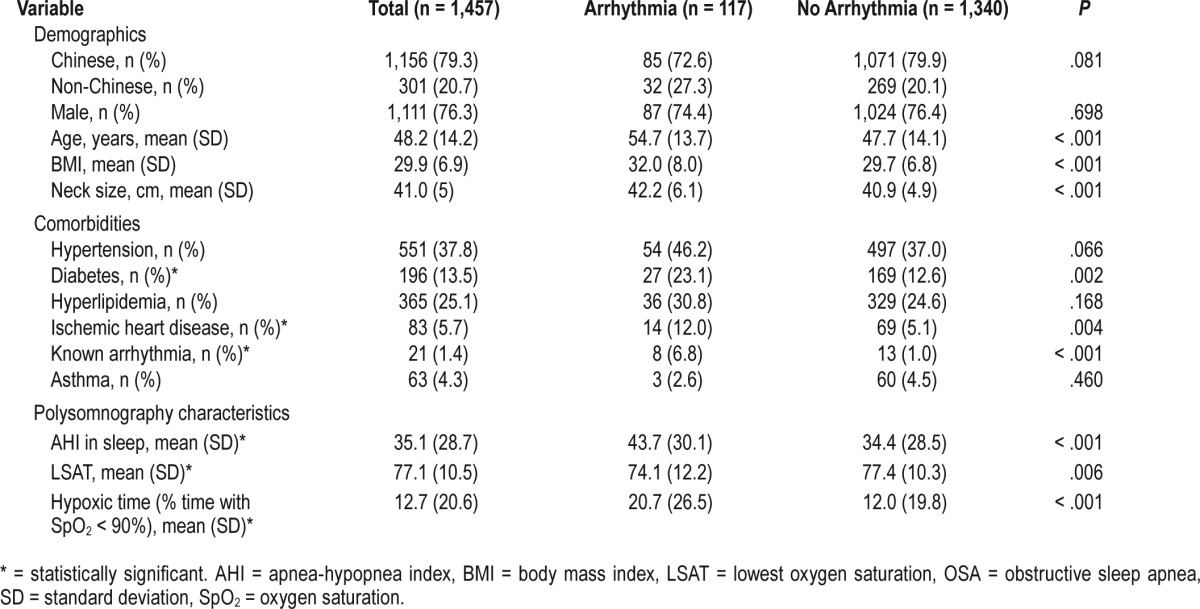
In our study, all subtypes of arrhythmias were more common in patients with OSA in comparison with those with primary snoring (Table 5), though none of the analyses were of statistical significance. The most common arrhythmias were ventricular arrhythmias (52.1%), followed by supraventricular arrhythmias (27.1%) and conduction delay arrhythmias (6.2%) (Table 5).
Table 5.
Arrhythmias according to subtypes.
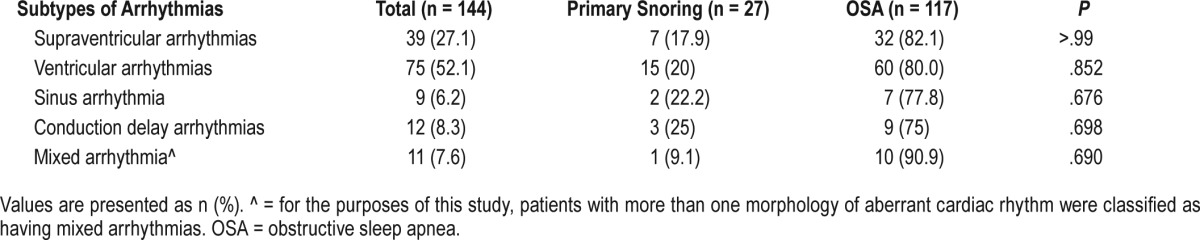
From the multivariate analysis, there is a 4% increase in risk with each year of age, this in turn, translates into a 20% increase with every 5 years of age (odds ratio [OR] 1.04, 95% confidence interval [CI] 1.02–1.05, P < .001) (Table 6). After adjustment for covariates, other variables such as AHI, LSAT, and hypoxic time were no longer statistically significant (Table 6). Similarly, the comorbidities such as hypertension, diabetes mellitus, and ischemic heart disease were no longer statistically significant (Table 6).
Table 6.
Predictors of arrhythmias among patients with OSA using multiple logistics regression analyses.
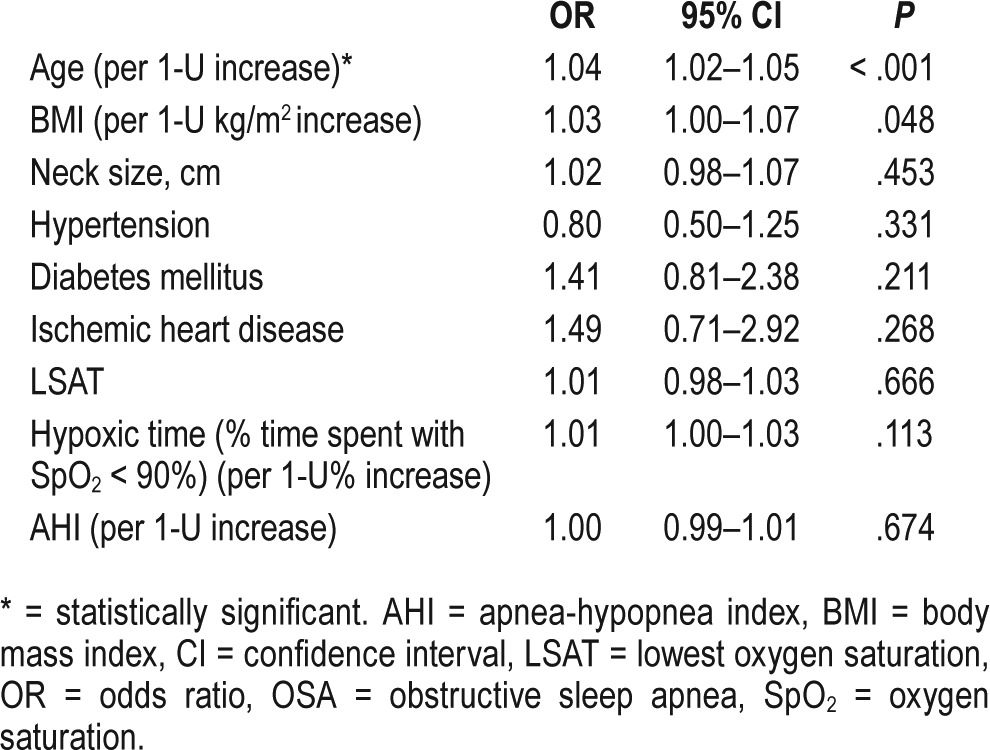
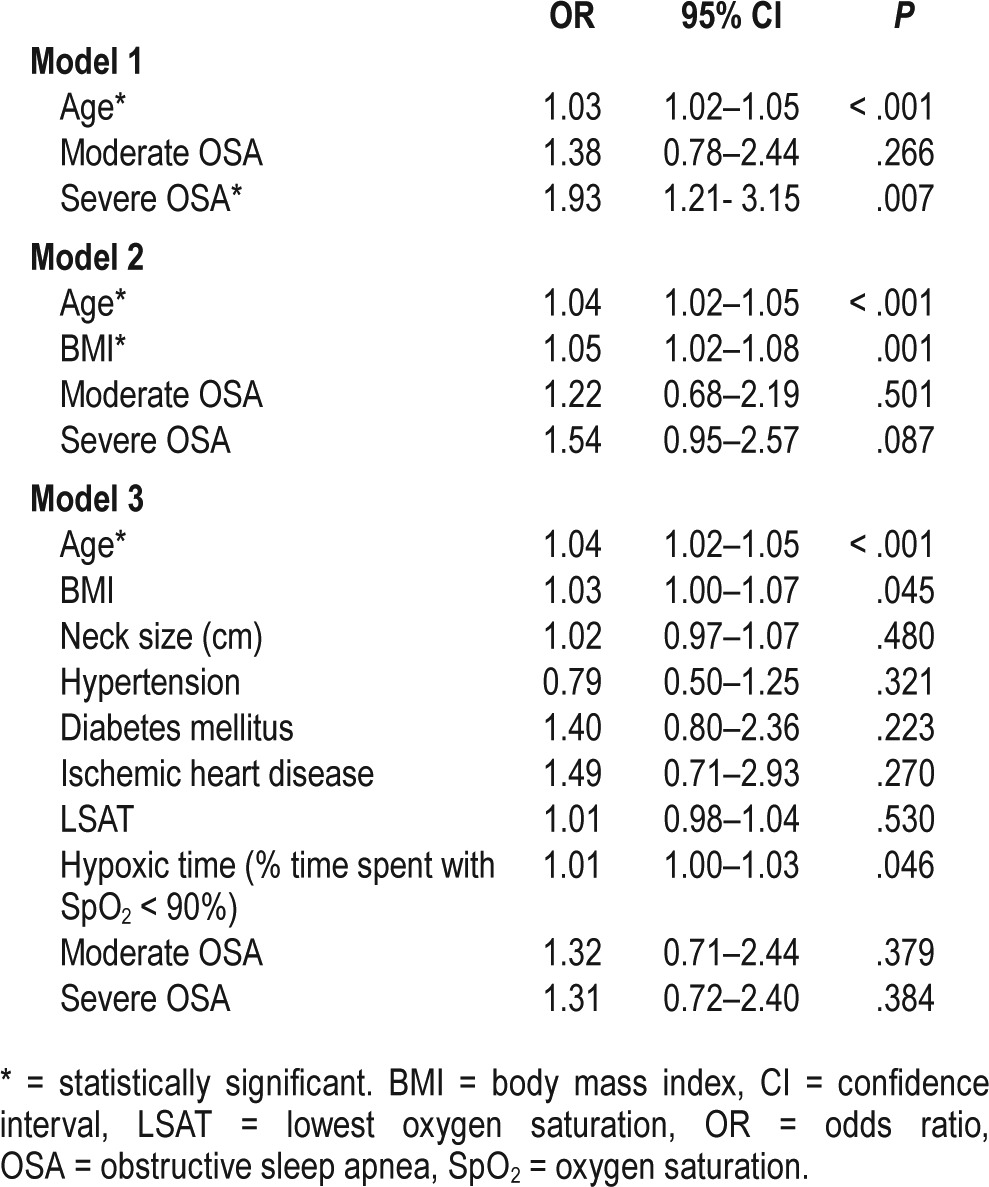
We further examined the effect modification by age. The OR of age was 1.03 (95% CI 1.02–1.05, P < .001) and the OR of severe OSA was 1.93 (95% CI: 1.21–3.15, P = .007) (Table 7), (ie, patients with severe OSA were almost two times more likely than their counterparts with mild sleep apnea to have cardiac arrhythmias). However, when other variables are taken into account in models 2 and 3, the severity of OSA is no longer statistically significant (Table 7). For instance, in model 2 there is a 4% risk increase per year of age increase (95% CI 1.02–1.05, P < .001) and 5% risk increase per unit BMI increase (95% CI 1.02–1.08, P < .001); however, severe OSA showed an OR of 1.54 (CI 0.95–2.57, P = .087) (Table 7). In all 3 models, moderate OSA did not increase the risk of having arrhythmias significantly compared to mild OSA (Table 7).
Table 7.
Effect of severe OSA on arrhythmias using multiple logistics regression analyses.
DISCUSSION
It is well known that OSA is associated with cardiovascular morbidity and mortality.3–6 The relationship between OSA and cardiac arrhythmias has increasingly been studied; however, the true significance and prevalence of such arrhythmias remain to be established. In particular, there has been a lack of studies done in the Asian population.
There is a markedly higher prevalence of OSA among the Singapore population1 and this could be attributed to differences in fat distribution per unit BMI and craniofacial characteristics, such as narrower retropalatal space, longer lower facial length.1 Asians with a similar degree of obesity were found to have a higher risk of OSA than Caucasians.15
Our study demonstrates an increased prevalence of cardiac arrhythmias among Asian patients with OSA as opposed to those who had primary snoring. However, the prevalence in our sample population is far lower than in existing studies in the Western population. According to one of the earliest studies by Guilleminault et al., 48% of subjects with OSA were found to have cardiac arrhythmias during sleep.7 Similarly, the Akershus Sleep Apnea Project, a large population-based study, demonstrated a prevalence of 12.2% for ventricular premature complexes (≥ 5 events/h) and 49.4% prevalence for atrial premature complexes (≥ 5 events/h).16
In our sample population, the most common arrhythmias were ventricular arrhythmias followed by supraventricular arrhythmias. Similarly, the Akershus Sleep Apnea Project demonstrated a higher prevalence of premature ventricular complexes among subjects with OSA, but not the other forms of arrhythmias such as atrial fibrillation and conduction delay arrhythmias.16 In the Sleep Heart Health Study, however, atrial arrhythmias were more common. Subjects with sleep-disordered breathing had four times the OR of atrial fibrillation, three times the odds of having nonsustained ventricular tachycardia and almost two times the odds of having complex ventricular ectopy.17 One possible reason that may account for the predominance of ventricular arrhythmias over atrial arrhythmias may be genetic differences between the Asian population as compared to the Caucasian population. There is emerging evidence that despite controlling for other comorbidities such as hypertension, diabetes mellitus, and coronary artery disease, Asian patients have a significantly lower prevalence of atrial fibrillation.18 Postulated mechanisms for this include ethnic variations in atrial size, atrial electrophysiological parameters and cardiac calcium ion channels.18 Further studies in this area are warranted.
From our multivariate analysis, the main predictor of cardiac arrhythmias among patients with OSA was increased age. Other comorbidities such as hypertension, diabetes, and existing cardiovascular disease were no longer statistically signifi-cant. In the Sleep Heart Health Study, a similar correlation is identified between increased age and nocturnal arrhythmias, controlling for smoking and comorbidities such as hypertension, hyperlipidemia, diabetes mellitus, and preexisting cardiovascular disease.17 This may suggest that the duration of OSA may affect one's risk of developing cardiac arrhythmias, supporting the need for prompt diagnosis and management of this problem.
From the multivariate analysis, polysomnograph variables such as AHI, LSAT, and hypoxic time were no longer statistically significant. However, adjusting for age alone, patients with severe OSA were shown to have a twofold risk of the development of arrhythmias. In comparison with other studies, the Sleep Heart Health Study demonstrated an increased frequency of arrhythmias noted among subjects with higher respiratory disturbance index and more time spent with oxygen saturation less than 90%.17 Similarly, in the Sleep Disorders in Older Men (MrOS Sleep) Study, there was a significant association between increasing AHI and arrhythmias (including complex ventricular ectopy and atrial fibrillation).19 The Akershus Sleep Apnea project also showed an increased prevalence of ventricular premature complexes with an increased AHI. In particular, mild and moderate OSA were associated with increased prevalence of ventricular arrhythmias, and a high AHI was significantly associated with frequent ventricular premature complexes.16 This may suggest that the severity of OSA may affect Asians differently in terms of predisposition to cardiac arrhythmias.
In particular, this may suggest that other alternative pathways of arrythmogenesis, such as metabolic dysfunction, may predominate in Asian patients, as other covariates appear to contribute to a greater extent than the severity of OSA. Existing studies have been performed in an attempt to establish the effect of ethnicity on comorbidities among patients with OSA. For instance, Leong et al. have shown a higher prevalence of type 2 diabetes mellitus among South Asian patients with OSA compared to white Europeans.20 Brady et al. similarly showed that South Asian patients with sleep-disordered breathing had higher glycated hemoglobin (HbA1c), lower high-density lipoprotein cholesterol, and a higher percentage of body fat than their Caucasian counterparts.21 Furthermore, existing epidemiological studies in the general population have suggested differences in the prevalence of arrhythmias, though more studies are required to establish the exact contributions of genetic variations.22 This suggests the pressing need for more studies to be done to establish the role ethnicity or genetic predisposition plays in the development of cardiovascular disease in OSA.
The clinical significance of cardiac arrhythmias in OSA lies in the possibility of more severe complications including sudden cardiac death. Notably, the severity of hypoxemia, a hallmark of OSA, is a strong predictor of ventricular arrhythmias and consequently, sudden cardiac death.23 Furthermore, multiple studies have suggested that treatment of OSA reduces the prevalence of arrhythmias,24,25 and this in turn may help reduce the cardiovascular morbidity associated with OSA. Although the clinical significance of other forms of arrhythmias remains to be established, this prompts the need for closer monitoring and enforcing treatment in patients with OSA.
Strengths
To our knowledge, this is the first large study in South East Asia that explored the relationship between OSA and cardiac arrhythmias. Our population is unique in that there is a multi-ethnic composition, unique to South East Asia. In comparison with studies done in Western countries, the severity of OSA appears to affect Asian patients differently, suggesting that ethnicity and genes may play a significant role.
In addition, with our secondary outcomes of our study, we aimed to identify possible predictors of arrhythmias among patients with OSA. In examining the effect modification by age and BMI, we find that comorbidities of our patients play a more significant role than the severity of OSA, contrary to existing studies done in the Caucasian population, suggesting alternative pathways of arrhythmogenesis. Perhaps this may, in the future, lay the foundation for screening criteria for cardiac arrhythmias among Asian patients with OSA.
Furthermore, there was blinded evaluation of ECG data, and each ECG was reviewed independent of respiratory events.
Limitations
This is a single-institution, retrospective study. The arrhythmias were evaluated via a single lead (lead II) electrocardiogram for the duration of sleep. This is inadequate to capture changes to the cardiac axis and the arrhythmias that may occur during the day were not captured. Furthermore, because of the retrospective nature of this study, some important confounders such as use of antiarrhythmic drugs, smoking, and drinking could not be accurately captured. In addition, the retrospective nature of this study makes it inadequate to establish the temporal relationship between OSA and arrhythmias. Prospective studies including long-term follow-up of patients with OSA and the inclusion of a control arm are necessary to ascertain the true incidence of cardiac arrhythmias among patients with OSA.
CONCLUSIONS
From this study, it is apparent that the prevalence of arrhythmias among patients with OSA is far greater in a Caucasian population as opposed to that in an Asian population. However, because of the aforementioned limitations, further studies are required in future to truly establish whether the difference may be attributable to ethnicity. Given the clinical significance of arrhythmias, this prompts the need for closer monitoring and enforcing treatment in patients with OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors declare that they have received no specific grant from any funding agency, or commercial or not-forprofit sectors. The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- ECG
electrocardiogram
- LSAT
lowest oxygen saturation
- OR
odds ratio
- OSA
obstructive sleep apnea
- SDB
sleep-disordered breathing
- SpO2
oxygen saturation
REFERENCES
- 1.Tan A, Cheung YY, Yin J, Lim WY, Tan LWL, Lee CH. Prevalence of sleep-disordered breathing in a multiethnic Asian population in Singapore: a community-based study. Respirology. 2016;21(5):943–950. doi: 10.1111/resp.12747. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea. Am J Cardiol. 1983;52(5):490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 8.Rossi VA, Stradling JR, Kohler M. Effects of obstructive sleep apnea on heart rhythm. Eur Respir J. 2013;41(6):1439–1451. doi: 10.1183/09031936.00128412. [DOI] [PubMed] [Google Scholar]
- 9.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 11.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 12.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 13.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42(8):1493–1531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 14.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva A, Buchanan P, Yee B, Grunstein R. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9(6):491–436. doi: 10.1016/j.smrv.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Namtvedt SK, Randby A, Einvik G, et al. Cardiac arrhythmias in obstructive sleep apnea (from the Akershus Sleep Apnea Project) Am J Cardiol. 2011;108(8):1141–1146. doi: 10.1016/j.amjcard.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neill J, Tayebjee MH. Why are South Asians seemingly protected against the development of atrial fibrillation? A review of current evidence. Trends Cardiovasc Med. 2017;27(4):249–257. doi: 10.1016/j.tcm.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS Sleep) study. Arch Intern Med. 2009;169(12):1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leong WB, Arora T, Jenkinson D, et al. The prevalence and severity of obstructive sleep apnea in severe obesity: the impact of ethnicity. J Clin Sleep Med. 2013;9(9):853–858. doi: 10.5664/jcsm.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady EM, Davies MJ, Hall AP, Talbot DC, Dick JL, Khunti K. An investigation into the relationship between sleep-disordered breathing, the metabolic syndrome, cardiovascular risk profiles, and inflammation between South Asians and Caucasians residing in the United Kingdom. Metab Syndr Relat Disord. 2012;10(2):152–158. doi: 10.1089/met.2011.0073. [DOI] [PubMed] [Google Scholar]
- 22.Freestone B, Lip GY. Ethnicity and arrhythmias. Card Electrophysiol Rev. 2003;7(1):92–95. doi: 10.1023/a:1023663712017. [DOI] [PubMed] [Google Scholar]
- 23.Gami A, Olson EJ, Shen W, et al. Obstructive sleep apnea and the risk of sudden cardiac death, a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 25.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60(9):781–785. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]


