Abstract
The lateral central nucleus of the amygdala (CeAL) and the dorsolateral bed nucleus of the stria terminalis (BNSTDL) coordinate the expression of shorter and longer-lasting fears, respectively. Less is known about how these structures communicate with each other during fear acquisition. One pathway, from the CeAL to the BNSTDL, is thought to communicate via corticotropin-releasing factor (CRF), but studies have yet to examine its function in fear learning and memory. Thus, we developed an adeno associated viral-based strategy to selectively target CRF neurons with the optogenetic silencer archaerhodopsin tp009 (CRF-ArchT) to examine the role of CeAL CRF neurons and projections to the BNSTDL during the acquisition of contextual fear. Expression of our CRF-ArchT vector injected into the amygdala was restricted to CeAL CRF neurons. Furthermore, CRF axonal projections from the CeAL clustered around BNSTDL CRF cells. Optogenetic silencing of CeAL CRF neurons during contextual fear acquisition disrupted retention test freezing 24 hours later, but only at later time-points (> 6 minutes) during testing. Silencing CeAL CRF projections in the BNSTDL during contextual fear acquisition produced a similar effect. Baseline contextual freezing, the rate of fear acquisition, freezing in an alternate context after conditioning and responsivity to foot-shock were unaffected by optogenetic silencing. Our results highlight how CeAL CRF neurons and projections to the BNSTDL consolidate longer-lasting components of a fear memory. Our findings have important implications for understanding how discrete amygdalar CRF pathways modulate longer-lasting fear in anxiety- and trauma-related disorders.
Keywords: central nucleus of the amygdala, bed nucleus of the stria terminalis, corticotropin releasing factor, contextual fear, optogenetics
Introduction
The neural mechanisms encoding aversive experiences into both short-term and longer-lasting fear and anxiety behaviors are unclear. Two structures that have received considerable attention in recent years for their role in fear and anxiety are the amygdala and bed nucleus of the stria terminalis (BNST), respectively 1, 2. Individuals diagnosed with post-traumatic stress disorder, phobias, and anxiety-related disorders often exhibit heightened amygdala and BNST activity to various types of threat 1, 3–5. Consistent with these findings, recent work has proposed that BNST dysfunction may lie at the heart of a number of psychiatric disorders 6. Despite substantial progress in identifying how fear and anxiety-like behaviors are expressed, less is known about how specific amygdala and BNST subdivisions and neurotransmitter systems contribute to initially acquiring fear.
Pre-clinical animal models of fear- and anxiety-like behaviors have been valuable for two key reasons. First, they have identified the functional importance of specific amygdala and BNST subdivisions in fear learning and memory. Second, they have provided insight into some of the core mechanisms that may regulate anxiety- and trauma-related dysfunction in humans. One mechanism that has received considerable attention for its role in fear and anxiety is corticotropin-releasing factor (CRF) 7, 8, a 41 amino-acid neuropeptide expressed in the lateral central nucleus of the amygdala (CeAL) and BNST 9, 10. Over the last few decades, a number of pharmacological studies have unraveled how CRF within the CeAL and BNST modulates fear and anxiety-like behaviors 11–13, but recent studies have yet to assess CRF’s function with novel approaches (e.g., optogenetics with cell-type specific targeting 14, 15). Antisense and viral knockdown studies have revealed that CeAL CRF is necessary for contextual fear memory consolidation 16 and stress-enhanced anxiety-like behaviors 17, but the functional importance of CeAL CRF neurons themselves during the formation of a fear memory is just beginning to receive attention 18. Indeed, CeAL CRF neurons are known to send axonal projections to the BNSTDL 19, 20 and these long-range projections have long been suspected to serve a critical function in fear- and anxiety-like behaviors 21, 22.
The BNST, like the CeAL, expresses CRF 23, 24 and lesions of the BNST disrupt the retention of contextual fear memories 25. The majority of preclinical work examining BNST and CRF function has focused on the expression of fear by examining enhanced startle behavior to light and long-duration cues (for reviews see 1, 21), with limited work evaluating its function in fear conditioning (for review see 26). Because both the BNST and CeAL have CRF expressing neurons, and the dorsolateral BNST (BNSTDL) and CeAL project to each other 27, the functional contributions of CeAL CRF neurons and projections to contextual fear learning and memory have been difficult to sort out. Understanding the function of CRF systems outside the HPA-axis is essential given their importance in anxiety- and trauma-related disorders – disorders which are often characterized by dysfunction of amygdala and BNST CRF systems 28.
Fear of phasic threats (i.e., short-lasting cues) is in part regulated by the CeA, whereas fear of sustained threats (i.e., long-lasting cues, lights, and contexts) is in part regulated by the BNST 1, 29, 30. However, the neuronal and molecular mechanisms that process these different types of threat are poorly understood. This is especially true with regard to how CeAL neurons and their projections to the BNSTDL might modulate fear learning and memory 30–32. More so, this focus is translationally relevant given that dysfunction in amygdala circuits may be a core feature in pathological fear and anxiety.
Therefore, in the present study, we focus on how CRF neurons in the CeAL and specific CeAL CRF projections to the BNSTDL modulate contextual fear learning and memory by selectively disrupting activity during fear acquisition. We developed an adeno-associated viral construct to selectively target CRF neurons with the optogenetic neural silencer archaerhodopsin tp009 (ArchT). We used immunohistochemical, in situ hybridization, and in vitro electrophysiological techniques to validate the selectivity and physiological characteristics of CeAL CRF-ArchT infected neurons. Finally, we used optogenetics to examine how silencing CeAL neurons and projections to the BNSTDL at the time of fear-acquisition affected the retention of contextual fear memory.
Materials and Methods
Subjects
Adult Male Sprague-Dawley rats (10–18 weeks of age) obtained from Envigo (Indianapolis, IN) were used for all experiments. Rats were maintained on a 12h light/dark cycle (lights on at 7:00 A.M.) at constant temperature with free access to food and water. Animals were randomly assigned to experimental conditions. Animals were pair-housed prior to implantation of fibers, after which they were single-housed. All behavioral experiments occurred between ~12:00 P.M. – 5:00 P.M. Given the nature of the optogenetic studies, blinding of the experimenter was not possible. All procedures were approved by the University of Delaware, or the NIDA IRP Institutional Animal Care and Use Committees (IACUC), in accordance with guidelines specified by the US National Institutes of Health Guide for the Care and Use of Experimental Animals.
Viral Vectors
Two viral plasmids were constructed: pAAV-CRF-ArchT-EGFP-WPRE-SV40 (abbreviated CRF-ArchT) and a control construct pAAV-CRF-EGFP-WPRE-HGH (abbreviated CRF-EGFP; Figure 1A). Both constructs contained a woodchuck hepatitis posttranscriptional regulatory element (WPRE) and a polyadenylation signal (SV40 or HGH) and were packaged into an AAV2/2 by the Penn Vector Core (Philadelphia, PA). More details about the viral constructs can be found in the Supplementary Methods.
Figure 1.
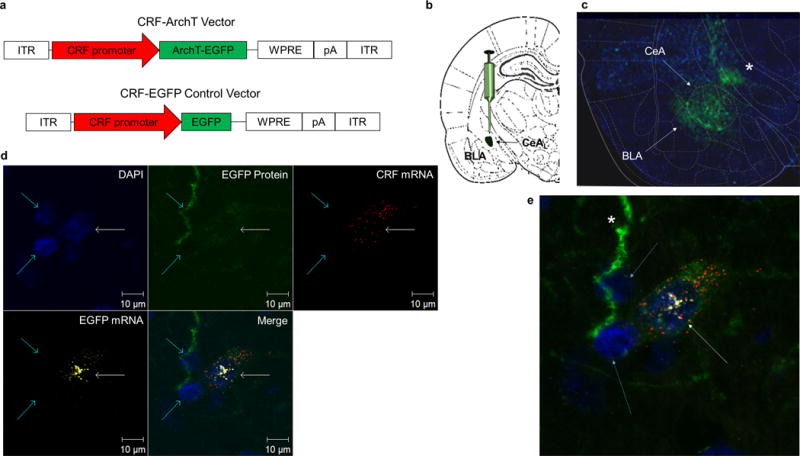
AAV2/2 CRF promoter driven ArchT vector selectively targets CRF cells. (a) CRF-ArchT and CRF-EGFP control vectors. (b) Schematic of CeAL viral injection site. (c) Immunohistochemical labeling shows CRF-ArchT-EGFP+ protein is restricted to the CeA. Note the fluorescence extending into the basolateral amygdala (BLA; white arrow) and towards the stria terminalis (asterisk). (d) In situ hybridization, DAPI staining, and intrinsic EGFP+ fluorescence across three neurons in the CeAL (two aqua arrows and one white arrow). Of the three cells with DAPI stained nuclei (blue stain), only one (white arrow) displays triple expression of cytoplasmic CRF-ArchT-EGFP+ protein (green) and mRNA (yellow-white) with CRF mRNA (red). (e) Magnification of panel d showing the CRF-ArchT-EGFP+ labeled CRF neuron (white arrow). Note the two adjacent cells (aqua arrows) which do not synthesize CRF mRNA also do not exhibit EGFP mRNA or protein. The green-labeled long process appears to be an EGFP+ axonal projection (asterisk).
Surgery
Rats received two surgeries spaced 4 weeks apart: one for viral infusions and another for implantation of the fiber optic ferrule assemblies. Animals were sacrificed following behavioral procedures to confirm viral expression and correct placement of cannula (see Supplementary Methods).
Contextual and Auditory Fear Conditioning
Contextual fear conditioning was conducted by providing five 0.6mA shocks spaced three minutes apart. An 18-min retention test was conducted 24 h after conditioning. Auditory fear conditioning used five 30-s tones co-terminating with foot-shock. A five tone retention test was provided in an alternate context 24-h after conditioning (see Supplementary Methods).
Shock responsivity testing
Shock responsivity testing was conducted similar to previous reports as detailed in the Supplementary Methods.
Immunohistochemistry and In Situ Hybridization
For confirming targeted expression of our construct, we used immunohistochemical and situ hybridization 33 techniques (described in Supplementary Methods).
Whole-Cell Patch Clamp
Slice preparation and recordings were conducted using procedures previously described and are presented in detail within the Supplementary Methods.
Statistical Analyses of fear conditioning and shock responsivity
Violations in homogeneity of variance were tested prior to statistical analyses. The number of animals in each group was selected based off pilot experiments (data not shown). Freezing during fear acquisition and retention of contextual fear conditioning was analyzed separately for test phase using a two-group (CRF-EGFP vs CRF-ArchT) between factor by 6 time bin within factor-repeated measure analysis of variance. A Holm-Bonferroni sequential correction test for non-independent samples 35–36 was used to compare freezing of the two groups at select time bins. One animal (CRF-ArchT) in the CeAL → BNSTDL CRF pathway experiment was removed for improperly placed fibers (see placement highlighted in blue in Supplementary Fig. 8).
Some animals were lost due to damaged head-stages and improper patch cord coupling (final group numbers shown in the Results section). Freezing at baseline during exposure to all contexts was assessed with an independent samples t-test to evaluate (1) if optogenetic stimulation itself could induce freezing before and after conditioning or (2) if stimulation in an alternate context could act as a retrieval cue. For auditory delay fear conditioning, we conducted analyses excluding outliers > 2 S.D. and computing a difference score for each CS. Mann-Whitney U tests were used to examine ordinal shock responsivity data. Electrophysiological data (pre vs. post laser effects) were analyzed using a one-way repeated measures ANOVA, followed by Dunnett’s post-hoc comparison.
Results
CRF-ArchT-EGFP Selectively Targets CeAL CRF+ Neurons
In order to selectively target CRF neurons with an inhibitory opsin, we reconstructed an AAV2/2 archaerhodopsin tp009 (ArchT) enhanced green fluorescent protein (EGFP) vector 37 using a ~2.2kb rat CRF promoter (CRF-ArchT; Fig. 1a; Supplementary Fig. 1–2). In parallel, we created a control construct that did not express ArchT (CRF-EGFP; Fig. 1a; Supplementary Fig. 1–2). Immunohistochemical labeling for EGFP confirmed that ArchT expression was restricted to the CeAL following injections into this structure with visible processes in the basolateral amygdala and coursing upwards to the stria terminalis (Fig. 1b–c; Supplementary Fig. 8a–b; n=4). Co-labeling of CRF with EGFP further showed that the CRF-ArchT-EGFP protein was produced in CeAL CRF+ neurons (Supplementary Fig. 3d).
Given differences in basal vs. physiological driven levels of CeAL CRF expression 38, poor antibody specificity 39, and the fact that ArchT is also expressed in axonal projections 37, we wanted to confirm that CRF-ArchT-EGFP protein expression was in fact restricted to CeAL CRF synthesizing cells. To further validate the CRF-ArchT-EGFP construct, we compared the expression pattern of CeAL CRF-ArchT-EGFP protein to that of CRF mRNA using radiolabeled in situ hybridization. CRF-ArchT-EGFP protein and CRF mRNA CeAL expression patterns were highly similar (Supplementary Fig. 3b–c; n=4). Additionally, using RNAscope in situ hybridization, we were able to better confirm that only cells that synthesized CRF also synthesized EGFP mRNA and, critically, expressed the CRF-ArchT-EGFP protein (Fig. 1d–e; n=2).
CRF-ArchT-EGFP is Selective for Other Types of CRF+ Neurons
Although the focus of the present paper was on optogenetic manipulation of CRF cells in the CeA, we also examined whether our viral construct could be expressed in other CRF populations. CRF cells in the CeA and BNST are GABAergic whereas CRF cells in the paraventricular nucleus (PVN) of the hypothalamus are glutamatergic 24. Injection of CRF-ArchT into the PVN demonstrated co-localization and selectivity of cellular expression in PVN CRF neurons (Supplementary Fig. 4). These data suggest that CRF-ArchT can be targeted to CRF+ neurons across the brain, irrespective of regional phenotypic and co-localized neurotransmitter differences.
Green Light Silences CRF-ArchT-EGFP Neurons
To validate that CRF-ArchT-EGFP was a viable neuronal silencer, we conducted in vitro whole-cell patch clamp on CeAL CRF-ArchT infected rats. Light-activated silencing of CeAL neurons by ArchT was confirmed in amygdala brain slices (Fig. 2). Action potentials elicited during 1-s photostimulation periods were compared to those preceding and following stimulation. Neuronal firing was significantly inhibited during laser illumination (one way repeated measures-ANOVA, F(2,6) = 17.03, p = 0.0034). Post hoc analysis found firing was decreased during laser illumination compared to both pre and post illumination.
Figure 2.
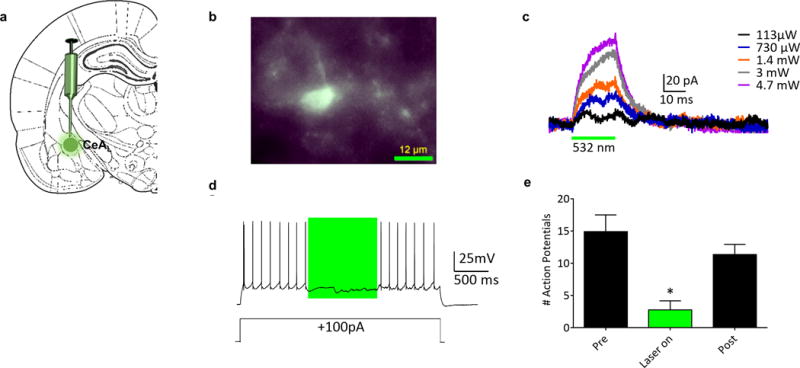
Confirmation of ArchT function in whole-cell recordings from CeAL Neurons in an in vitro slice preparation. (a) Schematic showing site of CRF-ArchT injection into CeAL. (b) Epifluorescent image demonstrating EGFP+ expression in a single CeAL neuron. (c) Voltage clamp recording (holding potential −60mV) of a neuron demonstrating outward currents elicited by photostimulation (indicated by green bar); the size of the current increased with increasing laser output. (d) Current clamp recording of a neuron demonstrating ArchT-mediated inhibition of firing induced by depolarizing current injection (+100 pA, 3 s) through the patch pipette. The photostimulation period is indicated by the green box. (e) Summary of recordings from CeAL neurons (n = 4, from 3 rats). Action potentials elicited during a 1-s photostimulation were compared to those preceding and following the stimulation. Neuronal firing was significantly inhibited by laser light. * denotes firing during laser-on was significantly different from pre and post illumination recording.
CeAL CRF+ Long-Range Projections to the BNSTDL cluster around BNSTDL CRF+ cells
To confirm that CeAL CRF neurons project to the BNSTDL as previously reported 19, 20, 40, we examined immunoreactivity in the BNSTDL. Following CRF-ArchT injection into the CeAL, immunohistochemical labeling confirmed the presence of EGFP in the BNSTDL (Fig. 5b–c, Supplementary Fig. 5b and 8e, n=4). Given that the BNSTDL is known to contain a number of CRF+ neurons 24 and the known role of BNSTDL CRF type one receptors 41 in fear and anxiety-like behaviors 21, 42 from our recent work, we examined if CeAL CRF fibers were present near BNSTDL CRF+ neurons (Supplementary Fig. 5; n=3). Co-labeling of BNSTDL sections for EGFP and CRF, following injections of CRF-ArchT into the CeAL, revealed that CeAL→BNSTDL long range CRF projections were in fact clustered around BNSTDL CRF producing cells.
Silencing CeAL CRF+ Neurons During Fear Acquisition Only Disrupts Later Time-Points of Fear Memory Retention
To explore the role of CeAL CRF+ neurons during the formation of a fear memory to a specific environment, we trained CRF-ArchT (n=13) and CRF-EGFP controls (n=11) in contextual fear conditioning (Fig. 3a–b). Optogenetic silencing of CeAL CRF+ neurons did not affect freezing between groups (Fig. 3c; F(1,22)=0.0005, ns) or the rate of acquisition (interaction; F(5,18)=0.44, ns). Both groups increased freezing with each subsequent shock, reaching ~80% freezing after 3 shocks, F(5,18=59.08, p<0.0001 (Fig. 3c). Thus, silencing CeAL CRF+ neurons did not affect acquisition.
Figure 3.
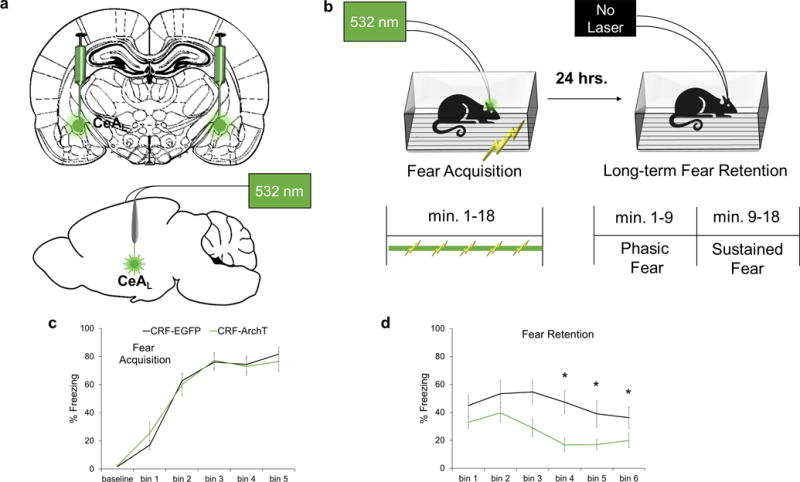
Silencing of CeAL CRF+ cells during acquisition disrupts later components of fear retention. (a) Schematic of CeAL viral injection site and fiber placement. (b) Parameters for contextual fear conditioning. (c) Laser silencing did not affect baseline activity/freezing or fear acquisition. (d) At fear retention, CRF-ArchT rats (n=13) exhibited reduced freezing at time bins 3, 4, and 5 relative to CRF-EGFP (n=11) controls. Each bin represents a three-minute period. *p<.05, error bars are ± S.E.M.
However, at retention testing 24-h later, CRF-ArchT animals showed reduced freezing relative to CRF-EGFP controls (Fig. 3d; F(1,22)=7.30, p<0.013). The interaction effect was marginally significant, F(5,18)=2.27, p<0.091, with freezing during the third, fourth and fifth time bins (minutes 6–15) lower in the CRF-ArchT group (Holm’s sequential Bonferroni, bin 3: p<0.046, bin 4: p<0.008, bin 5: p<0.046.
Laser silencing of CeAL CRF+ neurons in an alternate context after conditioning did not induce freezing in CRF-ArchT animals (n = 10) relative to CRF-EGFP controls (n = 9; (t(17)=.607, ns; Supplementary Fig. 6c) or alter shock responsivity (Supplementary Fig. 6d; p’s > .05). Additionally, laser silencing had a marginally disrupting effect on retention of auditory fear to a discrete 30-s tone CS in CRF-ArchT (n=11) relative to CRF-EGFP controls (n=10; Fig. 4). A repeated measures ANOVA on difference scores at each CS presentation (i.e., CS1 testing – CS1 training, etc.) with outliers > 2 S.D (n=3/group) removed revealed a significant interaction (F(4,52)=3.402, p <.016) with freezing during CS3 (p <.05), CS4 (p<.05), and CS5 (p<.05) in the CRF-ArchT group lower than controls. Holms-Bonferroni sequential correction confirmed effects at CS4: p< .05, and CS5: p< .05.
Figure 4.
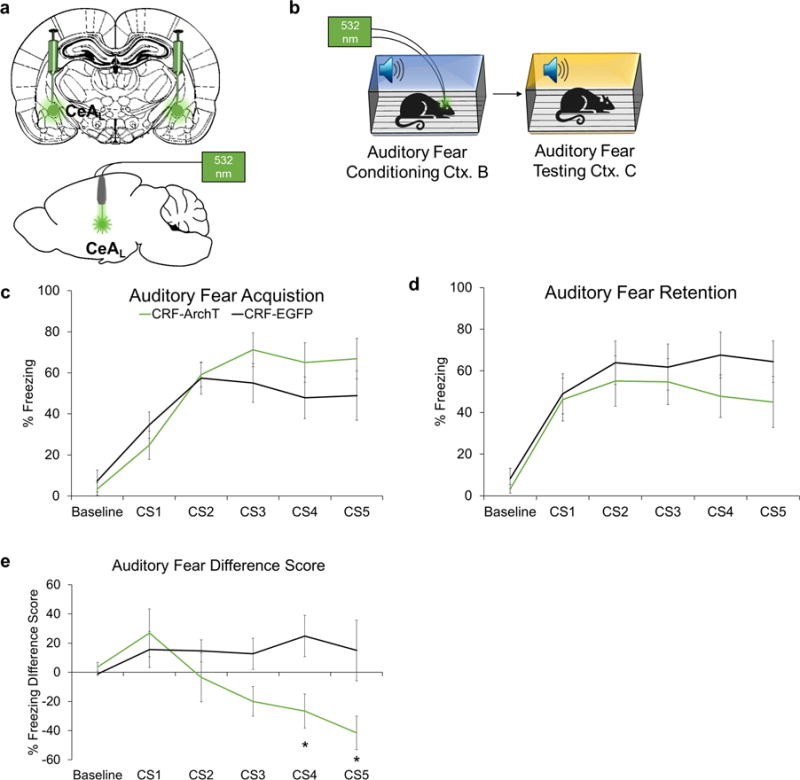
Effects of silencing CRF+ CeAL cells on retention of auditory fear conditioning. (a) Schematic of CeAL viral injection site and fiber placement. (b) Schematic of experimental timeline. Animals were trained with five 30-s CSs co-terminating with a 1-s 0.6 mA foot-shock. (c) Silencing of CRF+ CeAL neurons did not statistically affect auditory fear acquisition, but freezing tended to increase over the last few CS-shock pairings (d) At fear retention, CRF-ArchT (n=11) rats tended to have reduced freezing during the last two presentations of the CS relative to CRF-EGFP (n=10) controls (e) A difference score removing outliers > 2 S.D. and examining the change from training to testing (i.e., CS1 testing – CS1 training, etc.) revealed CRF-ArchT (n=8) animals exhibited a greater reduction in freezing relative to CRF-EGFP (n=7) controls during the last few CSs. A lower difference score indicates a greater reduction in freezing. *p<.05, error bars are ± S.E.M.
In summary, laser silencing during fear acquisition only disrupts freezing at later time-bins of contextual, and to a lesser extent auditory, fear retention 24-h later. However, short-term memory during acquisition, early time-points of long-term fear, and perception of environmental cues or pain responsivity are unaffected.
Silencing CeAL→ BNSTDL CRF+ Neuronal Projections Also Disrupts Later Time-Points of Fear Memory Retention
Next, we examined the role of the CeAL → BNSTDL CRF pathway in contextual fear conditioning (Fig. 5a–c) 19. Similar to silencing CeAL CRF+ neurons, optogenetic silencing of CeAL → BNSTDL CRF projections did not affect freezing at baseline (p > .05) and the rate of fear acquisition was normal (F(5,12) = 105.05, P<0.0001), with no between group effect (F(1,16) = 0.61, NS) nor interaction (F(5,12) = 0.97, NS; Figure 5d).
Figure 5.
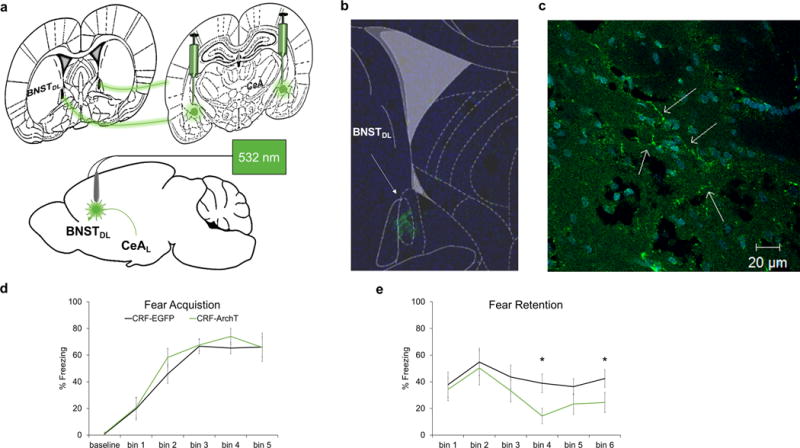
Silencing of the CeAL → BNSTDL CRF pathway during acquisition only disrupts later components of fear retention. (a) Schematic of CeAL viral injection site and BNSTDL fiber placement. (b) Rat atlas overlay showing EGFP expression in BNSTDL. (c) Axonal projections in BNSTDL (arrows). (d) Laser silencing with same parameters as shown in Fig.2b did not affect baseline activity/freezing or fear acquisition. (e) At fear retention CRF-ArchT (n=8) animals exhibited reduced freezing at time bins 4 and 6 relative to CRF-EGFP (n=10) controls. Each bin represents a three-minute period. *p<.05, error bars are ± S.E.M.
Also similar to silencing CeAL CRF+ neurons, silencing CeAL → BNSTDL CRF projections produced decreased freezing at retention testing (Fig. 5e). There was a highly significant repeated measure effect of time bins, suggesting changes in freezing as the testing session progressed (F(5,12)=105.05, p<0.0001), but no between group difference (F(1,16=0.61, ns) nor interaction effects (F(5,12)=0.97, ns). However, the decrease in freezing at retention was due to reduced freezing in CRF-ArchT animals at time bins 4 and 6 (Fig. 5e; Holm’s sequential Bonferroni procedure time bin 4: p<0.018, time bin 6: p<0.046).
Similar to CeAL experiments, freezing in an alternate context after conditioning (CRF-ArchT n=5 and CRF-EGFP controls n=5, levene’s test, p=.032, (tcorrected(4.763)=1.108, ns) and shock responsivity (CRF-ArchT n=8 and CRF-EGFP controls n=8, ns; Supplementary Fig. 7c–d) were unaffected.
Additionally, restricting silencing to 1-minute periods surrounding each shock (30s before, 1s during shock, and 29s after) produced the same effect as prolonged stimulation. CRF-ArchT animals (CRF-ArchTshort, n=5) were compared to animals that received laser silencing during the entire conditioning session (CRF-ArchTlong, n=8, from Fig. 3). Shorter periods of laser silencing did not differentially affect fear acquisition (between groups (F(1,12)=.344, ns), interaction (F(5,60)=.785, ns), but main effect of time bin (F(5,60)=37.996, p < .001) or fear retention (between groups (F(1,12)=1.020, ns), interaction (F(5,60)=.471, ns), but main effect of time bin (F(5,60)=2.931, p=.020). Exploratory post-hoc analyses confirmed the lack of a difference. Thus, long or short periods of CeAL→ BNSTDL CRF+ silencing during acquisition similarly disrupt later stages of retention.
Discussion
In this study, we demonstrate the fidelity of an AAV2/2 CRF-specific optogenetic neural silencer in selectively targeting CRF cells. Our CRF-ArchT construct should be a valuable addition to the optogenetic toolbox for fear, stress, and anxiety researchers to ask specific questions about the function of CRF neurons and projections. Our data provide strong support for the selectivity of CRF-ArchT within CRF+ cells. In particular, we show that: (1) the expression of CRF-ArchT parallels that of CRF mRNA in both the CeAL and PVN 33, (2) CRF-ArchT mRNA is only transcribed in CeAL cells that transcribe CRF, and (3) the induction of firing in CeAL CRF-ArchT cells is abolished with green light stimulation. Our use of a high-titer AAV2/2 explains, in part, why our construct successfully targeted phenotypically distinct CRF populations across the CeAL and PVN (i.e., GABAergic CeAL and glutamatergic PVN) 43, 44. Furthermore, we used a long promoter fragment 45, that has previously been used and validated 46, to selectively target CRF cells – an approach which has been successfully applied to targeting other peptidergic cells of the CeAL and PVN 47, 48. There have been mixed results in the literature with using transgenic approaches to target CRF+ cells 39, 40, 49 across mice and rats, but our data provide compelling evidence for the selectivity of our construct in CRF+ CeAL and PVN cells. Future studies will fully quantitate overlap of our CRF-ArchT construct with CRF neurons across the anterior/posterior axis, with other CeAL cellular markers (e.g., GABA, somatostatin, PKC-δ, TAC-2 50–55; and see 18, 40), and other CeAL CRF projections.
CeAL CRF Neurons Are Involved in Consolidating Sustained Fear
CeAL neurons are critical during the earliest stages of fear learning and memory 56. Our study shows that silencing CeAL CRF neurons during acquisition disrupts consolidation of longer-lasting components of a contextual fear memory, given that baseline activity, fear acquisition, freezing in a novel context after conditioning, or responding to varying foot-shock intensities were unaffected. Silencing CeAL CRF neurons did not affect fear acquisition to asymptote, suggesting CeAL CRF neurons are not critical for short-term memory. Given that freezing was disrupted 24 h later, but only beginning 6 minutes after the start of the retention test, CeAL CRF neurons appear to preferentially modulate longer-lasting components of long-term fear memories. Alternatively, it is possible that silencing CeAL CRF neurons during fear acquisition accelerates extinction learning 18. However, given that the rate of fear acquisition was unaffected, the most parsimonious conclusion is that the consolidation of longer-lasting components of fear were disrupted. Our optogenetic findings add to previous reports showing that neurotoxic lesions, functional inactivation, and CRF knockdown in the CeA prior to or during the acquisition phase of contextual fear disrupts fear retention 56–58.
CeAL→BNSTDL CRF Projections Represent a Critical Pathway in Consolidating Sustained Fear
Given that CeAL CRF projections target a number of other brain regions 19, it is unclear which specific CeAL CRF cells and/or pathways regulate this effect. We found that silencing CeAL→BNSTDL CRF axonal projections within the BNSTDL, similar to silencing CeAL CRF neurons within the CeAL, disrupted fear memory retention across a similar time course, indicating these projections are critical to modulating longer-lasting fragments of the fear memory. Because silencing is spatially selective to presynaptic boutons in the illuminated area and has no effects on action potentials, fibers of passage, or back propagation to the cell bodies in the CeAL 59, it can be concluded that only the CeAL→BNSTDL CRF ArchT expressing projections mimicked the silencing of CeAL CRF neurons. Whether CeAL projections to other regions have similar effects are for future studies.
Our findings agree with previous work indicating that the BNST is involved in consolidating long-term contextual fear memories 56, 60. Pharmacological work from our lab has shown that pre-training antagonism of CRF type 1 receptors in the BNSTDL blocks the retention, but not short-term acquisition, of contextual fear 42. Similarly, antisense knockdown of CRF in the CeAL before or immediately after contextual fear conditioning produces a similar effect 16, 57. However, these studies do not differentiate between the shorter and longer-lasting aspects of contextual fear memories – a key component of the present work. Our data support the hypothesis that CeAL →BNSTDL CRF projections regulate learning and memory of longer lasting fragments of contextually conditioned fear memories 1.
The present study does have some limitations. CeAL CRF cells are primarily GABAergic 61. Thus, the extent to which GABA and CRF within the CRF CeAL →BNST pathway contribute to fear learning and memory is unknown (for review see 26). However, previous work has shown that GABA-A(ɑ1) receptor deletion from CRF neurons, which abolishes the effects of GABA and enhances CRF across the CeA and BNST, impairs auditory fear extinction 61. Our study complements this work by showing that silencing CeAL CRF neurons during fear acquisition produces the opposite effect by disrupting retention of longer-lasting contextual and auditory fear memories. Given that we did not measure if CeAL →BNST CRF release decreased with silencing, an important future direction would be to assess CRF and GABA release in the CeAL→BNST pathway and within local BNST CRF neurons with optogenetic silencing to better understand how different sources of CRF and GABA influence longer-lasting fear behavior.
Recent evidence has also suggested that prolonged stimulation of ArchT in glutamatergic thalamocortical cells can stimulate presynaptic Ca2+ transmitter release 62, however it is unknown if prolonged stimulation of CeAL CRF+ neurons, which are GABAergic 63, produces a similar effect. Nonetheless, we found that curtailing ArchT silencing to 1-min periods (paralleling the low end of the Ca2+ ramp in glutamatergic cells observed by 62) produced a similar reduction in freezing relative to prolonged inhibition. Although we demonstrate successful laser silencing of CeAL CRF neurons in vitro, studies are needed to examine how silencing CeAL CRF neurons affect in vivo population activity. Finally, we did not test if silencing CeAL CRF projections affected corticosterone release or if other CeAL pathways could differentially modulate components of the fear memory 19, 64. These are important future questions for understanding how CeAL CRF projections (e.g., those mediating arousal and endocrine activity) may regulate freezing at earlier time-points during fear retention 19, 64, 65.
Conclusions
The mechanisms for transitioning from normal fear to pathological anxiety are still unclear 66, but the amygdala and BNST play a critical role (for reviews see 67, 68). Recent work in humans has shown that the amygdala is responsive to the onset of threat-predicting cues whereas the BNST is important for maintaining fear responses 2. This work complements decades of pre-clinical animal work suggesting that the CeA is important for phasic short-lasting fear whereas the BNST, in part, is critical for sustained long-lasting fear 1, 69. Our results shed light on how a select CRF network modulates sustained, long-lasting fear.
CeAL CRF neurons and their receptors coordinate a number of fear memory processes. For example, CRF type 1 receptors (CRFr1s) within the basolateral amygdala modulate the consolidation of inhibitory avoidance memories 70 and BNSTDL CRFr1s are important for the retention of contextual fear memories 26, 42. Furthermore, a recent study demonstrated that CeAL CRF neurons and receptors modulate weak, but not strong, fear conditioning 18. Our study adds an important piece to accumulating data over the last few decades on how CRF within the amygdala and BNST 1, 31, 65 regulate a number of fear/anxiety behaviors including startle behavior to lights, long-lasting cues, and contextual fear memories 16. Future studies are needed to understand how suppression and activation of other CeAL CRF pathways modulate different fear- and anxiety-like behaviors (e.g., fear potentiated startle, elevated plus maze, etc.) and may serve as a therapeutic target in individuals suffering from a variety of fear and anxiety disorders.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01HD07506603 to J.B.R. and an APA grant to A.A. Microscopy access was supported by grants from the NIH-NIGMS (P20 GM103446), the NSF (IIA-1301765) and the State of Delaware.
Footnotes
Author Contributions
A.A., J.B.R., and J.S. designed experiments, A.A. and A.D. performed behavioral experiments. A.A. performed molecular work. C.R.L. and A.F.H. performed the electrophysiology experiments. A.A., J.B.R., J.S., C.R.L., and A.F.H analyzed data and wrote the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann MJ, Boehme S, Becker MP, Tupak SV, Guhn A, Schmidt B, et al. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping. 2015;37(3):1091–1102. doi: 10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Münsterkötter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V, et al. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depression and Anxiety. 2015;32(9):656–663. doi: 10.1002/da.22382. [DOI] [PubMed] [Google Scholar]
- 4.Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research. 2012;46(8):1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebow M, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry. 2016;21(4):450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keck ME, Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22(5):835–844. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 8.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 9.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 10.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proceedings of the National Academy of Sciences. 1994;91(19):8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by a-helical CRF (9–41) Neuropsychopharmacology. 1989;2(4):285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- 12.Liang K, Melia K, Campeau S, Falls W, Miserendino M, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. The Journal of Neuroscience. 1992;12(6):2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swerdlow N, Geyer MA, Vale W, Koob G. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology. 1986;88(2):147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- 14.Gafford G, Ressler K. Mouse models of fear-related disorders: cell-type-specific manipulations in amygdala. Neuroscience. 2016;321:108–120. doi: 10.1016/j.neuroscience.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough K, Morrison F, Ressler K. Bridging the gap: Towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiology of Learning and Memory. 2016;135:27–39. doi: 10.1016/j.nlm.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiology of Learning and Memory. 2011;95(1):86–91. doi: 10.1016/j.nlm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biological Psychiatry. 2012;71(4):317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, et al. A central amygdala CRF circuit facilitates learning about weak threats. Neuron. 2016 doi: 10.1016/j.neuron.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Research. 1986;382(2):213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 20.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 21.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Structure and Function. 2008;213(1–2):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 22.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463(1):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 23.Morin S, Ling N, Liu X-J, Kahl S, Gehlert D. Differential distribution of urocortin-and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience. 1999;92(1):281–291. doi: 10.1016/s0306-4522(98)00732-5. [DOI] [PubMed] [Google Scholar]
- 24.Dabrowska J, Hazra R, Guo J-D, DeWitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Frontiers in Neuroscience. 2013;7 doi: 10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan G, Apergis J, Bush D, Johnson LR, Hou M, Ledoux J. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128(1):7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Gafford GM, Ressler KJ. GABA and NMDA receptors in CRF neurons have opposing effects in fear acquisition and anxiety in central amygdala vs. bed nucleus of the stria terminalis. Hormones and Behavior. 2015;76:136–142. doi: 10.1016/j.yhbeh.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gungor NZ, Yamamoto R, Pare D. Optogenetic study of the projections from the bed nucleus of the stria terminalis to the central amygdala. Journal of Neurophysiology. 2015;114(5):2903–2911. doi: 10.1152/jn.00677.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatric Clinics of North America. 2009;32(3):549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duvarci S, Bauer EP, Paré D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. The Journal of Neuroscience. 2009;29(33):10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammack SE, Todd TP, Kocho-Schellenberg M, Bouton ME. Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behavioral Neuroscience. 2015;129(5):673–678. doi: 10.1037/bne0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience. 1997;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. The Journal of Neuroscience. 1997;17(16):6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. Immediate early gene and neuropeptide expression following exposure to the predator odor 2, 5-dihydro-2, 4, 5-trimethylthiazoline (TMT) Behavioural Brain Research. 2013;248:85–93. doi: 10.1016/j.bbr.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 34.Root DH, Mejias-Aponte CA, Zhang S, Wang H-L, Hoffman AF, Lupica CR, et al. Single rodent mesohabenular axons release glutamate and GABA. Nature Neuroscience. 2014;17(11):1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdi H. Holm’s sequential Bonferroni procedure. Encyclopedia of Research Design. 2010;1 [Google Scholar]
- 36.Gaetano J. Holm-Bonferroni sequential correction: An EXCEL calculator (1.1) [Microsoft Excel workbook] 2013 doi: 10.13140/RG.2.1.4466.9927. Retrieved from https://www.researchgate.net/publication/236969037_Holm-Bonferroni_Sequential_Correction_An_EXCEL_Calculator. [DOI]
- 37.Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Frontiers in Systems Neuroscience. 2011;5 doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Distribution of corticotropin-releasing factor in rat brain. Federation Proceedings. 1985;44(1.1):215–219. [PubMed] [Google Scholar]
- 39.Cusulin JIW, Füzesi T, Watts AG, Bains JS. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One. 2013;8(5):e64943. doi: 10.1371/journal.pone.0064943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, et al. A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Frontiers in Neuroscience. 2015;9 doi: 10.3389/fnins.2015.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. The Journal of Neuroscience. 1995;15(10):6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asok A, Schulkin J, Rosen JB. Corticotropin releasing factor type-1 receptor antagonism in the dorsolateral bed nucleus of the stria terminalis disrupts contextually conditioned fear, but not unconditioned fear to a predator odor. Psychoneuroendocrinology. 2016;70:17–24. doi: 10.1016/j.psyneuen.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holehonnur R, Luong JA, Chaturvedi D, Ho A, Lella SK, Hosek MP, et al. Adeno-associated viral serotypes produce differing titers and differentially transduce neurons within the rat basal and lateral amygdala. BMC Neuroscience. 2014;15(1):1. doi: 10.1186/1471-2202-15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience. 2009;161(2):441–450. doi: 10.1016/j.neuroscience.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D-P Li. Identification of corticotropin-releasing hormone neurons in paraventricular nucleus in rats (876.6) The FASEB Journal. 2014;28(1 Supplement):876.876. [Google Scholar]
- 46.Przybycien-Szymanska MM, Mott NN, Pak TR. Alcohol dysregulates corticotropin-releasing-hormone (CRH) promoter activity by interfering with the negative glucocorticoid response element (nGRE) PloS One. 2011;6(10):e26647. doi: 10.1371/journal.pone.0026647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Jasnow AM, Rainnie DG, Maguschak KA, Chhatwal JP, Ressler KJ. Construction of cell-type specific promoter lentiviruses for optically guiding electrophysiological recordings and for targeted gene delivery. Viral Applications of Green Fluorescent Protein: Methods and Protocols. 2009:199–213. doi: 10.1007/978-1-59745-559-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Molet J, Gunn BG, Ressler K, Baram TZ. Diversity of reporter expression patterns in transgenic mouse lines targeting corticotropin-releasing hormone-expressing neurons. Endocrinology. 2015;156(12):4769–4780. doi: 10.1210/en.2015-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andero R, Daniel S, Guo J-D, Bruner RC, Seth S, Marvar PJ, et al. Amygdala-dependent molecular mechanisms of the Tac2 pathway in fear learning. Neuropsychopharmacology. 2016;41:2714–2722. doi: 10.1038/npp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 52.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 53.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468(7321):270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nature Neuroscience. 2013;16(3):332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu K, da Silva PG, Albeanu DF, Li B. Central amygdala somatostatin neurons gate passive and active defensive behaviors. The Journal of Neuroscience. 2016;36(24):6488–6496. doi: 10.1523/JNEUROSCI.4419-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of pavlovian fear conditioning. The Journal of Neuroscience. 2006;26(48):12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. The Journal of Neuroscience. 2009;29(22):7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning & Memory. 2001;8(3):148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Gaby M, Zhang Y, Wolf K, Schwiening CJ, Paulsen O, Shipton OA. Archaerhodopsin selectively and reversibly silences synaptic transmission through altered pH. Cell Reports. 2016;16(8):2259–2268. doi: 10.1016/j.celrep.2016.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis M, Walker DL. Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Structure and Function. 2014;219(6):1969–1982. doi: 10.1007/s00429-013-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gafford GM, Guo J-D, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA (A) α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proceedings of the National Academy of Sciences. 2012;109(40):16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nature Neuroscience. 2016;19(4):554–556. doi: 10.1038/nn.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Day HE, Curran EJ, Watson SJ, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: Evidence for their selective activation by interleukin‐1 β. The Journal of Comparative Neurology. 1999;413(1):113–128. [PubMed] [Google Scholar]
- 64.Petrovich G, Swanson L. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Research. 1997;763(2):247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- 65.Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15(1):353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 66.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- 67.Gungor NZ, Paré D. Functional heterogeneity in the bed nucleus of the stria terminalis. The Journal of Neuroscience. 2016;36(31):8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shackman AJ, Fox AS. Contributions of the central extended amygdala to fear and anxiety. The Journal of Neuroscience. 2016;36(31):8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avery S, Clauss J, Blackford J. The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41(1):126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proceedings of the National Academy of Sciences. 2002;99(21):13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. The Journal of Molecular Diagnostics. 2012;14(1):22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. Academic Press; Boston: 2007. p. 456. [Google Scholar]
- 73.Nielsen DM, Crnic LS. Automated analysis of foot-shock sensitivity and concurrent freezing behavior in mice. Journal of Neuroscience Methods. 2002;115(2):199–209. doi: 10.1016/s0165-0270(02)00020-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


