Abstract
Background
Acute diarrheal illness during deployment causes significant morbidity and loss of duty days. Effective and timely treatment is needed to reduce individual, unit, and health system performance impacts.
Methods
This critical appraisal of the literature, as part of the development of expert consensus guidelines, asked several key questions related to self-care and healthcare-seeking behavior, antibiotics for self-treatment of travelers’ diarrhea, what antibiotics/regimens should be considered for treatment of acute watery diarrhea and febrile diarrhea and/or dysentery, and when and what laboratory diagnostics should be used to support management of deployment-related travelers’ diarrhea. Studies of acute diarrhea management in military and other travelers were assessed for relevance and quality. Based on this critical appraisal, guideline recommendations were developed and graded by the Expert Panel using good standards in clinical guideline development methodology.
Results
New definitions for defining the severity of diarrhea during deployment were established. A total of 13 graded recommendations on the topics of prophylaxis, therapy and diagnosis, and follow-up were developed. In addition, four non-graded consensus-based statements were adopted.
Conclusions
Successful management of acute diarrheal illness during deployment requires action at the provider, population, and commander levels. Strong evidence supports that single-dose antimicrobial therapy is effective in most cases of moderate to severe acute diarrheal illness during deployment. Further studies are needed to address gaps in available knowledge regarding optimal therapies for treatment, prevention, and laboratory testing of acute diarrheal illness.
Keywords: traveler’s diarrhea, military health, clinical practice guidelines, travel medicine
SUMMARY OF RECOMMENDATIONS AND SUGGESTIONS
Diarrhea Definitions
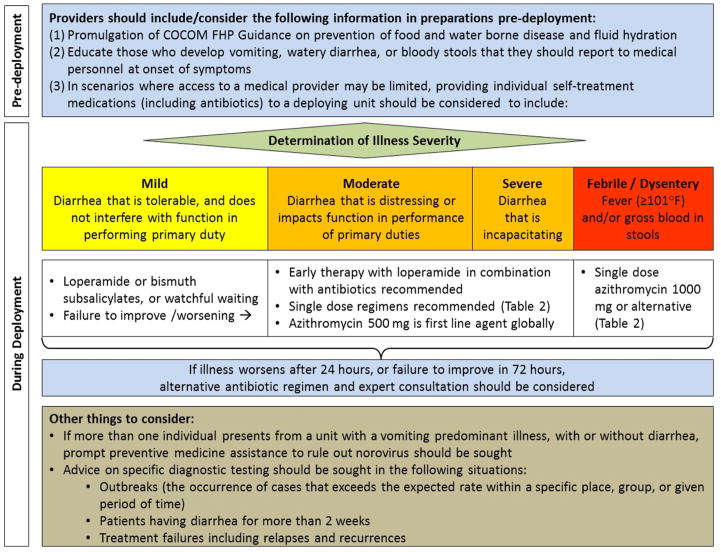
In military personnel reporting with an acute diarrheal illness, we define mild illness as no or minimal change in function, and moderate-severe illness as ranging from reduced to completely unable to function. Additionally, febrile diarrhea or dysentery is defined as diarrhea associated with fever or bloody stool.
General Recommendations on Force Health Protection Strategies
-
1
Providers should support and implement Combatant Command Force Health Protection (FHP) Guidance related to prevention of food and waterborne disease (Ungraded consensus-based statement)
-
2
In deployed settings, surveillance and early treatment are important. Therefore, those with vomiting, watery diarrhea, or bloody stools should report to medical personnel at onset of symptoms (Grade 1B)
-
3
In scenarios where access to a medical provider may be limited, providing individual self-treatment medications (including antibiotics) to a deploying unit should be considered (Grade 1B)
-
4
More than one individual presenting from a unit with a vomiting predominant illness, with or without diarrhea, should prompt preventive medicine assistance to investigate communicable etiologies, such as norovirus (Ungraded consensus-based statement)
Non-antibiotic Management of Acute Diarrheal Illness during Deployment
-
5
Prevention of dehydration is a critical aspect of diarrhea management. Early use of oral fluids and electrolytes should be encouraged (Grade 1A)
-
6
For those reporting mild watery diarrhea, the use of loperamide, bismuth subsalicylate, or watchful waiting are each reasonable options. Failure to improve or worsening of symptoms should prompt re-evaluation (Grade 1A)
Antibiotic Therapy of Acute Watery Diarrheal Illness during Deployment
-
7
For those reporting moderate or severe watery diarrhea, early antibiotic therapy combined with loperamide is recommended (Grade 1A)
-
8
For those who are treated for watery diarrhea, single-dose antibiotic therapy is recommended (Grade 1A)
-
9
Azithromycin 500 mg is recommended for use as a first-line agent for treatment of watery diarrhea in all regions of the world (Grade 1B)
-
10
Levofloxacin 500 mg is recommended as an alternative first-line agent for treatment of watery diarrhea, except in regions of the world where there is resistance (Grade 1A)
-
11
There are limited data suggesting single-dose rifaximin 1650 mg may be an alternative to the other single-dose antibiotic therapies in treatment of watery diarrhea where first-line agents cannot be used (Grade 1C, with remarks)
Antibiotic Therapy of Dysentery and Febrile Diarrhea
-
12
For those reporting fever greater than 101°F and/or bloody stools, single-dose azithromycin 1000 mg is recommended in all regions of the world (Grade 1B)
-
13
Loperamide should not be used as a standalone agent for dysentery or febrile diarrhea (Grade 1B)
-
14
When azithromycin 1000 mg is used for dysentery or febrile diarrhea, loperamide may be safely used in combination (Grade 2C)
-
15
Levofloxacin 500 mg for three days may be used as an alternative therapy to azithromycin in the treatment of dysentery/febrile diarrhea, except in areas where there is resistance (Grade 1B)
Follow-up and Diagnostic Testing
-
16
Among those that fail to improve in 72 hours or in whom illness worsens after 24 hours, alternative antibiotic regimens and expert consultation should be considered (Ungraded consensus-based statement).
-
17
Advice on specific diagnostic testing should be sought in the following situations: outbreaks, patients having diarrhea for more than 2 weeks, or treatment failures (Ungraded consensus-based statement)
INTRODUCTION
Infectious diarrhea has always been, and continues to be a problem in deploying troops and combat settings.1 The issue surrounding appropriate management of the disease likely dates back to World War II with the dawn of the antibiotic era. Some of the first randomized controlled treatment trials (RCTs) demonstrating antibiotic efficacy superior to placebos were conducted in the early 1980s, with consensus and expert-based treatment guidelines developed shortly thereafter.2 Studies have also evaluated various antibiotic regimens in combination with loperamide (an anti-motility agent) and shown through RCTs, improved efficacy compared to antibiotics alone when evaluating duration of post-treatment symptoms and clinical cure.3
The overall impact of travelers’ diarrhea (TD) during deployment of U.S. forces is substantial when one considers an average attack rate of 29% per month, and that for every episode, 3–5 days of illness ensues with an average of one complete duty day lost per episode.4 Given that it is estimated to cost over $2000 dollars per day to keep a troop in Afghanistan,5 timely and effective treatment could reduce the illness duration and time loss by 50 to 75%, resulting in a significant return on investment if optimized therapy were to be widely used. Beyond the potential for operational impact and disease burden mitigation, early treatment might also lessen the chronic health consequences (e.g., post-infectious functional bowel disorders and reactive arthritis), which are known to occur in approximately 10% of cases and can persist indefinitely in some individuals.6,7
No universal Department of Defense (DoD) treatment guidelines are available to offer global treatment recommendations to providers managing these illnesses during austere deployment settings. Furthermore, there are current gaps in our understanding of treatment with regards to which antibiotic classes should serve as front-line agents, safety and effectiveness of single-dose antibiotic/loperamide adjuncted regimens for ambulatory treatment of watery diarrhea and dysentery, and the best management practices at a population level.8
Despite a variety of therapeutic options, deployed providers continue to struggle with questions regarding best practice options for pharmacologic treatment.9,10 Our aim is to present practical guidance to deploying providers faced with common questions regarding the use of antibiotic and non-antibiotic therapies, as well as population health best practices to mitigate the burden of infectious diarrhea during deployment. We sought to apply a rigorous process to the review and assessment of evidence in order to make informed, evidence-based guideline recommendations. Unfortunately, rigorous data needed to address important questions faced by deployed providers in management of service members with TD are often absent or insufficient. Therefore, we present a hybrid document. When sufficiently strong evidence from RCTs or observational studies addressing a clinically important question is available, we based our guideline recommendation statements upon that data. When evidence is absent or insufficient to provide evidence-based guideline recommendation statements, we offer our best expert advice as consensus statements with the goal of helping deployed providers of all training levels navigate important therapeutic and population health management questions.
METHODS
The goal of this guideline project was to produce clinically relevant and useful recommendations on the management of acute diarrheal infections to forward deployed providers who treat service members who are at high risk. Healthcare providers should use these guidelines to assist with treatment choices that optimize benefits, minimize harm and burdens, and return the service member to duty as soon as possible. This guideline also considers other important aspects of force health protection (FHP) as they relate to management of diarrhea in the deployment setting.
In 2011, the Institute of Medicine (IOM) released new guideline standards that required significantly more scientific rigor and high-quality evidence to be considered trustworthy.11 Our Expert Panel is committed to upholding the IOM standards in guideline development. Nonetheless, the DoD does not have a formal institutionalized process for developing deployment health guidelines, and this project follows the Grades of Recommendations, Assessment, Development and Evaluation (GRADE) framework with noted limitations.12 Furthermore, the Expert Panel recognizes the need to communicate important messages within deployed military settings that lack the necessary associated evidence to be called a “guideline” by the new IOM standards. To provide guidance to frontline providers in such areas lacking sufficient evidence, consensus statements were developed. This hybrid methodology accommodates “very low” or “insufficient” levels of evidence, as well as recommendations that fall into the category of good clinical and public health practice, and incorporates guidelines and consensus statements. The following document reflects this hybrid approach and follows the standards to produce credible guidance for forward deployed providers and other members of the FHP team.
Composition and Selection of Expert Panel Members
For this guideline, a Chair was appointed based on leadership and experience in the area of diarrhea epidemiology and clinical trials. The Chair had the authority to nominate other panelists for specific roles, which included Infectious Disease and Preventive Medicine physician specialists from each of the three services (Navy, Army, and Air Force), representative forward deployed providers with recent operational medicine experience including a Family Practice provider, Independent Duty Medical Technicians, and a Special Operations Forces Physician Assistant. In addition, three non-DoD external panel members with Infectious Disease and Gastroenterology expertise were included. Conflict of interest for the external panel members (non-military) were reviewed and did not exclude them for participation in any of the voting related to recommendations.
Identifying and Reviewing the Evidence
Key Questions and Systematic Search: The Guideline Organizing Committee (GOC) developed a list of key clinical questions based on their knowledge of practice gaps:
How should military personnel with TD during deployment be directed with respect to self-care or seeking care?
Which service members should be prescribed antibiotics to self-treat TD?
What antibiotics/regimens should be considered for treatment of acute watery diarrhea?
What antibiotics/regimens should be considered for treatment of febrile diarrhea/dysentery?
When and what laboratory diagnostics should be used to support management of deployment TD?
To inform the evidence around these questions we provided all panelists with read-ahead materials, including existing TD management guidelines and systematic reviews, publications detailing the unique aspect of diarrhea in military deployment settings, primary references associated with the emerging problem of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, and current military documents describing FHP strategies related to the acute enteric infection health threat.3,6,7,13–33 In addition, prior to the closed panel session where guidelines were developed, the Uniformed Services University of the Health Sciences (USU) and the Naval Medical Research Center sponsored a one-day open session conference where experts in the field gave lectures focused on the key clinical questions identified along with presenting the findings from the Trial Evaluating Antibiotic Therapy in Travelers’ Diarrhea (TrEAT TD) study (ClinicalTrials.gov Identifier: NCT NCT01618591). These lectures and study results were considered as part of the evidence base to support recommendation development.
A formal systematic review with data extraction tables detailing all relevant articles for each of the key questions was not performed. Nevertheless, the Expert Panel did have access to the 2015 Committee to Advise on Tropical Medicine and Travel (CATMAT) Statement on Travellers’ Diarrhea from the Public Health Agency of Canada, which did have detailed summary tables on questions pertaining to treatment with antibiotics and non-antibiotics modalities.14 The data and evidence for key clinical questions were considered based on the provided read-ahead documentation, relevant didactic lectures, and subject matter expertise.
Drafting Recommendations
Focusing on key clinical questions outlined and deliberated on during the workshop, the closed panel reviewed the provided relevant publications on TD, considered the presentations from the open forum, and deliberated during the closed panel sessions to develop and agree upon consensus guideline recommendations. The closed panel discussion and deliberation was facilitated by an expert Chairperson in the field of management of diarrhea during deployment. The process for the development of each recommendation for a given key clinical question followed the general steps of recommendation formulation, grading the recommendation based on balance of risk harm and strength/quality of evidence supporting the recommendation, and discussion of the implications of the recommendation. Development and grading of recommendations utilized the Delphi process including features of anonymity, iteration, controlled feedback, and statistical group responses.34
Recommendation Formulation
The entire Expert Panel participated in the crafting of each of the recommendation statements. This process was achieved through the use of a facilitator (the Chair) who posed the key clinical research question and independently solicited from each of the panelists ideas on a recommendation statement. Points from each of the panelists were written down on a flip chart and grouped accordingly. Similar suggestions were grouped together where appropriate. From this process one or more recommendation statements emerged. In general, a statement would be collectively crafted and designed to include explicitly (or implicitly) the essential components of when, who, must/should/may/can, do what, and to whom. When the recommendation statement appeared to have no further discussion, the statement was put to an anonymous vote on the agreement of the statement using a Likert scale of strongly agree, weakly agree, weakly disagree, and strongly disagree (using the web-based PollEverywhere.com platform). If there was 80% or greater agreement (combining votes for strongly agree and weakly agree), the recommendation was accepted and moved forward for risk-harm and quality of evidence grading. In recommendations where there was less than 80% agreement achieved, the recommendation was revisited, modified, and re-voted upon until at least 80% agreement was reached. Recommendations that could not attain 80% agreement were abandoned.
Evidence Grading Process for Individual Recommendations
Upon consensus of each recommendation statement, the Expert Panel considered two dimensions on grading of the evidence. First, the balance of benefits to harms, risks, or burdens, including the confidence in the estimate of effect (e.g., “net benefit rating”) was deliberated. For this assessment, recommendations were considered strong when benefits clearly outweighed the harms or vice versa. In the latter case, there could be a strong negative recommendation (e.g., a strong recommendation not to use a specific drug or diagnostic method). Strong recommendations (Grade 1) included persuasive language such as “we recommend.” Nonetheless, when benefits and harms were closely balanced and it was possible additional research might change either the direction or strength of a recommendation, it was considered weak, and statements were worded as “we suggest.” When benefits were considered to clearly outweigh harms, most, if not all, service providers would choose the intervention; the recommendation is clearly Grade 1. In cases when there is considerable variability in provider preferences and tradeoffs between desirable and undesirable consequences are less clear, the recommendations are weaker (i.e., Grade 2).
The rating of the quality of the entire body of evidence for each recommendation was performed using the GRADE methodology.12 Ratings of the evidence started as high-quality and were downgraded based on criteria of study design, imprecision, indirectness (relative to the recommendation statement elements), inconsistency or heterogeneity of results across studies, and risk of reporting or publication bias. In general, study designs such as RCTs start as high-quality evidence, but are subject to downgrading based on these criteria. Observational studies start low, but may be upgraded if they meet design standards and (1) there is a large magnitude of effect, (2) there is a statistically significant effect even with the presence of bias, or (3) there is a dose-response gradient. A letter grade (A, B, or C) was assigned by the panel for the quality of evidence supporting each recommendation. A letter ‘A’ grade was evidenced from >1 properly randomized, controlled trial that met most (or all) of the criteria in terms of quality study design, precision, directness, consistency, and minimized risk of publication bias. A ‘B’ grade was considered appropriate based on evidence from ≥1 well-designed clinical trial, without randomization or from cohort or case-controlled analytic studies, multiple time-series, or dramatic results from uncontrolled experiments. A ‘C’ grade was relegated to evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees.
For both the net benefit and level of evidence ratings, a similar Delphi process was used to derive a consensus grading from the entire panel voting process. For each recommendation, the facilitator would lead a discussion about net benefit and level of evidence. Each participant was allowed to summarize his or her own opinions and thoughts. An anonymous voting method was used to record the participant’s individual grade selection. If at least 80% agreement was achieved, the consensus grade was accepted. If less than 80% agreement was obtained, the facilitator would lead further discussion of the recommendation and criteria to solicit clarifications and comments from the panelists. Upon completion of the rediscussion, another anonymous vote was taken. This process continued iteratively until at least 80% agreement was attained. In situations where 80% agreement could not be reached, recommendations with grades achieving 67 to 79% agreement were accepted, but reported with “additional remarks,” permitting those with minority opinions to address their concerns. Recommendations achieving less than 67% agreement are not included.
Finally, militarily relevant clinical and population health recommendations could not always be directly informed from the published data. For example, the Expert Panel recognized good clinical practice and standard FHP actions, which were considered important to be addressed. In such situations, the Expert Panel developed an ungraded consensus-based statement. Consensus in this instance was determined through open discussion and debate among the panel as well as putting the statement to a vote.
Review by External Reviewers
The deployment health guideline development Chair and co-chairs drafted initial recommendations, which were combined with the corresponding grade and presented to the entire Expert Panel in final draft form. Panelists were allowed to comment and suggest modifications and wording refinements. A final consensus document was reviewed and approved by all panelists. After the final manuscript was completed and endorsed by the GOC, the manuscript underwent peer review process by USU to consider content, methods, and adherence to GRADE process. Reviewers were self-nominated and vetted through the Director of the Infectious Disease Clinical Research Program and the Department of Preventive Medicine and Biostatistics at USU. Reviewers were military or civilian physicians not involved in the deployment health guideline development process and included other infectious diseases, preventive medicine, and gastroenterologists, as well as personnel in DoD Education and Training Commands, representing all levels of provider education and training who might use these deployment health guidelines.
Defining Diarrhea
In military personnel reporting with an acute diarrheal illness, we recommend defining mild illness as no or minimal change in function, and moderate-severe illness as reduced or completely unable to function. Additionally, febrile diarrhea or dysentery is defined as diarrhea associated with fever or bloody stool.
A classification of TD using functional impact for defining severity is advised rather than the traditional frequency-based algorithm that has been previously utilized.35 If passing numerous stools represents a functional impairment, it will be judged as more severe than an illness where less stools are passed. Moreover, a patient with febrile dysentery passing few stools is likely to be severely impaired due to systemic illness manifestations. The definition of dysentery should be discussed with future travelers and is defined as passage of stools that contain gross blood admixed with stool. It should be emphasized that normal appearing stools with streaks of blood on the toilet paper may well represent bleeding external hemorrhoids and not dysentery. Previous definitions have variably classified illness (with implied functional impacts) functionally as mild, moderate, or severe based on number of unformed stools passed in 24 hours (e.g., 1–2 stools mild, 3–5 stools moderate, and ≥ 6–9 stools for severe). Passage of a small number of stools with fever and severe cramps may be more disabling than passage of six watery diarrheal stools without cramps or pain. Furthermore, functional impairment may be dependent upon operational tempo as passage of multiple unformed stools during a period of low activity would not have as great an impact as when the tempo is high. We support an approach that matches the therapeutic intervention recommended, in terms of both safety and effectiveness, with the severity and impact of the illness. Therefore, we propose definitions based on functional impact. Prior to travel, the definitions of diarrhea should be discussed with service members, so they understand when to begin self-treatment, seek care, and what treatment modalities should be utilized. It is recognized that this is a departure from conventional definitions and will bring challenges to the interpretation and design of future trials related to TD, which rely on traditional severity based outcomes. Nonetheless, the Expert Panel feels that this classification system will likely lead to more tailored therapy for the individual.
General Recommendations on Force Health Protection Strategies
-
1
Providers should support and implement Combatant Command FHP Guidance related to prevention of food and waterborne disease (Ungraded consensus-based statement)
-
2
In deployed settings, surveillance and early treatment are important. Therefore, those with vomiting, watery diarrhea, or bloody stools should report to medical personnel at onset of symptoms (Grade 1B)
-
3
In scenarios where access to a medical provider may be limited, providing individual self-treatment medications (including antibiotics) to a deploying unit should be considered (Grade 1B)
-
4
More than one individual presenting from a unit with a vomiting predominant illness, with or without diarrhea, should prompt preventive medicine assistance to investigate communicable etiologies, such as norovirus (Ungraded consensus-based statement)
In World War II, Lieutenant General William Slim, Commander-in-Chief, 14th Army, said it best, “Good doctors are no use without good discipline. More than half the battle against disease is fought, not by the doctors, but by the regimental officers.”36 Thus, this guideline aims not only to address the practices of front-line medical providers and environmental health officers, but also emphasize the important doctrine that has been established and recognize the critical role in the non-medical leaders of the Military to assure policies and procedures are implemented to maintain the maximum fighting end-strength during any deployment. Military leaders should set, and providers should follow and implement, Combatant Command, theater Foreign Clearance Guide, and service specific FHP guidance pertinent to prevention of diarrheal illness. Observational studies have demonstrated when strict food and water controls and hygiene infrastructure is in place and access to unsafe food/beverage is restricted, rates of enteric infection can be minimized.37–40 Thus, efforts should be made whenever possible to emphasize the importance of procuring food and water from inspected military sources.
A systematic review of 52 epidemiological studies in military and similar populations found that a fraction (~20–25%) of deployed personnel who become ill with acute diarrhea seek medical attention.41 While diarrheal illness represents a spectrum of severity, recent studies have further supported lack of care seeking even among those with a disabling illness,39,42–50 and the consequent results of considerable loss-duty time and decreased performance, which could be mitigated by early and effective therapy. Thus, we recommend that guidance be given to deploying units that military service members should seek care from their attached medical providers when their illness is having an impact on their ability to perform their duties. Individuals with vomiting, fever, or dysentery should seek care immediately, independent of subjective illness impact. Studies are needed to evaluate the impact of such guidance, which will likely result in more patient volume; however, if coupled with appropriate therapy, this strategy should have an important reduction in lost duty days and improved daily effective end strength. If units or individuals are deployed to areas with a high potential risk for bacterial diarrhea without a medical provider, or with limited access to medical care, providing self-treatment to individual service members is recommended. After consulting with the relevant medical leads, modalities for self-treatment for TD should include rehydration, loperamide, and antibiotics (see treatment recommendations in this guideline for mild, moderate, severe diarrhea and dysentery). While it is currently not the policy to provide self-treatment to personnel at the unit level, individual provision of stand-by therapy is common in travel medicine and should meet standard of care in similar military deployment situations. Prior to deployment, units should consider their medication logistics and requirements needed to implement this guidance. Future studies should be designed to evaluate the effectiveness of this guidance in operational settings in reducing lost duty and decreased full mission capability days. It should also be noted that viral gastroenteritis in deployed settings, when uncontrolled, has demonstrated potential to impact medical treatment facilities.51 These second order effects of such infections are considerable and support the importance of surveillance and effective response.
Despite all efforts to control food, water, and environmental infrastructure, endemic and epidemic diarrhea is a perennial concern.1,8,52–54 The position of the DoD is to have people report for care when experiencing symptoms in order to capture information about potential illnesses and injuries. Among deployed personnel, this often means talking to a unit medic or corpsman, who can appropriately triage the patient. The disposition of having people report to medical with problems (even if it is an informal consultation) allows the early identification of population health problems. An “outbreak” is defined as the occurrence of a medical condition that exceeds the baseline/expected rate within a specific place or group of people over a given period of time.55 In high risk environments, diarrheal disease outbreaks can temporarily incapacitate a high percentage of personnel, and without appropriate intervention, this can have significant impact on military operations.56,57 As a result, health surveillance is a cornerstone to effective FHP and should be continuously conducted in order to implement early intervention and control strategies.58 While there is no minimum number of cases that constitutes a bacterial diarrheal/viral gastroenteritis outbreak, and a rate increase that should trigger reporting will vary according to the operational circumstances, we recommend that a low threshold be used to trigger an evaluation by public health with viral gastroenteritis (vomiting predominant illness) as it has a high potential force of infection and ability to impact large numbers. Thus, we emphasize that in accordance with service specific guidance, providers should investigate suspected disease outbreaks or occurrences capable of adversely affecting unit effectiveness or readiness (i.e., norovirus) and utilize reporting systems to ensure appropriate prevention and control actions are taken.59–61
Non-antibiotic Management of Acute Diarrheal Illness during Deployment
-
5
Prevention of dehydration is a critical aspect of diarrhea management. Early use of oral fluids and electrolytes should be encouraged (Grade 1A)
-
6
For those reporting mild watery diarrhea, the use of loperamide, bismuth subsalicylate, or watchful waiting are each reasonable options. Failure to improve or worsening of symptoms should prompt re-evaluation (Grade 1A)
Summary of Evidence
Acute diarrheal illness may lead to dehydration through excess loss of water and electrolytes in liquid stool. Diarrheal illness in the deployed setting may compound additional risk factors for dehydration particularly in hot and humid environments where high intensity activities and excessive sweating may lead to loss of critical electrolytes and fluids exceeding 10.5 L/day.62,63 Furthermore, dehydration may decrease one’s ability to perform mission-essential tasks in a timely and efficient manner.64 Prevention of dehydration is a critical aspect of diarrheal management and should start early in the course of illness.65 Early intake of fluids and electrolytes should be encouraged in an amount sufficient to maintain moist mucus membranes and adequate urine output.35
Oral rehydration therapy (ORT) involves balanced replacement of glucose, electrolytes, and fluids by mouth based on glucose-sodium cotransport mechanism in the small intestine where glucose facilitates absorption of sodium and water.66,67 Many experts suggest available foods and beverages, such as salty soups, crackers, fruit juices, and tea with sugar may provide sufficient fluid and electrolyte balance in otherwise healthy adults with TD.13,35,68 Few studies exist evaluating the efficacy of ORT in adults with travel-related diarrhea and there are currently no studies for ORT use in military populations. One investigator-blinded study evaluated use of ORT plus loperamide as compared to loperamide alone in U.S. adults with TD.69 Both groups were encouraged to maintain fluid and salt intake through soft drinks, soups, and saltine crackers. Average fluid intake did not vary significantly among groups and ORT showed no significant benefit. As suggested in this study, adequate hydration may be maintained through oral intake of available food and beverages during cases of mild to moderate diarrhea. For the military, it is essential for commanders to ensure sufficient food and fluid is available to ensure adequate hydration in individuals affected by episodes of infective diarrhea. If an individual is unable to maintain adequate oral intake, whether due to more severe illness, excessive vomiting, or extreme fluid losses, prompt medical attention should be sought to ensure appropriate hydration, which may include use of ORT and/or parenteral fluids as indicated.
Medical treatment may not be required in patients with non-severe, non-cholera-like diarrhea. Antisecretory and anti-motility drugs have been shown to reduce the overall number of stools in episodes of infective diarrhea, allowing some individuals to continue their planned schedule.69–73 Toxin-induced intestinal secretion is the major pathophysiologic mechanism leading to watery diarrhea in acute enteric infection - including TD. The antisecretory drug most frequently studied and shown to have value for therapy in secretory forms of diarrhea is bismuth subsalicylate (BSS), which reduces the number of stools passed by approximately 40%.74 The recommended dose of BSS for therapy of acute diarrhea is 30 mL (525 mg) of liquid formulation or two tablets (263 mg/tablet) chewed well every 30–60 minutes, not to exceed 8 doses in 24 hours. Potential side effects of BSS include production of black stools and black tongues from bismuth sulfide salts, which are transient and harmless.75 Importantly, BSS may significantly reduce the absorption of orally-administered doxycycline, leading to a reduction in the antibiotic’s bioavailability of up to 51%.76 This is a notable drug-drug interaction that is of concern in areas with high risk for malaria where doxycycline is an important prophylactic option. Therefore, care should be exercised when using BSS at the same time as doxycycline.
The major anti-motility drug available in DoD formularies and used for therapy of acute diarrhea is loperamide. In a comparative randomized trial in patients with TD, loperamide reduced the number of diarrheal stools passed when compared with BSS,73 and loperamide was shown to shorten duration of illness in both children77 and adults with acute diarrhea.78 The recommended dose of loperamide for adults with diarrhea is 4 mg initially followed by 2 mg after subsequently passed watery stools to a recommended maximum of 8 mg/d. Loperamide should not be given for more than 48 hours without physician review. The most valuable use of loperamide related to the self-treatment of TD is in combination with antibiotic therapy, where the combination has been shown to be superior to loperamide or antibiotics alone.3,70
A common complaint of loperamide therapy with acute diarrhea is post-treatment constipation; therefore, it is important to use the lowest dose of loperamide and wait 1–2 hours between interval dosing to provide antidiarrheal effects without the negative post-treatment effects of the drug. The main concern with respect to the use of loperamide in the deployment diarrhea setting is that anti-motility drugs have been associated with intestinal complications, such as toxic dilatation of the colon or prolonged illness when used in bacterial inflammatory colitis.79,80 There is some evidence that when used in combination with appropriate antibiotics this complication is very unlikely to occur.80
In summary, where medical treatment is not required (mild disease), antisecretory or anti-motility drugs may reduce complications associated with dehydration, particularly in deployed settings with limited access to medical care. Nevertheless, it should be noted that operational tempo or other considerations which dictate assurance of optimal force strength may prompt more aggressive therapy. Regardless, further care should be sought immediately if symptoms worsen or fail to improve with symptomatic therapies alone.
Antibiotic Therapy of Acute Watery Diarrheal Illness during Deployment
-
7
For those reporting moderate or severe watery diarrhea, early antibiotic therapy combined with loperamide is recommended (Grade 1A)
-
8
For those who are treated for watery diarrhea, single-dose antibiotic therapy is recommended (Grade 1A)
-
9
Azithromycin 500 mg is recommended for use as a first-line agent for treatment of watery diarrhea in all regions of the world (Grade 1B)
-
10
Levofloxacin 500 mg is recommended as an alternative first-line agent for treatment of watery diarrhea, except in regions of the world where there is concern for resistance (Grade 1A)
-
11
There are limited data suggesting single-dose rifaximin 1650 mg may be an alternative to the other single-dose antibiotic therapies in treatment of watery diarrhea where first-line agents cannot be used (Grade 1C, with remarks)
Summary of Evidence
Dating back to the seminal work of Kean and colleagues,81 the value of antibiotics related to the management of TD is strongly supported (Table 2 lists acceptable regimens). A systematic review of six RCTs demonstrated that antibiotics shorten the overall duration of TD to a little over 24 hours compared to more than 60 hours in placebo recipients.19 Furthermore, compared to antibiotics alone, combination therapy with loperamide shortens duration of illness to approximately half a day.3 Any antibiotic treatment is not without potential adverse consequences and use must be weighed relative to the benefits. Treatment emergent adverse events for commonly used antibiotics are rare; however, they do occur. Most recently, the U.S. Food and Drug Administration (FDA) released a statement on avoidance of fluoroquinolone use for non-complicated bacterial infections due to emerging and accumulating concerns of peripheral neuropathy, central nervous system, cardiac, dermatologic effects, and hypersensitivity reactions.82 Although the fluoroquinolone warning was targeted for non-complicated upper respiratory tract and urinary infections, the similar concern in treatment of TD may exist. Furthermore, the FDA also released a warning about the potential for cardiac anomalies (e.g., irregular heart rhythm) with use of azithromycin; however, the population at highest risk are those with known cardiovascular disease risk factors or disorders, such as long QT syndrome, low potassium or magnesium blood levels, slow heart rate, or use of certain antiarrhymia drugs.83 In the active military population and setting, such musculoskeletal and cardiac risks need to be balanced with the adverse consequences of TD. This Expert Panel reached consensus that oral antibiotic19 and loperamide-adjuncted3 regimens are well-tolerated, and in a deployment scenario, are judged to be outweighed by the risk of not-treating a potentially incapacitating and dehydrating illness.
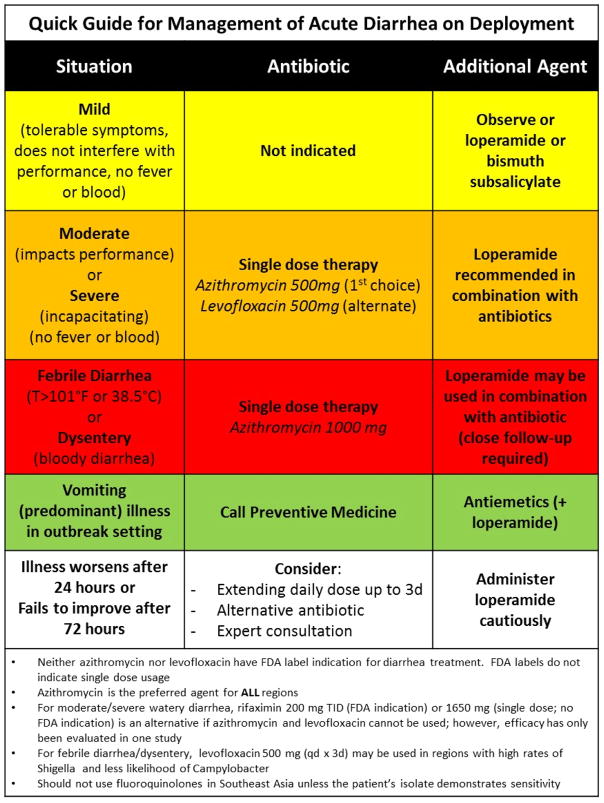
Table 2.
Acute watery diarrheal illness antibiotic treatment recommendationsa
| Antibioticb | Dose | Regimen Choice | Treatment duration | Recommendation Grade |
|---|---|---|---|---|
| Azithromycind,e | 500 mg by mouth | Primary | Single-doseb or up to 3 day coursee | Grade 1B |
| Levofloxacinf | 500 mg by mouth | Alternative | Single-doseb or up to 3 day course | Grade 1A |
| Rifaximing | 200 mg by mouth three times daily 1650 mg by mouth |
Alternative (diarrheagenic E. coli) | 3 days course Single-doseh |
Grade 1C, with remarks |
See Figure 1 for TD management algorithm
Antibiotic regimens may be combined with loperamide, 4 mg first dose, then 2 mg dose after each loose stool, not to exceed 16 mg in a 24 hour period (Grade 1A). Dosing interval of 1–2 hours should be recommended to avoid rebound constipation.
Use empirically as first-line in Southeast Asia and India to cover fluoroquinolone resistant Campylobacter or in other geographical areas if Campylobacter or resistant enterotoxigenic Escherichia coli are suspected.
Preferred regimen for dysentery or febrile diarrhea (Grade 1B)
If symptoms are not resolved after 24 hours, continue daily dosing for up to three days
Ciprofloxacin may be used interchangeably with levofloxacin (750 mg by mouth or 500 mg twice daily by mouth) as a single dose or up to a 3-day course
For watery diarrhea treatment only, do not use if clinical suspicion for Campylobacter, Salmonella, Shigella or other causes of invasive diarrhea.
Single-dose regimen can be used if access to other agents is limited.
For all antibiotics, current evidence supports that either single-dose therapy or treatment for up to three days are equivalently effective for TD due to noninvasive pathogens, which comprise the majority of cases.84,85 Given equivalence in efficacy, lower cost, increased ease of use, and probable improved safety profile, our recommendation for single-dose regimens is supported. Furthermore, we feel that concerns about significant alteration of the intestinal microbiome with antibiotic use may be minimized with single dose therapy; however, additional study is warranted. Nevertheless, it is recognized that in some cases of moderate or severe watery diarrhea (and certainly for invasive diarrhea to be further discussed below) longer durations of therapy may be necessary. Therefore, we recommend that a service member have appropriate follow-up (24 hours) for determination of continued therapy, or be provided with a 3-day course of antibiotics to continue self-treatment if a licensed medical provider is not available.
We recommend that azithromycin at a dose of 500 mg (refer to section on dysentery and febrile diarrhea for different dosing) be the first choice of treatment for all cases of TD, though alternatives are acceptable. Fluoroquinolones, such as ciprofloxacin or levofloxacin, have been the primary antibiotics of choice for most destinations;85–87 however, growing resistance and safety concerns with this class of antibiotics has emerged.88–90 While no studies have looked at the efficacy of azithromycin versus placebo, the four RCTs directly comparing azithromycin to a fluoroquinolones in the treatment of TD finds equivalent efficacy in three trials with azithromycin being superior in the RCT involving a high prevalence of fluoroquinolone resistant Campylobacter.91–94 Azithromycin, like all macrolide antibiotics, has motilin agonist activity that increases gut motility; however, the promotility effect with azithromycin is markedly less than with erythromycin. In studies for its FDA approval for respiratory and sexually transmitted infections, diarrhea, nausea and abdominal pain were the most common side effects of azithromycin. Gastrointestinal effects were seen in approximately 5% of patients administered 1.0 g of azithromycin.95 Patients in these studies were not being treated for diarrhea and while those with gastrointestinal symptoms may be more likely to experience gastrointestinal toxicity, the effects may be mitigated with the addition of concomitant loperamide. Furthermore, the literature on the use of fluoroquinolones in non-traveler settings has demonstrated risks of developing Clostridium difficile infections along with tendinopathies and arthropathies;96 however, such concerns have not been observed (nor well studied) in deployment settings. Despite being a relatively infrequent cause of diarrhea globally (though predominant in South and Southeast Asia), the majority of Campylobacter spp. are now fluoroquinolone resistant97 and there is evidence for treatment failures in these situations with fluoroquinolones. 94 Overall, this evidence supports that azithromycin is as effective as fluoroquinolones in providing relief from TD, and should be the antibiotic of choice. Surveillance for developing azithromycin resistance to common TD pathogens globally is needed to provide continued support for this recommendation in the future.
Rifaximin, a non-absorbable rifamycin-derived antibiotic, has been shown to be effective against diarrheagenic Escherichia coli, which appear to be the most common bacterial pathogen in the Western Hemisphere.98 In two studies evaluating rifaximin compared to placebo, rifaximin was associated with a higher percentage of travelers cured. A follow-up study conducted on a subset of patients with diarrhea due to enteroaggregative E. coli showed the 200 mg dose of rifaximin administered three times a day was more effective than placebo in decreasing median time to last unformed stool (22 versus 72 hours).99 Two additional studies directly compared rifaximin to ciprofloxacin with no significant difference with respect to cure or treatment failure.98,100 Another study failed to demonstrate an overall advantage when ciprofloxacin was compared to rifaximin in patients with TD in Mexico, Guatemala, and India. Nevertheless, a subgroup with invasive illness failed to show a benefit following treatment with rifaximin.101 In addition, well documented primary treatment failures leading to the requirement for rescue therapy have been observed with invasive infections (including Campylobacter infections).101,102
Most recently, the findings have been reported from TrEAT TD Study, which is a RCT comparing three single-dose antibiotic regimens with loperamide (ClinicalTrials.gov: NCT01618591).103 In this study, U.S. and U.K. adults with acute non-inflammatory diarrhea at four military deployment sites in Afghanistan, Djibouti, Kenya, and Honduras were randomized and received single-dose levofloxacin (500 mg; 111 persons), azithromycin (500 mg; 106 persons), and rifaximin (1650 mg, 107 persons) plus loperamide (labelled dosing). The primary outcome of clinical cure was evaluated in a non-inferiority trial design with azithromycin and rifaximin versus a levofloxacin standard. Clinical cure at 24 hours occurred in 80.2% of the levofloxacin arm, compared to 78.3% and 74.8% in the azithromycin and rifaximin arms, respectively. Compared to levofloxacin, non-inferiority was not shown with rifaximin. Furthermore, median time to last unformed stool among all three arms was not different (levofloxacin: 5.6 hours; azithromycin: 4.0 hours; rifaximin: 5.6 hours), treatment failures were uncommon (4.5%, 3.8%, and 1.9% in the levofloxacin, azithromycin, and rifaximin arms, respectively), and no safety concerns were identified. In addition to further supporting loperamide-adjuncted levofloxacin and azithromycin as safe and effective single-dose regimens, the use of single-dose rifaximin 1650 mg may be considered in situations where other agents are not available.
While recommendations for treatment of moderate-severe TD are well-evidenced and strong, there are some concerns with the use of empiric antibacterial therapy of TD that are important to understand. The first is that antibacterial drugs appear to complicate enteric disease caused by Shiga-like toxin producing E. coli (STEC) by increasing the risk of hemolytic uremic syndrome (HUS). Although this may occur in children, STEC is extremely rare as a pathogen in TD, and a meta-analysis did not show an association between antimicrobial therapy in adult patients with hemorrhagic colitis due to E. coli 0157:H7 and the subsequent development of HUS.104 Another theoretical concern with antibiotic utilization is that for non-typhoidal Salmonella strains there may be prolonged intestinal carriage. Specifically, a meta-analysis showed that antibiotic therapy does not appear to reduce the length of illness in immunocompetent adults and increases the period during which Salmonella was detected in stool.105 This would not necessarily be an argument against antibiotic use as short-term carriage appears to be of limited clinical significance to those who are affected. A more legitimate concern is that treatment with antibiotics will modify the microbiota, which may result in the development of C. difficile associated diarrhea or colitis.96,105 A recent publication reported patients who developed C. difficile colitis following treatment with ciprofloxacin;106 however, this does not appear to be a common adverse outcome associated with treated TD, and single-dose therapy should significantly minimize this risk.
Finally, it is important to address the growing concern about the effect of antibiotic therapy on acquisition and carriage of multidrug-resistant organisms, specifically ESBL-producing E. coli, in the setting of military diarrhea.17,107 While these resistant pathogens are usually not associated with symptoms in the average healthy traveller (or military service member), transient in duration, and of uncertain impact on the spread of disease in a community-wide and global scale,15 their potential importance warrants consideration in balancing the benefit of acute illness treatment with both individual and population health consequences. Current studies lack much needed systematically collected data on the differential impact of single-dose (and loperamide combined) antibiotic regimens.17,35,108,109 From a military perspective, antibiotic resistance is undoubtedly an important concern. Trauma-related infections among combat casualties are a substantial challenge110–112 with attendant morbidity and mortality113–118 and complicated by multidrug resistance.117,119–121 While needing further study, the multitude of exposures from environmental, as well as additional factors, such as daily antimalarial prophylaxis, stress to the immune system, perioperative antibiotic prophylaxis among injured personnel, and duration of deployment are strong contributors to multidrug-resistant organism colonization acquisition122 and, thus, the attributable fraction of TD treatment with single-dose antibiotics needs to be put into appropriate perspective and balanced against the clear FHP benefits of effective therapy for consequential moderate-severe TD infections. In summary, at present, the risk of acquired ESBL-producing bacteria to the individual service member during deployment and community versus the potential negative consequences of untreated TD has raised important awareness and calls for the development of more data to inform future management guidelines.
Antibiotic Therapy of Dysentery and Febrile Diarrhea
-
12
For those reporting fever greater than 101°F and/or bloody stools, single-dose azithromycin 1000 mg is recommended in all regions of the world (Grade 1B)
-
13
Loperamide should not be used as a standalone agent for dysentery or febrile diarrhea (Grade 1B)
-
14
When azithromycin 1000 mg is used for dysentery or febrile diarrhea, loperamide may be safely used in combination (Grade 2C)
-
15
Levofloxacin 500 mg for three days may be used as an alternative therapy to azithromycin in the treatment of dysentery/febrile diarrhea, except in areas where there is resistance (Grade 1B)
Summary of Evidence
Antibiotic therapy is recommended for treatment of dysentery, as well as acute watery diarrhea with high fever. Azithromycin is advised as the first-line agent for dysentery worldwide, given the potential etiologies of Campylobacter spp. and other fluoroquinolone-resistant pathogens.92,94,123,124 Azithromycin given as a single 1-gm dose or as 500 mg daily for three days was superior to levofloxacin 500 mg daily for three days for achieving clinical cure in Thailand in a setting with extremely high rates (exceeding 90%) of fluoroquinolone-resistant Campylobacter spp.94 Azithromycin was also shown to be effective in clinical trials of treatment of TD in Africa and Thailand and Mexico,91,92 as well as being effective for treatment of shigellosis.125,126 Fluoroquinolone-resistant travel-associated and domestic Campylobacter cases in industrialized countries have been increasingly reported and are not restricted to specific countries, such as Thailand.127–129 In addition, nalidixic acid and fluoroquinolone resistance in Shigella spp. and Salmonella spp. from India, and in a variety of enteric pathogens in sub-Saharan Africa has been recognized.130–133 Recent reports have also documented the emergence of azithromycin-resistant Shigella in men who have sex with men (MSM), along with an outbreak in the United States (not limited to MSM) and Canada, emphasizing the need for continued global surveillance and close follow-up to assess treatment response.134,135 Thus, current evidence would support azithromycin as the antibiotic of choice for global therapy of dysentery and febrile diarrhea.
Similar to the reasoning for single-dose therapy for moderate and severe diarrhea, azithromycin as a single 1 gm dose for treatment of dysentery and febrile watery diarrhea is generally well-tolerated with minimal side effects though dose-related gastrointestinal complaints have been observed.136 Drug-associated incidents or worsening nausea or vomiting may be exacerbated by the primary gastrointestinal infection,93,94 and are more common than in the treatment of non-gastrointestinal infections with rates of 3% and <1%, respectively.137–140 Furthermore, consideration of administering a dose of loperamide concomitantly with azithromycin may minimize the macrolide-associated promotility effects. Some experts believe that splitting the single 1 gram dose over the first day may achieve similar efficacy while inducing a lower frequency of nausea and related gastrointestinal side effects and increasing tolerability; however, this has not been specifically studied in a comparative trial.
A second option for the treatment of dysentery or febrile watery diarrhea is use of fluoroquinolones; however, important caveats exist. Fluoroquinolone antibiotics retain efficacy in much of the developing world and can be used effectively in such regions where the likelihood of fluoroquinolone-resistant Campylobacter spp. or Shigella spp. being the cause of dysentery is low, assuming there is good follow-up. Fluoroquinolones are generally well-tolerated. Gastrointestinal (nausea, vomiting, or diarrhea) and central nervous system (headache, dizziness, or insomnia) side effects are shared by all quinolones, but are generally mild and transient.141 The recent FDA warnings have been previously discussed, though the risk balance for treatment of dysentery or febrile diarrhea in a deployment setting favors treatment if azithromycin is not available.82 If situations do not allow the use of azithromycin (e.g., allergy or availability of supply), a 3-day fluoroquinolone course may be used, assuming that there is adequate follow-up to assess for treatment failure and further management.
The use of anti-motility agents in cases of severe diarrhea and dysentery is important to consider. Reported risks of loperamide therapy include ileus,142 so concern about theoretically increased likelihood of invasive disease when used as treatment monotherapy is warranted. Nonetheless, no studies have reported such outcomes in treatment of adults with TD, including severe disease,45,143–146 suggesting its safety profile may be acceptable, however, the Expert Panel recommends against use of loperamide as monotherapy in dysentery and febrile diarrhea given the clear aforementioned benefits of antibiotic therapy. An alternative question is to whether loperamide in combination with antibiotics in dysentery and febrile diarrhea improves treatment outcomes. While we lack robust data, there is some evidence to suggest that combination therapy may be effective and safely given in the setting of treatment of acute enteric invasive infections among adults. In a study by Murphy et al.,147 with the objective to evaluate the safety and efficacy of loperamide plus ciprofloxacin versus ciprofloxacin alone in the treatment of bacillary dysentery (in 88 Thai adults), the authors reported that compared to the non-adjuncted regimen, loperamide-adjuncted treatment decreased the number of unformed stools (median 2.0 vs 6.5) and shortened the duration of diarrhea (19 hours vs 42 hours). Importantly, the authors noted that there were no adverse outcomes in either group. These results in addition to the findings of other studies where invasive disease was treated with anti-motility agents such as loperamide148–151 suggest that loperamide-adjuncted antibiotic regimens may offer additional benefit to patients with inflammatory diarrhea. In summary, based on the available evidence, the consensus recommendation (weak) is that loperamide may be used in cases of febrile diarrhea and dysentery only in combination with antibiotics, assuming there is close follow-up to monitor for any adverse treatment outcomes. Further study is needed to understand the potential benefit of adjunctive loperamide in this specific clinical scenario.
Follow-up and Diagnostic Testing
-
16
Among those that fail to improve in 72 hours or in whom illness worsens after 24 hours, alternative antibiotic regimens and expert consultation should be considered (Ungraded consensus-based statement).
-
17
Advice on specific diagnostic testing should be sought in the following situations: outbreaks, patients having diarrhea for more than 2 weeks, or treatment failures (Ungraded consensus-based statement)
Summary of Evidence
While the differential diagnosis of acute diarrhea during a deployment setting will always have enteric pathogen infection at or near the top, clinical vigilance is a must and alternative etiologies need to be considered, particularly in cases that do not improve in 24 hours or resolution of illness in 72 hours does not occur despite effective therapy. For example, in febrile illness with diarrhea and/or vomiting as prominent features, the consideration of malaria may be appropriate if there is concern of lack of adherence to chemoprophylaxis.152–155 Clear instructions must be provided to patients regarding the expected response to antibiotic treatment, timing of illness resolution, and appropriate follow-up. Specifically, resolution of diarrhea or significant improvement by 24 hours does not warrant further evaluation. If the patient worsens within the first 24 hours following treatment or has continuation of symptoms for 72 hours (or relapse of symptoms) they should return for medical evaluation. In cases of severe, prolonged, or recurrent disease, referral or consultation with a higher level of care with enhanced diagnostics and therapeutic options should be considered. Other considerations may include diagnostic evaluation for potential etiologies (infectious and non-infections), as well as switching of antibiotic therapy to a different drug class (if resistance is a concern).
We recommend that specific laboratory investigation is not normally required in the majority of cases of acute watery diarrhea because it is usually self-limited, resolves rapidly with effective therapy, and there are limited diagnostics available, particularly in forward deployed settings without infrastructure. While studies evaluating the utility in supporting management of TD in a forward deployed setting are lacking, it is our expert opinion that diagnostics likely have a role in improving care for those clinical scenarios that include outbreaks, chronic diarrhea (more than 14 days), or treatment failures as less common or drug-resistant pathogens and non-infectious etiologies may be causative (Table 3). Improved diagnostics may enhance the ability to provide a more rapid resolution of symptoms for the individual, improve antibiotic stewardship, and enhance control measures to prevent and/or limit diarrhea outbreaks.
Table 3.
Differential Diagnoses for Persistent and Chronic Causes of Diarrhea in Returning Deployer
| Protozoa |
| Giardia lamblia |
| Cryptosporidium parvum |
| Isospora belli |
| Balantidium coli |
| Enterocytozoon bieneusi |
| Septata intestinalis |
| Cyclospora cayetanensis |
| Entamoeba histolytica |
| Dientamoeba fragilis |
| Helminths |
| Strongyloides stercoralis |
| Schistosoma species |
| Ascaris lumbricoides |
| Capillaria philippinensis |
| Trichuris trichiura |
| Trichinella spiralis |
| Bacteria |
| Enterobacteriaceae: E. coli (especially enteroadherent), |
| Shigella species, nontyphoidal Salmonella, Campylobacter species, |
| Yersinia enterocolitica |
| Vibrionaceae: Aeromonas species, Plesiomonas species |
| Clostridium difficile |
| Unknown pathogens/conditions |
| Brainerd diarrhea |
| Tropical sprue |
| Post-infectious processes |
| Post-infectious malabsorptive states |
| Disaccharide intolerance |
| Subacute tropical malabsorption |
| Bacterial overgrowth |
| Post-infectious irritable bowel syndrome |
| Post-dysenteric colitis |
| Chronic GI diseases unmasked by enteric infection |
| Idiopathic inflammatory bowel disease |
| Ulcerative colitis |
| Crohn disease |
| Microscopic colitis |
| Celiac sprue |
| Colorectal adenocarcinoma |
| AIDS |
Conventional diagnostic approaches to diarrheal disease require multiple procedures: bacterial culture, microscopy with and without stains or immunofluorescence, stool antigen tests for detection of protozoa, and electron microscopy or antigen based tests for detecting viral agents. Culture methods are laborious and time consuming with results often not available for 48 to 72 hours.156 Historically, a decision to obtain a stool culture in an individual with diarrhea has often been guided by the finding of fecal leukocytes or the presence of stool lactoferrin, but these are likely imprecise and probably unnecessary.157,158 Microscopy has been the principal diagnostic tool in parasitology, but is labor and time intensive, requires technical expertise, and lacks sensitivity and reproducibility. Multiple specimens are often required to reduce the day-to-day variability in parasite shedding.159
Culture independent molecular techniques are now available which provide results in hours rather than days and simultaneously identifies a multitude of bacterial, protozoan, and viral diarrheal pathogens, including those not commonly identified in clinical laboratories.160 Diarrheal disease by definition has a broad range of potential pathogens particularly well suited for multiplex molecular testing. One potential drawback of molecular technologies is the need to pre-define the particular microbes being sought. In addition, the significance of an identified organism may not be clear as these molecular technologies, which involve nucleic acid amplification limited to our existing knowledge of a microbes’ genome and do not discriminate between viable and non-viable organisms or incorporate qualitative versus quantitative discrimination of colonization versus infection. As a result they can detect microbes at non-pathogenic levels. To confound matters, further multiplex techniques are more frequently associated with increased detection of mixed infections and the relative importance of each pathogen may be unclear.27,161–171 Lastly, lack of isolates for subsequent antimicrobial susceptibility testing or molecular epidemiology with outbreak investigations limits the utility of these culture-independent testing platforms. Therefore, employment of these multiplex detection techniques when indicated should be encouraged; however, they require knowledgeable interpretation which considers clinical, epidemiological, and microbiological factors. Continued work in diarrheal pathogen identification is needed, and implementation of these new molecular diagnostic approaches in deployed settings is likely to assist in conserving the fighting strength.
DISCUSSION
This guideline is intended to help simplify the management of deployed service members with diarrheal illness. The goal is to encourage medical evaluation with appropriate, optimized treatment to maintain force strength during deployments. We have sought to consider the range of management activities, including commander, provider, and patient level perspectives, and applied rigorous methods of both literature review and broad-based, tri-service, and multi-provider perspectives. While implementation of guidelines must fall within the current policies of the Armed Services, we feel that the future of TD treatment during deployment needs to evolve to a directly observed therapy-based strategy at the corpsman/medic level to optimize force strength. This guideline is a critical step to reach that goal and adds to the evidence base necessary to inform policy in management of TD among globally deploying forces.
While this guideline is comprehensive, it does not address other important aspects related to acute diarrhea during deployment. Prophylaxis with antibiotics or other agents are not addressed, but may be a partial solution in some situations and have been advocated in some guidelines.14,35 Similarly, the use of probiotics and/or prebiotics for treatment and prevention has not been considered for multiple reasons, including lack of effectiveness.35 Furthermore, it is important to remember that the DoD has strict policy on use of agents for prevention in FHP. Vaccines, immunoglobulins, and drug prophylaxis can be given under FHP, but only in accordance with FDA-licensed products and regimens and for FDA-approved indications.170 Products not approved by the FDA for prevention can be given to service members only with voluntary informed consent under an institutional review board-approved protocol and in accordance with a current and FDA-approved investigational new drug application. Therefore, products such as antibiotics, probiotics, and prebiotics that do not have a FDA licensed indication for prevention of TD cannot be used. Future efforts to develop such modalities need to consider these restrictions to be fully implementable. Lastly, this guideline does not address the management of the chronic consequences of acute diarrheal infection during deployment,6,7,171 which in combination with the perennial concern of antibiotic resistance and potentially consequential dysbiotic treatment effects, demands continued efforts on primary prevention, including improved field sanitation, hygiene, food security, and vaccines.
In summary, management of acute diarrheal illness during deployment requires action at the provider, population, and commander levels to be most effective. The development of a comprehensive Deployment Health Guideline is only the beginning. Effective promulgation and adoption of these guidelines utilizing top-down and bottom-up approaches will require additional commitment on the part of many. Nevertheless, the effort to date, as well as moving forward, is necessary as diarrheal illness has proven to be a perennial deployment problem that degrades force capability. Therefore, successful evidence-based management is critically important until more effective interventions, such as vaccines are developed to prevent these infections.
Figure 1.
TD Management Algorithm during deployment
Figure 2.
Wallet card (quick guide) for management of Acute Diarrhea during military deployment
Table 1.
Summary of Recommendations
| General recommendations on force health protection strategies |
|
| Non-antibiotic management of acute diarrheal illness during deployment |
|
| Antibiotic Therapy of acute watery diarrheal illness during deployment |
|
| Antibiotic therapy of Dysentery and Febrile Diarrhea |
|
| Follow-up and diagnostic testing |
|
Acknowledgments
Funding sources: This work was supported by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health [Inter-Agency Agreement Y1-AI-5072]. This work was supported by a grant from the Bureau of Medicine and Surgery to the Uniformed Services University of the Health Sciences (USU Grant Agreement-HU0001-11-1-0022; USU Project No: G187V2)
We are very grateful to the entire expert panel for their hard work and dedication in producing this guideline. Specifically, we would like to thank CDR Andrew Baldwin, Dr. Bradley Connor, CPO Joseph Delacruz, Dr. Herbert Dupont, LTC Patrick Hickey, LTC James Pairmore, Dr. John Powers, and MSgt Melissa Worley for their participation in the expert panel. We would also like to give our sincerest gratitude to Ms. Leigh Carson for her expert assistance in supporting the guideline development meeting and editing of the guideline.
Footnotes
Disclaimer: The views expressed are those of the authors and do not reflect the official views or policies of the Uniformed Services University of the Health Science, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., U.S. Department of State, U.S. Department of Defense (DoD), the U.S. Departments of the Army, Navy or Air Force, or the United Kingdom Ministry of Defence (MOD). Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Copyright Statement: Several authors are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- 1.Riddle MS, Savarino SJ, Sanders JW. Gastrointestinal infections in deployed forces in the Middle East theater: an historical 60 year perspective. Am J Trop Med Hyg. 2015;93(5):912–7. doi: 10.4269/ajtmh.15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travelers’ diarrhea. National Institutes of Health Consensus Development Conference Statement. [accessed 17 February 2017];Natl Inst Health Consens Dev Conf Consens Statement. 1985 5(8):1–7. Available at https://consensus.nih.gov/1985/1985travelersdiarrhea048html.htm. [PubMed] [Google Scholar]
- 3.Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler’s diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(8):1007–14. doi: 10.1086/591703. [DOI] [PubMed] [Google Scholar]
- 4.Riddle MS, Tribble DR, Cachafiero SP, Putnam SD, Hooper TI. Development of a travelers’ diarrhea vaccine for the military: how much is an ounce of prevention really worth? Vaccine. 2008;26(20):2490–502. doi: 10.1016/j.vaccine.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Shaughnessy L. One soldier, one year: $850,000 and rising. [accessed 29 January 2017];CNN.com. 2012 Feb 28; Available at http://security.blogs.cnn.com/2012/02/28/one-soldier-one-year-850000-and-rising/
- 6.Connor BA, Riddle MS. Post-infectious sequelae of travelers’ diarrhea. J Travel Med. 2013;20(5):303–12. doi: 10.1111/jtm.12049. [DOI] [PubMed] [Google Scholar]
- 7.Verdu EF, Riddle MS. Chronic gastrointestinal consequences of acute infectious diarrhea: evolving concepts in epidemiology and pathogenesis. Am J Gastroenterol. 2012;107(7):981–9. doi: 10.1038/ajg.2012.65. [DOI] [PubMed] [Google Scholar]
- 8.Connor P, Porter CK, Swierczewski B, Riddle MS. Diarrhoea during military deployment: current concepts and future directions. Curr Opin Infect Dis. 2012;25(5):546–54. doi: 10.1097/QCO.0b013e3283582ebc. [DOI] [PubMed] [Google Scholar]
- 9.Riddle MS, Tribble DR, Jobanputra NK, Jones JJ, Putnam SD, Frenck RW, et al. Knowledge, attitudes, and practices regarding epidemiology and management of travelers’ diarrhea: a survey of front-line providers in Iraq and Afghanistan. Mil Med. 2005;170(6):492–5. doi: 10.7205/milmed.170.6.492. [DOI] [PubMed] [Google Scholar]
- 10.Hayat AM, Tribble DR, Sanders JW, Faix DJ, Shiau D, Armstrong AW, et al. Knowledge, attitudes, and practice of travelers’ diarrhea management among frontline providers. J Travel Med. 2011;18(5):310–7. doi: 10.1111/j.1708-8305.2011.00538.x. [DOI] [PubMed] [Google Scholar]
- 11.Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, editors. Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington (DC): The National Academies Press; 2011. [accessed 17 February 2017]. Availabel at https://www.nationalacademies.org/hmd/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx. [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steffen R, Hill DR, DuPont HL. Traveler’s diarrhea: a clinical review. JAMA. 2015;313(1):71–80. doi: 10.1001/jama.2014.17006. [DOI] [PubMed] [Google Scholar]
- 14.Committee to Advise on Tropical Medicine and Travel (CATMAT) Statement on Travellers’ Diarrhea. Public Health Agency of Canada; 2015. [accessed 17 February 2017]. Available at http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/15vol41/dr-rm41-11/ar-03-eng.php. [Google Scholar]
- 15.Ruppe E, Armand-Lefevre L, Estellat C, Consigny PH, El Mniai A, Boussadia Y, et al. High rate of acquisition but short duration of carriage of multidrug-resistant Enterobacteriaceae after travel to the tropics. Clin Infect Dis. 2015;61(4):593–600. doi: 10.1093/cid/civ333. [DOI] [PubMed] [Google Scholar]
- 16.Lubbert C, Straube L, Stein C, Makarewicz O, Schubert S, Mossner J, et al. Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae in international travelers returning to Germany. Int J Med Microbiol. 2015;305(1):148–56. doi: 10.1016/j.ijmm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Kantele A, Laaveri T, Mero S, Vilkman K, Pakkanen SH, Ollgren J, et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis. 2015;60(6):837–46. doi: 10.1093/cid/ciu957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alajbegovic S, Sanders JW, Atherly DE, Riddle MS. Effectiveness of rifaximin and fluoroquinolones in preventing travelers’ diarrhea (TD): a systematic review and meta-analysis. Syst Rev. 2012;1:39. doi: 10.1186/2046-4053-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Bruyn G, Hahn S, Borwick A. Antibiotic treatment for travellers’ diarrhoea. Cochrane Database Syst Rev. 2000;(3):CD002242. doi: 10.1002/14651858.CD002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuland EA, Sonder GJ, Stolte I, Al Naiemi N, Koek A, Linde GB, et al. Travel to Asia and traveller’s diarrhoea with antibiotic treatment are independent risk factors for acquiring ciprofloxacin-resistant and extended spectrum beta-lactamase-producing Enterobacteriaceae-a prospective cohort study. Clin Microbiol Infect. 2016;22(8):731.e1–7. doi: 10.1016/j.cmi.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Mathai D, Kumar VA, Paul B, Sugumar M, John KR, Manoharan A, et al. Fecal carriage rates of extended-spectrum beta-lactamase-producing Escherichia coli among antibiotic naive healthy human volunteers. Microb Drug Resist. 2015;21(1):59–64. doi: 10.1089/mdr.2014.0031. [DOI] [PubMed] [Google Scholar]
- 22.Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26(4):744–58. doi: 10.1128/CMR.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalani T, Tisdale MD, Maguire JD, Wongsrichanalai C, Riddle MS, Tribble DR. Detection of enteropathogens associated with travelers’ diarrhea using a multiplex Luminex-based assay performed on stool samples smeared on Whatman FTA Elute cards. Diagn Microbiol Infect Dis. 2015;83(1):18–20. doi: 10.1016/j.diagmicrobio.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zboromyrska Y, Hurtado JC, Salvador P, Alvarez-Martinez MJ, Valls ME, Mas J, et al. Aetiology of traveller’s diarrhoea: evaluation of a multiplex PCR tool to detect different enteropathogens. Clin Microbiol Infect. 2014;20(10):O753–9. doi: 10.1111/1469-0691.12621. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Kabir F, Manneh J, Lertsethtakarn P, Begum S, Gratz J, et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis. 2014;14(8):716–24. doi: 10.1016/S1473-3099(14)70808-4. [DOI] [PubMed] [Google Scholar]
- 26.Laaveri T, Pakkanen SH, Antikainen J, Riutta J, Mero S, Kirveskari J, et al. High number of diarrhoeal co-infections in travellers to Benin, West Africa. BMC Infect Dis. 2014;14:81. doi: 10.1186/1471-2334-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckmann C, Heininger U, Marti H, Hirsch HH. Gastrointestinal pathogens detected by multiplex nucleic acid amplification testing in stools of pediatric patients and patients returning from the tropics. Infection. 2014;42(6):961–70. doi: 10.1007/s15010-014-0656-7. [DOI] [PubMed] [Google Scholar]
- 28.Mutsch M, Pitzurra R, Hatz C, Steffen R. Post-infectious sequelae of travelers’ diarrhea: irritable bowel syndrome. J Travel Med. 2014;21(2):141–3. doi: 10.1111/jtm.12094_1. [DOI] [PubMed] [Google Scholar]
- 29.Lim PL, Han P, Chen LH, MacDonald S, Pandey P, Hale D, et al. Expatriates ill after travel: results from the Geosentinel Surveillance Network. BMC Infect Dis. 2012;12:386. doi: 10.1186/1471-2334-12-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youmans BP, Ajami NJ, Jiang ZD, Campbell F, Wadsworth WD, Petrosino JF, et al. Characterization of the human gut microbiome during travelers’ diarrhea. Gut Microbes. 2015;6(2):110–9. doi: 10.1080/19490976.2015.1019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalanka J, Salonen A, Fuentes S, de Vos WM. Microbial signatures in post-infectious irritable bowel syndrome--toward patient stratification for improved diagnostics and treatment. Gut Microbes. 2015;6(6):364–9. doi: 10.1080/19490976.2015.1096486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048. doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5(2):97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–80. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riddle MS, DuPont HL, Connor BA. ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults. Am J Gastroenterol. 2016;111(5):602–22. doi: 10.1038/ajg.2016.126. [DOI] [PubMed] [Google Scholar]
- 36.Slim WJ. Defeat into Victory: Battling Japan in Burma and India, 1942–1945. New York: David McKay Company; 1961. [Google Scholar]
- 37.Hameed JM, McCaffrey RL, McCoy A, Brannock T, Martin GJ, Scouten WT, et al. Incidence, etiology and risk factors for travelers’ diarrhea during a hospital ship-based military humanitarian mission: Continuing Promise 2011. PLoS One. 2016;11(5):e0154830. doi: 10.1371/journal.pone.0154830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riddle MS, Halvorson HA, Shiau D, Althoff J, Monteville MR, Shaheen H, et al. Acute gastrointestinal infection, respiratory illness, and noncombat injury among US military personnel during Operation Bright Star 2005, in Northern Egypt. J Travel Med. 2007;14(6):392–401. doi: 10.1111/j.1708-8305.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- 39.Riddle MS, Tribble DR, Putnam SD, Mostafa M, Brown TR, Letizia A, et al. Past trends and current status of self-reported incidence and impact of disease and nonbattle injury in military operations in Southwest Asia and the Middle East. Am J Public Health. 2008;98(12):2199–206. doi: 10.2105/AJPH.2007.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders JW, Putnam SD, Gould P, Kolisnyk J, Merced N, Barthel V, et al. Diarrheal illness among deployed U.S. military personnel during Operation Bright Star 2001--Egypt. Diagn Microbiol Infect Dis. 2005;52(2):85–90. doi: 10.1016/j.diagmicrobio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74(5):891–900. [PubMed] [Google Scholar]
- 42.Chern A, McCoy A, Brannock T, Martin GJ, Scouten WT, Porter CK, et al. Incidence and risk factors for disease and non-battle injury aboard the hospital ship USNS COMFORT during a Humanitarian Assistance and Disaster Response Mission, Continuing Promise 2011. Tropical Diseases, Travel Medicine and Vaccines. 2016;2(1):1–9. doi: 10.1186/s40794-016-0023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marimoutou C, Pommier de Santi V, Attrait X, Ollivier L, Michel R, Boutin JP. Self-reporting compared to prospective surveillance to evaluate the incidence of diarrhea among French Army personnel deployed to N’djamena, Chad. J Travel Med. 2011;18(3):217–20. doi: 10.1111/j.1708-8305.2011.00510.x. [DOI] [PubMed] [Google Scholar]
- 44.Brown JA, Riddle MS, Putnam SD, Schlett CD, Armstrong AW, Jones JJ, et al. Outcomes of diarrhea management in operations Iraqi Freedom and Enduring Freedom. Travel Med Infect Dis. 2009;7(6):337–43. doi: 10.1016/j.tmaid.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Letizia A, Riddle MS, Tribble D, Mostafa M, Monteville M, Armstrong A, et al. Effects of pre-deployment loperamide provision on use and travelers’ diarrhea outcomes among U.S. military personnel deployed to Turkey. Travel Med Infect Dis. 2014;12(4):360–3. doi: 10.1016/j.tmaid.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Monteville MR, Riddle MS, Baht U, Putnam SD, Frenck RW, Brooks K, et al. Incidence, etiology, and impact of diarrhea among deployed US military personnel in support of Operation Iraqi Freedom and Operation Enduring Freedom. Am J Trop Med Hyg. 2006;75(4):762–7. [PubMed] [Google Scholar]
- 47.Piyaphanee W, Kusolsuk T, Kittitrakul C, Suttithum W, Ponam T, Wilairatana P. Incidence and impact of travelers’ diarrhea among foreign backpackers in Southeast Asia: a result from Khao San road, Bangkok. J Travel Med. 2011;18(2):109–14. doi: 10.1111/j.1708-8305.2010.00484.x. [DOI] [PubMed] [Google Scholar]
- 48.Putnam SD, Sanders JW, Frenck RW, Monteville M, Riddle MS, Rockabrand DM, et al. Self-reported description of diarrhea among military populations in Operations Iraqi Freedom and Enduring Freedom. J Travel Med. 2006;13(2):92–9. doi: 10.1111/j.1708-8305.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 49.Reaves EJ, Kasper MR, Chimelski E, Klein ML, Valle R, Edgel KA, et al. Outbreak of gastrointestinal illness during Operation New Horizons in Pisco, Peru, July 2012. MSMR. 2012;19(11):17–9. [PubMed] [Google Scholar]
- 50.Riddle MS, Rockabrand DM, Schlett C, Monteville MR, Frenck RW, Romine M, et al. A prospective study of acute diarrhea in a cohort of United States military personnel on deployment to the Multinational Force and Observers, Sinai, Egypt. Am J Trop Med Hyg. 2011;84(1):59–64. doi: 10.4269/ajtmh.2011.10-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. Outbreak of acute gastroenteritis associated with Norwalk-like viruses among British military personnel--Afghanistan, May 2002. MMWR Morb Mortal Wkly Rep. 2002;51(22):477–9. [PubMed] [Google Scholar]
- 52.Connor P, Gutierrez RL. Update on military diarrhoea: current status and future plans. J R Army Med Corps. 2013;159(3):136–40. doi: 10.1136/jramc-2013-000091. [DOI] [PubMed] [Google Scholar]
- 53.Connor P. Military diarrhoea. J R Army Med Corps. 2009;155(3):233. [PubMed] [Google Scholar]
- 54.Connor P, Farthing MJ. Travellers’ diarrhoea: a military problem? J R Army Med Corps. 1999;145(2):95–101. doi: 10.1136/jramc-145-02-11. [DOI] [PubMed] [Google Scholar]
- 55.Armed Forces Health Surveillance Center. [accessed 23 January 2017];Armed Forces Reportable Medical Events Guidelines & Case Definitions. Available at http://www.health.mil/Reference-Center/Publications/2012/03/14/Armed-Forces-Reportable-Medical-Events-Guidelines.
- 56.Murray CK, Horvath LL. An approach to prevention of infectious diseases during military deployments. Clin Infect Dis. 2007;44(3):424–30. doi: 10.1086/510680. [DOI] [PubMed] [Google Scholar]
- 57.Aoun O, Roqueplo C, Rapp C. Spectrum and impact of health problems during deployment: a prospective, multicenter study of French soldiers operating in Afghanistan, Lebanon and Cote d’Ivoire. Travel Med Infect Dis. 2014;12(4):378–84. doi: 10.1016/j.tmaid.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Department of Defense. Directive 6490.02E. [accessed 25 October 2016];Comprehensive Health Surveillance. Available at http://www.dtic.mil/whs/directives/corres/pdf/649002e.pdf.
- 59.Navy and Marine Corps Public Health Center. [accessed 25 October 2016];Medical Surveillance and Medical Event Reporting Technical Manual. Available at http://www.public.navy.mil/surfor/Documents/6220_12_NMCPHC_TM.pdf.
- 60.Bureau of Medicine and Surgery. Medical Surveillance and Medical Event Reporting. Department of the Navy; [accessed 25 October 2016]. BUMED Instruction 6220.12C. Available at http://www.med.navy.mil/directives/ExternalDirectives/6220.12C.pdf. [Google Scholar]
- 61.Department of the Army. Pamphlet 40–11. [25 October 2016];Preventive Medicine. Available at http://8tharmy.korea.army.mil/safety/Toolbox/Resources/Publications/DAPam40_11.pdf;accessed.
- 62.Institute of Medicine. Nutrient Composition of Rations for Short-term, High-intensity Combat Operations. Washington DC: 2005. [accessed 17 Februar 2017]. Available at http://nationalacademies.org/hmd/reports/2005/nutrient-composition-of-rations-for-short-term-high-intensity-combat-operations.aspx. [Google Scholar]
- 63.Hawk D, Tribble DR, Riddle MS. Clinical treatment of nondysentery travelers’ diarrhea during deployment. Mil Med. 2010;175(3):140–6. doi: 10.7205/milmed-d-09-00190. [DOI] [PubMed] [Google Scholar]
- 64.Brown JD. Oral rehydration therapy for diarrhea. Mil Med. 1985;150(11):577–81. [PubMed] [Google Scholar]
- 65.Diarrhoeal Disease Control Programme. The selection of fluids and food for home therapy to prevent dehydration from diarrhoea: guidelines for developing a national policy. Geneva: 1993. [accessed 17 February 2017]. Available at http://apps.who.int/iris/bitstream/10665/62619/1/WHO_CDD_93.44.pdf. [Google Scholar]
- 66.World Health Organization. Oral rehydration salts: Production of the new ORS. Geneva: 2006. [accessed 17 February 2017]. Available at http://apps.who.int/iris/bitstream/10665/69227/1/WHO_FCH_CAH_06.1.pdf. [Google Scholar]
- 67.Casburn-Jones AC, Farthing MJ. Management of infectious diarrhoea. Gut. 2004;53(2):296–305. doi: 10.1136/gut.2003.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32(3):331–51. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 69.Caeiro JP, DuPont HL, Albrecht H, Ericsson CD. Oral rehydration therapy plus loperamide versus loperamide alone in the treatment of traveler’s diarrhea. Clin Infect Dis. 1999;28(6):1286–9. doi: 10.1086/514786. [DOI] [PubMed] [Google Scholar]
- 70.DuPont HL, Ericsson CD, Farthing MJ, Gorbach S, Pickering LK, Rombo L, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009;16(3):161–71. doi: 10.1111/j.1708-8305.2009.00300.x. [DOI] [PubMed] [Google Scholar]
- 71.Butler T. Loperamide for the treatment of traveler’s diarrhea: broad or narrow usefulness? Clin Infect Dis. 2008;47(8):1015–6. doi: 10.1086/591704. [DOI] [PubMed] [Google Scholar]
- 72.Ericsson CD. Nonantimicrobial agents in the prevention and treatment of traveler’s diarrhea. Clin Infect Dis. 2005;41(Suppl 8):S557–63. doi: 10.1086/432952. [DOI] [PubMed] [Google Scholar]
- 73.Johnson PC, Ericsson CD, DuPont HL, Morgan DR, Bitsura JA, Wood LV. Comparison of loperamide with bismuth subsalicylate for the treatment of acute travelers’ diarrhea. JAMA. 1986;255(6):757–60. [PubMed] [Google Scholar]
- 74.DuPont HL, Sullivan P, Pickering LK, Haynes G, Ackerman PB. Symptomatic treatment of diarrhea with bismuth subsalicylate among students attending a Mexican university. Gastroenterology. 1977;73(4 Pt 1):715–8. [PubMed] [Google Scholar]
- 75.Bierer DW. Bismuth subsalicylate: history, chemistry, and safety. Rev Infect Dis. 1990;12(Suppl 1):S3–8. doi: 10.1093/clinids/12.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 76.Ericsson CD, Feldman S, Pickering LK, Cleary TG. Influence of subsalicylate bismuth on absorption of doxycycline. JAMA. 1982;247(16):2266–7. [PubMed] [Google Scholar]
- 77.Kaplan MA, Prior MJ, McKonly KI, DuPont HL, Temple AR, Nelson EB. A multicenter randomized controlled trial of a liquid loperamide product versus placebo in the treatment of acute diarrhea in children. Clin Pediatr (Phila) 1999;38(10):579–91. doi: 10.1177/000992289903801003. [DOI] [PubMed] [Google Scholar]
- 78.Hanauer SB, DuPont HL, Cooper KM, Laudadio C. Randomized, double-blind, placebo-controlled clinical trial of loperamide plus simethicone versus loperamide alone and simethicone alone in the treatment of acute diarrhea with gas-related abdominal discomfort. Curr Med Res Opin. 2007;23(5):1033–43. doi: 10.1185/030079907x182176. [DOI] [PubMed] [Google Scholar]
- 79.DuPont HL, Hornick RB. Adverse effect of lomotil therapy in shigellosis. JAMA. 1973;226(13):1525–8. [PubMed] [Google Scholar]
- 80.Koo HL, Koo DC, Musher DM, DuPont HL. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48(5):598–605. doi: 10.1086/596711. [DOI] [PubMed] [Google Scholar]
- 81.Kean BH, Waters SR. The diarrhea of travelers. III. Drug prophylaxis in Mexico. N Engl J Med. 1959;261(2):71–4. doi: 10.1056/NEJM195907092610205. [DOI] [PubMed] [Google Scholar]
- 82.U.S. Food and Drug Administration. [accessed 25 October 2016];FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Available at http://www.fda.gov/Drugs/DrugSafety/ucm511530.htm.
- 83.U.S. Food and Drug Administration. [accessed 27 October 2016];FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. Available at http://www.fda.gov/downloads/Drugs/DrugSafety/UCM343347.pdf.
- 84.Salam I, Katelaris P, Leigh-Smith S, Farthing MJ. Randomised trial of single-dose ciprofloxacin for travellers’ diarrhoea. Lancet. 1994;344(8936):1537–9. doi: 10.1016/s0140-6736(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 85.Ericsson CD, DuPont HL, Mathewson JJ. Optimal dosing of ofloxacin with loperamide in the treatment of non-dysenteric travelers’ diarrhea. J Travel Med. 2001;8(4):207–9. doi: 10.2310/7060.2001.24244. [DOI] [PubMed] [Google Scholar]
- 86.Ericsson CD, Johnson PC, Dupont HL, Morgan DR, Bitsura JA, de la Cabada FJ. Ciprofloxacin or trimethoprim-sulfamethoxazole as initial therapy for travelers’ diarrhea. A placebo-controlled, randomized trial. Ann Intern Med. 1987;106(2):216–20. doi: 10.7326/0003-4819-106-2-216. [DOI] [PubMed] [Google Scholar]
- 87.Mattila L, Peltola H, Siitonen A, Kyronseppa H, Simula I, Kataja M. Short-term treatment of traveler’s diarrhea with norfloxacin: a double-blind, placebo-controlled study during two seasons. Clin Infect Dis. 1993;17(4):779–82. doi: 10.1093/clinids/17.4.779. [DOI] [PubMed] [Google Scholar]
- 88.European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2011. EFSA Journal. 2013;11:3196–555. doi: 10.2903/j.efsa.2020.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruiz J, Marco F, Oliveira I, Vila J, Gascon J. Trends in antimicrobial resistance in Campylobacter spp. causing traveler’s diarrhea. APMIS. 2007;115(3):218–24. doi: 10.1111/j.1600-0463.2007.apm_567.x. [DOI] [PubMed] [Google Scholar]
- 90.Bennish ML, Salam MA, Khan WA, Khan AM. Treatment of shigellosis: III. Comparison of one- or two-dose ciprofloxacin with standard 5-day therapy. A randomized, blinded trial. Ann Intern Med. 1992;117(9):727–34. doi: 10.7326/0003-4819-117-9-727. [DOI] [PubMed] [Google Scholar]
- 91.Adachi JA, Ericsson CD, Jiang ZD, DuPont MW, Martinez-Sandoval F, Knirsch C, et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis. 2003;37(9):1165–71. doi: 10.1086/378746. [DOI] [PubMed] [Google Scholar]
- 92.Kuschner RA, Trofa AF, Thomas RJ, Hoge CW, Pitarangsi C, Amato S, et al. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin Infect Dis. 1995;21(3):536–41. doi: 10.1093/clinids/21.3.536. [DOI] [PubMed] [Google Scholar]
- 93.Sanders JW, Frenck RW, Putnam SD, Riddle MS, Johnston JR, Ulukan S, et al. Azithromycin and loperamide are comparable to levofloxacin and loperamide for the treatment of traveler’s diarrhea in United States military personnel in Turkey. Clin Infect Dis. 2007;45(3):294–301. doi: 10.1086/519264. [DOI] [PubMed] [Google Scholar]
- 94.Tribble DR, Sanders JW, Pang LW, Mason C, Pitarangsi C, Baqar S, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44(3):338–46. doi: 10.1086/510589. [DOI] [PubMed] [Google Scholar]
- 95.Pfizer Laboratories. Azithromycin: Package insert and label information. Pfizer, Inc; 2016. [accessed 25 October 2016]. Available at http://labeling.pfizer.com/showlabeling.aspx?id=511. [Google Scholar]
- 96.Pepin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–60. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 97.Hoge CW, Gambel JM, Srijan A, Pitarangsi C, Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26(2):341–5. doi: 10.1086/516303. [DOI] [PubMed] [Google Scholar]
- 98.DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, et al. Rifaximin versus ciprofloxacin for the treatment of traveler’s diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis. 2001;33(11):1807–15. doi: 10.1086/323814. [DOI] [PubMed] [Google Scholar]
- 99.Infante RM, Ericsson CD, Jiang ZD, Ke S, Steffen R, Riopel L, et al. Enteroaggregative Escherichia coli diarrhea in travelers: response to rifaximin therapy. Clin Gastroenterol Hepatol. 2004;2(2):135–8. doi: 10.1016/s1542-3565(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 100.Steffen R, Sack DA, Riopel L, Jiang ZD, Sturchler M, Ericsson CD, et al. Therapy of travelers’ diarrhea with rifaximin on various continents. Am J Gastroenterol. 2003;98(5):1073–8. doi: 10.1111/j.1572-0241.2003.07283.x. [DOI] [PubMed] [Google Scholar]
- 101.Taylor DN, Bourgeois AL, Ericsson CD, Steffen R, Jiang ZD, Halpern J, et al. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74(6):1060–6. [PubMed] [Google Scholar]
- 102.Taylor DN, McKenzie R, Durbin A, Carpenter C, Haake R, Bourgeois AL. Systemic pharmacokinetics of rifaximin in volunteers with shigellosis. Antimicrob Agents Chemother. 2008;52(3):1179–81. doi: 10.1128/AAC.01108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riddle MS, Connor P, Fraser J, Porter CK, Swierczewski B, Hutley E, et al. Results from the Trial Evaluating Ambulatory Therapy of Travelers’ Diarrhea (TrEAT TD) Study: A randomized controlled trial comparing three single dose antibiotic regimens with loperamide. 65th Annual American Society of Tropical Medicine and Hygiene; Atlanta, GA. 2016; [accessed 17 February 2017]. Available at http://www.abstractsonline.com/pp8/#!/4114/presentation/1160. [Google Scholar]
- 104.Safdar N, Said A, Gangnon RE, Maki DG. Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis: a meta-analysis. JAMA. 2002;288(8):996–1001. doi: 10.1001/jama.288.8.996. [DOI] [PubMed] [Google Scholar]
- 105.Musher DM, Rubenstein AD. Permanent carriers of nontyphosa salmonellae. Arch Intern Med. 1973;132(6):869–72. [PubMed] [Google Scholar]
- 106.Norman FF, Perez-Molina J, Perez de Ayala A, Jimenez BC, Navarro M, Lopez-Velez R. Clostridium difficile-associated diarrhea after antibiotic treatment for traveler’s diarrhea. Clin Infect Dis. 2008;46(7):1060–3. doi: 10.1086/529380. [DOI] [PubMed] [Google Scholar]
- 107.Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis. 2016;63(3):310–8. doi: 10.1093/cid/ciw283. [DOI] [PubMed] [Google Scholar]
- 108.Kantele A, Mero S, Kirveskari J, Laaveri T. Increased risk for ESBL-producing bacteria from co-administration of loperamide and antimicrobial drugs for travelers’ diarrhea. Emerg Infect Dis. 2016;22(1):117–20. doi: 10.3201/eid2201.151272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, et al. Import and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17(1):78–85. doi: 10.1016/S1473-3099(16)30319-X. [DOI] [PubMed] [Google Scholar]
- 110.Eastridge BJ, Wade CE, Spott MA, Costanzo G, Dunne J, Flaherty S, et al. Utilizing a trauma systems approach to benchmark and improve combat casualty care. J Trauma. 2010;69(Suppl 1):S5–9. doi: 10.1097/TA.0b013e3181e421f3. [DOI] [PubMed] [Google Scholar]
- 111.Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2–7. [PubMed] [Google Scholar]
- 112.Ficke JR, Eastridge BJ, Butler F, Alvarez J, Brown T, Pasquina P, et al. Dismounted complex blast injury report of the Army Dismounted Complex Blast Injury Task Force. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S520–S34. [Google Scholar]
- 113.Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma. 2008;64(3 Suppl):S221–31. doi: 10.1097/TA.0b013e318163c40b. [DOI] [PubMed] [Google Scholar]
- 114.Blyth DM, Yun HC, Tribble DR, Murray CK. Lessons of war: Combat-related injury infections during the Vietnam War and Operation Iraqi and Enduring Freedom. J Trauma Acute Care Surg. 2015;79(4 Suppl 2):S227–S35. doi: 10.1097/TA.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hospenthal DR, Murray CK, Andersen RC, Bell RB, Calhoun JH, Cancio LC, et al. Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma. 2011;71(2 Suppl 2):S210–34. doi: 10.1097/TA.0b013e318227ac4b. [DOI] [PubMed] [Google Scholar]
- 116.Eardley WG, Brown KV, Bonner TJ, Green AD, Clasper JC. Infection in conflict wounded. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):204–18. doi: 10.1098/rstb.2010.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, et al. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: trauma infectious disease outcome study. J Trauma. 2011;71(1 Suppl):S33–S42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tribble DR, Li P, Warkentien TE, Lloyd BA, Schnaubelt ER, Ganesan A, et al. Impact of operational theater on combat and noncombat trauma-related infections. Mil Med. 2016;181(10):1258–68. doi: 10.7205/MILMED-D-15-00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calhoun JH, Murray CK, Manring MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. 2008;466(6):1356–62. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11(8):1218–24. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44(12):1577–84. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 122.Murray CK, Blyth D. Acquisition of multidrug-resistant organisms during travel. Mil Med. 2017 doi: 10.7205/MILMED-D-17-00067. XXXX. [DOI] [PubMed] [Google Scholar]
- 123.Al-Abri SS, Beeching NJ, Nye FJ. Traveller’s diarrhoea. Lancet Infect Dis. 2005;5(6):349–60. doi: 10.1016/S1473-3099(05)70139-0. [DOI] [PubMed] [Google Scholar]
- 124.Barrett J, Brown M. Travellers’ diarrhoea. BMJ. 2016;353:i1937. doi: 10.1136/bmj.i1937. [DOI] [PubMed] [Google Scholar]
- 125.Shanks GD, Smoak BL, Aleman GM, Oundo J, Waiyaki PG, Dunne MW, et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis. 1999;29(4):942–3. doi: 10.1086/520469. [DOI] [PubMed] [Google Scholar]
- 126.Khan WA, Seas C, Dhar U, Salam MA, Bennish ML. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Ann Intern Med. 1997;126(9):697–703. doi: 10.7326/0003-4819-126-9-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 127.Pollett S, Rocha C, Zerpa R, Patino L, Valencia A, Camina M, et al. Campylobacter antimicrobial resistance in Peru: a ten-year observational study. BMC Infect Dis. 2012;12:193. doi: 10.1186/1471-2334-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hakanen A, Jousimies-Somer H, Siitonen A, Huovinen P, Kotilainen P. Fluoroquinolone resistance in Campylobacter jejuni isolates in travelers returning to Finland: association of ciprofloxacin resistance to travel destination. Emerg Infect Dis. 2003;9(2):267–70. doi: 10.3201/eid0902.020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stockdale AJ, Beeching NJ, Anson J, Beadsworth MB. Emergence of extensive fluoroquinolone resistance in Campylobacter gastroenteritis in Liverpool, UK. J Infect. 2016;72(3):398–400. doi: 10.1016/j.jinf.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Chattaway MA, Aboderin AO, Fashae K, Okoro CK, Opintan JA, Okeke IN. Fluoroquinolone-resistant enteric bacteria in Sub-Saharan Africa: clones, implications and research needs. Front Microbiol. 2016;7:558. doi: 10.3389/fmicb.2016.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Al-Mashhadani M, Hewson R, Vivancos R, Keenan A, Beeching NJ, Wain J, et al. Foreign travel and decreased ciprofloxacin susceptibility in Salmonella enterica infections. Emerg Infect Dis. 2011;17(1):123–5. doi: 10.3201/eid1701.100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mensa L, Marco F, Vila J, Gascon J, Ruiz J. Quinolone resistance among Shigella spp. isolated from travellers returning from India. Clin Microbiol Infect. 2008;14(3):279–81. doi: 10.1111/j.1469-0691.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- 133.Pons MJ, Gomes C, Martinez-Puchol S, Ruiz L, Mensa L, Vila J, et al. Antimicrobial resistance in Shigella spp. causing traveller’s diarrhoea (1995–2010): a retrospective analysis. Travel Med Infect Dis. 2013;11(5):315–9. doi: 10.1016/j.tmaid.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 134.Bowen A, Grass J, Bicknese A, Campbell D, Hurd J, Kirkcaldy RD. Elevated risk for antimicrobial drug-resistant Shigella infection among men who have sex with men, United States, 2011–2015. Emerg Infect Dis. 2016;22(9):1613–6. doi: 10.3201/eid2209.160624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gaudreau C, Pilon PA, Cornut G, Marchand-Senecal X, Bekal S. Shigella flexneri with ciprofloxacin resistance and reduced azithromycin susceptibility, Canada, 2015. Emerg Infect Dis. 2016;22(11):2016–18. doi: 10.3201/eid2211.160883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hopkins S. Clinical toleration and safety of azithromycin. Am J Med. 1991;91(3A):40S–45S. doi: 10.1016/0002-9343(91)90401-i. [DOI] [PubMed] [Google Scholar]
- 137.Zuckerman JM, Qamar F, Bono BR. Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am. 2009;23(4):997–1026. ix–x. doi: 10.1016/j.idc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 138.Waugh MA. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J Antimicrob Chemother. 1993;31(Suppl E):193–8. doi: 10.1093/jac/31.suppl_e.193. [DOI] [PubMed] [Google Scholar]
- 139.Lister PJ, Balechandran T, Ridgway GL, Robinson AJ. Comparison of azithromycin and doxycycline in the treatment of non-gonococcal urethritis in men. J Antimicrob Chemother. 1993;31(Suppl E):185–92. doi: 10.1093/jac/31.suppl_e.185. [DOI] [PubMed] [Google Scholar]
- 140.Treadway G, Pontani D, Reisman A. The safety of azithromycin in the treatment of adults with community-acquired respiratory tract infections. Int J Antimicrob Agents. 2002;19(3):189–94. doi: 10.1016/s0924-8579(01)00490-3. [DOI] [PubMed] [Google Scholar]
- 141.Owens RC, Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(Suppl 2):S144–57. doi: 10.1086/428055. [DOI] [PubMed] [Google Scholar]
- 142.von Muhlendahl KE, Bunjes R, Krienke EG. Loperamide-induced ileus. Lancet. 1980;1(8161):209. doi: 10.1016/s0140-6736(80)90697-2. [DOI] [PubMed] [Google Scholar]
- 143.Oldfield EC., 3rd Is loperamide contraindicated in the treatment of bacillary dysentery? Am J Gastroenterol. 1995;90(2):327–8. [PubMed] [Google Scholar]
- 144.Meuris B. Observational study of travelers’ diarrhea. J Travel Med. 1995;2(1):11–15. doi: 10.1111/j.1708-8305.1995.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 145.Silberschmidt G, Schick MT, Steffen R, Kilpatrick ME, Murphy JR, Oyofo BA, et al. Treatment of travellers’ diarrhoea: zaldaride compared with loperamide and placebo. Eur J Gastroenterol Hepatol. 1995;7(9):871–5. [PubMed] [Google Scholar]
- 146.Wang HH, Shieh MJ, Liao KF. A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhea in adults. World J Gastroenterol. 2005;11(10):1540–3. doi: 10.3748/wjg.v11.i10.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murphy GS, Bodhidatta L, Echeverria P, Tansuphaswadikul S, Hoge CW, Imlarp S, et al. Ciprofloxacin and loperamide in the treatment of bacillary dysentery. Ann Intern Med. 1993;118(8):582–6. doi: 10.7326/0003-4819-118-8-199304150-00002. [DOI] [PubMed] [Google Scholar]
- 148.van Loon FP, Bennish ML, Speelman P, Butler C. Double blind trial of loperamide for treating acute watery diarrhoea in expatriates in Bangladesh. Gut. 1989;30(4):492–5. doi: 10.1136/gut.30.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ericsson CD, DuPont HL, Mathewson JJ, West MS, Johnson PC, Bitsura JA. Treatment of traveler’s diarrhea with sulfamethoxazole and trimethoprim and loperamide. JAMA. 1990;263(2):257–61. [PubMed] [Google Scholar]
- 150.Petruccelli BP, Murphy GS, Sanchez JL, Walz S, DeFraites R, Gelnett J, et al. Treatment of traveler’s diarrhea with ciprofloxacin and loperamide. J Infect Dis. 1992;165(3):557–60. doi: 10.1093/infdis/165.3.557. [DOI] [PubMed] [Google Scholar]
- 151.Taylor DN, Sanchez JL, Candler W, Thornton S, McQueen C, Echeverria P. Treatment of travelers’ diarrhea: ciprofloxacin plus loperamide compared with ciprofloxacin alone. A placebo-controlled, randomized trial. Ann Intern Med. 1991;114(9):731–4. doi: 10.7326/0003-4819-114-9-731. [DOI] [PubMed] [Google Scholar]
- 152.Robinson P, Jenney AW, Tachado M, Yung A, Manitta J, Taylor K, et al. Imported malaria treated in Melbourne, Australia: epidemiology and clinical features in 246 patients. J Travel Med. 2001;8(2):76–81. doi: 10.2310/7060.2001.24309. [DOI] [PubMed] [Google Scholar]
- 153.de Laval F, Oliver M, Rapp C, Pommier de Santi V, Mendibil A, Deparis X, et al. The challenge of diagnosing Plasmodium ovale malaria in travellers: report of six clustered cases in French soldiers returning from West Africa. Malar J. 2010;9:358. doi: 10.1186/1475-2875-9-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cordina CJ, Culleton R, Jones BL, Smith CC, MacConnachie AA, Coyne MJ, et al. Plasmodium knowlesi: clinical presentation and laboratory diagnosis of the first human case in a Scottish traveler. J Travel Med. 2014;21(5):357–60. doi: 10.1111/jtm.12131. [DOI] [PubMed] [Google Scholar]
- 155.Hussain WM, Bukhari SZ, Fatani MI, Karima TM, Madani TA, Badreddine S. Misdiagnosis of an imported case of malaria caused by Plasmodium falciparum. J Infect Dev Ctries. 2009;3(2):112–4. doi: 10.3855/jidc.58. [DOI] [PubMed] [Google Scholar]
- 156.Humphries RM, Linscott AJ. Laboratory diagnosis of bacterial gastroenteritis. Clin Microbiol Rev. 2015;28(1):3–31. doi: 10.1128/CMR.00073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee HM, Lee S, Lee BI, Jekarl DW, Song JY, Choi HJ, et al. Clinical significance of fecal lactoferrin and multiplex polymerase chain reaction in patients with acute diarrhea. Gut Liver. 2015;9(5):636–40. doi: 10.5009/gnl14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.DuPont HL. Guidelines on acute infectious diarrhea in adults. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1997;92(11):1962–75. [PubMed] [Google Scholar]
- 159.Libman MD, Gyorkos TW, Kokoskin E, Maclean JD. Detection of pathogenic protozoa in the diagnostic laboratory: result reproducibility, specimen pooling, and competency assessment. J Clin Microbiol. 2008;46(7):2200–5. doi: 10.1128/JCM.01666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raich TJ, Powell S. The changing landscape of diagnostic testing for diarrheal disease. MLO Med Lab Obs. 2014;46(4):36, 38. [PubMed] [Google Scholar]
- 161.Goldenberg SD, Bacelar M, Brazier P, Bisnauthsing K, Edgeworth JD. A cost benefit analysis of the Luminex xTAG Gastrointestinal Pathogen Panel for detection of infectious gastroenteritis in hospitalised patients. J Infect. 2015;70(5):504–11. doi: 10.1016/j.jinf.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 162.Drancourt M. Multiplex testing of diarrhoea breaks down microbial barriers. Lancet Infect Dis. 2014;14(8):663–4. doi: 10.1016/S1473-3099(14)70844-8. [DOI] [PubMed] [Google Scholar]
- 163.Halligan E, Edgeworth J, Bisnauthsing K, Bible J, Cliff P, Aarons E, et al. Multiplex molecular testing for management of infectious gastroenteritis in a hospital setting: a comparative diagnostic and clinical utility study. Clin Microbiol Infect. 2014;20(8):O460–7. doi: 10.1111/1469-0691.12476. [DOI] [PubMed] [Google Scholar]
- 164.Cheun HI, Chung BS, Ma DW, Goo BL, Cho SH, Ji MJ, et al. Development of a diagnostic kit to detect Cryptosporidium parvum and Giardia lamblia. Osong Public Health Res Perspect. 2013;4(3):146–51. doi: 10.1016/j.phrp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saigal K, Khurana S, Sharma A, Sehgal R, Malla N. Comparison of staining techniques and multiplex nested PCR for diagnosis of intestinal microsporidiosis. Diagn Microbiol Infect Dis. 2013;77(3):248–9. doi: 10.1016/j.diagmicrobio.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 166.Antikainen J, Kantele A, Pakkanen SH, Laaveri T, Riutta J, Vaara M, et al. A quantitative polymerase chain reaction assay for rapid detection of 9 pathogens directly from stools of travelers with diarrhea. Clin Gastroenterol Hepatol. 2013;11(10):1300–07. e3. doi: 10.1016/j.cgh.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 167.McAuliffe GN, Anderson TP, Stevens M, Adams J, Coleman R, Mahagamasekera P, et al. Systematic application of multiplex PCR enhances the detection of bacteria, parasites, and viruses in stool samples. J Infect. 2013;67(2):122–9. doi: 10.1016/j.jinf.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 168.Liu J, Kibiki G, Maro V, Maro A, Kumburu H, Swai N, et al. Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. J Clin Virol. 2011;50(4):308–13. doi: 10.1016/j.jcv.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buchan BW, Olson WJ, Pezewski M, Marcon MJ, Novicki T, Uphoff TS, et al. Clinical evaluation of a real-time PCR assay for identification of Salmonella, Shigella, Campylobacter (Campylobacter jejuni and C. coli), and shiga toxin-producing Escherichia coli isolates in stool specimens. J Clin Microbiol. 2013;51(12):4001–7. doi: 10.1128/JCM.02056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Department of Defense. [accessed 25 October 2016];DoD Instruction 6200.02: Application of Food and Drug Administration (FDA) Rules to Department of Defense Force Health Protection Programs. Available at http://www.dtic.mil/whs/directives/corres/pdf/620002p.pdf.
- 171.Porter CK, Thura N, Riddle MS. Quantifying the incidence and burden of postinfectious enteric sequelae. Mil Med. 2013;178(4):452–69. doi: 10.7205/MILMED-D-12-00510. [DOI] [PubMed] [Google Scholar]