Abstract
Objective
To determine the relationship between the production of cell-free plasma hemoglobin (PHb) and acute kidney injury (AKI) in infants and children undergoing cardiopulmonary bypass (CPB) for cardiac surgery.
Design
Prospective observational study
Setting
12-bed cardiac ICU in a university-affiliated children’s hospital
Patients
Children were prospectively enrolled during their pre-operative outpatient appointment with the following criteria: >1month to <18 years old, procedures requiring CPB, no preexisting renal dysfunction.
Interventions
None
Measurements and Main Results
Plasma and urine were collected at baseline (in a subset), the beginning and end of CPB, and 2h and 24h after CPB in 60 subjects. Levels of PHb increased during CPB and were associated (p<0.01) with CPB duration (R2=0.22), depletion of Hp at end and 24h after CPB (R2=0.12 and 0.15, respectively), LDH levels at end CPB (R2=0.27), and change in creatinine (R2=0.12). 43% of patients developed AKI. There was an association between PHb level and change in creatinine that varied by age (overall [R2=0.12, p<0.01], in age >2yrs [R2=0.22, p<0.01], and in <2yrs [R2=0.03, p=0.42]). Change in PHb and male gender were found to be risk factors for AKI (OR 1.02 and OR 3.78, p<0.05).
Conclusions
Generation of PHb during CPB and male gender are associated with subsequent renal dysfunction in low risk pediatric patients, especially in those >2yrs of age. Further studies are needed to determine whether specific subgroups of pediatric patients undergoing CPB would benefit from potential treatments for hemolysis and PHb-associated renal dysfunction.
Keywords: hemolysis, cell-free plasma hemoglobin, creatinine, acute kidney injury, cardiopulmonary bypass, pediatrics
INTRODUCTION
Cardiopulmonary bypass (CPB) during pediatric cardiac surgery facilitates the palliation or correction of congenital heart defects. However, the unfavorable sequelae of CPB-supported cardiac surgery remain complex and incompletely understood. Post-operative acute kidney injury (AKI) is a common complication of CPB reported in up to 52% of cardiac surgeries in pediatric studies, which usually include neonates and cyanotic lesions [1–3]. AKI independently predicts mortality and is associated with longer length of stays in critically ill pediatric patients [4–7]. The long-term sequelae of CPB-mediated AKI and its impact in the setting of multiple surgeries also remain to be defined.
The pathophysiology of AKI after CPB is likely multifactorial. Possible contributors include hypoperfusion or ischemia-reperfusion induced inflammation. Age, particularly neonates, and CPB duration are associated with increased risk of AKI [3, 8, 9]. Because AKI can occur without measurable hypoperfusion, its association with longer CPB durations suggest that CPB can directly injure the kidney. One potential mechanism for this is increased hemolysis resulting from longer CPB durations. Recent data suggest that cell-free plasma hemoglobin (PHb) increases nitric oxide (NO) consumption, augments oxidative damage, and causes vascular dysfunction [10–12].
The relative contribution of PHb to CPB-associated AKI in pediatric patients is unclear. Many patients with congenital heart disease have pre-surgical hemodynamic compromise, cyanosis, or other complications that can lead to AKI. In order to better understand the specific effects of CPB, we studied a group of pediatric patients undergoing semi-elective cardiac surgery. The primary objective of this study was to determine the relationship between the production of PHb and AKI, while accounting for other risk factors, in this relatively healthy pediatric population.
MATERIALS AND METHODS
This was a prospective study approved by the Institutional Review Board at the University of Pittsburgh. Patients were enrolled during their outpatient pre-surgery clinic visits at the Children’s Hospital of Pittsburgh (CHP) between May 2012 and September 2016. Inclusion criteria were age <18yrs and a scheduled procedure requiring CPB. Exclusion criteria were neonatal age, preexisting renal dysfunction, and pregnancy.
All CPB involved the use of a roller pump (Stockart SIII; Sorin Group, Arvada, CO). Blood flow was based on a cardiac index of 2.5–3 L/min/m2, cardiotomy suction catheters were used, and core temperatures were 32–35 °C. A circuit blood prime was used for patients <25kg or when the expected diluted hematocrit was <25%.
Blood and urine were collected at the start (StartCPB) and end of CPB (EndCPB) and 2h (2hREP) and 24h after reperfusion (24hREP). Blood samples were collected from the venous side of the CPB circuit during surgery or from a central venous or arterial catheter after reperfusion. In a subset of 40 subjects, baseline samples were collected upon insertion of a central venous catheter.
Demographic and clinical data collected included: age, gender, weight, surgical procedure, Kidney Disease: Improving Global Outcomes (KDIGO) score [13], the Risk Adjusted classification for Congenital Heart Surgery (RACHS-1) score [14], CPB duration, cross-clamp duration, mechanical ventilation days, and ICU (ICULOS) and hospital length of stay (HospLOS). Baseline creatinine levels were collected at the time of enrollment.
AKI was defined as an increase in serum creatinine (SCr) of ≥1.5 times baseline at any point during hospitalization as described in the KDIGO guidelines [13]. Urine output was intentionally not used in our definition due to variability in quantification (early removal of urinary catheters, use of absorbent diapers, and variable physician-dependent diuretic use).
PHb (normal <6mg/dL), haptoglobin (Hp), and LDH levels were determined in the CHP clinical labs. Lactate and SvO2 were obtained from the medical record and were generally only available for 24hREP. The vasoactive-inotropic score (VIS score) was calculated as previously described [15].
Statistical analysis
Categorical variables are presented as frequencies and compared with a chi-squared test or Fisher’s exact test when appropriate. Continuous variables are presented as median, interquartile range (IQR) and compared with the Mann-Whitney U test.
In order to account for the repeated measures and clustering of data within subjects across time points of PHb, Hp, and LDH, we used time-course analyses with general linear models and the longitudinal data modules of STATA (xt). Simple linear regressions were used to evaluate the association of changes in Hp and LDH with changes in PHb from StartCPB to EndCPB (ΔPHb).
Our primary outcome of AKI was analyzed as both a continuous (fold change in creatinine, foldΔCr) and categorical variable (Stage 0 = no AKI, Stage ≥1 = AKI). We used a simple linear regression to explore the relationship between foldΔCr and ΔPHb. As renal maturity does not fully occur until 2yrs [16], we included an interaction between ΔPHb and age. Backward stepwise regression analysis was used to identify risk factors associated with AKI. Risk factors with p≤0.1 in bivariate analysis were included in a multivariate logistic regression model. Keeping ΔPHb in the model, variables with the weakest adjusted associations with AKI (by Wald test and with a p to remove of 0.1) were removed from the multivariate model if their elimination did not significantly reduce the goodness of fit.
Pearson product correlations were completed for LOS data and variables from Table 1 that were different between groups. Effect size was calculated by Cohen’s d, the area under the receiver operating characteristic curve (AUC) and the Hosmer-Lemeshow (H-L) goodness of fit test.
Table 1.
Baseline characteristics by AKI
| Characteristic | All patients (n=60) | Non-AKI (34/60, 57%) | AKI (26/60, 43%) | p |
|---|---|---|---|---|
| Age (in Years) | 2.51 (0.48–10.52) | 5.12 (0.52–12.63) | 1.66 (0.38–4.74) | 0.10 |
| Weight (kg) | 12.3 (6.3–30.9) | 14.2 (6.3–40.4) | 9.9 (6.0–15.9) | 0.11 |
| Gender - Male | 30 (50%) | 13 (38%) | 17 (65%) | 0.04 |
| Change in PHb (mg/dL) | 43.8 (31.3–83.4) | 38.25 (18.5–60.6) | 53.9 (39.7–86.7) | 0.03 |
| CPB duration (min) | 78 (59–109) | 72.5 (48–91) | 97.5 (71–111) | 0.03 |
| Cross-clamp duration (min) | 38 (22.5–61.5) | 32 (23–52) | 52.5 (21–72) | 0.33 |
| Blood prime | 45/60 (75%) | 24/34 (71%) | 21/26 (81%) | 0.37 |
| RACHS-1 | 0.06 | |||
| Risk Category 1 | 6 (10%) | 6 (18%) | 0 (0%) | |
| Risk Category 2 | 23 (38%) | 14 (41%) | 9 (35%) | |
| Risk Category 3 | 22 (37%) | 9 (26%) | 13 (50%) | |
| Risk Category 4 | 9 (15%) | 5 (15%) | 4 (15%) | |
| VIS score (highest score) | ||||
| In first 24h after CPB | 10 (6–13) | 10 (5–12.5) | 12 (10–14) | 0.07 |
| 24–48h after CPB | 2 (0–5.5) | 0 (0–5) | 5 (1–7.5) | 0.01 |
| Lactate (highest) | ||||
| In first 24h after CPB | 3.1 (2.4–4.1) | 3.1 (2.3–4.3) | 3.1 (2.5–3.8) | 0.99 |
| SvO2 (lowest) | ||||
| In first 24h after CPB | 60.5 (50.5–72) | 67 (54–73) | 57.5 (44–64) | 0.02 |
| Mechanical ventilation (days) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0.09 |
| ICU LOS (days) | 2 (1–6) | 1 (1–3) | 4 (2–10) | <0.01 |
| Hospital LOS (days) | 5 (3–9) | 4 (3–6) | 9 (5–16) | <0.01 |
Continuous data described as median (IQR). All other data described as proportion (percentage).
An alpha-error rate of 0.05 was selected for analyses which were completed using STATA 14.0 (StataCorp, College Park, TX). Figures were created using GraphPad Prism 7.00 (GraphPad Software, San Diego, CA).
RESULTS
Demographic and clinical characteristics of 60 subjects are listed in Table 1. There was no mortality. Subjects with AKI were more often male, had higher ΔPHb, longer CPB duration, higher VIS scores 24REP-48hREP, lower SvO2 within 24hREP, and longer ICU and HospLOS.
Generation of cell-free plasma hemoglobin (PHb) during CPB and indicators of hemolysis
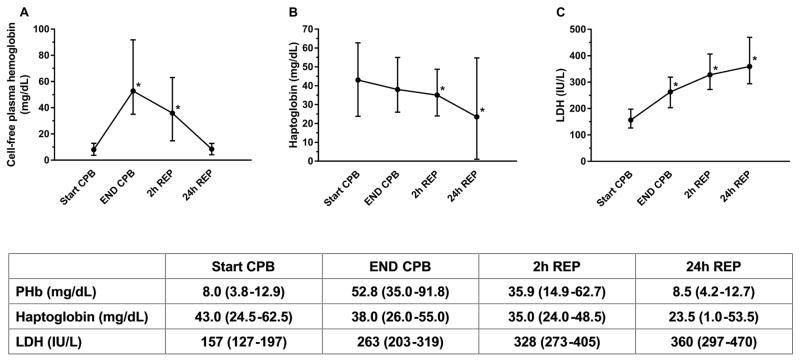
PHb levels increased during CPB and returned to StartCPB levels by 24hREP (Figure 1A). CPB duration was associated with ΔPHb (R2=0.22, p<0.01). In the subset of 40 subjects with baseline levels, there was no difference in PHb between baseline (median [IQR], 5.9mg/dL [2.9–13.0]) and StartCPB (7.8mg/dL [3.8–12.8]).
Figure 1.
(A) Cell-free plasma hemoglobin (PHb) levels increased during cardiopulmonary bypass (CPB) and returned to baseline by 24h reperfusion (REP). There was a difference (* p<.01) in PHb levels at EndCPB and 2hREP from StartCPB. (B) Haptoglobin levels (Hp) decreased during CPB and continued to fall for 24h after reperfusion. There was a significant difference (* p<.01) in Hp levels at 2hREP and 24hREP from StartCPB. (C) Lactate dehydrogenase (LDH) levels increased during CPB and continued to rise for 24h after reperfusion. There was a difference (* p<.01) in LDH levels at EndCPB, 2hREP, and 24hREP from StartCPB. Data is presented as median (IQR).
Median ΔPHb in 45 subjects with a blood prime and 15 subjects without was 48.8mg/dL (35.6–86.4) and 33.9mg/dL (15–47.9), respectively, with no difference (p>0.05) between groups.
Haptoglobin (Hp) levels decreased during CPB and declined until 24hREP (Figure 1B). Change in Hp levels from StartCPB to EndCPB and to 24hREP was associated with ΔPHb (R2=0.12, p<0.01 and R2=0.15, p<0.01, respectively).
LDH levels increased during CPB and rose until 24hREP (Figure 1C). Change in LDH levels from StartCPB to EndCPB was associated with ΔPHb (R2=0.27, p<0.01).
Evaluation of renal dysfunction
26/60 subjects (43%) met criteria for AKI [Stage 1 (18/26, 69%), Stage 2 (4/26, 15%), Stage 3 (4/26, 15%)] by change in SCr levels as defined by the KDIGO guidelines [13]. No subjects required renal replacement therapy.
Creatinine peaked within 48hREP in 92% of subjects (50 within 24hREP, 5 between 24hREP-48hREP, and 5 after 48hREP). Of the 26 patients with AKI, 17/26 (65%) developed AKI within 24hREP (65%), 4/26 (15%) between 24hREP-48hREP, and 5/26 (19%) after 48hREP.
Association of cell-free plasma hemoglobin with renal dysfunction
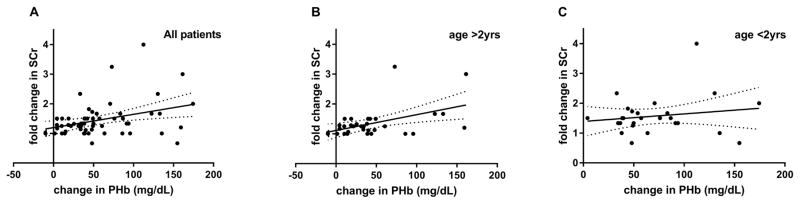
Overall, foldΔCr was associated with ΔPHb (R2=0.12, p<0.01, Figure 2A) which was also demonstrated in subjects >2yrs (R2=0.22, p<0.01, Figure 2B) but not in subjects <2yrs (R2=0.03, p=0.42, Figure 2C).
Figure 2.
(A) There was an overall association between foldΔCr with ΔPHb (R2=0.12, p<.01). This association persisted in patients aged >2yrs (R2=0.22, p<.01) (B) but was not significant in patients <2yrs (R2=0.03, p=0.42) (C).
Using AKI as a dichotomous outcome variable, age, male gender, ΔPHb, CPB duration, RACHS-1 score, highest VIS score within 24hREP and between 24hREP-48hREP, and highest lactate and lowest SvO2 within 24hREP were entered into a backward stepwise logistic regression analysis. ΔPHb (OR 1.02, p<0.05) and male gender (OR 3.78, p<0.05) were identified as risk factors for AKI (Table 2).
Table 2.
Factors independently associated with AKI by KDIGO
| Unadjusted analysis | Adjusted analysis* | |||
|---|---|---|---|---|
|
| ||||
| Risk Factor | OR (95% CI) | p | OR (95% CI) | p |
| Age (yrs) | 0.93 (.85–1.02) | 0.10 | ||
| Male gender | 3.05 (1.05–8.84) | 0.04 | 3.78 (1.17–12.25) | 0.03 |
| Change in PHb (mg/dL) | 1.01 (1.00–1.02) | 0.07 | 1.02 (1.00–1.03) | 0.03 |
| CPB duration (min) | 1.02 (1.00–1.03) | 0.03 | ||
| RACHS-1 score | 1.82 (0.96–3.43) | 0.07 | ||
| VIS score (highest) in first 24h after CPB | 1.13 (1.00–1.29) | 0.05 | ||
| VIS score (highest) 24–48h after CPB | 1.12 (0.99–1.26) | 0.06 | ||
| Lactate (highest) in first 24h after CPB | 0.98 (0.74–1.30) | 0.89 | ||
| SvO2 (lowest) in first 24h after CPB | 0.95 (0.92–0.99) | 0.02 | ||
Adjusted for (age, male gender, change in PHb, CPB duration, RACHS-1 score, highest VIS score in first 24h after CPB, highest VIS score 24–48h after CPB, lowest SvO2 in first 24h after CPB)
When repeating this analysis in subjects >2yrs, ΔPHb (OR 1.04, p<.05) and male gender (OR 26.01, p<.05) were again identified as risk factors for AKI (Table 3). None of the variables showed a relationship with AKI in the subset of subjects <2yrs (Supplemental Digital Content – Table 1).
Table 3.
Factors independently associated with AKI by KDIGO in subjects >2yrs
| Unadjusted analysis | Adjusted analysis* | |||
|---|---|---|---|---|
|
| ||||
| Risk Factor | OR (95% CI) | p | OR (95% CI) | p |
| Age (yrs) | 0.99 (.87–1.13) | 0.85 | ||
| Male gender | 6.22 (1.07–36.21) | 0.04 | 26.01 (1.13–599.36) | 0.04 |
| Change in PHb (mg/dL) | 1.02 (1.00–1.04) | 0.06 | 1.04 (1.00–1.07) | 0.03 |
| CPB duration (min) | 1.02 (1.00–1.04) | 0.04 | ||
| RACHS-1 score | 2.79 (1.06–7.37) | 0.04 | ||
| VIS score (highest) in first 24h after CPB | 1.25 (1.01–1.54) | 0.04 | ||
| VIS score (highest) 24–48h after CPB | 1.22 (0.95–1.55) | 0.12 | ||
| Lactate (highest) in first 24h after CPB | 1.15 (0.82–1.60) | 0.42 | ||
| SvO2 (lowest) in first 24h after CPB | 0.93 (0.86–1.01) | 0.07 | ||
Adjusted for (male gender, change in PHb, CPB duration, RACHS-1 score, highest VIS score in first 24h after CPB, lowest SvO2 in first 24h after CPB)
There were no differences in the variables of interest between males and females (Supplemental Digital Content-Table 2).
Length of stay
Subjects with AKI had longer ICULOS and HospLOS (median 4d vs. 1d and 9d vs. 4d, respectively). ICULOS correlated with HospLOS in both non-AKI and AKI groups (ρ=0.66 and 0.79, respectively). In the non-AKI group, ICULOS correlated with CPB duration, RACHS-1 score, highest VIS score (within 24hREP and 24hREP-48hREP), and days of mechanical ventilation whereas Hosp LOS correlated with RACHS-1 score and highest VIS score within 24hREP. In the AKI group, ICULOS correlated with lowest SvO2 within 24hREP and days of mechanical ventilation whereas HospLOS was only correlated with days of mechanical ventilation (Supplemental Digital Content – Table 3).
Effect size
In this study, overall, PHb was associated with AKI as measured by foldΔCr with a medium effect size as calculated by Cohen’s d = 0.50 which improved to 0.79 in the subset of subjects who were >2yrs of age. Likewise, the AUC=0.69 and the H-L test p=0.42 in our whole population improved to AUC=0.84 and the H-L test p=0.87 in the >2yrs subset.
DISCUSSION
Our study in patients with few pre-existing comorbidities suggests that: i) PHb levels rise during CPB; ii) AKI is common in low risk CPB patients; iii) PHb and male gender are associated with AKI; and iv) PHb is likely more important a risk factor for AKI in patients >2yrs.
PHb levels at EndCPB were higher than the upper limit measured during vaso-occlusive crisis in sickle cell patients (20μM [32mg/dL]) and the level associated with an 80% reduction of forearm blood flow in response to nitroprusside (6μM [9.7mg/dL]) [17, 18]. Thus, PHb produced during CPB is likely biologically active.
Consistent with prior studies, ΔPHb was associated with CPB duration [3, 8, 19], likely from the mechanical destruction of red cells. Release of PHb disrupts the biological effects of nitric oxide (NO) and contributes to oxidative injury [12, 20–22]. NO maintains normal vascular tone and is scavenged by PHb, which reduces its bioavailability and impairs vascular function. This has been shown in several hemolysis-associated conditions (sickle cell disease and renal replacement therapy) [17, 21–23] and in studies involving animal models [24] and stored blood [25]. PHb was associated with NO consumption and biomarkers of renal and intestinal injury in adult CPB [26]. Children who received NO during CPB had shorter durations of mechanical ventilation and ICULOS [12]. PHb’s role in oxidative stress lies, partly, in its peroxidase activity [27] which is influenced by reducing agents such as NO. In the presence of oxidizing agents, Hp bound hemoglobin also has peroxidase activity [27, 28]. Supporting this, PHb correlated with increased lipid peroxidation after CPB in both adults and children [11, 20].
There is conflicting evidence that transfusion of stored blood can lead to hemolysis [29–31] and so far, there is no evidence that a blood prime causes higher PHb levels. Many of our subjects (45/60, 75%) received a circuit blood prime with no difference in ΔPHb from those who did not. There was also no association between ΔPHb and RACHS-1 score. Thus, blood prime and surgery complexity did not contribute to PHb generation.
Most pediatric CPB studies include neonates, high RACHS-1 scores (5 and 6), pre-surgical ICU admissions, and cyanotic lesions. The presence of these comorbidities makes it difficult to define the specific contribution of PHb to AKI. A pediatric study involving 311 patients, which did not include neonates or high RACHS-1 scores, found a 42% incidence of AKI that was associated with younger age and longer CPB duration; PHb was not measured [9]. We attempted to create a homogenous sample with limited confounders by recruiting subjects through the cardiothoracic surgery clinic who are not neonates, generally without cyanosis, and well enough to be home without baseline renal dysfunction. 43% of our patients met criteria for AKI. Consistent with prior studies, a majority met criteria within 48hREP [2, 3, 9]. Thus, AKI was likely CPB-related and not secondary to later post-surgical complications. Our population did not include patients previously implicated to be at higher risk for AKI (i.e. neonates), yet we found a similar incidence (40–51%) [2, 3, 9]. This is an unexpected yet important finding and suggests that this phenomenon affects even low risk patients and likely represents a substantial problem.
PHb is an additional plausible contributor to CPB-associated AKI. In guinea pigs, hemolysis, vascular injury, and kidney dysfunction was attenuated by the Hb scavenger, haptoglobin [32]. In adult sickle cell patients, hemolysis is associated with progression of chronic kidney disease [33]. The production of PHb has been associated with renal injury in both adult [11, 26, 34] and pediatric [3] cardiac surgery.
We found an overall association between AKI and ΔPHb that was independent of risk factors such as age, CPB duration, surgical complexity, and markers of cardiac output and perfusion. However, unlike previous studies, we also evaluated whether this association changed in patients <2yrs with immature kidneys. In this age group, the relationship between AKI and ΔPHb is lost. In the analysis of our whole study population, ΔPHb was forced into the model because the relationship of ΔPHb with AKI was the primary objective and evidence from prior studies suggested that PHb has clinical relevance. However, in patients >2yrs, ΔPHb was significant on its own and was not forced into the model. Thus, ΔPHb is likely more important as a risk factor in patients >2yrs.
Another explanation is that SCr may not be an accurate measure of kidney function in the younger patient [35–37]. Prior CPB studies that found associations between younger age and AKI [2, 3, 38] included neonates who are not represented here. Our results suggest that in future studies of AKI using SCr, dedicated efforts may be required to investigate the relationship of PHb and AKI across the pediatric age spectrum.
Male gender was a risk factor for AKI. Females have a greater microvascular vasodilatory response to nitroglycerin [39] and males have a genetic predisposition to worse outcomes after trauma [40]. In adults with sickle cell, males have a greater risk for relative hypertension [41] and higher systemic and pulmonary vascular resistance [42]. In addition, both NO bioavailability and responses to exogenous NO were reduced in males [43]. Given that PHb is known to bind NO, this suggests that there may be a greater deleterious impact from similar levels of PHb in males. Only 16/60 (27%) of the patients in our cohort were >10yrs (the age of onset of secondary sexual characteristics in the U.S. [44, 45]) with no differences in proportion between genders. This suggests a non-hormonal mechanism that needs further exploration.
Consistent with other studies [4–7], patients with AKI had longer ICU and HospLOS. Thus, even in these low-risk patients, PHb-related AKI can have an impact on health care resources and cost. AKI can lead to renal replacement therapy, uremia, bleeding issues, and disturbances in pharmacokinetics, all of which can lead to longer LOS. AKI may also be an indicator of overall critical illness and probably does not solely contribute to LOS.
Recent evidence suggests that fluid overload after cardiac surgery leads to prolonged mechanical ventilation [46, 47]. In this study, subjects without AKI had LOS that were correlated with factors that suggested an increased need for cardiac support in the immediate postoperative period such as longer CPB duration, higher surgical complexity, higher VIS scores, and need for mechanical ventilation. However, subjects with AKI had LOS that did not correlate with this same profile and seemed to be more convincingly correlated with the duration of mechanical ventilation.
Our study has several limitations. Our results are not generalizable to all pediatric CPB. In addition, since CPB represents a complex therapy, there are likely many additional predictors of AKI. Nevertheless, our observations in comparatively healthy patients likely underestimates the potential effect of PHb which would suggest a much more robust impact on sicker or more complex patients undergoing CPB. In this study overall, PHb was associated with a higher incidence of AKI. The medium effect size in our whole population improved in the subset of subjects >2yrs. We found a similar result with a AUC for the full logistic regression model that improved in those >2yrs. A larger study involving all populations will be necessary to further elucidate these differences.
CONCLUSIONS
In conclusion, in low risk pediatric patients undergoing CPB, PHb levels increased during CPB, was associated with renal dysfunction in patients especially >2yrs of age, and may be more common in males. Studies of the impact of PHb on long term renal function and vulnerability are warranted. Future studies are also needed that target the evaluation of novel therapies to mitigate the effects of PHb on AKI after CPB.
Supplementary Material
Acknowledgments
Dr. Kim-Campbell was supported by the Ann E. Thompson Fellow Scholarship Award; UL1 TR000005 (University of Pittsburgh Clinical and Translational Science Institute), the Vascular Medicine Institute, the Hemophilia Center of Western Pennsylvania, and the Institute for Transfusion Medicine; and the NIH (T32HD040686 and 1K12HL109068). Dr. Gladwin is supported by R01 HL098032, R01 HL125886, and 2P01 HL103455. Dr. Bayır is supported by grants from the NIH (NS084604 and NS061817). We would like to acknowledge Goundappa K. Balasubramani, PhD from the Department of Epidemiology, Graduate School of Public Health and the Clinical and Translational Science Institute at the University of Pittsburgh for their statistical help. We would also like to thank the cardiothoracic surgery nurse practitioners and clinic staff, operating room staff, cardiac anesthesiologists, and perfusionists for their generous help in completing this study.
Footnotes
Copyright form disclosure: Drs. Kim-Campbell, Callaway, Gladwin, and Bayir received support for article research from the National Institutes of Health (NIH). Dr. Kim-Campbell’s institution received funding from the NIH (T32HD040686 and 1K12HL109068), UL1 TR000005 (University of Pittsburgh Clinical and Translational Science Institute), the Vascular Medicine Institute, the Hemophilia Center of Western Pennsylvania, and the Institute for Transfusion Medicine. Dr. Callaway’s institution received funding from National Heart, Lung, and Blood Institute K12 HL109068. Dr. Bayir’s institution received funding from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143:368–374. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Aydin SI, Seiden HS, Blaufox AD, et al. Acute kidney injury after surgery for congenital heart disease. Ann Thorac Surg. 2012;94:1589–1595. doi: 10.1016/j.athoracsur.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 3.Mamikonian LS, Mamo LB, Smith PB, et al. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children*. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014;15:e111–119. doi: 10.1097/PCC.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askenazi DJ, Ambalavanan N, Hamilton K, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2011;12:e1–6. doi: 10.1097/PCC.0b013e3181d8e348. [DOI] [PubMed] [Google Scholar]
- 5.Basu RK, Andrews A, Krawczeski C, et al. Acute kidney injury based on corrected serum creatinine is associated with increased morbidity in children following the arterial switch operation. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:e218–224. doi: 10.1097/PCC.0b013e3182772f61. [DOI] [PubMed] [Google Scholar]
- 6.Schneider J, Khemani R, Grushkin C, et al. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Critical care medicine. 2010;38:933–939. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 7.Fortenberry JD, Paden ML, Goldstein SL. Acute kidney injury in children: an update on diagnosis and treatment. Pediatr Clin North Am. 2013;60:669–688. doi: 10.1016/j.pcl.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Ricci Z, Pezzella C, Romagnoli S, et al. High levels of free haemoglobin in neonates and infants undergoing surgery on cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2014;19:183–187. doi: 10.1093/icvts/ivu129. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Critical care medicine. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006;81:S2347–2354. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 11.Billings FTt, Ball SK, Roberts LJ, 2nd, et al. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Checchia PA, Bronicki RA, Muenzer JT, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children--a randomized trial. J Thorac Cardiovasc Surg. 2013;146:530–536. doi: 10.1016/j.jtcvs.2012.09.100. [DOI] [PubMed] [Google Scholar]
- 13.Kellum JA, Lameire N for the KAKIGWG Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 15.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2010;11:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 16.Rubin MI, Bruck E, Rapoport M, et al. Maturation of Renal Function in Childhood: Clearance Studies. J Clin Invest. 1949;28:1144–1162. [PubMed] [Google Scholar]
- 17.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 18.Pohl U, Lamontagne D. Impaired tissue perfusion after inhibition of endothelium-derived nitric oxide. Basic Res Cardiol. 1991;86(Suppl 2):97–105. doi: 10.1007/978-3-642-72461-9_11. [DOI] [PubMed] [Google Scholar]
- 19.Vieira FU, Jr, Costa ET, Vieira RW, et al. The effect on hemolysis of the raceway profile of roller pumps used in cardiopulmonary bypass. ASAIO J. 2012;58:40–45. doi: 10.1097/MAT.0b013e31820a132c. [DOI] [PubMed] [Google Scholar]
- 20.Christen S, Finckh B, Lykkesfeldt J, et al. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med. 2005;38:1323–1332. doi: 10.1016/j.freeradbiomed.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Meyer C, Heiss C, Drexhage C, et al. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55:454–459. doi: 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt RT, McMahon L, Duffy SJ, et al. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am J Hematol. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- 23.Betrus C, Remenapp R, Charpie J, et al. Enhanced hemolysis in pediatric patients requiring extracorporeal membrane oxygenation and continuous renal replacement therapy. Ann Thorac Cardiovasc Surg. 2007;13:378–383. [PubMed] [Google Scholar]
- 24.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen Windsant IC, de Wit NC, Sertorio JT, et al. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. 2014;5:340. doi: 10.3389/fphys.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallelian F, Pimenova T, Pereira CP, et al. The reaction of hydrogen peroxide with hemoglobin induces extensive alpha-globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radic Biol Med. 2008;45:1150–1158. doi: 10.1016/j.freeradbiomed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Kapralov A, Vlasova II, Feng W, et al. Peroxidase activity of hemoglobin-haptoglobin complexes: covalent aggregation and oxidative stress in plasma and macrophages. J Biol Chem. 2009;284:30395–30407. doi: 10.1074/jbc.M109.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hult A, Malm C, Oldenborg PA. Transfusion of cryopreserved human red blood cells into healthy humans is associated with rapid extravascular hemolysis without a proinflammatory cytokine response. Transfusion. 2013;53:28–33. doi: 10.1111/j.1537-2995.2012.03710.x. [DOI] [PubMed] [Google Scholar]
- 31.Seheult JN, Triulzi DJ, Alarcon LH, et al. Measurement of haemolysis markers following transfusion of uncrossmatched, low-titer, group O+ whole blood in civilian trauma patients: initial experience at a level 1 trauma centre. Transfus Med. 2016 doi: 10.1111/tme.12372. [DOI] [PubMed] [Google Scholar]
- 32.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saraf SL, Zhang X, Kanias T, et al. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br J Haematol. 2014;164:729–739. doi: 10.1111/bjh.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 35.Askenazi DJ, Ambalavanan N. Goldstein SL Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009;24:265–274. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liborio AB, Branco KM. Torres de Melo Bezerra C Acute kidney injury in neonates: from urine output to new biomarkers. Biomed Res Int. 2014;2014:601568. doi: 10.1155/2014/601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallini F, Maggio L, Romagnoli C, et al. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol. 2000;15:119–124. doi: 10.1007/s004670000356. [DOI] [PubMed] [Google Scholar]
- 38.Krawczeski CD, Woo JG, Wang Y, et al. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–1015. e1001. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 39.McCue MC, Marlatt KL, Kelly AS, et al. Evaluation of gender differences in endothelium-independent dilation using peripheral arterial tonometry. Clin Physiol Funct Imaging. 2012;32:94–98. doi: 10.1111/j.1475-097X.2011.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperry JL, Zolin S, Zuckerbraun BS, et al. X chromosome-linked IRAK-1 polymorphism is a strong predictor of multiple organ failure and mortality postinjury. Ann Surg. 2014;260:698–703. doi: 10.1097/SLA.0000000000000918. discussion 703–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamarre Y, Lalanne-Mistrih ML, Romana M, et al. Male gender, increased blood viscosity, body mass index and triglyceride levels are independently associated with systemic relative hypertension in sickle cell anemia. PLoS One. 2013;8:e66004. doi: 10.1371/journal.pone.0066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detterich JA, Sangkatumvong S, Kato R, et al. Patients with sickle cell anemia on simple chronic transfusion protocol show sex differences for hemodynamic and hematologic responses to transfusion. Transfusion. 2013;53:1059–1068. doi: 10.1111/j.1537-2995.2012.03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gladwin MT, Schechter AN, Ognibene FP, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 44.Herman-Giddens ME, Steffes J, Harris D, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130:e1058–1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- 45.Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132:1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seguin J, Albright B, Vertullo L, et al. Extent, risk factors, and outcome of fluid overload after pediatric heart surgery*. Critical care medicine. 2014;42:2591–2599. doi: 10.1097/CCM.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 47.Lex DJ, Toth R, Czobor NR, et al. Fluid Overload Is Associated With Higher Mortality and Morbidity in Pediatric Patients Undergoing Cardiac Surgery. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016;17:307–314. doi: 10.1097/PCC.0000000000000659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.