Abstract
Platelets are anucleate cells in the blood at concentrations of 150,000 to 400,000 cells/µL and play a key role in hemostasis. Several studies have suggested that platelets contribute to cancer progression and cancer-associated thrombosis. In this review, we provide an overview of the biochemical and biophysical mechanisms by which platelets interact with cancer cells and review the evidence supporting a role for platelet-enhanced metastasis of cancer, and venous thromboembolism (VTE) in patients with cancer. We discuss the potential for and limitations of platelet counts to discriminate cancer disease burden and prognosis. Lastly, we consider more advanced diagnostic approaches to improve studies on the interaction between the hemostatic system and cancer cells.
Introduction
Platelets, present in the body at concentrations of 150,000 to 400,000 cells/µL, are anucleate cells that are derived from megakaryocytes in the bone marrow. The primary role of platelets is to seal sites of vascular damage or endothelial cell disruption as a means to prevent bleeding and promote wound healing.
Mechanistically, platelets are recruited to sites of vascular injury and adhere to newly exposed extracellular matrix proteins such as collagen, which bind platelet receptor GPIb-V-IX through von Willebrand factor (VWF)[1]. Once bound through GPIb-V-IX, the platelet receptor GPVI binds with collagen and activates via outside-in platelet signaling that results in calcium mobilization, cytoskeletal rearrangement, and release of α- and dense-granules, which contain the secondary platelet agonists adenosine phosphate (ADP) and thromboxane (TxA2). Additionally, upon activation, procoagulant phosphatidylserine is exposed on the platelet surface, which serves as a site of catalysis for generation of the serine protease thrombin. Thrombin, aside from its role in fibrin formation, activates protease-activated receptors (PARs) and triggers G-protein-coupled receptors to drive intracellular signaling cascade resulting in the rapid shift of platelet integrins to their active conformation to promote stable platelet adhesion and aggregation [2–4].
Both clinical and experimental evidence has also implicated an important role for platelets in cancer. The relationship between hemostatic components and cancer was first established in the mid-nineteenth century by Trousseau, who detected a correlation between excessive blood clotting and an occult carcinoma [5]. Moreover, Theodore Billroth found histological evidence of tumor cells colocalized in thrombi, suggesting there might be an interaction between hemostatic and metastatic systems [6]. The interplay of cancer cells and platelets has been demonstrated throughout the progression of cancer, including tumor growth, angiogenesis, metastasis, and cancer-associated thrombosis.
In this review, we discuss the contributions of platelets to cancer metastasis and associated venous thromboembolism (VTE). First we describe the biophysical and biochemical interactions between cancer cells and platelets to potentiate thrombocytosis, metastasis, and VTE. Next, we discuss and summarize clinical studies that investigated the relationship between platelet count and cancer metastasis or VTE. We consider the limitations and potential opportunities of monitoring platelet count in cancer patients to discriminate disease stage and patient prognosis to influence targeted treatment plans. Lastly, we discuss the use of advanced diagnostics and longitudinal electronical medical record clinical data supported by the Veterans Health Administration (VHA) and the Big Data Scientist Training Enhancement Program (BD-STEP) to study the interaction of hemostatic and metastatic components.
Contribution of platelets to cancer metastasis
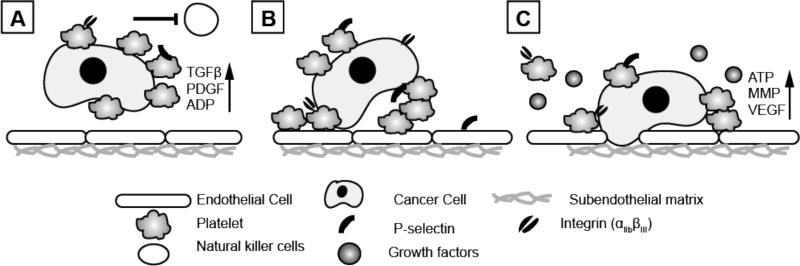
During the process of metastasis, cancer cells detach from a primary tumor site, intravasate the blood vessel lumen, travel through the circulatory system, and arrest at distant sites to form a metastatic niche [7]. Metastasizing tumor cells must endure the hydrodynamic shear forces inherent to the vasculature as well as evade immune system surveillance, which together, make the circulatory system a hostile environment. Experimental evidence indicates that platelets can exacerbate the process of cancer metastasis in a number of ways: shielding circulating tumor cells from recognition of natural killer cells, aiding tumor cell extravasation by encouraging and stabilizing tumor cell arrest in the vasculature and potentiating endothelial cell retraction, and stimulating tumor proliferation and angiogenesis by supplying the cancer cells with various growth factors [8–11]. Additionally, platelets can support the neovascularization of a tumor by providing a source of vascular endothelial growth factor (VEGF) contained in platelet α-granules[12]. In accordance with these findings, animal models have demonstrated a benefit of lowering platelet count in the inhibition of metastasis [13, 14].
Biophysical mechanisms of platelet-mediated cancer metastasis
Successful metastasis of tumor cells from a primary tumor may be affected by the biophysical-dependent interactions with platelets during their journey through the blood vessels. Circulating tumors cells (CTCs) must endure shear stress forces during the metastatic process, particularly during a phase in which they arrest on the luminal vessel wall prior to extravasation. Platelets are a potential mediator of tumor cell arrest, as they have the ability to tether, roll and arrest to a vessel wall at a wide range of shear stresses via interactions with shear-activated von Willebrand protein [13, 14]. Platelets express surface proteins such as αIIbβ3, a receptor involved in platelet aggregation, and P-selectin, a cell adhesion molecule. Both receptors have been shown to support and stabilize the adhesive interactions of colon carcinoma cells (LS174T and COLO205) with platelets cells in vitro up to wall shear stresses of 1.4 dyne/cm2 [15].
In addition to influencing tumor cells arrest, physical properties of the microenvironment also affect tumor cell and platelet interactions that lead to heteroaggregate formation under flow. Heteroaggregate formation may be an important aspect of cancer cell survival, as platelets may provide a protective cloak for cancer cells from immune cells during hematogenous dissemination. The formation of heteroaggregates is a function of the fluid-mechanical environment, with in vitro studies showing that interactions between CTC and platelets are most favored in a low shear stress environment [16]. Successful metastasis may also be highly dependent on physical features of the cancer cells. Several studies have made efforts to identify the biophysical properties of cancer cells, such as their morphology, size, mass, volume, density and density variations that would be advantageous in predicting their tumorigenic potential [17– 19]. In a computational model, it was demonstrated in a model coupling blood flow with advection-diffusion kinetics, that geometric measurements of CTCs and their respective aggregates drastically influenced the concentrations profiles of prothrombotic enzymes such as thrombin in flowing blood [20].
Platelet-derived soluble factors and receptors promote metastasis
Paracrine interactions between platelets and cancer cells facilitate the dissemination, survival and extravasation of cancer cells in the circulation. The α-granules of platelets are a source of transforming growth factor β (TGFβ), a secreted protein involved in the control of cell growth, cell proliferation, cell differentiation and apoptosis, and platelet derived growth factor (PDGF), a protein involved in cell growth and division and in promoting angiogenesis. These proteins both inhibit host immunosurveillance, and have thus been implicated in promoting tumor metastasis [21]. In addition to its role in protection cancer cells from a lethal immune response, TGFβ has been shown to enhance the invasive potential of cancer cells by activating the TGFβ/Smad and NF-KB signaling pathways in tumor cells necessary for the epithelial to mesenchymal transition [22]. Platelet dense granules secrete adenine nucleotides such as ADP and ATP, which have been shown to support the immune evasion and eventual extravasation of CTCs from the bloodstream, respectively. Specifically, platelet secretion of ATP, which can be induced by tumor cells, initiates a P2Y2 receptor-mediated signaling cascade in endothelial cells leading to the disaggregation of the endothelial barrier [23, 24] and creating a potential site for metastasizing tumor cells to arrest. Similarly, another molecule known as a metalloproteinase (MMP), released by both cancer cells or platelets, is known to degrade the extracellular matrix barrier and promote invasion and tumor extravasation [25] (Fig. 1).
Figure 1.
Platelets can support and encourage cancer cell metastasis by (A) shielding cancer cells from immune cells during vascular dissemination, (B) arresting cancer cells on the wall of a blood vessel, and (C) releasing growth factors that allow for extravasation of cancer cells through the endothelial cell and extracellular matrix barrier. (Key - TGFβ - transforming tumor growth factor β; MMP - matrix metalloproteases; VEGF - vascular endothelial growth factor; PDGF - platelet derived growth factor; ADP - adenosine diphosphate; ATP - adenosine triphosphate).
Platelet membrane expression of proteins has been associated with improved adhesion of cancer cells to vessel walls and the increased ability of cancer cells to withstand shear stresses in the circulation. Similarly, activated platelets express phosphatidylserine, which catalyzes the production of thrombin and in turn converts fibrinogen into fibrin. Fibrin formation has been implicated in strengthening heterotypic platelet-cancer aggregates, ensuring their survival and transport to secondary sites [26].
Activation of platelets by cancer cells
During the process of hematogenous dissemination, cancer cells may activate platelets in the bloodstream [23, 27], a phenomenon known as tumor cell-induced platelet activation (TCIPA). Platelet activation in turn results in the release of the aforementioned platelet-derived growth factors and the formation of a fibrin-rich tumor cell platelet aggregates. This process has been proposed to act as a positive feedback activation loop during cancer metastasis. Moreover, tumor cell-induced platelet activation may play a role in the development of cancer-associated thrombosis [26, 28].
Tumor cells can induce platelet activation via a combination of juxtacrine and paracrine routes, occurring by physical engagement of platelet receptors by tumor cells or via synthesis and/or release of soluble agonists, respectively. The major platelet receptors mediating adhesive interactions with tumor cells are αIIbβ3 integrins and the adhesive molecule P-selectin. αIIbβ3 is a receptor for fibrinogen, fibronectin and VWF while P-selectin functions as receptor for mucin-type glycoproteins or sulfated glycolipids expressed on the surface of many types of cancer cells [10, 15, 29, 30]. Deficiency or blockade of αIIbβ3 or P-selectin has been shown to inhibit TCIPA in vitro and reduce of experimental metastasis in vivo in several mouse models of cancer [31–39].
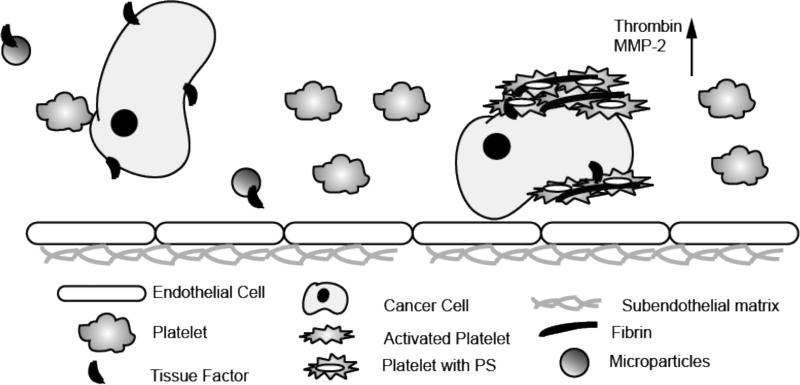
The major soluble mediators found to induce TCIPA are thrombin, ADP, TxA2 and matrix metalloproteases (MMPs). Thrombin can be generated directly by procoagulant tumor cells or via tumor cell-derived microvesicles expressing tissue factor (TF) or indirectly by host tissues which have been damaged due to tumor cell invasion or chemotherapy [26, 40]. Platelet activation by thrombin results from the cleavage of protease activated receptors (PARs) by thrombin, which leads to the rapid release of ADP from platelet dense granules and synthesis and release of TxA2. Subsequently, ADP and TXA2 synergize to amplify the response of the platelets to cancer cells by binding to the P2Y12 and TxA2 platelet receptors, respectively. There is also evidence that platelets can be directly activated by ADP released from cancer cells. The proteolytic enzyme, MMP-2, released by tumor cells as a result of activation has been shown to contribute to TCIPA in vitro using HT-1080 fibrosarcoma and A549 lung epithelial carcinoma cell lines [32, 41, 42]. Alternatively, osteosarcoma and prostate cancer cells have been shown to trigger TCIPA in vitro via engagement of the platelet podoplanin receptor CLEC-2 and via activation of the platelet IgG receptor FcγRIIa [43, 44]. Experimental TCIPA and the metastatic progression of tumors, including neuroblastoma, lung, breast and pancreatic carcinoma and melanoma has been shown to be impaired in animal models by treatment with anticoagulants like heparin or low molecular weight heparin, or the P2Y12 inhibitors such as ticagrelor and clopidogrel, or by aspirin, which inhibits TXA2 synthesis [40, 45–51]. Collectively, these observations suggest that platelet receptors and ligands may be targeted to suppress cancer-induced platelet responses during metastasis (Fig. 2).
Figure 2.
Cancer cells promote platelet activation and coagulation. Tissue factor is expressed on cancer cells and microparticles, which subsequently leads to the activation of FVII and FX via the extrinsic pathway. Inactive prothrombin is converted to thrombin, which then catalyzes the polymerization of fibrinogen to fibrin. Thrombin can also activate platelets through their PAR receptors (Key - PS - phosphatidylserine; MMP-2 - metalloproteases).
Cancer cell-induced thrombocytosis
Thrombocytosis, defined as a platelet count of >400,000 cells/µL of blood, was first associated with cancer progression in 1872 by Riess and remains a marker of poor prognosis in patients with solid tumors [52]. The mechanism underlying cancer cell-induced thrombocytosis remains ill-defined. It has been suggested that cancer cell-induced thrombocytosis results from the overproduction of thrombocytopoietic hormones acting on megakaryocytes and their precursors, such as thrombopoietin (TPO) which is synthetized and released in plasma by the liver. In vitro, three growth factors, including Interleukin (IL)-6, IL-1, and leukemia inhibitory factor, have been shown to be capable of promoting megakaryocytopoesis and subsequent thrombocytosis. Studies using mouse models of ovarian cancer have shown that tumor-derived IL-6 induces the synthesis and release of hepatic TPO. Moreover, high serum levels of IL-6 and TPO have been found to be associated with high platelet count in patients with ovarian cancer. However, inhibition of IL-6 and TPO production with small interfering RNA brought the platelet count back within normal range in an animal model of ovarian cancer, as did the clinical use of the anti-IL-6 antibody siltuximab in patients with ovarian cancer [53].
Clinical studies: Metastasis and platelets
The first observation of thrombocytosis accompanying metastasis was identified in 1964 in a clinical study by Levin and Conley in which they examined platelet counts of 14,000 patients admitted to Johns Hopkins hospital. Approximately 40% of the patients with malignant disease were found to have platelet counts greater than 400,000 cells/µL in the absence of iron deficiency and benign inflammation. A number of subsequent studies also found a relationship between thrombocytosis and cancer. For instance, a 1970 study by Silvis et al. showed that 60% of a cohort of 190 patients with lung cancer had thrombocytosis [54], and in 1974, Tranum et al. reported that thrombocytosis occurred in patients with solid tumors [55]. Multivariate analyses confirmed that thrombocytosis was an independent contributing factor to metastasis while accounting for age and gender. Other studies demonstrating the association of thrombocytosis and metastasis are highlighted in Table 1.
Table 1.
Clinical studies investigating the frequency of high platelet count in cancer patients and the role of high platelet count in patient outcomes
| Study | Study Type | Cancer Type/Stage |
Number of Patients |
Time of Platelet Count Measurement |
Cut off platelet count/µL |
Occurrence* (Number of patients (%)) |
Outcome** |
|---|---|---|---|---|---|---|---|
| Stone et al | Prospective | Epithelial ovarian/Stage III to IV | 619 | Time of diagnosis | 450,000 | 192 (31%) | OSŦ: 2.62 vs 4.65 years (P < 0.001) |
|
| |||||||
| Maraz et al | Retrospective | Lung/Stages I to IV | 398 | Perioperative; before surgery, 1st and 7th days after | 400,000 | 86 (22%) overall; 18.6% I, 19.3% II, 27.5% III, 28.6% IV | HR: 1.58 (P < 0.001), OS: 38 vs 63.1 months (P = <0.001) |
|
| |||||||
| Pederson et al | Retrospective | Lung/Stages I to IV | 1,115 | Time of diagnosis | 400,000 | 357 (32%) overall; 23% I & II, 37% IIIa & IV | RR: 4.24 (P < 0.001) |
| 269 | 1–3 months after resection | 400,000 | 24 (8.9%) | ||||
|
| |||||||
| Aoe et al | Retrospective | Lung/Stages I to IV | 611 | Preoperative | 400,000 | 98 (24%) | HR: 1.29 (P = 0.0348) |
|
| |||||||
| Taucher et al | Retrospective | Breast/I,II,IIIa | 4,300 | Time of diagnosis | 400,000 | 161 (3.7%) overall; 77 (3%) I, 77 (4.8%) II, 5 (3.8%) IIIa | RR: 1.73 (P = 0.0064), OS: 72 vs 99.5 months (p = 0.0054) |
|
| |||||||
| Herndon et al | Retrospective | Mesothelioma | 336 | No chemo, > 2 weeks after surgery | 400,000 | 184 (54.8%) | RR: 1.57 (P < 0.001) |
|
| |||||||
| Ishizuka et al | Retrospective | Colorectal/Stages I to IV | 453 | Preoperative | 300,000 | 226 (49.9%) overall; 159 (45.7%) I, II, III, 67 (63.8%) IV | OR: 1.64 (P = 0.039) (after surgery) |
|
| |||||||
| Bensalah et al | Retrospective | Renal/Stages I to IV | 804 | Preoperative | 450,000 | 63 (7.8%) overall; 16 (4%) T1 or 2, 47 (11.6%) T3 | RR: 1.8 ( P < 0.001) |
HR, hazard ratio; OR, odd ratio; OS, overall survival; RR, relative risk
Refers to the number and percentage of patients that had thrombocytosis.
Outcomes refers to patients that had thrombocytosis.
OS is showing survival of patients with thrombocytosis versus patients without thrombocytosis.
The reported occurrence of thrombocytosis in metastatic cancer patients varies widely in the literature, ranging from ~5–60%. Some of the disparities in reported results may be attributed to factors such as the type of cancer or the stage. For example, the percentage of patients with thrombocytosis was 3.7% for patients with breast cancer [56], whereas 24–63% [57] and 33–57% [53] in patients with renal or ovarian cancer, respectively. It has also been reported that patients with adenocarcinomas have an increased likelihood of metastasis and accompanying thrombocytosis; for instance, Zhang et al. documented that 26.6% of 308 cases of histopathologically-confirmed lung adenocarcinomas presented with accompanying thrombocytosis [58]. Pedersen et al. reported differences in the occurrence of thrombocytosis in cancer patients based on cancer histology, with squamous cell carcinoma at higher risk for thrombocytosis compared to non-squamous cell types [59, 60]. The occurrence of thrombocytosis in patients has been reported to vary depending on the stage of cancer, with later stage cancers being associated with increased platelet counts [59–61]. In a study of 398 lung cancer patients, the occurrence of platelet counts greater than 400,000 cells/µL was reported to be 28.6% in Stage IV patients versus 18.6% in Stage I patients [60]. While the occurrence and extent of thrombocytosis in metastatic cancer patients seems to be dependent on the particular malignancy, a common finding of all the studies is that thrombocytosis in cancer patients is a hallmark of poor prognosis and a low survival rate.
Multivariate analyses and Cox survival models have confirmed that thrombocytosis in cancer patients is an independent predictor of poorer outcomes [59]. Symbas et al. reported a shorter life expectancy in patients with renal cell cancer that presented with thrombocytosis [62]. In this study, the platelet counts were recorded for 259 patients with stage IV renal cell cancer who had undergone immunotherapy, chemotherapy, or hormonal therapy after surgery. Patients that had platelet counts greater than 400,000 cells/µL were found to have lower survival compared to those with a platelet count below the aforementioned threshold. Zhang et al. also demonstrated via log rank tests that there was a significant difference in survival between patients with and without thrombocytosis, as the former had an increased risk of bone metastases [58].
For the majority of the clinical studies, platelet counts were recorded at the time of diagnosis, before or after operations, or during chemotherapy treatment. Yet it is unknown at what point elevated platelet counts occur in the progression of disease; there is a possibility that it could precede cancer progression by months or even years. Several of the clinical studies, both retrospective and prospective, provided platelet counts at the times of diagnosis, prior to implementing an intervention. Once an intervention is initiated, it is likely that platelet counts become highly variable, and thus may not provide as much predictive power or would be a biased predictor due to unobserved heterogeneity. Platelet counts obtained following radiotherapy, chemotherapy, or surgical resection would likely be highly dependent on the intervention itself. For instance, in a study of stage IV renal cell cancer patients by Symbas et al., platelet count was reported to be affected by immunotherapy, chemotherapy, and hormonal therapy [62]. Moreover, in a study of 269 lung cancer patients undergoing surgery, Pederson et al. reported that after one to three months after the surgical resection, only 8.9% of the patients had platelet counts greater than 400,000 cells/µL as compared to incidences of 20–30% found before the operation. Yet only thrombocytosis prior to the treatment had an influence on the survival rate in surgically resected patients. Given the susceptibility of platelet counts to vary as a result of surgery, it is uncertain if the changes in the incidence of thrombocytosis could be attributed to differential surgery-related inflammation, or from the removal of the tumor.
One study on the association between thrombocytosis and outcomes in ovarian cancer patients suggested a role for cancer in the promotion of platelet production. A possible mechanism is the promotion of megakaryocytopoiesis by tumor-derived humoral agents, resulting in the enhancement of circulating platelets count. For instance, IL-6, a known potent stimulator of megakaryocytopoiesis, has been shown to be released from tumor cells in vitro and in vivo. Out of a 619 cohort of epithelial ovarian cancer patients, 192 patients were reported to have platelet counts above normal (> 450,000 cells/µL). Moreover, the IL-6 and thrombopoietin plasma levels were found to be significantly higher in a cohort of 46 patients with epithelial ovarian cancer and thrombocytosis as compared to a cohort of 104 patients with epithelial ovarian cancer and normal platelet counts. A potential mechanism to explain the author’s findings is that tumor-derived IL-6 stimulated the liver to produce increased TPO levels resulting in increased platelet production. The authors demonstrated that blocking IL-6 and TPO production with a small interfering RNA led to normalization of platelet counts in an animal model of ovarian cancer. Additionally, IL-6 was targeted with siltuximab (an anti-IL-6 antibody) and combined with paclitaxel (common chemotherapeutic agent for epithelial ovarian cancer), which significantly reduced tumor growth in the mouse models. From such work, it is conceivable that drugs reducing platelet counts or platelet activation might attenuate cancer progression and improve survival rate of patients. In the following section, we describe treatments that act on platelets and may interfere with cancer progression.
Treatment and prevention of cancer metastasis
Several therapeutic strategies targeted toward platelet production or activation have been evaluated for their efficacy and safety in inhibiting cancer metastasis. One such strategy that has shown promise is the long-term use of low dose aspirin. Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) inhibit cyclooxygenase (COX), the enzymes responsible for the synthesis of prostaglandins. An isoform of COX, known as COX-2 has been found to be overly expressed in cancer cell lines and has been implicated to play a role in carcinogenesis, tumor growth, apoptosis, and angiogenesis. Aspirin has been reported to reduce risk of colorectal cancer and some other cancers in clinical studies. In a 2012 meta-analysis of 5 randomized clinical trials assessing aspirin use in 17,285 participants, aspirin treatment reduced the risk of distant metastasis (0.64 HR, 95% CI 0.48–0.84, p = 0.001), especially in adenocarcinomas (0.54 HR, 95% CI 0.038–0.77, p = 0.0007) regardless of whether or not they had metastasis [63]. Benefits of regular aspirin use has been found to depend on the type of cancer and the duration of the treatment. For example, in a meta-analysis of 51 randomized clinical trials comprising a total of 77,549 participants, daily aspirin intake reduced cancer mortality (0.85 HR, 95% CI 0.76–0.96), notably demonstrating the greatest clinical benefit when assessed after 5 years [63]. Epidemiological studies of colorectal patients from 30 studies and 37,500 cases reported a 30% reduction in the risk of cancer after use of regular/high dose aspirin for at least 5 years. Aspirin was also found to have a favorable effect on cancers of the esophagus, stomach, breast, ovary, and lung, but was deemed ineffective for pancreatic, prostate, and bladder cancer, and could be harmful in kidney cancer. However, in randomized studies including the Physicians Health Study [64] and Women’s Health Study [65], no association was found between use of aspirin and incidence of total, breast or colorectal cancer. Similarly, in the Women’s Health Study, low-dose aspirin (100 mg) was not found to reduce the risk of breast or colorectal cancer in 39,942 women over the course of 10 years [65].
Beyond inhibiting platelet activation with aspirin, several studies have evaluated the effect of lowering overall platelet count on outcome in cancer patients. In a small-scale study of ovarian cancer patients, it was reported that clinical use of an anti-IL-6 antibody, siltuximab, reduced platelet count after 3 weeks of treatment, and inhibited tumor growth in 8 of 18 ovarian cancer patients receiving siltuximab [53]. Taken together, these studies suggest that pharmacological targeting of platelet activation or production may provide benefit in the treatment of cancer.
Role of platelets and cancer in VTE
The risk of a VTE is approximately seven times higher in patients with active cancer [66], yet it is unclear whether platelet-tumor cell interactions play a causative role in cancer-associated thrombosis. Several studies from the 1990’s reported that the incidence of thromboembolic episodes and thrombocytosis in cancer patients was not correlated [59, 67], while a number of clinical studies conducted in the early 2000s reported an association of cancer-associated thrombocytosis with VTE. Complicating epidemiological studies is the inherent difficulty in determining actual VTE rates, as only a fraction of VTE are clinically evident and therefore discovered. Moreover, Zakai et al. estimated that only actually 6% of DVTs are reported as symptomatic, and therefore the majority of incidences go unnoticed, resulting in an inaccurate prediction of VTE risk associated with cancer [68]. Clinical studies on thrombocytosis and VTE are summarized in Table 2. One such retrospective case control study compared the risk factors of VTE for medical inpatients with comparable rates of known malignancy in VTE cases (n=65, 25% with known malignancy) versus non VTE cases (n=123, 22% had no known malignancy) In this study, patients with platelet counts exceeding 350,000 cells/µL had a 2.5-fold risk of developing VTE during hospitalization [68]. Similarly, in the outpatient setting, the ANC Study Group Registry prospectively analyzed 3003 patients with specific tumor types (breast, lung, ovarian, sarcoma, colon, lymphoma) at the start of their new chemotherapy regimen. They found an incidence of VTE of 3.98% in patients with prechemotherapy platelet counts greater than or equal to 350,000 cells/µL compared to a 1.25% VTE incidence in patients with a platelet counts less than or equal to 200,000 cells/µL [69]. Another study investigating 665 patients with solid tumors of the breast, lung, gastrointestinal tract, pancreas, kidney, or prostate, demonstrated that with higher platelet count threshold of 443,000 cells/µL, probabilities of risk were more accurately discriminated: the risk for VTE below the threshold was 5.9% versus 34.3% for patients with platelet counts greater than the threshold [70].
Table 2.
Clinical studies investigating the effect of platelet count on the occurrence of VTE and patient outcomes
| Study | Study Type | Cancer Type | Patients | Treatments | Cut off platelet count/µL |
Follow up (median) |
Probability of VTE* | Outcome** |
|---|---|---|---|---|---|---|---|---|
| CATS, Simanek et al | Prospective | Multiple solid tumors | 665 | Chemotherapy | 443,000 | 12 months | 34.3% vs 5.9% | HR: 3.5 (P = 0.0032) |
| Khorana et al | Prospective | Breast, lung, ovarian, sarcoma, colon, and lymphomas | 3,003 | Chemotherapy | 350,000 | 2.4 months | 3.98% vs 1.25% | OR: 2.81 (P < 0.002) |
| Mandala et al | Prospective | Breast and gastrointestinal | 381 | Adjuvant chemotherapy | 300,000 | 35 months | Not Reported | HR: 1.65 (P < 0.0341) |
| Henry et al | Retrospective | Multiple | 187 | ESA therapy | 350,000 | Not applicable | Not Reported | OR: 2.9 (P = 0.036) |
HR, hazard ratio; OR, odd ratio; ESA, erythropoietic stimulating agents
Refers to the probability of a VTE in a cancer patients with and without thrombocytosis.
Outcomes refers to patients that had thrombocytosis.
The risk for VTE in patients with cancer has been found to have a temporal relationship with time of diagnosis, with incidence of VTE being the highest in the first few months after cancer diagnosis. However, it remains unknown if this phenomenon is representative of a naturally occurring biological mechanism, or whether VTE occurrences can be owed to interventions such as surgery or chemotherapy. Interestingly, it has been shown that VTE are the leading significant cause of death in patients undergoing chemotherapy. For instance, Zakai et al. reported that up to 21.5% of VTEs occur following non-surgical hospital admission in patients receiving chemotherapy. Though the actual process of chemotherapy can cause thrombocytopenia (low platelet count), as the treatment suppresses platelet count in the bone marrow [71], it has been determined that elevated pre-chemotherapy platelet counts are associated with worse VTE outcomes [69]. In addition to a high platelet count prior to the treatment, certain chemotherapy regimens have been shown to impose its own increased risks of VTE. In one such study, Blom et al. investigated 3,220 patients of various types of cancer and reported that chemotherapy and hormonal therapy lead to a 2.2-fold and 1.6-fold increased risk of VTE, respectively [72]. Chemotherapy combined with the use of immunomodulatory agents such as thalidomide or lenalidomide or anti-angiogenic vehicles are known to increase the risk of VTE, prompting the recommendation of thromboprophylaxis when this drug cocktail is administered [73]. Erythropoiesis-stimulating agents (ESA) such as erythropoietin and darbepoietin are given to patients to enhance hemoglobin and red blood cell production during chemotherapy treatments. Notably, ESA therapy has been associated with a 50% increase in the risk of VTE in cancer patients and can cause iron restricted erythropoiesis and thrombocytosis, all of which is enhanced when accompanied by chemotherapy and radiation [69, 74]. In a study of 187 cancer patients, iron substitution was found to reduce the incidence of thrombocytosis and VTE compared to control patients [75]. Surgery has also been suggested as a potential risk for VTE progression in cancer patients. Several studies have reported that patients with cancer who underwent surgical intervention had either negligible or up to a 4-fold increase risk for postoperative VTE [72]. In summary, these studies suggest that having a high platelet count coupled with interventional treatments might increase the risks for deleterious cardiovascular events and worsen patient outcomes.
When platelets become activated, they discharge prothrombotic molecules from their α-and dense-granules into the blood microenvironment, which may represent a mechanism of action linking increased platelet count with cancer-associated VTE. One of the molecules contained in α-granules, P-selectin, is a member of the selectin family of cell adhesion molecules. P-selectin can also be released from the Weibel-Palade bodies of endothelial cells [76], and is known to play a role in thrombus formation. As mentioned previously, P-selectin has been shown to mediate platelet and leukocyte adhesion to cancer cell lines in vitro [77]. Soluble levels of plasma P-selectin is a known biomarker of platelet activation. In clinical studies, levels of soluble P-selectin have been shown to correlate with acute VTE and risk of VTE in patients [78]. In another prospective study of 687 cancer patients, it was shown that the risk of VTE was 12% in patients with soluble P-selectin levels above the 75th percentile compared to 4% of patients with soluble P-selectin levels under the threshold (2.6 HR, 95% CI 1.4–4.9, P = 0.003) [79].
Platelet activation leads to the flipping of the platelet membrane to expose a procoagulant phosphatidylserine (PS)-rich surface. The PS surface acts as a site of catalysis for thrombin generation, with thrombin generation and the presence of PS-positive microparticles serving as biomarker for thrombosis. In the CATS study, it was found that elevated thrombin generation in patient plasma is associated with an increased risk of VTE [80], reporting that 11% of patients with elevated thrombin generation presented with a VTE and a worse prognosis (2.1 HR). While no association has been reported between procoagulant microparticles and occurrence of VTE, increased levels of PS-positive microparticles have been reported in cancer patients as compared to healthy controls [81].
VTE occurs in ~1–20% of cancer patients, and is dependent on the severity of the malignancies; the presence of cancer increases the risk of a VTE by 4–7 fold [66]. The association between cancer and thrombosis is bidirectional, such that a VTE occurrence is indicative of cancer progression and a worse prognosis than if the patients that do not experience VTE. More specifically, thrombocytosis is correlated with poor survival and a decreased response to surgery and chemotherapy in lung, breast, colorectal, renal, and gastric cancers [82]. Additionally, it has been found that a VTE in patients initially diagnosed with local regional stage cancer have lower survival rates. As such there has been a need for risk assessment to improve clinical decision making and determine which patients will benefit most from prophylaxis. The Khorana risk assessment for cancer patients at the time of chemotherapy initiation was developed to identify patients with high risk of developing VTE [83]. The Khorana score assigns point values for a range of parameters based on logistic regression coefficients attained in the model. The score assigns 2 points for high risk cancer sites for gastric or pancreatic, 1 point for lung, ovarian, bladder, and 1 point for platelet counts exceeding 350,000 cells/µL, hemoglobin less than or equal to 10 g/dL, leukocyte counts greater than 110,000 cells/µL and a body mass index greater than or equal to 35 kg/m2. Patients are stratified into three categories for risk: low, intermediate, and high. Khorana et al determine that patients that had scores of 0, 1–2, and 3, had VTE risks of 0.3%, 2% and 6.7%, respectively, during the 2.5 follow-up period [83]. The Khorana score has been validated in the Prophylaxis of Thromboembolism during Chemotherapy study [84] and was used to asses more than 12,000 patients in cohort studies including Vienna CATs. The score has been improved for chemotherapy patients by also accounting for chemotherapy associated risks such as platinum and gemcitabine-based chemotherapy [84], where treatment with cisplatin or carboplatin-based chemotherapy or gemcitabine adds 1 point or 2 points, respectively. Later studies have even begun to include additional biomarkers such as soluble P-selectin and D-dimer levels in an expanded risk assessment model known as the Vienna prediction score [79, 85], as it was shown that patients with a score of 4 had a cumulative probability of 20.4% of a VTE occurrence, while those with a score greater than 5 had 35% probability of a VTE, but quantitative P-selectin biomarker assays have been difficult to implement across several studies due to poor standardization [79, 86].
Several studies have determined that the type of cancer and the rate of growth are important predictors of VTE risk [87–89]. Buller et al. reported that mucinous adenocarcinomas are associated with a higher incidence of VTE [66, 90]. Additionally, it was demonstrated that patients with hematological malignancies such a lymphoma, leukemia, and myeloma had more incidences of VTE [88, 89]. Moreover, the SEER and Medicare data [91] demonstrated that recurrent thrombosis increased the risk of thrombosis in the subsequent two years and that patients with lung, stomach, or pancreas cancer were more likely to develop distant metastases within a year following a VTE episode. All in all, the development of a VTE in a cancer patient is a complex process that is related to the many factors involved in the specific characteristics of each cancer, the accompanying blood components, and treatment approaches.
Potential value and limitations of platelet counts
Platelet counts are routinely obtained in patients with cancer undergoing treatment, yet it is not yet clear how or if to incorporate these data into management of patients with cancer. Platelet counts might prompt decisions regarding the administration of anti-platelet therapies or anticoagulant prophylaxis. However, given the susceptibility of platelet count to change in response to a variety of endogenous and exogenous factors, there is a need to analyze temporal platelet count variations as a function of the cancer stage and the type of therapeutic treatment. Additionally, a limitation of only considering platelet counts as a measured biomarker is that they do not provide functional data on platelet activation, granule content or other mechanisms by which platelets and cancer cells may interact. Platelet function analyzers may provide information on platelet function with regards to hemostasis, but have not been validated with respect to cancer-platelet cell interactions or how to utilize this assay for managing patients with cancer. As more understanding of the interplay between platelets and cancer progression ensues, new opportunities to leverage existing assays to provide more insight, or development of novel assays to delineate cancer cell-platelet interaction within patients are needed. One such promising method is utilizing biological systems and multi-omics approaches, which will be discussed in the following section.
Big Data and Biological systems approaches
One approach to overcome the limitations of using platelet counts as biomarkers would be to leverage longitudinal electronic health information, ideally with information prior to cancer. The Department of Veterans Affairs (VA) treats more than 6 million patients annually and it routinely creates national databases from its electronic medical record, which was initiated in the early 1990s. Researchers can access information on laboratory tests, radiographic scans and procedures, thus offering researchers the ability to data mine and analyze a vast amount of longitudinal data from 100,000s to millions of patients. Lab counts are routinely obtained from veterans and sampled from healthy and unhealthy patients. This rich database enables researchers to determine temporal features of lab counts that have the potential to influence a patient’s survival, recurrence, or response to treatment. In addition, the VA has teamed up with the National Cancer Institute (NCI) to fund the Big Data Scientist Training Program (BD-STEP), an initiative that is designed to help train researchers interested in such these opportunities and aims to improve veteran health through evidence based research.
Although the VA captures information on platelets from blood tests, it is increasingly capturing genomic and proteomic information from patients. This is useful, because as noted above, the mechanisms by which platelets impact cancer phenotypes span diverse regulatory scales from genomic alterations and evolution through metabolic flux. Consequently, beyond collecting platelet counts as biomarkers, multi-scale and multi-omics approaches should also be considered to uncover the complex mechanisms underlying the interactions between platelets and cancer. In particular, the collective genomes, transcriptomes and proteomes of cancer cells have the potential to offer insight into complex platelet and cancer cell interactions that enable thrombocytosis, cancer metastasis and thrombosis. By integrating diverse multi-scale and multi-omic data (tissue-scale imaging, pathology, molecular characterization) of platelet cancer interactions it may ultimately be possible to use these diverse data to assist in predicting patient outcomes and inform clinical decision making. Bioinformatics approaches would be particularly useful in determining the mechanistic underpinnings of thrombocytosis. Though thrombocytosis is a common clinical scenario, the diagnostic process can be challenging, because it is associated with a multitude of reactive processes such as infection, cancer, tissue damage, or chronic inflammatory disorders, or a clonal disorder [92]. Tumor cell multi-omes could aid in discriminating their relative contribution to elevated platelet counts. Incorporation of genomic data has already been instrumental in detection of the cause of clonal thrombocytosis, as it is associated with a BCR-ABL1 fusion in chronic myeloid leukemia [92, 93]. An additional known point mutation in exon 14 of the Janus kinase 2 (JAK2) gene can result in the JAK2V617F protein which is associated with polycythemia vera, essential thrombocythemia, and primary myelofibrosis [94–98]. Multi-omics approaches could also be used to determine whether the etiologies of metastatic potential and thrombogenicty of the circulating tumor cells are from mutations in the genome or epigenetic or protein modifications incurred by the tumor cell microenvirnoment. All in all, multi-omic data would be useful in determining the mechanisms of thrombocytosis, metastasis and thrombocytosis, and also provide support for the use of targeted therapies.
Other hematological related molecules may also help contribute to predicting patient metastasis and VTEs in patients. In 2007, the American Society of Clinical Oncology made recommendations for tumor markers for patient prognosis in breast cancer, which included urokinase plasminogen activator (uPA) and plasminogen activator inhibitor (PAI-1). uPA and PAI-1 are known proteins involved in regulating clot digestion, but also play essential roles in tumor invasion and metastasis. Low levels of uPA and PAI-1 in tissue measured by enzyme-linked immunosorbent assays (ELISA) are associated with a low risk of recurrence [99] while their overexpression is associated with poor prognosis in early-stage breast cancer [100–102]. Other studies have confirmed that PAI-1 and uPA are strong prognostic factors independent of size, grade, and hormone receptor status in patients that did not receive adjuvant systemic therapy [102–104]. Additional molecules that might also be leveraged to predict cancer patients risk of VTEs include soluble P-selectin and microvesicles released from activated or apoptotic cell membranes once standardized measurement methods are available [105].
Conclusions
In the review, we summarized the biophysical and bidirectional biochemical interactions between cancer cells and platelets that are associated with the progression of cancer metastasis and thrombosis. We subsequently summarized studies that measured the correlation between thrombocytosis and cancer metastasis and thrombosis. We discussed some potential and limitations of leveraging platelet counts to predict the outcomes of cancer patients and describe the utility of multi-omics and longitudinal data mining approaches to be able to determine respective contributions of proteogenomic and temporal features in promoting lethal cancer and platelet interactions.
Acknowledgments
J.L. Sylman is supported by the Big Data Scientist Training Enhancement Program (BD-STEP) fellowship. This work was also supported by grants from the National Institutes of Health (R01HL101972) and the Altarum Institute. O.J.T. McCarty is an American Heart Association Established Investigator (13EIA12630000). The authors also acknowledge Jason Leonhard for his efforts in collating clinical research studies.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J. Thromb. Haemost. 2003;1:1335–42. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 2.Brass L. Understanding and evaluating platelet function. Hematol. Educ. Program Am. Soc. Hematol. Am. Soc. Hematol. Educ. Program. 2010;2010:387–96. doi: 10.1182/asheducation-2010.1.387. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman M, Monroe DM. Coagulation 2006: a modern view of hemostasis. Hematol. Oncol. Clin. North Am. 2007;21:1–11. doi: 10.1016/j.hoc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Auger JM, Kuijpers MJE, Senis YA, Watson SP, Heemskerk JWM. Adhesion of human and mouse platelets to collagen under shear: a unifying model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005;19:825–7. doi: 10.1096/fj.04-1940fje. [DOI] [PubMed] [Google Scholar]
- 5.Trousseau A, Hotel-Dieu . Clinique Médicale De L’hotel-dieu De Paris. Vol. 3. Nabu Press; 2011. [Google Scholar]
- 6.Tranum BL, Haut A. Thrombocytosis: platelet kinetics in neoplasia. J. Lab. Clin. Med. 1974;84:615–9. [PubMed] [Google Scholar]
- 7.Fidler IJ. Metastasis: Quantitative Analysis of Distribution and Fate of Tumor Emboli Labeled With 125I-5-Iodo-2′ -deoxyuridine. J. Natl. Cancer Inst. 1970;45:773–82. [PubMed] [Google Scholar]
- 8.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300. [PubMed] [Google Scholar]
- 9.Schafer AI. Thrombocytosis. N. Engl. J. Med. 2004;350:1211–9. doi: 10.1056/NEJMra035363. [DOI] [PubMed] [Google Scholar]
- 10.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33:231–69. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battinelli EM, Markens BA, Italiano JE. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–69. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 13.Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 14.Fogelson AL, Neeves KB. Fluid Mechanics of Blood Clot Formation. Annu. Rev. Fluid Mech. 2015;47:377–403. doi: 10.1146/annurev-fluid-010814-014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96:1789–97. [PubMed] [Google Scholar]
- 16.McCarty OJT, Jadhav S, Burdick MM, Bell WR, Konstantopoulos K. Fluid shear regulates the kinetics and molecular mechanisms of activation-dependent platelet binding to colon carcinoma cells. Biophys. J. 2002;83:836–48. doi: 10.1016/S0006-3495(02)75212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips KG, Kuhn P, McCarty OJT. Physical Biology in Cancer. 2. The physical biology of circulating tumor cells. Am. J. Physiol. - Cell Physiol. 2014;306:C80–8. doi: 10.1152/ajpcell.00294.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips KG, Velasco CR, Li J, Kolatkar A, Luttgen M, Bethel K, Duggan B, Kuhn P, McCarty OJT. Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Front. Oncol. 2012;2:72. doi: 10.3389/fonc.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King MR, Phillips KG, Mitrugno A, Lee T-R, de Guillebon AME, Chandrasekaran S, McGuire MJ, Carr RT, Baker-Groberg SM, Rigg RA, Kolatkar A, Luttgen M, Bethel K, Kuhn P, Decuzzi P, McCarty OJT. A physical sciences network characterization of circulating tumor cell aggregate transport. Am. J. Physiol. Cell Physiol. 2015;308:C792–802. doi: 10.1152/ajpcell.00346.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips KG, Lee AM, Tormoen GW, Rigg RA, Kolatkar A, Luttgen M, Bethel K, Bazhenova L, Kuhn P, Newton P, McCarty OJT. The thrombotic potential of circulating tumor microemboli: computational modeling of circulating tumor cell-induced coagulation. Am. J. Physiol. Cell Physiol. 2015;308:C229–236. doi: 10.1152/ajpcell.00315.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009;85:314–23. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet-cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br. J. Pharmacol. 2004;143:819–26. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan K, Cooke N, Kenny D. Living in shear: platelets protect cancer cells from shear induced damage. Clin. Exp. Metastasis. 2014;31:697–704. doi: 10.1007/s10585-014-9660-7. [DOI] [PubMed] [Google Scholar]
- 25.Santos-Martinez MJ, Medina C, Gilmer JF, Radomski MW. Matrix metalloproteinases in platelet function: coming of age. J. Thromb. Haemost. 2008;6:514–6. doi: 10.1111/j.1538-7836.2007.02876.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitrugno A, Tormoen GW, Kuhn P, McCarty OJT. The prothrombotic activity of cancer cells in the circulation. Blood Rev. 2016;30:11–9. doi: 10.1016/j.blre.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc. Natl. Acad. Sci. U. S. A. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit. Rev. Oncol. Hematol. 2004;50:187–96. doi: 10.1016/j.critrevonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Geng J-G. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch. Immunol. Ther. Exp. (Warsz.) 2006;54:75–84. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- 30.Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur. J. Intern. Med. 2013;24:393–400. doi: 10.1016/j.ejim.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Karpatkin S, Ambrogio C, Pearlstein E. The role of tumor-induced platelet aggregation, platelet adhesion and adhesive proteins in tumor metastasis. Prog. Clin. Biol. Res. 1988;283:585–606. [PubMed] [Google Scholar]
- 32.Alonso-Escolano D, Strongin AY, Chung AW, Deryugina EI, Radomski MW. Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. Br. J. Pharmacol. 2004;141:241–52. doi: 10.1038/sj.bjp.0705606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossi IM, Fitzgerald LA, Kendall A, Taylor JD, Sloane BF, Honn KV. Inhibition of human tumor cell induced platelet aggregation by antibodies to platelet glycoproteins Ib and IIb/IIIa. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. N. Y. N. 1987;186:378–83. doi: 10.3181/00379727-186-3-rc1. [DOI] [PubMed] [Google Scholar]
- 34.Bastida E, Almirall L, Ordinas A. Tumor-cell-induced platelet aggregation is a glycoprotein-dependent and lipoxygenase-associated process. Int. J. Cancer. 1987;39:760–3. doi: 10.1002/ijc.2910390617. [DOI] [PubMed] [Google Scholar]
- 35.Amirkhosravi A, Mousa SA, Amaya M, Francis JL. Antimetastatic effect of tinzaparin, a low-molecular-weight heparin. J. Thromb. Haemost. JTH. 2003;1:1972–6. doi: 10.1046/j.1538-7836.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 36.Borsig L. The role of platelet activation in tumor metastasis. Expert Rev. Anticancer Ther. 2008;8:1247–55. doi: 10.1586/14737140.8.8.1247. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig RJ, Boehme B, Podda M, Henschler R, Jager E, Tandi C, Boehncke W-H, Zollner TM, Kaufmann R, Gille J. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res. 2004;64:2743–50. doi: 10.1158/0008-5472.can-03-1054. [DOI] [PubMed] [Google Scholar]
- 38.Bane CE, Ivanov I, Matafonov A, Boyd KL, Cheng Q, Sherwood ER, Tucker EI, Smiley ST, McCarty OJT, Gruber A, Gailani D. Factor XI Deficiency Alters the Cytokine Response and Activation of Contact Proteases during Polymicrobial Sepsis in Mice. PloS One. 2016;11:e0152968. doi: 10.1371/journal.pone.0152968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9325–30. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 41.Jurasz P, Stewart MW, Radomski A, Khadour F, Duszyk M, Radomski MW. Role of von Willebrand factor in tumour cell-induced platelet aggregation: differential regulation by NO and prostacyclin. Br. J. Pharmacol. 2001;134:1104–12. doi: 10.1038/sj.bjp.0704343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medina C, Radomski MW. Role of Matrix Metalloproteinases in Intestinal Inflammation. J. Pharmacol. Exp. Ther. 2006;318:933–8. doi: 10.1124/jpet.106.103465. [DOI] [PubMed] [Google Scholar]
- 43.Mitrugno A, Williams D, Kerrigan SW, Moran N. A novel and essential role for FcγRIIa in cancer cell-induced platelet activation. Blood. 2014;123:249–60. doi: 10.1182/blood-2013-03-492447. [DOI] [PubMed] [Google Scholar]
- 44.Chang L-C, Chen T-C, Chen S-J, Chen C-L, Lee C-C, Wu S-H, Yen Y, Huang H-S, Lin J-J. Identification of a new class of WNT1 inhibitor: Cancer cells migration, G-quadruplex stabilization and target validation. Oncotarget. 2015 doi: 10.18632/oncotarget.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacchiarini L, Zucchella M, Milanesi G, Tacconi F, Bonomi E, Canevari A, Grignani G. Thromboxane production by platelets during tumor cell-induced platelet activation. Invasion Metastasis. 1991;11:102–9. [PubMed] [Google Scholar]
- 46.Franchini M, Montagnana M, Favaloro EJ, Lippi G. The bidirectional relationship of cancer and hemostasis and the potential role of anticoagulant therapy in moderating thrombosis and cancer spread. Semin. Thromb. Hemost. 2009;35:644–53. doi: 10.1055/s-0029-1242718. [DOI] [PubMed] [Google Scholar]
- 47.Dovizio M, Tacconelli S, Sostres C, Ricciotti E, Patrignani P. Mechanistic and pharmacological issues of aspirin as an anticancer agent. Pharm. Basel Switz. 2012;5:1346–71. doi: 10.3390/ph5121346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uluçkan O, Eagleton MC, Floyd DH, Morgan EA, Hirbe AC, Kramer M, Dowland N, Prior JL, Piwnica-Worms D, Jeong SS, Chen R, Weilbaecher K. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J. Cell. Biochem. 2008;104:1311–23. doi: 10.1002/jcb.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebremeskel S, LeVatte T, Liwski RS, Johnston B, Bezuhly M. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int. J. Cancer. 2015;136:234–40. doi: 10.1002/ijc.28947. [DOI] [PubMed] [Google Scholar]
- 50.White N, Ryten M, Clayton E, Butler P, Burnstock G. P2Y purinergic receptors regulate the growth of human melanomas. Cancer Lett. 2005;224:81–91. doi: 10.1016/j.canlet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 51.Boukerche H, Berthier-Vergnes O, Penin F, Tabone E, Lizard G, Bailly M, McGregor JL. Human melanoma cell lines differ in their capacity to release ADP and aggregate platelets. Br. J. Haematol. 1994;87:763–72. doi: 10.1111/j.1365-2141.1994.tb06736.x. [DOI] [PubMed] [Google Scholar]
- 52.Riess L. Pathology of Blood. Arch Anat Physiol Wissensch Med. 1872;39 [Google Scholar]
- 53.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupaimoole R, Armaiz-Pena GN, Pecot CV, Coward J, Deavers MT, Vasquez HG, Urbauer D, Landen CN, Hu W, Gershenson H, Matsuo K, Shahzad MMK, King ER, Tekedereli I, Ozpolat B, Ahn EH, Bond VK, Wang R, Drew AF, Gushiken F, Lamkin D, Collins K, DeGeest K, Lutgendorf SK, Chiu W, Lopez-Berestein G, Afshar-Kharghan V, Sood AK. Paraneoplastic Thrombocytosis in Ovarian Cancer. N. Engl. J. Med. 2012;366:610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silvis SE, Turkbas N, Doscherholmen A. Thrombocytosis in patients with lung cancer. JAMA. 1970;211:1852–3. [PubMed] [Google Scholar]
- 55.Tranum BL, Haut A. Thrombocytosis: platelet kinetics in neoplasia. J. Lab. Clin. Med. 1974;84:615–9. [PubMed] [Google Scholar]
- 56.Taucher S, Salat A, Gnant M, Kwasny W, Mlineritsch B, Menzel R-C, Schmid M, Smola MG, Stierer M, Tausch C, Galid A, Steger G, Jakesz R and Austrian Breast and Colorectal Cancer Study Group. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb. Haemost. 2003;89:1098–106. [PubMed] [Google Scholar]
- 57.Bensalah K, Leray E, Fergelot P, Rioux-Leclercq N, Tostain J, Guillé F, Patard J-J. Prognostic value of thrombocytosis in renal cell carcinoma. J. Urol. 2006;175:859–63. doi: 10.1016/S0022-5347(05)00526-4. [DOI] [PubMed] [Google Scholar]
- 58.ZHANG W, YU C, HUANG B, ZHOU F-L, HUANG H-D, LI Q. Correlation between bone metastasis and thrombocytosis in pulmonary adenocarcinoma patients. Oncol. Lett. 2015;9:762–8. doi: 10.3892/ol.2014.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur. Respir. J. 1996;9:1826–30. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 60.Maráz A, Furák J, Varga Z, Kahán Z, Tiszlavicz L, Hideghéty K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33:1725–9. [PubMed] [Google Scholar]
- 61.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J. Surg. Oncol. 2012;106:887–91. doi: 10.1002/jso.23163. [DOI] [PubMed] [Google Scholar]
- 62.Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. BJU Int. 2000;86:203–7. doi: 10.1046/j.1464-410x.2000.00792.x. [DOI] [PubMed] [Google Scholar]
- 63.Rothwell PM, Wilson M, Price JF, Belch JFF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet Lond. Engl. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 64.Stürmer T, Glynn RJ, Lee IM, Manson JE, Buring JE, Hennekens CH. Aspirin use and colorectal cancer: post-trial follow-up data from the Physicians’ Health Study. Ann. Intern. Med. 1998;128:713–20. doi: 10.7326/0003-4819-128-9-199805010-00003. [DOI] [PubMed] [Google Scholar]
- 65.Cook NR, Lee I, Gaziano J, et al. Low-dose aspirin in the primary prevention of cancer: The women’s health study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 66.Buller HR, van Doormaal FF, van Sluis GL, Kamphuisen PW. Cancer and thrombosis: from molecular mechanisms to clinical presentations. J. Thromb. Haemost. JTH. 2007;5(Suppl 1):246–54. doi: 10.1111/j.1538-7836.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- 67.Buss DH, Cashell AW, O’Connor ML, Richards F, Case LD. Occurrence, etiology, and clinical significance of extreme thrombocytosis: a study of 280 cases. Am. J. Med. 1994;96:247–53. doi: 10.1016/0002-9343(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 68.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J. Thromb. Haemost. JTH. 2004;2:2156–61. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 69.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–9. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 70.Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, Steger G, Jaeger U, Zielinski C, Pabinger I. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) J. Thromb. Haemost. JTH. 2010;8:114–20. doi: 10.1111/j.1538-7836.2009.03680.x. [DOI] [PubMed] [Google Scholar]
- 71.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981–6. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blom JW, Vanderschoot JPM, Oostindiër MJ, Osanto S, van der Meer FJM, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J. Thromb. Haemost. JTH. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 73.Carrier M, Le Gal G, Tay J, Wu C, Lee AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J. Thromb. Haemost. JTH. 2011;9:653–63. doi: 10.1111/j.1538-7836.2011.04215.x. [DOI] [PubMed] [Google Scholar]
- 74.Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kuzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–24. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 75.Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. Am. J. Hematol. 2012;87:308–10. doi: 10.1002/ajh.22262. [DOI] [PubMed] [Google Scholar]
- 76.Fijnheer R, Frijns CJ, Korteweg J, Rommes H, Peters JH, Sixma JJ, Nieuwenhuis HK. The origin of P-selectin as a circulating plasma protein. Thromb. Haemost. 1997;77:1081–5. [PubMed] [Google Scholar]
- 77.Chen M, Geng J-G. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch. Immunol. Ther. Exp. (Warsz.) 2006;54:75–84. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- 78.Blann AD, Noteboom WM, Rosendaal FR. Increased soluble P-selectin levels following deep venous thrombosis: cause or effect? Br. J. Haematol. 2000;108:191–3. doi: 10.1046/j.1365-2141.2000.01813.x. [DOI] [PubMed] [Google Scholar]
- 79.Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C, Pabinger I. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) Blood. 2008;112:2703–8. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 80.Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, Zielinski C, Pabinger I. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:2099–103. doi: 10.1200/JCO.2010.32.8294. [DOI] [PubMed] [Google Scholar]
- 81.Thaler J, Ay C, Weinstabl H, Dunkler D, Simanek R, Vormittag R, Freyssinet J-M, Zielinski C, Pabinger I. Circulating procoagulant microparticles in cancer patients. Ann. Hematol. 2011;90:447–53. doi: 10.1007/s00277-010-1111-1. [DOI] [PubMed] [Google Scholar]
- 82.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 83.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern. Emerg. Med. 2012;7:291–2. doi: 10.1007/s11739-012-0784-y. [DOI] [PubMed] [Google Scholar]
- 85.Ay C, Dunkler D, Marosi C, Chiriac A-L, Vormittag R, Simanek R, Quehenberger P, Zielinski C, Pabinger I. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–82. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 86.Pabinger I, Ay C. Risk of venous thromboembolism and primary prophylaxis in cancer. Should all patients receive thromboprophylaxis? Hämostaseologie. 2012;32:132–7. doi: 10.5482/ha-1173. [DOI] [PubMed] [Google Scholar]
- 87.Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, White RH. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:1112–8. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 88.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25:70–6. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 89.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 90.Mandalà M, Barni S, Prins M, Labianca R, Tondini C, Russo L, Milesi A, Cremonesi M, Zaccanelli M, Regonesi C, Moro C, Falanga A. Acquired and inherited risk factors for developing venous thromboembolism in cancer patients receiving adjuvant chemotherapy: a prospective trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO. 2010;21:871–6. doi: 10.1093/annonc/mdp354. [DOI] [PubMed] [Google Scholar]
- 91.Marks MA, Engels EA. Venous Thromboembolism and Cancer Risk among Elderly Adults in the U.S. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014;23:774–83. doi: 10.1158/1055-9965.EPI-13-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bleeker JS, Hogan WJ. Thrombocytosis: Diagnostic Evaluation, Thrombotic Risk Stratification, and Risk-Based Management Strategies. Thrombosis. 2011;2011:e536062. doi: 10.1155/2011/536062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skoda RC. Thrombocytosis. Hematol. Am. Soc. Hematol. Educ. Program. 2009:159–67. doi: 10.1182/asheducation-2009.1.159. [DOI] [PubMed] [Google Scholar]
- 94.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR and Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet Lond. Engl. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 95.James C, Ugo V, Le Couédic J-P, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 96.Kralovics R, Passamonti F, Buser AS, Teo S-S, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N. Engl. J. Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 97.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJP, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Fröhling S, Döhner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 98.Goerttler PS, Steimle C, März E, Johansson PL, Andreasson B, Griesshammer M, Gisslinger H, Heimpel H, Pahl HL. The Jak2V617F mutation, PRV-1 overexpression, and EEC formation define a similar cohort of MPD patients. Blood. 2005;106:2862–4. doi: 10.1182/blood-2005-04-1515. [DOI] [PubMed] [Google Scholar]
- 99.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 2007;25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 100.Duffy MJ. Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer: from pilot to level 1 evidence studies. Clin. Chem. 2002;48:1194–7. [PubMed] [Google Scholar]
- 101.Foekens JA, Schmitt M, van Putten WL, Peters HA, Kramer MD, Jänicke F, Klijn JG. Plasminogen activator inhibitor-1 and prognosis in primary breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994;12:1648–58. doi: 10.1200/JCO.1994.12.8.1648. [DOI] [PubMed] [Google Scholar]
- 102.Look MP, van Putten WLJ, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Fernö M, Eppenberger-Castori S, Sweep CGJF, Ulm K, Peyrat J-P, Martin P-M, Magdelenat H, Brünner N, Duggan C, Lisboa BW, Bendahl P-O, Quillien V, Daver A, Ricolleau G, Meijer-van Gelder ME, Manders P, Fiets WE, Blankenstein MA, Broët P, Romain S, Daxenbichler G, Windbichler G, Cufer T, Borstnar S, Kueng W, Beex LVAM, Klijn JGM, O’Higgins N, Eppenberger U, Jänicke F, Schmitt M, Foekens J. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J. Natl. Cancer Inst. 2002;94:116–28. doi: 10.1093/jnci/94.2.116. [DOI] [PubMed] [Google Scholar]
- 103.Harbeck N, Schmitt M, Kates RE, Kiechle M, Zemzoum I, Jänicke F, Thomssen C. Clinical utility of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 determination in primary breast cancer tissue for individualized therapy concepts. Clin. Breast Cancer. 2002;3:196–200. doi: 10.3816/CBC.2002.n.023. [DOI] [PubMed] [Google Scholar]
- 104.Zemzoum I, Kates RE, Ross JS, Dettmar P, Dutta M, Henrichs C, Yurdseven S, Höfler H, Kiechle M, Schmitt M, Harbeck N. Invasion factors uPA/PAI-1 and HER2 status provide independent and complementary information on patient outcome in node-negative breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003;21:1022–8. doi: 10.1200/JCO.2003.04.170. [DOI] [PubMed] [Google Scholar]
- 105.Key NS, Khorana AA, Mackman N, McCarty OJT, White GC, Francis CW, McCrae KR, Palumbo JS, Raskob GE, Chan AT, Sood AK. Thrombosis in cancer: Research priorities identified by a national cancer institute/national heart, lung, and blood institute strategic working group. Cancer Res. 2016;76:3671–5. doi: 10.1158/0008-5472.CAN-15-3100. [DOI] [PubMed] [Google Scholar]