Abstract
Objective
The role of TGF-β signaling in abdominal aortic aneurysm (AAA) formation is controversial. Others reported that systemic blockade of TGF-β by neutralizing antibodies accelerated AAA development in angiotensin II-infused mice. This result is consistent with other studies suggesting that TGF-β signaling prevents AAA. Development of a therapy for AAA that exploits the protective actions of TGF-β would be facilitated by identification of the mechanisms through which TGF-β prevents AAA. We hypothesized that TGF-β signaling prevents AAA by its actions on aortic medial smooth muscle cells.
Approach and Results
We compared the prevalence, severity, and histopathology of angiotensin II-induced AAA among control mice (no TGF-β blockade), mice with antibody-mediated systemic neutralization of TGF-β, and mice with genetically based smooth muscle-specific loss of TGF-β signaling. Surprisingly, we found that systemic—but not smooth muscle-specific—TGF-β blockade significantly increased the prevalence of AAA, and tended to increase AAA severity, adventitial thickening and aortic wall macrophage accumulation. In contrast, abdominal aortas of mice with smooth muscle-specific loss of TGF-β signaling differed from controls only in having a thinner media. We examined thoracic aortas of the same mice. Here we found that smooth muscle-specific loss of Tgfbr2—but not systemic TGF-β neutralization—significantly accelerated development of aortic pathology, including increased prevalence of intramural hematomas, medial thinning, and adventitial thickening.
Conclusion
Our results suggest that TGF-β signaling prevents both abdominal and thoracic aneurysmal disease but does so by distinct mechanisms. Smooth muscle-extrinsic signaling protects the abdominal aorta and smooth muscle-intrinsic signaling protects the thoracic aorta.
Keywords: aorta, angiotensin II, aneurysm, genetically altered mice, growth factor
Subject Terms: Aneurysm, Animal Models of Human Disease, Aortic Dissection, Genetically Altered and Transgenic Models, Vascular Disease
The prevalence of abdominal aortic aneurysms (AAA) in adults older than 50 is estimated at 5%–7% in men and 1.3% in women.1 Patients with AAA are usually asymptomatic and often present with sudden death. When an asymptomatic AAA is detected, the patient is typically monitored until aortic diameter reaches 5.5 cm, at which point percutaneous or open surgery to replace the dilated segment is recommended.2, 3 Both procedures are expensive, often complicated, and are sometimes unfeasible. Medical therapies that would slow or reverse AAA progression are highly desirable; however, no such therapies are currently available. A better understanding of the molecular mechanisms of AAA pathogenesis would facilitate the development of medical therapies.
The role of transforming growth factor-beta (TGF-β) in AAA pathogenesis is controversial, with data supporting both pathogenic and protective roles.4, 5 A pathogenic role for TGF-β is supported by detection of elevated TGF-β1 in human and experimental animal AAA tissue.6–9 However, these associations do not reveal causality and increased TGF-β1 expression in AAA tissue could be a homeostatic response that limits aortic damage. A protective role for TGF-β expression in AAA tissue is supported by data showing that overexpression of TGF-β1 in the aortic wall limits AAA expansion in rats.10, 11 Moreover, systemic neutralization of TGF-β significantly exacerbates AAA prevalence and severity in Ang II-infused mice.11, 12 In addition, we recently reported that loss of physiologic TGF-β signaling in aortic smooth muscle cells (SMC) of young mice caused significant abdominal aortic dilation and inflammation.13 Taken together, the preponderance of experimental data support a protective role for TGF-β in AAA development and suggest that this protective role might be exploited for the development of human therapies.
Development of a therapy based on the protective actions of TGF-β on AAA growth would be facilitated by identification of the mechanisms through which TGF-β prevents AAA. In the first study cited above,10 TGF-β1 overexpression decreased accumulation of monocyte/macrophages and T cells, suppressed aortic wall metalloproteinase activity, and increased intimal accumulation of SMC, collagen, and elastin. In the second study,11 systemic TGF-β neutralization stimulated accumulation of circulating monocytes in the aortic wall and promoted medial SMC apoptosis. Therefore, both studies suggest that TGF-β signaling may act both on circulating inflammatory cells to suppress their activation and on aortic SMC to preserve their health. In contrast, our study suggests that disruption of TGF-β signaling that is confined to SMC is sufficient to cause medial cell loss, aortic dilation, and inflammatory cell infiltration in the aortic wall.13
We hypothesized that TGF-β signaling in aortic SMC—not immune cells—is a primary protector of abdominal aortic health. According to this hypothesis, destructive inflammatory responses generated by systemic neutralization of TGF-β in the Ang-II AAA model11 are all caused by stimulation of the immune system by SMC that are injured by withdrawal of TGF-β signaling. We used the Ang II-induced AAA model14 and mice with conditional alleles for the type II TGF-β receptor (TBRII)13, 15 to test whether SMC-specific loss of TGF-β signaling accelerates AAA formation equivalently to systemic neutralization of TGF-β activity. Because Ang-II infusion also causes thoracic aortic dilation,16, 17 we also examined thoracic aortas of the experimental mice. The pathogenesis of thoracic and aortic aneurysms appears to differ,18 and some propose that TGF-β signaling in the thoracic aorta is pathogenic, not protective.19 We reasoned that close examination of the abdominal and thoracic aortas of the same mice—not done in previous studies in this model11, 12, 20—might reveal site-specific roles for TGF-β signaling.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
Neutralization of Systemic TGF-β Activity and Knockdown of TBRII in Aortic SMC
To test whether the TGF-β neutralizing antibody 2G7 blocked TGF-β1 activity in vivo, we measured active and total TGF-β1 activity in serum of mice injected intraperitoneally with 10, 15, or 20 mg/kg of 2G7 or with vehicle (n=4 per group comprised of 2 male and 2 female mice). Active TGF-β1 was nearly undetectable in serum of vehicle-treated mice and was undetectable in serum of mice treated with all of the 2G7 doses (data not shown). TGF-β1 activity in acid-activated serum (i.e., the total activity of both active and latent serum TGF-β1) was reduced by 2G7 in a dose-dependent manner (Figure I in the online-only Data Supplement). The reduction in TGF-β1 activity from the 10 mg/kg dose was similar in males and females (data not shown). Because the 10 mg/kg dose significantly lowered serum TGF-β1 and because previous work showed high mortality in Ang II-infused mice injected with higher doses of 2G7,11 we used the 10 mg/kg dose for the remainder of the in vivo studies. We measured TBRII protein in extracts of aortic media of tamoxifen-injected Acta2-CreERT2 0/0 (control) and Acta2-CreERT2 +/0 mice. TBRII was significantly reduced in Acta2-CreERT2 +/0 mice (42%; P=0.02; Figure II in the online-only Data Supplement).
Ang II-induced Abdominal Aortic Pathology is Exacerbated by Systemic TGF-β Blockade, Not by SMC-Specific Loss of TGF-β Signaling
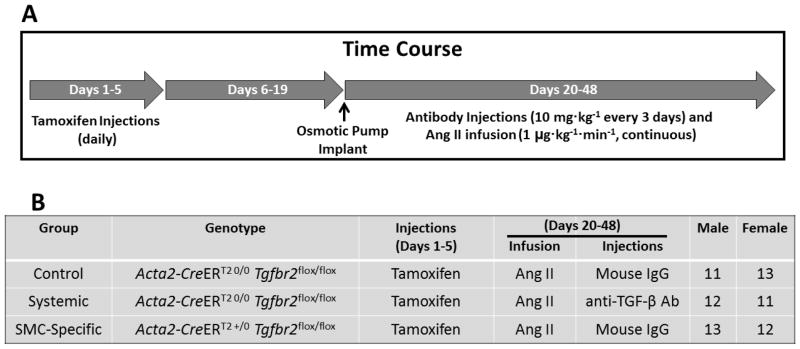
We compared the development of abdominal aortic aneurysms in 3 groups of Ang II-infused Tgfbr2flox/flox mice, all of which also received tamoxifen injections (Figure 1): 1) Acta2-CreERT2 0/0 mice injected with mouse IgG (the control group); 2) Acta2-CreERT2 0/0 mice injected with the 2G7 antibody (systemic TGF-β blockade); and 3) Acta2-CreERT2 +/0 mice injected with mouse IgG (SMC-specific loss of TGF-β signaling). The 3 groups included nearly equal numbers of male and female mice (Figure 1B). Of 75 mice enrolled (25 per group), 71 survived to completion of the study. One mouse treated with the 2G7 antibody died from apparent abdominal aortic rupture. This mouse was considered to have had abdominal aortic pathology of the highest severity (grade 4; see below) but was not included in other analyses. Two other 2G7-treated mice died prematurely, as did one mouse in the control group. The cause of these 3 deaths was uncertain and aortas of these mice were not analyzed. There were no statistically significant differences in survival among the 3 groups (Figure 2A).
Figure 1.
Experimental time course and groups. (A) Mice are enrolled at 6 weeks of age and receive daily tamoxifen injections for 5 days. Two weeks after the last injection, mice are implanted with osmotic minipumps that release angiotensin II (Ang II) for 28 days. During the Ang II infusion, antibodies are injected every three days. Mice are euthanized on day 48. (B) Genotypes, treatments, and number of mice enrolled in each of the 3 experimental groups. The numbers of male and female mice reflect those that completed the study.
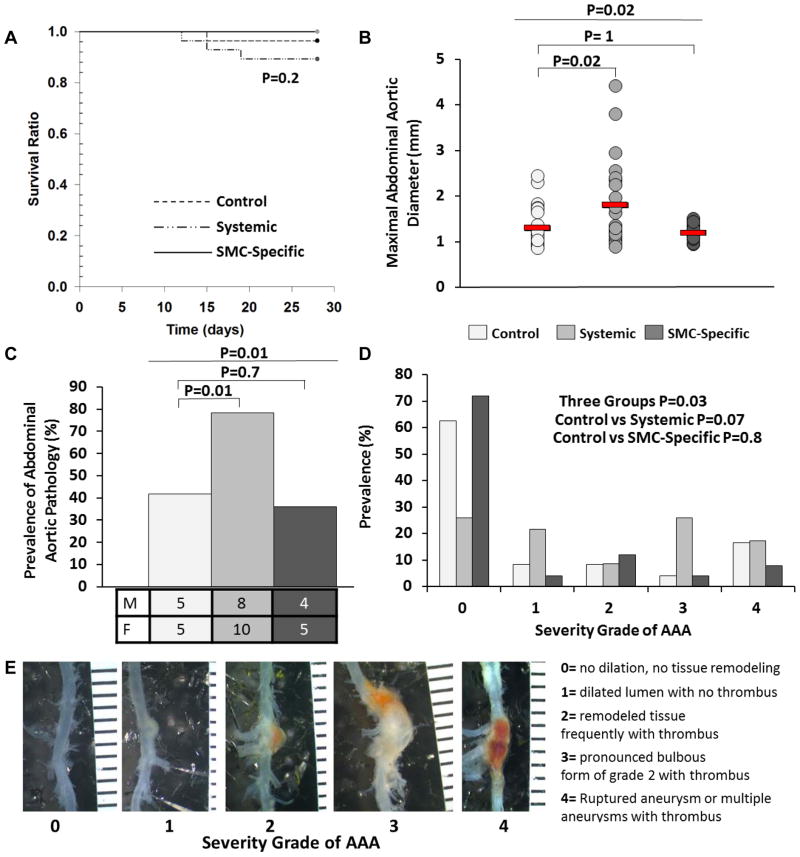
Figure 2.
Systemic TGF-β signaling blockade—not SMC-specific loss of TGF-β signaling—exacerbates angiotensin II-induced gross abdominal aortic pathology. (A) Survival of mice in 3 experimental groups: control mice (no TGF-β inhibition); mice with systemic inhibition of TGF-β activity; and mice with SMC-specific loss of TGF-β signaling. (B–D) After 28 days of angiotensin II infusion, aortas (n=20–25 per group) were removed from surviving mice, photographed, and examined. (B) Maximal abdominal aortic diameters (adventitia to opposite side of adventitia), measured on images of explanted aortas. Red lines indicate group means; data points are individual mice. (C) Prevalence of abdominal aortic pathology and number of mice affected (shown by sex). (D) Severity of abdominal aortic pathology. (E) Scale for grading abdominal aortic pathology, with examples. (A) P value is from log-rank test. (B) P values are from Kruskal-Wallis one-way ANOVA (overall P value comparing the 3 groups is above), with Dunn’s correction for the pair-wise comparisons. (C,D) P values are from chi-square tests. (E) Ruler is in mm.
We assessed gross abdominal aortic pathology in the remaining 71 mice. Compared to controls, mean maximal aortic external diameter (from one side of the adventitia to the opposite side) was significantly increased in mice treated with 2G7 (43%; P=0.02; Figure 2B). In contrast, SMC-specific deletion of TBRII had no significant effect on maximal external abdominal aortic diameter (P=1.0 versus controls). Similarly, 2G7 injection—but not SMC-specific loss of TBRII—significantly increased the prevalence of abdominal aortic pathology (with pathology defined as presence of aortic dilation, blood in the aortic wall, or both). Pathology was present in abdominal aortas of 17 of 22 surviving mice injected with the 2G7 antibody (18 of 23 including the mouse with aortic rupture; 78%) compared to 10 of 24 surviving control mice (42%; P=0.01 versus 2G7 recipients; Figure 2C) and 9 of 25 mice with SMC-specific loss of TBRII (36%; P=0.7 versus controls). The prevalence of abdominal aortopathy for all three groups was nearly identical between males and females. Independent assessment of the severity of abdominal aortic pathology (the 0 – 4 severity scale is illustrated in Figure 2E) revealed a borderline significant increase in severity in mice treated with 2G7. The majority of control mice (62%) had grade 0 pathology, with the remainder divided among grades 1 – 4 (Figure 2D). The distribution of severity grades was similar in mice with SMC-specific loss of TBRII (P=0.8 versus controls). In contrast, far fewer mice treated with 2G7 had grade 0 pathology (26%); 43% had either grade 3 or 4 pathology (P=0.07 versus controls).
Ang II-induced Thoracic Aortic Pathology is Exacerbated by SMC-Specific Loss of TGF-β Signaling, Not by Systemic TGF-β Blockade
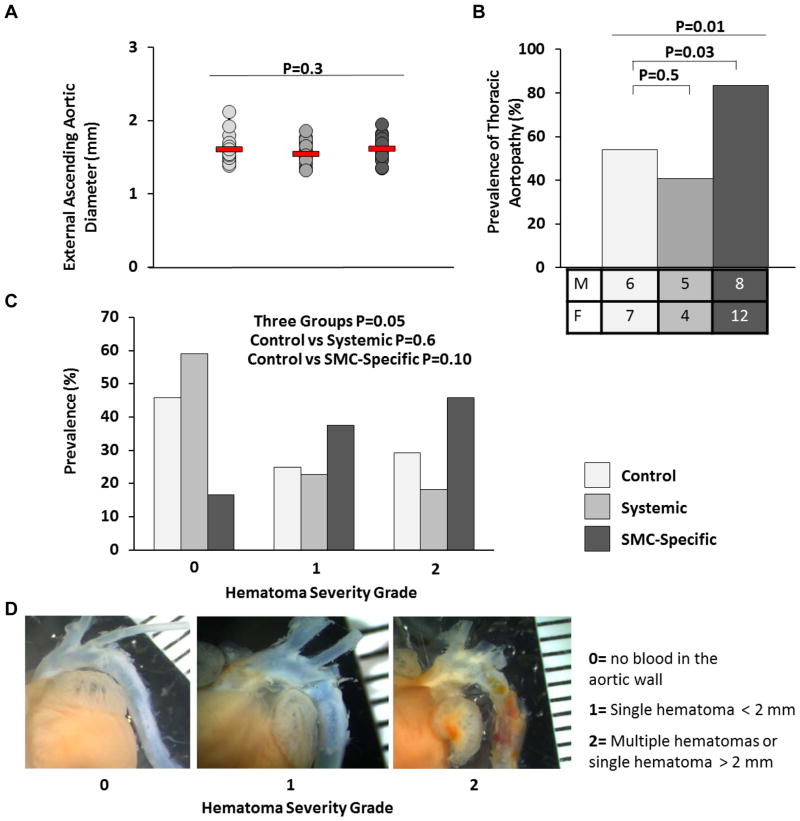
Ang II treatment also causes aneurysmal disease of the thoracic aorta.16, 17 To test whether systemic TGF-β signaling blockade or SMC-specific loss of TGF-β signaling exacerbate thoracic aortic pathology in Ang II-infused mice, we measured ascending aortic diameter (from one side of the adventitia to the opposite side, with all measurements made just proximal to the brachiocephalic artery takeoff) in the same mice in which we analyzed abdominal aortas. None of the ascending aortas appeared dilated, and there were no significant differences among the 3 groups in external aortic diameter (P=0.3; Figure 3A). However, SMC-specific loss of TGF-β signaling significantly increased the prevalence of aortic wall hematomas and also tended to increase the severity of hematomas. Bleeding was present in the thoracic aortic wall of 20 of 24 mice with SMC-specific loss of TGF-β signaling (83%; results in one mouse in this group were inconclusive and it was omitted) compared to 13 of 24 surviving control mice (54%; P=0.03; Figure 3B) and 9 of the 22 surviving mice with systemic TGF-β signaling blockade (41%; P=0.5 versus controls). The prevalence of thoracic aortopathy was nearly identical between males and females in controls and mice with systemic TGF-β signaling blockade, but slightly higher in females than males with SMC-specific TGF-β signaling blockade. We independently assessed the severity of aortic intramural bleeding (the 0 – 2 severity scale is illustrated in Figure 3D). Bleeding was grade 0 in ~45% of aortas of control mice with ~25% of mice with grade 1 and ~30% with grade 2 bleeding (Figure 3C). Mice with systemic TGF-β signaling blockade had a similar distribution of bleeding severity (P=0.7 versus controls). Far fewer mice with SMC-specific TGF-β blockade had grade 0 bleeding (< 20%); 80% had either grade 1 or grade 2 bleeding; however, this difference was not statistically significant; P=0.1 versus controls).
Figure 3.
SMC-specific loss of TGF-β signaling—not systemic TGF-β signaling blockade—exacerbates angiotensin II-induced gross thoracic aortic pathology. After 28 days of angiotensin II infusion, aortas were removed from: control mice (no TGF-β inhibition); mice with systemic inhibition of TGF-β activity; and mice with SMC-specific loss of TGF-β signaling. (A) Ascending aortic diameters (adventitia to opposite side of adventitia), measured on images of explanted aortas. Red lines indicate group means; data points are individual mice. (B) Prevalence of thoracic aortic pathology and number of mice affected (shown by sex). (C) Severity of thoracic aortic pathology. (D) Scale for grading thoracic aortic pathology, with examples. (A) P value is from one-way ANOVA; (n=18–21 per group). (B–C) P values are from chi-square tests (n=22–24 per group). (B) Overall P value comparing the 3 groups is above. (E) Ruler is in mm.
Systemic Blockade of TGF-β Signaling and SMC-Specific Loss of TGF-β Signaling Have Tissue-Specific Effects on Abdominal Aortic Adventitia and Media
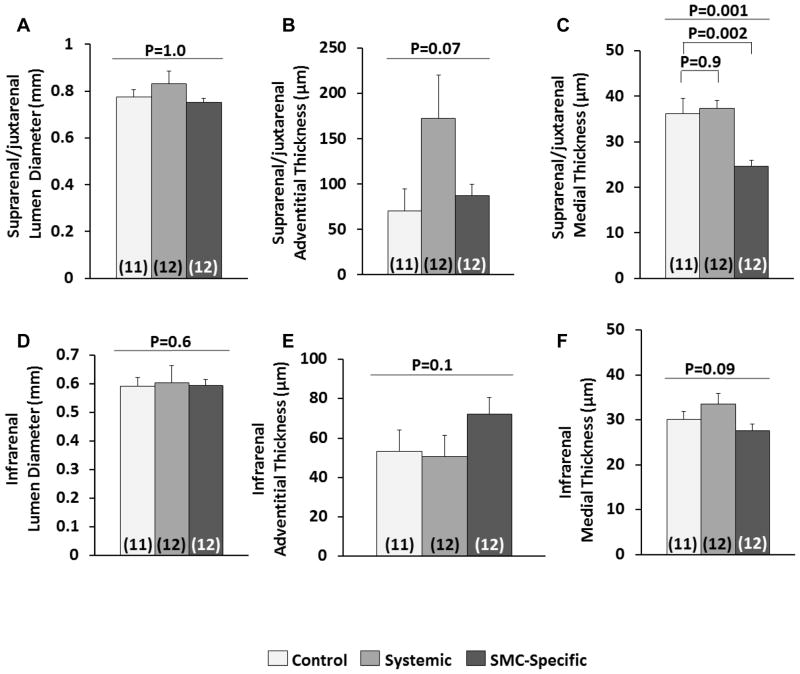
The different effects of the 2 interventions on the abdominal and thoracic aorta were surprising. Moreover, the results in the abdominal aorta did not support our hypothesis that worsened abdominal aortic pathology in Ang II-infused mice with systemic TGF-β blockade is caused by loss of salutary effects of TGF-β on aortic medial SMC. To determine whether SMC-specific loss of TGF-β signaling caused any abdominal aortic medial pathology in Ang II-infused mice and to gain further insights into why systemic TGF-β signaling blockade—but not SMC-specific loss of TGF-β signaling—accelerates gross abdominal aortic pathology, we performed histologic analyses of a subset of abdominal aortas (12 per group; chosen randomly and without regard to presence or absence of gross pathology). Because the most severe pathology was in the area adjacent to and cranial to the renal arteries, we analyzed suprarenal/juxtarenal aortas and infrarenal aortas separately (Figure III in the online-only Data Supplement).
Despite significantly increased external abdominal aortic diameters in mice with systemic TGF-β signaling blockade (Figure 2B), the mean internal (i.e., luminal) suprarenal/juxtarenal aortic diameters of mice in this group were not increased (P=1.0 for comparison among the 3 groups; Figure 4A and Figure IV in the online-only Data Supplement). Rather, increased external aortic diameters in mice with systemic TGF-β signaling blockade appeared due entirely to adventitial expansion and not to luminal expansion or accumulation of blood within dissected aortic media (Figure 4B and Figure IV J–L in the online-only Data Supplement). However, adventitial expansion did appear at least partially due to medial rupture with extravasated blood contained within the adventitia and thrombosed in situ (Figure IVJ and IVK in the online-only Data Supplement). The average suprarenal/juxtarenal adventitial thickness was 70±24 μm in control mice, and was increased 2.5-fold to 170±48 μm in mice with systemic TGF-β signaling blockade (the increase had only borderline statistical significance; P=0.07 by ANOVA of the 3 groups). Suprarenal/juxtarenal adventitial thickness in mice with SMC-specific TGF-β blockade was similar to controls (87±12 μm). In contrast to the adventitia, mean medial thickness in the suprarenal/juxtarenal aorta of mice with systemic TGF-β signaling blockade did not differ significantly from control mice (36±3 μm versus 37±2 μm, respectively; P=0.9; Figure 4C). However, mice with SMC-specific loss of TGF-β signaling had significant medial thinning in the suprarenal/juxtarenal aorta (25±1 μm; P=0.002 versus control mice; Figure 4C).
Figure 4.
Systemic TGF-β signaling blockade and SMC-specific loss of TGF-β signaling have tissue-specific effects on the abdominal aorta. Planimetry was performed on transverse histologic sections of suprarenal/juxtarenal (A–C) and infrarenal (D–F) segments of abdominal aortas. Luminal, outer medial, and outer adventitial circumferences were measured. All other parameters were calculated assuming circular geometry in vivo. Aortas were from control mice (no TGF-β inhibition), mice with systemic inhibition of TGF-β activity, and mice with SMC-specific loss of TGF-β signaling. All mice received angiotensin II infusions. The number of mice per group is indicated in each bar. Bar heights are means; variance is SEM. (A, B, D and E) P values are from Kruskal-Wallis one-way ANOVA (overall P value is shown). (C and F) P values are from one-way ANOVA (overall P value is above) with Dunnett’s correction for the pair-wise comparisons (C).
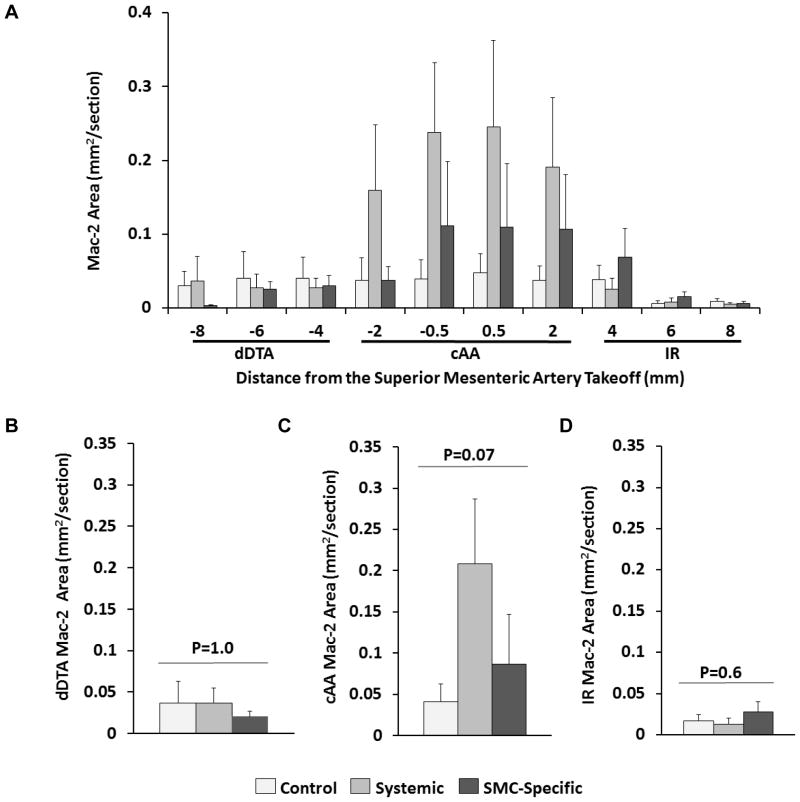
The thickened abdominal aortic adventitia of mice with systemic TGF-β signaling blockade included occasional thrombi and apparent inflammatory cells. To identify these cells, we stained sections for the Mac-2 marker. The level of Mac-2 staining was highly variable, both among animals in the same group and among aortic sections in the same animal. Qualitatively, most sections had at least some Mac-2 staining, with the highest levels of staining in sections with adventitial expansion (Figure VD in the online-only Data Supplement). Among the 3 groups, sections of aortas of mice with systemic TGF-β signaling blockade had the highest levels of Mac-2 staining, with Mac-2 positivity concentrated in a 4-mm region surrounding the takeoff of the superior mesenteric artery (Figure 5A–5D). In contrast, aortic sections of mice with SMC-specific loss of TGF-β signaling had less Mac-2 staining, and control mice had only low levels of Mac-2 staining. Differences among the 3 groups in this 4-mm region were large (2.5–4-fold, although they were of only borderline statistical significance; P=0.07; Figure 5C). Others reported that Ang II treatment alone causes inflammation and expansion of the suprarenal aorta.21 However, in the present study, systemic TGF-β blockade increased both adventitial thickness (Figure 4B) and macrophage accumulation far above levels in the Ang II-infused controls.
Figure 5.
Systemic TGF-β inhibition increases macrophage accumulation in a focal region of the abdominal aorta. (A) Area staining for the Mac-2 antigen was measured on transverse sections of aortas from: control mice (no TGF-β inhibition); mice with systemic inhibition of TGF-β activity; and mice with SMC-specific loss of TGF-β signaling. All mice had 28 days of angiotensin II infusion. Sections were from the distal descending thoracic aorta (dDTA), cranial abdominal aorta (cAA), and infrarenal aorta (IR). (B–D) Mean Mac-2-stained area per section for each of the 3 aortic regions shown in (A). Data from the 1–2 mm tissue steps in (A) were pooled for each aortic region (dDTA, cAA, and IR) and used for region-specific analyses. (A – D) Bar heights are means; variance is SEM. (B–D) P values are from Kruskal-Wallis one-way ANOVA; n=8–11 per group
Analyses of sections of infrarenal aortas showed no significant differences among the groups in lumen diameter, adventitial thickness or medial thickness, although there were modest trends towards thicker adventitias and thinner medias in mice with SMC-specific loss of TGF-β signaling (Figure 4D–4F). Little Mac-2 staining was present in any of the infrarenal aortic sections (Figure 5A and 5D). These data suggest that the primary consequence of systemic blockade of TGF-β signaling on abdominal aortas of Ang II-infused mice is the initiation of severe focal adventitial inflammation; systemic TGF-β blockade has relatively little effect on medial SMC. In contrast, SMC-specific loss of TGF-β signaling in Ang II-infused mice primarily damages medial SMC and does not elicit a severe inflammatory reaction.
SMC-Specific Loss of TGF-β Signaling, but Not Systemic TGF-β Blockade, Alters Thoracic Aortic Structure
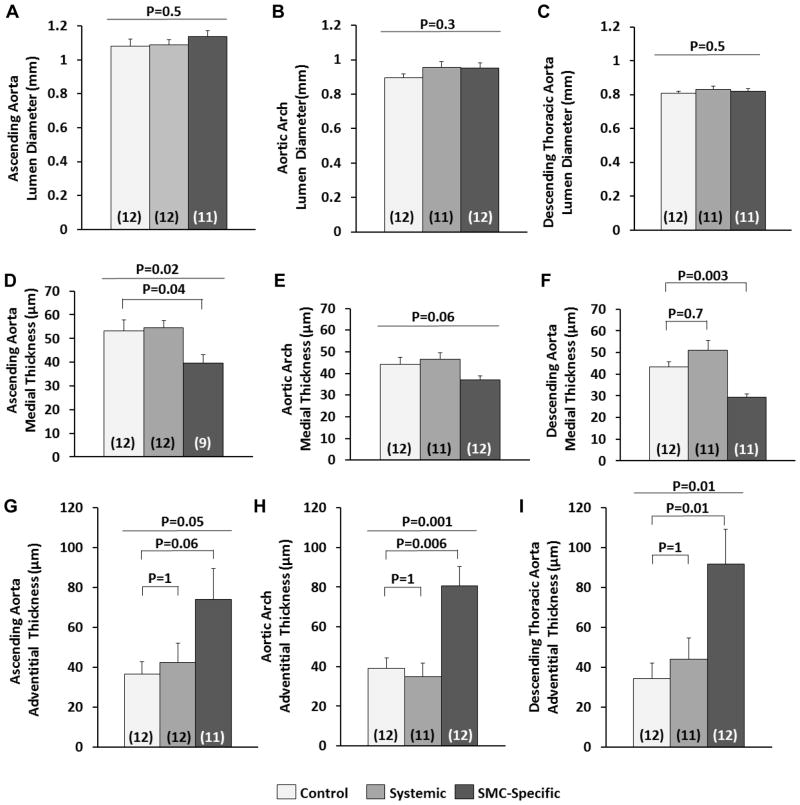
To determine whether systemic blockade of TGF-β signaling caused any thoracic aortic pathology in Ang II-infused mice and to gain further insights into why SMC-specific loss of TGF-β signaling—but not systemic TGF-β signaling blockade—accelerates thoracic aortic pathology, we performed histologic analyses of thoracic aortas of the same mice used for abdominal aortic analyses (12 per group). We examined sections from the ascending aorta, aortic arch, and proximal descending aorta (Figure III in the online-only Data Supplement). Neither SMC-specific loss of TGF-β signaling nor systemic TGF-β signaling blockade altered thoracic aortic lumen diameter (Figure 6A–6C). SMC-specific loss of TGF-β signaling caused significant medial thinning in all 3 regions of the thoracic aorta (16%–33% thinner media than controls; P=0.04 and P=0.003 for the ascending and proximal descending aorta, respectively; Figure 6D–6F). Aortas from mice with SMC-specific loss of TGF-β signaling also had significant thickening of the thoracic aortic adventitia (2–3-fold in all 3 regions; P≤0.04; Figure 6G–6I) with increased cellularity. Thoracic aortic sections had only low levels of Mac-2 expression, with no differences among the 3 groups in any of the aortic regions (Figure VI in the online-only Data Supplement). These data are similar to those obtained by gross examination of experimental aortas in showing significant effects of SMC-specific loss of TGF-β signaling—but not systemic TGF-β blockade—on the thoracic aorta.
Figure 6.
SMC-specific loss of TGF-β signaling—but not systemic TGF-β signaling blockade—alters thoracic aortic architecture. Planimetry was performed on transverse histologic sections of ascending aorta (A, D, and G), aortic arch (B, E, and H), and descending thoracic aorta (C, F, and I). Aortas were from control mice (no TGF-β inhibition), mice with systemic inhibition of TGF-β activity, and mice with SMC-specific loss of TGF-β signaling. All mice received angiotensin II infusions. Luminal, outer medial, and outer adventitial circumferences were measured. All other parameters were calculated assuming circular geometry in vivo. The number of mice per group is indicated in each bar. Bar heights are means; variance is SEM. (A, B, C–E) P values are from one-way ANOVA (overall P value is above) with Dunnett’s correction for the pairwise comparisons (D). (F–I) P values are from Kruskal-Wallis one-way ANOVA (overall P value is above) with Dunn’s correction for the pair-wise comparisons.
Systemic Neutralization of TGF-β1, -β2 and -β3 Activity in Ang II-Infused Mice
As our study neared completion, a concern was raised about whether the 2G7 antibody blocked serum TGF-β2 and TGF-β3 (in addition to TGF-β1). The ability of 2G7 to neutralize TGF-β isoforms in vivo in the setting of Ang II infusion was also questioned. To address both of these concerns, we implanted Ang II pumps in 2 groups of mice (9–10 per group), treated one group with 2G7 (10 mg/kg) and the other with the same dose of mouse IgG. After 1 week, 2G7 significantly reduced serum total (i.e., acid-activatable) TGF-β1 (60% decrease; Figure XA in the online-only Data Supplement; P=0.002). TGF-β3 activity was undetectable in serum of either group. Surprisingly, 2G7 did not reduce serum total TGF-β2 activity (Figure XB in the online-only Data Supplement; P=0.9). We then tested whether 2G7 or another putative “pan-neutralizing anti-TGF-β antibody” 1D11 (also widely used in aortopathy research)12, 22–24 could neutralize all 3 TGF-β isoforms in vitro. Both 2G7 and 1D11 neutralized TGF-β1 and TGF-β3; whereas, neither antibody had any activity against TGF-β2 (Figure XD – XF in the online-only Data Supplement).
Systolic Blood Pressure Measurements in Control and Experimental Mice
Because alterations in TGF-β signaling can affect arterial blood pressure,25 we measured blood pressure in separate cohorts of control and experimental mice (9–12 per group). Two weeks after beginning Ang II infusion, control mice had significantly lower systolic blood pressure (101±8 mmHg) than mice with systemic TGF-β signaling blockade (129±6 mmHg; P=0.04) and a statistically insignificant decrease in systolic blood pressure compared to mice with SMC-specific loss of TGF-β signaling (121±8 mmHg; P=0.1; Supplemental Fig. XIA in the online-only Data Supplement). However, after 4 weeks of Ang II infusion systolic blood pressure did not differ significantly among the 3 groups (P=0.3; Supplemental Fig. XIB in the online-onlyData Supplement). Systolic blood pressure in mice with SMC-specific loss of TGF-β signaling did not differ significantly from controls at either time point and was similar to blood pressure in mice with systemic TGF-β signaling blockade.
Discussion
We tested the hypothesis that TGF-β signaling in aortic SMC is a primary protector of aortic health and that abdominal aortic expansion, tissue destruction, and inflammation in Ang II-infused mice with systemic antibody-mediated TGF-β neutralization11 are all consequences of loss of salutary physiologic TGF-β signaling in aortic medial SMC. Our results do not support this hypothesis. Instead we found that: 1) systemic—but not SMC-specific—blockade of TGF-β signaling significantly increased the prevalence of AAA and tended to increase AAA severity; 2) systemic—but not SMC-specific—blockade of TGF-β signaling tended to increase abdominal aortic adventitial expansion and macrophage accumulation; 3) SMC-specific blockade of TGF-β signaling caused significant thinning of the abdominal aortic media but had negligible effects on inflammation and expansion of the abdominal aortic adventitia. Therefore, systemic blockade of TGF-β signaling acts on cells other than SMC to accelerate AAA in Ang II-infused mice and the protective effects of TGF-β on AAA in this model are not mediated primarily by its actions on SMC. Our study supports a protective effect of TGF-β in the abdominal aorta and breaks new ground in helping to identify the cell type in which TGF-β acts to prevent AAA.
Another important and novel aspect of our study is that—in addition to tracking the prevalence of abdominal and thoracic aortic rupture—we also examined abdominal and thoracic aortic dimensions and histopathology in the same experimental mice. This design provides ideal internal controls (thoracic aorta for abdominal aorta and vice versa) that allow confident conclusions about site-specific actions of TGF-β. In thoracic aortas we found: 1) SMC-specific—but not systemic—blockade of TGF-β signaling significantly increased the prevalence of aortic hematomas and tended to increase their severity; 2) SMC-specific—but not systemic—blockade of TGF-β signaling caused significant medial thinning and adventitial thickening; and 3) systemic blockade of TGF-β signaling had negligible effects on the thoracic aorta. Therefore, in Ang II-infused mice, thoracic aortic pathology is accelerated by SMC-specific—but not systemic—blockade of TGF-β signaling. When combined with results in the abdominal aorta, our results show region- and tissue-specific roles for TGF-β in preventing aortic pathology.
Our finding that TGF-β signaling protects against AAA development is consistent with several reports published both before and after initiation of the present study. These include 3 studies showing that infusion of neutralizing antibodies to TGF-β accelerates AAA formation in Ang II-infused mice,11, 12, 20 as well as a report that whole-body knockout of Smad3 (a mediator of TGF-β signaling) increases AAA severity in a model of calcium chloride-induced AAA.26 In contrast, one study showed that injection of neutralizing antibodies to TGF-β diminished Ang II-induced AAA.6 This latter study suggests that TGF-β signaling contributes to Ang II-induced AAA formation; however, the salutary effect of antibody injection was found only in mice deficient in Cxcl10 (not in wild-type mice) and therefore may not reveal the normal physiologic role of TGF-β. Moreover, this study used a low dose of a polyclonal rabbit anti-TGF-β antibody that was recently shown to be of limited efficacy when given at a far higher dose.12 In addition, neutralization of serum TGF-β by the polyclonal antibody was not documented in this study.6
Physiologic TGF-β signaling appears to protect against AAA; however, neither antibody-mediated systemic TGF-β neutralization nor whole-body deletion of a gene (e.g., Smad3) can identify the site at which protective TGF-β signaling occurs. Because the earliest events in Ang II-induced AAA formation include macrophage accumulation in the suprarenal aortic media and aortic medial dissection,27, 28 because systemic TGF-β neutralization in Ang-II-infused mice is accompanied by early medial SMC apoptosis,11 and because ablation of TGF-β signaling in SMC causes severe medial pathology and macrophage infiltration,13, 29 we hypothesized that ablation of TGF-β signaling that was confined to SMC would accelerate Ang II-induced AAA formation. According to this model, medial SMC deprived of trophic TGF-β signaling would attract monocyte/macrophages by release of chemoattractant apoptotic bodies30 or by release of extracellular matrix fragments31, 32 generated by SMC proteolytic activity.33 Subsequent macrophage-mediated proteolytic tissue destruction, potentially mediated by MMP-1211 would accelerate AAA formation. Surprisingly, AAA prevalence/severity and aortic wall macrophage accumulation were unaffected by SMC loss of TGF-β signaling in Ang II-infused mice. The only discernable effect of ablation of SMC TGF-β signaling on Ang II-mediated AAA formation was moderate medial thinning. We conclude that physiologic TGF-β signaling protects against AAA primarily via actions on cell types other than SMC.
If TGF-β does not prevent AAA via actions on SMC, via which cell type(s) are the protective effects of TGF-β mediated? Monocyte/macrophages are among the cell types on which TGF-β appears to act to mitigate Ang II-induced AAA formation;11 however, elimination of monocyte/macrophages does not completely block the pathogenic effects of TGF-β neutralization in this model.11 Moreover, enhanced monocyte/macrophage accumulation in aortas of mice treated with TGF-β neutralizing antibody is exquisitely localized to a 4-mm segment of suprarenal adventitia (Figure 5A). These observations suggest that TGF-β protects against Ang-II-induced AAA via actions on a cell type present in a focal area of the adventitia.27 Candidate cell types on which TGF-β may exert protective actions include fibroblasts,21, 34 adipocytes,35, 36 as well as resident immune cells and progenitor cells.37 Future work will be aimed at identifying this cell type. In addition to the target cell type, the source of protective TGF-β ligand is also unknown. Our study does not reveal whether the ligand is produced locally (i.e. by cells in the abdominal aortic wall), whether it is produced outside the aorta (for example in the bone marrow) and acts on the abdominal aortic wall, or whether it is produced outside the aorta and acts on extravascular cells (e.g., immune cells) before their arrival to the abdominal aorta. Resolving this question will be challenging because of potential involvement of multiple TGF-β ligands and numerous candidate cell types.
Ang II infusion in mice also causes ascending thoracic aortic aneurysms,16, 17, 38 and systemic neutralization of TGF-β appears to worsen this pathology. For example, Wang et al reported that systemic neutralization of TGF-β in Ang II-infused mice caused ascending aortic rupture in 15% of mice.11 Chen et al recently confirmed a moderate (25%) prevalence of ascending aortic rupture in Ang-II-infused mice treated with neutralizing antibodies to TGF-β.12 However, the effects of TGF-β neutralization on thoracic aortic structure and inflammation are not well studied. Ascending aortic dimensions in surviving mice were not reported in the study by Wang et al,11 and TGF-β neutralization inconsistently increased thoracic aortic dimensions in the study by Chen et al.12 Neither study presented data obtained by histologic analysis of thoracic aortic sections. Here we report that treatment of mice with neutralizing antibodies to TGF-β did not cause thoracic aortic rupture and had no significant effects on gross aortic dimensions, microscopic aortic dimensions, or macrophage accumulation. Absence of thoracic aortic rupture in our study could be due to our use of a lower antibody dose than was used by Wang et al,11 or our use of a different antibody than was used by Chen et al.12 However, the antibody dose used in the present study clearly exacerbated Ang-II-induced AAA while having no detectable effects on thoracic aortas of the same mice. These data suggest that SMC-extrinsic TGF-β signaling is a more important protector of Ang II-mediated pathology in the abdominal aorta than in the thoracic aorta. Two other potential causes for disparate results between our study and those of Wang and Chen are genetic drift among C57BL/6 colonies and variability in intestinal microbiota among institutions. Experimental evidence for a potential role of the microbiome has been reported in settings of allograft rejection and vascular disease, with immunomodulation as the pathway that hypothetically connects the microbiome with biological responses at other locations.39–41 Because Ang II-induced AAA depends to a large extent on infiltrating monocytes,11 microbiome-mediated effects on immunity could, at least in part, account for the differences among the studies.
In contrast to our finding that systemic TGF-β neutralization did not alter thoracic aortic pathology, we found that TGF-β signaling blockade confined to SMC had significant pathogenic effects on the thoracic aorta including increased intramural hematomas, medial thinning, and adventitial thickening. These data suggest that TGF-β signaling in SMC is a primary protector of Ang II-mediated pathology in the thoracic aorta, but not in the abdominal aorta. Our results are consistent with others’ work showing fundamental differences between Ang II-induced pathology in thoracic versus abdominal aortas, including variations in patterns of elastolysis, response to hyperlipidemia, pattern of luminal expansion, and role of endothelial AT1a receptors.17, 38, 42, 43 Our results are also consistent with data showing variability in the effects of TGF-β on SMC from different segments of the aorta.44, 45 Both Ang II-mediated disease and the mechanisms of TGF-β protection appear to differ between the thoracic and abdominal aorta.
The results of the present study, including our finding that loss of SMC TGF-β signaling has only minimal effects on Ang II-induced abdominal aortic pathology, may appear to conflict with our previous report that SMC-specific Tgfbr2 deletion in otherwise normal mice causes severe thoracic and abdominal aortopathy.13 However, the two studies are fundamentally different. In the present study all mice, including those in the control group, were treated with Ang II; whereas, in the former study none of the mice received Ang II. Ang II causes both thoracic and abdominal aortopathy;14, 16 therefore, the present study investigates the role of SMC-specific loss of Tgfbr2 on Ang II-induced aortopathy, not on normal aortic homeostasis. A potential explanation for different consequences of loss of SMC TGF-β signaling in the presence or absence of Ang II is that loss of TGF-β signaling impairs activation of several downstream protein kinases;5, 13, 29 whereas, Ang II is a potent activator of many of the same SMC protein kinases.46 Accordingly, stimulation of SMC protein kinase signaling by Ang II could mitigate abdominal aortopathy that would normally result from loss of SMC TGF-β signaling. Because Ang II has more potent effects in the abdominal aorta than in the thoracic aorta,14 site-specific effects of Ang II could explain why Ang II mitigates abdominal aortopathy more than thoracic aortopathy in mice with SMC Tgfbr2 deletion. These hypotheses require prospective testing.
We considered whether our finding that the 2G7 antibody does not neutralize TGF-β2 should alter interpretation of our results. The only manner in which to address this definitively would be to repeat our experiments with a different murine antibody that is reliably shown to block activity of all TGF-β isoforms. Lacking such a reagent, we can only speculate. For 2 reasons, we believe that our primary conclusion—that TGF-β protects the thoracic and abdominal aorta by different mechanisms—remains valid. First, regardless of whether 2G7 blocks TGF-β2, it does block TGF-β1 and TGF-β3 and in so doing worsens pathology in the abdominal aorta but not the thoracic aorta. Second, SMC-specific loss of TGF-β signaling reveals a far greater protective role for TGF-β signaling in the thoracic than in the abdominal aorta, and this result is independent of 2G7 activity. Nevertheless, our finding that neither 2G7 nor 1D11 had any TGF-β2-inhibitory activity is surprising and must be considered in the design and interpretation of experiments in which these reagents are used.
Our study has other limitations. First, it is possible that higher doses of the 2G7 antibody would have yielded different results. This concern merits particular attention in relation to the thoracic aorta, in which antibody injections did not alter aortic pathology (despite accelerated abdominal aortic pathology in the same mice). A more potent neutralizing antibody regimen might more effectively ablate TGF-β signaling within the thoracic aortic media and consequently reproduce the effects (reported herein) of SMC Tgfbr2 deletion on thoracic aortic pathology. Second, it is possible that Tgfbr2 deletion in SMC has secondary cell-autonomous compensatory effects13 that —rather than loss of TGF-β signaling per se—are responsible for the increased thoracic aortic pathology. The associated difficulty in discriminating primary from secondary effects in identifying the precise drivers of phenotypic changes in knockout cells and mice is an inherent limitation of knockout approaches. A similar concern applies to systemic inhibition of TGF-β ligands: vascular cells might compensate for decreased ligand availability, for example, by upregulating receptor expression, with uncertain downstream effects. These possibilities should be explored in future studies.
A third limitation of our study is that our western blot data raise the possibility of incomplete deletion of Tgfbr2 in aortic SMC. The 42% reduction in western blot signal for TBRII differs from the near-complete ablation of the receptor that we documented (in 2 independent studies) by western blotting of aortic media of the same line of tamoxifen-treated bi-transgenic mice.13, 47 In one of these studies we also documented that aortic SMC of these mice have impaired canonical and non-canonical TGF-β signaling.47 The apparently lower degree of receptor loss in the present study is likely due to adventitial cell contamination of the aortic medial peels (a technical issue). This explanation is supported by measurements of mRNA in medial peels from mice from the blood-pressure cohort of the present study: mice with SMC-specific Tgfbr2 deletion had significantly higher levels of Myh11, Tagln, and LoxL1 mRNA than control mice (data not shown). We reported previously that all 3 of these genes are upregulated in aortic media of mice with near-complete deletion of TBRII in SMC.13 Upregulation of all of these genes in aortic medial SMC supports our conclusion that we achieved significant deletion of SMC TBRII in the present study. A related issue is that Tgfbr2 deletion might have been significantly less complete in the abdominal aorta, accounting for the lack of effect in that region. We cannot exclude this possibility, but we believe it is unlikely because: 1) we and others13, 48 found equivalent Acta2-Cre activity throughout the entire aorta using the cell-specific R26R reporter gene; 2) In the absence of Ang II infusion, SMC-specific deletion of Tgfbr2 with this same Cre-Lox system causes significant abdominal aortic pathology;13 and 3) in the present study there was significant medial thinning in the abdominal aorta of mice with SMC-specific loss of TGF-β signaling (Figure 4C).
Finally, we note that Gao et al reported that SMC-specific deletion of Tgfbr2 prevented aneurysmal pathology after application of elastase to the infrarenal aorta of 8-week-old mice.49 This result suggests that SMC TGF-β signaling is pathogenic in this location and model and contrasts with results from the same group showing that SMC-specific deletion of Tgfbr2 alone causes severe aortopathy.29 One must hypothesize model or site-specific effects to reconcile this result with the large body of data—including the present study—suggesting that TGF-β signaling prevents abdominal aortic aneurysmal disease.10–12, 20, 26
In conclusion, TGF-β appears to protect the abdominal and thoracic aorta from Ang II via different mechanisms, involving SMC-extrinsic and SMC-intrinsic signaling, respectively. Although it remains uncertain as to whether our findings can be translated to human biology, results at both locations reveal beneficial effects of physiologic TGF-β signaling on aortic health and emphasize the potential risks to human health of either systemic or vascular wall-targeted TGF-β signaling blockade.11, 13, 29
Supplementary Material
Highlights.
We compared the effects of systemic and smooth muscle cell (SMC)-specific blockade of transforming growth factor beta (TGF-β) signaling on the development of aortic aneurysmal pathology in angiotensin II-infused mice.
Systemic TGF-β neutralization significantly increased the prevalence of abdominal—but not thoracic—aortic aneurysmal disease.
SMC-specific blockade of TGF-β signaling significantly increased the prevalence of thoracic—but not abdominal—aortic aneurysmal disease.
Systemic TGF-β neutralization tended to increase macrophage accumulation in a focal (~4 mm) segment of abdominal aortic adventitia.
Acknowledgments
We thank Rachel Enstrom, Alexandra Smith, and Dr. Bradley Wacker for their technical support. We also thank Drs. Cecilia Giachelli and Mark Majesky for helpful advice. We thank Dr. Ziad Mallat for providing hybridoma cells that produce the anti-TGF-β antibody and the Immunology and Inflammation Core of the Diabetes Research Center for assistance in antibody purification.
Sources of Funding
This work was supported by a grant from the National Heart Lung and Blood Institute (R01HL116612) and a gift from the John L. Locke Jr. Charitable Trust. Dr. Angelov was supported by T32HL007828. Antibody purification was partly supported by a grant from the National Institute of Diabetes and Digestive Kidney Disease (P30 DKO17047).
Abbreviations
- TGF-β
transforming growth factor-beta
- Tgfbr2
transforming growth factor-beta receptor 2 gene
- TBRII
type II TGF-β receptor protein
- Ang II
angiotensin II
- SMC
smooth muscle cell
- AAA
abdominal aortic aneurysm
Footnotes
Disclosures
The authors have no conflicts to disclose.
References
- 1.Guirguis-Blake JM, Beil TL, Senger CA, Whitlock EP. Ultrasonography screening for abdominal aortic aneurysms: A systematic evidence review for the u.S. Preventive services task force. Ann Intern Med. 2014;160:321–329. doi: 10.7326/M13-1844. [DOI] [PubMed] [Google Scholar]
- 2.Pande RL, Beckman JA. Abdominal aortic aneurysm: Populations at risk and how to screen. J Vasc Interv Radiol. 2008;19:S2–8. doi: 10.1016/j.jvir.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 3.LeFevre ML Force USPST. Screening for abdominal aortic aneurysm: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2014;161:281–290. doi: 10.7326/M14-1204. [DOI] [PubMed] [Google Scholar]
- 4.Lin F, Yang X. Tgf-beta signaling in aortic aneurysm: Another round of controversy. J Genet Genomics. 2010;37:583–591. doi: 10.1016/S1673-8527(09)60078-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Lu H, Rateri DL, Cassis LA, Daugherty A. Conundrum of angiotensin ii and tgf-beta interactions in aortic aneurysms. Curr Opin Pharmacol. 2013;13:180–185. doi: 10.1016/j.coph.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King VL, Lin AY, Kristo F, Anderson TJ, Ahluwalia N, Hardy GJ, Owens AP, 3rd, Howatt DA, Shen D, Tager AM, Luster AD, Daugherty A, Gerszten RE. Interferon-gamma and the interferon-inducible chemokine cxcl10 protect against aneurysm formation and rupture. Circulation. 2009;119:426–435. doi: 10.1161/CIRCULATIONAHA.108.785949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rush C, Nyara M, Moxon JV, Trollope A, Cullen B, Golledge J. Whole genome expression analysis within the angiotensin ii-apolipoprotein e deficient mouse model of abdominal aortic aneurysm. BMC Genomics. 2009;10:298. doi: 10.1186/1471-2164-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spin JM, Hsu M, Azuma J, Tedesco MM, Deng A, Dyer JS, Maegdefessel L, Dalman RL, Tsao PS. Transcriptional profiling and network analysis of the murine angiotensin ii-induced abdominal aortic aneurysm. Physiol Genomics. 2011;43:993–1003. doi: 10.1152/physiolgenomics.00044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle AJ, Redmond EM, Gillespie DL, Knight PA, Cullen JP, Cahill PA, Morrow DJ. Differential expression of hedgehog/notch and transforming growth factor-beta in human abdominal aortic aneurysms. J Vasc Surg. 2015;62:464–470. doi: 10.1016/j.jvs.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai J, Losy F, Guinault AM, Pages C, Anegon I, Desgranges P, Becquemin JP, Allaire E. Overexpression of transforming growth factor-beta1 stabilizes already-formed aortic aneurysms: A first approach to induction of functional healing by endovascular gene therapy. Circulation. 2005;112:1008–1015. doi: 10.1161/CIRCULATIONAHA.104.523357. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. Tgf-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin ii-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Tgf-beta neutralization enhances angii-induced aortic rupture and aneurysm in both thoracic and abdominal regions. PLoS One. 2016;11:e0153811. doi: 10.1371/journal.pone.0153811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu JH, Wei H, Jaffe M, Airhart N, Du L, Angelov SN, Yan J, Allen JK, Kang I, Wight TN, Fox K, Smith A, Enstrom R, Dichek DA. Postnatal deletion of the type ii transforming growth factor-beta receptor in smooth muscle cells causes severe aortopathy in mice. Arterioscler Thromb Vasc Biol. 2015;35:2647–2656. doi: 10.1161/ATVBAHA.115.306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e–deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leveen P, Larsson J, Ehinger M, Cilio CM, Sundler M, Sjostrand LJ, Holmdahl R, Karlsson S. Induced disruption of the transforming growth factor beta type ii receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100:560–568. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin ii infusion promotes ascending aortic aneurysms: Attenuation by ccr2 deficiency in apoe−/− mice. Clin Sci (Lond) 2010;118:681–689. doi: 10.1042/CS20090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rateri DL, Davis FM, Balakrishnan A, Howatt DA, Moorleghen JJ, O’Connor WN, Charnigo R, Cassis LA, Daugherty A. Angiotensin ii induces region-specific medial disruption during evolution of ascending aortic aneurysms. Am J Pathol. 2014;184:2586–2595. doi: 10.1016/j.ajpath.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. doi: 10.1161/01.CIR.0000154569.08857.7A. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klink A, Heynens J, Herranz B, Lobatto ME, Arias T, Sanders HM, Strijkers GJ, Merkx M, Nicolay K, Fuster V, Tedgui A, Mallat Z, Mulder WJ, Fayad ZA. In vivo characterization of a new abdominal aortic aneurysm mouse model with conventional and molecular magnetic resonance imaging. J Am Coll Cardiol. 2011;58:2522–2530. doi: 10.1016/j.jacc.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook JR, Clayton NP, Carta L, Galatioto J, Chiu E, Smaldone S, Nelson CA, Cheng SH, Wentworth BM, Ramirez F. Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for marfan syndrome. Arterioscler Thromb Vasc Biol. 2015;35:911–917. doi: 10.1161/ATVBAHA.114.305150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo EM, Loch DC, Habashi JP, et al. Angiotensin ii-dependent tgf-beta signaling contributes to loeys-dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124:448–460. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granata A, Serrano F, Bernard WG, McNamara M, Low L, Sastry P, Sinha S. An ipsc-derived vascular model of marfan syndrome identifies key mediators of smooth muscle cell death. Nat Genet. 2017;49:97–109. doi: 10.1038/ng.3723. [DOI] [PubMed] [Google Scholar]
- 25.Zacchigna L, Vecchione C, Notte A, et al. Emilin1 links tgf-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Dai X, Shen J, Annam NP, Jiang H, Levi E, Schworer CM, Tromp G, Arora A, Higgins M, Wang XF, Yang M, Li HJ, Zhang K, Kuivaniemi H, Li L. Smad3 deficiency promotes vessel wall remodeling, collagen fiber reorganization and leukocyte infiltration in an inflammatory abdominal aortic aneurysm mouse model. Sci Rep. 2015;5:10180. doi: 10.1038/srep10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin ii-infused, apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty A, Cassis L. Angiotensin ii-mediated development of vascular diseases. Trends Cardiovasc Med. 2004;14:117–120. doi: 10.1016/j.tcm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest. 2014;124:755–767. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torr EE, Gardner DH, Thomas L, Goodall DM, Bielemeier A, Willetts R, Griffiths HR, Marshall LJ, Devitt A. Apoptotic cell-derived icam-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 2012;19:671–679. doi: 10.1038/cdd.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postlethwaite AE, Kang AH. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976;143:1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunninghake GW, Davidson JM, Rennard S, Szapiel S, Gadek JE, Crystal RG. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981;212:925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- 33.Reilly CF, McFall RC. Platelet-derived growth factor and transforming growth factor-b regulate plasminogen activator inhibitor-1 synthesis in vascular smooth muscle cells. J Biol Chem. 1991;266:9419–9427. [PubMed] [Google Scholar]
- 34.Tieu BC, Ju X, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Brasier AR, Tilton RG. Aortic adventitial fibroblasts participate in angiotensin-induced vascular wall inflammation and remodeling. J Vasc Res. 2011;48:261–272. doi: 10.1159/000320358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin ii-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daugherty A, Cassis LA, Lu H. Complex pathologies of angiotensin ii-induced abdominal aortic aneurysms. J Zhejiang Univ Sci B. 2011;12:624–628. doi: 10.1631/jzus.B1101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: A dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of ang ii type 1a receptors attenuates ang ii-induced ascending aortic aneurysms in ldl receptor−/− mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei YM, Chen L, Wang Y, Stefka AT, Molinero LL, Theriault B, Aquino-Michaels K, Sivan AS, Nagler CR, Gajewski TF, Chong AS, Bartman C, Alegre ML. The composition of the microbiota modulates allograft rejection. J Clin Invest. 2016;126:2736–2744. doi: 10.1172/JCI85295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chukkapalli SS, Rivera MF, Velsko IM, Lee JY, Chen H, Zheng D, Bhattacharyya I, Gangula PR, Lucas AR, Kesavalu L. Invasion of oral and aortic tissues by oral spirochete treponema denticola in apoe(−/−) mice causally links periodontal disease and atherosclerosis. Infect Immun. 2014;82:1959–1967. doi: 10.1128/IAI.01511-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizoguchi T, Kasahara K, Yamashita T, Sasaki N, Yodoi K, Matsumoto T, Emoto T, Hayashi T, Kitano N, Yoshida N, Amin HZ, Hirata KI. Oral administration of the lactic acid bacterium pediococcus acidilactici attenuates atherosclerosis in mice by inducing tolerogenic dendritic cells. Heart Vessels. 2017 doi: 10.1007/s00380-017-0949-8. [DOI] [PubMed] [Google Scholar]
- 42.Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin ii-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10. doi: 10.1111/j.1749-6632.2011.06332.x. [DOI] [PubMed] [Google Scholar]
- 43.Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, Daugherty A. Depletion of endothelial or smooth muscle cell-specific angiotensin ii type 1a receptors does not influence aortic aneurysms or atherosclerosis in ldl receptor deficient mice. PLoS One. 2012;7:e51483. doi: 10.1371/journal.pone.0051483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- 45.Jaffe M, Sesti C, Washington I, Du L, Dronadula N, Chin MT, Stolz DB, Davis EC, Dichek DA. Transforming growth factor beta signaling in myogenic cells regulates vascular morphogenesis, differentiation, and matrix synthesis. Arterioscler Thromb Vasc Biol. 2012;32:e1–e11. doi: 10.1161/ATVBAHA.111.238410. [DOI] [PubMed] [Google Scholar]
- 46.Mehta PK, Griendling KK. Angiotensin ii cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 47.Wei H, Hu JH, Angelov SN, Fox K, Yan J, Enstrom R, Smith A, Dichek DA. Aortopathy in a mouse model of marfan syndrome is not mediated by altered transforming growth factor beta signaling. J Am Heart Assoc. 2017:6. doi: 10.1161/JAHA.116.004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wendling O, Bornert JM, Chambon P, Metzger D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis. 2009;47:14–18. doi: 10.1002/dvg.20448. [DOI] [PubMed] [Google Scholar]
- 49.Gao F, Chambon P, Offermanns S, Tellides G, Kong W, Zhang X, Li W. Disruption of tgf-beta signaling in smooth muscle cell prevents elastase-induced abdominal aortic aneurysm. Biochem Biophys Res Commun. 2014;454:137–143. doi: 10.1016/j.bbrc.2014.10.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.