Abstract
Objective
To comprehensively evaluate the independent associations and potential interactions of vitamin D-related biomarkers including total and bioavailable 25-hydroxyvitamin D (25OHD), vitamin D binding protein (VDBP), and parathyroid hormone (PTH) with risk of coronary heart disease (CHD).
Approach and Results
We prospectively identified incident cases of nonfatal myocardial infarction and fatal CHD among women in the Nurses’ Health Study during 20 years of follow-up (1990–2010). Using risk-set sampling, 1~2 matched controls were selected for each case. The analysis of 25OHD and PTH included 382 cases and 575 controls; the analysis of VDBP included 396 cases and 398 controls. After multivariate adjustment, plasma levels of total 25OHD, bioavailable 25OHD, and PTH were not significantly associated with CHD risk. VDBP was associated with a lower CHD risk with an extreme-quartile odds ratio (OR) of 0.60 (95% confidence interval [CI]: 0.39, 0.92; P-trend=0.02). When examining the biomarkers jointly, a significant, inverse association between 25OHD and CHD was observed among participants with higher PTH levels (P for interaction=0.02). The OR (95% CI) comparing the highest quartile of 25OHD to lowest was 0.43 (0.23, 0.82; P-trend=0.003) when PTH levels were above population median (35.3 pg/mL), whereas among the rest of participants the corresponding OR (95% CI) was 1.28 (0.70, 2.36; P-trend=0.43).
Conclusions
Our data suggest that higher 25OHD levels were associated with a lower CHD risk when PTH levels were high, whereas no association was observed for participants with low PTH levels. VDBP but not bioavailable 25OHD was independently associated with lower CHD risk.
Keywords: Vitamin D, biomarker, coronary artery disease, epidemiology
Journal subject terms: Biomarkers, Epidemiology, Risk Factors, Women, Coronary Artery Disease
Graphical Abstract
INTRODUCTION
An increasing number of healthy US adults take vitamin D supplements, primarily for bone health1. Vitamin D status has been also implicated in heart health through inhibition of vascular smooth muscle cell proliferation, systemic anti-inflammatory effects, enhancement in insulin secretion and sensitivity, and regulatory effects on the renin-angiotensin-aldosterone system2. The most representative measure for vitamin D status is serum 25-hydroxyvitamin D (25OHD) because it reflects the total stored quantity from both endogenous and exogenous sources, and has a fairly long circulating half-life3. Despite potential pleiotropic cardioprotective mechanisms, prospective observational studies on the association between circulating 25OHD and risk of cardiovascular outcomes have generated mixed results3–7.
The majority of 25OHD and the activated vitamin D are bound to vitamin D binding protein (VDBP), a group-specific component of serum globulin, and transported to target tissues8. Beyond transport of vitamin D metabolites, VDBP could also modulate innate immunity and inflammatory responses9–11 and may be linked to a reduced risk of coronary heart disease (CHD)12, 13. The smaller amount of albumin-bound and the free, unbound 25OHD is often referred to as ‘bioavailable’ fraction. The free hormone hypothesis suggests that protein-bound hormones are relatively inactive, whereas those liberated from binding proteins are free to exert biological activity14. It was previously demonstrated that bioavailable 25OHD was more tightly associated with bone density than total levels in healthy individuals15. Moreover, parathyroid hormone (PTH) is also involved in the regulation of vitamin D metabolism and maintenance of calcium homeostasis. Elevated PTH has been associated with increased CHD risk in a meta-analysis of prospective studies16.
Previous studies have largely analyzed these vitamin D-related biomarkers separately, and little is known about whether the associations of 25OHD, VDBP, and PTH are mutually independent. In addition, it is unclear whether these biomarkers may interact with each other in relation to CHD risk. Because lower 25OHD levels represent decreased vitamin D stores, whereas elevated PTH levels partly reflect true deficiency or inadequate biological activity of vitamin D17, 18; we hypothesized that the potential associations of lower vitamin D levels with CHD would be stronger in the presence of concomitant elevated PTH. Therefore, we comprehensively assessed the independent and joint associations of circulating levels of total and bioavailable 25OHD, VDBP, and PTH with risk of CHD in a nested case-control study within a large prospective cohort, the Nurses’ Health Study (NHS).
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Distributions of demographic, lifestyle, and metabolic risk factors according to quartiles of plasma levels of 25OHD, VDBP, and PTH among controls assessed at blood draw are shown in Table 1. Higher circulating 25OHD levels were associated with lower BMI and higher physical activity levels, diet quality, dietary intake of vitamin D and calcium, and alcohol intake. Participants with higher 25OHD levels were more likely to use postmenopausal hormone and multivitamin. Higher 25OHD levels were also associated with a more favorable lipid profile, including slightly lower total cholesterol and triglycerides as well as higher HDL cholesterol. Circulating VDBP was associated with younger age, lower BMI, but also lower diet quality and calcium intake. Participants with higher VDBP levels were less likely to have a family history of MI. Similarly to 25OHD, VDBP also showed an association with an elevated level of HDL cholesterol. In contrast, higher PTH levels were associated with higher BMI, total cholesterol, LDL cholesterol, and creatinine. Those with higher PTH levels displayed an increased likelihood of having hypertension and hypercholesterolemia. Among controls, total 25OHD was positively correlated with bioavailable 25OHD (r=0.62) and VDBP (r=0.18) but inversely correlated with PTH (r=−0.24), while VDBP and PTH were very weakly correlated (r=−0.001) (Table 2).
Table 1.
Baseline characteristics at blood draw by levels of vitamin D-related biomarkers among controls*
| Quartiles of 25-hydroxyvitamin D |
P for trend † |
Quartiles of vitamin D binding protein |
P for trend † |
Quartiles of parathyroid hormone |
P for trend † |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||
| Range | 19.8–51.2 | 51.4–62.9 | 62.9–75.0 | 75.0–416.4 | 73.8–204.0 | 204.5–251.4 | 251.6–302.7 | 303.2–452.2 | 9.1–28.4 | 28.5–35.4 | 35.4–45.4 | 45.9–210.5 | |||
| Age (year)‡ | 62.1±7.3 | 63.9±8.1 | 63.4±7.5 | 62.2±7.8 | 0.90 | 66.1±7.3 | 64.4±7.9 | 64.6±6.9 | 61.9±8.4 | <0.001 | 60.3±7.8 | 63.3±7.2 | 64.2±7.3 | 63.8±7.9 | <0.001 |
| White (%) | 97.9 | 99.3 | 100 | 100 | 0.02 | 100 | 100 | 100 | 99 | 0.18 | 99.3 | 99.3 | 99.3 | 99.3 | 0.99 |
| Body mass index (kg/m2) | 26.0±4.8 | 25.3±4.5 | 25.6±4.9 | 24.5±3.4 | 0.006 | 26.9±5.3 | 25.5±4.9 | 24.5±3.9 | 24.8±3.3 | <0.001 | 24.6±4.3 | 24.9±4.7 | 25.8±4.6 | 26.2±4.1 | <0.001 |
| Physical activity (MET-hr/week) | 14.0±14.5 | 16.4±14.2 | 20.5±19.7 | 18.1±18.0 | 0.01 | 17.3±18.1 | 18.0±17.3 | 16.8±16.5 | 18.7±17.3 | 0.64 | 18.2±17.9 | 16.6±15.8 | 18.9±15.8 | 15.3±18.0 | 0.23 |
| Alternate Healthy Eating Index score | 36.5±8.9 | 39.0±9.2 | 39.5±8.1 | 39.4±8.1 | 0.004 | 40.1±8.5 | 39.8±8.9 | 35.9±8.3 | 38.0±7.4 | 0.01 | 38.8±8.5 | 38.7±8.1 | 39.1±9.1 | 37.9±8.9 | 0.39 |
| Dietary vitamin D intake (IU/day) | 194.5±105.1 | 200.4±89.2 | 228.0±97.3 | 253.3±115.5 | <0.001 | 217.7±101.8 | 227.0±105.9 | 221.7±97.7 | 213.9±119.9 | 0.74 | 220.7±102.9 | 227.3±96.1 | 220.9±114.2 | 207.4±104.6 | 0.18 |
| Dietary calcium intake (mg/day) | 673.9±189.3 | 711.0±195.8 | 747.2±238.2 | 767.7±222.0 | <0.001 | 752.4±214.6 | 728.8±203.7 | 712.0±227.7 | 688.9±199.9 | 0.03 | 751.8±215.3 | 732.3±208.9 | 716.8±230.9 | 699.5±201.5 | 0.03 |
| Alcohol (g/d) | 5.5±7.9 | 6.8±11.0 | 8.9±12.7 | 8.3±10.5 | 0.01 | 5.6±8.2 | 6.9±9.3 | 7.0±10.3 | 7.6±10.5 | 0.15 | 6.7±9.4 | 7.6±11.6 | 8.1±11.1 | 7.1±10.7 | 0.82 |
| Never smoker (%) | 32.2 | 38.2 | 34.0 | 43.1 | 0.11 | 40.4 | 29.0 | 41.0 | 37.4 | 0.89 | 31.5 | 39.6 | 36.8 | 39.6 | 0.23 |
| Past smoker (%) | 30.1 | 41.0 | 43.1 | 39.6 | 0.09 | 44.4 | 46.0 | 36.0 | 35.4 | 0.09 | 39.9 | 38.9 | 42.4 | 32.6 | 0.32 |
| Current smoker (%)‡ | |||||||||||||||
| 1–14 cigarettes/d | 18.2 | 6.3 | 9.7 | 9.0 | 0.04 | 7.1 | 10.0 | 10.0 | 15.2 | 0.08 | 10.5 | 9.7 | 9.0 | 13.9 | 0.41 |
| 15–24 cigarettes/d | 11.2 | 9.0 | 9.0 | 2.8 | 0.01 | 3.0 | 9.0 | 9.0 | 9.1 | 0.13 | 6.3 | 9.0 | 6.3 | 10.4 | 0.34 |
| ≥25 cigarettes/d | 8.4 | 5.6 | 4.2 | 4.9 | 0.17 | 5.1 | 6.0 | 4.0 | 3.0 | 0.39 | 11.2 | 2.8 | 5.6 | 3.5 | 0.02 |
| Hypertension (%) | 36.4 | 37.5 | 30.6 | 31.9 | 0.25 | 37.4 | 32.0 | 46.0 | 31.3 | 0.85 | 25.2 | 35.4 | 36.8 | 38.9 | 0.02 |
| Hypercholesterolemia (%) | 42.0 | 48.6 | 52.1 | 44.4 | 0.56 | 55.6 | 54.0 | 51.0 | 43.4 | 0.08 | 40.6 | 43.8 | 51.4 | 51.4 | 0.03 |
| Diabetes (%) | 6.3 | 7.6 | 5.6 | 6.3 | 0.81 | 6.1 | 7.0 | 3.0 | 6.1 | 0.69 | 8.4 | 6.3 | 8.3 | 2.8 | 0.11 |
| Parental MI before age 65 years (%) | 19.6 | 20.8 | 22.2 | 19.4 | 0.95 | 31.3 | 22.0 | 21.0 | 19.2 | 0.05 | 16.1 | 20.1 | 22.2 | 23.6 | 0.10 |
| Fasting status (%)‡ | 67.8 | 73.6 | 78.5 | 73.6 | 0.18 | 78.8 | 82.0 | 82.0 | 68.7 | 0.10 | 67.1 | 73.6 | 75.0 | 77.8 | 0.04 |
| Postmenopausal (%) | 90.9 | 91.0 | 92.4 | 88.9 | 0.66 | 93.9 | 93.0 | 93.0 | 90.9 | 0.44 | 83.9 | 91.7 | 93.1 | 94.4 | 0.002 |
| Postmenopausal hormone use (%)§ | 59.2 | 71.8 | 66.9 | 75.0 | 0.02 | 60.2 | 73.1 | 62.4 | 75.6 | 0.11 | 71.7 | 67.4 | 70.9 | 63.2 | 0.23 |
| Use of aspirin (%) | 49.7 | 54.9 | 54.2 | 61.1 | 0.07 | 40.4 | 46.0 | 49.0 | 51.5 | 0.11 | 57.3 | 59.7 | 50.0 | 52.8 | 0.21 |
| Use of multivitamin (%) | 32.6 | 52.1 | 49.6 | 52.2 | 0.003 | 51.1 | 54.6 | 40.0 | 45.9 | 0.19 | 52.6 | 39.4 | 46.3 | 48.2 | 0.76 |
| Biomarkers | |||||||||||||||
| 25-hydroxyvitamin D (nmol/L) | 41.3±7.8 | 57.5±3.5 | 68.6±3.5 | 89.8±29.9 | <0.001 | 58.1±14.8 | 63.3±13.6 | 65.9±41.5 | 68.8±19.3 | 0.003 | 69.8±19.7 | 65.9±17.8 | 62.3±17.0 | 59.9±34.5 | <0.001 |
| Vitamin D binding protein (mg/L) | 241.1±73.3 | 238.7±61.7 | 257.3±79.3 | 267.8±75.4 | 0.006 | 156.3±35.7 | 227.1±13.3 | 273.5±14.3 | 345.0±38.4 | <0.001 | 259.3±79.8 | 250.1±70.5 | 247.6±66.6 | 246.4±76.7 | 0.28 |
| Parathyroid hormone (pg/mL) | 43.1±16.9 | 39.3±19.2 | 37.3±12.4 | 34.5±11.7 | <0.001 | 39.2±15.1 | 39.0±13.2 | 41.3±22.1 | 35.8±12.7 | 0.27 | 24.0±3.5 | 31.8±2.1 | 40.1±2.8 | 58.3±17.5 | <0.001 |
| Total cholesterol (mg/dL) | 232.1±38.1 | 223.6±37.4 | 227.6±47.1 | 221.5±32.3 | 0.05 | 230.7±44.6 | 228.2±43.9 | 228.4±31.4 | 222.9±42.0 | 0.19 | 216.7±37.8 | 229.1±36.7 | 228.2±45.6 | 230.6±34.7 | 0.01 |
| LDL cholesterol (mg/dL) | 135.8±34.9 | 127.6±33.6 | 132.7±36.4 | 128.0±34.6 | 0.14 | 129.8±36.7 | 138.7±37.6 | 132.8±35.8 | 126.3±34.5 | 0.35 | 120.9±32.8 | 131.9±37.0 | 132.8±35.8 | 138.4±32.1 | <0.001 |
| HDL cholesterol (mg/dL) | 58.5±15.9 | 62.4±18.6 | 59.2±17.7 | 63.9±19.5 | 0.04 | 58.7±15.5 | 57.4±14.6 | 63.8±21.4 | 64.4±18.3 | 0.005 | 62.1±17.9 | 62.6±19.0 | 60.8±17.7 | 58.5±17.6 | 0.05 |
| Triglycerides (mg/dL) | 135.5±101.9 | 115.2±72.3 | 125.7±68.7 | 114.1±55.9 | 0.05 | 116.0±77.8 | 116.2±56.1 | 120.7±89.7 | 128.1±69.6 | 0.22 | 115.5±62.4 | 117.8±59.1 | 128.0±92.8 | 129.1±86.9 | 0.09 |
| High-sensitivity C-reactive protein (mg/dL) | 0.40±0.55 | 0.36±0.42 | 0.39±0.44 | 0.30±0.34 | 0.10 | 0.36±0.42 | 0.28±0.26 | 0.28±0.33 | 0.39±0.41 | 0.61 | 0.37±0.56 | 0.32±0.38 | 0.39±0.45 | 0.37±0.37 | 0.67 |
| Creatinine (mg/dL) | 0.73±0.15 | 0.74±0.16 | 0.74±0.15 | 0.76±0.18 | 0.10 | 0.73±0.13 | 0.79±0.21 | 0.74±0.19 | 0.74±0.14 | 0.92 | 0.71±0.12 | 0.73±0.16 | 0.75±0.14 | 0.77±0.21 | 0.002 |
| Albumin (g/dL) | 4.6±0.31 | 4.5±0.33 | 4.6±0.31 | 4.6±0.30 | 0.37 | 4.5±0.26 | 4.5±0.27 | 4.5±0.30 | 4.6±0.32 | 0.15 | 4.6±0.30 | 4.5±0.29 | 4.5±0.32 | 4.6±0.35 | 0.58 |
| Hemoglobin A1c (%) | 5.7±0.63 | 5.6±0.51 | 5.7±0.41 | 5.6±0.58 | 0.35 | 5.7±0.69 | 5.6±0.40 | 5.6±0.42 | 5.6±0.49 | 0.10 | 5.6±0.56 | 5.6±0.51 | 5.7±0.60 | 5.7±0.47 | 0.63 |
Values are mean±SD for continuous variables or % for categorical variables.
N=575 for 25-hydroxyvitamin D and parathyroid hormone and N=398 for vitamin D binding protein.
P for trend was assessed by F test and modeling median values in each quartiles continuously for variables expressed as mean±SD or Cochran-Armitage trend test for variables expressed as percentages.
Matching factors.
Among postmenopausal women only.
Table 2.
Partial Spearman correlation coefficients between vitamin D-related biomarkers among controls*
| Median | Total 25OHD (nmol/L) | Bioavailable 25OHD (nmol/L) | VDBP (mg/L) | PTH (pg/mL) |
|---|---|---|---|---|
|
| ||||
| 62.6 | 7.5 | 250.9 | 35.9 | |
| Total 25OHD | 1.00 | 0.62† | 0.18† | −0.24† |
| Bioavailable 25OHD | 1.00 | −0.59† | −0.18† | |
| VDBP | 1.00 | −0.001 | ||
| PTH | 1.00 | |||
Abbreviations: 25OHD, 25-hydroxyvitamin D; VDBP, vitamin D binding protein; PTH, parathyroid hormone.
N=368. Partial correlation coefficients were adjusted for age at blood draw (years, continuous), smoking status (never, past, or current smoker), fasting status (yes/no), time of blood draw (collection 1: 06/1989-10/1989, 11/1989-03/1990, 04/1990-07/1990, or 08/1990-03/1991; collection 2: 03/2000-07/2000, 08/2000-12/2000, 01/2001-05/2001, or 06/2001-01/2002), BMI (kg/m2: <25.0, 25.0–27.4, 27.5–29.9, 30.0–34.9, ≥35.0), postmenopausal status and hormone use (premenopausal or postmenopausal (never, past, or current menopausal hormone use), physical activity (tertiles), alcohol intake (g/d: 0, 0.1–4.9, 5.0–14.9, ≥15.0), aspirin use (yes/no), MI family history (yes/no), and AHEI score (tertiles).
P<0.001.
In Table 3, we present the associations of circulating total and bioavailable 25OHD, VDBP, and PTH with risk of developing CHD. In crude models that were adjusted for matching factors (results not shown), total 25OHD and VDBP were associated with lower CHD risk. After further adjustment for BMI, lifestyle factors, and diet (model 1), the association for 25OHD was attenuated and no longer statistically significant, whereas the association for VDBP remained robust. Compared with the lowest quartile of VDBP, the OR (95% CI) in the highest quartile was 0.60 (0.39, 0.92; P-trend=0.02). PTH and bioavailable 25OHD were not significantly associated with CHD risk, and the multivariate-adjusted OR comparing highest vs. lowest quartile was 0.68 (95% CI: 0.46, 1.01; P-trend=0.06) for PTH and 1.23 (95% CI: 0.79, 1.90; P-trend=0.19) for bioavailable 25OHD. For total and bioavailable 25OHD as well as VDBP, the associations did not materially change with additional adjustment for clinical factors that could be either confounders or mediators, including baseline history of hypertension or diabetes, HDL cholesterol, LDL cholesterol, C-reactive protein, and creatinine (results not shown). However, the association for PTH became statistically significant after clinical factors were controlled for; the largest change was seen with additional adjustment for HDL cholesterol and LDL cholesterol. Comparing to the lowest quartile of PTH, the OR (95% CI) in the highest quartile was 0.58 (0.37, 0.89; P-trend=0.01). Of note, additional mutual adjustment for other biomarkers (model 2) did not appreciably alter any of these associations, with the OR (95% CI) in the highest quartile of 0.98 (0.61, 1.58; P-trend=0.66) for total 25OHD, 0.55 (0.35, 0.87; P-trend=0.01) for VDBP, 0.70 (0.44, 1.19; P-trend=0.13) for PTH, and 0.87 (0.52, 1.45; P-trend=0.76) for bioavailable 25OHD, compared to the lowest quartile of the biomarkers, respectively.
Table 3.
Odds ratio (95% CI) of coronary heart disease by levels of vitamin D-related biomarkers
| Quartiles of biomarker levels | P for trend | Per SD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 2 | 3 | 4 | |||
| Total 25-hydroxyvitamin D (nmol/L) | ||||||
| Median | 43.4 | 57.5 | 68.5 | 83.6 | ||
| Case/control | 119/143 | 98/144 | 83/144 | 82/144 | ||
| Model 1* | 1.00 (ref) | 0.91 (0.63, 1.33) | 0.76 (0.51, 1.13) | 0.84 (0.56, 1.27) | 0.30 | 0.95 (0.82, 1.09) |
| Model 2† | 1.00 (ref) | 0.92 (0.60, 1.41) | 0.75 (0.48, 1.16) | 0.98 (0.61, 1.58) | 0.66 | 1.00 (0.85, 1.17) |
| Vitamin D binding protein (mg/L) | ||||||
| Median | 166.3 | 228.5 | 270.8 | 336.7 | ||
| Case/control | 131/99 | 101/100 | 93/100 | 71/99 | ||
| Model 1* | 1.00 (ref) | 0.76 (0.51, 1.14) | 0.74 (0.49, 1.11) | 0.60 (0.39, 0.92) | 0.02 | 0.87 (0.75, 1.02) |
| Model 2† | 1.00 (ref) | 0.71 (0.47, 1.09) | 0.68 (0.44, 1.04) | 0.55 (0.35, 0.87) | 0.01 | 0.84 (0.71, 0.98) |
| Parathyroid hormone (pg/mL) | ||||||
| Median | 24.2 | 31.9 | 39.8 | 53.7 | ||
| Case/control | 98/143 | 100/144 | 101/144 | 83/144 | ||
| Model 1* | 1.00 (ref) | 0.90 (0.61, 1.32) | 0.90 (0.61, 1.32) | 0.68 (0.46, 1.01) | 0.06 | 0.94 (0.82, 1.07) |
| Model 2† | 1.00 (ref) | 0.90 (0.59, 1.39) | 0.91 (0.58, 1.41) | 0.70 (0.44, 1.19) | 0.13 | 0.96 (0.82, 1.11) |
| Bioavailable 25-hydroxyvitamin D (nmol/L) | ||||||
| Median | 5.1 | 6.8 | 8.3 | 11.1 | ||
| Case/control | 93/92 | 77/92 | 102/92 | 104/92 | ||
| Model 1* | 1.00 (ref) | 0.84 (0.54, 1.31) | 1.16 (0.76, 1.78) | 1.23 (0.79, 1.90) | 0.19 | 1.10 (0.94, 1.29) |
| Model 2† | 1.00 (ref) | 0.76 (0.48, 1.20) | 0.96 (0.61, 1.53) | 0.87 (0.52, 1.45) | 0.76 | 0.99 (0.82, 1.19) |
Model 1 was adjusted for age at blood draw (years, continuous), smoking status (never, past, or current smoker), fasting status (yes/no), and time of blood draw (collection 1: 06/1989-10/1989, 11/1989-03/1990, 04/1990-07/1990, or 08/1990-03/1991; collection 2: 03/2000-07/2000, 08/2000-12/2000, 01/2001-05/2001, or 06/2001-01/2002), BMI (kg/m2: <25.0, 25.0–27.4, 27.5–29.9, 30.0–34.9, ≥35.0), postmenopausal status and hormone use (premenopausal or postmenopausal (never, past, or current menopausal hormone use), physical activity (tertiles), alcohol intake (g/d: 0, 0.1–4.9, 5.0–14.9, ≥15.0), aspirin use (yes/no), MI family history (yes/no), and AHEI score (tertiles). Additional adjustment for sun exposure (≥8h/wk outdoor in the summer with sunscreen, ≥8h/wk outdoor in the summer without sunscreen, or no), dietary vitamin D intake (IU/day, continuous), vitamin D supplement use (IU/day, continuous) or multivitamin use (yes/no) did not appreciably change the results.
Model 2 was further mutually adjusted for the other biomarkers of interest. We did not simultaneously adjust for total and bioavailable 25-hydroxyvitamin D due to the strong correlation between the two.
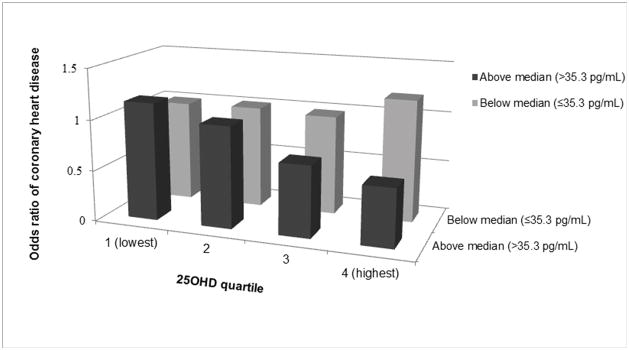
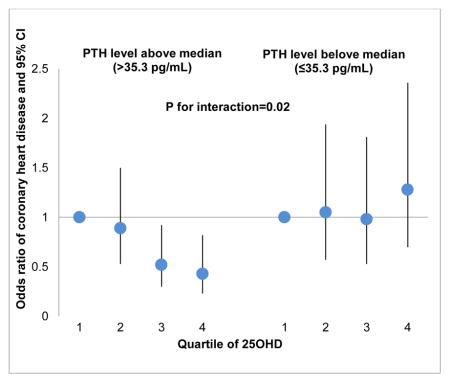
We next evaluated the potential interactions among these vitamin D-related biomarkers in relation to the risk of CHD (Table 4). We found a significant interaction between PTH and 25OHD on the disease risk (P for interaction=0.02). Among participants who had higher PTH levels, higher 25OHD levels were associated with a lower risk of developing CHD; conversely, we observed a non-significant association among participants with lower PTH levels. Comparing the highest quartile of 25OHD to the lowest, the multivariable-adjusted OR (95% CI) was 0.43 (0.23, 0.82; P-trend=0.003) when PTH levels were above population median (35.3 pg/mL), and 1.28 (0.70, 2.36; P-trend=0.43) otherwise. Consistent with stratified analyses, when joint categories of 25OHD and PTH levels were evaluated (Figure 1), inverse associations between 25OHD and CHD risk were present only among participants with high PTH levels, but not among the other participants. However, we did not observe similar interaction using clinical cutoff points of 50 nmol/L for 25OHD and 65 pg/mL for PTH. No significant interaction was observed among other biomarkers.
Table 4.
Odds ratio (95% CI) of total 25-hydroxyvitamin D, bioavailable 25-hydroxyvitamin D, and vitamin D binding protein with coronary heart disease according to median level of parathyroid hormone
| Quartiles of biomarkers levels | P for trend | Per SD | P for interaction | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | ||||
| Total 25-hydroxyvitamin D (nmol/L) | |||||||
| Below median of parathyroid hormone (≤35.3 pg/mL) | |||||||
| Median | 44.8 | 58.2 | 67.5 | 85.2 | |||
| Case/Control | 42/56 | 47/69 | 47/72 | 59/86 | |||
| Model | 1.00 (ref) | 1.05 (0.57, 1.94) | 0.98 (0.53, 1.81) | 1.28 (0.70, 2.36) | 0.43 | 1.10 (0.86, 1.40) | 0.02 |
| Above median of parathyroid hormone (>35.3 pg/mL) | |||||||
| Median | 42.8 | 57.3 | 69.1 | 81.7 | |||
| Case/Control | 77/87 | 51/75 | 36/72 | 23/58 | |||
| Model | 1.00 (ref) | 0.89 (0.53, 1.50) | 0.52 (0.30, 0.92) | 0.43 (0.23, 0.82) | 0.003 | 0.77 (0.58, 1.01) | |
| Vitamin D binding protein (mg/L) | |||||||
| Below median of parathyroid hormone (≤35.3 pg/mL) | |||||||
| Median | 175.2 | 228.5 | 269.3 | 338.3 | |||
| Case/Control | 61/45 | 51/41 | 44/44 | 36/47 | |||
| Model | 1.00 (ref) | 0.86 (0.46, 1.61) | 0.82 (0.44, 1.53) | 0.63 (0.33, 1.22) | 0.17 | 0.88 (0.69, 1.11) | 0.86 |
| Above median of parathyroid hormone (>35.3 pg/mL) | |||||||
| Median | 158.7 | 227.2 | 270.5 | 334.6 | |||
| Case/Control | 69/47 | 42/51 | 42/48 | 31/45 | |||
| Model | 1.00 (ref) | 0.51 (0.28, 0.94) | 0.61 (0.33, 1.12) | 0.42 (0.21, 0.83) | 0.02 | 0.81 (0.64, 1.02) | |
| Bioavailable 25-hydroxyvitamin D (nmol/L) | |||||||
| Below median of parathyroid hormone (≤35.3 pg/mL) | |||||||
| Median | 5.3 | 6.7 | 8.4 | 11.5 | |||
| Case/Control | 40/38 | 35/43 | 57/46 | 60/50 | |||
| Model | 1.00 (ref) | 0.97 (0.48, 1.96) | 1.27 (0.65, 2.47) | 1.48 (0.75, 2.93) | 0.18 | 1.21 (0.94, 1.56) | 0.30 |
| Above median of parathyroid hormone (>35.3 pg/mL) | |||||||
| Median | 5.0 | 6.8 | 8.2 | 11.0 | |||
| Case/Control | 53/54 | 42/49 | 45/46 | 44/42 | |||
| Model | 1.00 (ref) | 0.83 (0.45, 1.52) | 0.99 (0.53, 1.84) | 0.98 (0.52, 1.85) | 0.92 | 0.98 (0.80, 1.21) | |
Model was adjusted for age at blood draw (years, continuous), smoking status (never, past, or current smoker), fasting status (yes/no), time of blood draw (collection 1: 06/1989-10/1989, 11/1989-03/1990, 04/1990-07/1990, or 08/1990-03/1991; collection 2: 03/2000-07/2000, 08/2000-12/2000, 01/2001-05/2001, or 06/2001-01/2002), BMI (kg/m2: <25.0, 25.0–27.4, 27.5–29.9, 30.0–34.9, ≥35.0), postmenopausal status and hormone use (premenopausal or postmenopausal (never, past, or current menopausal hormone use), physical activity (tertiles), alcohol intake (g/d: 0, 0.1–4.9, 5.0–14.9, ≥15.0), aspirin use (yes/no), MI family history (yes/no), and AHEI score (tertiles). Further adjustment for parathyroid hormone levels (pg/mL, continuous) did not appreciably change the results.
Figure 1.
Joint associations of 25-hydroxyvitamin D and parathyroid hormone with risk of CHD. Multivariate model was adjusted for age at blood draw, smoking status, fasting status, time of blood draw, BMI, postmenopausal status and hormone use, physical activity, alcohol intake, aspirin use, MI family history, and AHEI score. Participants with a PTH level below the median and 25OHD level in the lowest quartile were set as the reference group.
DISCUSSION
In this prospective study of healthy US women, although higher plasma levels of 25OHD were correlated with a lower risk profile, they were not independently associated with CHD incidence after multivariate adjustment. VDBP showed a significant association with lower CHD incidence, independent of established demographic, lifestyle and dietary risk factors. PTH was associated with lower risk of CHD only when clinical biomarkers were further controlled for. In addition, we found that PTH status modified the relation between 25OHD and CHD risk, and a significant, inverse association was observed only among women with elevated PTH levels.
Despite experimental evidence supporting a cardioprotective role of vitamin D and potential mechanisms linking vitamin D deficiency and CHD risk2, results from prospective investigations remain inconsistent3–7. A meta-analysis of prospective studies of 25OHD and cardiovascular event demonstrated a generally linear, inverse association up to 60 nmol/L but revealed no further reductions at higher 25OHD levels19. In contrast, 25OHD was not prospectively associated with a comprehensive set of biochemical, electrocardiographic and echocardiographic measurements of cardiac structure and function among older adults20. These discordant findings may be partly explained by the fact that a spectrum of cardiovascular outcomes was considered in prior studies, which may have distinct etiologies with relevance to 25OHD biology. Other sources of heterogeneity may include study population (e.g., age, gender, and ethnicity), categories of 25OHD used, and confounders considered. Our findings suggest that previously observed associations are very likely to be confounded by CHD risk factors and lifestyle variables associated with 25OHD. In support of this notion, by using Mendelian randomization approach and eliminating confounding, genetically determined 25OHD was not found to be associated with risk of incident CHD21 or cardiovascular mortality22. Therefore, the causality and the effects of vitamin D supplement on CHD are yet to be clarified in ongoing and future randomized controlled trials. The lack of an association between bioavailable 25OHD and CHD implied that bioavailable 25OHD might not exert higher biological activity regarding association with disease as expected according to the free hormone hypothesis. In addition, a mean affinity constant between 25OHD and VDBP was used to estimate bioavailable 25OHD23, 24, without accounting for the potential affinity differences according to VDBP genotype25. Additional information on the polymorphisms will be useful for a more accurate estimation of bioavailable 25OHD.
Compared with accumulating epidemiological data on 25OHD, relatively little is known regarding the effects of VDBP on cardiovascular health. In a recent study, higher plasma levels of VDBP isotopes were associated with increased risk of cardiovascular mortality, but not incident cardiovascular events26. However, this study was limited by low statistical power with an inclusion of only 17 cases and did not assess whether the association was independent of other vitamin D-related biomarkers. Our results showed that the observed inverse association between VDBP and CHD remained robust after further adjustment for 25OHD and PTH levels. Experimental evidence suggests VDBP could modulate immunity by macrophage activation and reduce inflammation and attenuate clot formation through the removal of actin from circulation11. Proteomics analyses also revealed that among MI patients, VDBP reduced aggregation rate and prolonged coagulation time13, and plasma levels of VDBP were inversely correlated with the number of affected arteries12. Taken together, our results provide novel evidence that VDBP or its metabolic determinants may reduce CHD risk beyond its link to 25OHD, suggesting that VDBP should be a target for additional experimental, observational, and interventional studies.
Although elevated PTH levels have been linked to hypertension, valvular and myocardial calcifications, as well as left ventricular hypertrophy16, 27, the independent role of PTH in heart health among people with normal renal function remains controversial. A meta-analysis of 10 prospective studies reported that PTH levels were associated with a 1.45-fold increase (95% CI: 1.24, 1.71) in the incidence of cardiovascular events. However, there was considerable between-study heterogeneity regarding study population, PTH assays, outcome definitions, and adjustment for confounders, with most individual cohorts showing null associations28. In contrast to any positive association, PTH levels were inversely associated with incident heart failure, peripheral artery disease, and CVD mortality in a large US cohort of middle-aged adults29. In our analysis, PTH was not significantly associated with CHD in the multivariate model, but the association became significant with further adjustment for some clinical factors in particular HDL and LDL cholesterol. The potentially non-linear, inverse association was largely driven by the highest PTH quartile. Our findings add to the literature and highlight the need to comprehensively investigate the pathways through which PTH especially in high levels might affect the pathogenesis of CHD.
To our knowledge, this is the first report of a significant interaction between PTH status and 25OHD in relation to CHD risk. Vitamin D regulates intestinal calcium absorption, and the observed interaction between vitamin D and PTH excess is supported by biology because PTH increases in response to endogenous deficiency of vitamin D through negative feedback18. A low 25OHD level in the context of elevated PTH may indicate a physiologic vitamin D insufficiency, while the lack of relation between 25OHD and CHD for those with PTH below the median could be because the lower vitamin D levels with low PTH are not reflecting true deficiency or at least not causing hypocalcaemia and systemic response. The only previous study evaluating the potential interaction between these two biomarkers did not detect an additive interaction and the combination of a low 25OHD and PTH excess was not significantly associated with greater risk of cardiovascular outcomes17. However, that study focused on older adults with a mean age of 74 years, and a cutoff value of 65 pg/mL for PTH levels was applied to keep consistent with the definition of primary hyperparathyroidism. Further research is warranted to confirm our findings in other cohorts and explore the PTH threshold for associations between 25OHD and cardiovascular outcomes.
The prospective design, high follow-up rate, and use of plasma biomarkers minimized major sources of bias, such as selection bias or recall bias. We comprehensively evaluated the independent and synergistic associations of biomarkers of vitamin D status with CHD. We controlled for major covariates that could influence biomarker levels and CHD risk as well as metabolic risk factors. Potential limitations of the current analysis, however, should be considered. First, we measured VDBP levels using monoclonal sandwich immunoassay (R&D Systems)30, which has been shown to quantify VDBP differentially by isoform and systematically underestimate VDBP levels among people with Gc1F/Gc1F genotype, compared to using a polyclonal assay or liquid chromatography–tandem mass spectrometry (LC-MS/MS) methodology31, 32. Although the majority (99.1%) of our study population is white, among whom the prevalence of Gc1F homozygotes is low30, 32, indicating our results might not be substantially influenced by these issues, we acknowledge that future studies of VDBP need to employ assays that are not affected by VDBP genotype. Second, although the homogeneity of socioeconomic status and ethnicity helps to control for confounding, the results might not be generalizable to other populations. We acknowledge that 25OHD levels and their association with incident CHD vary by race33, 34. Third, plasma biomarkers were assessed only once at baseline, and changes over time would have caused misclassification and potentially attenuated true associations. Lastly, although we adjusted for multiple important risk factors for CHD, residual confounding because of unmeasured or imprecisely measured confounding cannot be excluded.
In conclusion, our results indicate that a low 25OHD level in the presence of elevated PTH is a risk factor for CHD among largely healthy US women. High circulating VDBP is independently associated with reduced risk of CHD, whereas PTH and bioavailable 25OHD levels are not significantly associated. Our findings emphasize the importance of simultaneously considering the interrelationships of vitamin D-related biomarkers when evaluating vitamin D status and CHD risk.
Supplementary Material
HIGHLIGHTS.
In this prospective study of US women, although higher plasma levels of 25OHD were correlated with a lower cardiovascular risk profile, they were not independently associated with incidence of CHD after multivariate adjustment.
PTH status modified the relation between 25OHD and CHD risk, and a significant, inverse association was observed only among women with elevated PTH levels, whereas no association was observed for those with low PTH levels.
VDBP showed a significant association with lower CHD incidence, independent of established demographic, lifestyle and dietary risk factors.
Acknowledgments
Sources of Funding: The cohorts were supported by grants of UM1 CA186107, R01 CA49449, R01 HL034594 from the National Institutes of Health. The current study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States – Israel Binational Science Foundation Grant 2011036. Dr. Qi was a recipient of the American Heart Association Scientist Development Award (0730094N).
Abbreviations
- 25OHD
25-hydroxyvitamin D
- VDBP
vitamin D binding protein
- PTH
parathyroid hormone
- CHD
coronary heart disease
- NHS
Nurses’ Health Study
- MI
myocardial infarction
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- CV
coefficient of variation
- BMI
body mass index
- AHEI
Alternative Healthy Eating Index
- SD
standard deviation
- CI
confidence interval
- OR
odds ratio
Footnotes
Author contributions: Dr. Qi and Dr. Manson have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: LQ, JEM. Statistical analysis: LQ, WM. Statistical advice and interpretation of data: LQ, WM, YH, YZ. Drafting of the manuscript: LQ, WM. Critical revision of the manuscript for important intellectual content and approval of the final manuscript for submission: All authors.
Disclosure: None.
References
- 1.Ebeling PR. Vitamin d and bone health: Epidemiologic studies. BoneKEy reports. 2014;3:511. doi: 10.1038/bonekey.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnatz PF, Manson JE. Vitamin d and cardiovascular disease: An appraisal of the evidence. Clin Chem. 2014;60:600–609. doi: 10.1373/clinchem.2013.211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin d deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin d and risk of myocardial infarction in men: A prospective study. Archives of internal medicine. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, Ronnemaa T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2005;15:188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin d levels and the risk of mortality in the general population. Archives of internal medicine. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cawthon PM, Parimi N, Barrett-Connor E, Laughlin GA, Ensrud KE, Hoffman AR, Shikany JM, Cauley JA, Lane NE, Bauer DC, Orwoll ES, Cummings SR Osteoporotic Fractures in Men Research G. Serum 25-hydroxyvitamin d, parathyroid hormone, and mortality in older men. The Journal of clinical endocrinology and metabolism. 2010;95:4625–4634. doi: 10.1210/jc.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leong A, Rehman W, Dastani Z, et al. The causal effect of vitamin d binding protein (dbp) levels on calcemic and cardiometabolic diseases: A mendelian randomization study. PLoS Med. 2014;11:e1001751. doi: 10.1371/journal.pmed.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JS, Chen H, Chun R, Ren S, Wu S, Gacad M, Nguyen L, Ride J, Liu P, Modlin R, Hewison M. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin d synthesis and action: The human innate immune response. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(Suppl 2):V20–24. doi: 10.1359/jbmr.07s214. [DOI] [PubMed] [Google Scholar]
- 10.Chun RF. New perspectives on the vitamin d binding protein. Cell biochemistry and function. 2012;30:445–456. doi: 10.1002/cbf.2835. [DOI] [PubMed] [Google Scholar]
- 11.Gomme PT, Bertolini J. Therapeutic potential of vitamin d-binding protein. Trends in biotechnology. 2004;22:340–345. doi: 10.1016/j.tibtech.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Rocchiccioli S, Andreassi MG, Cecchettini A, Carpeggiani C, L’Abbate A, Citti L. Correlation between vitamin d binding protein expression and angiographic-proven coronary artery disease. Coronary artery disease. 2012;23:426–431. doi: 10.1097/MCA.0b013e328358781c. [DOI] [PubMed] [Google Scholar]
- 13.Gasparri C, Curcio A, Torella D, Gaspari M, Celi V, Salituri F, Boncompagni D, Torella M, Gulletta E, Cuda G, Indolfi C. Proteomics reveals high levels of vitamin d binding protein in myocardial infarction. Frontiers in bioscience. 2010;2:796–804. doi: 10.2741/e140. [DOI] [PubMed] [Google Scholar]
- 14.Mendel CM. The free hormone hypothesis: A physiologically based mathematical model. Endocrine reviews. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 15.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin d-binding protein modifies the vitamin d-bone mineral density relationship. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease--a review. European heart journal. 2004;25:1776–1787. doi: 10.1016/j.ehj.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin d, parathyroid hormone, and cardiovascular events among older adults. Journal of the American College of Cardiology. 2011;58:1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saliba W, Barnett O, Rennert HS, Lavi I, Rennert G. The relationship between serum 25(oh)d and parathyroid hormone levels. Am J Med. 2011;124:1165–1170. doi: 10.1016/j.amjmed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD. Circulating 25-hydroxy-vitamin d and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ballegooijen AJ, Visser M, Kestenbaum B, Siscovick DS, de Boer IH, Gottdiener JS, deFilippi CR, Brouwer IA. Relation of vitamin d and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the cardiovascular health study) The American journal of cardiology. 2013;111:418–424. doi: 10.1016/j.amjcard.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian randomization studies do not support a role for vitamin d in coronary artery disease. Circ Cardiovasc Genet. 2016;9:349–356. doi: 10.1161/CIRCGENETICS.116.001396. [DOI] [PubMed] [Google Scholar]
- 22.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin d concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. doi: 10.1136/bmj.g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin d is more tightly linked to mineral metabolism than total vitamin d in incident hemodialysis patients. Kidney international. 2012;82:84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin d in serum and its regulation by albumin and the vitamin d-binding protein. The Journal of clinical endocrinology and metabolism. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 25.Arnaud J, Constans J. Affinity differences for vitamin d metabolites associated with the genetic isoforms of the human serum carrier protein (dbp) Hum Genet. 1993;92:183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 26.Melander O, Modrego J, Zamorano-Leon JJ, Santos-Sancho JM, Lahera V, Lopez-Farre AJ. New circulating biomarkers for predicting cardiovascular death in healthy population. J Cell Mol Med. 2015;19:2489–2499. doi: 10.1111/jcmm.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rostand SG, Drueke TB. Parathyroid hormone, vitamin d, and cardiovascular disease in chronic renal failure. Kidney international. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 28.van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. American heart journal. 2013;165:655–664. 664 e651–655. doi: 10.1016/j.ahj.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Alonso A, Misialek JR, Michos ED, Selvin E, Eckfeldt JH, Coresh J, Pankow JS, Lutsey PL. Parathyroid hormone concentration and risk of cardiovascular diseases: The atherosclerosis risk in communities (aric) study. American heart journal. 2014;168:296–302. doi: 10.1016/j.ahj.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin d-binding protein and vitamin d status of black americans and white americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin d-binding protein concentrations quantified by mass spectrometry. N Engl J Med. 2015;373:1480–1482. doi: 10.1056/NEJMc1502602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB Chronic Renal Insufficiency Cohort study i. Comparison of two elisa methods and mass spectrometry for measurement of vitamin d-binding protein: Implications for the assessment of bioavailable vitamin d concentrations across genotypes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31:1128–1136. doi: 10.1002/jbmr.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin d concentrations of young american black and white women. Am J Clin Nutr. 1998;67:1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 34.Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25-hydroxyvitamin d concentration with coronary heart disease events. Jama. 2013;310:179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.