Abstract
Characterization of adeno-associated viral vector (AAV) mediated gene delivery to the enteric nervous system (ENS) was recently described in mice and rats. In these proof-of-concept experiments, we show that intravenous injections of clinically relevant AAVs can transduce the ENS in guinea pigs and non-human primates. Neonatal guinea pigs were given intravenous injections of either AAV8 or AAV9 vectors that contained a green fluorescent protein (GFP) expression cassette or PBS. Piglets were euthanized three weeks post-injection and tissues were harvested for immunofluorescent analysis. GFP expression was detected in myenteric and submucosal neurons along the length of the gastrointestinal tract in AAV8 injected guinea pigs. GFP positive neurons were found in dorsal motor nucleus of the vagus and dorsal root ganglia. Less transduction occurred in AAV9 treated tissues. Gastrointestinal tissues were analyzed from young cynomolgus macaques that received systemic injection of AAV9 GFP. GFP expression was detected in myenteric neurons of the stomach, small and large intestine. These data demonstrate that ENS gene delivery translates to larger species. This work develops tools for the field of neurogastroenterology to explore gut physiology and anatomy using emerging technologies such as optogenetics and gene editing. It also provides a basis to develop novel therapies for chronic gut disorders.
Introduction
Gene delivery is an effective basic and translational tool to manipulate neurological behavior, inflammatory responses and degeneration that has only recently been explored for application to the enteric nervous system (ENS)(1–3). Efficiency is enhanced with the use of viral or non-viral vectors to facilitate nucleic acid uptake and stability. Viral vectors are commonly used in the central nervous system for basic and pre-clinical studies and have an excellent safety profile in clinical testing(4–6). Delivery of vectors can involve direct injection of target structures, which can be invasive and challenging if transduction is required in neuronal networks that span a large area. It was shown recently that direct injections of adeno-associated viruses (AAV) into the colon, small intestine or cecum of rats and mice showed capsid and dose dependent targeting of myenteric and submucosal neurons and glia near the injection site(2). We and others have recently shown that widespread ENS transduction is achieved in mice following a one-time systemic delivery of AAV8 or 9(1, 3). AAV9 targeted up to 57% of myenteric neurons from the stomach to the colon in mice following an intravenous AAV9 injection(1). Similar transduction efficiency was also achieved in the murine submucosal plexus(3).
Guinea pigs are commonly used to model gut function and study gut physiology but, to our knowledge, no data exist on AAV transduction in the guinea pig ENS(7, 8). Here we show that systemic injection of AAV8 or AAV9 targets myenteric and submucosal neurons in guinea pigs. Transgene expression occurred primarily in ChAT positive (myenteric plexus, MP) and VIP positive (submucosal plexus, SMP) cells from the esophagus through the colon. Further, we demonstrate ENS transduction within the stomach, small intestine and large intestine of systemically injected cynomolgus macaques following intravenous AAV9 injection. These proof-of-principle data describe new tools to manipulate ENS gene expression in larger species and provide the foundation for future translation of an ENS directed gene therapy.
Results
Systemic AAV Targets Guinea Pig Myenteric and Submucosal Plexuses
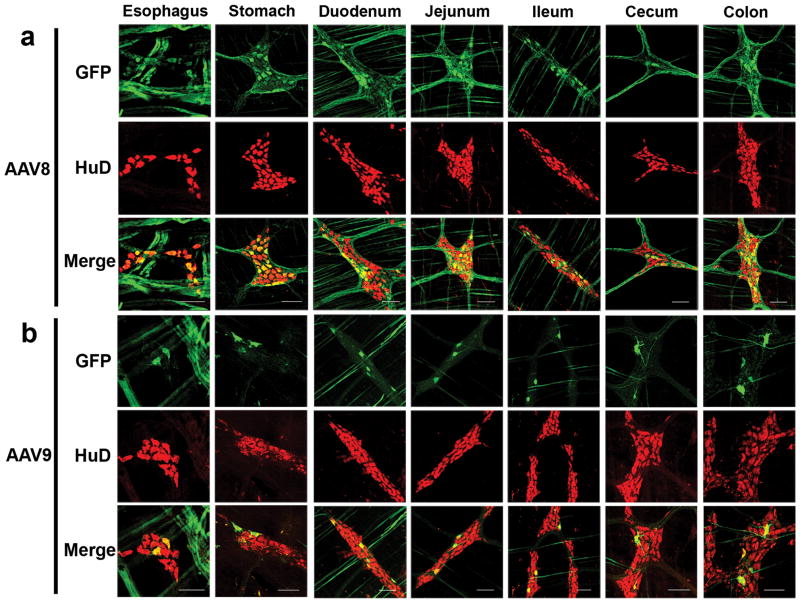
To examine AAV transduction efficiency and distribution in guinea pigs, we injected postnatal day 2 (P2) guinea pigs intravenously with either AAV8 or AAV9 vectors containing identical self-complementary (sc) GFP expression cassettes under the control of the chicken-β actin (CB) promoter which is designed to provide broad, sustained transgene expression. A pregnant sow (gestational day 50–53) was purchased and the litter of piglets was used in the study. Neither the sow nor the piglets were screened for anti-AAV antibodies. Two animals each received 2x1012 vector genomes (vg) (2.8–3.4x1013vg/kg) of either scAAV8-CB-GFP or scAAV9-CB-GFP through the saphenous or cephalic vein while one animal received a PBS injection (Table 1). Four of five injected animals completed the 21-day study. One AAV8 injected animal (#101, Table 1) was euthanized at P5 due to dehydration and weight loss. Tissues from #101 were collected and analyzed identically to the remaining vector treated animals. The remaining animals were euthanized at P21 and eyes, cochleae, brain, spinal cord, liver, lungs, spleen, heart, skeletal muscle (triceps, quadriceps, and gastrocnemius), bladder, gonads, kidneys, and the entire gastrointestinal tract were harvested for analysis. Our previous work showed dose dependent transduction of mouse myenteric plexus following AAV9 injection that reached up to 57% of myenteric neurons at the highest dose tested (1.3x1014vg/kg)(1). Dissection of the gastrointestinal tracts from AAV8 and AAV9 injected guinea pigs showed clear GFP expression in the myenteric plexus from the esophagus through the colon (Figure 1). GFP expression in the plexuses was fairly uniform throughout the given tissue, as opposed to “hot spots” of single ganglion transduction. For AAV8 treated animals, the average number of GFP positive cells in the myenteric plexus were distributed from most to fewest in the duodenum, jejunum, stomach, ileum, colon and cecum; esophagus was not quantified. In AAV9 treated animals the average number of GFP positive cells were distributed from most to least in the duodenum, colon, jejunum, cecum, stomach and ileum. More replicates are required to determine if the distribution of GFP expression is reproducible. Differences in viral receptor expression likely explain the pattern of transgene expression within the tissues. In our study, GFP positive cells were more abundant in AAV8 treated animals than AAV9 (Table 2). GFP signal in both groups was most abundant in embryonic lethal abnormal vision (ELAV)-like proteins (HuD) positive cells indicating primarily neuronal transduction(9). Anti-Hu antibody labels exclusively neurons in the ENS and has been tested in prior studies in guinea pigs and macaques (10–13). On average, neuronal transduction efficiency ranged from 3% in the cecum to 16% in the duodenum of the AAV8 treated animals. AAV9 mediated GFP expression ranged from 1.5% in the ileum to 5.5% in the duodenum. Percentages of GFP immunopositive HuD positive MP neurons are listed in Table 2.
Table 1.
Guinea Pig Experimental Design
| Identifier | Gender | Weight at time of injection (g) | AAV Serotype | Injection Site | Total AAV dose (vg) | AAV dose per unit body weight (vg/kg) | Age at TOD |
|---|---|---|---|---|---|---|---|
| 101 | F | 72 | AAV8 | Saphenous Vein | 2.00 x 1012 | 2.80 x 1013 | P5 |
| 102 | M | 64 | AAV8 | Saphenous Vein | 2.00 x 1012 | 3.10 x 1013 | P21 |
| 103 | F | 72 | AAV9 | Saphenous Vein | 2.00 x 1012 | 2.80 x 1013 | P21 |
| 104 | M | 58 | AAV9 | Cephalic Vein | 2.00 x 1012 | 3.40 x 1013 | P21 |
| 105 | F | 65 | PBS | Saphenous Vein | P21 |
Fig 1. Systemic administration of AAV vectors targets guinea pig myenteric neurons along the gastrointestinal tract.
Three weeks post injection, sections from guinea pig gastrointestinal tract were dissected and labeled with anti-GFP (green) and anti-HuD (red, neuron marker) antibodies. Representative sections from AAV8 (a) and AAV9 (b) treated animals are shown. No GFP expression was detected in the PBS injected animal (not shown). Scale bars = 100μm
Table 2.
Percent GFP expression in HuD positive neurons within individual regions of the guinea pig ENS
| Animal Number | Myenteric Plexus | Submucosal Plexus | ||||||
|---|---|---|---|---|---|---|---|---|
| AAV8 | AAV9 | AAV8 | AAV9 | |||||
| 101 | 102 | 103 | 104 | 101 | 102 | 103 | 104 | |
| Stomach | 0.64 | 16.78 | 2.55 | 0.97 | ||||
| Duodenum | 11.28 | 21.05 | 4.87 | 6.08 | 9.50 | 17.20 | 4.09 | 6.76 |
| Jejunum | 7.63 | 14.05 | 4.57 | 2.55 | 22.53 | 34.43 | 0.52 | 1.03 |
| Ileum | 5.08 | 8.00 | 1.72 | 1.38 | 7.50 | 39.80 | 3.06 | 1.38 |
| Cecum | 2.53 | 3.89 | 3.19 | 2.56 | 1.20 | 28.13 | 4.74 | 1.18 |
| Colon | 2.96 | 10.32 | 4.28 | 3.24 | 22.34 | 23.92 | 2.61 | 2.94 |
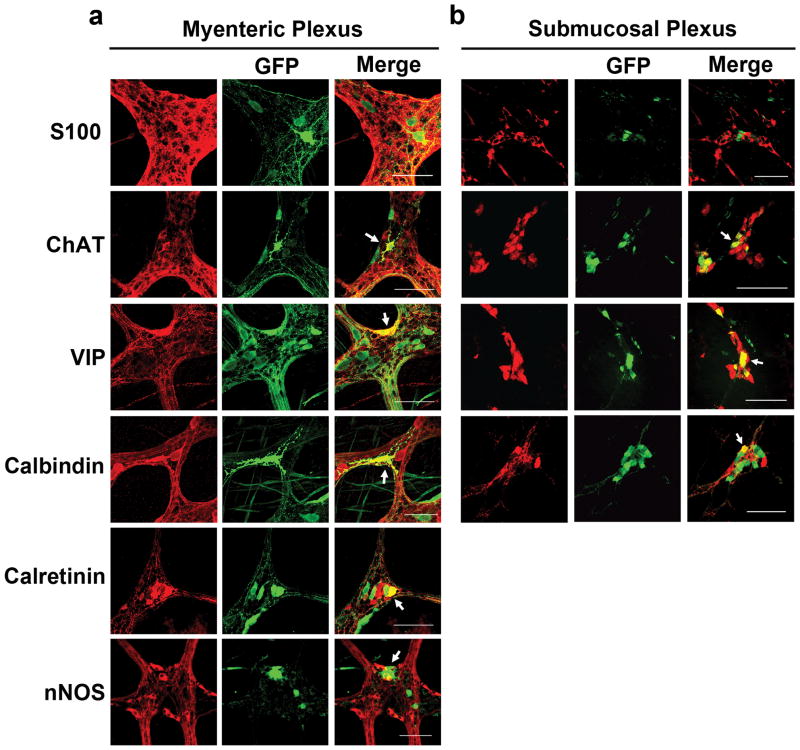
GFP positive cells were present in the SMP of vector treated guinea pigs from the duodenum through colon (Figure 2). As in the myenteric plexus, GFP expression was found in HuD positive neurons and not S100 positive intraganglionic glia (Figure 3a–b). Transduction of intraganglionic glia was not observed with either serotype in the myenteric or submucosal plexuses, though this is likely due to promoter usage (1). SMP transduction in AAV8 treated animals ranged from a high of approximately 28% in the jejunum to a low of 13% in the duodenum. AAV9 was less efficient at targeting SMP cells with a high of 5% in the duodenum to a low of 1% in the jejunum. Percentages of GFP immunopositive HuD positive SMP neurons for each animal are listed in Table 2.
Fig 2. Systemic injection of AAV vectors targets guinea pig submucosal plexus neurons in all GI regions examined.
Representative sections from AAV8 (a) and AAV9 (b) treated guinea pigs shows GFP expression (green) in submucosal plexus neurons (red, HuD). GFP was not detected in the SMP of the PBS treated animal (not shown). Scale bars = 100μm
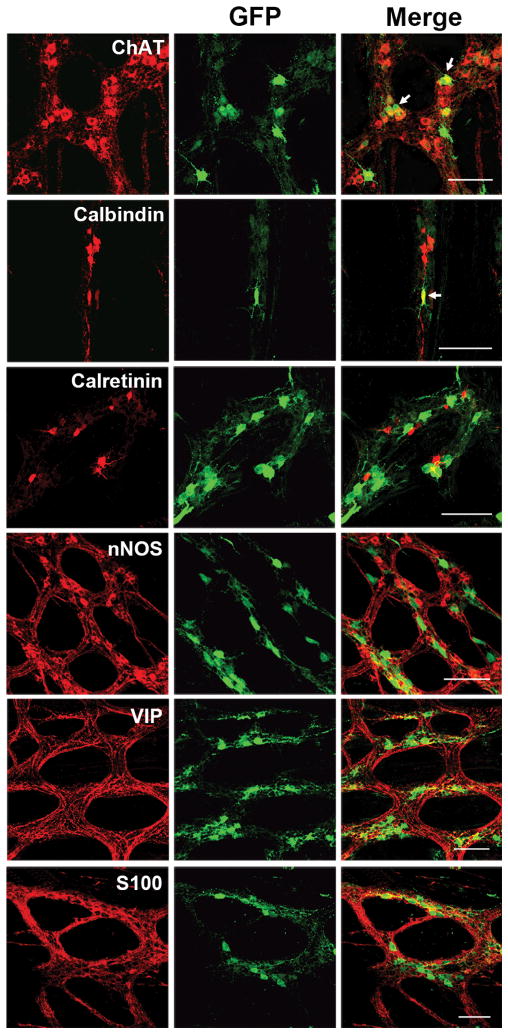
Fig 3. AAV vectors target multiple types of neurons in the guinea pig ENS.
All images are taken from tissues of AAV8 treated animals. Immunolabeling in the myenteric plexus (a) reveals that AAV targets ChAT, VIP, calbindin, calretinin and nNOS neurons. We did not observe AAV transduction of S100 positive intraganglionic glial cells in either the MP (a) or SMP (b).
Chemical Coding of Transduced ENS Neurons
In order to determine if AAV8 or AAV9 had a preference for any specific type of ENS neuron, longitudinal muscle-myenteric plexus (LMMP) preparations from colons of treated animals were immunolabeled for the neuron subtype markers ChAT, calbindin, calretinin, VIP and nNOS. In the MP, neuronal subtypes expressing ChAT include excitatory motor neurons projecting to circular and longitudinal muscles, ascending and descending interneurons, and some sensory neurons(14). VIP and nNOS labeling occurs in inhibitory motor neurons projecting to circular and longitudinal muscles(15). Calretinin expression occurs predominantly in ascending interneuron populations and less frequently in excitatory motor neurons(16). Calbindin expression is commonly found in descending interneurons and sensory neurons(15, 17). Classes of cholinergic (ChAT positive) and non-cholinergic (VIP positive) secretormotor and vasodilator neurons are found in the SMP(18). Calbindin immunoreactivity is detected in both secretomotor and vasodilator intrinsic primary afferent neurons(19). Representative images from AAV8 animals are in Figure 3. Counts within the specific cell types can be found in Table 3. For example, of the VIP cells counted, a mean of 31% were GFP positive in AAV8 MP tissues. Piglet #101 that was euthanized at P5, had nearly 13% of VIP neurons expressed in GFP immunopositive neurons, while in piglet #102, euthanized at P21, had approximately 48% of VIP positive neurons expressing GFP. In AAV9 tissues, between 12–13% of VIP cells were GFP positive. Within calbindin positive cells, AAV8 targeted between 2–6% and AAV9 targeted between 0.9–3%. Representative images of ChAT, VIP and calbindin labeling in the SMP of AAV8 treated animals are shown in Figure 3b. The proportions of GFP positive ChAT, VIP and calbindin SMP neurons counted in the ileum of vector treated animals are in Table 3. AAV8 targeted between 10–23% of VIP cells, 5–10% of calbindin cells and 12–29% of ChAT positive cells. AAV9 GFP expression was detectable in between 1–3% of SMP VIP positive and 0–1% of calbindin positive neurons respectively. GFP was not detectable in ChAT positive SMP neurons in tissues from AAV9 treated animals.
Table 3.
Percent GFP expression in subpopulations of chemically coded neurons within the Guinea Pig ENS
| Animal Number | Myenteric Plexus | Submucosal Plexus | ||||||
|---|---|---|---|---|---|---|---|---|
| AAV8 | AAV9 | AAV8 | AAV9 | |||||
| 101 | 102 | 103 | 104 | 101 | 102 | 103 | 104 | |
| ChAT | 4.25 | 14.07 | 6.35 | 3.05 | 12.82 | 29.97 | 0.00 | 0.00 |
| VIP | 13.33 | 47.62 | 12.00 | 13.33 | 10.75 | 23.58 | 3.92 | 1.85 |
| Calbindin | 1.89 | 6.17 | 2.69 | 0.96 | 5.41 | 10.00 | 1.98 | 0.00 |
| Calretinin | 2.17 | 25.83 | 16.02 | 4.03 | ||||
| nNOS | 1.04 | 14.88 | 2.72 | 6.21 | ||||
Systemic Injection of AAV8 and AAV9 Targets Neurons That Innervate the GI Tract
Projections to the enteric nervous system can originate from neurons in the vagal nucleus, sacral spinal cord and dorsal root ganglia(20). Therefore we examined innervating tissues for evidence of GFP expression in treated and control guinea pigs. Entire brains were sliced into coronal sections, collected in series and immunolabeled for GFP and cellular markers. Sections through the medulla of vector treated guinea pigs showed GFP expression within ChAT positive neuronal cells in the MN X nucleus (Figure 4). Brains of AAV8 treated guinea pigs had GFP positive cells scattered throughout. Representative images from cortex, hippocampus, striatum and cerebellum are shown in S1 Figure. Co-labeling with markers for neurons (NeuN), oligodendrocytes (CC1) and astrocytes (GFAP) revealed AAV8 targeting of all three cell types (S2 Figure). Brains from AAV9 treated animals also had scattered GFP positive cells throughout, but expression was confined to neurons and astrocytes (S1–S2 Figure). Dorsal root ganglia (DRG) were harvested and immunolabeled for GFP expression from vector treated animals. GFP expression was detected in cells with neuronal morphology within DRG of both AAV8 (Figure 4) and AAV9 (not shown) treated animals. In the spinal cords of vector treated animals, GFP expression was detected in fibers in dorsal horns and dorsal columns in the cervical, thoracic and lumbar regions of the spinal cord (S3 Figure). This pattern of expression is consistent with DRG neuronal transduction. GFP expression was absent from S100 positive cells in DRG indicating a lack of gene expression within satellite glial cells(21).
Fig 4. Systemic AAV targets central nervous system neurons that project to the ENS in guinea pigs.
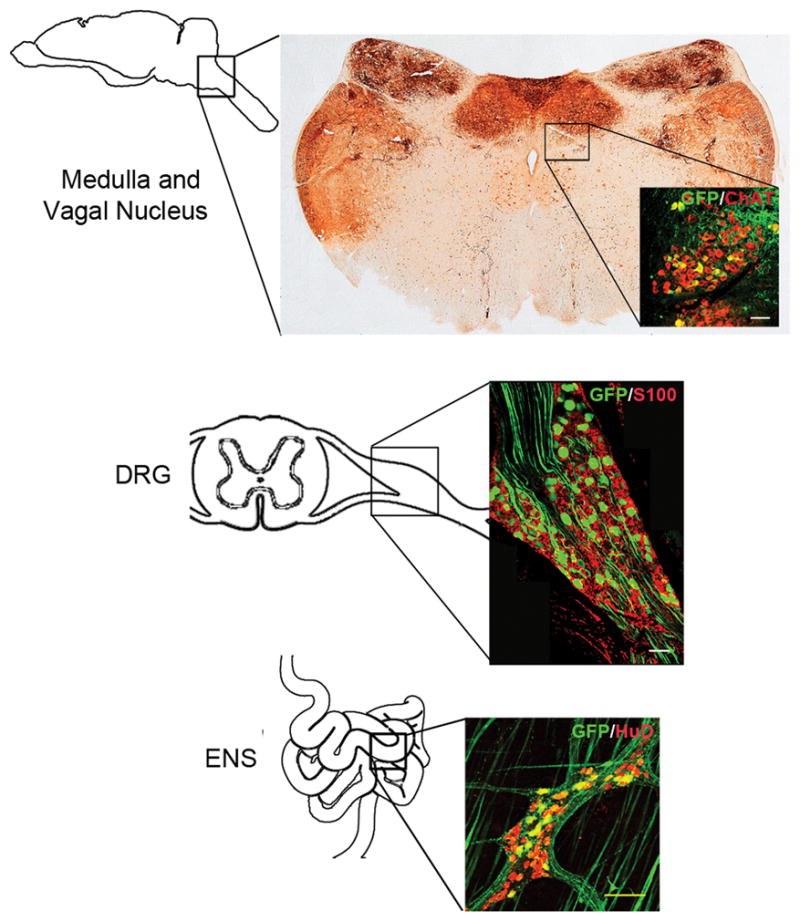
All images are taken from AAV8 treated guinea pigs. Diagrams show the approximate locations of immunolabeled sections collected from the dorsal motor nucleus of the vagus, dorsal root ganglion and myenteric plexus. Sections from the medulla show extensive GFP signal in fiber tracts and cell bodies in cranial nerve nuclei including cranial nerve X. GFP signal (green) co-localizes with ChAT (red, Medulla inset) and HuD labeling (red, ENS inset) but fails to co-localize with S100 (red, DRG inset). Scale bars = 100μm
Systemic Injection of AAV Vectors in Guinea Pigs Results in Widespread Gene Expression Throughout the Body
Gene delivery studies in mice, rats and non-human primates show that systemic AAV targets multiple tissues including liver and skeletal and cardiac muscle, but we are unaware of distribution studies in guinea pigs(22–24). Therefore we examined GFP immunofluorescence in multiple tissues from AAV8, AAV9 and control treated guinea pigs. Scattered GFP positive cells were detectable in lung, kidney, spleen and liver of both vector treated cohorts but not the control treated animal (S4 Figure). AAV mediated gene delivery to skeletal muscle is well documented in rodents, dogs, non-human primates and humans with multiple AAV serotypes(25). To determine whether skeletal muscle was transduced in guinea pigs, gastrocnemius and triceps muscles from control and vector treated animals were examined for GFP expression. Surprisingly, GFP expression in skeletal muscle was detected only sporadically and only in one AAV9 treated animal (S4 Figure). Cardiac muscle also had evidence of GFP positive cells in AAV8 and AAV9 treated animals. Besides the ENS, guinea pigs are used in studies of vision and hearing(26–29). AAV vectors transduce retinal and cochlear tissues in other species following systemic or direct injections(30–33). However, we found no evidence that systemic injection of either AAV8 or AAV9 resulted in GFP expression in guinea pig retina or cochlea (not shown). No GFP expression was detected in any tissues from the PBS treated animal.
Systemic AAV9 Transduces the Myenteric Plexus in Cynomolgus Macaques
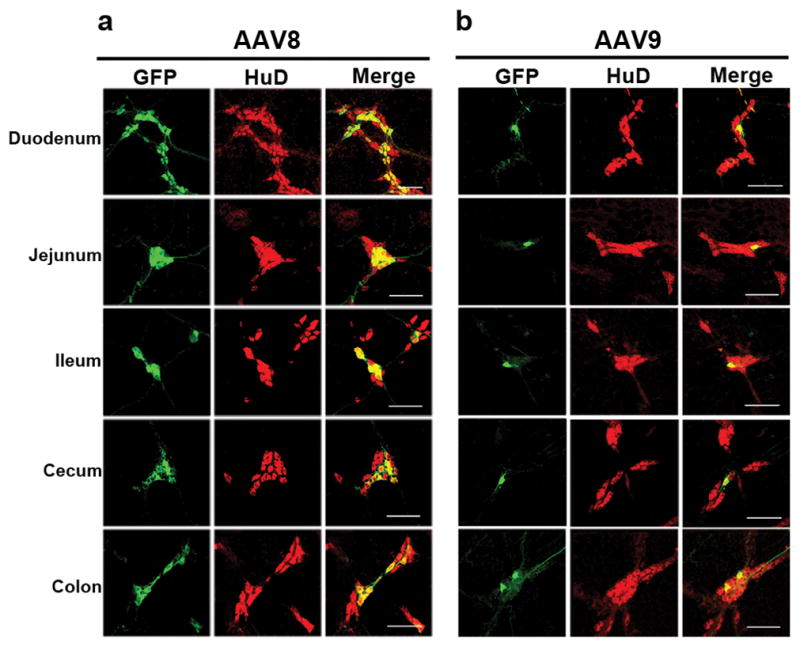
The targeting of ENS cells throughout the GI tract in guinea pigs lays the foundation for the development of novel therapeutic strategies to modulate barrier function, inflammation or visceral pain using gene delivery(34–36). An important step in therapeutic development is the translation of therapeutic delivery to higher order species. It has been shown that systemic AAV9 targets enteric neurons in the gut of non-human primates, but those data were obtained from intestinal cross sections and provide no information on distribution along the GI tract or the types of neurons targeted(33). Therefore, we obtained archived tissues samples from previous work to better characterize AAV9 transduction in non-human primates(22). Archived samples from AAV8 treated non-human primates were not available. Cynomolgus macaques (n=3) received systemic injections of 1–3×1014vg/kg scAAV9 containing the same expression construct as the treated guinea pigs. Animals were euthanized approximately 21–24 days post injection and flushed with PBS and 4% paraformaldehyde pH 7.4. Tissue segments were collected from the stomach, small intestine and large intestine and were held in 4% paraformaldehyde pH 7.4 until analysis. GI tissue sampling during harvest was not uniform across animals leading to sampling of different intestinal regions. Therefore we did not quantify ENS transduction in NHP tissues. LMMP preparations from the stomach, small intestine and large intestine reveal GFP expression within HuD positive cells (Figure 5). There was no evidence of GFP expression in S100 cells (not shown) or longitudinal muscle. Together, these data indicate a consistent neuronal pattern of transduction across species with AAV9. Based on the sections examined, GFP positive cells were more abundant in the small and large intestine compared to the stomach. GFP expression was most often found in ChAT positive neurons and co-localization with other neuronal markers calbindin, calretinin, nNOS and VIP were infrequent or never observed (Figure 6).
Fig 5. Systemic AAV9 targets myenteric plexus neurons in cynomolgus macaques.
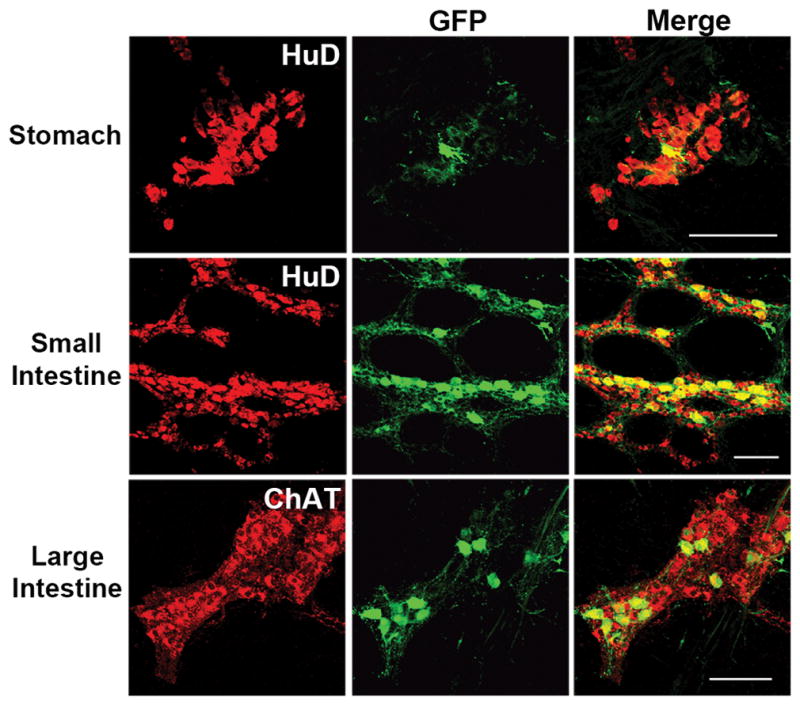
Immunolabeling of longitudinal muscle-myenteric plexus (LMMP) preparations from stomach, small intestine and large intestine show GFP expression (green) in HuD or ChAT (red) neurons approximately three weeks post injection. Scale bars = 100μm
Fig 6. ChAT and Calbindin myenteric neurons are targeted by systemic AAV9 in non-human primates.
Sections from AAV9 treated cynomolgus macaque small intestine, prepared by LMMP dissection to expose the myenteric plexus, were labeled for GFP (green) and ChAT, Calbindin, Calretinin, nNOS, VIP or S100 (all in red, first column) and examined for co-localization by confocal microscopy. GFP was detected within ChAT positive and Calbindin positive neurons but not within other cell types. Scale bars = 100μm
Discussion
Our previous work applied systemic delivery of multiple AAV serotypes to newborn and juvenile mice to determine transduction efficiency of the myenteric plexus(1). Those data showed that efficient targeting of myenteric neurons was achieved with AAV8 and AAV9 vectors in all regions examined (stomach to colon). Neuronal targeting included ChAT, calretinin and calbindin positive cells, but not VIP or nNOS cells. Transgene expression in S100 positive enteric glial cells was rare but influenced by viral capsid and expression cassette(1). These findings were largely replicated and furthered by showing transduction in the submucosal plexus in mice(3). Here we demonstrate proof of concept that systemic delivery of AAV vectors can target the enteric nervous system in guinea pigs and non-human primates. Across our studies, mice and guinea pigs were given similar vector doses (~3x1013vg/kg) with ~16–20% of myenteric neurons targeted in mouse colon and 4–6% in guinea pigs(1, 3). At least for AAV8, this difference in efficiency is partially due to one AAV8 guinea pig not completing the study since a longer time post injection increases the accumulation of GFP and therefore the sensitivity of detection. Guinea pig #101 may have died prematurely because high GFP expression can cause acute liver toxicity. GFP expression was detected in the liver from #101 in native and stained tissues, but no further toxicological analysis was performed. Buckinx et al. reported 22–29% targeting of myenteric neurons in mice following IV injection of ~1.3x1013vg/kg AAV8 or AAV9(3). The different efficiencies may be due to their use of the CMV promoter versus the CB promoter in our studies. Future work should directly examine the role of the viral expression cassette in enhancing or narrowing the pattern of transduction by comparing the CMV and CB promoters and including cell type specific promoters(37, 38). Buckinx and group also reported SMP neuron transduction in mice that ranged from 23–28% in the ileum and 5–12% in the colon following IV AAV8 or AAV9 injections(3). In the current study, SMP transduction ranged from 13–28% in the small intestine and 14–23% in the cecum and colon for AAV8 treated guinea pigs. AAV9 treated guinea pigs had considerably fewer transduced neurons in the SMP in all regions examined (~1–5%). Despite subtle differences between the current and Buckinx studies, the transduction efficiency for AAV8 in both enteric plexuses is remarkably similar across species. It is unclear why SMP was more efficiently targeted than MP in AAV8 treated guinea pigs but not AAV9 treated animals. This may be due to different distributions of co-receptors for AAV8 and AAV9(39, 40). It is also important to note that the ENS transduction in this study is from animals that received AAV injections during their first few days (guinea pigs) or weeks (NHP) of life. We previously reported different transduction patterns in the CNS of mice that received injections as neonates or adults(41). Interestingly, ENS transduction in mice remained consistent across age(1). Transgene expression should persist to adulthood in non-dividing cells, future work should document transduction when vector is administered to adult animals.
AAV9 has been extensively studied for its ability to cross the blood brain barrier and efficiently transduce the CNS of mice, rats, cats and non-human primates(22, 24, 41–43). The data remain consistent across groups and species and supported the Phase I/II testing of systemic AAV9 delivery for the pediatric neuromuscular disease spinal muscular atrophy (SMA) (clinicaltrials.gov #NCT02122952). Therefore the drop in AAV9 CNS and ENS transduction in guinea pigs compared to mice is surprising though species differences in AAV transduction have been reported for other serotypes including AAV8(44, 45). Preliminary data from the SMA clinical trial indicate safety and therapeutic efficacy at doses above those tested here (2x1014vg/kg) suggesting that systemically injected AAV9 targets the CNS in humans(46). The safety of the higher AAV9 dose currently in clinical testing supports increasing the viral dose in animals which should enhance ENS transduction. Increased viral doses are also supported by our earlier work in mice where doses ranging from 6.7x1013 to 1x1014vg/kg transduced 25–57% of myenteric neurons depending on gastrointestinal region examined(1). These data are important because they are the foundation for the use of AAV gene delivery as a novel tool and/or therapeutic for the ENS.
Currently, viral gene delivery is used sparingly to target the ENS. This is partially because means of viral delivery to ENS cells has not been thoroughly examined. In the ENS, AAV gene delivery can be used for classical experiments like neuronal tracing and with emerging technologies like optogenetics and gene editing(47–49). Our work shows that it is now feasible to bring these technologies into large animal models of the ENS. An advantage of vector mediated gene delivery is that the ease of use and kinetics of AAV mediated gene delivery offer temporal control of transgene expression in target cells. AAV vectors infect both dividing and non-dividing cells but only persist in non-dividing cells(50). Once inside, recombinant AAV genomes largely persist as stable episomes that can produce transgene expression for the life of the cell(51). In the CNS, AAV vectors infect a variety of cells including neurons, astrocytes, oligodendrocytes, endothelial cells and ependymal cells(41, 52, 53). We previously reported extensive CNS astrocyte transduction in mice and non-human primates using the identical AAV9 construct as this study(22, 41). Here we show targeting of CNS astrocytes, neurons and oligodendrocytes with AAV8 in guinea pigs (S1 and S2 Figures). In the ENS, transduction was limited to neurons, with VIP and ChAT staining being the most abundant within plexuses. The limited enteric glial transduction observed across species remains puzzling. The emerging roles of enteric glia in gut homeostasis and pathology make them an important target cell(34, 54). The ability to customize the AAV vector through changes to the viral capsid and/or expression cassette are important tools for future targeting of enteric glia and other types of enteric neurons. Further investigation of AAV promoter and capsid type is required to alter expression in specific cell types, however our work demonstrates that delivery is feasible in larger animal models. In addition to the >100 naturally occurring capsids available for testing, unbiased evolution strategies that rapidly screen entire libraries for enhanced transduction of a target cell are now available (55, 56). As mentioned above, the expression cassette encoded by the recombinant AAV genome can include cell type specific promoters and/or miRNA binding sites to further specify transgene expression once inside a target cell(38). More work is required to identify capsid/expression cassette combinations that target more cells within the ENS. Novel capsid evolution or mutagenesis techniques could also be applied for a more directed enhancement of ENS transduction. This work is the initial steps in examining viral gene therapy potential in the ENS.
A crucial step to therapy development is to ensure that the therapy reaches the intended target. In this work, we advance gene delivery for the ENS by providing proof-of-concept data that non-invasive, widespread targeting of ENS cells is possible in guinea pigs and non-human primates. In primates, GFP expression was detected in ChAT positive and calbindin positive neurons suggesting that vector targeted intrinsic primary afferent neurons, descending interneurons and/or circular muscle excitatory motor neurons. It is likely that the transduction in non-human primates shown here represents an underestimation because tissue collection and storage in the original study was not optimized for gut dissection. Unfortunately, this also limited analysis of SMP transduction. Future studies should include the SMP and more extensive co-labeling, tracing and potentially cell extraction to better characterize transduction. Finally, NHP GI tissues were not uniformly harvested which limited our ability to quantify transduction.
In conclusion, we have shown that AAV gene delivery to the ENS is translatable from mice to the commonly used guinea pig and cynomolgus macaque. The potential exists for this approach to allow for spatial and temporal control of target gene expression and/or knockdown within a percentage of cells in the ENS, and describes an important tool for the study and treatment of the ENS in larger species.
Materials and Methods
Ethics Statement
This study was approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee (Protocol #2011A00000100R1). All animal procedures (non-surgical injection and euthanasia) were performed to minimize animal discomfort. Piglet injection procedures were performed under 1% isoflurane anesthesia and are described below. Piglet euthanasia procedures were performed in consultation with OSU animal care veterinary staff and are described below.
Animals and Tissue Samples
A pregnant guinea pig sow (50–60 days gestation), purchased from Hilltop Lab (Scottdale, Pennsylvania), delivered 3 female and 2 male guinea piglets. Piglets were intravenously injected on postnatal day 2 (P2) with AAV vectors described in “Vector Production and Purification” (also Table 1). Following vector injection, piglets remained with the sow until P21. Guinea pigs were given standard food and water ad libitum in a 12h light/dark cycle rooms in the vivarium. Cynomologus macaque (Macaca fasciculata, n = 3) GI tissues were from the laboratory of Dr. Brian Kaspar (Nationwide Children’s Hospital) and arrived fixed in 4% paraformaldehyde pH 7.4. For a detailed description of monkey procedures and AAV9 transduction outside the ENS refer to Bevan et al., 2011(22). Briefly, primates were injected in the saphenous vein with 3x1014 vg/kg of AAV9 CB GFP at ages P1, P30, or P90. All subjects were euthanized and transcardially perfused 21 to 24 days post vector injection.
Vector Production and Purification
AAV vectors used in these studies were identical to those previously described(1). Briefly, AAVs were produced by the University of Massachusetts Medical School Viral Vector Core. Self complementary AAV (scAAV) genomes were engineered to encode for green fluorescent protein (GFP) transgene under the control of the chicken beta actin/cytomegalovirus hybrid (CB) promoter, flanked by AAV2 inverted terminal repeats. Virus was packaged in HEK293 cells with plasmids containing AAV2 rep genes and AAV8 or 9 cap genes. Cesium chloride gradient was used to purify the vector and titers were determined by qPCR. Vector titers for scAAV8 and scAAV9-CB-GFP were 1x1013 vector genomes (vg)/ml.
AAV Injections in Neonatal Guinea Pigs
Neonatal guinea pigs were injected on P2 in the saphenous or cephalic vein with either scAAV8-CB-GFP (n = 2) or scAAV9-CB-GFP (n = 2) of 2x1012 vg. Injections were performed by the vet staff. Piglets were anesthetized with isoflurane prior to injection. Injection areas were then shaved and swabbed with isopropyl alcohol. A 28 gauge needle was used to inject 200μl of virus into each piglet, resulting in final doses of 2.8–3.1x1013 vg/kg for scAAV8-CB-GFP and 2.8–3.4x1013 vg/kg for scAAV9-CB-GFP piglets. The first piglet injected with scAAV8 CB GFP was found laterally recumbent on P5 due to indeterminate cause and was euthanized as described below. All other treated piglets completed the study without incident.
Guinea Pig Euthanasia and Tissue Preparation
All piglets were euthanized by ketamine/xylazine IP injection and transcardiac perfusion with a PBS rinse and 4% paraformaldehyde (PFA) pH 7.4 for fixation by lab staff according to IACUC approved protocols. Tissues collected from piglets included eyes, cochleae, brain, spinal cord with dorsal root ganglia, liver, lungs, spleen, heart, skeletal muscle (triceps, quadriceps, and gastrocnemius), bladder, gonads, kidneys, and the entire gastrointestinal tract. The gastrointestinal tract was pinned flat in Zamboni’s fixative prior to further dissection. Dissection of the ENS is described in the following section.
Brains were post-fixed in 4% paraformaldehyde pH 7.4 for 48 hours before cryoprotection then sliced into 40μm coronal sections on a sliding microtome. Paraformaldehyde fixed sections from the cervical, thoracic, and lumbar spinal cord were sliced at 40μm on a vibratome. Paraformaldehyde fixed liver, lung, kidney, spleen, heart and all skeletal muscles were cut 40μm thick on a vibratome. Bladder, testes, and ovaries were flash frozen and sliced at 25μm on a cryostat, and sections were collected on slides. For cochlear tissue analysis, the fixed cochleae were initially removed from the temporal bones of the guinea pigs. Under a dissection microscope, the cochleae were dissected in PBS. The bony portion of each cochlea was removed to expose the membranous portion of the cochlea underneath. The membranous portion included the basilar membrane with the organ of Corti on top and the lateral wall, which consists of the stria vascularis and spiral ligament. The basilar membrane is anchored to the central bony core of the cochlea, the modiolus. The modiolus was sectioned horizontally to create three segments of modiolus, basilar membrane, and lateral wall. The three segments were from the basal, middle, and apical turns of the cochlea. Cochleae were then fixed in formalin and sliced at 15μm on a cryostat.
Whole Mount Preparations of the ENS
Guinea Pig
Whole mount longitudinal muscle-myenteric plexus (LMMP) and submucosal plexus (SMP) preparations from guinea pig tissue were dissected as previously described(1). Briefly, the entire gastrointestinal tract from the esophagus to the rectum was removed from euthanized guinea pigs and placed in room temperature (RT) PBS. Sections from all regions of the GI tract including the esophagus, stomach, duodenum, jejunum, ileum, cecum and both proximal and distal sections of the colon were placed in a dish lined with a silicone elastomer and filled with cold PBS, opened along the line of the mesenteric attachment, and pinned flat so that the mucosal layer was closest to the surface. Tissues were fixed overnight at 4°C in Zamboni’s fixative. The next day, tissues were rinsed 5x in RT PBS. For LMMP preparations, the mucosa, submucosa, and circular muscle layers were gently removed with fine-tipped forceps under a stereoscope to revel the myenteric plexus atop the longitudinal muscle. For SMP preparations, the mucosa was gently peeled away and the tissue was flipped over and re-pinned. Afterward the longitudinal muscle, MP, and circular muscle were removed to expose the SMP. Dissected sections went on to immunofluorescent labeling.
Cynomologus Macaque
Whole mount LMMP preparations were dissected like the guinea pig. NHP gastrointestinal tissues were previously fixed in 4% paraformaldehyde pH 7.4 in their original tubular orientation. Pieces of the small intestine, large intestine, and stomach were rinsed in PBS and pinned flat. For LMMP preparations, muscosal, submucosal and circular muscle layers were removed to expose the MP. Dissected sections were then immunofluorescently labeled. The protocol for immunolabeling is below.
Immunolabeling
Immunofluorescent or chromogen staining was used to detect GFP expression in tissues. Chromogen detection was the preferred method of staining in tissues with high levels of autofluorescence. All tissues were stained as free-floating sections except those sliced on the cryostat (see Tissue Preparation). All antibodies and their concentrations used for each tissue are listed in S1 Table.
Tissues were washed with PBS prior to blocking (blocking solution: 10% normal donkey serum, 1% Triton-X 100, PBS) for 2 hours at RT then incubated in primary antibody diluted in blocking solution (24–72 hours) at 4°C. Next, tissues were rinsed three times with PBS before incubation in secondary antibody in blocking solution for 2 hours at RT. Following final PBS rinses, sections were mounted or coverslipped using PVA/DABCO mounting media.
For labeling of macaque GI tissue, antigen retrieval was performed by incubating tissues in 1mM ethylenediaminetetraacetic acid (EDTA) for 20 minutes at 90°C. Tissues were then rinsed in cold tap water and incubated overnight in blocking solution at 4°C. Primary antibody (diluted in blocking solution) incubation occurred over the following night. After PBS rinses, the tissue was re-incubated in blocking solution at RT for 2 hours prior to secondary antibody (diluted in blocking solution) incubation for 2 hours at RT. Sections were rinsed then mounted and coverslipped using PVA/DABCO mounting media.
For chromogen staining: tissues were rinsed three times in fresh PBS and incubated in blocking solution (10% normal donkey serum, 1% Triton-X 100, PBS) for 2 hours at RT. All antibody incubations were performed in blocking solution. Primary antibody was applied overnight and tissues were rinsed in PBS the following day. Secondary antibody was applied for 2 hours at RT, followed by three rinses in PBS. Tissues were then incubated in ABC solution per the kit instructions (Vector Labs, Burlingame, CA) for 2 hours at RT and tissues were rinsed again with PBS. Finally, tissues were developed using 3′,3′-Diaminobenzidine. Free floating tissue sections were mounted on glass slides, dehydrated and coverslipped with Cytoseal (Thermo Scientific).
Quantification of ENS Neurons
Quantification of neurons in the submucosal and myenteric plexus of the guinea pig was completed as previously described(1). Briefly, fluorescently stained whole mount LMMP or SMP tissues were viewed with a Zeiss fluorescent microscope equipped with filters for CY3, CY5, and FITC fluorophores. Cells were counted by an experienced investigators (SEG, CJC, MGN) blind to the treatment groups. Prior to quantification, levels of autofluorescence and background fluorescence were examined in stained and unstained naïve and PBS injected tissues. No auto- or background fluorescence was apparent in enteric neurons or glial cells in piglet or primate tissues, therefore, neurons were considered GFP positive if they expressed any level of FITC fluorescence. MP and SMP ganglia were viewed with a 40X objective for counting. GFP positive and HuD positive neurons were counted in the MP of the stomach, duodenum, jejunum, ileum, cecum, and colon. In the SMP, GFP positive and HuD positive neurons were counted in all GI regions except the stomach. Transduction efficiency of enteric neurons positive for VIP, calretinin, calbindin, nNOS, or ChAT and GFP were recorded in the MP of ileal sections only. Total neuron counts are recorded in S2 Table. Data is reported as the percentage of GFP positive neurons ± standard error of the mean (SEM).
Sample Size and Data Analysis
Due to the nature of this exploratory study and the consequent low sample size, statistical analysis was deemed inappropriate and was not completed. The investigator responsible for quantification of transduced neurons was blind to which animal was being investigated and what vector type each animal had been injected with. There was a total of five guinea piglets used in this study (Table 1) and gastrointestinal tissue from three macaques was examined. All animals were included in this study, and the piglet to receive either vector type or a control injection was chosen at random. A single piglet receiving an AAV8 injection was euthanized due to failing health on postnatal day 5. Due to this abnormality between samples, data is listed for each animal individually in Tables 1 and 2.
Supplementary Material
Acknowledgments
Support: This work was partially supported by NIH T32# 5T32NS077984-02 (SEG) and NIH RC2 #NS69476-01 (BKK). Christian Mueller from UMASS Medical School in Worchester, MA provided viral vector. Brian K. Kaspar from Nationwide Children’s Hospital in Columbus, OH generously donated macaque tissues.
This work was partially supported by NIH T32# 5T32NS077984-02 (SEG) and NIH RC2 #NS69476-01 (BKK). The authors thank Emily Armstrong (Ohio State University, Columbus, OH) for assistance with tissue processing. Also, the authors thank Christian Mueller (UMASS Medical School, Worcester, MA) for providing vector and Brian K. Kaspar (Nationwide Children’s Hospital, Columbus, OH) for donation of the cynomologus macaque tissues.
Footnotes
Conflict of Interest
Brian K. Kaspar is a shareholder, scientific founder and Chief Scientific Officer of Avexis. Kevin D. Foust is currently an employee of Avexis. Other authors have nothing to disclose.
References
- 1.Gombash SE, Cowley CJ, Fitzgerald JA, Hall JC, Mueller C, Christofi FL, et al. Intravenous AAV9 efficiently transduces myenteric neurons in neonate and juvenile mice. Front Mol Neurosci. 2014;7:81. doi: 10.3389/fnmol.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benskey MJ, Kuhn NC, Galligan JJ, Garcia J, Boye SE, Hauswirth WW, et al. Targeted Gene Delivery to the Enteric Nervous System Using AAV: A Comparison Across Serotypes and Capsid Mutants. Mol Ther. 2015;23(3):488–500. doi: 10.1038/mt.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckinx R, Van Remoortel S, Gijsbers R, Waddington SN, Timmermans JP. Proof-of-concept: neonatal intravenous injection of adeno-associated virus vectors results in successful transduction of myenteric and submucosal neurons in the mouse small and large intestine. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2016;28(2):299–305. doi: 10.1111/nmo.12724. [DOI] [PubMed] [Google Scholar]
- 4.Ojala DS, Amara DP, Schaffer DV. Adeno-associated virus vectors and neurological gene therapy. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2015;21(1):84–98. doi: 10.1177/1073858414521870. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg MS, Samulski RJ, McCown TJ. Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology. 2013;69:82–8. doi: 10.1016/j.neuropharm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nature reviews Genetics. 2011;12(5):341–55. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 7.Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302(1):59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- 8.Furness JB. The Enteric Nervous System. Blackwell Publishing; 2006. [Google Scholar]
- 9.Lomax AE, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. Journal of the autonomic nervous system. 1999;76(1):45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Gao N, Hu HZ, Liu S, Gao C, Kim G, et al. Immunoreactivity of Hu proteins facilitates identification of myenteric neurones in guinea-pig small intestine. Neurogastroenterol Motil. 2002;14(2):197–204. doi: 10.1046/j.1365-2982.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- 11.Orandle MS, Veazey RS, Lackner AA. Enteric ganglionitis in rhesus macaques infected with simian immunodeficiency virus. J Virol. 2007;81(12):6265–75. doi: 10.1128/JVI.02671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neddens J, Buonanno A. Expression of the neuregulin receptor ErbB4 in the brain of the rhesus monkey (Macaca mulatta) PLoS One. 2011;6(11):e27337. doi: 10.1371/journal.pone.0027337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GD, Wang XY, Hu HZ, Fang XC, Liu S, Gao N, et al. Angiotensin receptors and actions in guinea pig enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289(3):G614–26. doi: 10.1152/ajpgi.00119.2005. [DOI] [PubMed] [Google Scholar]
- 14.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Progress in histochemistry and cytochemistry. 2010;44(4):173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Furness JB. Types of neurons in the enteric nervous system. Journal of the autonomic nervous system. 2000;81(1–3):87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 16.Bergner AJ, Stamp LA, Gonsalvez DG, Allison MB, Olson DP, Myers MG, Jr, et al. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. The Journal of comparative neurology. 2014;522(3):514–27. doi: 10.1002/cne.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa M, Furness JB, Gibbins IL. Chemical coding of enteric neurons. Prog Brain Res. 1986;68:217–39. doi: 10.1016/s0079-6123(08)60241-1. [DOI] [PubMed] [Google Scholar]
- 18.Steele PA, Brookes SJ, Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience. 1991;45(1):227–39. doi: 10.1016/0306-4522(91)90119-9. [DOI] [PubMed] [Google Scholar]
- 19.Quinson N, Robbins HL, Clark MJ, Furness JB. Calbindin immunoreactivity of enteric neurons in the guinea-pig ileum. Cell Tissue Res. 2001;305(1):3–9. doi: 10.1007/s004410100395. [DOI] [PubMed] [Google Scholar]
- 20.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Advances in experimental medicine and biology. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 21.de Levy BF, do Cunha JC, Chadi G. Cellular analysis of S100Beta and fibroblast growth factor-2 in the dorsal root ganglia and sciatic nerve of rodents. focus on paracrine actions of activated satellite cells after axotomy. The International journal of neuroscience. 2007;117(10):1481–503. doi: 10.1080/15569520701502716. [DOI] [PubMed] [Google Scholar]
- 22.Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19(11):1971–80. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 24.Wang DB, Dayton RD, Henning PP, Cain CD, Zhao LR, Schrott LM, et al. Expansive gene transfer in the rat CNS rapidly produces amyotrophic lateral sclerosis relevant sequelae when TDP-43 is overexpressed. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(12):2064–74. doi: 10.1038/mt.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Zhong L, Nahid MA, Gao G. The potential of adeno-associated viral vectors for gene delivery to muscle tissue. Expert opinion on drug delivery. 2014;11(3):345–64. doi: 10.1517/17425247.2014.871258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Experimental brain research. 2011;210(3–4):347–52. doi: 10.1007/s00221-010-2499-5. [DOI] [PubMed] [Google Scholar]
- 27.Woolf NK. Guinea pig model of congenital CMV-induced hearing loss: a review. Transplantation proceedings. 1991;23(3 Suppl 3):32–4. discussion 4. [PubMed] [Google Scholar]
- 28.Bundoc VG, Keane-Myers A. Animal models of ocular allergy. Current opinion in allergy and clinical immunology. 2003;3(5):375–9. doi: 10.1097/00130832-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Deng HW, Tian Y, Zhou XJ, Zhang XM, Meng J. Effect of Bilberry Extract on Development of Form-Deprivation Myopia in the Guinea Pig. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2016;32(4):196–202. doi: 10.1089/jop.2015.0053. [DOI] [PubMed] [Google Scholar]
- 30.Budenz CL, Wong HT, Swiderski DL, Shibata SB, Pfingst BE, Raphael Y. Differential effects of AAV.BDNF and AAV.Ntf3 in the deafened adult guinea pig ear. Scientific reports. 2015;5:8619. doi: 10.1038/srep08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang GP, Guo JY, Peng Z, Liu YY, Xie J, Gong SS. Adeno-associated virus-mediated gene transfer targeting normal and traumatized mouse utricle. Gene therapy. 2014;21(11):958–66. doi: 10.1038/gt.2014.73. [DOI] [PubMed] [Google Scholar]
- 32.Byrne LC, Lin YJ, Lee T, Schaffer DV, Flannery JG. The expression pattern of systemically injected AAV9 in the developing mouse retina is determined by age. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(2):290–6. doi: 10.1038/mt.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattar CN, Waddington SN, Biswas A, Johana N, Ng XW, Fisk AS, et al. Systemic delivery of scAAV9 in fetal macaques facilitates neuronal transduction of the central and peripheral nervous systems. Gene therapy. 2013;20(1):69–83. doi: 10.1038/gt.2011.216. [DOI] [PubMed] [Google Scholar]
- 34.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. The Journal of clinical investigation. 2015;125(3):918–25. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Giovangiulio M, Verheijden S, Bosmans G, Stakenborg N, Boeckxstaens GE, Matteoli G. The Neuromodulation of the Intestinal Immune System and Its Relevance in Inflammatory Bowel Disease. Frontiers in immunology. 2015;6:590. doi: 10.3389/fimmu.2015.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckley MM, O’Halloran KD, Rae MG, Dinan TG, O’Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. The Journal of physiology. 2014;592(23):5235–50. doi: 10.1113/jphysiol.2014.279968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portales-Casamar E, Swanson DJ, Liu L, de Leeuw CN, Banks KG, Ho Sui SJ, et al. A regulatory toolbox of MiniPromoters to drive selective expression in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(38):16589–94. doi: 10.1073/pnas.1009158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Leeuw CN, Korecki AJ, Berry GE, Hickmott JW, Lam SL, Lengyell TC, et al. rAAV-compatible MiniPromoters for restricted expression in the brain and eye. Molecular brain. 2016;9(1):52. doi: 10.1186/s13041-016-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. Journal of virology. 2006;80(19):9831–6. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011;286(15):13532–40. doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature biotechnology. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(7):1187–96. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Human gene therapy. 2012;23(4):382–9. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506(7488):382–6. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neuroscience research. 2015;93:144–57. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac L, Kissel JT, et al. Gene Therapy for Spinal Muscular Atrophy Type 1 Shows Potential to Improve Survival and Motor Functional Outcomes. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24(S1):S190. [Google Scholar]
- 47.Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain research. 1998;793(1–2):169–75. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimano T, Fyk-Kolodziej B, Mirza N, Asako M, Tomoda K, Bledsoe S, et al. Assessment of the AAV-mediated expression of channelrhodopsin-2 and halorhodopsin in brainstem neurons mediating auditory signaling. Brain research. 2013;1511:138–52. doi: 10.1016/j.brainres.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaj T, Epstein BE, Schaffer DV. Genome Engineering Using Adeno-associated Virus: Basic and Clinical Research Applications. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24(3):458–64. doi: 10.1038/mt.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Podsakoff G, Wong KK, Jr, Chatterjee S. Efficient gene transfer into nondividing cells by adeno-associated virus-based vectors. Journal of virology. 1994;68(9):5656–66. doi: 10.1128/jvi.68.9.5656-5666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Cherel Y, Chenuaud P, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. Journal of virology. 2008;82(16):7875–85. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(7):3428–32. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Jonquieres G, Frohlich D, Klugmann CB, Wen X, Harasta AE, Ramkumar R, et al. Recombinant Human Myelin-Associated Glycoprotein Promoter Drives Selective AAV-Mediated Transgene Expression in Oligodendrocytes. Front Mol Neurosci. 2016;9:13. doi: 10.3389/fnmol.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9(11):625–32. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 55.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. Journal of virology. 2004;78(12):6381–8. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nature biotechnology. 2016;34(2):204–9. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.