Summary
Long‐term storage and transport of post‐harvest carrots (Daucus carota L.) require a low‐temperature, high‐relative‐humidity environment, usually with low ventilation. Following long‐term storage, a slimy exudate (oozing) often appears on the carrots, leading to severe spoilage. We characterized the environmental conditions leading to these symptoms and identified the causative agent. Simulation of non‐ventilated storage conditions revealed accumulation of CO 2 (to 80%) and ethanol (to 1000 ppm); then, a transparent exudate appeared on the carrot surface which, upon ventilation, developed into tissue browning and soft rot. Peels from oozing carrots contained over 10‐fold the total bacterial counts of healthy carrots. The total peel microbiome was determined by 16S rDNA sequencing. During oozing stage, the surface of carrots incubated in a CO 2‐rich (98%) environment harboured a bacterial population dominated by Lactobacillales and Enterobacteriales, differing markedly from those incubated in air. Three prevalent bacterial isolates from the oozing carrots were identified as Pantoea agglomerans, Rahnella aquatilis and Leuconostoc mesenteroides. Inoculation of carrot discs with L. mesenteroides, but not the others, induced oozing under high CO 2, suggesting that this bacterium is responsible for oozing of stored carrots. These findings should enable development of approaches to preventing carrot spoilage during long‐term storage.
Introduction
Carrot (Daucus carota L.) is an economically important staple food worldwide. Optimal storage conditions consist of refrigeration at 1°C and 98% relative humidity (Phan et al., 1973; Seljåsen et al., 2001). These conditions prevent moisture loss from the carrots, help to retain their quality for up to 7 months and enable overseas export (Godfrey and Marshall, 2002). During harvest, transport and processing carrot tissue damage triggers non‐microbial and microbial spoilage during storage and a subsequent negative impact on sensory quality (Martínez‐Hernández et al., 2015). Post‐harvest diseases can be triggered, and they are considered as an important limiting factors to long‐term storage of carrot products (Martínez‐Hernández et al., 2015). It has been suggested that spoilage development in stored carrots is related to the presence of organic debris or soil on the roots, the degree of wounding from mechanical harvesting and pre‐storage washing, and the length of the storage period (Goodliffe and Heale, 1977; Godfrey and Marshall, 2002). Bacterial soft rot of stored carrots, for instance, caused by the bacterial pathogens, is known to contaminate carrots in the field before harvest and readily spread in the washing water during the post‐harvest handling (Klaiber et al., 2005). Another type of rot, induced by carrot brushing, is termed ‘black root rot’ and is caused by two fungi, Thielaviopsis basicola and Chalaripsis thielavioides. These fungi causing large black superficial patches on the carrot roots infect them through wounds or abrasions (Weber and Tribe, 2004; Eshel et al., 2009). Other types of common post‐harvest pathogens of carrots include other fungi, such as Botrytis cinerea, Rhizoctonia carotae (Geeson et al., 1988) and Sclerotinia sclerotiorum (Liew and Prange, 1994), and the bacterial pathogens Pectobacterium (Erwinia) carotovorum (Michalik et al., 1992), (Erwinia) chrysanthemi (Farrar et al., 2000), Pseudomonas viridiflava (Wells et al., 1998) and Pseudomonas marginalis (Hunter and Cigna, 1981).
A variety of methods has been proposed to overcome post‐harvest carrot diseases in order to minimize losses. These methods are not only applicable for the whole stored carrot, but also for other derived products such as minimally processed, sliced and shredded carrots (Tzortzakis, 2016; Villafañe, 2017). Generally, these approaches can be divided into physical, chemical, biological and combined, based on the tool being used. The physical methods include treatments like low‐dose irradiation or microwaving (Kamat et al., 2005; Martínez‐Hernández et al., 2015), steam application (Gan‐Mor et al., 2011) and modified atmosphere (Larsen and Wold, 2016). The chemical treatments include usually washing with chlorinated water, and final treatment with fungicide (iprodione, etc.) is a widely used method of sanitizing brushed and fresh‐cut carrots, thus reducing their initial microbial loads (Eshel et al., 2009). As the potential risks of the latter group are accumulating and safety regulations limit the use of these chemical products, more and more biological applications are entering the market. Among these applications are different kinds of edible chitosan coatings (reviewed by Villafañe, 2017). Lastly combined methods are encouraging the synergetic use of several tools to maximize their efficiency (Eshel et al., 2009; Eshel, 2011).
In Israel, carrots usually undergo minimal processing, which includes removal of inedible parts, washing and brushing to remove soil and the outer peel of the root (Eshel et al., 2009). Exported carrots are transported overseas in large containers at 4–6°C. In recent years, some shipments from Israel have been rejected due to development of oozing followed by soft rot during shipment, causing high economic losses to the farmers. The aims of this study were to characterize the environmental conditions that may trigger the oozing symptoms in stored carrots and to isolate and identify the causative agent.
Results
Non‐ventilated storage conditions induce oozing symptoms
To determine the effect of ventilation on symptom development, whole carrots were stored in either sealed (non‐ventilated) or open chambers. After 9–12 days of storage, a transparent exudate (oozing) was observed on the surface of the stored carrots in the non‐ventilated chambers (Fig. 1A, B), and the average percentage of oozing carrots increased sharply to 80% after 17 days in the non‐ventilated chambers (Fig. 1A). In the ventilated chambers, no symptoms were observed (Fig. 1A), but sprouting of leaves were observed in all carrots (Fig. 1B). Oozing carrots became brownish within 24 h after opening the non‐ventilated chambers (Fig. 1B). After further incubation in ambient atmosphere for another 48 h, soft rot developed, with the brownish carrot tissues becoming soft and ‘slimy’ (Fig. 1B). As the oozing phenomenon frequently results from microbial activity (Davis and Nu, 2007; Horst, 2013), it could be assumed that the elevated level of CO2 in the non‐ventilated chamber induced specific microbial activity that resulted in the oozing phenotype.
Figure 1.
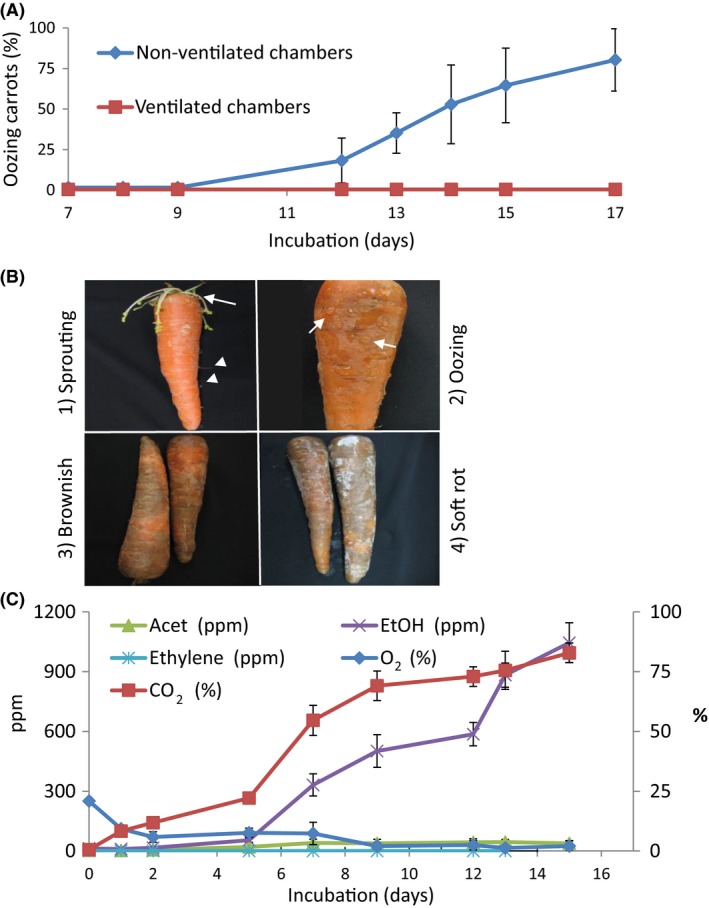
Non‐ventilated storage conditions induce CO 2 and ethanol volatile accumulation followed by oozing symptoms.
A. Percentage of oozing carrots in non‐ventilated chambers as compared to ventilated chambers.
B. Typical carrot symptoms in the sequence of appearance: (1) sprouting (arrow) and rooting (arrow‐head) of carrots during storage under ambient air; (2) oozing (arrows) carrots in non‐ventilated chambers after 9–12 days; (3) brownish colour appearing on stored oozing carrots after ventilation for 24 h; (4) soft rot after oozing followed by ventilation for 3 days.
C. Changes of volatile levels in non‐ventilated chambers. Error bars, ± SD, n = 5. Acet, acetaldehyde; EtOH, ethanol.
CO2 and ethanol volatiles accumulate under non‐ventilated conditions
To determine the effect of storage conditions on volatile composition and a possible correlation to carrot spoilage, the presence of CO2, oxygen, acetaldehyde, ethanol and ethylene was determined in the headspace of the non‐ventilated chambers containing stored carrots for up to 15 days. Accumulation of CO2 was observed as early as day 1 of storage, reaching 80% after 15 days (Fig. 1C). A sharp, ca. threefold increase in CO2 concentration occurred between days 5 and 9. Ethanol concentration began to increase after 5 days of storage to 1000 ppm on day 15 (Fig. 1C). Conversely, O2 concentration decreased gradually until day 9 and remained constant at 2% until day 15. Acetaldehyde concentration remained low throughout the storage period. No ethylene was detected during storage (Fig. 1C).
CO2 induces oozing in carrots
The apparent correlation between CO2 accumulation, O2 reduction and carrot oozing suggested that anaerobic conditions may induce carrot oozing and spoilage. To determine whether oozing symptoms are caused specifically by CO2, non‐ventilated chambers containing carrots were flushed once a day, for 7 days, with CO2, N2 or air. Only the CO2 atmosphere resulted in carrot oozing, starting after 3–4 days of incubation. The percentage of oozing carrots under the high CO2 atmosphere reached 86.9 ± 15.4% on day 7 (Fig. 2). The N2 or ambient air atmosphere had no visible effect on oozing (Fig. 2). After 7 days, the chambers were opened and the carrots were examined daily for symptom development. Within 24 h, all the carrots that had been stored in 98% CO2 atmosphere, as opposed to none of the carrots in 98% N2 or air, became brownish. These data suggested that high CO2 level, rather than lack of O2, induces oozing and the consequent development of brownish colour following air ventilation.
Figure 2.
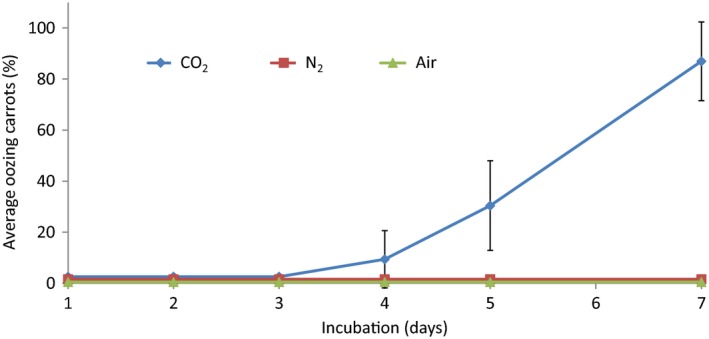
Only CO 2 flushing causes oozing of stored carrot in non‐ventilated conditions. CO 2, N2 and air were flushed daily into the chambers for 7 days, and then, incubation chambers were ventilated for 24 h. Error bars, ± SD, n = 5.
Oozing symptoms correlate with bacterial count
To determine the microorganisms associated with the oozing and brownish colour symptoms, peel extracts derived from healthy, oozing and brownish carrots were serially diluted and plated on plate count agar (PCA) and potato dextrose agar (PDA)–chloramphenicol to isolate bacteria and fungi respectively. Fungal isolates were not consistently cultured from oozing and brownish carrot samples (data not shown), suggesting that these symptoms result from the presence of bacterial pathogens. The total count of mesophilic aerobic bacteria on oozing carrots was more than 10‐fold higher [7.3 × 108 colony‐forming units (CFU) g−1] than that on healthy carrots (1.8 × 107 CFU g−1) (Fig. 3). The number of bacteria on brownish carrots increased to 2.7 × 109 CFU g−1 (Fig. 3).
Figure 3.
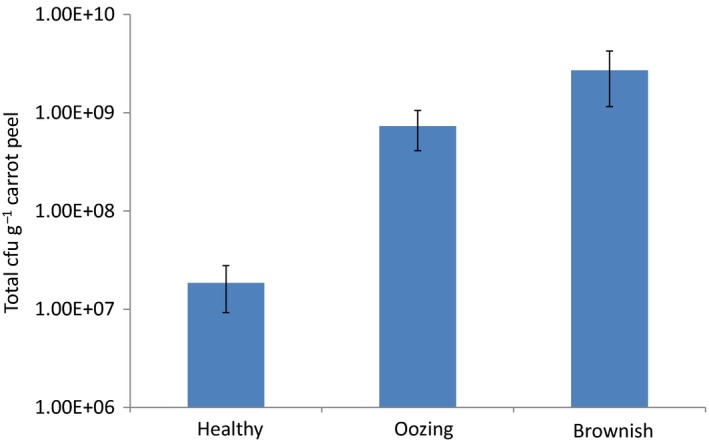
Heterotrophic bacterial counts on carrot peels derived from healthy and symptomatic carrots. Bacteria were plated on PCA and incubated at 28°C for 48 h. Error bars, ± SD, n = 5.
CO2‐rich environment modifies carrot microbiome
To determine the effect of high ambient CO2 on the carrot microbiome, the microbial population of carrots exposed to a 98% CO2 environment was analysed in four phenological stages: ‘healthy’ (time 0), ‘pre‐oozing’ (a day before visible oozing), ‘oozing’ and ‘brownish’ (24‐h ventilation after oozing).
A heat map was used to show the relative normalized abundance within each sample of the most abundant microbial orders (Fig. 4). Carrots incubated in air were dominated by Rickettsiales and a lower level of Pseudomonadales, Actinomycetales, Rhizobiales and Burkholderiales in the healthy and pre‐oozing stages (Fig. 4). In later stages of incubation, low severity of oozing and brownish increased levels of Enterobacteriales, Pseudomonadales, Actinomycetales, Flavobacteriales, Sphingobacteriales and Xanthomonadales (Fig. 4). In contrast, carrots incubated in the CO2‐rich environment were dominated by Lactobacillales and Enterobacteriales, mainly during oozing and brownish stages (Fig. 4).
Figure 4.
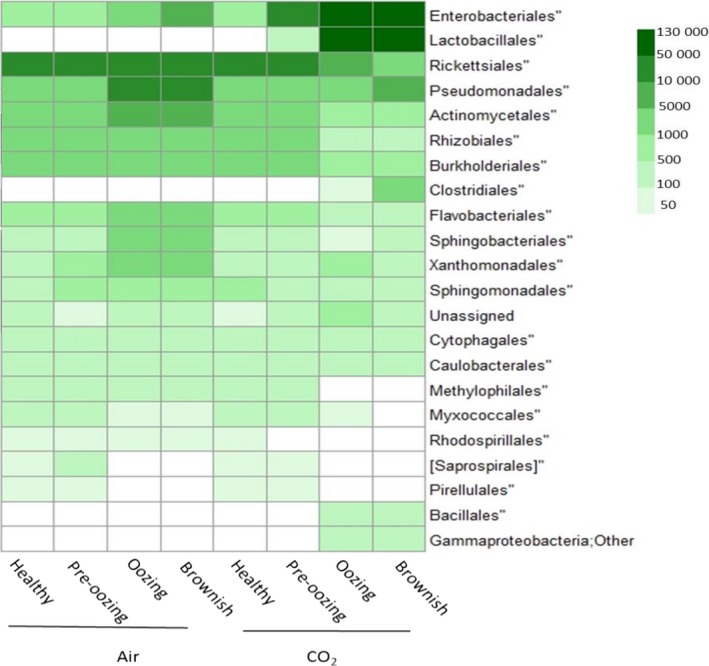
Heat map of average normalized counts suggesting that CO 2 environment changes the microbiome on the carrot surface. The microbial population of carrots exposed to air or 98% CO 2 environment was analysed at four phenological stages: healthy (time 0), pre‐oozing (a day before visible oozing), oozing and brownish (24‐h ventilation after oozing). The scale of the heat map represents abundance in major bacterial population distribution at the order level (L4). The abundance of the different bacteria at the order level was normalized using the trimmed mean of M‐values normalization method (TMM), and then, all replicates were averaged to create the mean normalized level of the bacterial population.
The alpha diversity value, representing mean species diversity in each treatment, reflected minor differences for the air‐exposed samples and significant differences for the CO2‐exposed samples over time (Table 1). During the oozing process occurring in the CO2‐rich environment, the number of operational taxonomic units (OTUs) decreased (Table 1). The air samples and the pre‐oozing CO2 samples showed higher species richness (OTUs) than the oozing and brownish CO2 samples (Table 1). The former group was more diverse as well, according to its higher Shannon diversity index compared to the CO2‐oozing and CO2‐brownish samples (Table 1). Similarly, the single rarefaction curves (not shown) computed for each sample by Chao1 richness estimator showed similar trends per sample (Table 1).
Table 1.
Average alpha diversity for carrot samples exposed to air or CO2
| OTUs (97%) | Coverage | Shannon | Chao | |||||
|---|---|---|---|---|---|---|---|---|
| Average | STDEV | Average | STDEV | Average | STDEV | Average | STDEV | |
| Air | ||||||||
| Healthy | 4847 aba | 652.02 | 0.96 | 0.008 | 4.490 ab | 0.57 | 11 034.146 a | 1270.90 |
| Pre‐oozing | 5030 a | 699.88 | 0.96 | 0.008 | 4.3163 a | 0.59 | 11 306.765 a | 1470.05 |
| Oozing | 6534 a | 382.76 | 0.97 | 0.003 | 5.2607 ab | 0.52 | 13 869.607 a | 765.38 |
| Brownish | 6479 a | 810.61 | 0.97 | 0.002 | 4.884 ab | 0.44 | 13 642.255 a | 1488.41 |
| CO2 | ||||||||
| Healthy | 5070 a | 358.18 | 0.96 | 0.004 | 4.773 ab | 0.56 | 11 204.286 a | 732.59 |
| Pre‐oozing | 5643 a | 677.81 | 0.96 | 0.004 | 4.720 ab | 0.66 | 12 686.648 a | 1618.00 |
| Oozing | 4199 c | 253.24 | 0.98 | 0.002 | 4.109 b | 0.18 | 7517.282 b | 554.90 |
| Brownish | 4326 bc | 385.80 | 0.98 | 0.002 | 4.154 ab | 0.25 | 7765.346 b | 855.83 |
Different letters indicate significant difference based on one way ANOVA (P < 0.05), followed by Tukey HSD test.
The bacterial community compositions differed considerably between the air‐ and CO2‐stored carrots, when carrot oozing began. A sharp difference in community structure between the CO2‐oozing and brownish samples and all other samples are clearly illustrated by the PCA biplot and the topology of the weighted UniFrac UPGMA tree (Figs 5 and S1 respectively). All of the air samples, and the CO2‐healthy and CO2‐pre‐oozing samples, are clustered tightly in the biplot graph, indicating a similar community composition in these samples. The CO2‐oozing and CO2‐brownish samples, on the other hand, are clustered separately, albeit not as tightly as the other samples (Fig. 5). The UniFrac tree supports the PCA graph, with the CO2‐oozing and CO2‐brownish samples forming one cluster, and the other samples forming a separate cluster, indicating a distinct difference between the bacterial community compositions of healthy and oozing carrots (Fig. S1).
Figure 5.

PCA biplot calculated with factomineR. The microbial population of carrots exposed to air or 98% CO 2 was analysed at four phenological stages: healthy (time 0), pre‐oozing (a day before visible oozing), oozing and brownish (24‐h ventilation after oozing).
Isolation of microorganisms from oozing carrots
Cultivation of oozing and brownish carrot samples resulted in the appearance of three prevalent bacterial morphotypes on the agar media, which differed in colour and size. To determine whether each of these three apparent morphotypes make up a homogeneous population, five colonies of each morphotype were further isolated to pure cultures and subjected to molecular identification by 16S rRNA gene sequence analysis. The three morphotypes were identified as Pantoea agglomerans (99% similarity, GenBank accession number NR041978), Rahnella aquatilis (99% similarity, GenBank accession number NR074921) and Leuconostoc mesenteroides (100% similarity, GenBank accession number NR074957). P. agglomerans and R. aquatilis are Gram‐negative bacteria of the family Enterobacteriaceae, while L. mesenteroides is a Gram‐positive lactic acid bacterium of the family Leuconostocaceae (Krieg and Holt, 1994). These findings are in agreement with the microbiome analysis as L. mesenteroides belongs to the order Lactobacillales, while P. agglomerans and R. aquatilis are members of the order Enterobacteriales.
Effect of CO2 on growth of P. agglomerans, R. aquatilis and L. mesenteroides
The presence of abundant bacterial populations of P. agglomerans, R. aquatilis and L. mesenteroides on oozing and brownish carrot tissues under high levels of CO2 suggested a growth advantage for these bacteria in the CO2‐rich atmosphere. To test this, serial dilutions of the three bacteria were plated on carrot agar medium and incubated for 5 days at 20°C in a 98% CO2 atmosphere. L. mesenteroides showed rich growth, while R. aquatilis grew poorly and P. agglomerans did not grow at all under these conditions. All three strains showed comparable growth levels upon ventilation in ambient air for 3 days (Table 2).
Table 2.
Growth of bacterial isolates derived from oozing and brownish carrot peels
| Most closely related hit in GenBank | % Identity (> 500 bp) | Bacterial taxa (class, family) | Level of growth: CO2 incubation | Level of growth: air incubation (after CO2) |
|---|---|---|---|---|
| Pantoea agglomerans (NR041978) | 99 | Gammaproteobacteria, Enterobacteriaceae | − | +++ |
| Rahnella aquatilis (NR074921) | 99 | Gammaproteobacteria, Enterobacteriaceae | + | +++ |
| Leuconostoc mesenteroides (NR0974957) | 100 | Bacilli, Leuconostocaceae | +++ | +++ |
(−) no growth; (+) poor growth of few colonies; (+++) colonies cover of the entire Petri dish.
L. mesenteroides induces oozing on carrot discs
As only L. mesenteroides grew in the CO2‐rich atmosphere, this bacterium might be the causative agent of the oozing symptoms. To examine the role of each strain in oozing, Koch's postulates were tested by inoculating carrot discs with each of the three strains, as well as with fluid from the exudate, followed by incubation in a controlled 98% CO2 atmosphere or ambient air for 5 days. Only L. mesenteroides and the carrot exudate, and not P. agglomerans or R. aquatilis, induced oozing symptoms on the carrot discs in the CO2 atmosphere (Fig. 6). None of the bacterial strains, nor the fluid exudate, were able to induce oozing symptoms following incubation in ambient air. R‐2A Broth, used as a control, did not induce any symptoms either in both of the conditions (Fig. 6). To confirm that L. mesenteroides is indeed the causative agent of the oozing, Koch's postulates were completed by reisolating L. mesenteroides from the oozing fluid that developed on the L. mesenteroides‐inoculated carrot discs.
Figure 6.

Representative images showing inoculated carrot discs.
A.Control, no bacteria.
B. R. aquatilis.
C. P. agglomerans.
D. L. mesenteroides.
E. Oozing fluid, after 5 days at 20°C.
These findings supported the notion that high CO2 level supports the growth of the anaerobic bacterium L. mesenteroides. However, it could be that the high CO2 level stressed the carrot tissue, making it more susceptible to bacterial proliferation, regardless of atmospheric composition. To test this notion, carrot discs were pre‐exposed to 98% CO2 from 5 h to 5 days before bacterial inoculation as indicated above. Following inoculation, the discs were stored in ambient air for an additional 5 days. None of the discs displayed any oozing symptoms, suggesting that previous CO2 stress alone is not sufficient to support oozing symptoms under ambient air in the presence of each of the three species.
Discussion
Non‐ventilated conditions modify microbiome composition
In the current study, we developed a small‐scale model system that simulates the environmental conditions during long‐term storage of commercially brushed carrots under non‐ventilated conditions. During storage of carrots, which are harvested at full metabolic activity (Godfrey and Marshall, 2002), non‐ventilated conditions allow the build‐up of CO2 concentration, while O2 concentration gradually decreases (Fig. 1). These environmental changes can induce the development of oozing, previously reported in stored ‘ready‐to‐use’ grated carrots packed in polymeric films, where the combination of high CO2 and low O2 concentrations was shown to trigger spoilage (Carlin et al., 1990a,b). Spoilage because of growth of lactic acid bacteria (LAB) is well documented in cold‐stored, vacuum‐packaged or modified atmosphere‐packaged meat, poultry and processed meat products (Vihavainen et al., 2008). In these products, LAB spoilage is often characterized by deteriorations in odour, flavour, colour or appearance (reviewed by Remenant et al., 2015). However, it is important to mention that high CO2 can have a dual effect on different microbial populations. Modified atmosphere may significantly inhibit spoilage organisms as well as desirable microflora in post‐harvest produce, due to the non‐selective antimicrobial effect of CO2 (Farber et al., 2003; Caleb et al., 2013).
The main changes observed in the carrot microbiome during storage in the non‐ventilated environment were increased abundance of Enterobacteriales and Lactobacillales and decreased abundance of Rickettsiales. These changes are likely triggered by alterations in the atmosphere's gaseous composition (increased CO2, low O2). Elevated CO2 levels have been previously reported to affect the composition of microbial communities on other agricultural produce (Gill, 1996; de Oliveira et al., 2010; Lo et al., 2016). The antimicrobial effect of CO2 has been reported to affect bacterial and viral population density, and gene expression of some foodborne‐pathogen toxins (Francis et al., 1999; Bidawid et al., 2000; Artin et al., 2008). Whereas aerobic bacteria such as pseudomonads are inhibited by moderate to high levels of CO2, other microorganisms, such as the facultative anaerobic LAB and yeast, can be stimulated under these conditions (Enfors and Molin, 1980; Amanatidou et al., 1999; Soliva‐Fortuny and Martín‐Belloso, 2003; Oliveira et al., 2010).
Rickettsiales are small aerobic bacteria in the class Alphaproteobacteria. They have a diverse host range as endosymbionts, including arthropods, vertebrates and plants (Weinert et al., 2009). Rickettsia‐related organisms were found in diseased plants from various species (including in carrots) although it is not clear whether they are the actual disease agents (Franova et al., 2000; Streten et al., 2005; Luis‐Pantoja et al., 2015). Reduction in the abundance of Rickettsiales in a CO2‐rich atmosphere suggests that they are sensitive to high CO2 concentration, similar to other Gram‐negative bacteria.
Storage of carrots in a non‐ventilated or CO2‐rich atmosphere resulted in decreased population richness and diversity (Figs 4, 5, 6). This might be associated with growth limitation in CO2‐rich atmosphere, as reported, for example, during storage of meat under modified atmosphere (Stoops et al., 2015). Similarly, decreased species richness and diversity have also been reported during storage of spinach and fresh‐cut lettuce under modified atmosphere (Lopez‐Velasco et al., 2011; Di Carli et al., 2016).
Expression of antibacterial compounds, known to be secreted by many LAB species (Cotter et al., 2013), may also have contributed to the decreased species richness and diversity. Other causes for the population changes might include competition for carrot‐derived nutrients and adaptation to the changing environment.
L. mesenteroides is the causative agent of oozing
Induction of oozing was associated with increased bacterial counts on the root surface (Fig. 3), and the abundance of three dominant species, P. agglomerans, R. aquatilis and L. mesenteroides, all of which could potentially be involved in carrot spoilage. However, only L. mesenteroides induced oozing on infected carrot discs under high CO2 atmosphere (Fig. 6). Nevertheless, inoculation of carrot discs with exudate derived from oozing whole roots resulted in more extensive symptoms than that induced by L. mesenteroides alone (Fig. 6). This might indicate the involvement of additional microorganisms in the soft rot of naturally infected roots, which were absent in our model of sterilized carrot discs. Indeed, rotten vegetables are frequently colonized by secondary bacterial or fungal pathogens which may even mask the primary spoilage pathogen (Godfrey and Marshall, 2002).
The other two species isolated from the oozing carrots are also considered to be natural soil and plant flora. P. agglomerans is abundant in soil and plants (Monier and Lindow, 2005; Son et al., 2006) and colonizes numerous types of vegetables and fruits, including sprouts, spinach, lettuce, pepper, strawberries (Leff and Fierer, 2013), potato tubers (Sturz et al., 1999) and onion (Dutta et al., 2014). Similarly, R. aquatilis is a common soil and plant inhabitant (Kim et al., 1997; Calvo et al., 2007; Chen et al., 2007). Hausdorf et al. (2011) found both P. agglomerans and R. aquatilis in wash water from an industrial carrot washing and packing plant. It is possible that these bacteria were merely secondary colonizers that flourished in the nutrient‐rich exudate generated by L. mesenteroides.
Leuconostoc mesenteroides is a lactic acid bacterium (LAB) associated with the fermentation of vegetables and other products (Hemme and Foucaud‐Scheunemann, 2004; Wouters et al., 2013). It has been isolated from lettuce (Leff and Fierer, 2013) and fresh‐cut celery (Robbs et al., 1996), as well as from grated carrots (Carlin et al., 1990a) and spoiled vacuum‐packaged vegetable sausages (Vihavainen et al., 2008). Known causative agents of soft rot in stored carrot include Pseudomonas viridiflava and Pseudomonas marginalis (Godfrey and Marshall, 2002; Almeida et al., 2013), Pectobacterium (Erwinia) carotovorum and Pectobacterium (Erwinia) chrysanthemi (Towner and Beraha, 1976), Rhizoctonia carotae (Geeson et al., 1988) and Sclerotinia sclerotiorum (Liew and Prange, 1994).
To the best of our knowledge, our study is the first to implicate L. mesenteroides in oozing of partially processed carrot roots during storage under non‐ventilated conditions, although its involvement in soft rot of ‘ready‐to‐use’ grated carrots packaged in polymeric films is known (Carlin et al., 1990a). It is likely that L. mesenteroides is an innocuous inhabitant of the carrot root surface, which became an opportunistic pathogen due to the commercial brushing process followed by high respiration and low ventilation of the transported carrot. Brushing or grating of the root surface results in loss of the epidermal tissue, which exposes the inner nutrient‐rich tissues to the intrinsic microbial flora.
High levels of CO2 can inactivate certain microorganisms, including bacteria, yeast, fungi and their spores (reviewd by Damar and Balaban, 2006). It is likely that nutrients derived from the bruised tissue in combination with high CO2 atmosphere provided a selective growth advantage to L. mesenteroides. Once the chambers are opened and ventilated, the ambient air together with nutrients that are available in the exudates may provide conditions favouring the growth of other species present in the same ecological niche, such as P. agglomerans and R. aquatilis.
None of the three predominant species were able to cause oozing symptoms on carrot discs under ambient air conditions (Fig. 6). While L. mesenteroides presented rich growth on carrot agar medium under high CO2 and ambient air environments (Table 2), oozing symptoms only occurred on carrot discs with high CO2 levels (Fig. 6). Thus, the pathogen grows and initiates oozing mainly in a CO2‐rich environment. It can be assumed that high CO2 concentration induces virulence genes required for the production and secretion of exudate by L. mesenteroides, as other studies have suggested that CO2 regulates the expression of virulence factors involved in bacterial pathogenesis (Finlay and Falkow, 1989; Miller et al., 1989). Similarly, the aggressiveness of the plant pathogens Colletotrichum gloeosporioides and Erysiphe cichoracearum was found to increase in the presence of elevated CO2 concentrations (Chakraborty and Datta, 2003; Lake and Wade, 2009). Interestingly, a recent study has indicated increased quorum‐sensing‐mediated virulence in soft rot caused by Pectobacterium carotovorum under elevated temperature and CO2 levels (Das and Chaudhary, 2014). Transcriptome profiling of L. mesenteroides under low‐ and high‐CO2 atmospheres may shed light on the genes and mechanisms responsible for the oozing phenomenon in L. mesenteroides‐infected carrot under non‐ventilated conditions.
In summary, this study pinpoints a case in which consumer preference for aesthetic carrot appearance combined with technological advances (washing and mechanical brushing) and trade globalization have led to the emergence of an economically important disease by a seemingly opportunistic pathogen that until now had only rarely been associated with carrot spoilage. Characterization of the conditions associated with the shipping of stored carrots and better control of the ventilation conditions will probably open the way to developing intervening means to prevent spoilage of shipments, and the associated financial losses.
Experimental procedures
Plant material, processing and storage conditions
Carrot roots (Daucus carota L.) cv. Nairobi were grown in the northern Negev (in southern Israel). They were harvested in the spring (April), washed in water to remove soil residue and brushed in a commercial machine using spiral‐wound coil brushes made of 0.5‐mm polyester filaments, which resulted in peeling the epidermis from the roots. After brushing, carrots were hydro‐cooled in a 4°C water bath, packed in commercial 12‐kg polyethylene bags and stored at 1°C for 2 months until further analysis.
Storage experiments and analysis of volatiles
Glass chambers (2 l) equipped with a rubber septum were washed and disinfected by spraying with 70% ethanol for 1 min and wiping with a paper towel. The chambers were left open for 5 min so that the ethanol residue would evaporate. Brushed carrots (9–12) were placed in each chamber, occupying approximately 75% of its volume. Chambers were hermetically sealed or air‐ventilated by keeping the upper septum open. To enhance spoilage symptoms, the chambers were kept in the dark at 20°C for a further 15–17 days. The chambers were then opened and exposed to ambient atmosphere for up to 48 h. Visual symptoms were monitored daily throughout the storage period.
Volatiles were sampled from the headspace of each chamber by inserting a syringe through the septum. CO2 levels were determined by gas chromatography using a thermal conductivity detector (Series 580; GOW‐MAC Instrument Co, Bethlehem, PA, USA). Oxygen levels were determined by Oxybaby gas analyser (model 6; WITT‐Gasetechnik GmbH and Co KG, Witten, Germany). Acetaldehyde and ethanol levels were determined with a Varian 3300 gas chromatograph (Hewlett‐Packard, Bloomington, IL, USA). Ethylene levels were determined with a Varian 3300 gas chromatograph (Walnut Creek, CA, USA).
In some experiments, the carrot‐containing chambers were flushed daily for 7 days with CO2, N2 or air to maintain a controlled atmosphere. The level of the gas in each chamber was confirmed daily, immediately after flushing, as described above. The gas levels were as follows: 98% CO2, 98% N2, and for air: 78.9% N2, 20.8% O2 and 0.3% CO2.
Microbial analysis
During storage in the non‐ventilated chambers, an exudate appeared on the carrot surface, a phenomenon known as oozing. Carrots that changed their colour to brown, 24 h after opening and ventilating the chamber, were termed ‘brownish’. Peels of ‘healthy’, ‘oozing’ and ‘brownish’ carrots (five carrots from each chamber) were individually sampled using a sterile kitchen peeler and were transferred into sterile 15‐ml polypropylene tubes. Peel (5 g) was ground in a laboratory blender in 50 ml of saline in a sterile cup. Microbial enumeration was performed by standard serial dilutions and plating in triplicate on three media: (i) PCA (Difco, Sparks, MD, USA); (ii) carrot agar, a medium which was prepared by mincing four carrots in 1 l distilled water supplemented with 1.5% Bacto Agar (Difco); (iii) PDA (Difco) with addition of 0.25 mg ml−1 chloramphenicol. All plates were incubated at 28°C for 2 or 7 days (for bacteria and fungi respectively), and the number of CFU was determined. The prevalent bacterial colonies, based on colony phenotype, were isolated and subcultured on PCA plates to obtain pure cultures. Isolated colonies from pure cultures were kept in 30% glycerol stocks and maintained at −80°C.
Microbiome sample collection
Ten 2‐l glass chambers were filled with carrots (cv. Nairobi). Five chambers were sealed and flushed daily with 100% CO2 and the other five were left open (ventilated). To enhance symptom appearance, the chambers were kept in the dark at 20°C, as above, for 21 days. Microbial samples were taken from the carrot surface at four time points by swabbing. The first sample (‘healthy’) was taken at the beginning of the experiment (time 0). A second sample was taken after 18 days of incubation (‘pre‐oozing’), and a third sample was taken after 20 days (‘oozing’). After the third sampling, all of the chambers were opened and exposed to ambient air. The final sample was taken 24 h later, on day 21 (‘brownish’). Each sample included five repeats of 1.0‐ml sterilized double‐distilled water (sddw) used to resuspend the swab from one carrot. The samples were stored immediately after sampling at −80°C until further use.
DNA was extracted from the different samples using Exgene Soil DNA Isolation kit (GeneAll Biotechnology, Seoul, Korea). DNA concentration was determined in a Nanodrop ND1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The DNA samples were stored at −80°C until use.
16S rRNA sequencing analysis
Amplicons for barcoded paired‐end Illumina Miseq sequencing (Illumina, San Diego, CA, USA) were generated using the primer set 515F (5′‐GTGCCAGCMGCCGCGGTAA‐3′) and 806R (5′‐GGACTACVSGGGTATCTAAT‐3′) that amplifies the bacterial V4 hypervariable region as described previously (Caporaso et al., 2012). Overall, 4 765 126 amplicons were sequenced. All sequence pre‐processing, analysis of OTUs and classifications were performed using QIIME version 1.9.0 (Caporaso et al., 2010a). In the first step, PEAR software version 0.9.6 (Zhang et al., 2014) was used to merge the fastq files, and then, quality and length trimming was performed using CLC Bio software (http://www.clcbio.com/). Chimeras were removed using USEARCH (Edgar, 2010), and finally, taxonomic assignment was performed with the script assign_taxonomy.py against Greengenes QIIME datafiles version 13_8 (DeSantis et al., 2006). OTUs were generated using a 97% sequence‐identity threshold. OTUs with less than two observations, and samples with fewer than 150 observations were filtered from the OTU table. Phylogenetic analysis was performed by aligning the representative sequences with PyNAST (Caporaso et al., 2010b) and then creating the tree using the FASTTREE algorithm (Price et al., 2009). Community profiles were compared using the weighted UniFrac metric (Hamady et al., 2010), and a UPGMA tree was constructed with QIIME based on the UniFrac metric.
Molecular identification of isolated bacteria
Identification of bacterial isolates was performed by sequencing of the 16S rRNA gene. Briefly, five colonies of each prevalent isolate were picked with a sterile bacteriological needle and resuspended separately in 10 μl of filtered sddw. The suspension was heated at 95°C for 5 min and then centrifuged for 10 min at 13 800 g to remove intact bacterial cells and large debris. The supernatant containing the DNA was used as a template for PCR amplification using the universal bacterial primers 8–27: 5′‐AGAGTTTGATCCTGGCTCAG‐3′ and 1492: 5′‐GGTTACCTTGTTACGACT‐3′ (Lampert et al., 2006) as follows: 5 min of denaturation at 94°C; 30 cycles of 1 min at 94°C, 1 min at 52°C and 1 min at 72°C; termination at 72°C for 10 min. The amplified DNA fragments were purified from an agarose gel using the Zymoclean Gel DNA Recovery kit (The Epigenetics Company; Zymo Research Corp., Irvine, CA, USA) and sent for sequencing (Hylabs Ltd., Rehovot, Israel). Sequence data were compared to the 16S rRNA gene database in the National Center for Biotechnology Information (NCBI) GenBank database, using the BLAST program (Altschul et al., 1990).
Laboratory models for carrot inoculation
Carrots were surface‐sterilized by dipping in 1% sodium hypochlorite solution (Bio‐Lab, Jerusalem, Israel) for 1 min and immediately washed by dipping in sddw for 30 s. Surface‐sterilized carrots were peeled or horizontally cut by sterile scalpel blade to sample the cortex or central core tissues respectively. The presence of microorganisms in these tissues was determined as described above for the peels.
Carrot discs (ca. 5 mm width and 30–35 mm diameter) were placed in a sterile Petri dish containing a 0.22‐μm filter paper (47 mm; Millipore, Merck) soaked with 600 μl sddw. A 50‐μl aliquot of either pure culture of the tested strain (108 CFU ml−1) in R2 Broth (Hi Media Laboratories Pvt., Mumbai, Maharashtra, India) grown at 28°C for 24 h, or carrot exudate collected from oozing carrots and kept at 4°C for 24 h, was placed at the centre of each disc. The Petri dishes were placed in the above‐described glass chambers, which were flushed daily with 98% CO2 or ambient air, and kept at 20°C for up to 5 days, as described above for intact carrots.
In another set of experiments, sterile carrot discs were pre‐exposed to 98% CO2 for various periods of time ranging from 5 h to 1–5 days before inoculation with bacteria or with exudate fluid. The inoculated discs were left in ambient air for another 5 days at 20°C. All of the carrots discs were examined daily for oozing and browning symptoms.
Conflict of interest
None declared.
Supporting information
Fig. S1. Unweighted UniFrac distance Jackknife dendrogram of bacterial communities.
Acknowledgements
This work was supported by grant 430‐0522‐14 of the Chief Scientist, Ministry of Agriculture, Israel. This manuscript is contribution no. 781/17 from ARO, the Volcani Center, P.O Box 15159 Rishon LeZion, 7528809, Israel.
Microbial Biotechnology (2017) 10(6), 1677–1689
Funding information
This work was supported by grant 430‐0522‐14 of the Chief Scientist, Ministry of Agriculture, Israel.
References
- Almeida, I. , Maciel, K. , Neto, J.R. , and Beriam, L. (2013) Pseudomonas viridiflava in imported carrot seeds. Aust Plant Dis Notes 8: 17–19. [Google Scholar]
- Altschul, S.F. , Gish, W. , Miller, W. , Myers, E.W. , and Lipman, D.J. (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Amanatidou, A. , Smid, E. , and Gorris, L. (1999) Effect of elevated oxygen and carbon dioxide on the surface growth of vegetable‐associated micro‐organisms. J Appl Microbiol 86: 429–438. [DOI] [PubMed] [Google Scholar]
- Artin, I. , Carter, A.T. , Holst, E. , Lövenklev, M. , Mason, D.R. , Peck, M.W. , and Rådström, P. (2008) Effects of carbon dioxide on neurotoxin gene expression in nonproteolytic Clostridium botulinum type E. Appl Environ Microbiol 74: 2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidawid, S. , Farber, J. , and Sattar, S. (2000) Contamination of foods by food handlers: experiments on hepatitis A virus transfer to food and its interruption. Appl Environ Microbiol 66: 2759–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caleb, O.J. , Mahajan, P.V. , Al‐Said, F.A.‐J. , and Opara, U.L. (2013) Modified atmosphere packaging technology of fresh and fresh‐cut produce and the microbial consequences—a review. Food Bioprocess Technol 6: 303–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo, J. , Calvente, V. , de Orellano, M.E. , Benuzzi, D. , and de Tosetti, M.I.S. (2007) Biological control of postharvest spoilage caused by Penicillium expansum and Botrytis cinerea in apple by using the bacterium Rahnella aquatilis . Int J Food Microbiol 113: 251–257. [DOI] [PubMed] [Google Scholar]
- Caporaso, J.G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F.D. , Costello, E.K. , et al (2010a) QIIME allows analysis of high‐throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J.G. , Bittinger, K. , Bushman, F.D. , DeSantis, T.Z. , Andersen, G.L. , and Knight, R. (2010b) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26: 266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J.G. , Lauber, C.L. , Walters, W.A. , Berg‐Lyons, D. , Huntley, J. , Fierer, N. , et al (2012) Ultra‐high‐throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin, F. , Nguyen‐the, C. , Chambroy, Y. , and Reich, M. (1990a) Effects of controlled atmospheres on microbial spoilage, electrolyte leakage and sugar content of fresh ‘ready‐to‐use'grated carrots. Int J Food Sci Technol 25: 110–119. [Google Scholar]
- Carlin, F. , Nguyen‐the, C. , Hilbert, G. , and Chambroy, Y. (1990b) Modified atmosphere packaging of fresh”,ready‐to‐use” grated carrots in polymeric films. J Food Sci 55: 1033–1038. [Google Scholar]
- Chakraborty, S. , and Datta, S. (2003) How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Phytol 159: 733–742. [DOI] [PubMed] [Google Scholar]
- Chen, F. , Guo, Y. , Wang, J. , Li, J. , and Wang, H. (2007) Biological control of grape crown gall by Rahnella aquatilis HX2. Plant Dis 91: 957–963. [DOI] [PubMed] [Google Scholar]
- Cotter, P.D. , Ross, R.P. , and Hill, C. (2013) Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11: 95–105. [DOI] [PubMed] [Google Scholar]
- Damar, S. , and Balaban, M.O. (2006) Review of dense phase CO2 technology: microbial and enzyme inactivation, and effects on food quality. J Food Sci 71: R1–R11. [Google Scholar]
- Das, N.R. , and Chaudhary, A. (2014) Effect of elevated temperature and CO2 on quorum sensing mediated virulence in soft rot causing Pectobacterium carotovorum pv. carotovorum. Ind J Hort 71: 227–231. [Google Scholar]
- Davis, R.M. and Nu, J. (2007) Integrated approaches for carrot pests and diseases management In: General Concepts in Integrated Pest and Disease Management: Springer. 149–188. [Google Scholar]
- DeSantis, T.Z. , Hugenholtz, P. , Larsen, N. , Rojas, M. , Brodie, E.L. , Keller, K. , et al (2006) Greengenes, a chimera‐checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carli, M. , De Rossi, P. , Paganin, P. , Del Fiore, A. , Lecce, F. , Capodicasa, C. , et al (2016) Bacterial community and proteome analysis of fresh‐cut lettuce as affected by packaging. FEMS Microbiol Lett 363, fnv209. [DOI] [PubMed] [Google Scholar]
- Dutta, B. , Barman, A. , Srinivasan, R. , Avci, U. , Ullman, D. , Langston, D. , and Gitaitis, R. (2014) Transmission of Pantoea ananatis and P. agglomerans, causal agents of center rot of onion (Allium cepa), by onion thrips (Thrips tabaci) through feces. Phytopathology 104: 812–819. [DOI] [PubMed] [Google Scholar]
- Edgar, R.C. (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461. [DOI] [PubMed] [Google Scholar]
- Enfors, S.O. , and Molin, G. (1980) Effect of high concentrations of carbon dioxide on growth rate of Pseudomonas fragi, Bacillus cereus and Streptococcus cremoris. J Appl Bacteriol 48: 409–416. [DOI] [PubMed] [Google Scholar]
- Eshel, D. (2011) Non‐chemical approaches for postharvest quality management of underground vegetables. Stewart Postharvest Rev 7: 1–7. [Google Scholar]
- Eshel, D. , Regev, R. , Orenstein, J. , Droby, S. , and Gan‐Mor, S. (2009) Combining physical, chemical and biological methods for synergistic control of postharvest diseases: a case study of Black Root Rot of carrot. Postharvest Biol Technol 54: 48–52. [Google Scholar]
- Farber, J. , Harris, L. , Parish, M. , Beuchat, L. , Suslow, T. , Gorney, J. , et al (2003) Microbiological safety of controlled and modified atmosphere packaging of fresh and fresh‐cut produce. Compr Rev Food Sci Food Saf 2: 142–160. [Google Scholar]
- Farrar, J. , Nunez, J. , and Davis, R. (2000) Influence of soil saturation and temperature on Erwinia chrysanthemi soft rot of carrot. Plant Dis 84: 665–668. [DOI] [PubMed] [Google Scholar]
- Finlay, B.B. , and Falkow, S. (1989) Common themes in microbial pathogenicity. Microbiol Rev 53: 210–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, G.A. , Thomas, C. and O'beirne, D. (1999) The microbiological safety of minimally processed vegetables. Int J Food Sci Technol 34, 1–22. [Google Scholar]
- Franova, J. , Karešová, R. , Navrátil, M. , Válová, P. , and NebesáŔová, J. (2000) A carrot proliferation disease associated with rickettsia‐like organisms in the Czech Republic. J Phytopathol 148: 53–55. [Google Scholar]
- Gan‐Mor, S. , Regev, R. , Levi, A. , and Eshel, D. (2011) Adapted thermal imaging for the development of postharvest precision steam‐disinfection technology for carrots. Postharvest Biol Technol 59: 265–271. [Google Scholar]
- Geeson, J. , Browne, K. , and Everson, H. (1988) Storage diseases of carrots in East Anglia 1978–82, and the effects of some pre‐and post‐harvest factors. Ann App Biol 112: 503–514. [Google Scholar]
- Gill, C. (1996) Extending the storage life of raw chilled meats. Meat Sci 43: 99–109. [DOI] [PubMed] [Google Scholar]
- Godfrey, S. , and Marshall, J. (2002) Identification of cold‐tolerant Pseudomonas viridiflava and P. marginalis causing severe carrot postharvest bacterial soft rot during refrigerated export from New Zealand. Plant Pathol 51: 155–162. [Google Scholar]
- Goodliffe, J. , and Heale, J. (1977) Factors affecting the resistance of stored carrot roots to Botrytis cinerea . Ann App Biol 85: 163. [Google Scholar]
- Hamady, M. , Lozupone, C. , and Knight, R. (2010) Fast UniFrac: facilitating high‐throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorf, L. , Fröhling, A. , Schlüter, O. , and Klocke, M. (2011) Analysis of the bacterial community within carrot wash water. Can J Micobiol 57: 447–452. [DOI] [PubMed] [Google Scholar]
- Hemme, D. , and Foucaud‐Scheunemann, C. (2004) Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int Dairy J 14: 467–494. [Google Scholar]
- Horst, R.K. (2013) Bacterial diseases In: Westcott's Plant Disease Handbook: Springer. 69–90. [Google Scholar]
- Hunter, J. , and Cigna, J. (1981) Bacterial Blight Incited in Parsnip by Pseudomonas marginalis and Pseudomonas viridiflava . Phytopathology 71: 1238–1241. [Google Scholar]
- Kamat, A. , Ghadge, N. , Ramamurthy, M. , and Alur, M. (2005) Effect of low‐dose irradiation on shelf life and microbiological safety of sliced carrot. J Sci Food Agric 85: 2213–2219. [Google Scholar]
- Kim, K.Y. , Jordan, D. , and Krishnan, H.B. (1997) Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatite. FEMS Microbiol Lett 153: 273–277. [Google Scholar]
- Klaiber, R.G. , Baur, S. , Wolf, G. , Hammes, W.P. , and Carle, R. (2005) Quality of minimally processed carrots as affected by warm water washing and chlorination. Innov Food Sci Emerg Technol 6: 351–362. [Google Scholar]
- Krieg, N. and Holt, J. (1994) Bergey's manual of determinative bacteriology The Williams and Wilkins Company. In: Library of Congress, Baltimore, MA. [Google Scholar]
- Lake, J.A. , and Wade, R.N. (2009) Plant–pathogen interactions and elevated CO2: morphological changes in favour of pathogens. J Exp Bot 60: 3123–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert, Y. , Kelman, D. , Dubinsky, Z. , Nitzan, Y. , and Hill, R.T. (2006) Diversity of culturable bacteria in the mucus of the Red Sea coral Fungia scutaria. FEMS Microbiol Ecol 58: 99–108. [DOI] [PubMed] [Google Scholar]
- Larsen, H. , and Wold, A.‐B. (2016) Effect of modified atmosphere packaging on sensory quality, chemical parameters and shelf life of carrot roots (Daucus carota L.) stored at chilled and abusive temperatures. Postharvest Biol Technol 114: 76–85. [Google Scholar]
- Leff, J.W. , and Fierer, N. (2013) Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS ONE 8: e59310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew, C.L. , and Prange, R.K. (1994) Effect of ozone and storage temperature on postharvest diseases and physiology of carrots (Daucus carota L.). J Am Soci Hort Sci 119: 563–567. [Google Scholar]
- Lo, R. , Xue, T. , Weeks, M. , Turner, M.S. , and Bansal, N. (2016) Inhibition of bacterial growth in sweet cheese whey by carbon dioxide as determined by culture‐independent community profiling. Int J Food Microbiol 217: 20–28. [DOI] [PubMed] [Google Scholar]
- Lopez‐Velasco, G. , Welbaum, G. , Boyer, R. , Mane, S. , and Ponder, M. (2011) Changes in spinach phylloepiphytic bacteria communities following minimal processing and refrigerated storage described using pyrosequencing of 16S rRNA amplicons. J App Microbiol 110: 1203–1214. [DOI] [PubMed] [Google Scholar]
- Luis‐Pantoja, M. , Ramos‐González, P. , Naranjo, M. , Hernández‐Rodríguez, L. , Rodríguez, J. , and Pérez‐López, E. (2015) Rickettsia‐related bacteria associated with papaya plants showing bunchy top disease in Cuba. J Gen Plant Pathol 81: 166–168. [Google Scholar]
- Martínez‐Hernández, G.B. , Amodio, M.L. and Colelli, G. (2015) Potential use of microwave treatment on fresh‐cut carrots: physical, chemical and microbiological aspects. J Sci Food Agric 96: 2063–2072. [DOI] [PubMed] [Google Scholar]
- Michalik, B. , Simon, P.W. , and Gabelman, W.H. (1992) Assessing susceptibility of carrot roots to bacterial soft rot. HortScience 27: 1020–1022. [Google Scholar]
- Miller, J.F. , Mekalanos, J.J. , and Falkow, S. (1989) Coordinate regulation and sensory transduction in the control of bacterial virulence. Science 243: 916. [DOI] [PubMed] [Google Scholar]
- Monier, J.‐M. , and Lindow, S. (2005) Spatial organization of dual‐species bacterial aggregates on leaf surfaces. Appl Environ Microbiol 71: 5484–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, M. , Usall, J. , Solsona, C. , Alegre, I. , Viñas, I. , and Abadias, M. (2010) Effects of packaging type and storage temperature on the growth of foodborne pathogens on shredded ‘Romaine’ lettuce. Food Microbiol 27: 375–380. [DOI] [PubMed] [Google Scholar]
- de Oliveira, L.L. , Costa, R.B. , Okada, D.Y. , Vich, D.V. , Duarte, I.C.S. , Silva, E.L. , and Varesche, M.B.A. (2010) Anaerobic degradation of linear alkylbenzene sulfonate (LAS) in fluidized bed reactor by microbial consortia in different support materials. Biores Technol 101: 5112–5122. [DOI] [PubMed] [Google Scholar]
- Phan, C. , Hsu, H. , and Sarkar, S. (1973) Physical and chemical changes occurring in the carrot root during storage. Can J Plant Sci 53: 635–641. [Google Scholar]
- Price, M.N. , Dehal, P.S. , and Arkin, A.P. (2009) FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evo 26: 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenant, B. , Jaffrès, E. , Dousset, X. , Pilet, M.‐F. , and Zagorec, M. (2015) Bacterial spoilers of food: behavior, fitness and functional properties. Food Microbiol 45: 45–53. [DOI] [PubMed] [Google Scholar]
- Robbs, P. , Bartz, J. , McFie, G. , and Hodge, N. (1996) Causes of decay of fresh‐cut celery. J Food Sci 61: 444–448. [Google Scholar]
- Seljåsen, R. , Bengtsson, G.B. , Hoftun, H. , and Vogt, G. (2001) Sensory and chemical changes in five varieties of carrot (Daucus carota L) in response to mechanical stress at harvest and post‐harvest. J Sci Food Agricult 81: 436–447. [Google Scholar]
- Soliva‐Fortuny, R.C. and Martín‐Belloso, O. (2003) New advances in extending the shelf‐life of fresh‐cut fruits: a review. Trends Food Sci Technol 14, 341–353. [Google Scholar]
- Son, H.‐J. , Park, G.‐T. , Cha, M.‐S. , and Heo, M.‐S. (2006) Solubilization of insoluble inorganic phosphates by a novel salt‐and pH‐tolerant Pantoea agglomerans R‐42 isolated from soybean rhizosphere. Bioresource Technol 97: 204–210. [DOI] [PubMed] [Google Scholar]
- Stoops, J. , Ruyters, S. , Busschaert, P. , Spaepen, R. , Verreth, C. , Claes, J. , et al (2015) Bacterial community dynamics during cold storage of minced meat packaged under modified atmosphere and supplemented with different preservatives. Food Microbiol 48: 192–199. [DOI] [PubMed] [Google Scholar]
- Streten, C. , Herrington, M.E. , Hutton, D.G. , Persley, D.M. , Waite, G.K. , and Gibb, K.S. (2005) Plant hosts of the phytoplasmas and rickettsia‐like‐organisms associated with strawberry lethal yellows and green petal diseases. Australas Plant Pathol 34: 165–173. [Google Scholar]
- Sturz, A. , Christie, B. , Matheson, B. , Arsenault, W. , and Buchanan, N. (1999) Endophytic bacterial communities in the periderm of potato tubers and their potential to improve resistance to soil‐borne plant pathogens. Plant Pathol 48: 360–369. [Google Scholar]
- Towner, D. , and Beraha, L. (1976) Core‐rot: a bacterial disease of carrots. Plant Dis Rep 60: 357–359. [Google Scholar]
- Tzortzakis, N. (2016. ) Ozone: A Powerful Tool for the Fresh Produce Preservation In: Postharvest Management Approaches for Maintaining Quality of Fresh Produce: Springer. 175‐207. [Google Scholar]
- Vihavainen, E.J. , Murros, A.E. , and Björkroth, K.J. (2008) Leuconostoc spoilage of vacuum‐packaged vegetable sausages. J Food Prot 71: 2312–2315. [DOI] [PubMed] [Google Scholar]
- Villafañe, F. (2017) Edible coatings for carrots. Food Rev Int 33: 84–103. [Google Scholar]
- Weber, R.W.S. , and Tribe, H.T. (2004) Moulds that should be better known: Thielaviopsis basicola and T. thielavioides, two ubiquitous moulds on carrots sold in shops. Mycologist 18: 6–10. [Google Scholar]
- Weinert, L.A. , Werren, J.H. , Aebi, A. , Stone, G.N. , and Jiggins, F.M. (2009) Evolution and diversity of Rickettsia bacteria. BMC Biol 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, J.M. , Liao, C.‐H. , and Hotchkiss, A.T. (1998) In vitro inhibition of soft‐rotting bacteria by EDTA and nisin and in vivo response on inoculated fresh cut carrots. Plant Dis 82: 491–495. [DOI] [PubMed] [Google Scholar]
- Wouters, D. , Grosu‐Tudor, S. , Zamfir, M. , and De Vuyst, L. (2013) Bacterial community dynamics, lactic acid bacteria species diversity and metabolite kinetics of traditional Romanian vegetable fermentations. J Sci Food Agricult 93: 749–760. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Kobert, K. , Flouri, T. , and Stamatakis, A. (2014) PEAR: a fast and accurate Illumina Paired‐End reAd mergeR. Bioinformatics 30: 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Unweighted UniFrac distance Jackknife dendrogram of bacterial communities.


