Abstract
Pathogens adapt and evolve in response to pressures exerted by host environments, leading to generation of genetically diverse variants. Treponema pallidum subspecies pallidum displays a substantial amount of interstrain diversity. These variants have been identified in various parts of the world, indicating transmission linkage between geographical regions. Genotyping is based on molecular characterisation of various loci in the syphilis treponeme genome, but still require further development and continued research, as new bacterial types are continually being detected. The goal for studying the molecular epidemiology of Treponema pallidum variants is the global monitoring of the transmission of genetically distinct organisms with different drug sensitivities and, potentially, different virulence proprieties.
Introduction
Mistakenly believed to be a disease of the past, syphilis is one of the most common sexually transmissible infections worldwide1 and a current concern for global health. Late-stage syphilis can be characterised by serious clinical manifestations, including blindness, dementia and paralysis. Vertical transmission of the disease to the fetus during pregnancy can result in stillbirth, spontaneous abortion and neonatal death, and syphilis-affected individuals are at greater risk to acquire HIV. The estimated worldwide yearly incidence of syphilis in 1999 was ~12 million, with an overall prevalence of at least 25 million.1 Within the last 10 years, the incidence of syphilis in the US and Europe has increased, particularly among men who have sex with men.2–5 This is a worrying trend as syphilis is easily preventable with the use of condoms, can be quickly diagnosed with a variety of laboratory tests and can effectively be treated by administration of penicillin. The disappointing results of existing public health efforts to control syphilis worldwide emphasise the need for more effective methods of syphilis prevention and control strategies.
Treponema pallidum subsp. pallidum (T. pallidum hereafter), the aetiological agent of syphilis, is an obligate human pathogen, and therefore constantly exposed to host defenses throughout its lifecycle. Genetic variation forms the basis of antigenic evolution that facilitates survival and adaptation of pathogens to host immune mechanisms. At the same time, however, genetic variability allows development of molecular methods that allow differentiation and categorisation of treponemal strains. The same efforts that aim to find and improve typing systems for T. pallidum will eventually facilitate the differentiation of subspecies pallidum from closely related pathogens that belong to other subspecies, such as T. pallidum subspecies pertenue that causes yaws, and subspecies endemicum that causes endemic syphilis (or bejel).
Treponema pallidum was initially thought to be a genetically uninteresting organism because of a ‘stable’ intractable chromosome lacking significant genetic variation, despite the impressive range of disease manifestations in its natural host. Over the last 15 years, whole genome and targeted sequencing efforts have uncovered a vast reservoir of genome plasticity among and within strains and organisms in patient lesions. Such genetic diversity is the basis of current efforts for developing typing methods of utility for several purposes, including: (i) surveillance of global changes in strain prevalence in particular geographical regions, which can reveal important insight into population-induced pressures on genetic mutation; (ii) monitoring of the global migration of syphilis carriers; and (iii) the identification of new genetic variants that may have increased virulence or developed drug resistance. This review will focus on the epidemiology of genetic variants of T. pallidum, the methods currently utilised for genetic typing of T. pallidum strains and the impact of increased genetic diversity.
Treponema pallidum strain
In bacteria, genetic variation can facilitate adaptation to new habitats. The development of T. pallidum typing methods was based on the use of strains propagated in the New Zealand White rabbit. This raised concerns that rabbit passage may have mutational effects on the T. pallidum genome and thus be a confounding factor when interpreting sequence differences of syphilis genotypes. In this hypothetical scenario, treponemes of human origin may sense a new niche when inoculated into rabbit tissues and begin adaptive sequence changes. The earliest known causative strain was isolated from the cerebrospinal fluid from a patient with neurosyphilis in Washington D.C. in 1912.6 Named after the physician who isolated the bacteria, the Nichols strain of T. pallidum remains the most extensively studied isolate to date and is widely utilised in experimental studies. The Nichols stain was initially isolated in brown–white rabbits6 and since then, has been propagated in hosts of different genetic backgrounds including monkeys and other species of rabbits. Currently, the New Zealand White species of rabbit remains the animal of choice for laboratory study, as syphilis pathogenesis within this particular species closely resembles the natural progression within human infection. This underscores similar pathogenic and virulence mechanisms within the human and the rabbit, which argues in favour that no sequence changes can be attributed to rabbit passage. Nonetheless, whether rabbit passage induce mutations in T. pallidum and that such changes significantly influences current genotyping strategies remains to be determined.
Genotyping strains of Treponema pallidum: the ‘CDC’ and ‘enhanced CDC’ methods
Strain typing is a powerful tool for determining the diversity of pathogens and the epidemiology of infections. The earliest method was developed in 1998 by Pillay et al. and has been classically termed the Centers for Disease Control and Prevention (‘CDC) method’7. Later, it served as the basis for an ‘enhanced CDC method’ developed by Marra et al. in 2010.8 These DNA-based typing tools have helped to distinguish the molecular diversity of this spirochete within human clinical specimens, but have not yet been applied in syphilis prevention and control. The CDC method is based on the characterisation of the genes encoding the acidic repeat protein (arp) and the T. pallidum repeat proteins E, G, and J (tprEGJ). The quantification by gel electrophoresis of the number of 60-base pair repeats in the arp gene will determine types 1–14. For tprEGJ, restriction fragment length polymorphism (RFLP) analysis is determined using polymerase chain reaction (PCR)-amplified tprEGJ products digested with the MseI restriction enzyme. The distinct banding patterns produced by MseI digestion determine types a–i. Therefore, a strain containing 12 arp repeats and a ‘f’ banding pattern in the tprEGJ RFLP will be termed strain subtype ‘12f’.
The CDC method has been applied to specimens obtained from different tissues and from different geographical locations around the world.7,9–14 However, a shortcoming of this early subtyping system is its relative inability to discriminate among the most common strain types; nearly 50% of US strains are of a single type, thus limiting usefulness of typing in the investigation of outbreaks.15 For example, Sutton et al. examined T. pallidum DNA isolated from blood and genital ulcer swabs during an outbreak of syphilis in Phoenix, strain AZ42.16 Approximately half of the samples were CDC subtype 14f. Nine other types were identified, including 14d. Types 14d and 14f have been identified in diverse geographic settings, including North and South Carolina, Lisbon, Scotland, South Africa and China.9,11–14
To overcome limitations on discriminatory power, an ‘enhanced CDC method’ for typing of T. pallidum strains, as defined by analysis of six loci, has been more recently developed.8 Eight syphilis typing targets were initially examined: (i) the number of 60 bp repeats in the acidic repeat protein gene; (ii) RFLP analysis of Subfamily II tpr genes; (iii) RFLP analysis of the tprC gene; (iv) determination of which tprD allele is in the tprD gene locus; (v) presence of a 51 bp insertion between tp0126 and tp0127 genes; and (vi) sequence analysis of an 84 bp region of tp0548. By adding tp0548 to the CDC method, Marra et al.8 were able to separate 14 CDC subtypes into 25 different strain types. T. pallidum strain type designation is based on the number of arp repeats, the tprE, G and J RFLP pattern (a–p) and the sequence signature for tp0548 (a–i); for example, strain type 14d/f. By using this enhanced approach, Marra et al. were able to demonstrate epidemiological relevance by showing a change in circulating strain types in Seattle over a 10-year period, with introduction and disappearance of specific strain types in the region.8 Further analysis of the geographical distribution of the redefined CDC-tp0548 subtypes revealed a commonality of prevalent strains from Seattle, Washington and San Francisco, California, which suggested a transfer of syphilis between these two geographical locations. Importantly, the 14d/f type was significantly more common in patients with neurosyphilis. Thus, a specific lineage of T. pallidum could be associated with a particular clinical syndrome, which suggests that infection with particular T. pallidum strains may have higher propensity for development of specific clinical outcomes predicting biological risk. Figure 1 below shows segregation of T. pallidum subtypes based on tp0548 sequencing.
Fig. 1.
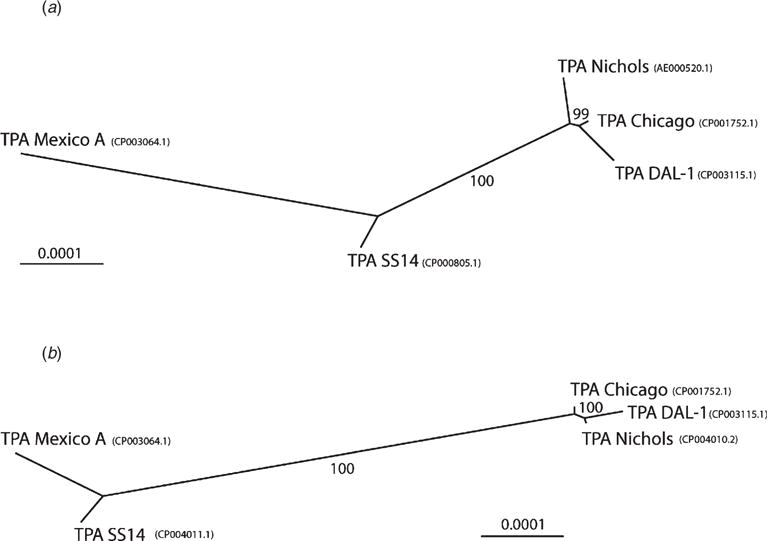
Unrooted trees constructed from whole genome sequences of syphilis strains. Trees were constructed using the Neighbour-Joining method using the Tamura-Nei genetic distance model and 1000 bootstrap replicates. The numbers above the branches show bootstrap support and the bar scale represents 0.0001 substitutions per target site. (a) A tree constructed from the alignment of genomes from strains Chicago (CP001752.1), DAL-1 (CP003115.1) and Mexico A (CP003064.1) with original versions of whole genome sequences of TPA Nichols (AE000520.1) and SS14 (CP000805.1). (b) A tree constructed from the alignment of genomes from strains Chicago (CP001752.1), DAL-1 (CP003115.1) and Mexico A (CP003064.1) with the improved whole genome sequences of Nichols-RS (CP004010.2) and SS14-RS (CP004011.1). Reprinted with permission from PLoS One; Pe trošová H et al. PLoS One, 2013.
There has recently been some contention on the validity of the ‘CDC method’ in its ability to accurately type T. pallidum strains. Mikalová et al. collected two samples from the same patient from a cohort of seropositive syphilis patients and conducted CDC method typing as well as sequencing-based typing using the tp0136, tp0548, 23S rDNA, arp and tpr loci.17 The authors found that arp and tpr loci-based typing showed subtype discrepancy in 61% of paired samples, whereas typing based on sequence showed no discrepancies. There were also higher discrepancies in CDC-based subtyping between whole blood and swab samples from the same patient compared with that of sequence-based subtyping. However, the results from these data are subject to scrutiny as the sample size of typable paired samples was small (n = 18). Such discordant results may have profound implications in molecular typing of T. pallidum strains of syphilis and awaits further confirmation.
Treponema pallidum genotypes in maternal populations
Microbial infections are multifactorial processes in which strain type can play a decisive role in the outcome. From the lengthy list of human pathogens, only a handful are capable of crossing the placenta to reach the fetus, among them is T. pallidum. The risk of congenital syphilis is extremely high in women with primary or secondary syphilis during pregnancy. Virtually every infected pregnant woman in the primary and secondary stages will potentially transmit the infection to the fetus.18 The outcome of congenital syphilis is, however, not uniform, ranging from healthy babies to fetal loss. In clear contrast to molecular epidemiological studies within the general population and in high-risk groups, molecular strain types associated with clinical outcomes or distinct biological measures in the babies due to vertical transmission have remained unexplored.
Other potential typing schemes
Multiple additional strains have been isolated in various geographical locations. The complete genome sequences have been elucidated for the Nichols,19 the Mexico A,20 the Seattle81–4,21 the Street strain 14 (SS14),22 the DAL-123 and the Chicago strains.24 SS14 was isolated by John Clark in 197724 and the Nichols strain was isolated in 1913 by Nichols the latter utilised early on to identify T. pallidum antigens in experimental settings.25 In 2013, re-sequencing of the whole genomes of the SS14 and Nichols strains corrected multiple sequence errors and also confirmed or identified new genetic changes between these two isolates;25 350 substitutions, 16 insertions and 17 deletions.
Multilocus analyses using the tp0136, tp0548 and 23S rRNA genes16,26 or the enhanced CDC typing scheme divides T. pallidum strains into two clusters: SS14-like and Nichols-like26,28–30 organisms. The SS14-like strains included SS14, Grady, Mexico A and the Philadelphia 1,31 while the Nichols-like group included Nichols, Bal 73–1, DAL-1,32 MN-3, Philadelphia 2, Haiti B and Madras strains.19,25,27–29 This suggests two genetically divergent pathways in the evolution of T. pallidum. Such observations need further confirmation, with isolates from multiple geographical regions and from samples obtained directly from patients without rabbit passage, to assert the relevance of these two lineages in terms of virulence, outcomes measures and evolution.
A recent study by Centurion-Lara et al. shows the potential of other target combinations for strain typing.33 Sequences of entire open reading frames (ORFs) of Subfamilies I, II and III of the tpr gene family segregate strains of T. pallidum. Among the strains analysed, four different genotypes, Nichols, Street 14, Mexico A and Seattle 81–4, as determined by the ‘enhanced CDC’ typing system, also fall into four different molecular types by tpr sequencing. The correlation strongly supports the feasibility of developing a typing system of equal or greater discriminative power based upon alternate chromosomal loci.
Global distribution of molecular subtypes
Various studies have examined the distribution of T. pallidum subtypes in diverse geographical locations. In 2011, two separate articles summarised the global distribution of T. pallidum subtypes15,34 determined by either the CDC or the enhanced CDC methods. These studies demonstrated that interstrain variability at the arp and tprEGJ locus has remained relatively unchanged, which suggests that the prevalent subtypes have evolved genotypically to a degree that makes them successful pathogens in modern times. The general global prevalence of subtypes 14d and 14f also makes it difficult to use modern subtype data to determine the origin of human T. pallidum strains, but increased prevalence of 14d may suggest some linked transmission of this strain.
Development of other typing methods
More traditional typing methods such as identification of serovars or susceptibility testing are currently ineffective for T. pallidum due to inability to culture T. pallidum in vitro, the inability to genetypically track this organism and the poor understanding of antibody responses to T. pallidum antigenic variants. In the past decade, however, DNA-based typing methods have been a source for development of typing methods for syphilis, with a couple of instances where biological relevance has been established: neurosyphilis and macrolide resistance. These DNA typing methods have also been useful in identifying or ruling out recurrences, re-infections with the same strain, as well as to layout distribution maps by geographical regions and identify movement of genotypes among human populations. From the vast array of methods that use DNA as targets, only a few (electrophoresis of PCR-amplified DNA fragments, sequencing and RFLP analysis) have entered the syphilis typing pipeline. Barriers that prevent more widespread use of molecular techniques for T. pallidum typing include inherent constraints of amounts of T. pallidum DNA in lesions requiring prior amplification of organisms in rabbits and technical impediments in designing specific probes (ribotyping).
Despite the discriminative power of the current expanded typing system, researchers are still ineffective when attempting to predict outcome measures during syphilis infections, underscoring the need for more discriminatory typing schemes. Renewed interest is focussed now on multiloci sequence typing (MLST) and efforts are being driven by: (i) the potential of MLST high resolution power35,36 of carefully selected number of allelic genes; (ii) the ease of designing Internet-accessible databases and the improved cost-effectiveness of using whole genome sequencing from rabbit-propagated isolates; and (iii) the more recently developed methods utilising high throughput whole genome sequencing of organisms directly from lesions. MLST amenable discreet chromosomal regions visualised from whole genome sequence alignments of T. pallidum33 has shed light into possible amenable markers for MLST development. This method is conceivably useful for predicting distinctive outcomes measures, tracking sources of infections and strain displacement by interventions, evolution and findings answers for questions related to virulence or other fundamental biological properties.
Consequences of genetic diversity
Intrastrain or interstrain genetic mutation affects virulence, which can increase chances to develop better adaptations to evade host responses and better transmission between hosts. Two examples of genetic variation with links to clinical outcomes are: (i) the genotype 14d/f, a surrogate of biological risk of developing neurosyphilis; and (ii) resistance to macrolide compounds. In 2000, Stamm and Bergen identified point mutations in a clinical isolate of T. pallidum that was associated with increased resistance to erythromycin and associated macrolides.27 The mutations were located in the adenosine of the cognate residue of the 23S rRNA gene found in Escherichia coli, at positions A2058 and A2059. A guanosine replacement at these positions, which were found in SS14 strains but not in Nichols strains,27 resulted in antibiotic resistant treponemes via inhibition of macrolide binding to the bacterial 50S rRNA subunit.37 Indeed, macrolide-resistant syphilis cases have been increasingly reported over time in various parts of the world.38,39
Since penicillin was introduced for the treatment of syphilis, the number of cases decreased dramatically in the US from 1944 to 1975. Rates of overall syphilis had declined by almost 90%,39 but T. pallidum has re-emerged in recent epidemics in the US, Eastern and Western Europe and China.39–41 The majority of these syphilis cases have occurred in men who have sex with men in the US and in Western Europe. In contrast, syphilis transmission in heterosexual populations is observed in China more prevalently than in the US and Europe.
Identification and treatment of syphilis cases are key components in controlling the spread of syphilis within and among communities, where tracking and treatment of contacts have a significant impact in transmission control. However, the use of benzathine penicillin G (BPG) as a parentally administered drug has disadvantages. Moreover, the introduction of a single dose of orally administered azithromycin has garnered apparent favourable popularity, especially for post-exposure treatment of partners. Initially, azithromycin was characterised as being equally or even more effective than BPG for the treatment of early syphilis;42–45 however, treatment failures became rapidly evident and reported in San Francisco soon after the introduction of oral azithromycin interventions.42,46–49
Resistance of T. pallidum to macrolides has been also mapped to distinct genotypes affecting the rRNA genes. In such patients, the 23S rRNA A2058G mutation was identified.40 Several reports documented the A2058 23S mutation around the globe, with increased frequencies among infected individuals. In 2004, Lukehart et al. identified the 23S rRNA A2058G mutation conferring resistance to azithromycin treatment in clinical as well as experimental settings.40 These results were later affirmed in separate studies that also identified the 23S rRNA A2058G mutation in azithromycin treatment failures in patients.47,48 In 2009, another 23S rDNA mutation was identified (A2059G) in the Czech Republic that led to a failed treatment case using a macrolide antibiotic.42 A recent report conducted from the same hospital in the Czech Republic involving a cohort of syphilis patients between 2011 and 2013 identified mutations associated with macrolide resistance in nearly 67% of all typable samples,50 which reflects an increase for what had been reported for that region between 2004 and 2010 (37%).51 While it is possible that improved methods for sensitivity of identifying macrolide resistance mutations may account for this increased prevalence in this region, it is also plausible that the increased incidence of syphilis permits genetic drift in T. pallidum, leading to more strains harboring macrolide-resistant alleles. In addition, this study from the Czech Republic showed that samples from men who have sex with men (MSM) patients exhibited a higher frequency of containing macrolide resistance mutations compared with non-MSM patients.51 Other studies from England and Australia have also reported a high incidence of macrolide resistance mutations in samples derived from MSM;52,53 the latter reporting up to 97% of samples with the A2058G mutation originating from this particular group.
It is difficult to establish any physiological reasons why macrolide resistance mutations may be occurring more frequently in MSM patients compared with non-MSM patients. However, it is possible that increased numbers of cases occurring in MSM generates higher probability of identifying such mutations within this demographic. Specifically, one study from the US found no statistical association between sexual orientation and incidence of macrolide resistance mutations, despite reporting the A2058G mutation appeared more frequently in MSM samples.54 Additionally, it is possible that a higher incidence of syphilis within MSM populations requires increased use of macrolides for treatment, which inadvertently increases selective pressure for evolution of resistant strains within this particular demographic. Indeed, the prevalence of the 23S rRNA mutation was higher among individuals who had taken macrolides 12 months before enrolment in a clinical study in Seattle compared with individuals who had not received macrolide treatment.55 Additionally, single-dose azithromycin remained highly effective at treating syphilis in patients in developing nations such as Tanzania, where azithromycin may not have been widely utilised in the past.43 This is not to say that macrolide resistance will be limited to the US or solely in MSM populations, as 23S rRNA mutations have been already reported in countries such as Australia and Taiwan.53,56 As macrolide resistant T. pallidum strains continue to emerge globally, appropriate management requires re-evaluation of antibiotic usage for the treatment and control of syphilis, as well as investigation into alternative methods of therapy.
Conclusions
The heterogeneity and the rise of new variants of T. pallidum promotes renewed efforts to gain a better insight into essential mechanisms of the biology of T. pallidum, including translational research aimed at the development of new drugs and personalised treatments with better compliance. Indeed, proper surveillance of these variants also depends on the development of reliable genotyping methods. We have highlighted the usefulness of the CDC enhanced typing system as the currently most widely applied method for T. pallidum typing, but also emphasised the need for more discriminatory approaches such as MLST to address biological and epidemiological questions. These techniques are required to improve public health strategies aimed at prevention and control of syphilis disease. Among others, these include correlation of specific genetic variants with particular outcome measures, longitudinal studies to determine strain movement among populations, identification of high-risk demographic groups, identification of sources of infection, tracking and treatment of contacts and personalised choice of antibiotics for individual cases. MLST approaches developed on the basis of whole genome sequences will also have the advantage in terms of studying evolutionary relationships and the identification of adaptive changes of relevance in pathogenesis and virulence.
The obvious drawback of DNA-based typing method is the absence of information about phenotypic surrogates; for example, even a refined MLST will not inform about important targets for antibody responses during infection. However, with the simultaneous concurrence of other disciplines such as bioinformatics and molecular immunology, key regions of a protein encoded by a MLST DNA region can be unveiled, which would facilitate the design of protective immunogens across T. pallidum variants. Ultimately, a better understanding of T. pallidum intra- and interstrain variability will facilitate better personalised patient care, prevention and control of syphilis transmission among human populations.
Acknowledgments
This project was supported by a NIH Research Training Grant # R25TW009346 funded by the Fogarty International Center, National Institute of Mental Health and the Office of the Director.
Footnotes
Conflicts of interest
None declared.
References
- 1.Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74(Suppl 1):S12–6. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Primary and secondary syphilis - United States, 2000–2001. MMWR Morb Mortal Wkly Rep. 2002;51:971–3. [PubMed] [Google Scholar]
- 3.Sasse A, Defraye A, Ducoffre G. Recent syphilis trends in Belgium and enhancement of STI surveillance systems. Euro Surveill. 2004;9:6–8. [PubMed] [Google Scholar]
- 4.Righarts AA, Simms I, Wallace L, Solomou M, Fenton KA. Syphilis surveillance and epidemiology in the United Kingdom. Euro Surveill. 2004;9:21–5. [PubMed] [Google Scholar]
- 5.Marcus U, Bremer V, Hamouda O. Syphilis surveillance and trends of the syphilis epidemic in Germany since the mid-90s. Euro Surveill. 2004;9:11–4. [PubMed] [Google Scholar]
- 6.Nichols HJ. Observations on a strain of Spirochaeta pallida isolated from the nervous system. J Exp Med. 1914;19:362–71. doi: 10.1084/jem.19.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillay A, Liu H, Chen CY, Holloway B, Sturm AW, Steiner B, et al. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis. 1998;25:408–14. doi: 10.1097/00007435-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Marra C, Sahi S, Tantalo L, Godornes C, Reid T, Behets F, et al. Enhanced molecular typing of Treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J Infect Dis. 2010;202:1380–8. doi: 10.1086/656533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope V, Fox K, Liu H, Marfin AA, Leone P, Sena AC, Chapin J, Fears MB, Markowitz L. Molecular subtyping of Treponema pallidum from North and South Carolina. J Clin Microbiol. 2005;43:3743–6. doi: 10.1128/JCM.43.8.3743-3746.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton MY, Liu H, Steiner B, Pillay A, Mickey T, Finelli L, Morse S, Markowitz LE, St Louis ME. Molecular subtyping of Treponema pallidum in an Arizona County with increasing syphilis morbidity: use of specimens from ulcers and blood. J Infect Dis. 2001;183:1601–6. doi: 10.1086/320698. [DOI] [PubMed] [Google Scholar]
- 11.Cole MJ, Chisholm SA, Palmer HM, Wallace LA, Ison CA. Molecular epidemiology of syphilis in Scotland. Sex Transm Infect. 2009;85:447–51. doi: 10.1136/sti.2009.036301. [DOI] [PubMed] [Google Scholar]
- 12.Martin IEGW, Yang Y. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin Infect Dis. 2009;49:515–21. doi: 10.1086/600878. [DOI] [PubMed] [Google Scholar]
- 13.Molepo J, Pillay A, Weber B, Morse SA, Hoosen AA. Molecular typing of Treponema pallidum strains from patients with neurosyphilis in Pretoria, South Africa. Sex Transm Infect. 2007;83:189–92. doi: 10.1136/sti.2006.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florindo C, Reigado V, Gomes JP, Azevedo J, Santo I, Borrego MJ. Molecular typing of Treponema pallidum clinical strains from Lisbon, Portugal. J Clin Microbiol. 2008;46:3802–3. doi: 10.1128/JCM.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121:4584–92. doi: 10.1172/JCI57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton MY, Liu H, Steiner B, Pillay A, Mickey T, Finelli L, Morse S, Markowitz LE, St Louis ME. Molecular subtyping of Treponema pallidum in an Arizon County with increasing syphilis morbidity: use of specimens from ulcers and blood. J Infect Dis. 2001;183:1601–6. doi: 10.1086/320698. [DOI] [PubMed] [Google Scholar]
- 17.Mikalová L, Pospisilova P, Woznicova V, Kuklova I, Zakoucka H, Smajs D. Comparison of CDC and sequence-based molecular typing of syphilis treponemes: tpr and arp loci are variable in multiple samples from the same patient. BMC Microbiol. 2013;13:178. doi: 10.1186/1471-2180-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74:12–6. [PubMed] [Google Scholar]
- 19.Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–88. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 20.Petrosova H, Zobanikova M, Cejkova D, Mikalova L, Pospisilova P, Strouhal M, et al. Whole genome sequence of Treponema pallidum ssp. pallidum, strain Mexico A, suggests recombination between yaws and syphilis strains. PLoS Negl Trop Dis. 2012;6:e1832. doi: 10.1371/journal.pntd.0001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacani L, Iverson-Cabral SL, King JC, Molini BJ, Lukehart SA, Centurion-Lara A. Complete genome sequence of the Treponema pallidum subsp. pallidum Sea81–4 Strain. Genome Announc. 2014;2:e00333–14. doi: 10.1128/genomeA.00333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matejkova P, Strouhal M, Smajs D, Norris SJ, Palzkill T, Petrosino JF, et al. Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol. 2008;8:76. doi: 10.1186/1471-2180-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zobanikova M, Mikolka P, Cejkova D, Pospisilova P, Chen L, Strouhal M, et al. Complete genome sequence of Treponema pallidum strain DAL-1. Stand Genomic Sci. 2012;7:12–21. doi: 10.4056/sigs.2615838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacani L, Jeffrey BM, Molini BJ, Le HT, Lukehart SA, Centurion-Lara A, et al. Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J Bacteriol. 2010;192:2645–6. doi: 10.1128/JB.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamm LV, Kerner TC, Jr, Bankaitis VA, Bassford PJ., Jr Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun. 1983;41:709–21. doi: 10.1128/iai.41.2.709-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrosova H, Pospisilova P, Strouhal M, Cejkova D, Zobanikova M, Mikalova L, et al. Resequencing of Treponema pallidum ssp. pallidum strains Nichols and SS14: correction of sequencing errors resulted in increased separation of syphilis treponeme subclusters. PLoS ONE. 2013;8:e74319. doi: 10.1371/journal.pone.0074319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamm LV, Bergen HL. A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob Agents Chemother. 2000;44:806–7. doi: 10.1128/AAC.44.3.806-807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nechvatal L, Petrosova H, Grillova L, Pospisilova P, Mikalova L, Strnadel R, et al. Syphilis-causing strains belong to separate SS14-like or Nichols-like groups as defined by multilocus analysis of 19 Treponema pallidum strains. Int J Medical Microbiology. 2014;304:645–53. doi: 10.1016/j.ijmm.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Mikalova L, Strouhal M, Cejkova D, Zobanikova M, Pospisilova P, Norris SJ, et al. Genome analysis of Treponema pallidum subsp. pallidum and subsp. pertenue strains: most of the genetic differences are localized in six regions. PLoS ONE. 2010;5:e15713. doi: 10.1371/journal.pone.0015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacani L, Chattopadhyay S, Centurion-Lara A, Jeffrey BM, Le HT, Molini BJ, et al. Footprint of positive selection in Treponema pallidum subsp. pallidum genome sequences suggests adaptive microevolution of the syphilis pathogen. PLoS Negl Trop Dis. 2012;6:e1698. doi: 10.1371/journal.pntd.0001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harper KN, Ocampo PS, Steiner BM, George RW, Silverman MS, Bolotin S, et al. On the origin of the treponematoses: a phylogenetic approach. PLoS Negl Trop Dis. 2008;2:e148. doi: 10.1371/journal.pntd.0000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendel GD, Jr, Sanchez PJ, Peters MT, Harstad TW, Potter LL, Norgard MV. Identification of Treponema pallidum in amniotic fluid and fetal blood from pregnancies complicated by congenital syphilis. Obstet Gynecol. 1991;78:890–5. [PubMed] [Google Scholar]
- 33.Centurion-Lara A, Giacani L, Godomes C, Molini BJ, Brinck Reid T, Lukehart SA. Fine analysis of genetic diversity of the tpr gene family among treponemal species, subspecies and strains. PLoS Negl Trop Dis. 2013;7:e2222. doi: 10.1371/journal.pntd.0002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng RR, Wang AL, Li J, Tucker JD, Yin YP, Chen XS. Molecular typing of Treponema pallidum: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2011;5:e1273. doi: 10.1371/journal.pntd.0001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiden MC. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–5. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spratt BG, Maiden MC. Bacterial population genetics, evolution and epidemiology. Philos Trans R Soc Lond B Biol Sci. 1999;354:701–10. doi: 10.1098/rstb.1999.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franceschi F, Kanyo Z, Sherer EC, Sutcliffe J. Macrolide resistance from the ribosome perspective. Curr Drug Targets Infect Disord. 2004;4:177–91. doi: 10.2174/1568005043340740. [DOI] [PubMed] [Google Scholar]
- 38.Katz KA, Klausner JD. Azithromycin resistance in Treponema pallidum. Curr Opin Infect Dis. 2008;21:83–91. doi: 10.1097/QCO.0b013e3282f44772. [DOI] [PubMed] [Google Scholar]
- 39.Stamm LV. Global challenge of antibiotic-resistant Treponema pallidum. Antimicrob Agents Chemother. 2010;54:583–9. doi: 10.1128/AAC.01095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukehart SA, Godomes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, Engelman J, Mitchell SJ, Rompalo AM, Marra CM, Klausner JD. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–8. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 41.Marra CM, Colina AP, Godornes C, Tantalo LC, Puray M, Centurion-Lara A, Lukehart SA. Antibiotic selection may contribute to increases in macrolide resistant Treponema pallidum. J Infect Dis. 2006;194:1771–3. doi: 10.1086/509512. [DOI] [PubMed] [Google Scholar]
- 42.Matejkova P, Flasarova M, Zakoucka H, Borek M, Kremenova S, Arenberger P, et al. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J Med Microbiol. 2009;58:832–6. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 43.Riedner G, Rusizokla M, Todd J, Maboko L, Hoelscher M, Mmbando D, Samky E, Lyamuya E, Mabey D, Grosskurth H, Hayes R. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–44. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 44.Kiddugavu MK, Kiwanuka N, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, Makumbi F, Li X, Reynolds SJ, Quinn TC, The Rakai Study Group. Gray RH. Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda. Sex Transm Dis. 2005;32:1–6. doi: 10.1097/01.olq.0000148297.48590.d8. [DOI] [PubMed] [Google Scholar]
- 45.Hook EW, Behets F, Van Damme K, Ravelomanana N, Leone P, Sena AC, Martin D, Langley C, McNeil L, Wolff M. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J Infect Dis. 2010;201:1729–35. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention Azithromycin treatment failures in syphilis infections–San Francisco, California 2002–2003. MMWR Morb Mortal Wkly Rep. 2004;53:197–8. [PubMed] [Google Scholar]
- 47.Chen JC. Update on emerging infections: news from the Centers for Disease Control and Prevention Brief report: azithromycin treatment failures in syphilis infections–San Francisco, California, 2002–2003. Ann Emerg Med. 2004;44:232–3. doi: 10.1016/j.annemergmed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell SJ, Engelman J, Kent CK, Lukehart SA, Godornes C, Klausner JD. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004. Clin Infect Dis. 2006;42:337–45. doi: 10.1086/498899. [DOI] [PubMed] [Google Scholar]
- 49.Klausner JD, Kohn RP, Kent CK. Azithromycin versus penicillin for early syphilis. N Engl J Med. 2006;354:203–5. doi: 10.1056/NEJMc052777. author reply 203–5. [DOI] [PubMed] [Google Scholar]
- 50.Grillova L, Petrosova H, Mikalova L, Strnadel R, Dastychova E, Kuklova I, et al. Molecular typing of Treponema pallidum in the Czech Republic during 2011 to 2013: increased prevalence of identified genotypes and of isolates with macrolide resistance. J Clin Microbiol. 2014;52:3693–700. doi: 10.1128/JCM.01292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flasarova M, Pospisilova P, Mikalova L, Valisova Z, Dastychova E, Strnadel R, et al. Sequencing-based molecular typing of Treponema pallidum strains in the Czech Republic: all identified genotypes are related to the sequence of the SS14 strain. Acta Derm Venereol. 2012;92:669–74. doi: 10.2340/00015555-1335. [DOI] [PubMed] [Google Scholar]
- 52.Tipple C, McClure MO, Taylor GP. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex Transm Infect. 2011;87:486–8. doi: 10.1136/sextrans-2011-050082. [DOI] [PubMed] [Google Scholar]
- 53.Read P, Jeoffreys N, Tagg K, Guy RJ, Gilbert GL, Donovan B. Azithromycin-resistant syphilis-causing strains in Sydney, Australia: prevalence and risk factors. J Clin Microbiol. 2014;52:2776–81. doi: 10.1128/JCM.00301-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su JR, Pillay A, Hook EW, Ghanem KG, Wong W, Jackson D, Smith LD, Pierce E, Philip SS, Wilson S, Golden MR, Workowski KA, Chi KH, Parrish DD, Chen CY, Weinstock HS. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex Transm Dis. 2012;39:794–8. doi: 10.1097/OLQ.0b013e31826f36de. [DOI] [PubMed] [Google Scholar]
- 55.Marra CM, Colina AP, Godornes C, Tantalo LC, Puray M, Centurion-Lara A, et al. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J Infect Dis. 2006;194:1771–3. doi: 10.1086/509512. [DOI] [PubMed] [Google Scholar]
- 56.Wu BR, Yang CJ, Tsai MS, Lee KY, Lee NY, Huang WC, et al. Multicentre surveillance of prevalence of the 23S rRNA A2058G and A2059G point mutations and molecular subtypes of Treponema pallidum in Taiwan, 2009–2013. Clin Micro Infect. 2014;20:802–7. doi: 10.1111/1469-0691.12529. [DOI] [PubMed] [Google Scholar]


