Abstract
United States soldiers are returning from the Greater Middle East with respiratory illnesses ranging from new onset asthma to constrictive bronchiolitis. The etiologies of the diseases are unknown. A study was conducted to determine the possible role of local mineral dust in the development of abnormal respiratory illnesses in soldiers during and after deployment in Iraq. A dust sample obtained in proximity to a burn pit in Camp Victory, Iraq (Camp Victory dust) was characterized both chemically and mineralogically. For comparison, a dust sample from Fort Irwin, California (Fort Irwin dust) was also collected. The ability of the dust samples to generate reactive oxygen species (ROS) was quantified, as well as their ability to generate an inflammatory stress response (ISR) in human lung epithelial cells. Both samples are composed of common silicate and carbonate minerals and contain heavy metals with concentration ranges expected for mineral dust. The ISR generated by each sample was within the range of inert material with the minimal stress generated associated with the carbonate phases. The findings based on this one sample suggest that the origin of the disease is not driven by the particles ability to generate ROS. However, it is likely that particle overload and associated complications, or endotoxin contributes extensively to pathogenesis.
Keywords: dust, Middle East, U.S. soldiers, inhalation, lung injury
Key Points
U.S. soldiers are returning from the Greater Middle East with respiratory illnesses of unknown etiology
Dust samples originating form Camp Victory, Iraq and Fort Irwin, California are evaluated geochemically and toxicologically
Geochemical features of the Iraqi dust (e.g., mineralogy and ability to generate ROS) are unlikely sources of pulmonary stress
1. Introduction
Soldiers are experiencing lingering health effects associated with their deployment to Iraq. Apart from combat‐related injuries, a significant number of soldiers returning from the Greater Middle East have lung illnesses ranging from new onset asthma to constrictive bronchiolitis [King et al., 2011; Szema et al., 2011]. The rate of asthma development among soldiers is higher for troops that have been deployed in Iraq than those remaining stateside (6.6% versus 4.3%, respectively) [Szema et al., 2011, 2012]. In fact, 14% of medic visits in Iraq were due to respiratory complaints [Kilpatrick, 2011]. More recent studies show that the rates of sinusitis, respiratory symptoms, and asthma are also significantly elevated among stateside U.S. military personnel after deployment to Iraq [Abraham et al., 2014; Barth et al., 2014]. However, this issue is not limited to U.S. military personnel. A study investigating Polish coalition soldiers also found acute respiratory tract diseases to be prevalent and correlated this finding with environmental factors, such as extreme temperature fluctuations, unsanitary conditions, and sand and dust storms [Korzeniewski et al., 2013].
The conditions in the Greater Middle East are dramatically different from the usual conditions that soldiers are exposed to in the United States. Average rainfall in Iraq is low (216 mm per annum versus 715 mm per annum for Iraq and United States, respectively), leading to dry soil and dust‐filled air [Bank, 2013]. Specifically, the average particulate matter (PM) 10 and PM2.5 mass in the Greater Middle East is an order of magnitude higher than in southwestern United States [Engelbrecht et al., 2009a]. Recurrent dust storms, predominately composed of geological dust, smoke from burning trash, and industrial processing facilities, can place as much as 10,000 μg/m3 of PM10 in the air, which is 154 times higher than the exposure standard for soldiers [Engelbrecht et al., 2009a; Lyles et al., 2008; Morman and Plumlee, 2013]. Although masks are made available to military personnel, they are often not worn due to the heat and because they clog quickly, due to the high concentration of PM. Given that the inhalation of PM10 particulate dust can trigger asthma in the absence of allergic sensitization and low levels of ambient pollution are capable of interfering with normal biological pathways, this level of exposure is of great concern [Park et al., 2005; Pope et al., 2011].
According to Falvo et al., six different factors may be contributing to the higher incidences of respiratory distress among U.S. military personnel: airborne hazards, tobacco smoke, individual susceptibility, exercise ventilation, and stress and violence [Falvo et al., 2015]. The current study focuses on airborne hazards, specifically natural dust (e.g., sand, soil, and associated microflora) collected near a burn pit used to incinerate organic and inorganic waste, including spent ammunition and jet fuel. Since the lung illnesses observed are inflammatory in nature and therefore more indicative of an overabundance of reactive oxygen species (ROS) within lungs, the ability of the dust sample to contribute to this process is examined [Vandivier et al., 2006].
In general, a material can generate inflammation within the body in a number of ways. One proposed pathway to inflammation is particle‐induced formation of ROS via reactions with surface defects and/or particle‐mediated Fenton chemistry [Schoonen et al., 2006]. In Fenton chemistry, dissolved molecular oxygen is reduced stepwise by dissolved ferrous iron, leading to the formation of superoxide, hydrogen peroxide, and hydroxyl radical. In particle‐mediated Fenton chemistry the reduction of dissolved molecular oxygen can take place at iron atoms exposed on particle surfaces or in solution with iron released by the particle. It is now widely recognized that metals other than iron, such as chromium, manganese, vanadium, and copper, can also facilitate Fenton chemistry. Apart from directly increasing the ROS level in cells, particle‐derived metals may also affect the homeostasis between prooxidant and antioxidant within cells by reacting with antioxidants, thereby tipping the balance in favor of prooxidants.
A second pathway to particle‐induced inflammation is driven by the mere exposure to particulate matter, regardless of its reactivity. Particle exposures leading to a lung burden in excess of 1 μg/L impair alveolar macrophage (AM)‐mediated lung clearance. This condition, called particle overload, leads to a decrease or even cessation of the phagocytosis of particles. This particle‐induced suppression of phagocytosis can also diminish the ability to clear dead or dying cells from the lung through phagocytosis, a process referred to as efferocytosis. A lack of efficient efferocytosis exacerbates the inflammation by generating secondary necrotic cellular death. If the particulate material is cytotoxic, phagocytosis may be compromised at exposure levels below the threshold triggering impairment when exposed to unreactive material. [Oberdorster, 1995]
In this study we evaluate the inflammatory stress response (ISR) of epithelial lung cells to a dust sample collected in Iraq. Defined as cellular ROS upregulation normalized by cellular viability, the ISR is an indicator of particle toxicity. This qualitative technique is utilized to determine whether the Iraqi material elicits an unusually strong cellular response compared to dust collected at an arid location in the United States and standard reference soils.
2. Materials and Methods
2.1. Dust Sample Acquisition, Characterization, and Treatment
Two natural dust samples were investigated. The primary dust sample came from Camp Victory, Iraq (Camp Victory dust (CVD)), and was collected near a burn pit. For comparison, a second dust sample was collected from Fort Irwin, California (Fort Irwin dust (FID)). Fort Irwin is located in the Mojave Desert, midway between Las Vegas, Nevada and Los Angeles, California. Silica glass beads from Corpuscular and two National Institute of Standards and Technology (NIST) soils (San Joaquin #2709 and Montana #2710) were evaluated along with the two dust samples. The San Joaquin soil contains baseline trace elements whereas the Montana sample contains elevated trace elements. Full descriptions of the two soil reference materials is provided by NIST [National Institute of Standards and Technology, 2002a, 2002b].
The inorganic components of both CVD and FID dust samples were fully characterized. Morphology was examined using the LEO Gemini 1550 (Figure 1). The mineralogy of each dust samples was determined using a Scintag PAD X‐ray diffractometer under the following conditions: CuKα1, 40 kV, 25 mA, 5°–75° 2θ, step 0.02° 2θ, and counting times of 5–10 s per step. Complete chemical analyses were performed using X‐ray fluorescence (XRF) at the Geoanalytical Laboratory at Washington State University. The XRF analysis is based on a lithium borate fused glass bead of the sample. In order to determine the origin of toxicity within the dust, carbonate phases were dissolved by exposing a subsample of each dust to a 1.0 M HCl solution for about 12 h with agitation. The acid‐treated material was reanalyzed in‐house with a Bruker S4Pioneer XRF using the pressed pellet method. For normalization of exposure experiments, the surface area was determined using a Quantachrome NOVA 5‐point BET analyzer using ultrahigh purity N2 gas.
Figure 1.
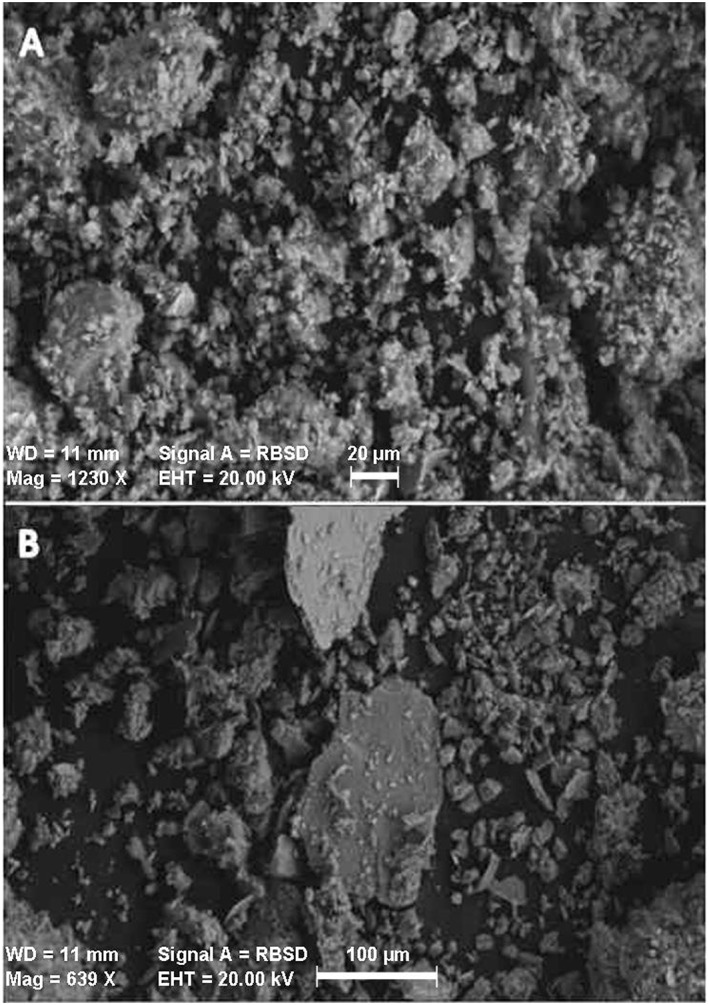
Scanning electron microscope images of (a) irradiated Camp Victory, Iraq, dust and (b) Fort Irwin, CA, dust.
2.2. Particle‐Derived Reactive Oxygen Species Formation
The formation of hydroxyl radical (˙OH) and hydroxyl radical plus hydrogen peroxide (˙OH + H2O2) was determined upon dispersal of the dust in water. The analysis for ˙OH and ˙OH + H2O2 was conducted using a protocol developed by Cohn et al. [2009] which is based on the reaction of 3′‐(p‐aminophenyl) fluorescein (APF) with ˙OH forming fluorescein [Setsukinai et al., 2003]. The APF probe has a very high specificity for ˙OH. By adding horse radish peroxidase (HRP) any H2O2 will be converted and leads to the formation of fluorescein. Hence, by adding HRP the concentration of ˙OH + H2O2 is determined. Following the protocol outlined in Cohn et al. [2009] we measured both the production of ˙OH and ˙OH + H2O2 after 24 h of incubation.
2.3. Endotoxin Quantification
The presence of endotoxin within the CVD and FID was quantified by generating a 0.1 m2/mL slurry in sterile autoclaved DI water. The slurry was rotated end‐over‐end for 1 h at STP and centrifuged to remove the particles. The supernatant was then analyzed using the Pierce™ LAL Chromogenic Endotoxin Quantification Kit.
2.4. Culturing and Plating the A549 Human Lung Epithelial Cell Line
The A549 human lung epithelial cell line was utilized. The cells were cultured and plated using a previous published protocol [Harrington et al., 2012]. The cell growth media consisted of predominately Ham's F12K Media with 10% fetal bovine serum and 1% 1X penicillin/streptomycin. Trypsin with ethylenediaminetetraacetic acid was used to lyse the cells for passage and plating. Cell quantification was determined with Trypan blue stain on a hemocytometer.
To maximize replicates and experimental conditions, 96‐well microplates were utilized. When plating, columns 3 through 10 were loaded with 8 × 104 cells/mL, covered with a microplate lid, and allowed to incubate at 37°C in culturing media until confluent (approximately 2 days). Columns 1, 2, 11, and 12 were kept cell free for normalization. In order to avoid airborne bacteria, sterile hoods were always employed when working with the cells and microplate lids were utilized when not in the hood. Temperature, relative humidity, and carbon dioxide (CO2) concentration in air were kept constant (37°C, 95% and 5% CO2 in air, respectively) in the incubator.
2.5. Inflammatory Stress Response Measurements
A previously published protocol was used to determine the inflammatory stress response generated by particle contaminates [Harrington et al., 2012]. Cell growth media was discarded upon experimental initiation, and columns 1–6 were filled with 200 μL 50 μM 2′,7′‐dichlorofluoroscein‐diacetate (DCFH‐DA) from Sigma Aldrich in Hank's Buffered Salt Solution (HBSS), and columns 7–12 were filled with 200 μL HBSS, and then placed in the incubator for 20 min. All liquid contents were again discarded, and 200 μL of the contaminant slurry was added to selected wells. The range in dust particle loadings was derived from serial dilutions of two stock solutions. Initial experiments were performed using a stock solution of 0.002 m2/mL, and follow‐up experiments were performed using a stock solution of 0.05 m2/mL. A row of wells were kept contaminant free for normalization (control). After the addition of the contaminant slurry, 20 μL of 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium (MTS) from Promega was added to wells in columns 7–12. Plates were then placed in the incubator for 30 min until the first analysis. Duplicate plates allowed for eight wells in each experimental condition.
Since each plate contained fluorometric (DCFH‐DA) and colorimetric (MTS) probes, two plate readers were utilized. A Thermo Scientific's Fluoroskan Ascent was used to determine the fluorescence developed as a result of the addition of DCFH‐DA, while a Molecular Devices' SpectraMax 340PC384 was used to determine the color associated with the MTS probe. Plates were analyzed systematically over 24 h. For all times outside of incubator, the microplates were kept in foil to keep out light. The pH of the plate wells was determined with a Sensorex Combination pH Electrode (1.27 cm in diameter) attached to a Fisher Scientific Accumet Portable pH meter.
3. Results
The two dust samples were predominately composed of the same mineral assemblage with quartz, calcite, and feldspar representing the bulk mineralogy (Table 1). Camp Victory dust (CVD) also contained aragonite and dolomite, and Fort Irwin dust also contained chlorite and illite. The elemental analysis of each dust sample did not show any anonymously high heavy metals concentrations. In fact, except for the elevated chromium and nickel concentrations in CVD, these dust samples are similar to NIST standard reference material #2709A, a soil collected in San Joaquin Valley, California. Along with glass beads, the San Joaquin Valley soil acts as a negative control [Harrington et al., 2012]. The slightly elevated chromium and nickel levels in CVD might be related to the presence of a suite of accessory minerals derived from igneous rocks. By treating dust samples with 1.0 M HCl overnight and removing dissolved constituents, manganese was determined to be mostly associated with the carbonate phases in both dusts (see Table 1). The surface areas of both dust samples were high at 26.429 m2/g and 16.335 m2/g (CVD and FID, respectively).
Table 1.
Mineralogical and Elemental Analysis for Camp Victory and Fort Irwin Dust
| Normalized Major Elements (Weight %) | Trace Elements (ppm) | Acid Treated/Untreated Elemental Ratios | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mineralogy |
Camp Victory |
Fort Irwin |
Camp Victory |
Fort Irwin |
Camp Victory |
Fort Irwin |
|||
| Camp Victory | SiO2 | 50.46 | 60.37 | Ni | 186 | 19 | SiO2 | 0.96 | 0.95 |
| Quartz | TiO2 | 0.807 | 0.753 | Cr | 277 | 32 | TiO2 | 1.12 | 1.07 |
| Calcite | Al2O3 | 12.77 | 14.68 | Cu | 40 | 35 | Al2O3 | 0.89 | 0.92 |
| Feldspar | FeOb | 6.57 | 6.10 | V | 113 | 84 | FeOb | 1.02 | 1.01 |
| Aragonitea | MnO | 0.130 | 0.119 | Zn | 82 | 115 | MnO | 0.54 | 0.65 |
| Dolomitea | MgO | 6.69 | 3.62 | Pb | 18 | 29 | MgO | 0.70 | 0.81 |
| Fort Irwin | CaO | 18.76 | 8.19 | Th | 5 | 16 | CaO | 0.15 | 0.24 |
| Quartz | Na2O | 1.94 | 2.52 | Ba | 273 | 704 | Na2O | 0.65 | 0.86 |
| Calcite | K2O | 1.66 | 3.40 | U | 2 | 2 | K2O | 1.06 | 1.02 |
| Chlorite | P2O5 | 0.215 | 0.249 | P2O5 | 0.24 | 0.23 | |||
| Feldspar | |||||||||
| Illitea | |||||||||
Possible.
Total iron.
CVD generated 25% of the ˙OH and 28% of the ˙OH + H2O2 of FID when dispersed in solution for 24 h (Figures 2 and 3). When normalized to surface area these values dropped, with CVD having generated 16% of the ˙OH and 17% of the ˙OH + H2O2 of FID. Both the CVD and FID contained endotoxin in quantities that exceeded the detection limit of the probe (1 EU/mL).
Figure 2.
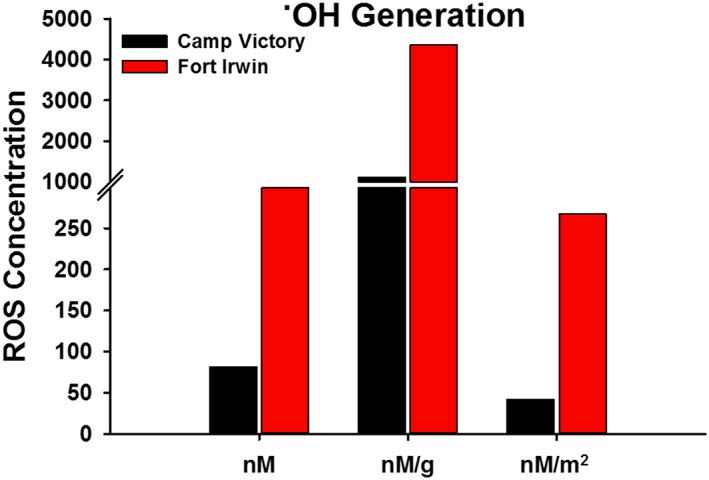
˙OH generated by Camp Victory, Iraq, dust and Fort Irwin, CA, dust. Concentrations are based on a 24 h incubation in phosphate‐buffered solution at a pH of 7.4 with APF. Data represented as total ˙OH formed and also normalized by weight and surface area of the particles.
Figure 3.
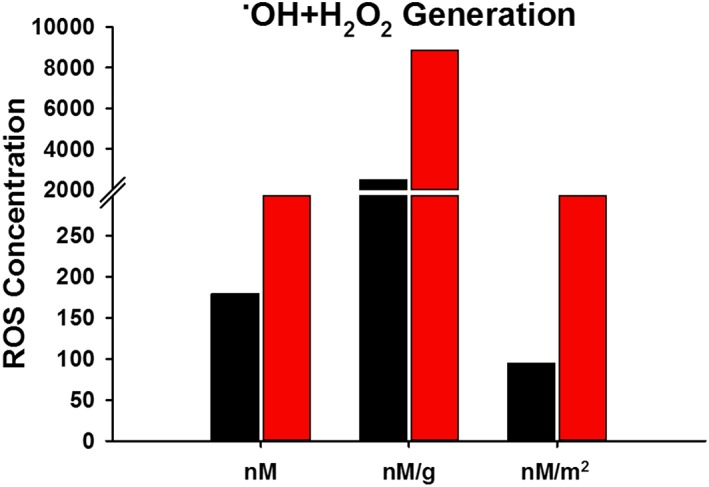
˙OH + H2O2 generated by Camp Victory, Iraq, dust and Fort Irwin, CA, dust. Concentrations based on a 24 h incubation in phosphate‐buffered solution at a pH of 7.4 with APF and HRP. Data represented as total ˙OH + H2O2 formed and also normalized by weight and surface area of the particles.
The initial particle loading utilized for this study was 0.002 m2/mL. This loading was chosen for comparison with other inflammatory stress response studies [Harrington et al., 2012]. At the highest loading for this particle loading range, the greatest ISR generated by CVD was 159% of the control. FID generated an ISR 220% higher than the control. Given that there was no significant loss of cell viability the increase in ISR compared to the control was derived by the upregulation of ROS. Both of these ISR values were within the range caused by inert glass beads and anatase [Harrington et al., 2012]. Besides the standard particle loading, we also conducted a set of experiments at higher loadings to simulate the high exposure burden encountered by personnel in the Middle East theater of operations. The ISR resulting from exposure to CVD dust increased to 3312% of the control at a particle loading of 0.05 m2/mL (Figure 4). The ISR generated by FID dust increased to 2433% of the control. The ISR generated by both dust samples generally increased with time and was predominately attributed to an upregulation in cellular‐derived ROS. The ISR generated by the acid‐treated CVD and FID was 288 and 550% of the control, respectively, both occurring early into exposure (20 min and 1 h, respectively). Unlike with the untreated dust samples, the ISR was predominately driven by necrotic cellular death.
Figure 4.
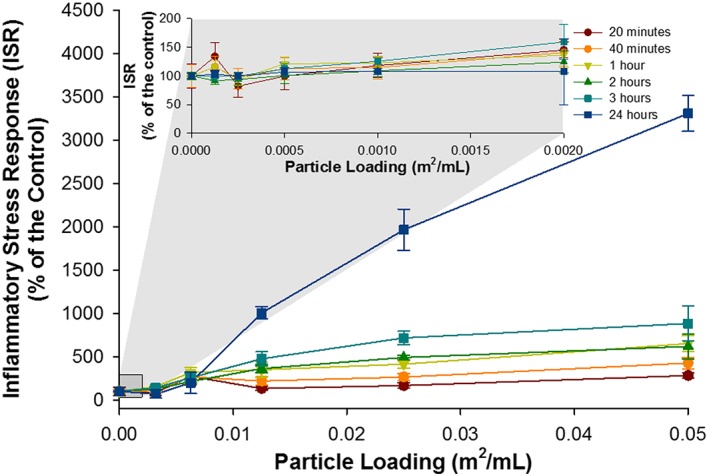
The ISR generated by Camp Victory, Iraq, dust over a range of particle loadings and times points. Inset displays the ISR generated by Camp Victory dust at low particle loadings. For some data points, the error bars are hidden by the symbols.
For comparison, ISR experiments were performed on glass beads and two NIST soil standards using the elevated loading of 0.05 m2/mL. Due to extreme loss of cell viability at the 0.05 m2/mL loading for both NIST standards, the 0.025 m2/mL loading was used for comparison with the dust samples. The ISR generated by the Montana NIST soil was 36,600% of the control at the 24 h time point, progressively increasing with time (Figures 5 and 6). The ISR generated by the San Joaquin NIST soil was 2952% of the control at the 8 h time point and decreased to 1954% of the control at 24 h. The ISR values for both NIST soils were driven up both cellular ROS upregulation and extreme cell death at the highest particle loadings. The ISR generated by the inert glass beads was the highest at 8 h (280% of the control) and exhibited only a small amount of cellular death. The ISR generated by CVD and FID at the lower loading of 0.025 m2/mL was 1967 and 1412% of the control, respectively (Figures 7 and 8).
Figure 5.
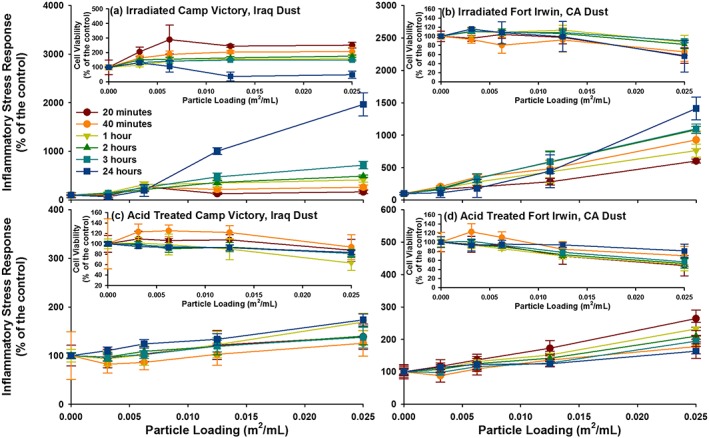
ISR generated by the four study materials are represented, with the cell viability for each in the inset. (a) Irradiate Camp Victory, Iraq, dust; (b) irradiated Fort Irwin, CA, dust; (c) acid‐treated Camp Victory, Iraq, dust; and (d) acid‐treated Fort Irwin, CA, dust. For some data point, the error bars are hidden by the symbols.
Figure 6.
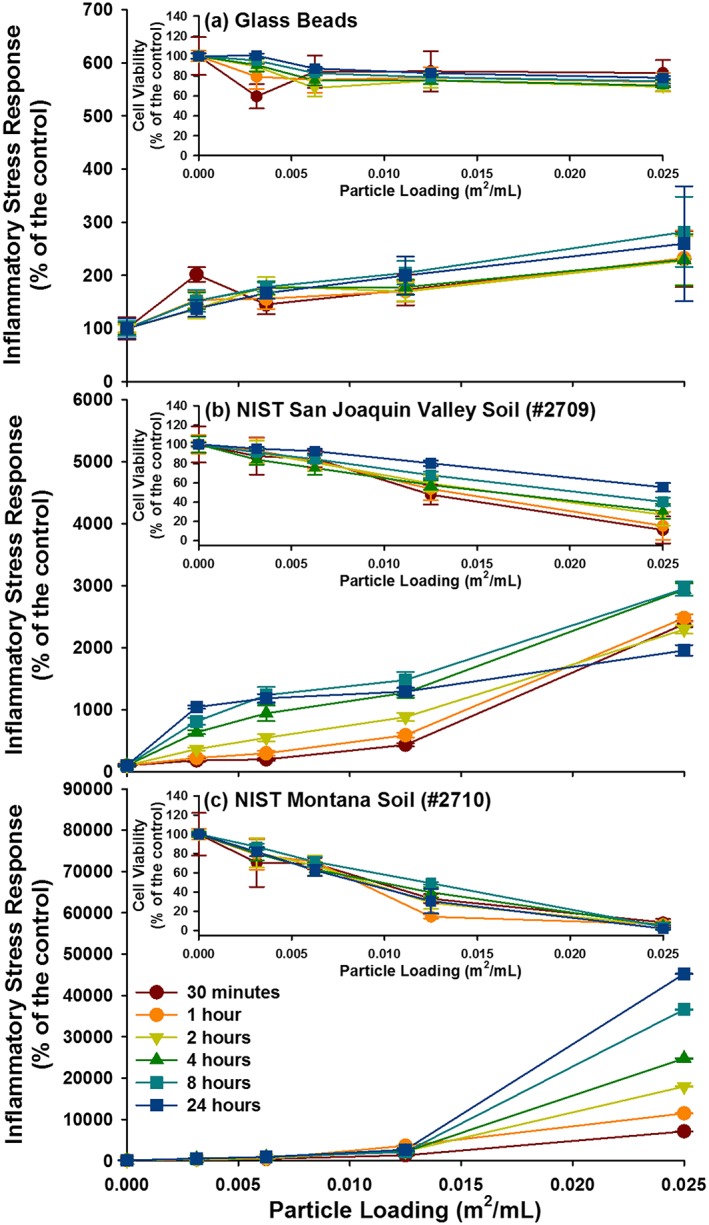
ISR generated by the three reference materials are represented, with the cell viability for each in the inset. (a) Glass beads, (b) NIST San Joaquin Valley soil (#2709), and (c) NIST Montana soil (#2710). For some data point, the error bars are hidden by the symbols.
Figure 7.
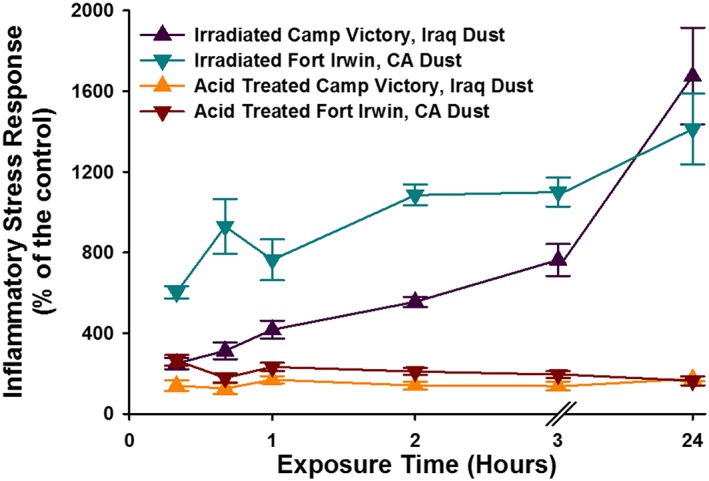
The temporal evolution of the ISR generated by elevated particle loadings (0.025 m2/mL) for Camp Victory and Fort Irwin dust samples. Also represented are the dust samples that were treated with acid to remove carbonate phases. For some data points, the error bars are hidden by the symbols.
Figure 8.
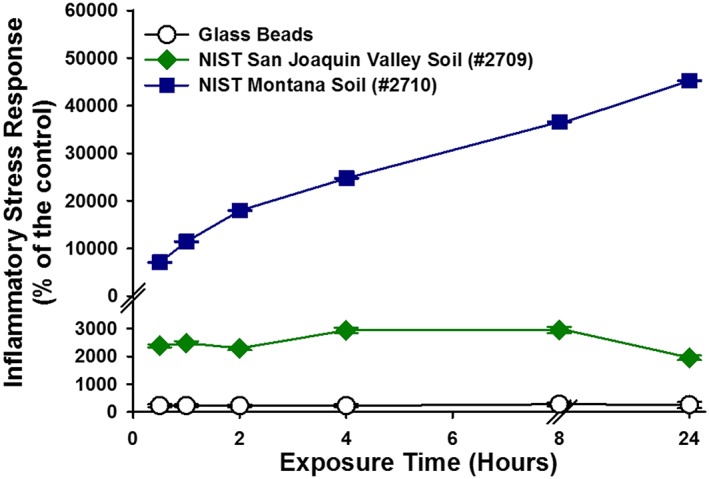
The temporal evolution of the ISR generated by elevated particle loadings (0.025 m2/mL) for reference material samples: glass beads, NIST San Joaquin Valley soil (#2709), and NIST Montana soil (#2710). For some data points, the error bars are hidden by the symbols.
4. Discussion
The CVD sample investigated in this study is similar to other dust samples collected throughout the Greater Middle East; containing the same characteristic elevation in calcium carbonate respective to worldwide dust [Engelbrecht et al., 2009a]. However, as with other grab samples, there are some differences in the concentration of trace metals between CVD and ambient air samples from the Middle East. Specifically, the CVD contains relatively more chromium and nickel and less lead and zinc than the average ambient PM sample [Engelbrecht et al., 2009a, 2009b]. The FID, while similar to CVD, is composed of higher proportions of silica. It is difficult to evaluate how much of the combusted burn pit material is within the CVD sample. However, the relatively low concentration of heavy metals and the lack of elevated amorphous material within the sample, which would indicate glass sinters, suggest that it is not a major component.
The dust collected at Fort Irwin appears to elicit a stronger cellular response than the Iraqi dust, but both are far less reactive than the two soil reference materials that were also tested (Table S1 in the supporting information). Both the CVD and FID dust show little loss of cell viability. The higher initial ISR values for FID is possibly related to the higher ROS formation in experiments with FID. While ROS formation upon dispersal in water in cell‐free experiments does not always translate to ROS upregulation within epithelial cells, it is noted that FID showed a significantly higher production of geochemically derived ROS in water compared to CVD. However, the ISR values determined for FID and CVD are lower than those determined for either of the NIST soils when the results for the experiments with 0.025 m2/L loadings are compared (near‐complete loss of cell viability in experiments with the Montana NIST soil prevents a comparison at 0.05 m2/L loading). For example, CVD dust generates an ISR that is twentyfold lower than that measured for the Montana soil (NIST 2710).
Besides the magnitude of the cellular response to the challenge, it is also useful to evaluate the temporal changes in ISR values. Changes in ISR over time provide insight into how cells respond to the exposure. For example, although exposure to chalcopyrite (CuFeS2) leads to an significant loss of cell viability initially, the cell survival increased over time and the ISR values drop [Harrington et al., 2014]. As noted above, the ISR generated by CVD was lower than the NIST soil with baseline trace elements (2709), but, unlike this NIST soil, the ISR steadily increased with time over 24 h. This rising response indicates that the inflammation is likely to continue to increase, whereas cells challenged with the NIST 2709 manage to adapt to the challenge. Furthermore, CVD generates an ISR value that is nearly sevenfold that for glass beads at their peak ISR value. Therefore, the CVD is not inert, and we conclude, on the basis of the experiments described here, that the material is considered slightly cytotoxic, on par with the average natural dust or soil. It is also noted that while the majority of the ISR is generated by the carbonate phases and metals incorporated in carbonates (e.g., manganese), the origin of the ISR between the untreated and acid‐treated material differs. The ISR generated by the untreated dust samples is driven by the upregulation of ROS, possibly related to the ROS generated by the material. Whereas the minimal ISR generated by the acid‐treated material is driven by cellular death, indicating other immunosuppressive properties.
Based on this in vitro technique and comparison with other standard samples, the geochemical components of the dust are considered nonhazardous if exposures are limited to the World Health Organizations AQ Guidelines for daily total suspended particle threshold of 120 μg/m3. However, it must be noted that the elevated loading of 0.05 m2/mL, which parallels a PM exposure of 1982 μg/m3, is only 20% the PM10 value present in a single dust storm [Engelbrecht et al., 2009a; Lyles et al., 2008]. Therefore, while this particle loading is elevated relative to previous ISR experiments, the total particle burden in the lungs is likely underrepresented. Furthermore, this study evaluates an acute exposure. U.S. soldiers deployed in Iraq and Afghanistan are not only exposed to a single high‐intensity dust storm but also to elevated ambient dust levels and multiple dust storms over the course of months to years [Lyles et al., 2008].These acute to moderate exposure durations are also most likely to induce cardiovascular events on top of respiratory issues [Pope et al., 2011].
While it is likely that high and persistent dust exposure is the main factor in the development of decreased lung function and respiratory illnesses seen in U.S. soldiers, other factors may contribute, for example, CVD's small particle size and complex morphology. The specific surface area of CVD is a factor of 2.5 higher than both NIST reference materials utilized in the present study. The fine particle size could lead to a deeper deposition of the particles in the human lung than many other soils or dusts. Furthermore, the particles are sharp and appear to be aggregated into clumps. It is not clear whether this aggregation persists after deposition in the lung. Should the aggregates remain intact then many may be too large for phagocytosis. Due to the high PM10 values in the Greater Middle East, particle overload likely contributes to the observed illnesses and the small particle size of individual particles will exacerbate the disruption of AM‐mediated phagocytosis and efferocytosis. The porous aggregates may also harbor a plethora of bacterial, fungal, and viral colonies as indicated by other work on Iraqi dust samples [Lyles et al., 2008]. The present study determined that both CVD and FID contained high concentrations of endotoxin. It is important to note that although the A549 lung epithelial cell line is a good indicator of proinflammatory activity, particularly geochemical based, their lack of lipopolysaccharide receptors (CD14 and CD18) is a limiting factor in determining overall hazard of a material [Palmberg et al., 1998; Wright, 1991]. Knowing this, the low ISR generated by the CVD and FID only indicates low geochemical‐based toxicity and not low overall toxicity of the dust.
In the end, it is a very complex situation without a straightforward answer. However, the most consistent trend is that U.S. and coalition soldiers stationed in the Middle East that were exposed to high concentrations of PM for a moderate period have developed respiratory issues. Given the lack of a definitive trend for the other susceptibility and vulnerability factors, they are likely more additive or synergistic contributors, rather than the main driver of pathogenesis [Falvo et al., 2015].
5. Conclusions
There is nothing unusual about the CVD in terms of its chemistry, mineralogy, ability to generate ROS upon dispersal in water, or induce cellular inflammation or cytotoxicity in A549 lung epithelial cells. However, CVD is slightly cytotoxic and generated an ISR that increased with increasing particle loading and time. The slight inflammatory nature that exists seems to be attributable to the carbonate phases and elements associated with the carbonate phases, which includes manganese. While it is possible that this dust does not represent the bulk dust present in Iraq, given the highly elevated PM10 and PM2.5 levels in the Middle East, it is likely that particle overload is the underlying cause for the health problems among U.S. personnel, who served in Iraq and not the geochemical composition[Frank and Ottoboni, 2011]. Additionally, organic contamination in Greater Middle East dusts, such as the endotoxin found in CVD, will also contribute to pathogenesis. However, in order to definitively determine the cause of the lung injury, more experiments need to be performed not only with other Iraqi dust samples but also with an array of toxicological techniques utilizing in vivo mouse exposures.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
This work was supported by the Minerals, Metals, Metalloids and Toxicity (3MT) program at Stony Brook University, which is funded by NSF‐IGERT, NIH R01 NS42168 (SET), and NIH/NIEHS 2 T32 ES07324‐16 (T.G.). Data can be accesses in Stony Brook University's data repository called Academic Commons at http://commons.library.stonybrook.edu/geo-articles/. A.D.H. helped design the study, performed the experiments, and drafted the manuscript. M.P.S. and K.G. performed experiments. A.M.S. acquired dust samples and helped design the study. T.G., S.E.T., and M.A.A.S. helped design the experiments, supervised the study, and edited the manuscript. All authors have read and approved the final manuscript.
Harrington, A. D. , Schmidt M. P., Szema A. M., Galdanes K., Tsirka S. E., Gordon T., and Schoonen M. A. A. (2017), The role of Iraqi dust in inducing lung injury in United States soldiers—An interdisciplinary study, GeoHealth, 1, 237–246, doi: 10.1002/2017GH000071.
This article was corrected on 15 JUL 2019. The online version of this article has been modified to include a Conflict of Interest statement.
References
- Abraham, J. H. , et al. (2014), A retrospective cohort study of military deployment and postdeployment medical encounters for respiratory conditions, Mil. Med., 179(5), 540–546, doi: 10.7205/milmed-d-13-00443. [DOI] [PubMed] [Google Scholar]
- Bank, T. W. (2013), World Development Indictors: Average precipitation in depth (mm per year).
- Barth, S. K. , Dursa E. K., Peterson M. R., and Schneiderman A. (2014), Prevalence of respiratory diseases among veterans of Operation Enduring Freedom and Operation Iraqi Freedom: Results from the National Health Study for a New Generation of U.S. Veterans, Mil. Med., 179(3), 241–245, doi: 10.7205/milmed-d-13-00338. [DOI] [PubMed] [Google Scholar]
- Cohn, C. A. , Pedigo C. E., Hylton S. N., Simon S. R., and Schoonen M. A. A. (2009), Evaluating the use of 3′‐(p‐aminophenyl) fluorescein for determining the formation of highly reactive oxygen species in particle suspensions, Geochem. Trans., 10, 8–16, doi: 10.1186/1467-4866-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht, J. P. , McDonald E. V., Gillies J. A., Jayanty R. K. M., Casuccio G., and Gertler A. W. (2009a), Characterizing mineral dusts and other aerosols from the Middle East. Part 1: Ambient sampling, Inhalation Toxicol., 21(4), 297–326, doi: 10.1080/08958370802464273. [DOI] [PubMed] [Google Scholar]
- Engelbrecht, J. P. , McDonald E. V., Gillies J. A., Jayanty R. K. M., Casuccio G., and Gertler A. W. (2009b), Characterizing mineral dusts and other aerosols from the Middle East. Part 2: Grab samples and re‐suspensions, Inhalation Toxicol., 21(4), 327–336, doi: 10.1080/08958370802464299. [DOI] [PubMed] [Google Scholar]
- Falvo, M. J. , Osinubi O. Y., Sotolongo A. M., and Helmer D. A. (2015), Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans, Epidemiol. Rev., 37, 116–130, doi: 10.1093/epirev/mxu009. [DOI] [PubMed] [Google Scholar]
- Frank, P. , and Ottoboni M. A. (2011), The Dose Makes the Poison: A Plain‐Language Guide to Toxicology, 3rd ed., John Wiley, Hoboken, N. J. [Google Scholar]
- Harrington, A. D. , Tsirka S. E., and Schoonen M. A. A. (2012), Quantification of particle‐induced inflammatory stress response: A novel approach for toxicity testing of Earth materials, Geochem. Trans., 13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington, A. D. , Smirnov A., Tsirka S. E., and Schoonen M. A. A. (2014), Metal‐sulfide mineral ores, Fenton Chemistry and Disease – Particle Induced Inflammatory Stress Response in Lung Cells, Int. J. Hyg. Environ. Health, 218(1), 19–27, doi: 10.1016/j.ijheh.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick, M. E. (2011), Deputy Director for Force Health Protection and Readiness Programs in the Office of the Assistant Secretary of Defense for Health Affairs, edited by A. M. Szema.
- King, M. S. , et al. (2011), Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan, N. Engl. J. Med., 365(3), 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski, K. , Nitsch‐Osuch A., Konarski M., Guzek A., Prokop E., and Bieniuk K. (2013), Prevalence of acute respiratory tract diseases among soldiers deployed for military operations in Iraq and Afghanistan, Adv. Exp. Med. Biol., 788, 117–124, doi: 10.1007/978-94-007-6627-3_18. [DOI] [PubMed] [Google Scholar]
- Lyles, M. B. , Fredrickson H. L., Bednar A. J., Fannin H. B., Griffin D., and Sobecki T. M. (2008), Medical geology: Dust exposure and potential health risks in the Middle East, Geochim. Cosmochim. Acta, 72(12), A576–A576. [Google Scholar]
- Morman, S. A. , and Plumlee G. S. (2013), The role of airborne mineral dusts in human disease, Aeolian Res., 9, 203–212, doi: 10.1016/j.aeolia.2012.12.001. [DOI] [Google Scholar]
- NIST (2002a), Certificate of Analysis—Standard Reference Material 2709 (San Joaquin Soil), edited by D. O. Commerce , p. 3, National Institute of Standards & Technology, Washington, D. C. [Google Scholar]
- NIST (2002b), Certificate of Analysis—Standard Reference Materail 2710 (Montana Soil), edited by D. O. Commerce , p. 3, National Institute of Standards & Technology, Washington, D. C. [Google Scholar]
- Oberdorster, G. (1995), Lung particle overload: Implications for occupational exposures to particles, Regul. Toxicol. Pharm., 21(1), 123–135. [DOI] [PubMed] [Google Scholar]
- Palmberg, L. , Larsson B. M., Malmberg P., and Larsson K. (1998), Induction of IL‐8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust, Thorax, 53(4), 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. W. , Lim Y. H., Kyung S. Y., An C. H., Lee S. P., Jeong S. H., and Ju Y. S. (2005), Effects of ambient particulate matter on peak expiratory flow rates and respiratory symptoms of asthmatics during Asian dust periods in Korea, Respirology, 10(4), 470–476, doi: 10.1111/j.1440-1843.2005.00728.x. [DOI] [PubMed] [Google Scholar]
- Pope, C. A. , Brook R. D., Burnett R. T., and Dockery D. W. (2011), How is cardiovascular disease mortality risk affected by duration and intensity of fine particulate matter exposure? An integration of the epidemiologic evidence, Air Qual. Atmos. Health, 4(1), 5–14, doi: 10.1007/s11869-010-0082-7. [DOI] [Google Scholar]
- Schoonen, M. A. A. , Cohn C. A., Roemer E., Laffers R., Simon S. R., and O'Riordan T. (2006), Mineral‐induced formation of reactive oxygen species, in Medical Mineralogy and Geochemistry, edited by Sahai N. and Schoonen M. A. A., pp. 179–221, Miner. Soc. of Am., Chantilly, Va. [Google Scholar]
- Setsukinai, K. , Urano Y., Kakinuma K., Majima H., and Nagano T. (2003), Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish different species, J. Biol. Chem., 278, 3170–3175. [DOI] [PubMed] [Google Scholar]
- Szema, A. M. , Salihi W., Savary K., and Chen J. J. (2011), Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury, J. Occup. Environ. Med., 53(9), 961–965, doi: 10.1097/JOM.0b013e31822c9f05. [DOI] [PubMed] [Google Scholar]
- Szema, A. M. , Schmidt M. P., Lanzirotti A., Harrington A. D., Lyubsky S., Reeder R. J., and Schoonen M. A. A. (2012), Titanium and iron in lung of a soldier with nonspecific interstitial pneumonitis and bronchiolitis after returning from Iraq, J. Occup. Environ. Med., 54(1), 1–2, doi: 10.1097/JOM.0b013e31824327ca. [DOI] [PubMed] [Google Scholar]
- Vandivier, R. W. , Henson P. M., and Douglas I. S. (2006), Burying the dead: The impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease, Chest J., 129(6), 1673–1682, doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- Wright, S. D. (1991), Multiple receptors for endotoxin, Curr. Opin. Immunol., 3(1), 83–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


