Abstract
Background and purpose
Damage of the blood-brain barrier (BBB) increases the incidence of neurovascular complications, especially for cerebral hemorrhage after tPA therapy. Currently there is no effective method to evaluate the extent of BBB damage to guide tPA use. Herein, we investigated whether blood levels of tight junction proteins could serve as biomarker of BBB damages in acute ischemic stroke (AIS) in both rats and patients. We examined whether this biomarker could reflect the extent of BBB permeability during cerebral ischemia/reperfusion, and the effects of normobaric hyperoxia (NBO) on BBB damage.
Methods
Rats were exposed to NBO (100%O2) or normoxia (21%O2) during middle cerebral artery occlusion. BBB permeability was determined. Occludin and claudin-5 in blood and cerebromicrovessels were measured. AIS patients were assigned to oxygen therapy or room air for 4 hours, and blood occludin and claudin-5 were measured at different time points after stroke.
Results
Cerebral ischemia/reperfusion resulted in the degradation of occludin and claudin-5 in microvessels, leading to increased BBB permeability in rats. In blood samples, occludin increased with 4-h ischemia and remained elevated during reperfusion, correlating well with its loss from ischemic cerebral microvessels. NBO treatment both prevented occludin degradation in microvessels and reduced occludin levels in blood, leading to improved neurological functions in rats. In AIS patients receiving intravenous tPA thrombolysis, the blood occludin was already elevated when patients arrived at hospital (within 4.5 h since symptoms appeared) and remained at a high level for 72 hours. NBO significantly lowered the level of blood occludin and improved neurological functions in AIS patients.
Conclusions
Blood occludin may be a clinically viable biomarker for evaluating BBB damage during ischemia/reperfusion. NBO therapy has the potential to reduce blood occludin, protect BBB and improve outcome in AIS patients with intravenous tPA thrombolysis.
Keywords: occludin, normobaric hyperoxia, blood-brain barrier, acute ischemic stroke
Introduction
Early thrombolytic therapy is critical for the treatment of acute ischemic stroke patients. However, thrombolysis significantly increases the risk of hemorrhagic transformation (HT) in AIS patients1. Studies have shown that ischemia/reperfusion-induced BBB damage is the main reason for HT after vascular recanalization2, 3. Therefore, it is vital to find a reliable biomarker to reflect the progress of BBB permeability changes during cerebral ischemia, helping to evaluate the risk of HT and decide whether to apply thrombolysis.
To date, several proteins in serum (MMP-9, c-FN, PAI-1, TAFI, GFAP, NSE and S100B) have been reported to be linked to the brain injury after cerebral ischemia4,5. However, there has been no reliable biomarker to accurately reflect the change of BBB permeability during cerebral ischemia.
Tight junction proteins (TJPs), including claudin-5 and occludin, that seal the gap between endothelial cells are important structural components to maintain BBB integrity6. The loss of TJPs causes BBB damage and HT after cerebral ischemia7, 8. Our recent study demonstrated that the fragments of cleaved occludin may release into circulation and the levels of blood occludin correlate well with the extent of BBB damage in a permanent cerebral ischemic model of rats, suggesting that occludin may serve as a potential biomarker for evaluating BBB damage during the ischemic phase9. In this study, we investigated the relationship between TJPs (claudin-5 and occludin) and BBB damage using an ischemia-reperfusion rat model, which better mimics the ischemia-recanalization in clinic. Then we examined the changes of claudin-5 and occludin in serum of ischemic stroke patients designated to receive intravenous tPA thrombolysis.
An ideal biomarker should also respond to the protective effects of therapy. Studies in animals and patients have shown that short duration of normobaric hyperoxia (NBO) treatment is highly neuroprotective if started early after ischemia onset10–13. Animal experiments by our lab and others have suggested that NBO can protect BBB by reducing the degradation of TJPs in ischemic microvessels8, 14, 15. In this study, we also investigated whether NBO treatment could reduce the level of claudin-5 or occludin in serum both in ischemia-reperfusion rats and in AIS patients with intravenous tPA thrombolysis.
Materials and methods
Animal preparation
The Laboratory Animal Care and Use Committee of the Capital Medical University approved all animal experiments. Male Sprague-Dawley rats weighing 290–320g were anesthetized with 2% isoflurane and subjected to middle cerebral artery occlusion (MCAO) followed by reperfusion using the suture occlusion model, as we previously described16. Rats in sham group received the same surgical procedures as those in model group, but the suture was not advanced beyond the internal carotid bifurcation.
NBO treatment of rats
Animals recovered rapidly from anesthesia within 5-min post MCAO surgery, and were placed into an anesthesia box that was ventilated (5L/min) with air (21% O2, normoxia) or 100% O2 (NBO) until 10-min before the end of MCAO.
Animal study design
A total of 128 rats were used in this study, of which 11 were excluded from our analysis due to incomplete occlusion or subarachnoid hemorrhage. To investigate the change of blood claudin-5 and occludin during cerebral ischemia and reperfusion with or without NBO treatment, 36 rats with successful MCAO were randomly assigned into claudin-5 and occludin group with each having three subgroups (n=6): sham group, MCAO+normoxia, and MCAO+NBO. In each group, blood samples were collected at 5 time points: 0-h (basal level), 2-h MCAO, 4-h MCAO, 4-h MCAO+5-min reperfusion and 4-h MCAO+2-h reperfusion. For measurement of occludin and claudin-5 on microvessels, another 28 rats were randomly assigned into the following groups (n=4): sham group, MCAO+normoxia and MCAO+NBO group with each having three subgroups: 2-h MCAO, 4-h MCAO, and 4-h MCAO+2-h reperfusion. Brain tissues were collected for microvessel isolation and protein assays. To evaluate the degree of BBB damage and neurological deficits, another 20 rats were randomly assigned into 4 groups (n=5): 2-h MCAO+2-h reperfusion (+/− NBO), and 4-h MCAO+2-h reperfusion (+/− NBO). To assess 24-h mortality rate, another 33 rats were randomly assigned into 4 groups: 2-h MCAO+normoxia (n=8); 2-h MCAO+NBO (n=8); 4-h MCAO+normoxia (n=8); and 4-h MCAO+NBO (n=9).
Evaluation of BBB permeability by Evan’s blue leakage
Evan’s blue dye (EB, 2 % in PBS, 3 mL/kg) (Sigma, USA) was administered intravenously in the tail vein at the onset of reperfusion. At the end of 2-h reperfusion, EB in vessels were removed by transcardially perfusing with PBS. The brain was removed and sectioned to visualize EB extravasation. BBB permeability was assessed by detecting EB contents in ischemic hemispheric tissue, as previously reported17.
Brain tissue collection
The rats were transcardially perfused with 250 mL cold PBS. Brains were quickly removed and sectioned to 2-mm thick coronary slices, 2 mm away from the tip of the frontal lobe. Ischemic hemispheric tissue was then collected from each brain slice for cerebral microvessel isolation.
Cerebral microvessels isolation
Cerebral microvessels were isolated from ischemic hemisphere, as described in our previous study15. Briefly, the hemispheric brain tissue was homogenized in ice-cold PBS. The homogenates were filtered through a 41-μm-nylon mesh. Microvessels that retained on the mesh were purified with Dextran T-500 and stored at −80 °C for Western blot.
Western blot analysis of occludin and claudin-5 protein in cerebral microvessels
Isolated cerebral microvessels were incubated in 100 μl RIPA lysis buffer (CST, USA) on ice and centrifuged at 16,000g for 15 min at 4°C. The supernatants were collected and the protein concentrations were determined. Occludin and claudin-5 proteins levels were measured by Western blot, as described in our previous studies7, 15. Anti-occludin and anti-claudin-5 were from Invitrogen (USA) and anti-β-actin was from Santa Cruz (USA).
Blood sample collection and ELISA assay
One milliliter of blood were taken from the left femoral vein at each time point: before MCAO, 2h-MCAO, 4h-MCAO, 4h-MCAO with 5min-reperfusion, and 4h-MCAO with 2h-reperfusion. Serum was separated by centrifugation at 3000 rpm for 10 minutes at 4°C. About 200 μl serum could be harvested to measure the levels of occludin and claudin-5 using commercially available ELISA kits for rat samples (occludin: USCN, China; claudin-5: USCN, China).
Measurement of neurological deficits and mortality rate
Neurological deficits of rats were determined in an double-blinded manner with Zea-Longa scores at 2-h MCAO, 4-h MCAO, and 4-h MCAO with 2-h reperfusion in each group. Mortality was assessed in 2-h MCAO+24-h reperfusion and 4-h MCAO+24-h reperfusion groups with or without NBO treatment.
Human study I
Study design
All procedures performed in studies involving human participants were in accordance with the Institutional Ethics Committee of Xuanwu Hospital, Capital Medical University. To investigate the changes of occludin and claudin-5 levels in human blood samples after AIS, we recruited 8 AIS patients who were admitted within 4.5 hours after stroke onset at Xuanwu Hospital between June 15, 2016 and November 23, 2016. In addition, 8 healthy people were randomly chosen from the physical examination population age between 40 and 69 years from the check-center in Xuanwu Hospital on December 15, 2016.
The inclusion and exclusion criteria of AIS patients
The inclusion criteria were: (1) Age>18, presenting<4.5 hours after witnessed symptom onset; (2) Eligible for intravenous thrombolysis; (3) 4 < NIHSS score <25; (4) Pre-admission modified Rankin scale (mRS) score <1. (5) Acute ischemic stroke was confirmed by CT or MRI the following day. The exclusion criteria were: (1) Active chronic obstructive pulmonary disease; (2)﹥3 L/min oxygen required to maintain peripheral arterial oxygen saturation >95% per stroke management guidelines18; (3) rapidly improving neurological deficits; (4) medically unstable; (5) pregnancy; (6) inability to obtain informed consent. All enrolled AIS patients received intravenous tPA thrombolytic therapy and standard clinical treatment (anticoagulant, antiplatelet). The general conditions of patients are shown in Supplemental Table I.
Measurement of blood sample from AIS patients and healthy people
Blood samples (4ml) from 8 AIS patients and 8 healthy subjects were collected to measure blood occludin and claudin-5 at the following time points: at admission, 24 hours (range: 20–28 hours), and 72 hours (range: 68–76 hours). Sera (100μl) were then separated to measure the levels of occludin and claudin-5 using commercially available ELISA kits for human samples (occludin: USCN, China; claudin-5: USCN, China).
Human study II
Study design
To investigate the changes of claudin-5 and occludin in AIS patients with or without NBO treatment, another 18 AIS patients between November 23, 2016, and January 10, 2107 were recruited and randomly divided into NBO and normoxia group. The criteria for inclusion and exclusion and the methods to measure blood sample were the same as in Human Study I.
NBO treatment in AIS patients
As soon as diagnosis of AIS was made based on clinical symptoms and CT, patients in NBO group were immediately given oxygen inhalation for 4 hours by oxygen facemask at a flow rate of 10 L/min. Normoxia group inhaled room air.
Measurement of neurological functions by NIHSS scores
National Institutes of Health Stroke Scale (NIHSS) score were measured to determine the relationship between the outcome and biomarker levels after NBO treatment. NIHSS score were recorded at admission, 24 hours (range, 20–28 hours), 72 hours (range, 68–76 hours), and 1 week (range, 6–8 days).
Statistical analysis
The data are presented as means ±SEM. Statistical analysis was carried out using ANOVA and χ2. Repeated measures ANOVAs were used to analyze differences in related variables. A value of P<0.05 was considered statistically significant.
Results
NBO-treated rats significantly slowed ischemic BBB damage
We compared the extent of BBB damage with or without NBO treatment after 2-h or 4-h ischemia followed by 2-h reperfusion. EB extravasation into brain tissue was shown in Fig. 1. As expected, EB contents in non-ischemic hemispheric tissue were very low in all groups. Cerebral ischemia/reperfusion considerably increased EB leakage into the ischemic hemisphere after 2-h ischemia/reperfusion in normoxic group, and EB leakage was further elevated when ischemia duration was increased to 4 h. Importantly, NBO treatment significantly reduced Evan’s blue leakage in 4-h ischemia/reperfusion rats when compared with the normoxic rats (Fig. 1). These results indicate that NBO treatment can attenuate BBB disruption after cerebral ischemia/reperfusion.
Figure 1. NBO-treated rats showed significantly less Evan’s blue leakage after 4-h of MCAO with 2-h reperfusion.
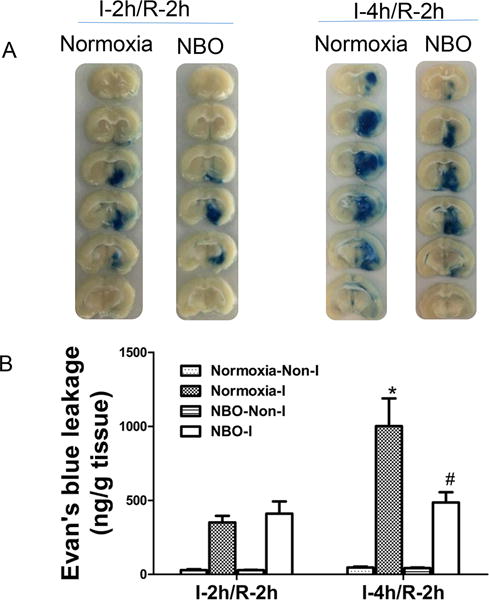
A). Representative brain slices show EB leakage in ischemic hemisphere after 2-h or 4-h MCAO with 2-h reperfusion. B). Quantification of EB extravasation in brain tissue of ischemic hemisphere (I) and non-ischemic hemisphere (Non-I). N=5, *P<0.05 versus Normoxia-I in I-2h/R-2h group; #P<0.05 versus Normoxia-I in I-4h/R-2h group. Data are expressed as means ± SEM.
NBO reduces the loss of TJPs in microvessels in cerebral ischemia rats
To investigate the kinetics of TJPs loss during cerebral ischemia/reperfusion under NBO or normoxia condition, we extracted cerebral microvessels from ischemic hemispheric tissue to determine the levels of occludin (Fig. 2A) and claudin-5 (Fig. 2B) in the isolated microvessels. Western blot for occludin showed that rats in sham group have high level of occludin. Although two-hours of ischemia did not induce detectable loss of occludin proteins in ischemic cerebral microvessels, there were significant reductions observed in 4-h MCAO and 4-h MCAO plus 2-h reperfusion. Of note, NBO-treatment significantly prevented occludin in microvessels from degradation both in 4-h MCAO and in 4-h MCAO with 2-h reperfusion. Similar results were observed for claudin-5 in microvessels (Fig. 2B). These results indicate NBO can slow the loss of occludin and claudin-5 proteins in ischemic microvessels during cerebral ischemia/reperfusion.
Figure 2. NBO reduced the loss of TJPs in isolated microvessels.
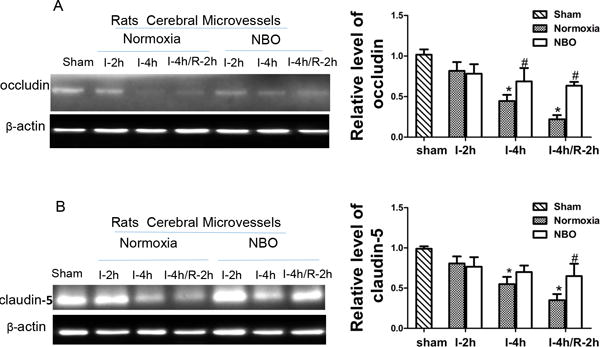
A). Representative western blots and quantitative analysis of occludin in isolated microvessels following indicated ischemia/reperfusion (I/R) time. B). Representative western blots and the quantitative analysis of the level of claudin-5 in isolated microvessels. N=4, *p<0.05 versus sham group rats; #p<0.05 versus normoxic rats with same duration of I/R. Data are expressed as means ± SEM.
NBO reduces the levels of occludin in blood sample of rats
Blood occludin in sham rats was constantly at low level at all 5 time points, showing that the microvessels were healthy and intact. The levels of blood occludin were doubled after 4-h cerebral ischemia, and then slightly fell after reperfusion but still remained significant higher than that of sham group (Fig. 3A). It is noteworthy that NBO could prevent ischemia-induced occludin increase in blood, keeping the level close to the baseline of sham group. Unlike occludin, we did not observe significant changes of blood claudin-5 in response to different cerebral ischemia durations, neither did NBO significantly affect blood claudin-5 level during ischemia/reperfusion (Fig. 3B). These results indicate that blood occludin levels mirrored the extent of BBB injury during ischemia/reperfusion, and NBO could protect BBB by suppressing occludin degradation from the microvessels that leads to reduced occludin in the blood circulation.
Figure 3. Changes of blood TJPs and effects of NBO following MCAO.
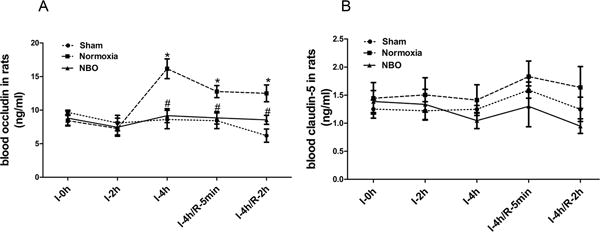
A) Levels of occludin in blood of ischemic rats. 4-h MCAO induced a significant increase of blood occludin and remained high in subsequent 5min and 2h reperfusion; NBO-treatment reduced blood occludin. N=6, *p<0.05 versus I-0h in sham group, #p<0.05 versus the normoxia rats with the same ischemic duration. B). Levels of claudin-5 in blood. No significant difference of blood claudin-5 level was observed among three groups. Data were presented as means ± SEM.
Effects of NBO on neurological scores and mortality in ischemic stroke rats
As a consequence of protecting the integrity of BBB, we expect that NBO could improve the functional outcomes in animals subjected to ischemia-reperfusion. Neurological functions in NBO or normoxia groups were measured after 2-h ischemia, 4-h ischemia and 4-h ischemia+2-h reperfusion. There was no significant difference between NBO and control groups after 2-h ischemia. However, NBO treatment improved neurological scores at 4-h MCAO and 4-h ischemia+2-h reperfusion, compared with rats in the normoxia group (Fig. 4A).
Figure 4. NBO improved the neurological score and decreased 24-h mortality rate in ischemic stroke rats.
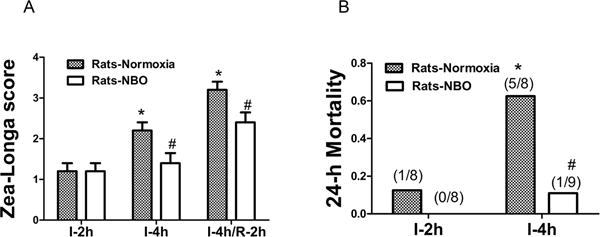
A). Neurological scores were measured at 2 h (I-2h), 4h ischemia (I-4h) and 4h ischemia plus 2 h reperfusion (I-4h/R-2h). NBO treatment significantly improved neurological scores in I-4h and I-4h/R-2h groups. N=5. B). Morality rate at 24-h post reperfusion. NBO significantly reduced 24-h mortality. N=9. *p<0.05, versus I-2h in normoxic group; #p<0.05, versus normoxia rats with the same duration of I/R. Data were presented as means ± SEM.
Mortality in both groups was determined at the end of 24-h reperfusion following 2-h or 4-h ischemia (Fig. 4B). Four-hour ischemia in normoxia resulted in a higher mortality to 62.5% (5/8) than 2-h ischemia at 12.5% (1/8). NBO significantly decreased the mortality in 4h ischemia/reperfusion rats to 11.1% (1/9). These results suggest that NBO can improve the outcome of ischemia/reperfusion rats.
The change of blood occludin and claudin-5 levels in AIS patients
Blood samples from 8 AIS patients were collected when they arrived at hospital, as well as 24 h (range, 20~28 h) and 3 d (range, 66~78 h) since symptoms appeared. Eight healthy people were chosen from Check-center for physical examination. There were no significant differences in mean age, gender, and underlying conditions (such as diabetes and myocardial infarction) in these two groups, but there was a significant difference in history of hypertension (Supplemental Table I). ELISA assay showed that the basal level of occludin was relatively low in serum of healthy volunteers. However, blood occludin in AIS patients significantly increased at admission (about 4.5h since symptoms appeared), and remained at high level up to 72 h since stroke onset (Fig. 5A). There was a trend of slight, but not statistically significant, increase in blood claudin-5 in AIS patients after stroke (Fig. 5B). These results provide direct clinical evidence that blood occludin increased in response to cerebral ischemia.
Figure 5. Blood TJPs levels in AIS patients and healthy volunteers.

A). Occludin level in blood of stroke patients at indicated time after stroke onset. B). Claudin-5 level in blood. N=8. *p<0.05 versus normal group. Data were presented as means ± SEM.
NBO reduced blood occludin and improved neurological functions in AIS patients
We further investigated whether NBO treatment can reduce blood occludin in AIS patients. There was no intracranial hemorrhage, subarachnoid hemorrhage or death occurred in either group when they were in hospital. There was no significant difference in the average age, gender, admission time, underlying conditions (such as diabetes, myocardial infarction, arterial fibrillation, history of stroke) and NIHSS scores at admission (Supplemental Table II).
ELISA assay demonstrated that blood occludin was elevated within 24 h since stroke took place and stayed at a high level till the end of this trial (1 week since stroke happened). Four-hour NBO treatment significantly reduced occludin in 24-h blood samples, when compared with patients in normoxic group. The levels of occludin in NBO-treated patients continuously went down and the effects persisted until the end of this trial (Fig. 6A).
Figure 6. NBO reduced blood occludin and improved neurological functions in AIS patients.
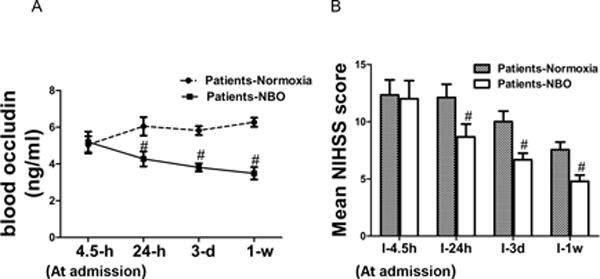
A). Blood occludin levels in normoxia and NBO-treated AIS patients. N=8. B). NIHSS scores of AIS patients. N=9. #P<0.05 versus normoxia group at the same time point. Data were presented as means ± SEM.
Neurological functions in patients in NBO and normoxia groups were compared using NIHSS scores. Neurological function in normoxia group slowly recovered during the first week, while NBO treatment accelerated the recovery at all three time points within 1 week (Fig. 6B). These clinical data illustrated the protective effects of NBO on BBB and neurological functions at early phase of AIS, as reflected by the reduced occludin levels in blood and NIHSS scores.
Discussion
The present study reports that blood occludin increased as a result of ischemia/reperfusion-induced BBB damage, not only in ischemic rats but also in AIS patients with intravenous tPA thrombolysis. Moreover, NBO could downregulate the level of blood occludin and significantly improve neurological outcomes during ischemia/reperfusion in both rats and human.
To date, intravenous tPA thrombolysis is only approved by FDA for treatment of ischemic stroke patients within a strict temporal window19. This one-size-fits-all time window prevents stroke patients with a low risk of ICH from receiving the benefits of tPA’s. Therefore, it is important to seek biomarkers, which could accurately evaluate the extent of BBB damage during cerebral ischemia/reperfusion, to guide tPA thrombolysis.
Some studies have reported about pretreatment biomarkers to guide stroke thrombolysis. However, to date, no reliable biomarkers are able to accurately predict the extent of BBB damage before tPA administration. Our recent study showed that elevated blood occludin level may be a promising biomarker for evaluating BBB during the period of ischemia in ischemic model of rats. In the present study, we investigated the level of blood occludin and claudin-5 during ischemia and reperfusion in both rats and human.
Blood occludin in rats significantly increased at 4-h MCAO, and remained at significantly higher level than sham group at 4-h MCAO with 5-min and 2-h reperfusion. At the same time, occludin was lost from the microvessels isolated from ischemic brain tissue. On the contrary, there was no significant change in blood claudin-5 after cerebral ischemia/reperfusion. These data suggest that increased blood occludin may reflect the extent of BBB damage during ischemia/reperfusion in ischemic model of rats.
We also investigated the changes of blood occludin and claudin-5 in AIS patients, who underwent intravenous tPA thrombolysis. We found that compared with healthy people, occludin was significantly increased in AIS patients’ blood samples, which were collected upon their arrival at hospital. These data suggest that ischemia-induced BBB damages are detectable when AIS patients arrived at hospital (within 4.5 after stroke) using blood occludin as a biomarker. Blood occludin in AIS patients remained at a high level at 24 h and 3 d, suggesting either that ischemia/reperfusion induced a sustained BBB damage process, or that its clearance from blood is very limited. Our findings imply that blood occludin is a promising, clinically relevant biomarker for evaluating the microvessel damage of individual patient and potentially guiding tPA thrombolysis.
We further examined whether ischemia/reperfusion-induced occludin degradation and its fragments in blood could be suppressed if given a BBB-protective intervention (i.e., NBO). Many recent studies showed that NBO given during cerebral ischemia is vaso-protective in animals. NBO can inhibit MMP-9 activity in the ischemic brain, thereby preventing occludin degradation, Evan’s blue extravasation and hemispheric swelling8, 15, 16, 20, 21. In the present study, we demonstrate that NBO treatment effectively slowed the degradation of occludin on microvessels during ischemia/reperfusion and reduced blood occludin fragments in ischemic rats. Our data in AIS patients confirm that NBO can suppress the level of occludin and its fragments in blood at 24 h, 3 d and 1 w after stroke. These data from stroke patients suggest that NBO could reduce the level of blood occludin, which reflect the decreased BBB damage in these patients.
In this study, we observed NBO’s effects on neurological outcome in animals and in patients. Our results indicate that NBO treatment significantly improved neurological outcome and reduced mortality in the ischemic model of rats. Moreover, NBO can improve neurological function of AIS patients, which is consistent with previous clinical pilot studies where NBO transiently improved stroke scale scores, reduced mortality and comorbidities in severe acute ischemic stroke patients, and attenuated ischemic lesion growth12, 13, 22. In the present study, we observed NBO’s neuro-protective effects in AIS patients who underwent thrombolysis after admission and these benefits persisted at 3 days and 1 week. These findings from a pilot study with very limited number of patients provide an important basis for further examining the potential application of NBO for BBB protection in the clinic. Larger scale clinical studies are warranted to investigate the long-term effects and optimum duration of NBO in patients with thrombolysis.
In conclusion, the results from our study suggest that blood occludin has the potential to be a biomarker of BBB damage during both ischemia and reperfusion period for both animal and human stroke. More importantly, our findings indicate that NBO may be a clinically relevant approach to protect BBB by reducing occludin degradation from microvasculature, leading to improved neurological functions for both animal and human stroke. Consequently, NBO has the potential to serve as an efficacious adjuvant therapy for tPA thrombolysis in ischemic stroke in the clinic.
Supplementary Material
Acknowledgments
Fundings: This work was partially supported by grants from National Natural Science Foundation of China (81571175, 81620108011), Beijing Nova Program (Z141107001814045), and U.S. National Institutes of Health (P30GM103400).
Footnotes
Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique identifier: NCT02974283
Disclosure/conflict of interest: None
Reference List
- 1.Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. The ninds t-pa stroke study group. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, von Kummer R, Hamann GF. Ischaemic damage of brain microvessels: Inherent risks for thrombolytic treatment in stroke. Journal of neurology, neurosurgery, and psychiatry. 1998;65:1–9. doi: 10.1136/jnnp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin X, Liu J, Yang Y, Liu KJ, Yang Y, Liu W. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiology of disease. 2012;48:309–316. doi: 10.1016/j.nbd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics. 2011;8:349–360. doi: 10.1007/s13311-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glushakova OY, Glushakov AV, Miller ER, Valadka AB, Hayes RL. Biomarkers for acute diagnosis and management of stroke in neurointensive care units. Brain Circ. 2016;2:28–47. doi: 10.4103/2394-8108.178546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, et al. Early inhibition of mmp activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. Journal of cerebral blood flow and metabolism. 2013;33:1104–1114. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. Journal of neuroscience. 2012;32:3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Hendren J, Qin XJ, Shen J, Liu KJ. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting mmp-9-mediated occludin degradation in focal cerebral ischemia. Journal of neurochemistry. 2009;108:811–820. doi: 10.1111/j.1471-4159.2008.05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan R, Yu K, Weatherwax T, Zheng H, Liu W, Liu KJ. Blood occludin level as a potential biomarker for early blood brain barrier damage following ischemic stroke. Scientific reports. 2017;7:40331. doi: 10.1038/srep40331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. Journal of cerebral blood flow and metabolism. 2007;27:1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial po2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. Journal of cerebral blood flow and metabolism. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- 12.Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- 13.Wu O, Benner T, Roccatagliata L, Zhu M, Schaefer PW, Sorensen AG, et al. Evaluating effects of normobaric oxygen therapy in acute stroke with mri-based predictive models. Medical gas research. 2012;2:5. doi: 10.1186/2045-9912-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Annals of neurology. 2005;57:571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Cetin O, et al. Normobaric hyperoxia inhibits nadph oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. Journal of neurochemistry. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Hendren J, Qin XJ, Liu KJ. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009;40:2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soejima Y, Ostrowski RP, Manaenko A, Fujii M, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hyperglycemia enhanced hemorrhagic transformation after transient mcao in rats. Medical gas research. 2012;2:9. doi: 10.1186/2045-9912-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the stroke council of the american stroke association. Stroke. 2003;34:1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 19.Jadhav AP, Jovin TG. Endovascular therapy for acute ischemic stroke: The standard of care. Brain Circ. 2016;2:178–82. doi: 10.4103/2394-8108.195283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Chen Q, Liu J, Liu KJ. Normobaric hyperoxia protects the blood brain barrier through inhibiting nox2 containing nadph oxidase in ischemic stroke. Medical gas research. 2011;1:22. doi: 10.1186/2045-9912-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang J, Qi Z, Liu W, Wang P, Shi W, Dong W, et al. Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia. Stroke. 2015;46:1344–1351. doi: 10.1161/STROKEAHA.114.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu EH, Liu CS, Tan TY, Chang KC. Venturi mask adjuvant oxygen therapy in severe acute ischemic stroke. Archives of neurology. 2006;63:741–744. doi: 10.1001/archneur.63.5.741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


