Abstract
Background
Atherosclerotic renal artery stenosis (ARAS) reduces renal blood flow (RBF) and amplifies stenotic kidney (SK) hypoxia. Revascularization with percutaneous transluminal renal angioplasty and stenting (PTRA) often fails to recover renal function possibly due to ischemia reperfusion injury (IRI) developing after PTRA. Elamipretide is a mitochondrial-targeted-peptide that binds to cardiolipin and stabilizes mitochondrial function. We tested the hypothesis that Elamipretide plus PTRA would improve renal function, oxygenation and RBF in patients with ARAS undergoing PTRA.
Methods and Results
Inpatient studies were performed in patients with severe ARAS scheduled for PTRA. Patients were treated before and during PTRA with Elamipretide (0.05 mg/kg/hour-IV-infusion, n=6) or placebo (n=8). SK-cortical/medullary perfusion and RBF were measured using contrast-enhanced multidetector-CT, and renal oxygenation by 3T BOLD-MRI before and 3 months after PTRA. Age and basal GFR did not differ between groups. BOLD-imaging demonstrated increased fractional hypoxia 24 hours after angiography and stenting in placebo (+47%) vs Elamipretide (−6%). These were reverted to baseline 3-months later. SK-RBF rose (202± 29 to 262±115 ml/min, P=0.04) 3 months after PTRA in the Elamipretide-treated group only. Over 3-months, systolic blood pressure decreased, and eGFR increased (P=0.003) more in the Elamipretide group than in the placebo group (P= 0.11).
Conclusions
Adjunctive Elamipretide during PTRA was associated with attenuated post-procedural hypoxia, increased RBF and improved kidney function in this pilot trial. These data support a role for targeted mitochondrial protection to minimize procedure-associated ischemic injury and to improve outcomes of revascularization for human ARAS.
Keywords: Renal artery stenosis, hypoxia, mitochondria and revascularization
Introduction
Atherosclerotic renal artery stenosis (ARAS) reduces renal blood flow (RBF) and ultimately accentuates tissue hypoxia1. Although the kidneys can adapt to moderate reductions in blood flow without major loss of oxygenation2, severe reductions in RBF eventually lead to vascular rarefication, inflammatory injury and tissue fibrosis, which has been designated “ischemic nephropathy”3. Severe degrees of vascular occlusion are associated with overt cortical hypoxia1, 4, oxidative stress and loss of renal function5–7. Many of these changes fail to reverse after restoring vessel patency alone8, 9.
The clinical benefits of revascularization procedures to restore blood flow in ARAS remain ambiguous. While some patients treated with stent revascularization achieve lower blood pressures and/or reduced medication requirements, kidney function infrequently improves and sometimes declines10–12. These observations raise concerns that abrupt reperfusion may induce a form of “ischemia reperfusion injury” (IRI) in the kidney.
Experimental studies in swine ARAS suggest that renal revascularization triggers the release of inflammatory cytokines such as monocyte chemoattractant protein (MCP)-113 from the stenotic kidney (SK). MCP-1 is a primary mediator of inflammation, fibrosis and microvascular rarefaction induced by hypertension14. Abrupt reperfusion can amplify tissue injury by upregulating inflammation and oxidative stress15 characterized by rapid swelling and fragmentation of mitochondria in the renal proximal tubule16, sustained energetic deficits17 and activation of cell death pathways18. Increased mitochondrial reactive oxygen species (ROS) production is thought to be a major mechanism in the pathogenesis of IRI19. ROS cause peroxidation and loss of cardiolipin20, a phospholipid found in the inner mitochondrial membrane, leading to mitochondrial dysfunction21. Kidney ischemia is recognized to induce disruptions in mitochondrial function and ATP generation that can be partially abrogated by agents that stabilize mitochondrial cardiolipin22, 23. Experimental studies have shown that cardiolipin protection before and during angioplasty (PTRA) attenuates IRI-induced apoptosis and oxidative stress in animal models13
Elamipretide (also known as MTP-131 or Bendavia) is a small peptide that targets the mitochondrial matrix independent of membrane potential, preventing peroxidation of cardiolipin15, 24, 25. Elamipretide has demonstrated potential for attenuating IRI in experimental models of acute kidney injury (AKI) and improving kidney outcomes and restoring renal function after PTRA in experimental ARAS13. However, its capability to modify outcomes after restoration RBF in humans with chronic renal ischemia is unknown. In this pilot study, we tested the hypothesis that Elamipretide infusion immediately before and during renal revascularization would improve renal function, oxygenation and RBF in ARAS patients undergoing PTRA with stenting.
Methods
Patient selection
In this phase 2a, randomized, double-blinded, placebo-controlled pilot study (Clinicaltrial.gov: NCT01755858), we enrolled fourteen patients with severe ARAS (estimated by renal artery Doppler ultrasound velocity acceleration (average Peak systolic Velocity >375 cm/sec) between December 2012 to September 2015. Patients were scheduled for renal revascularization for clinical indications including resistant hypertension (systolic hypertension >150 mm Hg and/or the use of at least two BP drugs) and/or declining renal function. Exclusion criteria were the following: serum creatinine >2.5 mg/dL, renal disease requiring dialysis, significant medical conditions (cancer, angina or stroke) within the six months before administration of the drug and serum sodium < 135 mmol/L on the day of the PTRA. Patients were admitted into the Clinical Research Unit at St. Mary’s Hospital, Rochester, MN for a 3-day inpatient protocol on two occasions (before and 3 months after renal artery revascularization), as previously described 26. All received agents blocking the renin-angiotensin system during these studies (ACE inhibitors or ARBs). Since many patients had bilateral stenosis, 21 SKs were stented and available for analysis. Three patients had unilateral atrophic kidneys, leaving 4 non-stenotic (contralateral kidneys (CLK)) available for analysis. Dietary intake was regulated at 150 mEq of sodium with an isocaloric diet prepared on site. Informed, written consent was obtained as approved by the institutional review board of the Mayo Clinic. During PTRA, patients were assigned randomly to either Elamipretide ((0.05 mg/kg/hour, N=6) or identically prepared placebo (N=8) infusion, which started 30 minutes before PTRA. All study personnel and patients were blinded to treatment.
Renal function and blood pressure measurements
The morning of first study day included measurement of GFR by iothalamate clearance (iothalamate meglumine, Conray, Mallinckrodt) after oral hydration (20 mL/kg) over three 30-minute timed collection periods27, 28. Blood pressure was measured by automated oscillometric recordings including 3 values taken 3 times daily (an automated oscillometric unit, Omron blood pressure, measured blood pressure at 5, 7, and 9 minutes after a 5-minute rest).
Renal oxygenation determined by Blood Oxygen Level Dependent (BOLD) MRI
On the afternoon of the first and third days, BOLD MRI examinations were performed on a [GE Twin Speed Signa EXCITE] 3.0T system (GE Medical Systems, Waukesha, WI) using a 12-channel torso phased array coil26. BOLD imaging consisted of a 2D fast spoiled gradient echo sequence with multiple echo times (TEs). Parametric images of R2* were generated by fitting signal intensity versus TE data to an exponential function on a voxel-by-voxel basis and solving for R2*29. After the first BOLD acquisition, furosemide (20 mg) was administered intravenously and flushed with 20 mL of saline. BOLD measurements for each kidney were repeated 15 minutes later. Analysis of BOLD data was performed by drawing parenchymal regions of interest (ROIs) on 2–4 slices through the midpole hilar region of each kidney on representative T2*-weighted images and then transferring the ROI to the corresponding R2* parametric image as previously described1. To determine the portion of measured kidney area for which tissue hypoxia was present, we evaluated “fractional tissue hypoxia” by measuring the percentage of voxels from the whole kidney ROI with R2* values above 20 sec−1 (on coronal images) which mainly represents the medulla, taking the average of all available slices30. BOLD imaging was repeated 24 hours after renal artery stent placement and during the return protocol admission three months later.
Cortical and medullary tissue perfusion and blood flow measured by Multidetector Computed Tomography (MDCT)
On the second study day, the common femoral vein was cannulated with a 6F sheath and blood samples drawn from the right and left renal veins with a 5F pigtail Cobra catheter (Cook, Inc, Bloomington, IN). The catheter was then advanced into the right atrium for central venous injection of contrast for flow studies using MDCT. For assessment of perfusion MDCT imaging was performed using a dual-source 64-slice helical MDCT scanner (SOMATOM Definition, Siemens Medical Solutions) after a bolus injection of iopamidol 370 (0.5 mL/kg up to a maximum of 40 mL). Fifteen minutes after completion of the perfusion study, a kidney volume study (5-mm thick slices) was performed in the helical mode to determine both cortical and medullary regional volumes. To calculate regional perfusions and volumes, images were reconstructed and displayed with the Analyze™ software package (Biomedical Imaging Resource, Mayo Clinic, MN, USA). ROIs were selected from cross-sectional images from the aorta, renal cortex, and medulla. Average tissue attenuation in each region was plotted over time and fitted by curve-fitting algorithms to obtain measures of renal perfusion and function as described previously31, 32.
Elamipretide infusion and safety monitoring
After completing CT imaging, patients were returned to the angiography suite where Elamipretide or placebo infusion started for 30 minutes. All procedures were performed with use of the same technique. The right common femoral artery was accessed with 18 gauge, 7-cm angiographic needle (Cook, Bloomington, Indiana). A 6F or 7F vascular sheath (Terumo, Somerset, New Jersey) was placed in the artery over a Bentsen guide wire (Cook). A 6F or 7F internal mammary guide catheter (Boston Scientific, Natick, Massachusetts) was used to engage the origin of the renal artery. Angiography was performed with 4 to 8 mL of Visipaque 320 (GE Healthcare, Princeton, New Jersey) to determine the extent of stenosis. Patients were typically given a bolus of heparin intravenously (50 U/kg of body weight) prior to the placement of the stent. The Elamipretide or placebo infusion continued for 3 hours. After the stent deployment, a completion angiogram was performed to exclude segmental or intra-renal occlusion. The total contrast volume for both MDCT and angiography was less than ~100–120 mL.
Patients were followed for the next 24 hours after PTRA with serial measurements of vital signs, serum sodium, MCP-1, interleukin-10(IL-10), kidney injury molecule (KIM-1), tumor necrosis factor (TNF-α), Insulin-like growth factor binding protein-7 (IGFBP-7) or Tissue Inhibitor of Metalloproteinases-2 (TIMP-2), and neutrophil-gelatinase associated lipocalin (NGAL). Collected samples were centrifuged, and the supernatant stored at −80°C until measurement. NGAL, KIM-1(ng/mL) were tested by ELISA according to manufacturer’s protocol (BioPorto Diagnostics, Cat# KIT 036 and R&D systems, Cat# DKM100, respectively).The samples for IGFBP-7 were diluted 1:2 and tested by ELISA (R&D systems, Cat# KIT DY1334–05). The samples for TIMP-2 were diluted 1:500 and tested by ELISA (SIGNA-ALDRICH, lot # RAB0472). Levels of TNF-α and MCP-1 were measured by luminex (Millipore, cat #: MPXHCYTO-60K). Signals were read by the Bio-plex 200 systems (BIO-RAD).
Statistical analysis
Results were expressed using mean values and standard deviation (SD) or median values (interquartile range), as appropriate. Qualitative variables were expressed as number (percentage). Since several patients had bilateral disease and stenting, our analysis accounted for the clustering within subjects by running repeated measures linear regression with a random intercept for each patient. Chi-squared test or Fisher’s exact test was used for categorical variables as appropriate. Comparisons between individual kidneys before and after treatment and changes over time in the two treatment groups were performed using repeated measures models. Percent (%) change in SK-fractional hypoxia was calculated as: [((3 month SK-%R2*>20 − baseline SK-%R2*> 20)/baseline SK-%R2*> 20) × 100%]. Statistical significance was accepted for P ≤ 0.05. Statistical analysis was performed using JMP software package version 8.0 (SAS Institute Inc., Cary, NC). This trial is registered with ClinicalTrials.gov, number: NCT01755858.
Results
Demographic comparison between ARAS patients
Complete data were available for 14 ARAS patients included in the Elamipretide (N=6) and placebo (N=8) groups. The demographic and clinical features of the patients studied are summarized in Table 1. Age, gender, serum creatinine, GFR and blood pressure were not different between groups.
Table 1.
Clinical, laboratory, and demographic data of EH and ARAS patients.
| Elamipretide (N=6) |
Placebo (N=8) |
P value | |
|---|---|---|---|
| Gender (%Men)* | 3 (50%) | 4 (50%) | 0.99 |
| Age (years) | 66.7 ± 6.8 | 72.5 ± 8.1 | 0.17 |
| Creatinine (mg/dl) | 1.58 ± 0.36 | 1.83 ± 0.52 | 0.32 |
| Iothalamate clearance (mL/min) | 46.8 ± 24.5 | 43.6 ± 12.3 | 0.99 |
| Statins (yes/no) (%)* | 5/1 (83%) | 5/3 (63%) | 0.58 |
| Number of anti-HTN drugs# | 3.5 (2, 5) | 4 (2, 6) | 0.44 |
| SBP(mmHg) | 154.2 ±16.3 | 155.2 ± 19.5 | 0.92 |
| DBP (mmHg) | 79.2 ± 9.1 | 72.5 ± 15.4 | 0.33 |
| Weight (kg) | 90.7 ± 35.3 | 83.8 ± 14.6 | 0.66 |
| BMI (kg/m2) | 33 ± 12.5 | 29.4 ± 3.9 | 0.51 |
| Hematocrit % | 37.3 ± 2.6 | 36.2 ± 2.5 | 0.42 |
Mean±SD, GFR=glomerular filtration rate, Anti-HTN=antihypertensive, SBP=systolic blood pressure, DBP=diastolic blood pressure, ARAS= atherosclerotic Renal artery stenosis, N= number of patients.
Median (range) reported due to skewed data, P value obtained from Wilcoxon rank sum test,
Fisher’s exact test.
Safety monitoring: Sequential measurements after Elamipretide infusion
All patients tolerated the single Elamipretide infusion without identified adverse clinical effects including: fever, headache, vomiting, hematuria or allergic reactions. Over the 24 hours following infusion of Elamipretide, there were no changes in serum creatinine or urine cytology.
Adjunctive Elamipretide during PTRA was associated with attenuated Post Procedural Hypoxia
Tissue oxygenation levels defined both by cortical R2* values and fractional hypoxia did not differ between groups at baseline. 24 hours after PTRA and contrast enhanced-CT, overt cortical and fractional tissue hypoxia developed in both groups in 9 patients (64%). The degree of post-stenting hypoxia was attenuated in the Elamipretide group (fractional hypoxia % R2*> 20 sec−1 from 45 ± 17 to 52.4, P= 0.42) whereas a rise was observed in the placebo group (50.9 ± 18.6 to 67.7 ± 27.1, P= 0.03) (Table 2). When expressed as percent (%) change from baseline, the SK-fractional hypoxia in the Elamipretide group was unchanged at 24 hours (−5.9%) but increased in the placebo group (47%). These levels of tissue hypoxia reversed to baseline levels at 3 months in both groups (Supplemental figure 1). Representative BOLD images (R2* parametric maps) illustrating the change in hypoxia in a SK from each treatment group before and 24 hours after PTRA are illustrated in Figure 1.
Table 2.
BOLD MRI for SK before and 24 hours after revascularization using Fractional Tissue Hypoxia and Cortical regions of interest (ROI) measurements
| Single Kidney | Elamipretide (N=10) | Placebo (N=11) | |||
|---|---|---|---|---|---|
|
| |||||
| Baseline | After 24 hours | baseline | After 24 hours | ||
| Cortex R2* (sec−1) | Pre-furosemide | 19.4 ± 2.7 | 20.1 ± 4.4 | 19.9 ± 3.4 | 24.4 ± 6.2* |
| Post-furosemide | 18.3 ±2.6# | 19.8 ± 5.1 | 19.5 ± 3.9 | 23.7 ± 6.8#* | |
| Percent change in cortex R2* at 24 hrs | Pre-furosemide | −3 (−10, 24.8) | 13 (−0.5, 31.9) | ||
| Post-furosemide | −0.8 (−14.9, 37) | 11.1 (−0.02, 39) | |||
| Fractional Hypoxia (% R2* > 20 sec−1) | Pre-furosemide | 45 ± 17 | 52.4 ± 28.9 | 50.9 ± 18.6 | 67.7 ± 27.1* |
| Post-furosemide | 31.9 ± 12.8# | 44.2 ± 26.4# | 41 ± 22.8# | 58.3 ± 36.6 | |
| Percent change in fractional hypoxia %> 20 * at 24 hrs | Pre-furosemide | −5.9 (−34.5, 96.2) | 47 (−2.2, 74.9) | ||
| Post-furosemide | 13 (−31, 133.5) | 44 (−48.4, 109.7) | |||
P<0.05 vs. baseline and
p< 0.05 vs pre furosemide (from repeated measures regression model)
Figure 1.
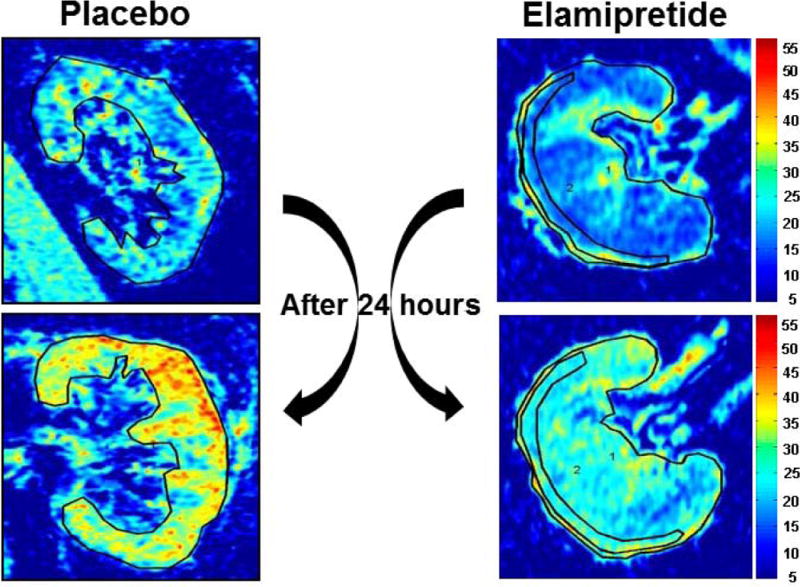
Examples of R2* parametric maps (coronal plane) for subjects with ARAS at baseline and 24 hours after contrast imaging and PTRA, obtained using the same color scale for R2*, demonstrating the transient widespread tissue hypoxia developed 24 hours after contrast imaging and renal stenting. Adjunctive Elamipretide during PTRA attenuated post procedural hypoxia
Adjunctive Elamipretide during PTRA was associated with increased RBF and Cortical Perfusion after 3 months
Results from quantitative MDCT measurements of hemodynamics for individual SKs at baseline and after three months are summarized in Table 3. The total SK volume did not change in either placebo or Elamipretide groups while total RBF and cortical blood flow increased only in the Elamipretide group, Figure 2. Cortical perfusion in the SKs rose in the Elamipretide group (from 1.99±0.8 to 2.9±1 ml/min/ml tissue, P =0.04) but remained unchanged in the placebo group.
Table 3.
Multidetector CT Measurements of individual kidney volume, tissue perfusion, blood flow and iothalamate filtration of SK
| Single Kidney- SK | Elamipretide (N=10) | Placebo (N=11) | ||
|---|---|---|---|---|
|
| ||||
| Baseline | 3 months | baseline | 3 months | |
| Total kidney volume (CT), mL | 124.7 ± 50 | 127.8 ± 54 | 115.6 ± 46.7 | 112.5 ± 46.9 |
| Cortical volume, mL | 71.6 ± 30.3 | 76 ± 32 | 68.8 ± 30.6 | 71.3 ± 30.6 |
| Medullary volume, mL | 53.1 ± 22 | 51.8 ± 23 | 46.8 ± 17.4 | 41.2 ± 17.5 |
| Cortical perfusion, mL/min per mL of tissue | 1.99 ± 0.8 | 2.9 ± 1.04* | 2.6 ± 0.9 | 2.4 ± 0.39 |
| Medullary perfusion, mL/min per mL of tissue | 0.75 ± 0.37 | 0.95 ± 0.4 | 0.92 ± 0.4 | 0.89 ± 0.2 |
| Total renal blood flow, mL/min | 202 ± 129 | 261.7 ± 115* | 234 ± 133 | 234 ± 99 |
| Cortical flow, mL/min | 157 ± 99 | 212 ± 93.1* | 189 ± 111 | 194.9 ± 87 |
| Medullary flow, mL/min | 44.6 ± 36.9 | 49.7 ± 35 | 45.1 ± 27.7 | 39 ± 15 |
P<0.05 vs. baseline (paired t test and Wilcoxon signed-rank test).
Figure 2.
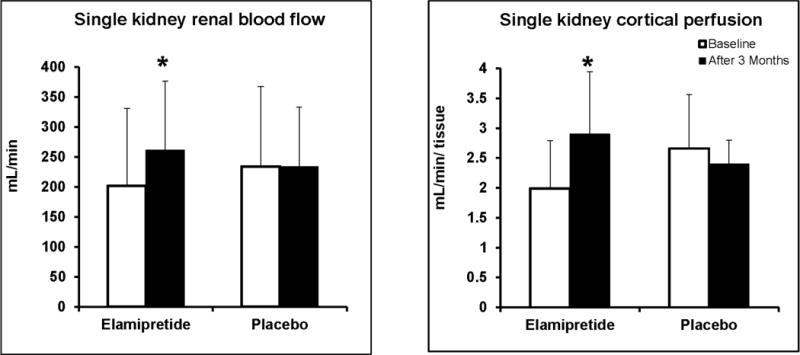
Adjunctive Elamipretide during PTRA was associated with increased cortical Perfusion (mL/min/tissue) (A) and RBF (mL/min) (B) at 3 months (P< 0.05)
Adjunctive Elamipretide during PTRA was associated with increased GFR after 3 Months
Serum creatinine decreased 3 month after PTRA (from 1.58±0.36 to 1.4±1.34 mg/dL, P= 0.0005) in the Elamipretide group and was unchanged in the placebo group (from 1.8±0.5 to 1.7±0.4 mg/dL, P=0.13). As a result, eGFR increased (from 40.7±13.4 to 46.5±15 ml/min/1.73m2, P=0.002) in the Elamipretide group and from 34.4±9.5 to 37±10.5 ml/min/1.73m2, P=0.24) in the placebo group (Figure 3). Systolic blood pressure decreased (from 154±16 to 133±16.8 mm/Hg, P=0.03) in the Elamipretide group but less (from 154±18.5 to 143.7±26, P=0.06) in the placebo group.
Figure 3.
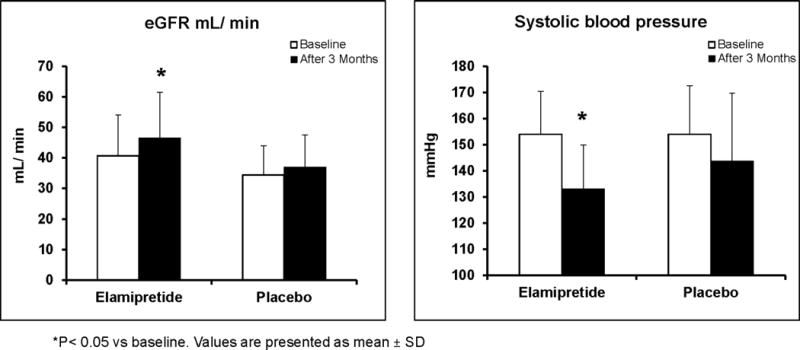
Adjunctive Elamipretide during PTRA was associated with a greater rise in eGFR and decline in systolic blood pressure after 3 Months as compared to placebo-treated subjects.
Adjunctive Elamipretide during PTRA was associated with transient increased cell-cycle arrest markers IGFBP-7 * TIMP-2
24 hours after PTRA (reperfusion), peripheral vein levels of IGFBP-7*TIMP-2 increased only in the Elamipretide group (Figure 4) but decreased again at 3 months, while NGAL levels decreased at 24 hours in both groups then rose up again to baseline levels at 3 months in both groups (Supplemental table-1). There were no changes in the IL-10, MCP-1 and TNF-α levels.
Figure 4.
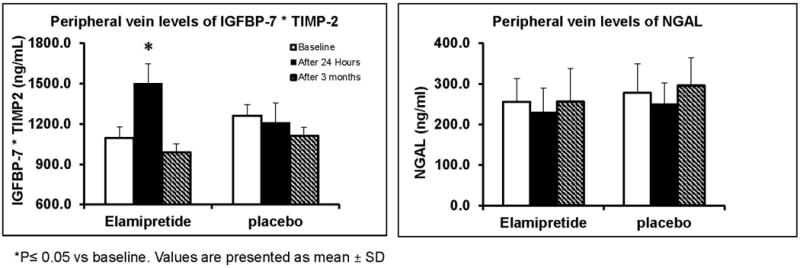
Adjunctive Elamipretide during PTRA was associated with increased peripheral vein levels of IGFBP-7*TIMP-2 at 24 hours after stenting (P< 0.05) that fell down after 3 months. NGAL levels fell transiently after 24 hours in both groups.
Discussion
The results of this phase 2a, randomized, double-blinded, placebo-controlled pilot study indicate that adjunctive intravenous Elamipretide (a mitochondria targeted peptide) during PTRA attenuated renal hypoxia developing 24 hours after contrast imaging and renal artery stent revascularization. Adjunctive Elamipretide was associated with increased cortical perfusion and RBF in the stented kidney after 3 months in patients with ARAS as compared with placebo. These changes in the Elamipretide treated patients were associated with reductions in systolic blood pressure and greater increase in total GFR. The results of this study support further development of mitochondrial-targeted therapies to limit procedure-associated hypoxia and potentially to improve kidney functional outcomes of revascularization in patients with ARAS.
Renal stenting alone often fails to restore kidney function in ARAS, as reported in multiple recent clinical trials11, 12. Our experimental studies in swine models of RAS demonstrate loss of microvascular structures33 that is magnified in the atherosclerotic environment5, 34, 35 and correlates with loss of GFR8, 36. Loss of cortical structural integrity appears in part related to oxidative stress, ATP depletion and mitochondrial damage23, 33, which do not reverse after revascularization alone8, 9. Although restoring blood flow may be needed to prevent further ischemic damage, abrupt restoration of blood flow also may injure cells that are hovering between life and death15. Abrupt reperfusion accentuates tissue injury by upregulating inflammatory signals like MCP-1, oxidative stress, or other injurious pathways13 that define IRI. Reperfusion also increases production of mitochondrial Ca2+ and ROS, opening the mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane, leading to release of cytochrome-C into the cytosol and cardiolipin peroxidation37, 38. Cardiolipin is a bisphosphatidyl glycerol lipid exclusively distributed in the inner mitochondrial membrane that regulates multiple mitochondrial activities, including electron transport chain assembly and function, ATP biosynthesis, and apoptosis39. The changes instigated during IRI in turn may induce mitochondrial dysfunction, apoptosis, inflammation, fibrosis, and renal dysfunction.
Elamipretide is a tetrapeptide that selectively concentrates in the inner mitochondrial membrane, where it binds to and stabilizes cardiolipin and prevents its peroxidation23. This facilitates electron transport and inhibits mPTP opening, attenuating apoptosis and experimental myocardial IRI23, 40. Infusion of Elamipretide during revascularization of the stenotic renal artery in swine ARAS reduces oxidative stress, tubular damage and inflammation, thereby improving revascularization outcomes. 13. Previous studies in human ARAS have identified development of widespread transient renal hypoxia lasting at least 24 h following contrast imaging and PTRA41. Our results extend the experimental studies of mitochondrial protection with Elamipretide to human subjects. These data demonstrate that Elamipretide reduced post-procedural hypoxia, as measured by BOLD MRI 24 hours after PTRA.
Remarkably, peripheral vein levels of IGFBP7 and TIMP-2 rose after imaging and PTRA in the Elamipretide group. IGFBP7 and TIMP-2 have been proposed as markers in early diagnosis and prognostic prediction in AKI42, 43. Both participate in G1 cell cycle arrest. When cell damage occurs from ischemia or sepsis, renal tubular cells enter a short period of G1 cell-cycle arrest that prevents cells from dividing until the damage has been repaired44. We speculate that the rise of IGFBP7 and TIMP-2 at 24 hours in the Elamipretide group may reflect a protective role in the face of contrast and procedural hazards. The precise actions and role of NGAL in ARAS remain incompletely understood. While often presented as a marker of acute injury, NGAL itself is an anti-inflammatory cytokine that may limit tissue injury45. Administration of NGAL provides structural and functional protection in animal models and participates in regeneration and repair processes observed after injury46. We interpret our results demonstrating a rise of G1 cell-cycle arrest markers and transient reductions in peripheral NGAL after imaging and PTRA in patients with chronic renal ischemia may have limited reperfusion injury. These changes in the Elamipretide group were associated with reduced acute changes in tissue hypoxia and no evidence of tissue injury (as reflected by NGAL and creatinine).
Taken together, we interpret the enhanced kidney perfusion, blood flow and recovery of GFR in the Elamipretide group after renal artery stenting to support a role for mitochondrial protection in this condition. These data extend results in experimental swine ARAS studies, in which mitochondrial biogenesis was upregulated in poststenotic kidneys of pigs treated with Elamipretide, whereas oxidative stress, apoptosis, microvascular loss, and tissue injury were ameliorated four weeks after revascularization13.
Administration of Elamipretide protects against reperfusion injury in several models of cardiac injury 47. It also improves post- myocardial infarction (MI) cardiac function, prevents infarct expansion and adverse left ventricular remodeling, and reduced ROS and cardiomyocytes apoptosis in the non-infarcted MI border in rats48. Experimental studies of Elamipretide infusion during renal revascularization improves myocardial mitochondrial biogenesis, cardiac function, and oxygenation, and attenuates myocardial remodelling 4 weeks later49. Elamipretide also prevents cardiac remodelling and diastolic dysfunction in a mouse model of Ang-II-induced cardiomyopathy40. These studies suggest a potential for this compound to attenuating hypertensive myocardial injury. Despite efficacy in animal models, administration of Elamipretide 10 min prior to PCI to first- time anterior ST-Elevation Myocardial Infarction (STEMI) patients was not associated with a decrease in myocardial infarct size as assessed by AUC0-72 of creatinine kinase-MB enzyme46. The reasons for the lack of benefit after cardiac reperfusion are not clear. These differences underscore potential differences in injury associated with cardiac and renal reperfusion, as well as major species effects
This study has limitations. It has relatively small number of patients. Enrolled patients were selected for revascularization based on clinical criteria. Revascularization and contrast injection were performed as part of a single procedure and either or both could contribute to development of tissue hypoxia after 24 hours. Half the patients had bilateral stenosis with slightly less renal function. The BOLD MRI parameter, R2* utilized as a marker for tissue PO2 can be affected slightly by variations in R2 (=1/T2) due to changes in water content50, although our patients were uniformly hydrated. Although the second BOLD MRI was done 24 hours after contrast administration, we cannot exclude the possibility that some contrast retention within the kidney could affect the R251. Studies of water loading in normal volunteers demonstrated a change in cortical R2 of 0.72 sec−1 while R2* fell by 1.36 sec−1. The observed cortical changes in R2* in our patients were considerably greater (averaged 4.5 sec−1) making it likely that R2 changes alone would be minor. Future studies might include R2 mapping to allow for better interpretation of changes observed with R2*50.
Conclusion
Our results indicate that transient hypoxia developing after renal artery stenting was attenuated by Elamipretide in patients with atherosclerotic renal artery stenosis. Adjunctive Elamipretide before and during PTRA were associated with increased RBF and cortical perfusion, and eGFR by three months later, that were not apparent in control subjects. These pilot data support a role for targeted mitochondrial protection to improve outcomes of PTRA for human ARAS
Supplementary Material
What is Known
Reduced kidney function from atherosclerotic renal artery stenosis often fails to recover after revascularization. Elamipretide is a mitochondria targeted peptide shown to protect against experimental ischemic renal injury and improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis.
What the Study Adds
Elamipretide during PTRA attenuated renal hypoxia developing 24 hours after contrast imaging and renal artery stent revascularization. Cortical blood flow and eGFR increased in the Elamipretide group when measured 3 months later.
Adjunctive Elamipretide during PTRA was associated with increased peripheral venous levels of G1 cell-cycle arrest markers “IGFBP-7*TIMP-2” at 24 hours after stenting.
The rise of IGFBP7 and TIMP-2 at 24 hours in the Elamipretide group may reflect a protective role in the face of contrast and procedural hazards
Acknowledgments
Sources of Funding: This project was partly supported by a grant from Stealth Peptides, Inc. R01 DK100081, DK10232, DK106427 and R01 DK 73608 from the National Institute for Digestive, Diabetic and Kidney Diseases (NIDDK), as well as Clinical and Translational Science Award (Grant UL1 RR024150) from NIH/National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not represent the official views of the NIDDK or the NIH.
Footnotes
Clinical Trial Registration: Clinicaltrial.gov: NCT01755858. URL:https://clinicaltrials.gov/ct2/show/NCT01755858
Disclosures: None.
References
- 1.Saad A, Crane J, Glockner JF, Herrmann SM, Friedman H, Ebrahimi B, Lerman LO, Textor SC. Human renovascular disease: Estimating fractional tissue hypoxia to analyze blood oxygen level-dependent mr. Radiology. 2013;268:770–778. doi: 10.1148/radiol.13122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55:961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112:1362–1374. doi: 10.1161/CIRCULATIONAHA.104.492348. [DOI] [PubMed] [Google Scholar]
- 4.Gloviczki ML, Lerman LO, Textor SC. Blood oxygen level-dependent (bold) mri in renovascular hypertension. Curr Hypertens Rep. 2011;13:370–377. doi: 10.1007/s11906-011-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 6.Chade AR, Krier JD, Rodriguez-Porcel M, Breen JF, McKusick MA, Lerman A, Lerman LO. Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol. 2004;286:F1079–1086. doi: 10.1152/ajprenal.00385.2003. [DOI] [PubMed] [Google Scholar]
- 7.Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv. 2013;6:428–435. doi: 10.1161/CIRCINTERVENTIONS.113.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in swine renal artery stenosis. Circ Cardiovasc Interv. 2012;5:720–728. doi: 10.1161/CIRCINTERVENTIONS.112.972596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv. 2013;6:428–435. doi: 10.1161/CIRCINTERVENTIONS.113.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safian RD, Madder RD. Refining the approach to renal artery revascularization. JACC Cardiovasc Interv. 2009;2:161–174. doi: 10.1016/j.jcin.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 12.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr, Dworkin LD. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, Lerman LO. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension. 2012;60:1242–1249. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Zhu X, Chade AR, Jordan KL, Lavi R, Daghini E, Gibson ME, Guglielmotti A, Lerman A, Lerman LO. Monocyte chemoattractant proteins mediate myocardial microvascular dysfunction in swine renovascular hypertension. Arterioscler Thromb Vasc Biol. 2009;29:1810–1816. doi: 10.1161/ATVBAHA.109.190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilakantan V, Hilton G, Maenpaa C, Van Why SK, Pieper GM, Johnson CP, Shames BD. Favorable balance of anti-oxidant/pro-oxidant systems and ablated oxidative stress in brown norway rats in renal ischemia-reperfusion injury. Mol Cell Biochem. 2007;304:1–11. doi: 10.1007/s11010-007-9480-z. [DOI] [PubMed] [Google Scholar]
- 16.Hall AM, Rhodes GJ, Sandoval RM, Corridon PR, Molitoris BA. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. 2013;83:72–83. doi: 10.1038/ki.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72:1493–1502. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- 20.Wiswedel I, Gardemann A, Storch A, Peter D, Schild L. Degradation of phospholipids by oxidative stress–exceptional significance of cardiolipin. Free Radic Res. 2010;44:135–145. doi: 10.3109/10715760903352841. [DOI] [PubMed] [Google Scholar]
- 21.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res. 2014;103:461–472. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates atp recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound ss-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24:1250–1261. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweetwyne MT, Pippin JW, Eng DG, Hudkins KL, Chiao YA, Campbell MD, Marcinek DJ, Alpers CE, Szeto HH, Rabinovitch PS, Shankland SJ. The mitochondrial-targeted peptide, ss-31, improves glomerular architecture in mice of advanced age. Kidney Int. 2017;91:1126–1145. doi: 10.1016/j.kint.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szeto HH, Liu S, Soong Y, Birk AV. Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Renal Physiol. 2015;308:F11–21. doi: 10.1152/ajprenal.00366.2014. [DOI] [PubMed] [Google Scholar]
- 26.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC. Comparison of 1.5 and 3 t bold mr to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009;44:566–571. doi: 10.1097/RLI.0b013e3181b4c1e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Textor SC, Turner ST. Renal vascular response to sodium loading in sons of hypertensive parents. Hypertension. 1991;17:982–988. doi: 10.1161/01.hyp.17.6.982. [DOI] [PubMed] [Google Scholar]
- 28.Wilson DM, Bergert JH, Larson TS, Liedtke RR. Gfr determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis. 1997;30:646–652. doi: 10.1016/s0272-6386(97)90488-1. [DOI] [PubMed] [Google Scholar]
- 29.Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, Grande JP, Gomez SI, Romero JC. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19:780–788. doi: 10.1681/ASN.2007040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad A, Crane J, Glockner JF, Herrmann SMS, Friedman H, Ebrahimi B, Lerman LO, Textor SC. Human renovascular disease: Estimating fractional tissue hypoxia to analyze blood oxygen level–dependent mr. Radiology. 2013;268:770–778. doi: 10.1148/radiol.13122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerman LO, Taler SJ, Textor SC, Sheedy PF, 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49:846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]
- 32.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector ct: Comparison with electron-beam ct. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 33.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney. Role of increased oxidative stress. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 34.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 35.Urbieta-Caceres VH, Lavi R, Zhu XY, Crane JA, Textor SC, Lerman A, Lerman LO. Early atherosclerosis aggravates the effect of renal artery stenosis on the swine kidney. Am J Physiol Renal Physiol. 2010;299:F135–140. doi: 10.1152/ajprenal.00159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol. 2011;300:F1394–1401. doi: 10.1152/ajprenal.00697.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 38.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saad A, Wang W, Herrmann SM, Glockner JF, McKusick MA, Misra S, Bjarnason H, Lerman LO, Textor SC. Atherosclerotic renal artery stenosis is associated with elevated cell cycle arrest markers related to reduced renal blood flow and postcontrast hypoxia. Nephrol Dial Transplant. 2016;31:1855–1863. doi: 10.1093/ndt/gfw265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmele T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, Letourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 44.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Gorlich D, Kellum JA, Zarbock A. Urinary timp-2 and igfbp7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roudkenar MH, Halabian R, Bahmani P, Roushandeh AM, Kuwahara Y, Fukumoto M. Neutrophil gelatinase-associated lipocalin: A new antioxidant that exerts its cytoprotective effect independent on heme oxygenase-1. Free Radic Res. 2011;45:810–819. doi: 10.3109/10715762.2011.581279. [DOI] [PubMed] [Google Scholar]
- 46.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 47.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, Gorman JH, 3rd, Sloan RC, Frasier CR, Watson CA, Bostian PA, Kypson AP, Brown DA. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective peptide. J Am Heart Assoc. 2012;1:e001644. doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai W, Shi J, Gupta RC, Sabbah HN, Hale SL, Kloner RA. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 2014;64:543–553. doi: 10.1097/FJC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 49.Eirin A, Williams BJ, Ebrahimi B, Zhang X, Crane JA, Lerman A, Textor SC, Lerman LO. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J Hypertens. 2014;32:154–165. doi: 10.1097/HJH.0b013e3283658a53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vivier PH, Storey P, Chandarana H, Yamamoto A, Tantillo K, Khan U, Zhang JL, Sigmund EE, Rusinek H, Babb JS, Bubenheim M, Lee VS. Renal blood oxygenation level-dependent imaging: Contribution of r2 to r2* values. Invest Radiol. 2013;48:501–508. doi: 10.1097/RLI.0b013e3182823591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenhard DC, Frisk AL, Lengsfeld P, Pietsch H, Jost G. The effect of iodinated contrast agent properties on renal kinetics and oxygenation. Invest Radiol. 2013;48:175–182. doi: 10.1097/RLI.0b013e31827b70f9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


