Abstract
Background and Purpose
There are few studies of spinal microvascular pathologies in older adults. We characterized spinal cord microvascular pathologies and examined their associations with other spinal and brain postmortem indices and parkinsonism in older adults.
Methods
We documented 3 features of microvascular pathologies in spinal cord and brain specimens from 165 deceased older participants. We also measured spinal white matter pallor. Parkinsonian signs were assessed with a modified version of the motor section of the Unified Parkinson’s Disease Rating Scale. We examined the associations of spinal arteriolosclerosis with other spinal and brain postmortem indices and parkinsonism proximate to death using regression models which controlled for age and sex.
Results
Microinfarcts and cerebral amyloid angiopathy were not observed within the spinal cord parenchyma. Spinal arteriolosclerosis was observed at all spinal levels (C7, T7, L4, S4) examined and was more severe posteriorly than anteriorly (Posterior 4.3 SD=0.72 versus Anterior 3.9 SD=0.74; t=14.58, p<0.001). Arteriolosclerosis was more severe in the spinal cord than in the brain [Cord 4.10 (SD=0.70); Brain 3.5 (0.98), t=10.39, p<0.001]. The severity of spinal arteriolosclerosis was associated with spinal white matter pallor (r=0.47, p<0.001). Spinal arteriolosclerosis accounted for about 3% of the variation in parkinsonism in models controlling for age, sex, brain arteriolosclerosis and cerebrovascular disease pathologies. Further models showed that the association of spinal arteriolosclerosis and parkinsonism was not mediated via spinal white matter pallor.
Conclusions
While the regional distribution of microvascular pathologies vary within the CNS, spinal arteriolosclerosis is common and may contribute to the severity of spinal white matter pallor and parkinsonism in older adults.
Keywords: spinal cord, microvascular pathology, small vessel disease, parkinsonism
INTRODUCTION
There is increasing recognition that microvascular brain pathologies may contribute to late-life motor impairment.1, 2 To date antemortem imaging and autopsy studies of microvascular pathologies in older adults have focused on the brain, while the spinal cord has been virtually unstudied.3 Thus, it is unknown to what degree microvascular pathologies which are common in the brain occur in the spinal To date antemortem imaging and autopsy studies of microvascular pathologies in older adults have focused on the brain, while the spinal cord has been virtually unstudied.3 Thus, it is unknown to what degree microvascular pathologies which are common in the brain occur in the spinal cord of older adults.4
To fill these gaps in our knowledge about the spinal cord, we assessed 3 microvascular pathologies including microinfarcts, cerebral amyloid angiopathy and arteriolosclerosis in spinal specimens from well-characterized older individuals participating in the Rush Memory and Aging Project.5 First, we examined the distribution of microvascular pathologies in spinal cord. Next, we examined the inter-correlation of spinal and brain arteriolosclerosis, then its associations with other indices of cerebrovascular disease and degenerative brain pathologies.
Next we sought to determine if the spinal arteriolosclerosis is associated with potential deleterious tissue or clinical consequences. First, we examined its association with spinal white matter pallor. Our prior work has shown that several indices of brain pathology when considered together only account for a small minority of the variance of parkinsonism.6, 7 Motor control networks in the cortical and subcortical brain regions extend to networks located within the spinal cord, and damage to either or both can impair motor function.8, 9 So in further analyses we examined if spinal arteriolosclerosis was independently associated with the severity of parkinsonism proximate to death.6
METHODS
Subjects
Participants were from the Memory and Aging Project approved by the Institutional Review Board of Rush University Medical Center. Each subject signed an informed consent for annual exam and donation of brain and spinal cord at the time of death.5
MAP began in 1997 and its structured postmortem exam was limited to the brain. The current study prospectively collecting spinal microvascular pathologies from decedents undergoing autopsy was added in 2012. Decedents in the current study were older at baseline, had more severe parkinsonism and lower cognitive function compared to living MAP participants. At baseline, these two groups did not differ with respect to sex, years of education or history of the number of vascular diseases and vascular risk factors (results not shown).
Parkinsonism and other Clinical Covariates
A uniform structured clinical evaluation was performed each year and included a 26-item modified version of the motor portion of the Unified Parkinson’s Disease Rating Scale and cognitive testing.6 Date of birth, sex, vascular diseases and risk factors were collected through a participant interview (Supplemental Methods).5
Neuropathological Examination
A uniform gross and microscopic postmortem examination was conducted to collect brain indices of macroinfarcts, atherosclerosis, microvascular pathologies (see below) and neurodegenerative pathologies including Alzheimer’s disease pathology, Lewy body disease pathology, and nigral neuronal loss as previously described (Supplemental Methods). Postmortem indices described below were collected from 4 levels of the spinal cord (C5/6, T7, L4/5 and S4/5). Neuropathologic evaluation was performed blinded to the clinical data, reducing the potential for bias.
Microvascular Pathology
Microscopic Infarcts
Hematoxylin and eosin- stained 6 micron sections were used to identify microscopic infarcts in midfrontal, midtemporal cortex, anterior cingulate, inferior parietal, entorhinal and occipital cortices, hippocampus, anterior basal ganglia, anterior thalamus, cerebellum, substantia nigra and 4 levels of the spinal cord. Age and location was recorded for each microscopic infarct. Only chronic microinfarcts were included in our analyses.10
Cerebral Amyloid Angiopathy (CAA)
CAA pathology was assessed in 4 levels of the spinal cord and 4 brain regions: midfrontal, midtemporal, inferior parietal, and calcarine cortices. The presence of CAA was assessed by immunohistochemistry using monoclonal antihuman-amyloid-β, 4G8 (1:9000, Covance Labs, Madison, WI). For each region, meningeal and parenchymal vessels were assessed for amyloid deposition and scored from 0 to 4 for the degree of circumferential deposition and the number of vessels involved.11 We also documented the presence or absence of capillary CAA and double-barreling in all regions examined.11
Arteriolosclerosis
We evaluated the small arterioles within the spinal cord and brain with a semiquantitative grading system from 0 (none) to 6 (severe) as previously described.10 Figure 1 shows the spectrum of arteriolosclerosis observed within the spinal cord and Figure 2 shows the similarity of arteriolosclerosis in the spinal cord and brain. We examined vessels within 4 levels of the spinal cord, in the white matter of the anterior and posterior watershed regions and the anterior basal ganglia.10 The weighted kappa statistics for interrater agreement for assessment of the severity of arteriolosclerosis was 0.47 reflecting moderate agreement.
Figure 1. Spinal Arteriolosclerosis.
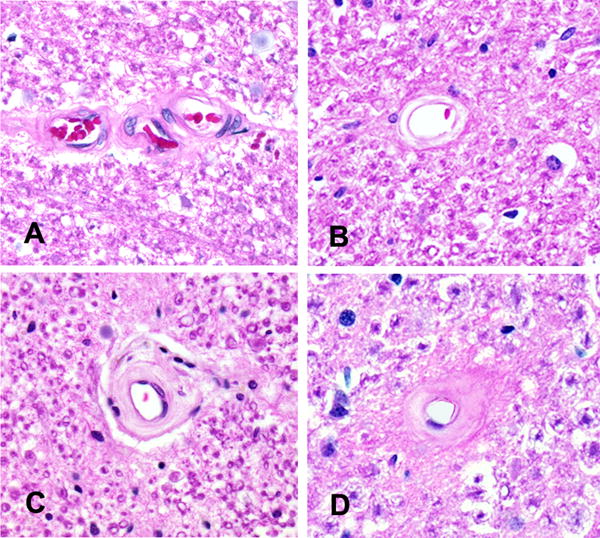
The spectrum of arteriolosclerosis observed in the cervical spinal cord is illustrated: no arteriolosclerosis, grade 0 (A), mild, grade 2 (B), moderate, grade 4 (C) and severe arteriolosclerosis, grade 6 (D). Original magnification × 200.
Figure 2. Spinal and Brain Arteriolosclerosis.
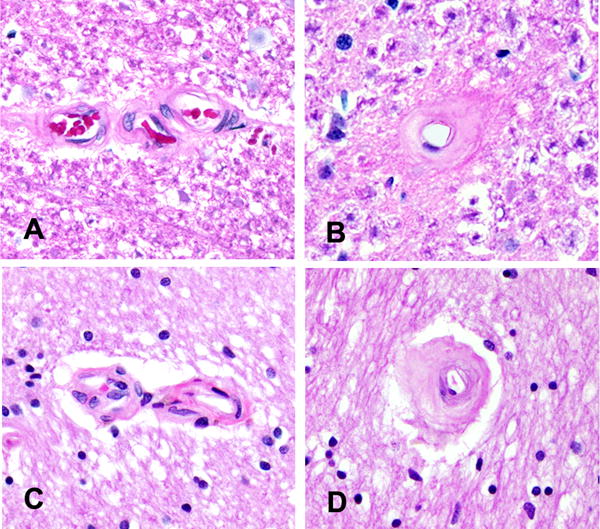
Photomicrographs depict the similarity of the arteriolosclerosis grades in the cervical spinal cord and anterior watershed region of the brain. Spinal cord photomicrographs from Figure 1 show no arteriolosclerosis, grade 0 (A) and severe arteriolosclerosis, grade 6 (B). Similar caliber vessels in the brain show no arteriolosclerosis, grade 0 (C) and severe arteriolosclerosis, grade 6 (D). Original magnification × 200.
Spinal White Matter Pallor
At each of the 4 levels of the spinal cord a semiquantitative scale from 0 (none) to 6 (severe) was used to assess the degree of pallor of the posterior column (gracillus) and the lateral corticospinal tracts bilaterally. In the cervical cord, pallor of the cuneatus was also recorded. These individual measures were used to summarize pallor for each cord level. Figure 3 illustrates the locations of the spinal regions in which white matter pallor was measured and also illustrates the spectrum of white matter pallor observed in the cervical spinal cord. The weighted kappa statistic for interrater agreement for assessment of the severity of white matter pallor was 0.86 reflecting substantial agreement.
Figure 3. Spinal White Matter Pallor.
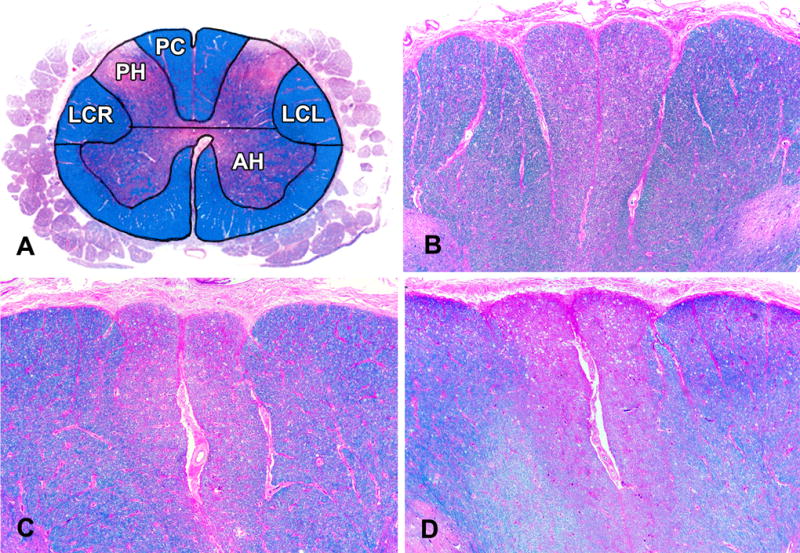
The locations of the spinal regions (PC, LCR and LCL) in which we measured white matter pallor in the cervical spinal cord (A) are shown in relation to spinal grey matter. The spectrum of white matter pallor observed in the cervical spinal cord is illustrated: mild, grade 2, (B) moderate, grade 4 (C) and severe, grade 6 (D) white matter pallor in both gracile fasiculi of the posterior columns (PC) in the Luxol Fast blue-hematoxylin and eosin stained sections. The pallor extends into the cuneate fasiculi in the cross section (D) of the cervical cord. LCR= right lateral column and LCL= left lateral column, AH= anterior horn, PH=posterior horn. Original magnification B–D × 25.
Statistical Approach
We examined the bivariate associations of spinal arteriolosclerosis and other spinal and brain post mortem indices and vascular disease and risk factors. The global parkinsonian sign score had a positively skewed distribution and was subjected to a square root transformation; the transformed scores were used in all analyses. We used a series of regression models to document the association of post-mortem indices with the severity of parkinsonian proximate to death and included terms for age, sex, and for the time from the last valid exam proximate to death. To quantify interrater reliability, weighted kappas assigned partial weight (0.9) to adjacent raters. All analyses were carried out using SAS/STAT software Version 9.3 (SAS Institute Inc., Cary, NC) on a Hewlett Packard ProLiant ML350 server running LINUX.12 Statistical significance is recognized when p<0.05.
RESULTS
Microvascular pathologies in the spinal cord
There were 165 individuals included in these analyses and their clinical characteristics proximate to death and their postmortem indices are included in Table 1.
Table 1.
Clinical and Postmortem Characteristics (N=165)
| Variable | Mean (SD) Or N (%) |
|---|---|
| Demographics | |
| Age at death (yrs) | 91.2 (5.91) |
| Sex (female) | 121(73 %) |
| White, non-Hispanic | 162 (98 %) |
| Education (yrs) | 14.6 (3.04) |
| MMSE (score of 30) | 20.2 (9.47) |
| Vascular Diseases & Risk Factors | |
| BMI | 25.6 (5.29) |
| Hypertension | 104 (63%) |
| Diabetes | 34 (21%) |
| Smoking (current or former) | 62 (38%) |
| Claudication | 41 (25%) |
| Myocardial infarction | 28 (17%) |
| Congestive heart failure | 25 (16%) |
| Stroke | 25 (16%) |
| Postmortem | |
| Time from last exam (yrs) | 1.15 (1.56) |
| Time of Death to autopsy (hrs) | 8.8 (5.23) |
| Cerebrovascular brain pathologies | |
| Macroinfarcts present | 59 (36%) |
| Microinfarcts present | 54 (33%) |
| Atherosclerosis (moderate-severe) | 12 (7%) |
| Cerebral amyloid angiopathy (moderate-severe) | 51 (31%) |
| Arteriolosclerosis (moderate-severe) | 40 (24%) |
| Degenerative brain pathologies | |
| Lewy body disease present | 45 (27%) |
| Nigral neuronal loss (moderate-severe) | 22 (13%) |
| Alzheimer’s disease (Reagan moderate to high) | 117 (71%) |
While microinfarcts are common in the brain (Table 1), none were observed in the spinal cord.
CAA was common in the brain (Table 1) and was documented in a minority of meningeal vessels (cervical 9/160, 6%; thoracic 7/152, 5%; lumbar 7/158, 4%; sacral 7/140, 5%). Parenchymal, double-barreling and capillary CAA were not observed in the spinal cord.
Arteriolosclerosis was common in the brain (Table 1) and was observed at all 4 levels of the spinal cord. The severity in the spinal cord ranged from 2 to 6 with an average value of 4.17 (SD=0.83). In more than 50% of participants, the severity of anterior and posterior spinal arteriolosclerosis was rated the same. When the ratings differed, posterior spinal arteriolosclerosis was almost always more severe (Table I). These findings and further analyses support combining severity measures into a summary spinal measure (Supplemental Methods). The average severity for spinal arteriolosclerosis based on all sites examined was 4.10 (SD=0.70). Spinal arteriolosclerosis was not associated with age of death (r=0.12, p=0.131) or sex (t163=0.04, p=0.967).
Associations of spinal arteriolosclerosis with other cerebrovascular and degenerative indices of brain pathology
Spinal and brain arteriolosclerosis showed a moderate association (r = 0.23, p=0.004), but spinal arteriolosclerosis was more severe than brain arteriolosclerosis [Spinal 4.1 SD= (0.70) versus Brain: 3.5 (SD=0.98), t= 10.38, p<0.001]. Figure I contrasts the heterogeneity of arteriolosclerosis in spinal cord and brain.
In contrast, spinal arteriolosclerosis was not related to other cerebrovascular pathologies (macroinfarcts, microinfarcts, atherosclerosis, cerebral amyloid angiopathy) or to indices of other degenerative brain pathologies (AD pathology, Lewy bodies and nigral neuronal loss). (all p’s> 0.200; Table II).
Clinical risk factors, spinal and brain arteriolosclerosis
Brain arteriolosclerosis were not associated with clinical vascular diseases and risk factors, although spinal arteriolosclerosis was associated with higher BMI and smoking (Table III).
Spinal arteriolosclerosis and spinal white pallor
At all 4 levels of the spinal cord, white matter pallor was more severe in the posterior column as compared to the lateral corticospinal tract. In the cervical region within the posterior column the cuneatus was intermediate in severity between the gracilis and lateral corticospinal tract at the same level (Table IV).
Mean white matter pallor ranged from 2.7 at the sacral level to 3.7 in the lumbar cord and was intermediate for the cervical and thoracic levels. Further analyses supported combining severity assessments from all 4 spinal cord levels into a composite white matter pallor severity measure (Supplemental Methods). The average white matter pallor for all 4 spinal cord levels was 3.4 (SD=0.61) and was not associated with age at death (r=0.08, p=0.303) or sex (t163=−0.65, p=0.520). A scatter plot (Figure 4a) illustrates that the severity of spinal arteriolosclerosis was related to the severity of white matter pallor (r=0.47, p<0.001). Although structural spinal abnormalities can cause spinal damage, spinal arteriolosclerosis was not related to the history of cervical radiculopathy (r=0.08, p=0.328).
Figure 4. Spinal Arteriolosclerosis is Associated with White Matter Pallor and Parkinsonism in Older Adults.
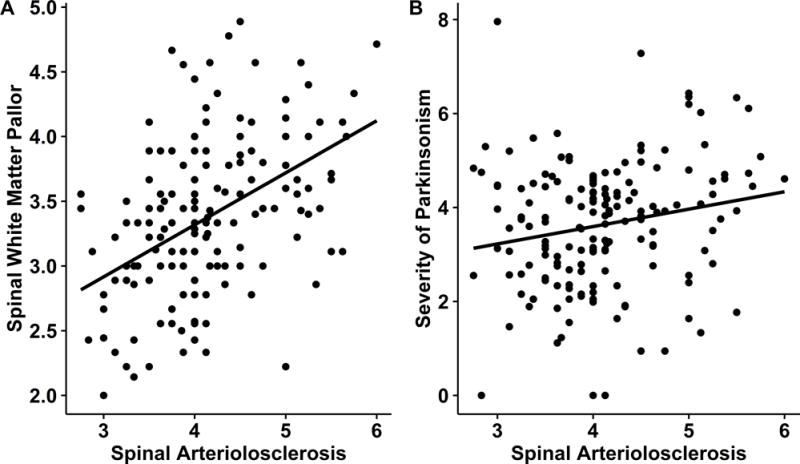
On the left (A), a scatter plot illustrates that more severe spinal arteriolosclerosis is associated with more severe spinal white matter pallor. On the right (B) a scatter plot shows that more severe spinal arteriolosclerosis is related to more severe parkinsonism proximate to death.
Spinal arteriolosclerosis and parkinsonism proximate to death
The severity of spinal arteriolosclerosis was associated with the severity of clinical parkinsonism proximate to death, is illustrated in a scatter plot in Figure 4b. Spinal arteriolosclerosis accounted for an additional 3% of the of the variance of parkinsonism when controlling for age, sex and time from the last exam to death (Table 2, Model A). In contrast brain arteriolosclerosis was not associated with parkinsonism in a model alone. (Table 2, Models B). In contrast, spinal arteriolosclerosis was independently associated with parkinsonism in models which included terms for brain arteriolosclerosis (Table 2, Model C) other cerebrovascular disease pathologies (Table 2, Model D) and other degenerative brain pathologies (Table 2, Model E).
Table 2.
Spinal Arteriolosclerosis, Other Brain Pathologies and Parkinsonism Proximate to Death
| Postmortem Indices | Term | Model A | Model B | Model C | Model D | Model E | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | S.E | Sig. | Est. | S.E | Sig. | Est. | S.E | Sig. | Est. | S.E | Sig | Est. | Est. | Est. | ||
| Arteriolosclerosis | Spinal arteriolosclerosis | 0.361 | 0.148 | 0.016 | 0.316 | 0.151 | 0.038 | 0.330 | 0.148 | 0.027 | 0.356 | 0.14 | 0.018 | |||
| Brain arteriolosclerosis | 0.196 | 0.106 | 0.432 | 0.146 | 0.108 | 0.177 | ||||||||||
| Brain Cerebrovascular Pathologies | Macroinfarcts | 0.192 | 0.222 | 0.389 | ||||||||||||
| Microinfarcts | −0.352 | 0.222 | 0.116 | |||||||||||||
| Cerebral Amyloid (CAA) | −0.081 | 0.093 | 0.386 | |||||||||||||
| Atherosclerosis | 0.208 | 0.184 | 0.259 | |||||||||||||
| Brain Degenerative Pathologies | Lewy body pathology | 0.053 | 0.282 | 0.852 | ||||||||||||
| Nigral neuronal loss | 0.115 | 0.157 | 0.465 | |||||||||||||
| AD pathology | 0.150 | 0.168 | 0.373 | |||||||||||||
| Adjusted R- squared | 2.98% | 1.44% | 3.48% | 3.80% | 2.22% | |||||||||||
Each model (A–E) represents a separate regression model with the Estimate (Est.), Standard Error (S.E) and p-Value (Sig.) for each predictor with parkinsonism as the outcome. Models A and B contrast the associations of spinal and brain arteriolosclerosis alone with parkinsonism. Additional models include terms for other brain pathologies with spinal arteriolosclerosis (C–E). All models included terms for age, sex and time from last exam to death (results are not shown). The bottom row shows the Adjusted R- squared which is an estimate of the percentage of the variance of parkinsonism accounted for by all the terms for postmortem indices included in each model (A–E) after subtracting the percentage variance due to age, sex and the time from the last exam to death.
The pathologic basis for parkinsonism in older adults without a clinical diagnosis of PD may vary from individual’s with a clinical diagnosis of PD.6 In a sensitivity analysis, we repeated the analyses described above to establish that spinal arteriolosclerosis remained associated with the severity of parkinsonism when we excluded 6 cases with a diagnosis of clinical PD proximate to death (Table V, Models A–E).
Prior antemortem brain imaging studies have suggested that small vessel disease manifested by white matter brain abnormalities is associated with late-life motor impairments.13 Thus, the association of spinal arteriolosclerosis with clinical parkinsonism could be attenuated when a term for spinal white pallor is included in the same model. In a regression model which controlled for age and sex, spinal white matter pallor was not associated with parkinsonism proximate to death (Estimate 0.220, S.E., 0.174, p=0.207). Spinal arteriolosclerosis remained associated with parkinsonism in a model including a term for spinal white matter pallor (Estimate 0.347, S.E., 0.168, p=0.040).
DISCUSSION
Microvascular pathologies in the spinal cord has been almost entirely unstudied. We documented indices of microvascular pathologies in spinal cord and brain specimens from 165 deceased older participants with motor assessments proximate to death. Microinfarcts and cerebral amyloid angiopathy were common in brain, but were not observed within the spinal cord parenchyma. In contrast, arteriolosclerosis was common at all levels of the spinal cord and associated with brain arteriolosclerosis. Support for the deleterious effects of spinal arteriolosclerosis is suggested by its association with the severity of white matter pallor and its independent association with the severity of parkinsonism proximate to death. These data suggest that while the regional distribution of microvascular pathologies may vary within the CNS, spinal arteriolosclerosis is common and may contribute to the severity of spinal white matter pallor and parkinsonism in older adults.
The increased recognition of the importance of microvascular pathologies and late-life motor impairment in older adults derives almost exclusively from brain imaging and postmortem studies of the brain, while studies of the spinal cord of well-characterized older adults are lacking.1, 14–16 There are a handful of prior studies which have examined postmortem indices of spinal cord in older adults, but these examined a small numbers of cases, or have generally focused on larger arteries such as the anterior spinal artery or meningeal arteries.3, 17, 18 The current study provides novel data about the accumulation of microvascular pathologies in spinal cords of older adults and lends support to several observations made in past smaller autopsy studies. While microinfarcts and CAA were common in the brains of older adults,6, 7, 10 microinfarcts were not observed within the spinal cord segments which we examined. Thus, although an individual might have clinical vascular diseases and risk factors and documented brain microinfarcts, no spinal microinfarcts were observed as noted in prior smaller studies.17 Moreover, CAA, which is common in brains from older adults, did not affect intramedullary spinal vessels, but was noted in 5% of meningeal vessels.
In contrast to microinfarcts and CAA, we observed arteriolosclerosis in both the brain and at all levels of the spinal cord. In fact, the severity of small vessel disease in the same individual appeared more severe in the spinal cord than in the brain. While it can be difficult to conclusively differentiate between arterioles and venules, both are important components of the microvascular network underlying the blood-brain/spinal barrier.19,20 The dissociation between the presence of spinal arteriolosclerosis and absent spinal microinfarcts, underscores the necessity for further work to explicate alternative mechanisms through which arteriolosclerosis might contribute to age-related spinal cord changes including but not limited to neuronal loss and demyelination.3, 17, 18 Some have suggested that the lack of spinal microinfarcts derived from the extensive collaterals present in the spinal cord as compared to subcortical brain regions, but this suggestion does not account for the lack of microinfarcts in known spinal watershed regions. The absence of microinfarcts in the spinal cord despite arteriolosclerosis suggests that microinfarcts are not the inevitable consequence of arteriolosclerosis and underscore important gaps in our understanding about the pathogenesis of microinfarcts within the CNS.14 Nonetheless, in the absence of microinfarcts, i.e. frank spinal tissue damage, other local mechanisms leading to spinal cord degeneration such as oxidative stress, inflammation or other non-ischemic mechanisms as well as spinal changes secondary to brain lesions will need to be explored.21–24 The current data suggest that microvascular pathologies within the CNS are heterogeneous and further studies are needed to elucidate which factors account for the observed regional differences.3, 17, 18, 25
Documenting spinal arteriolosclerosis does not inform on its tissue or clinical consequences. The severity of spinal arteriolosclerosis was associated with the degree of spinal white matter pallor. Posterior column demyelination documented in some prior reports has been considered secondary due to axonal or regional nerve root loss.26 The current study focused on microvascular pathologies and It will be important to assess the loss of axons and ganglion cells to determine if the pallor which was observed is primary or secondary demyelination.18 In the absence of microinfarcts, non-ischemic mechanisms which may be mediated via extracellular matrix inflammation may underlie the association of spinal arteriolosclerosis and white matter pallor.24
Further evidence suggesting that spinal arteriolosclerosis may have a deleterious effect on health, is the finding in the current analyses that it is independently associated with the severity of parkinsonism proximate to death. This finding was robust and remained significant when controlling for brain arteriolosclerosis, several vascular brain pathologies and spinal white matter pallor. Although spinal arteriolosclerosis was associated with both spinal white matter pallor and parkinsonism, spinal white matter pallor was not associated with parkinsonism proximate to death. These results suggest that other unmeasured cellular elements or factors may link spinal arteriolosclerosis with the severity of parkinsonism proximate to death. These data also suggest that there may be several mechanisms through which spinal arteriolosclerosis contributes to tissue damage and adverse health consequences. These findings need to be replicated by others and longitudinal studies are needed to determine the directionality of these cross sectional associations.
In the current study, spinal arteriolosclerosis alone accounted for about 3% of the variance of parkinsonism when controlling for brain arteriolosclerosis, other cerebrovascular indices and multiple degenerative brain pathologies. To appreciate these findings, it is worth noting that although our prior work has shown that together brain pathologies accounted for less than 10% of the variance of parkinsonism proximate to death in older adults without Parkinson’s disease.6, 7, 10 Since motor control systems extend beyond the brain through the CNS to the spinal cord the additional variance of parkinsonism accounted for by spinal arteriolosclerosis in the current study is not surprising. Moreover, it suggests that other motor related structures within and outside the CNS such as cerebellum or muscle are likely to make separate contributions to the severity of parkinsonism in older adults.7, 27 The current spinal data extends prior brain imaging and autopsy studies by providing evidence that indices of vascular pathology in both spinal cord as well as brain may contribute to parkinsonism in many older adults.7, 10, 28 Thus, the clinical phenotype of parkinsonism in older adults is not simply an early manifestation of PD, but this common clinical phenotype derives from diverse neuropathologies including AD, PD and CNS cerebrovascular pathologies.6, 29–32 Thus, like clinical AD dementia, the phenotype of parkinsonism in older adults may be commonly caused by mixed neuropathologies.16 Finally, the finding that spinal arteriolosclerosis may be a common unrecognized cause of parkinsonism in older adults, suggests that the health burden of CNS microvascular pathologies may be underestimated in our aging population.1, 33 Approaches which allow for antemortem identification and treatment of at risk individuals are needed to prevent late-life motor impairment.2, 14
There are several limitations to the current study. The cases we studied are from a selected cohort. It will be important to investigate these findings in more diverse cohorts. While we examined three features of microvascular pathologies which can only be assessed via histopathology, several other features including expanded Virchow-Robin spaces and microbleeds were not systematically assessed. Modest interrater agreement for severity of arteriolosclerosis is a limitation. Identifying unmeasured confounding pathologies is always a concern in observational studies. Although spinal white matter pallor was assessed and did not link the association of spinal arteriolosclerosis with parkinsonism, further work is needed to evaluate whether the preferential posterior column involvement may manifest as sensory loss that might contribute to other clinical impairments such as urinary incontinence, balance or increased motor variability in older adults. Moreover, spinal white matter assessments in this study were not compared to rostral white matter measures in the brain and brainstem or with musculoskeletal or clinical measures of cervical myelopathy. More precise measures to quantify white matter tissue integrity and tractography are needed to more fully assess the tissue and clinical consequences of spinal arteriolosclerosis.
There are several strengths to the study, including the community-based cohort with large numbers of women and men coming to autopsy following high rates of clinical follow-up and high autopsy rates. Uniform structured clinical procedures were used that included a detailed assessment of parkinsonian signs that has been widely used in other studies.
Supplementary Material
Acknowledgments
We thank all the participants in the Memory and Aging Project. We also thank the staff of the Rush Alzheimer’s Disease Center.
Disclosures: ASB and SN are supported by grants from the NIH; VV: NIH, Judy Goldberg Foundation; SEL: NIH/NIA; JAS: NIH and Advisory Board-Eli Lily; DAB: NIH, has received honoraria for non-industry sponsored lectures; has served as a paid consultant to Danone, Inc., Wilmar Schwabe GmbH & Co., Eli Lilly, Inc., Schlesinger Associates, and the Gerson Lehrman Group.
Funding Sources For Study: Supported by the NIH grants R01AG17917, R01NS708009, R01AG43379 the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
References
- 1.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular Contributions to Cognitive Impairment and Dementia. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg SM. Small Vessels, Big Problems. The New England Journal of Medicine. 2006;354:1451–1453. doi: 10.1056/NEJMp068043. [DOI] [PubMed] [Google Scholar]
- 3.Jellinger K. Spinal cord arteriosclerosis and progressive vascular myelopathy. J Neurol Neurosurg Psychiatry. 1967;30:195–206. doi: 10.1136/jnnp.30.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, et al. Aging, the Central Nervous System, and Mobility. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68:1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and Findings From the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA. Parkinsonism in Older Adults and Its Association With Adverse Health Outcomes and Neuropathology. J Gerontol A Biol Sci Med Sci. 2016;71:549–556. doi: 10.1093/gerona/glv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71:258–266. doi: 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine AJ, Lewallen KA, Pfaff SL. Spatial organization of cortical and spinal neurons controlling motor behavior. Curr Opin Neurobiol. 2012;22:812–821. doi: 10.1016/j.conb.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothwell JC. Overview of neurophysiology of movement control. Clin Neurol Neurosurg. 2012;114:432–435. doi: 10.1016/j.clineuro.2011.12.053. [DOI] [PubMed] [Google Scholar]
- 10.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular Disease Pathology and Parkinsonian Signs in Old Age. Stroke. 2011;42:3183–3189. doi: 10.1161/STROKEAHA.111.623462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85:1930–1936. doi: 10.1212/WNL.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAS/STAT® User’s Guide, Version 9.3 [computer program] Version 9.3. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 13.Pantoni L, Fierini F, Poggesi A. Impact of cerebral white matter changes on functionality in older adults: An overview of the LADIS Study results and future directions. Geriatrics & gerontology international. 2015;15(Suppl 1):10–16. doi: 10.1111/ggi.12665. [DOI] [PubMed] [Google Scholar]
- 14.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017 doi: 10.1093/brain/awx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017 doi: 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Hashizume Y, Yoshida M, Inagaki T, Kameyama T. Pathological changes of the spinal cord in centenarians. Pathology International. 1999;49:118–124. doi: 10.1046/j.1440-1827.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- 18.Bailey AA. Changes with age in the spinal cord. AMA archives of neurology and psychiatry. 1953;70:299–309. doi: 10.1001/archneurpsyc.1953.02320330024003. [DOI] [PubMed] [Google Scholar]
- 19.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm I, Nyul-Toth A, Suciu M, Hermenean A, Krizbai IA. Heterogeneity of the blood-brain barrier. Tissue barriers. 2016;4:e1143544. doi: 10.1080/21688370.2016.1143544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson JE, Hosny O, Wharton SB, Heath PR, Holden H, Fernando MS, et al. Microarray RNA Expression Analysis of Cerebral White Matter Lesions Reveals Changes in Multiple Functional Pathways. Stroke. 2009;40:369–375. doi: 10.1161/STROKEAHA.108.529214. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy R, Shekhtman A, Schmidt AM. The multiple faces of RAGE – opportunities for therapeutic intervention in aging and chronic disease. Expert opinion on therapeutic targets. 2016;20:431–446. doi: 10.1517/14728222.2016.1111873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garbuzova-Davis S, Haller E, Tajiri N, Thomson A, Barretta J, Williams SN, et al. Blood-Spinal Cord Barrier Alterations in Subacute and Chronic Stages of a Rat Model of Focal Cerebral Ischemia. J Neuropathol Exp Neurol. 2016;75:673–688. doi: 10.1093/jnen/nlw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond) 2017;131:425–437. doi: 10.1042/CS20160604. [DOI] [PubMed] [Google Scholar]
- 25.Hashizume Y, Yoshida M, Wang Y, Kume A, Kameyama T, Ando T, et al. Pathology of spinal vascular disease. Neuropathology. 1997;17:58–66. [Google Scholar]
- 26.Wang Y, Hashizume Y, Yoshida M, Inagaki T, Kameyama T. Pathological changes of the spinal cord in centenarians. Pathol Int. 1999;49:118–124. doi: 10.1046/j.1440-1827.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- 27.Buchman AS, Nag S, Shulman JM, Lim AS, VanderHorst VG, Leurgans SE, et al. Locus coeruleus neuron density and parkinsonism in older adults without Parkinson’s disease. Mov Disord. 2012;27:1625–1631. doi: 10.1002/mds.25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis ED, Brickman AM, DeCarli C, Small SA, Marder K, Schupf N, et al. Quantitative Brain Measurements in Community-Dwelling Elderly Persons With Mild Parkinsonian Signs. Arch Neurol. 2008;65:1649–1654. doi: 10.1001/archneurol.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchman AS, Leurgans SE, Yu L, Wilson RS, Lim AS, James BD, et al. Incident parkinsonism in older adults without Parkinson disease. Neurology. 2016;87:1036–1044. doi: 10.1212/WNL.0000000000003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korczyn AD. Vascular parkinsonism–characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11:319–326. doi: 10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- 31.Vizcarra JA, Lang AE, Sethi KD, Espay AJ. Vascular Parkinsonism: deconstructing a syndrome. Mov Disord. 2015;30:886–894. doi: 10.1002/mds.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg D, Lang AE, Postuma RB, Maetzler W, Deuschl G, Gasser T, et al. Changing the research criteria for the diagnosis of Parkinson’s disease: obstacles and opportunities. The Lancet Neurology. 2013;12:514–524. doi: 10.1016/S1474-4422(13)70047-4. [DOI] [PubMed] [Google Scholar]
- 33.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. Journal of Neurology, Neurosurgery & Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


