Abstract
Objectives
PSMA (prostate-specific membrane antigen) is overexpressed in prostate cancer cells and is reported to be a promising target for antibody-based radioligand therapy in patients with metastasized prostate cancer. Since PSMA expression is not restricted to prostate cancer, the underlying study investigates PSMA expression in non-small cell lung cancer (NSCLC).
Material and methods
Immunohistochemistry was used to identify PSMA expression in n = 275 samples of NSCLC tissue specimens. By means of CD34 co-expression, the level of PSMA expression in tumor associated neovasculature was investigated. The impact of PSMA expression on clinicopathologic parameters and prognosis was evaluated.
Results
PSMA tumor cell expression in NSCLC is as low as 6% and was predominantly found in squamous cell carcinoma (p = 0.002). Neovascular PSMA expression was found in 49% of NSCLC. High neovascular PSMA expression was associated with higher tumor grading (G3/G4) (p < 0.001). Neither for PSMA tumor cell expression, nor for PSMA neovascular cell expression prognostic effects were found for the investigated NSCLC cases.
Conclusion
Here, we report on the expression of PSMA in NSCLC tissue samples. Against the background of a potential treatment with radiolabeled PSMA ligands, our data might serve for the future identification of patients who could benefit from this therapeutic option.
Introduction
The fact that patients are often diagnosed with late-stage and/or metastatic disease contributes to the high mortality of lung cancer patients. Since lung cancer is the most frequent cause of cancer-related death, it is necessary to improve both our diagnostic and our therapeutic armamentarium to overcome its high lethality and its poor prognosis[1]. PSMA (prostate-specific membrane antigen) is a type II transmembrane glycoprotein with influence on the activity of folate hydrolase and neurocarboxypeptidase[2, 3]. Initially, upregulation of PSMA was found in prostate cancer cells. Consequently, PSMA-based imaging technologies for the detection of metastatic disease in advanced-stage prostate cancer patients were developed and PSMA-based radioligand therapy was introduced as a therapeutic regimen in metastasized prostate cancer[3–10].
In addition, PSMA is expressed in the endothelium of tumor-associated neovasculature of various solid malignancies (i.e. breast, lung and urothelial cancer), possibly due to tumor-associated angiogenic factors[11–15]. Functionally, PSMA might facilitate endothelial cell sprouting and invasion through its regulation of lytic proteases that have the ability to cleave the extracellular matrix[6, 16]. Moreover, the expression of PSMA in malignancy-associated neovasculature bears the possibility of specific antibody-based therapies.
The current study has its focus on the expression of PSMA in a comprehensive lung cancer study cohort of n = 275 patients. In the context of PSMA-targeted anti-tumor therapies, both neovascular and tumor cell PSMA expression were evaluated.
Methods
Study population
Lung cancer tissue samples and the corresponding clinical follow-up data of n = 275 curatively resected NSCLC patients (217 NSCLC patients from the Thoracic Departments in Ostercappeln, Germany; 58 NSCLC patients from the University Hospital Mainz) were collected and examined. Patients with stage IV, neoadjuvant treatment, R1 or R2 resection status, or with non-specified tumor histology (e.g. NSCLC not otherwise specified) were excluded from our analysis. All tissue samples are embedded in tissue microarrays. The original patient data set contains 304 patient samples. Due to loss of tissue sections from the arrays, immunohistochemical evaluation was not possible in 29 cases. Hence, 275 NSCLC patients were included for further evaluation. Approval of the study by the Ethical Committees in Mainz [837.188.99 (2133)] and Münster [Az 2016-445-f-S and Reg.Nr.:4XMüller1] were obtained. All data were fully anonymized before they were accessed and the local ethics committee waived the requirement for informed consent. Clinical TNM staging (including clinical examination, CT scans, sonography, endoscopy, MRI, bone scan) was based on IUCC/AJCC recommendations. In terms of definite tumor staging, pathological exploration was carried out post-surgically. Primary pulmonary lesions were pathologically classified based on the WHO 2004 guidelines; 121 specimens were classified as squamous cell carcinoma, 112 as adenocarcinoma and 42 as large cell carcinoma. Survival time was either computed from the date of histological diagnosis to death or censored at the date of last contact. Baseline information of the NSCLC population is shown in Table 1.
Table 1. Baseline characteristics of the NSCLC study population.
| Parameter | n | % of non-missing values |
|---|---|---|
| Age (mean) | 66.1 years | |
| Sex | ||
| Female | 66 | 24 |
| Male | 209 | 76 |
| Tumor cell PSMA expression | ||
| SI 0 | 258 | 94 |
| SI 1 | 11 | 4 |
| SI 2 | 5 | 2 |
| SI 3 | 1 | 1 |
| Neovascular PSMA expression | ||
| SI 0 | 140 | 51 |
| SI 1 | 92 | 34 |
| SI 2 | 39 | 14 |
| SI 3 | 4 | 2 |
| Performance status (WHO ECOG | ||
| ECOG 0 | 47 | 18 |
| ECOG 1 | 202 | 77 |
| ECOG 2 | 14 | 5 |
| Tumor Histology | ||
| Adenocarcinoma | 112 | 41 |
| Large cell carcinoma | 42 | 15 |
| Squamous cell carcinoma | 121 | 44 |
| Tumor stage | ||
| UICC Tumor stage I | 188 | 68 |
| UICC Tumor stage II | 60 | 22 |
| UICC Tumor stage III | 27 | 10 |
| Grading | ||
| G1 | 6 | 2 |
| G2 | 94 | 35 |
| G3 | 138 | 51 |
| G4 | 31 | 12 |
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 4-μm-thick paraffin sections using the peroxidase-conjugated avidin-biotin method. Primary antibodies included a monoclonal mouse anti-PSMA antibody (clone 3E6, Ventana, Germany, 1:50 dilution), monoclonal anti-CD34 antibody (clone QBEnd10, Ventana, Germany, ready to use concentration of 0.8μg/ml) and anti-CD31 antibody (clone JC70, Cell Marque, United States, concentration of 0.61μg/ml). In brief, sections were deparaffinized in xylene and rehydrated through graded ethanol at room temperature. Incubation with the primary antibodies was performed for 30 minutes at room temperature. After washing, the paraffin sections were incubated with biotinylated secondary antibodies. Immunoreactions were visualized using a 3-amino-9-ethylcarbazole as a substrate (Ventana Optiview DAB IHC detection KIT, Germany). Prostate carcinoma tissue sections served as a positive control. Several anti-PSMA antibodies are currently available and the most commonly used subclones include 7E11 and 3E6. We decided to use clone 3E6 as it targets an extracellular epitope of PSMA. For imaging or therapeutic purposes only antibodies targeting extracellular domains can be potentially used as they have to be internalized by living cells.
Assessment of PSMA expression
PSMA expression was evaluated by two pathologists (BH and KS). In lung cancer, tumor cells and associated neovascular endothelium were analyzed separately, and the identity of vascular structures was confirmed by CD34 coexpression, a marker for endothelial cells among others[17–19]. Any reactivity in either tumor cells or neoplastic vessels was considered positive. Staining intensity was scored semiquantitatively as negative (0), weak (1 = barely perceptible staining at high power (400x) magnification), moderate (2 = readily apparent at low power (40x) magnification) or strong (3). The fraction of PSMA positive cells was assessed as < 5% or ≥ 5%. In the case of heterogeneous staining, the predominant pattern was recorded. For further analysis, labeling indices were defined. A weak (1) or moderate (2) staining intensity in ≤ 5% of the neovasculature and a weak (1) staining intensity in > 5% of the neovasculature was allocated to the “low expression” group (PSMA labelling index = 1), whereas a moderate (2) staining intensity in > 5% of the neovasculature and a strong (3) staining intensity in ≤ or > 5% of the neovasculature were assigned to the “strong expression” group (PSMA labelling index = 2). This scoring system has been previously established in soft tissue tumors[20, 21].
Statistics
SPSS (SPSS Statistics, Version 24.0 released 2016, IBM Corp., Armonk, United States) was used for all statistical analyses. The study population was described by standard descriptive statistical measures. For categorical variables, absolute and relative frequencies are reported. Continuous variables are described by mean, standard deviation, median and inter-quartile range (IQR). Survival time is defined from first diagnosis until death. Univariate overall survival analysis was performed using the Kaplan-Meier method and log rank tests. We considered potential prognostic factors that are tolerably complete (less than ten missing values, and with at least ten cases) to prevent statistical problems emerging from low sample size and extreme values. Patients with missing values in the cofactors were excluded from the analysis. All statistical tests were performed as exploratory analyses on a local significance level of 0.05. Since multiplicity adjustment was not carried out, no distinct overall significance level was ascertained. Hence, our findings may be used to set up new hypotheses.
Results
PSMA expression in NSCLC tissues
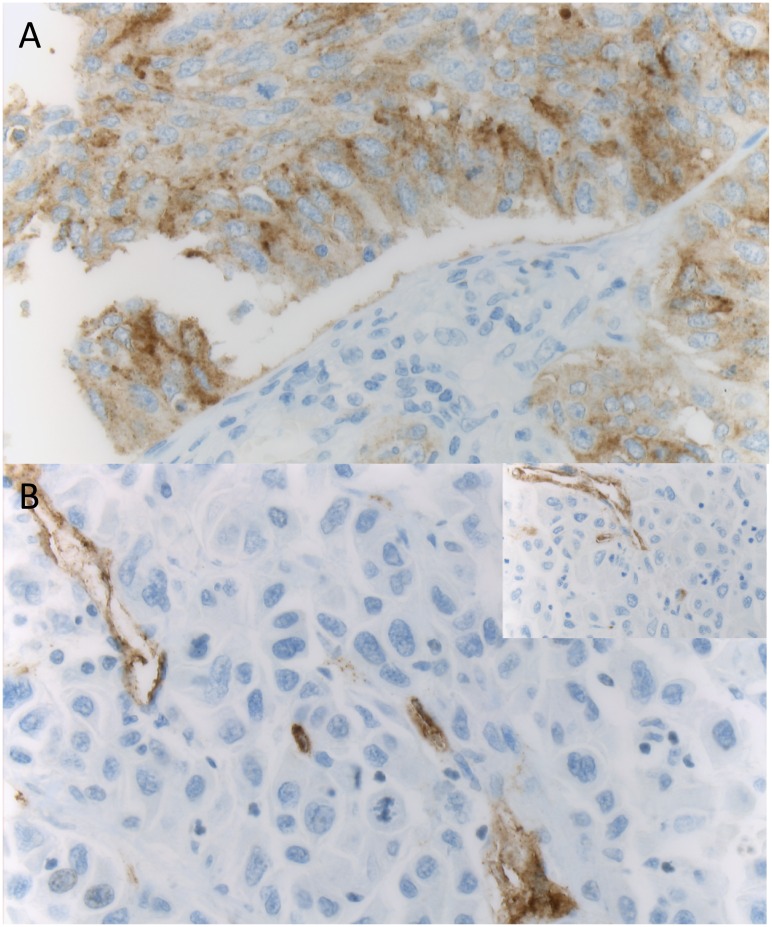
Baseline characteristics of n = 275 NSCLC patients with available immunohistochemical staining results are summarized in Table 1. A positive immunostaining (i.e. 2+/3+, according to the scoring system as described above) of tumor tissues with the monoclonal PSMA antibody was found in n = 17 cases (6%). Likewise, a positive immunostaining (i.e. 1+/2+/3+, according to the scoring system as described above) of neovascular PSMA expression was found in n = 135 cases (49%). In the 14 cases with PSMA-positive squamous lung cancer cells the tumor associated neovasculature was expressing PSMA in 8 cases. A representative example for PSMA expression is demonstrated in Fig 1.
Fig 1. PSMA expression in non-small cell lung cancer (NSCLC).
A (100x, anti-PSMA) displays strong PSMA expression in tumor cells. In B (100x, anti-PSMA), neovascular PSMA expression is shown. Neovasculature was identified by CD34 coexpression (insert, anti-CD34).
Clinicopathological correlations
With regard to clinicopathological parameters, positive correlations for tumor cell PSMA expression (i.e. 2+/3+) were found for tumor histology. In total n = 17 patients displayed tumor cell PSMA expression. Among these patients, n = 14 patients suffered from squamous cell carcinoma (82%; p = 0.002, two-sided Fisher’s exact test). In contrast, tumor cell PSMA expression in adenocarcinoma and in large cell carcinoma was low (Table 2). With regard to tumor histology, neovascular PSMA expression also displayed a positive correlation. Here n = 69 patients with squamous cell carcinoma (51%) expressed PSMA in tumor-associated neovasculature, whereas n = 88 patients with non-squamous cell carcinoma (63%) were negative for neovascular PSMA expression (p = 0.021, two-sided Fisher’s exact test). Furthermore, there was a positive correlation between high histological grade and PSMA expression, with PSMA-positive neovasculature found more frequently in high-grade or undifferentiated tumors. Of those, n = 99 tumor samples (75%) displayed positive neovascular PSMA expression (p < 0.001, two-sided Fisher’s exact test). The relationship of of neovascular PSMA staining and grading depending on the histological subtype is shown in supplementary S1 Table. Neither for tumor stage, nor for patient age relevant associations could be found (two-sided Fisher’s exact test for all: p > 0.05; Table 2). Of interest, of the n = 17 patients with positive tumor cell PSMA expression n = 13 patients were male (76%; p > 0.05, two-sided Fisher’s exact test) and n = 112 patients with positive neovascular PSMA expression (83%) were male (p = 0.011, two-sided Fisher’s exact test; Table 2).
Table 2. Correlations of clinicopathological variables with PSMA in NSCLC patients.
| Parameter | Tumor cell PSMA expression | Neovascular PSMA expression | ||||
|---|---|---|---|---|---|---|
| SI 0/1 | SI 2/3 | p-value* | SI 0/1 | SI 2/3 | p-value* | |
| Age | 0.793 | 0.899 | ||||
| <70 years | 168 | 10 | 90 | 88 | ||
| ≥70 years | 89 | 6 | 49 | 46 | ||
| Sex | 1.000 | 0.011 | ||||
| Male | 196 | 13 | 97 | 112 | ||
| Female | 62 | 4 | 43 | 23 | ||
| Performance status | 1.000 | 0.108 | ||||
| ECOG 0 | 45 | 2 | 29 | 18 | ||
| ECOG 1–3 | 203 | 13 | 104 | 112 | ||
| Tumor Histology | 0.002 | 0.021 | ||||
| Non squamous cell carcinoma | 151 | 3 | 88 | 66 | ||
| Squamous cell carcinoma | 107 | 14 | 52 | 69 | ||
| 0.011 | 0.001 | |||||
| Non adenocarcinoma | 148 | 15 | 69 | 94 | ||
| Adenocarcinoma | 110 | 2 | 71 | 41 | ||
| 0.484 | 0.180 | |||||
| Non large cell carcinoma | 217 | 16 | 123 | 110 | ||
| Large cell carcinoma | 41 | 1 | 17 | 25 | ||
| Tumor stage | 0.284 | 0.605 | ||||
| UICC Tumor stage I | 174 | 14 | 98 | 90 | ||
| UICC Tumor stage II-III | 84 | 3 | 42 | 45 | ||
| Grading | 1.000 | <0.001 | ||||
| G1/G2 | 94 | 6 | 67 | 30 | ||
| G3/G4 | 159 | 10 | 70 | 99 | ||
*p-value according to two-sided Fisher’s exact test
Univariate prognostic effects
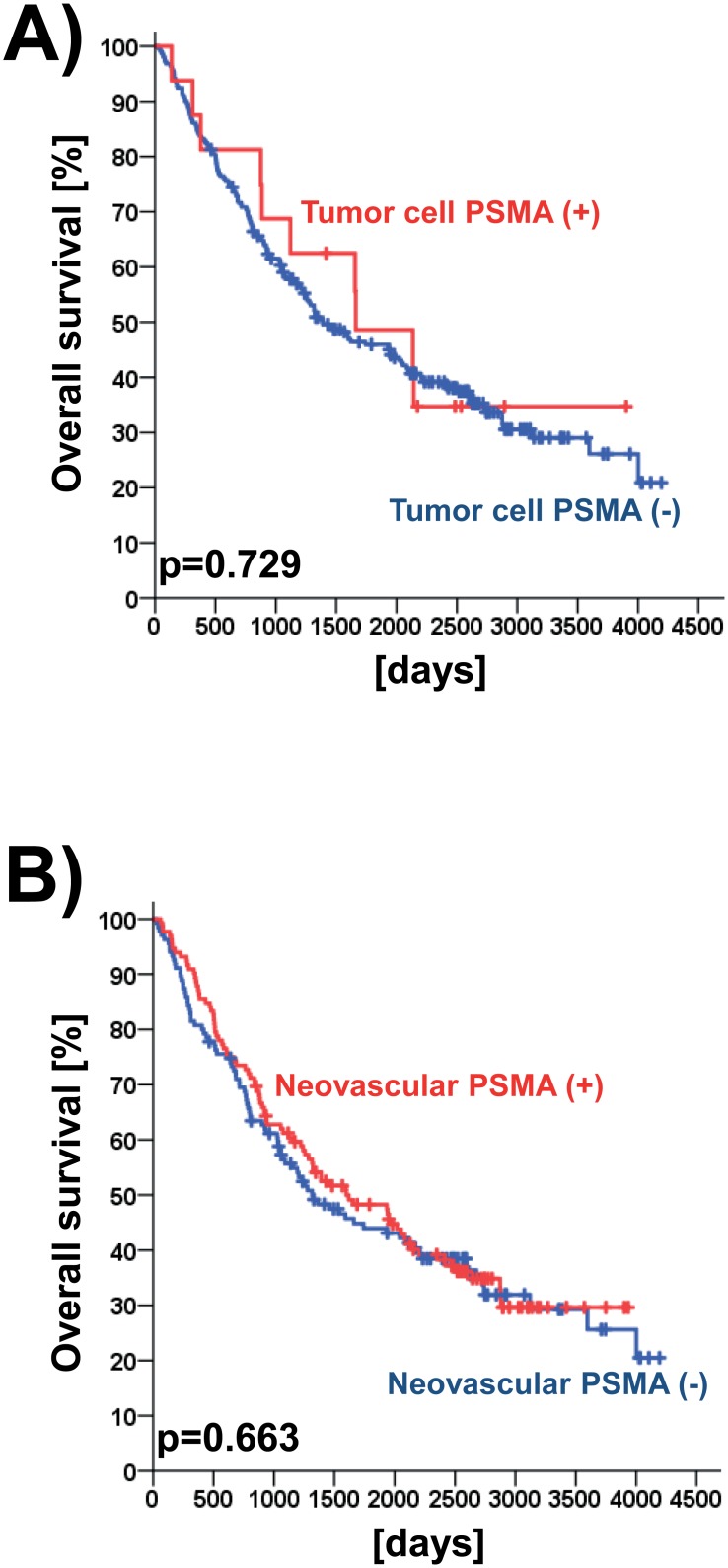
In the entire NSCLC study collective, neither tumor cell PSMA expression nor neovascular PSMA expression had a prognostic effect (both analyses p > 0.05; log rank test). Besides, no subgroup depending prognostic (i.e. tumor histology, grading and tumor stage) effects were found (p > 0.05; log rank test; Table 3). To visualize prognostic effects, Kaplan-Meier curves were generated (Fig 2A and 2B).
Table 3. Univariate log rank test results for the association of PSMA with overall survival for defined subgroups.
The investigated study collective consisted of n = 275 tissue microarray-embedded NSCLC specimens (immunohistochemical analysis).
| Parameter | Tumor cell PSMA expression | Neovascular PSMA expression |
|---|---|---|
| SI 0/1 vs. SI 2/3 | SI 0/1 vs. SI 2/3 | |
| All investigated patients | 0.729 | 0.663 |
| Tumor Histology | ||
| Non squamous cell carcinoma | 0.149 | 0.767 |
| Squamous cell carcinoma | 0.986 | 0.169 |
| Tumor stage | ||
| UICC Tumor stage I | 0.763 | 0.201 |
| UICC Tumor stage II-III | 0.607 | 0.341 |
| Grading | ||
| G1/G2 | 0.207 | 0.347 |
| G3/G4 | 0.427 | 0.065 |
Fig 2. Prognostic impact of PSMA expression in non-small cell lung cancer (NSCLC).
Univariate prognostic models are shown for the immunohistochemical staining results (Fig 2A). Likewise prognostic models are given for neovascular PSMA expression of all patients as detected by immunohistochemistry (Fig 2B).
Discussion
To overcome the poor prognosis of lung cancer patients, it is crucial to both improve the prognostic stratification of patients at the diagnostic level and to expand our therapeutic armamentarium. In this context, molecular targeted therapies on the basis of tumor-associated antigens are a promising approach.
Among suitable candidates for targeted therapy concepts, the type II transmembrane protein PSMA might be a promising target antigen in lung cancer[22]. Two facts contribute to this strategy: (1) Upon ligand binding and subsequent endocytosis, PSMA is transported directly into the cells[23]. (2) Whereas strong PSMA expression is found in prostatic cancer cells (including advanced-stage prostate carcinomas)[24], normal tissue displays rather low expression levels[22]. With regard to these facts, enhanced uptake levels and retention in the tumor favor the local enrichment of antitumoral compounds. Hence, PSMA might serve as an ideal candidate for targeted radiopharmaceutical therapies[22]. Besides prostate carcinoma, PSMA expression has been detected in other malignancies[11–15]. Functionally, PSMA has been shown to be involved in angiogenesis and endothelial cell sprouting[6, 16], but its exact role in tumor-associated angiogenesis remain to be further clarified[25].
To identify the prognostic relevance of PSMA, we performed a systematic analysis of a comprehensive NSCLC study cohort (n = 275) with focus on the PSMA expression in both neovascular and tumor cells. In our study collective, PSMA tumor cell expression was found in n = 17 cases (6%).
To our knowledge, there is only one additional study that reports on PSMA expression in lung cancer. Wang et al. describe high PSMA expression rates both for tumor neovasculature endothelial cells (85%) and for tumor cells (54%) in n = 150 NSCLC patients. Besides, PSMA-positive endothelial cells were found more often in early-stage NSCLC in contrast to advanced NSCLC tissues[12].
Similar to the published results, we detected strong neovascular PSMA expression levels in 49% of the investigated cases. However, the rate of NSCLCs with PSMA expression on tumor cells was much lower in our cohort (6%). The observed differences for PSMA expression on tumor cells might be due to a difference in the monoclonal antibodies that had been used in the different studies, heterogeneity of the investigated NSCLC study cohort and/ or differences in interpretation. In our study we used an anti-PSMA antibody (subclone 3E6) targeting the extracellular region of PSMA. In contrast to other subclones, it might be better used to predict the likely hood of success of PSMA imaging and therapy.
With regard to PMSA expression, it is difficult not to misinterpret antibody reaction products in macrophages as positive tumor cells. Of interest, PSMA tumor cell expression was detected more often in squamous cell carcinomas than in non-squamous NSCLC samples.
In our cohort, neovascular PSMA expression was detected in 49% of the investigated tumors. Overall, neovascular PSMA expression was found to be associated with higher histologic grade (p<0.001). Recently, we reported on PSMA expression patterns in soft tissue tumors[20]. In the latter study, higher histologic grade (e.g., poor tumor differentiation) had also been associated with higher neovascular PSMA expression. However, the biological relevance of this observation to be further clarified. According to one hypothesis, it might be due to intratumoral hypoxia as a result of rapid tumor growth. Since PSMA facilitates endothelial cell invasion during angiogenic sprouting, PSMA upregulation might enhance tumor vascularization in this setting[6, 16]. As a result, targeting PSMA-expressing neovessels might represent a promising therapeutic option in rapidly growing solid tumors.
For patients with metastatic prostate cancer, the PSMA-targeted radionuclide therapy has been shown to be a therapeutic and diagnostic option[4, 26, 27]. In the light of our findings, patients with PSMA expressing NSCLC tumors might benefit from PSMA-targeted radionuclide therapies. However, given the low frequency of PSMA expressing tumors (6% in our cohort, predominantly squamous cell carcinomas), prospective studies should focus on the immunohistochemical pre-evaluation in order to identify candidates with possible benefit from these targeted therapeutic approaches. Moreover, our findings of PSMA expression in tumor-associated neovasculature of 49% of the investigated cases might point towards a possible anti-angiogenic effects of PSMA-targeted radionuclide therapy in these patients.
In line with this theory, PSMA-targeted therapies in different non-prostate cancers have been recently published. Both, radionuclide based PSMA antibodies and PSMA-targeted docetaxel-containing nanoparticle were used and preliminary evaluated[28, 29].
In conclusion, our study reports the expression of PSMA in a subset of NSCLCs, especially in tumor-associated neovasculature. Expression does not seem to “drive” disease, but the presence of PSMA in these selected cases might represent a potential therapeutic target. With focus on the treatment with radiolabeled PSMA ligands, our data might serve for the identification of patients who might benefit from these novel and promising therapeutic approaches.
Supporting information
(DOCX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The laboratories of W.E.B. and G.L. are supported by Deutsche Forschungsgemeinschaft (DFG EXC1003, Cluster of Excellence “Cells in Motion”).
References
- 1.Global Burden of Disease Cancer C, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The Global Burden of Cancer 2013. JAMA oncology. 2015;1(4):505–27. doi: 10.1001/jamaoncol.2015.0735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(2):749–53. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 1996;2(9):1445–51. . [PubMed] [Google Scholar]
- 4.Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI research. 2015;5(1):114 doi: 10.1186/s13550-015-0114-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kratochwil C, Giesel FL, Stefanova M, Benesova M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016. doi: 10.2967/jnumed.115.171397 . [DOI] [PubMed] [Google Scholar]
- 6.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. Journal of cellular biochemistry. 2004;91(3):528–39. doi: 10.1002/jcb.10661 . [DOI] [PubMed] [Google Scholar]
- 7.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–61. . [DOI] [PubMed] [Google Scholar]
- 8.Evangelista L, Briganti A, Fanti S, Joniau S, Reske S, Schiavina R, et al. New Clinical Indications for F/C-choline, New Tracers for Positron Emission Tomography and a Promising Hybrid Device for Prostate Cancer Staging: A Systematic Review of the Literature. European urology. 2016. doi: 10.1016/j.eururo.2016.01.029 . [DOI] [PubMed] [Google Scholar]
- 9.Foss CA, Mease RC, Cho SY, Kim HJ, Pomper MG. GCPII imaging and cancer. Current medicinal chemistry. 2012;19(9):1346–59. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. The Journal of urology. 2003;170(5):1717–21. doi: 10.1097/01.ju.0000091655.77601.0c . [DOI] [PubMed] [Google Scholar]
- 11.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer research. 1999;59(13):3192–8. . [PubMed] [Google Scholar]
- 12.Wang HL, Wang SS, Song WH, Pan Y, Yu HP, Si TG, et al. Expression of prostate-specific membrane antigen in lung cancer cells and tumor neovasculature endothelial cells and its clinical significance. PloS one. 2015;10(5):e0125924 doi: 10.1371/journal.pone.0125924 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernicke AG, Edgar MA, Lavi E, Liu H, Salerno P, Bander NH, et al. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Archives of pathology & laboratory medicine. 2011;135(11):1486–9. doi: 10.5858/arpa.2010-0740-OA . [DOI] [PubMed] [Google Scholar]
- 14.Godeiro KD, Frota AC, Antecka E, Odashiro AN, Maloney S, Fernandes B, et al. Prostate-specific membrane antigen is undetectable in choroidal neovascular membrane. Journal of carcinogenesis. 2006;5:21 doi: 10.1186/1477-3163-5-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clinical cancer research: an official journal of the American Association for Cancer Research. 1999;5(10):2674–81. . [PubMed] [Google Scholar]
- 16.Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Molecular and cellular biology. 2006;26(14):5310–24. doi: 10.1128/MCB.00084-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75(12):2417–26. . [PubMed] [Google Scholar]
- 18.Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Laboratory investigation; a journal of technical methods and pathology. 1990;62(6):690–6. . [PubMed] [Google Scholar]
- 19.Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, Schlingemann RO. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 2012;15(1):151–63. doi: 10.1007/s10456-011-9251-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitkotter B, Trautmann M, Grunewald I, Bogemann M, Rahbar K, Gevensleben H, et al. Expression of PSMA in tumor neovasculature of high grade sarcomas including synovial sarcoma, rhabdomyosarcoma, undifferentiated sarcoma and MPNST. Oncotarget. 2016. doi: 10.18632/oncotarget.13994 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schildhaus HU, Riegel R, Hartmann W, Steiner S, Wardelmann E, Merkelbach-Bruse S, et al. Lysine-specific demethylase 1 is highly expressed in solitary fibrous tumors, synovial sarcomas, rhabdomyosarcomas, desmoplastic small round cell tumors, and malignant peripheral nerve sheath tumors. Human pathology. 2011;42(11):1667–75. doi: 10.1016/j.humpath.2010.12.025 . [DOI] [PubMed] [Google Scholar]
- 22.Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(1):9–15. doi: 10.1158/1078-0432.CCR-15-0820 . [DOI] [PubMed] [Google Scholar]
- 23.Rajasekaran SA, Anilkumar G, Oshima E, Bowie JU, Liu H, Heston W, et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Molecular biology of the cell. 2003;14(12):4835–45. doi: 10.1091/mbc.E02-11-0731 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perner S, Hofer MD, Kim R, Shah RB, Li H, Moller P, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Human pathology. 2007;38(5):696–701. doi: 10.1016/j.humpath.2006.11.012 . [DOI] [PubMed] [Google Scholar]
- 25.Chang SS. Overview of prostate-specific membrane antigen. Reviews in urology. 2004;6 Suppl 10:S13–8. . [PMC free article] [PubMed] [Google Scholar]
- 26.Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of Intraprostatic Tumor Extent with 68Ga-PSMA Distribution in Patients with Prostate Cancer. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2016;57(4):563–7. doi: 10.2967/jnumed.115.169243 . [DOI] [PubMed] [Google Scholar]
- 27.Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of Intraprostatic Tumor Extent with (6)(8)Ga-PSMA Distribution in Patients with Prostate Cancer. J Nucl Med. 2016;57(4):563–7. Epub 2016/01/16. doi: 10.2967/jnumed.115.169243 . [DOI] [PubMed] [Google Scholar]
- 28.Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(5):540–7. doi: 10.1200/JCO.2006.07.8097 . [DOI] [PubMed] [Google Scholar]
- 29.Von Hoff DD, Mita MM, Ramanathan RK, Weiss GJ, Mita AC, LoRusso PM, et al. Phase I Study of PSMA-Targeted Docetaxel-Containing Nanoparticle BIND-014 in Patients with Advanced Solid Tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(13):3157–63. doi: 10.1158/1078-0432.CCR-15-2548 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.