Abstract
Combining anticatabolic agents with parathyroid hormone (PTH) to enhance bone mass has yielded mixed results in osteoporosis patients. Toward the goal of enhancing the efficacy of these regimens, we tested their utility in combination with loss of the transcription factor Nmp4 because disabling this gene amplifies PTH-induced increases in trabecular bone in mice by boosting osteoblast secretory activity. We addressed whether combining a sustained anabolic response with an anticatabolic results in superior bone acquisition compared with PTH monotherapy. Additionally, we inquired whether Nmp4 interferes with anticatabolic efficacy. Wild-type and Nmp4−/− mice were ovariectomized at 12 weeks of age, followed by therapy regimens, administered from 16 to 24 weeks, and included individually or combined PTH, alendronate (ALN), zoledronate (ZOL), and raloxifene (RAL). Anabolic therapeutic efficacy generally corresponded with PTH + RAL = PTH + ZOL > PTH + ALN = PTH > vehicle control. Loss of Nmp4 enhanced femoral trabecular bone increases under PTH + RAL and PTH + ZOL. RAL and ZOL promoted bone restoration, but unexpectedly, loss of Nmp4 boosted RAL-induced increases in femoral trabecular bone. The combination of PTH, RAL, and loss of Nmp4 significantly increased bone marrow osteoprogenitor number, but did not affect adipogenesis or osteoclastogenesis. RAL, but not ZOL, increased osteoprogenitors in both genotypes. Nmp4 status did not influence bone serum marker responses to treatments, but Nmp4−/− mice as a group showed elevated levels of the bone formation marker osteocalcin. We conclude that the heightened osteoanabolism of the Nmp4−/− skeleton enhances the effectiveness of diverse osteoporosis treatments, in part by increasing hyperanabolic osteoprogenitors. Nmp4 provides a promising target pathway for identifying barriers to pharmacologically induced bone formation.
Loss of the transcription factor Nmp4 improves the efficacy of osteoporosis therapies in mice.
A coveted but elusive goal in osteoporosis therapy is to replace the bone lost to this disease while reducing fracture risk (1), and parathyroid hormone (PTH; teriparatide) as well as the PTH-related protein analog, abaloparatide, are the only Food and Drug Administration–approved compounds capable of stimulating new bone formation. However, the rate of PTH-induced net bone gain diminishes within months of initiating treatment (2, 3), and therapy duration is currently restricted to 24 months (4). These limitations constrain PTH efficacy for treating this chronic degenerative bone disease.
One approach to extend the duration of PTH anabolic efficacy is through combination therapy. Combination treatment is based upon the hypothesis that the joint actions of the osteoanabolic PTH with the action of a drug that attenuates bone resorption such as bisphosphonates, raloxifene (RAL), or denosumab will lead to more bone and lower fracture risk than monotherapy with either agent alone. Unfortunately, combining PTH therapy with an anticatabolic agent has yielded mixed results (5–8) with about as many studies showing improvement with combination therapies (9–13) as those showing no significant advantage (14–17). In most clinical studies, the efficacy of combination treatment is based on biomarkers and bone mineral density (BMD). Reduced efficacy is attributed to a blunting of the PTH anabolic action by the added anticatabolic treatment (7).
Disabling nuclear matrix protein 4 (Nmp4, also known as Ciz, Zfp384) may present a means of modifying the skeletal response to PTH combination therapy. Nmp4 is a transcription factor that represses certain genes and enhances transcription of others (18, 19). These mice harboring a loss-of-function mutation in the Nmp4 gene (Nmp4−/− mice) have an unremarkable skeletal phenotype until challenged with any one of several anabolic stimuli (e.g., PTH, Bmp2), which elicits enhanced and accelerated bone formation (18, 20–23). Moreover, ovariectomy does not nullify the large osteogenic response (18) to PTH. Nmp4−/− mice are healthy under typical vivarium conditions. They show normal growth and development and do not exhibit shortened life expectancy compared with their wild-type (WT) littermates. The female mice are fertile; however, a small percentage of males show sporadic infertility due to increased occurrence of spermatogenic apoptosis (24). Of interest, a recent report demonstrates that disabling Nmp4 suppresses the induction of serum transfer-induced arthritis (25). Therefore, the Nmp4−/− phenotype exhibits several promising preclinical assets (18, 20–22). Nmp4−/− mice harbor more osteoprogenitors (CFU-FAlk phos+) than WT animals (18, 21, 23), and Nmp4−/− mesenchymal stem/progenitor cells (MSPCs) exhibit an accelerated and enhanced mineralization (18). Bioinformatic profiling of our genome-wide ChIP-seq data, combined with array analysis, identified a network of genes outlining an antianabolic axis that suppresses osteoblast activity (18). Many genes showing enhanced messenger RNA expression in the null cells are osteoblast secretory proteins, including osteocalcin (Oc), osteopontin (Opn), collagen (Col1a1), and Bmp2 (18). Consistent with these findings, Nmp4−/− mice show enhanced levels of serum OC under PTH therapy (21). This enhanced secretory activity is supported by elevated ribosome biogenesis and an expanded and sustained unfolded protein response (UPR) that enlarges the capacity of the endoplasmic reticulum to synthesize and deliver bone matrix in these professional secretory cells (26). The ability of these mice to have a robust and sustained response to PTH therapy makes them a useful model to test aspects of combination therapy.
Our previous studies have revealed that Nmp4 inhibition represents an attractive strategy to enhance anabolic therapy in bone. However, it remains to be determined whether Nmp4 inhibition can enhance the efficacy of anticatabolic therapies in the skeleton. Therefore, the goal of this study was twofold, as follows: (1) to test the hypothesis that combining a sustained anabolic response to PTH with an anticatabolic agent results in superior bone acquisition compared with PTH monotherapy alone; and (2) to test the hypothesis that Nmp4 does not interfere with the efficacy of antiresorptive agents. To test these hypotheses, we evaluated the efficacy of combining PTH therapies in ovariectomized mice (normal and Nmp4-null) with one of three anticatabolic drugs: the nitrogen-containing bisphosphonates alendronate (ALN) and zoledronate (ZOL) and the selective estrogen receptor modulator (SERM) RAL. Our findings demonstrate that loss of Nmp4 significantly enhances the response of combining PTH with anticatabolics and intriguingly improves the skeletal effects of the RAL monotherapy, but not the bisphosphonate monotherapies. The sustained anabolic effect may be driven, in part, by an expansion in the bone marrow pool of hyperanabolic osteoprogenitors. Nevertheless, disabling the Nmp4 antianabolic bone axis provides a potential strategy for improving diverse existing osteoporosis treatments.
Materials and Methods
Mice
WT and Nmp4−/− mice were generated, as previously described (18, 20). Mice were maintained in our colony at Indiana University Bioresearch Facility, Indiana University School of Dentistry. Animals for these experiments were randomly selected from litters produced by heterozygous × heterozygous, Nmp4−/− × WT, WT × WT, and Nmp4−/− × Nmp4−/− breeding pairs. Local Institute Animal Care and Use Committee have approved all husbandry practices and experimental procedures described in the current study.
Bilateral ovariectomy surgery
The surgeries on 12-week-old virgin mice were performed, as previously described in detail (18). To keep the study to 16 treatment groups, we did not perform sham surgeries because we have previously shown ovariectomy induces significant bone loss in both genotypes and there is no difference between the baseline skeletal phenotypes in healthy and ovariectomized mice (18, 20).
Therapies
At 16 weeks of age, the ovariectomized mice were sorted into 16 treatment groups by weight and genotype. All mice were housed typically two to four per cage under standard conditions with ad libitum access to water and regular chow (Laboratory Rodent Diet 5001; LabDiet, St. Louis, MO). Each mouse received two sequential 100-µL injections per day containing the drugs or vehicle(s) 7 days/week for 8 weeks (see Fig. 1 for full details). ZOL and ALN were synthesized by the Indiana University–Purdue University Indianapolis Chemistry Core Facility, verified by nuclear magnetic resonance spectroscopy, and have been previously shown to produce the expected effects on bone remodeling (27, 28). Mice receiving PTH were injected subcutaneously with synthetic human PTH 1–34 acetate salt (Bachem Americas, Inc., Torrance, CA) at 30 µg/kg/d, daily, a dose frequently used in mice to study PTH bone anabolic action in vivo. Doses of anticatabolic agents were based on human clinical doses. The standard ALN dose for treatment of osteoporosis is typically given as either a daily (10 mg) or weekly (70 mg) dose. Based on a 60-kg individual, this is ∼1.17 mg/kg/wk. The human dose is oral and has an estimated bioavailability of ∼0.6%, meaning that the absorbed dose is approximately 7 μg/kg/wk. We dosed via injection, assuming 100% absorption; thus, we delivered ALN at 1 μg/kg/d (12, 29, 30). RAL is typically given clinically as a 60 mg daily dose. Based on a 60-kg patient, the dose would be 1 mg/kg/d. The assumption is 100% absorption; thus, the full dose is used when injecting (30, 31). ZOL is typically given yearly at a dose of 5 mg. Based on a 60-kg patient, the dose is 0.083 mg/kg. Our single dose of 80 μg/kg approximates this amount (9, 32).
Figure 1.
WT and Nmp4−/− mice were ovariectomized (ovx’d) at 12 weeks of age. At 16 weeks of age, the mice were sorted into 16 treatment groups by weight and genotype. Each mouse received two sequential 100-µL injections per day (q.d.) containing the drugs or vehicle(s), as shown for 8 weeks. Mice were euthanized, and the bones were processed for analysis at 24 weeks of age. WT and Nmp4−/− mice were administered the following treatments: (1) Vehicle-control: inject subcutaneously (subq.) 100 µL 0.2% bovine serum albumin/0.1% 1.0 µN HCl in 0.9% NaCl (BHN diluent for PTH/ALN) + 100 µL 20% hydroxypropyl-β-cyclodextrin (HBC diluent for RAL diluent). (2) Daily ALN: inject subq. 100 µL ALN at 1 μg/kg/d + 100 µL HBC. (3) ZOL: on day 1 of treatment, inject intraperitoneally (i.p.) 100 µL ZOL at 80 μg/kg in phosphate-buffered saline. On day 2 forward, inject subq. 100 µL BHN + 100 µL HBC. (4) Daily RAL: inject subq. 100 µL RAL at 1 mg/kg/d + 100 µL BHN. (5) Daily PTH: inject subq. 100 µL synthetic human PTH 1–34 acetate salt (Bachem Americas, Inc.), at 30 µg/kg/d + 100 µL HBC. (6) Daily PTH + ALN: inject subq. 100 µL PTH/ALN + 100 µL HBC. (7) Single-dose ZOL followed by daily PTH: on day 1 of treatment inject i.p. 100 µL ZOL. On day 2 forward, inject subq. 100 µL PTH + 100 µL HBC. (8) Daily PTH + RAL: inject subq. 100 µL RAL + 100 µL PTH.
Dual energy X-ray absorptiometry
The postcranial skeleton and spine (L3–L5) areal BMD (mg/cm2) and bone mineral content (g) were evaluated in vivo using a PIXImus II densitometer, as previously described (20, 21).
Microcomputed tomography
Trabecular and cortical bone architectures were analyzed, as we have previously described in detail (18, 21). Briefly, after tissue preparation, the distal femur metaphysis and the L5 vertebral body were scanned using a Skyscan 1172. All scans were conducted at a 6-µm scan resolution. Three-dimensional reconstructions using Skyscan software provided the following parameters: trabecular bone volume per total volume (BV/TV; %), trabecular number (Tb.N; mm−1), trabecular thickness (Tb.Th; mm), and trabecular spacing (Tb.Sp; mm). Additionally, the Skyscan software provided the following data for femoral diaphysis cortical bone: periosteal perimeter (mm), endocortical perimeter (mm), total area (mm2), bone area (mm2), marrow area (mm2), cortical porosity (%), cortical thickness (mm), minimum moment of inertia (mm4), maximum moment of inertia (mm4), and polar moment of inertia (mm4).
Serum biochemistry
Serum osteocalcin was evaluated as a bone formation marker using enzyme-linked immunosorbent assay BTI Mouse Osteocalcin EIA Kit (Biomedical Technologies, Stoughton, MA). Serum C-terminal telopeptides (CTX) were assessed as an indicator for resorption using the RatLapsTM enzyme-linked immunosorbent assay (Immunodiagnostic System, Scottsdale, AZ). Serum osteoprotegerin and serum receptor activator of nuclear factor-κB ligand (RANKL) were determine using Mouse Osteoprotegerin/TNFRSF11B Immunoassay Kit and the Mouse TRANCE/RANK L/TNFSF11 Immunoassay Kit, respectively (R&D Systems, Minneapolis, MN).
Immunohistochemistry
Osterix was detected on formalin-fixed, paraffin-embedded sections by using primary antibodies from Abcam (human anti-SP7/osterix; ab 94744; Cambridge, MA). We followed the protocol described by Nissenson et al. (33, 34), with some modifications. Briefly, slides were deparaffinized at room temperature in Coplin jars in three washes of xylene and rehydrated in a decreasing ethanol gradient. Endogenous peroxidases were deactivated with 3% H2O2 for 5 minutes, and sections were blocked in phosphate-buffered saline (PBS) supplemented with 1.5% goat serum (Gibco BRL) for 30 minutes at room temperature. Sections were incubated with primary antibody (1:25 dilution) in blocking solution overnight at 4°C. Sections were then washed in PBS and incubated with the biotinylated goat anti-rabbit IgG (VectaStain Elite ABC Kit; Vector Laboratories, Burlingame, CA) for 45 minutes at room temperature. After washing with PBS, sections were incubated with VECTASTAIN® ABC Reagent for 30 minutes at room temperature, followed by washing in buffer for 5 minutes. Incubating sections in peroxidase substrate solution, according to the manufacturer’s instructions, achieved color development. Finally, counterstaining was accomplished by staining with 0.2% methyl green for 60 to 90 seconds, followed by dehydration in a series of ethanol and xylene changes, and mounted using coverslips with xylene-based mounting media.
Tartrate-resistant acid phosphatase (TRAP) staining was performed using a modified protocol based on the method of Erlebacher and Derynck (35). In brief, formalin-fixed, paraffin-embedded sections were deparaffinized, followed by rehydration via a sequential ethanol wash. Subsequently, slides were transferred to 0.2 M acetate buffer (pH 5.0) for 20 minutes at room temperature and then placed in medium containing napthol AS-MX phosphate (0.5 mg/mL; Sigma-Aldrich, St. Louis, MO; N4875) and fast red TR salt (1.1 mg/mL; Sigma-Aldrich; E6760) in acetate buffer for 60 minutes at 37°C before counterstaining with toluidine blue. Slides dried for 24 hours. Aqueous-base mounting media was added on top of the sample, and coverslip was applied.
Adipocytes were stained in deparaffinized slides that had been rehydrated using a sequential ethanol wash. Sections were then incubated in Sudan Black B solution for 3 hours. Subsequently, the slides were rinsed thoroughly in two changes of 70% isopropyl alcohol, followed by six changes of distilled water. The slides were counterstained in nuclear fast red solution for 10 minutes and rinsed again with two changes of distilled water. Slides were coverslipped with an aqueous-based mounting medium.
The stained bone marrow cells were counted using the Bioquant imaging software (Nashville TN). Bone marrow osteoprogenitors were counted within a 0.75- to 1-mm2 area ∼1 mm below the growth plate of the distal femur. Small, round cells within the marrow exhibiting a brown nucleus indicating positive staining for osterix were counted as osteoprogenitors, and then the count normalized to the tissue area selected. Adipocytes were counted within a 1.75- to 2-mm2 area adjacent to the growth plate at the distal femur. Empty-appearing cells >30 μm in diameter and exhibiting a membrane positively stained with Sudan Black were counted and then normalized to tissue area. Finally, to determine the osteoclast surface, a 1.75- to 2-mm2 area adjacent to the growth plate at the distal femur was selected; the surface of all TRAP+ cells and the total trabecular surface in this region were measured. The ratio of the two, Trap+ cell surface/bone surface (Trap+ S/BS), was then calculated.
Flow cytometry
Cellular marker profiles from bone marrow were assessed using the antibodies CD45, CD146, CD105, and nestin (BD Biosciences, San Jose, CA), as previously described (18, 22). Stained cells were analyzed on a FACSCalibur (BD Biosciences), and results were quantified using FlowJo Version 8.8.6 software (Tree Star).
Statistical analysis
Statistical packages JMP version 7.0.1 (SAS Institute, Cary, NC) and the Statistical Analysis System version 9.4 (SAS Institute) were used for analyses.
To test the hypothesis that combining a sustained anabolic response with an anticatabolic agent results in superior bone acquisition compared with PTH monotherapy, we compared the anabolic therapies PTH + RAL, PTH + ZOL, PTH + ALN, and PTH with each other and with vehicle control (VEH). To test our second hypothesis that Nmp4 does not interfere with the efficacy of antiresorptive agents, we compared the anticatabolic treatments ALN, ZOL, and RAL with each other and with VEH. All data were first analyzed for outliers using the interquartile range method to evaluate statistical dispersion (36). Data were then analyzed with a two-way analysis of variance for effects of genotype and treatment, followed by a Tukey-Kramer post hoc test for comparison of more than two groups or Student t post hoc test for comparing WT and Nmp4−/− parameters as two groups. Statistical significance was set at P ≤ 0.05. In these analyses, all experimental data were grouped by either genotype or treatment to determine whether either or both impacted the value of the endpoint parameter as well as whether genotype influenced the response to treatment (genotype × treatment interaction). Finally, to determine whether there was an interaction between PTH and any of the anticatabolic drugs, we performed a series of two-way analyses of variance using PTH and the antiresorptive drug in question as the independent variables.
Results
Effect of combination treatments using anabolic agents on bone
PTH + RAL and PTH + ZOL synergistically enhanced therapeutic bone restoration; loss of Nmp4 further improved the actions of these treatments on femoral trabecular bone.
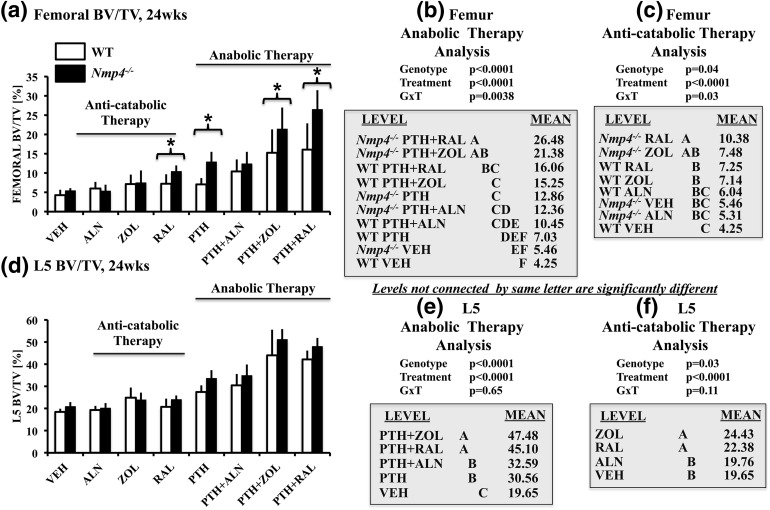
We have previously shown that loss of Nmp4 improves femoral trabecular bone response to PTH in both healthy and ovariectomized mice without compromising gains in cortical bone (18, 20–22). To address the contribution of Nmp4 in regulating the response of trabecular bone to concurrent PTH combination therapies, we evaluated the distal femoral BV/TV of mice under these treatments. Mice administered the PTH + RAL and PTH + ZOL treatments yielded the highest femoral BV/TV in both WT and null mice, and these combination therapies surpassed femoral BV/TV obtained with the PTH monotherapy [Fig. 2(a) and 2(b); treatment effect, P < 0.0001]. Moreover, PTH showed a greater-than-additive (synergistic) interaction with RAL and ZOL at this site (Table 1). In contrast, the femoral BV/TV values of mice under the PTH + ALN treatment were equivalent to those values of the PTH monotherapy cohorts [Fig. 2(a); Table 1].
Figure 2.
(a–c) Femoral BV/TV and (d–f) L5 BV/TV for all the experimental cohorts (age 24 weeks) comparing ovariectomized WT and Nmp4−/− mice. (b and e) We compared the anabolic therapies PTH + RAL, PTH + ZOL, PTH + ALN, and PTH with each other and with VEH. (c and f) We compared the anticatabolic treatments ALN, ZOL, and RAL with each other and with VEH. Statistical analyses were performed using two-way analyses of variance, setting genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05. The asterisk denotes genotype × treatment interaction. The data represent mean ± standard deviation, n = 7 to 12 mice per group. See text for explanation of results.
Table 1.
Identification of Synergy Between PTH and Anticatabolic Drugs
| Therapy | P Value PTH Treatment | P Value Anticatabolic Treatment | P Value PTH × Anticatabolic Interaction |
|---|---|---|---|
| Femur BV/TV | |||
| PTH + ALN [WT] | P < 0.0001 | P = 0.0001 | P = 0.19 |
| PTH + ALN [Nmp4−/−] | P < 0.0001 | P = 0.55 | P = 0.91 |
| PTH + ZOL [WT] | P < 0.0001 | P < 0.0001 | P = 0.02 |
| PTH + ZOL [Nmp4−/−] | P < 0.0001 | P < 0.0001 | P = 0.01 |
| PTH + RAL [WT] | P < 0.0001 | P < 0.0001 | P = 0.0139 |
| PTH + RAL [Nmp4−/−] | P < 0.0001 | P < 0.0001 | P = 0.001 |
| L5 BV/TV | |||
| PTH + ALN [WT] | P < 0.0001 | P = 0.05 | P = 0.31 |
| PTH + ALN [Nmp4−/−] | P < 0.0001 | P = 0.84 | P = 0.38 |
| PTH + ZOL [WT] | P < 0.0001 | P < 0.0001 | P = 0.02 |
| PTH + ZOL [Nmp4−/−] | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| PTH + RAL [WT] | P < 0.0001 | P < 0.0001 | P = 0.0002 |
| PTH + RAL [Nmp4−/−] | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| WB BMD | |||
| PTH + ALN [WT] | P < 0.0001 | P = 0.04 | P = 0.65 |
| PTH + ALN [Nmp4−/−] | P < 0.0001 | P = 0.50 | P = 0.64 |
| PTH + ZOL [WT] | P < 0.0001 | P = 0.0003 | P = 0.8258 |
| PTH + ZOL [Nmp4−/−] | P < 0.0001 | P = 0.0034 | P = 0.3620 |
| PTH + RAL [WT] | P < 0.0001 | P < 0.0001 | P = 0.0007 |
| PTH + RAL [Nmp4−/−] | P < 0.0001 | P < 0.0001 | P = 0.1807 |
| Cortical bone area | |||
| PTH + ALN [WT] | P = 0.0001 | P = 0.0867 | P = 0.9113 |
| PTH + ALN [Nmp4−/−] | P < 0.0001 | P = 0.2717 | P = 0.3244 |
| PTH + ZOL [WT] | P < 0.0001 | P = 0.0663 | P = 0.8497 |
| PTH + ZOL [Nmp4−/−] | P < 0.0001 | P = 0.1095 | P = 0.5698 |
| PTH + RAL [WT] | P < 0.0001 | P = 0.0081 | P = 0.0407 |
| PTH + RAL [Nmp4−/−] | P = 0.0009 | P < 0.0001 | P = 0.1487 |
A series of two-way analyses of variance were used to compare the efficacy of the PTH monotherapy, a specific anticatabolic monotherapy, and the combination of the two drugs. Statistical significance was set at P ≤ 0.05.
Loss of Nmp4 improved the gains in femoral BV/TV of PTH + RAL, PTH + ZOL, and PTH treatments [genotype × treatment interaction, P = 0.0038; Fig. 2(a) and 2(b)]. The three-dimensional microcomputed tomography (micro-CT) images of the distal femur illustrate the differences in trabecular bone between the WT and Nmp4−/− VEH cohorts and various treatments (Fig. 3). Similarly, the two-dimensional micro-CT images show the more extensive bone formation in the Nmp4−/− mice treated with PTH or PTH + RAL compared with the WT animals [Supplemental Fig. 1(A–D) (7.7MB, tif) ].
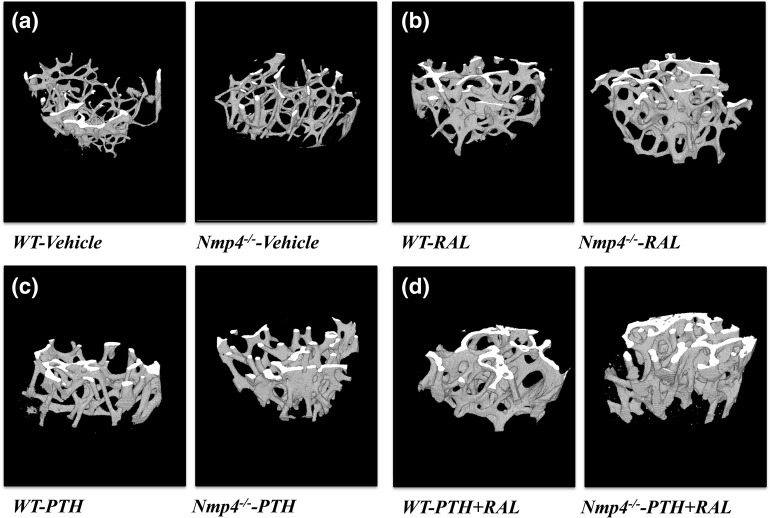
Figure 3.
The three-dimensional micro-CT images of the femoral distal femur from WT and Nmp4−/− mice (24 weeks of age). Mice were ovariectomized at 12 weeks of age and treated with the indicated therapies from 16 weeks to 24 weeks. (a) VEH; (b) RAL monotherapy; (c) PTH monotherapy; (d) PTH + RAL combination therapy.
Loss of Nmp4 had similar effects on femoral trabecular architecture (Tb.N, Tb.Th, Tb.Sp), as observed for BV/TV. Null mice showed significantly higher Tb.N under the PTH + RAL, PTH + ZOL, and PTH therapies compared with the WT cohorts (Table 2). Similarly, the Nmp4−/− mice exhibited a lower Tb.Sp under PTH + RAL and PTH + ZOL compared with WT mice (Table 2). Finally, loss of Nmp4 enhanced increases in Tb.Th under PTH + ZOL and PTH.
Table 2.
Femoral Trabecular Parameters: Anabolic Therapies
| Group | Femoral Tb.N | Femoral Tb.Th | Femoral Tb.Sp | |||
|---|---|---|---|---|---|---|
| VEH: WT/Nmp4−/− | 0.91 ± 0.23/1.18 ± 0.09 | 0.046 ± 0.006/0.045 ± 0.003 | 0.331 ± 0.023/0.285 ± 0.011 | |||
| PTH: WT/Nmp4−/− | 1.27 ± 0.17/1.99 ± 0.21 | 0.058 ± 0.002/0.065 ± 0.002 | 0.345 ± 0.038/0.264 ± 0.023 | |||
| PTH+ALN: WT/Nmp4−/− | 1.71 ± 0.43/1.96 ± 0.45 | 0.059 ± 0.004/0.063 ± 0.004 | 0.275 ± 0.020/0.263 ± 0.029 | |||
| PTH+ZOL: WT/Nmp4−/− | 2.07 ± 0.65/2.74 ± 0.47 | 0.072 ± 0.008/0.081 ± 0.006 | 0.282 ± 0.039/0.235 ± 0.034 | |||
| PTH+RAL: WT/Nmp4−/− | 2.16 ± 0.78/3.23 ± 0.49 | 0.075 ± 0.005/0.081 ± 0.008 | 0.263 ± 0.041/0.202 ± 0.032 | |||
| Anabolic therapy | G: | P < 0.0001 | G: | P < 0.0001 | G: | P < 0.0001 |
| T: | P < 0.0001 | T: | P < 0.0001 | T: | P < 0.0001 | |
| GxT: | P = 0.02 | GxT: | P = 0.0427 | GxT: | P = 0.0123 | |
| Nmp4−/− PTH+RAL | A | Nmp4−/− PTH+RAL | A | WT PTH | A | |
| Nmp4−/− PTH+ZOL | AB | Nmp4−/− PTH+ZOL | A | WT VEH | A | |
| WT PTH+RAL | BC | WT PTH+RAL | AB | Nmp4−/− VEH | B | |
| WT PTH+ZOL | C | WT PTH+ZOL | BC | WT PTH+ZOL | B | |
| Nmp4−/− PTH | C | Nmp4−/− PTH | CD | WT PTH+ALN | BC | |
| Nmp4−/− PTH+ALN | C | Nmp4−/− PTH+ALN | DE | Nmp4−/− PTH | BC | |
| WT PTH+ALN | CD | WT PTH+ALN | DE | WT PTH+RAL | BC | |
| WT PTH | DE | WT PTH | E | Nmp4−/− PTH+ALN | BC | |
| Nmp4−/− VEH | DE | Nmp4−/− VEH | F | Nmp4−/− PTH+ZOL | CD | |
| WT VEH | E | WT VEH | F | Nmp4−/− PTH+RAL | D | |
The micro-CT analyses of the femoral architecture in mice treated with the anabolic therapies. The data were analyzed using two-way analyses of variance using genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05, and levels not connected by the same letter are significantly different. The data represent mean ± standard deviation, n = 7 to 12 mice per group.
Abbreviations: G, genotype; T, treatment.
We next interrogated the impact of the therapies on vertebral trabecular bone. As we observed with the femoral BV/TV, the comparative efficacies of the anabolic treatment groups were PTH + RAL = PTH + ZOL > PTH + ALN = PTH > VEH [treatment effect, P < 0.0001; Fig. 2(d) and 2(e)]. PTH exhibited a greater-than-additive (synergistic) interaction with RAL and ZOL at this site [Fig. 2(b); Table 1].
The loss of Nmp4 did not further enhance the efficacy of the anabolic treatments for restoring L5 BV/TV [Fig. 2(d) and 2(e)]. However, the null mice showed an enhanced increase in Tb.Th under PTH + RAL, PTH + ZOL, and the PTH monotherapy (Table 3). The three-dimensional micro-CT images (Fig. 4) and two-dimensional micro-CT [Supplemental Fig. 2(A–D) (5.3MB, tif) ] of the L5 vertebrae show comparative improvements that we observed in the trabecular architecture with various treatments.
Table 3.
L5 Trabecular Parameters: Anabolic Therapies
| Group | L5 Tb.N | L5 Tb.Th | L5 Tb.Sp | |||
|---|---|---|---|---|---|---|
| VEH: WT/Nmp4−/− | 3.71 ± 0.21/3.79 ± 0.15 | 0.051 ± 0.003/0.054 ± 0.003 | 0.250 ± 0.013/0.239 ± 0.017 | |||
| PTH: WT/Nmp4−/− | 5.82 ± 0.64/6.26 ± 0.49 | 0.047 ± 0.002/0.054 ± 0.002 | 0.191 ± 0.020/0.182 ± 0.021 | |||
| PTH+ALN: WT/Nmp4−/− | 6.24 ± 0.93/6.49 ± 0.62 | 0.049 ± 0.002/0.053 ± 0.003 | 0.188 ± 0.025/0.170 ± 0.017 | |||
| PTH+ZOL: WT/Nmp4−/− | 8.24 ± 0.1.87/9.0 ± 0.85 | 0.053 ± 0.003/0.057 ± 0.002 | 0.156 ± 0.044/0.135 ± 0.023 | |||
| PTH+RAL: WT/Nmp4−/− | 7.79 ± 0.65/8.05 ± 0.81 | 0.052 ± 0.002/0.061 ± 0.002 | 0.172 ± 0.003/0.159 ± 0.027 | |||
| Anabolic therapy | G: | P = 0.0483 | G: | P < 0.0001 | G: | P = 0.0091 |
| T: | P < 0.0001 | T: | P < 0.0001 | T: | P < 0.0001 | |
| PTH+ZOL | A | GxT: | P = 0.0010 | VEH | A | |
| PTH+RAL | A | Nmp4−/− PTH+RAL | A | PTH | B | |
| PTH+ALN | B | Nmp4−/− PTH+ZOL | B | PTH+ALN | B | |
| WT PTH | B | Nmp4−/− PTH | BC | PTH+RAL | BC | |
| VEH | C | Nmp4−/− VEH | BCD | PTH+ZOL | C | |
| GxT: | P = 0.76 | WT PTH+ZOL | CD | GxT: | P = 0.94 | |
| Nmp4−/− PTH+ALN | CD | |||||
| WT PTH+RAL | CD | |||||
| WT VEH | DE | |||||
| WT PTH+ALN | EF | |||||
| WT PTH | F | |||||
The micro-CT analyses of the L5 trabecular architecture in mice treated with the anabolic therapies. The data were analyzed using two-way analyses of variance comparing the anabolic therapies and the anticatabolic monotherapies. Statistical significance was set at P ≤ 0.05, and levels not connected by the same letter are significantly different. The data represent mean ± standard deviation, n = 7 to 12 mice per group.
Abbreviations: G, genotype; T, treatment.
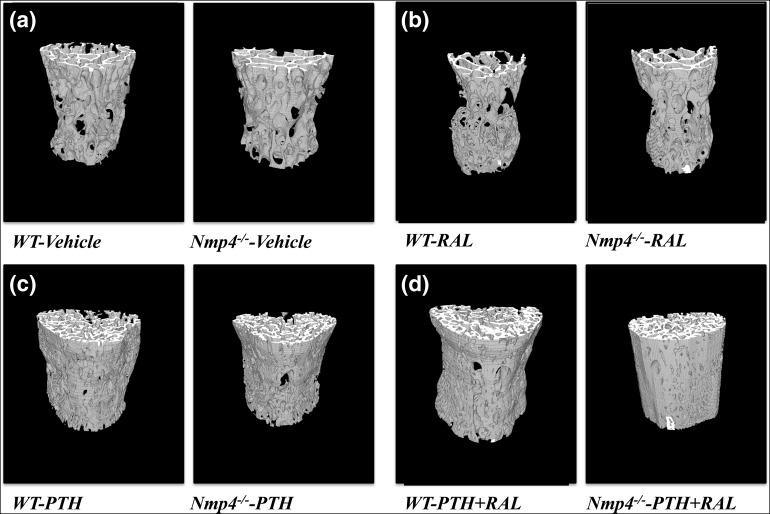
Figure 4.
Three-dimensional micro-CT images of the L5 vertebra from WT and Nmp4−/− mice (24 weeks of age). Mice were ovariectomized at 12 weeks of age and treated with the indicated therapies from 16 weeks to 24 weeks. (a) Vehicle control; (b) RAL monotherapy; (c) PTH monotherapy; (d) PTH + RAL combination therapy.
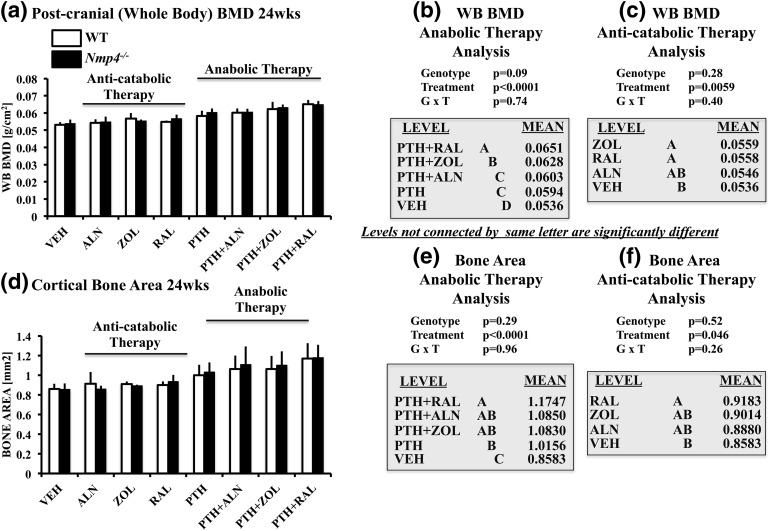
Overstimulation of the PTH receptor has been reported to increase trabecular bone but decrease cortical bone formation in transgenic mice (37). Therefore, to address whether the cortical bone gains in the Nmp4−/− mice were compromised under the present experimental therapies, we evaluated postcranial whole body (WB) BMD and femoral cortical geometry. Both WT and Nmp4−/− mice administered PTH + RAL yielded the highest WB BMD, exhibiting a strong treatment effect (P < 0.0001) but without a genotype × treatment interaction [Fig. 5(a) and 5(b)]. The PTH + RAL and PTH + ZOL cohorts exceeded the WB BMD observed in the PTH monotherapy cohorts, but only the PTH and RAL drugs showed a synergistic interaction and only for the WT mice (Table 1). Furthermore, PTH + RAL was the only combination treatment that significantly improved femoral cortical area over the PTH monotherapy [Fig. 5(d) and 5(e)], and the drugs showed a significant interaction in the WT animals for this parameter (Table 1). Finally, the anabolic treatments were equally efficacious for improving other aspects of cortical geometry (Table 4). Therefore, although the loss of Nmp4 did not further improve the combination treatments’ restorative efficacy, the gain in trabecular bone [Fig. 2(a) and 2(b)] did not compromise the improved gains in the cortical compartment.
Figure 5.
(a–c) Postcranial (WB) BMD and (d–f) cortical bone area of femoral diaphysis for all the experimental cohorts comparing ovariectomized WT and Nmp4−/− mice (age 24 weeks). (b and e) We compared the anabolic therapies PTH + RAL, PTH + ZOL, PTH + ALN, and PTH with each other and with VEH. (c and f) We compared the anticatabolic treatments ALN, ZOL, and RAL with each other and with VEH. Statistical analyses were performed using two-way analyses of variance, setting genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05. The data represent mean ± standard deviation, n = 5–12 mice per group. See text for explanation of results.
Table 4.
Cortical Geometry: Anabolic Therapies
| Group | Cortical Thickness | Marrow Area | Total Area | Endocortical Perimeter | Periosteal Perimeter | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VEH: WT/Nmp4−/− | 0.190 ± 0.016/0.184 ± 0.007 | 0.964 ± 0.096/0.958 ± 0.026 | 1.841 ± 0.109/1.834 ± 0.092 | 3.843 ± 0.185/3.861 ± 0.121 | 5.243 ± 0.167/5.242 ± 0.175 | |||||
| PTH: WT/Nmp4−/− | 0.201 ± 0.009/0.196 ± 0.009 | 1.029 ± 0.090/0.992 ± 0.069 | 2.028 ± 0.118/2.024 ± 0.096 | 2.028 ± 0.118/3.955 ± 0.088 | 5.508 ± 0.172/5.476 ± 0.130 | |||||
| PTH+ALN: WT/Nmp4−/− | 0.200 ± 0.014/0.197 ± 0.007 | 1.046 ± 0.082/0.907 ± 0.129 | 2.074 ± 0.078/2.015 ± 0.145 | 4.104 ± 0.214/4.023 ± 0.302 | 5.633 ± 0.076/5.497 ± 0.235 | |||||
| PTH+ZOL: WT/Nmp4−/− | 0.207 ± 0.009/0.198 ± 0.011 | 1.012 ± 0.084/0.957 ± 0.092 | 2.074 ± 0.159/2.061 ± 0.126 | 4.038 ± 0.151/4.301 ± 0.477 | 5.595 ± 0.246/5.543 ± 0.220 | |||||
| PTH+RAL: WT/Nmp4−/− | 0.206 ± 0.015/0.203 ± 0.020 | 0.891 ± 0.136/0.822 ± 0.149 | 2.061 ± 0.116/2.005 ± 0.111 | 3.958 ± 0.404/3.860 ± 0.262 | 5.550 ± 0.225/5.546 ± 0.327 | |||||
| Anabolic therapy | G: P = 0.0501 | G: P = 0.0028 | G: P = 0.23 | G: P = 0.9952 | G: P = 0.28 | |||||
| T: P = 0.0003 | T: P < 0.0001 | T: P < 0.0001 | T: P = 0.0029 | T: P < 0.0001 | ||||||
| PTH+RAL | A | PTH | A | PTH+ZOL | A | PTH+ZOL | A | PTH+ZOL | A | |
| PTH+ZOL | A | PTH+ZOL | A | PTH+ALN | A | PTH+ALN | AB | PTH+ALN | A | |
| PTH | A | PTH+ALN | A | PTH+RAL | A | PTH | AB | PTH+RAL | A | |
| PTH+ALN | A | VEH | A | PTH | A | PTH+RAL | B | PTH | A | |
| VEH | B | PTH+RAL | B | VEH | B | VEH | B | VEH | B | |
| GxT: P = 0.92 | GxT: P = 0.92 | GxT: P = 0.89 | GxT: P = 0.18 | GxT: P = 0.86 | ||||||
The micro-CT analyses of the femoral cortical geometry in mice treated with the anabolic therapies. The data were analyzed using two-way analyses of variance to compare the anabolic therapies and the anticatabolic monotherapies. Statistical significance was set at P ≤ 0.05, and levels not connected by the same letter are significantly different. The data represent mean ± standard deviation, n = 7 to 12 mice per group.
Abbreviations: G, genotype; T, treatment.
Effects of anticatabolic treatments on bone
RAL and ZOL promoted modest bone restoration; loss of Nmp4 further augmented RAL-induced increases in femoral trabecular bone.
The Nmp4 skeletal phenotype appears to be largely driven by the hyperanabolic activity of osteogenic cells (18, 22, 38). Nevertheless, the response of the Nmp4−/− mice to antiresorptive therapy alone had not been reported; therefore, we analyzed the therapeutic efficacy of the SERM RAL and the bisphosphosphonates ALN and ZOL on ovariectomized WT and null mice. The RAL and ZOL monotherapies significantly improved femoral and L5 BV/TV [Fig. 2(c) and 2(f)], as well as WB BMD and cortical bone area [Fig. 5(c) and 5(f)]. However, RAL was particularly notable in that it was the only anticatabolic that increased femoral Tb.Th in both genotypes (Table 5) and enhanced cortical area over the VEH cohorts [Fig. 5(f)], and the only antiresorptive that increased femoral cortical thickness and decreased femoral marrow area (Table 6). However, its impact on L5 trabecular architecture was unexceptional (Table 7).
Table 5.
Femoral Trabecular Parameters: Anticatabolic Therapies
| Group | Femoral Tb.N | Femoral Tb.Th | Femoral Tb.Sp | |||
|---|---|---|---|---|---|---|
| VEH: WT/Nmp4−/− | 0.91 ± 0.23/1.18 ± 0.09 | 0.046 ± 0.006/0.045 ± 0.003 | 0.331 ± 0.023/0.285 ± 0.011 | |||
| ALN: WT/Nmp4−/− | 1.17 ± 0.25/1.14 ± 0.29 | 0.051 ± 0.006/0.046 ± 0.004 | 0.302 ± 0.007/0.294 ± 0.023 | |||
| ZOL: WT/Nmp4−/− | 1.38 ± 0.38/1.47 ± 0.47 | 0.052 ± 0.003/0.049 ± 0.007 | 0.316 ± 0.032/0.282 ± 0.029 | |||
| RAL: WT/Nmp4−/− | 1.21 ± 0.36/1.74 ± 0.13 | 0.060 ± 0.003/0.059 ± 0.005 | 0.313 ± 0.023/0.274 ± 0.011 | |||
| Anticatabolic therapy | G: | P = 0.0024 | G: | P = 0.0701 | G: | P < 0.0001 |
| T: | P < 0.0001 | T: | P < 0.0001 | T: | P < 0.2017 | |
| GxT: | P = 0.0306 | RAL | A | GxT: | P = 0.04 | |
| Nmp4−/− RAL | A | ZOL | B | WT VEH | A | |
| Nmp4−/− ZOL | AB | ALN | B | WT ZOL | AB | |
| WT ZOL | AB | VEH | B | WT RAL | AB | |
| WT RAL | BC | GxT: | P = 0.22 | WT ALN | ABC | |
| Nmp4−/− VEH | BC | Nmp4−/− ALN | BC | |||
| WT ALN | BC | Nmp4−/− VEH | BC | |||
| Nmp4−/− ALN | BC | Nmp4−/− ZOL | BC | |||
| WT VEH | C | Nmp4−/− RAL | C | |||
The micro-CT analyses of the femoral architecture in mice treated with the anticatabolic therapies. The data were analyzed using two-way analyses of variance using genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05, and levels not connected by the same letter are significantly different. The data represent mean ± standard deviation, n = 7 to 12 mice per group.
Abbreviations: G, genotype; T, treatment.
Table 6.
Cortical Geometry: Anticatabolic Therapies
| Group | Cortical Thickness | Marrow Area | Total Area | Endocortical Perimeter | Periosteal Perimeter | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VEH: WT/Nmp4−/− | 0.190 ± 0.016/0.184 ± 0.007 | 0.964 ± 0.096/0.958 ± 0.026 | 1.841 ± 0.109/1.834 ± 0.092 | 3.843 ± 0.185/3.861 ± 0.121 | 5.243 ± 0.167/5.242 ± 0.175 | |||||
| ALN: WT/Nmp4−/− | 0.187 ± 0.013/0.181 ± 0.012 | 0.968 ± 0.050/0.963 ± 0.085 | 1.865 ± 0.099/1.836 ± 0.112 | 3.870 ± 0.143/3.874 ± 0.161 | 5.251 ± 0.105/5.204 ± 0.162 | |||||
| ZOL: WT/Nmp4−/− | 0.189 ± 0.010/0.192 ± 0.007 | 1.033 ± 0.091/0.994 ± 0.072 | 1.944 ± 0.080/1.879 ± 0.078 | 4.116 ± 0.183/3.925 ± 0.158 | 5.477 ± 0.170/5.308 ± 0.140 | |||||
| RAL: WT/Nmp4−/− | 0.202 ± 0.010/0.206 ± 0.010 | 0.856 ± 0.076/0.892 ± 0.054 | 1.757 ± 0.063/1.827 ± 0.084 | 3.761 ± 0.123/3.820 ± 0.125 | 5.153 ± 0.098/5.271 ± 0.191 | |||||
| Anticatabolic therapy | G: P = 0.62 | G: P = 0.83 | G: P = 0.72 | G: P = 0.46 | G: P = 0.52 | |||||
| T: P < 0.0001 | T: P < 0.0001 | T: P = 0.0094 | T: P = 0.0010 | T: P = 0.0073 | ||||||
| RAL | A | ZOL | A | ZOL | A | ZOL | A | ZOL | A | |
| ZOL | B | ALN | A | ALN | AB | ALN | B | VEH | B | |
| VEH | B | VEH | A | VEH | AB | VEH | B | ALN | B | |
| ALN | B | RAL | B | RAL | B | RAL | B | RAL | B | |
| GxT: P = 0.92 | GxT: P = 0.58 | GxT: P = 0.25 | GxT: P = 0.12 | GxT: P = 0.10 | ||||||
The micro-CT analyses of the femoral cortical geometry in mice treated with the anticatabolic therapies. The data were analyzed using two-way analyses of variance to compare the anabolic therapies and the anticatabolic monotherapies. Statistical significance was set at P ≤ 0.05, and levels not connected by the same letter are significantly different. The data represent mean ± standard deviation, n = 7 to 12 mice per group.
Abbreviations: G, genotype; T, treatment.
Table 7.
L5 Trabecular Parameters: Anticatabolic Therapies
| Group | L5 Tb.N | L5 Tb.Th | L5 Tb.Sp | |||
|---|---|---|---|---|---|---|
| VEH: WT/Nmp4−/− | 3.71 ± 0.21/3.79 ± 0.15 | 0.051 ± 0.003/0.054 ± 0.003 | 0.250 ± 0.013/0.239 ± 0.017 | |||
| ALN: WT/Nmp4−/− | 3.81 ± 0.29/3.66 ± 0.27 | 0.051 ± 0.003/0.055 ± 0.003 | 0.247 ± 0.008/0.241 ± 0.018 | |||
| ZOL: WT/Nmp4−/− | 5.08 ± 0.89/4.42 ± 0.54 | 0.049 ± 0.004/0.053 ± 0.001 | 0.232 ± 0.028/0.233 ± 0.021 | |||
| RAL: WT/Nmp4−/− | 4.01 ± 0.40/4.44 ± 0.34 | 0.049 ± 0.003/0.054 ± 0.002 | 0.245 ± 0.026/0.230 ± 0.019 | |||
| Anticatabolic therapy | G: | P = 0.4554 | G: | P < 0.0001 | G: | P = 0.12 |
| T: | P < 0.0001 | T: | P = 0.31 | T: | P = 0.23 | |
| ZOL | A | GxT: | P = 0.87 | GxT: | P = 0.64 | |
| RAL | B | |||||
| VEH | C | |||||
| ALN | C | |||||
| GxT: | P = 0.0067 | |||||
The micro-CT analyses of the L5 trabecular architecture in mice treated with the anticatabolic therapies. The data were analyzed using two-way analyses of variance to compare the anabolic therapies and the anticatabolic monotherapies. Statistical significance was set at P ≤ 0.05, and levels not connected by the same letter are significantly different. The data represent mean ± standard deviation, n = 7 to 12 mice per group.
Abbreviations: G, genotype; T, treatment.
Unexpectedly, loss of Nmp4 enhanced RAL-induced increases in femoral BV/TV compared with WT mice [genotype × treatment interaction, P = 0.03; Fig. 2(c)]. Moreover, under the RAL monotherapy, the null cohorts showed significantly higher femoral Tb.N and exhibited a lower femoral Tb.Sp compared with WT mice (Table 5). Disabling Nmp4 did not amplify the response to the bisphosphonates. This would suggest that Nmp4 suppresses a SERM-mediated pathway(s) mediating femoral trabecular bone restoration in our preclinical model.
The effects of combination treatments using anabolic agents on osteoprogenitor cells
The combination of PTH, RAL, and loss of Nmp4 significantly expanded the bone marrow osteoprogenitor pool but had no similar impact on the number of marrow adipocytes or TRAP+ cells.
To address the cellular basis underlying the improved femoral trabecular bone response of Nmp4−/− mice over the WT animals, we counted various cell types in formalin-fixed, paraffin-embedded bone marrow sections using immunohistochemical analysis. We also used flow cytometry to obtain bone marrow cellular profiles. Based on the micro-CT imaging results, we largely limited our focus to VEH; the RAL, ZOL, and PTH monotherapies; and the PTH + RAL and PTH + ZOL combination treatments.
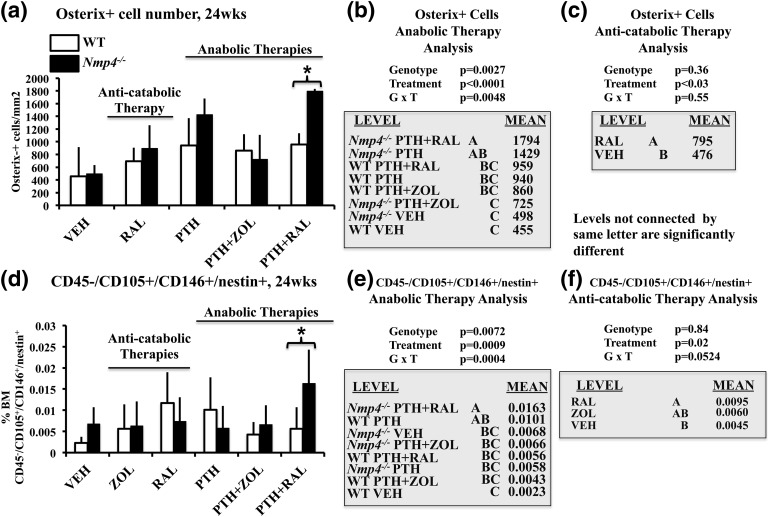
We counted bone marrow cells that were positive for the early osteoblast-specific transcription factor osterix (39) as one method to identify osteoprogenitors [Supplemental Fig. 3(A–F) (17MB, tif) ]. Nmp4−/− mice under the PTH + RAL therapy harbored more osteoprogenitors than the WT cohorts treated with this regimen [genotype × treatment effect, P = 0.0048; Fig. 6(a) and 6(b)]. Additionally, the null mice harbored significantly more bone marrow osteoprogenitors under the PTH + RAL therapy than under the PTH + ZOL treatment, but the WT cohorts did not exhibit this dichotomy [Fig. 6(a) and 6(b)]. As a complementary approach, we performed flow cytometry analysis of WT and Nmp4−/− bone marrow CD45−/CD105+/CD146+/nestin+ cells, which have been used as markers to identify MSPC/osteoprogenitors (40, 41) [Fig. 6(d) and 6(e)]. As observed with immunohistochemistry (IHC) staining, the Nmp4−/− mice under the PTH + RAL therapy exhibited a significantly expanded population of these cells compared with WT mice treated with this combination regimen [treatment × genotype, P = 0.0004; Fig. 6(d) and 6(e)]. Moreover, as determined with the IHC analysis, the flow cytometry analysis revealed that the null mice harbored significantly more bone marrow CD45−/CD105+/CD146+/nestin+ cells under the PTH + RAL therapy than under the PTH + ZOL treatment, and that the WT cohorts did not exhibit this contrast [Fig. 6(d) and 6(e)].
Figure 6.
(a–c) Bone marrow osterix+ cells and (d–f) flow cytometry analysis of the means of the frequency of femoral bone marrow CD45−/CD105+/CD146+/CD105+/nestin+ cells in ovariectomized WT and Nmp4−/− mice (24 weeks of age). (b and e) We compared the anabolic therapies PTH + RAL, PTH + ZOL, and PTH with each other and with VEH using either osterix+ expression or the expression profile of CD45−/CD105+/CD146+/CD105+/nestin+ as the endpoints. (c) We compared the number of osterix+ cells in the WT and Nmp4−/− RAL monotherapy cohorts. (f) We compared the anticatabolic treatments ZOL and RAL with each other and with VEH using the expression profile of CD45−/CD105+/CD146+/CD105+/nestin+ as the endpoint. Statistical analyses were performed using two-way analyses of variance, setting genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05. The asterisk denotes genotype × treatment interaction. The data represent mean ± standard deviation, n = 4 to 6 osterix+ cells and n = 7 to 12 mice per group for flow cytometry. See text for explanation of results.
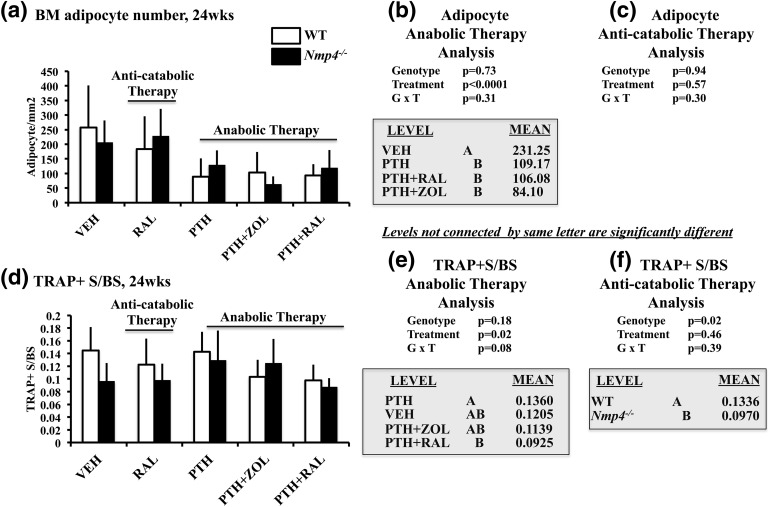
We next addressed whether the anabolic treatment regimens and/or loss of Nmp4 altered bone marrow adiposity because, in addition to osteoprogenitors, MSPCs can differentiate into bone marrow fat cells. Treatments that included PTH (i.e., PTH monotherapy, PTH + ZOL, and PTH + RAL significantly decreased the number of bone marrow adipocytes) [Fig. 7(a) and 7(b)]. Bone marrow sections from the VEH cohorts exhibited a high number of adipocytes throughout the marrow and near the growth plate, but adipocyte numbers were strikingly lower in marrow from cohorts that included PTH in the treatment regimen [Supplemental Fig. 4(A) and 4(B) (18.8MB, tif) ]. However, unlike the case with the osteoprogenitors, loss of Nmp4 did not alter the frequency of these cells in the bone marrow, and therefore did not appear to have a direct or indirect effect on adipogenic lineage commitment in our model.
Figure 7.
(a–c) Bone marrow (BM) adipocytes and (d–f) TRAP+ S/BS for WT and Nmp4−/− mice (24 weeks of age). (b and e) We compared the anabolic therapies PTH + RAL, PTH + ZOL, and PTH with each other and with VEH using either adipocyte number or TRAP+ S/BS as the endpoints. (c and f) We compared the number of adipocytes or the TRAP+ S/BS in the WT and Nmp4−/− RAL monotherapy cohorts. Statistical analyses were performed using two-way analyses of variance, setting genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05. The data represent mean ± standard deviation, n = 5 fields from six mice per cohort for the adipocytes and n = 6 mice per group (TRAP+ S/BS). See text for explanation of results.
We assessed femoral TRAP+ S/BS as an indicator of osteoclast activity and whether Nmp4 regulated the number of these cells under the anabolic treatments [Fig. 7(d) and 7(e)]. In contrast to the osteoprogenitor profiles, loss of Nmp4 did not impact the number of TRAP+ cells under the PTH + RAL regimen or any of the other anabolic treatments [genotype × treatment, P = 0.08; Fig. 7(b)]. The PTH + RAL WT/Nmp4−/− cohorts had fewer TRAP+ cells than the PTH groups, but both were equivalent to PTH + ZOL- and VEH-treated mice [treatment effect, P = 0.02; Fig. 7(e)].
Effects of anticatabolic treatments on osteoprogenitor cells
We next addressed the impact of RAL, ZOL, and Nmp4 on the bone marrow osteoprogenitor pool in the anticatabolic arm of the study. The RAL monotherapy significantly elevated the number of osteoprogenitors compared with VEH cohorts, as determined by both IHC and flow cytometry analyses, but loss of Nmp4 did not impact the response of these cell populations to this antiresorptive [Fig. 6(c) and 6(f)]. ZOL monotherapy did not significantly alter the number of CD45−/CD105+/CD146+/nestin+ cells compared with the VEH cohorts [Fig. 6(f)]. RAL monotherapy had no impact on adipocyte number [P = 0.57; Fig. 7(c)] or TRAP+ S/BS [treatment effect, P = 0.46; Fig. 7(f)] in our preclinical model. The statistical comparison between the RAL monotherapy and VEH cohorts indicated that the null mice harbored fewer TRAP+ cells than WT animals [genotype effect, P = 0.02; Fig. 7(f)]. This genotype difference was not observed when analyzing the anabolic cohorts [Fig. 7(e)].
We conclude that the combination of PTH, RAL, and loss of Nmp4 is strongly restorative or nurturing for bone marrow osteoprogenitors, and that the substitution of ZOL for RAL abrogates this tonic effect in the null mice. Moreover, the loss of Nmp4 does not influence the number of bone marrow adipocytes or TRAP+ cells under the strongly anabolic PTH + RAL therapy.
Effects of treatments on bone turnover markers
Nmp4 status did not influence serum profile response to any treatment.
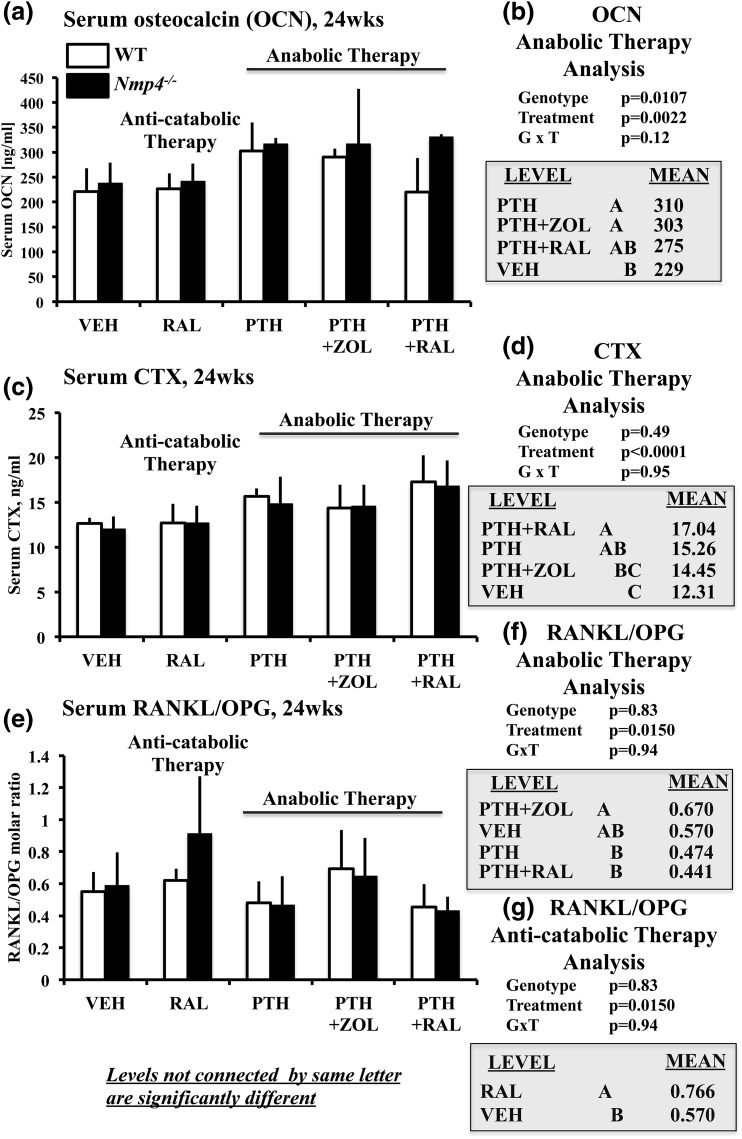
To address the impact of Nmp4 on serum bone formation and resorption makers of select therapies, we evaluated osteocalcin, CTX, and the RANKL/osteoprotegerin (OPG) ratio in mice under the PTH, RAL, PTH + RAL, PTH + ZOL, and VEH therapies. Loss of Nmp4 elevated levels of the serum bone formation marker osteocalcin in mice under the anabolic therapies; however, there was no genotype × treatment interaction [genotype effect, P = 0.01; genotype × treatment, P = 0.12; Fig. 8(a) and 8(b)]. The treatment groups PTH and PTH + ZOL exhibited significantly elevated serum osteocalcin compared with VEH controls, whereas the PTH + RAL cohorts were not significantly different from either VEH or the other two groups [treatment effect, P = 0.0022; Fig. 8(b)].
Figure 8.
(a and b) Serum osteocalcin (OCN), (c and d) serum CTX, and (e and f) serum RANKL/OPG for WT and Nmp4−/− mice (24 weeks of age). (b, d, and f) We compared the anabolic therapies PTH + RAL, PTH + ZOL, and PTH with each other and with VEH using the three serum parameters as endpoints. (g) We compared VEH with RAL monotherapy using RANKL/OPG as the endpoint. Statistical analyses were performed using two-way analyses of variance, setting genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05. The data represent mean ± standard deviation, n = 6 to 7 (OCN, CTX, RANKL/OPG). See text for explanation of results.
Disabling Nmp4 had no impact on serum CTX and serum RANKL/OPG [no genotype effect or genotype × treatment interaction; Fig. 8(c–g)]. Analysis by treatment groups showed that mice from the PTH + RAL therapy exhibited significantly elevated serum CTX levels compared with mice from the PTH + ZOL and VEH treatments [Fig. 8(d)]. The PTH + ZOL cohorts exhibited a significantly larger RANKL/OPG ratio than PTH and PTH + RAL, but none of these treatments were statistically different from VEH [Fig. 8(f)].
The RAL monotherapy did not alter serum osteocalcin or CTX (data not shown). This treatment did, however, elevate RANKL/OPG [Fig. 8(g)]. Therefore, only serum osteocalcin, and not the resorption markers, could broadly discriminate between the genotypes [genotype effect, P = 0.0107; Fig. 8(b)]. However, Nmp4 status did not influence how any of the treatments altered serum marker profiles (no genotype × treatment interactions; Fig. 8). Finally, we noticed that the parameters of osteoclast size and bone resorption did not necessarily move in a parallel manner in our analysis. We have reported on this phenomenon before in healthy, nonovariectomized Nmp4−/− mice (21). This dissociation between CTX levels and osteoclast area may be due in part to the fact that CTX is a systemic measurement, whereas osteoclast area is a local measurement. An example of a similar observation is described in the Prx1-Cre; RBPjkf/f (PRBP) mouse (42). Finally, Weinstein et al. (43) report that long-term ALN treatment is associated with an increase in the number of osteoclasts, and an increase in osteoclast size in healthy postmenopausal women.
Discussion
The blunting of PTH bone-forming efficacy may be a principal limitation of osteoporosis combination therapies (7). We have previously demonstrated that deletion of Nmp4 enhances PTH-induced trabecular bone formation in experimental mice and, as such, the goals of this study were to determine whether combining this sustained anabolic response with an anticatabolic results in superior bone acquisition compared with PTH monotherapy. Additionally, we inquired whether Nmp4 interferes with anticatabolic efficacy. In principle, PTH combination therapies have the potential to maximize skeletal mass while maintaining a tonic level of remodeling by boosting bias toward bone formation and minimizing loss from resorption. We evaluated seven therapies against a control, including three anticatabolics singly and in concurrent combination with PTH in a preclinical osteoporosis model, comparing skeletal improvement in WT and Nmp4−/− mice. The PTH + RAL and PTH + ZOL combination treatments outperformed the PTH monotherapy throughout the skeleton, and loss of Nmp4 further leveraged the potency of these bone-restoring osteoanabolic regimens in some of the trabecular compartments. Unexpectedly, the Nmp4−/− mice also exhibited an enhanced femoral BV/TV response to RAL monotherapy. These improvements in the restoration of the trabecular bone compartment did not come at the expense of gains in cortical bone. Altogether, this is an exciting proof of principle, in scenarios of heightened osteoanabolism, that combination treatment can be more effective than PTH alone.
The bone-forming efficacy of the concurrent PTH combination treatments in this study generally corresponded with PTH + RAL = PTH + ZOL > PTH + ALN = PTH > VEH, which parallels the results observed in several individual clinical studies, thus supporting the medical relevance of our findings and the potential clinical impact of disabling Nmp4. Deal et al. (44) compared the efficacy of PTH monotherapy with concurrent PTH + RAL in 117 postmenopausal women over a 6-month period. BMD in the concurrent group was numerically higher than the PTH monotherapy at the spine and hip, but this increase was only statistically significant at the hip (44). In the PTH and alendronate study (16), treatments of naive women were randomized to three groups: (1) PTH monotherapy; (2) ALN monotherapy; and (3) concurrent PTH + ALN. Quantitative computed tomography revealed severe blunting of BMD increases to PTH in the PTH + ALN group. Finally, patients on concurrent PTH + ZOL therapy were compared with patients on ZOL alone or PTH monotherapy (9). Contrary to the results obtained with ALN in the PTH and alendronate study, greater increases in BMD were observed with concurrent PTH + ZOL treatment than the monotherapies (7, 9, 16, 44). In the current study, PTH and RAL showed a synergistic drug interaction throughout the analyzed skeletal sites, including the WB BMD, cortical bone area, femur, and L5 trabecular bone volume. PTH + ZOL generally equaled the therapeutic performance of PTH + RAL with respect to bone gain, but the synergistic interaction of PTH and ZOL was more limited with respect to skeletal sites. PTH + ALN was the least efficacious of the concurrent combination therapies and did not outperform the PTH monotherapy.
The loss of Nmp4 further improved the gains in femoral trabecular bone obtained with the concurrent PTH + RAL and PTH + ZOL combination treatments and with the RAL monotherapy, resolving our primary queries as to whether the heightened osteoanabolism of the Nmp4−/− skeleton would boost the restorative response to diverse osteoporosis treatments. We observed a strong genotype × treatment interaction under the anabolic therapies of PTH, PTH + RAL, and PTH + ZOL for femoral BV/TV and for multiple trabecular bone architectural parameters. The improved RAL monotherapy-induced increases in femoral BV/TV and other trabecular architectural parameters were unanticipated because the Nmp4−/− skeletal phenotype is distinguished by the exaggerated response to anabolic cues (18, 22, 23, 38, 45). And, indeed, in the current study, the Nmp4−/− mice showed no improved response with the bisphosphonates.
The hyperanabolic phenotype of the Nmp4−/− bone marrow osteoprogenitors and their progeny most likely drives the improved responses to the osteoporosis therapies observed in the current study. Moreover, loss of Nmp4 had no effect on the cellular or serum parameters of the resorption arm in cohorts under the anabolic therapies. The elevated expressions of c-MYC and GADD34 in the Nmp4−/− cells (26) may underlie their precocious and enhanced mineralization in culture (18). c-MYC is a potent inducer of ribosome biogenesis (46), and the Nmp4−/− MSPCs show significantly elevated global protein synthesis (26), consistent with the increased bone matrix production. The subsequent increase in the load of endoplasmic reticulum client proteins typically triggers the UPR, which in turn diminishes global protein synthesis via the phosphorylation of eIF2α (47). Upon resolution of endoplasmic reticulum stress, GADD34 serves as a feedback mechanism to dephosphorylate eIF2α, facilitating resumption of protein synthesis. However, in the Nmp4−/− cells, the high expression of GADD34 maintains elevated matrix synthesis throughout UPR activation without initiating apoptosis (26).
In the current study, the PTH + RAL therapy significantly expanded the bone marrow pool of the Nmp4−/− hyperanabolic osteoprogenitors. Although the RAL monotherapy increased the number of these cells in both genotypes, the heightened osteoanabolism of the Nmp4−/− osteoprogenitors and their progeny is consistent with the enhanced RAL-induced increase in femoral BV/TV compared with the WT mice. RAL has been shown to have tonic effects on osteogenic cells both in vivo and in vitro, depending on the model system, and the concentration and duration of exposure to this drug (48–55). The ZOL monotherapy had no impact on this bone marrow population in our study, but the substitution of ZOL for RAL in the concurrent combination regimen abrogated the expansion of the Nmp4−/− osteoprogenitor pool, suggesting that the PTH + ZOL combination lacked the boost to this cell population provided by combining PTH with RAL. Bisphosphonates have both tonic as well as toxic effects on osteogenic cells, depending on various factors (56–60). Therefore, Nmp4 may play a role in osteogenic cell response to anticatabolics without influencing the impact of these drugs on the resorption arm. This may explain why treatment with the amino-bisphosphonate ALN was less successful at enhancing bone mass than the amino-bisphosphonate ZOL when applied either alone or in combination with PTH. There are a number of factors that may explain the differences between ALN and ZOL efficacy in our study. ZOL is a significantly more potent bisphosphonate than ALN due in part to its stronger binding to both farnesyl pyrophosphate synthase enzyme and bone (61). Interestingly, the frequency of dosing may also contribute to the differences obtained in our investigation and prior studies. Specifically, osteoblasts may take up bisphosphonates by pinocytosis, leading to the inhibition of the mevalonate pathway, and it has been speculated that the more frequent dosing with ALN compared with ZOL may increase exposure of osteoblasts to bisphosphonates from the interstitial fluid (7). However, in the present model, the PTH + ZOL therapy was equally effective as the PTH + RAL treatment at increasing femoral BV/TV. This observation is consistent with a previous study showing that intermittent PTH significantly increased the luciferase activity of tagged bone marrow stromal cells used to generate bony ossicles implanted in immunocompromised mice, but combining ZOL with PTH treatment reduced this hormone-mediated increase in luciferase activity without attenuating the PTH-induced increase in total bone (62). Therefore, it is likely that osteoprogenitors alone do not drive the heightened pharmacologically induced osteoanabolism observed in the Nmp4−/− mouse, and that bone lining cells, osteoblasts, and perhaps osteocytes also contribute to this phenotype. Studies using conditional deletion of this gene are required to fully interrogate the cellular hierarchy of the Nmp4−/− skeletal phenotype.
The implication regarding osteoporosis treatment is that disabling Nmp4 will boost whatever anabolic activity is associated with any particular therapy. Suppression of sclerostin with the drug romozosumab, a bone formation inhibitor, represents a route to bone anabolism and is proof of principle that impeding osteogenic inhibitors is a powerful approach to therapy (1). Nmp4 is another kind of inhibitor in that its inactivation boosts the response potency to osteoanabolics, but, unlike romozosumab, does not impact baseline skeletal phenotype.
The expansion of the Nmp4−/− osteogenic reserve did not appear to occur at the expense of marrow adipogenic potential. Bone marrow fat cells derive from heterogeneous populations of MSPCs, not all of which have the capacity for committing to the adipogenic lineage (63, 64). Our present data revealed no genotype effect for adipocyte number. However, these data showed a strong treatment effect in that PTH-based therapies reduced bone marrow adipogenesis in both the WT and Nmp4−/− mice. This is consistent with the previous observations that osteoporosis patients as well as ovariectomized rats exhibit an enhanced fat in the marrow (65, 66) and that PTH attenuates marrow adiposity in both rats (65) and in postmenopausal osteopenic women (67).
The improved gains in the Nmp4−/− L5 trabecular architecture were more moderate than those observed in the femur, although loss of Nmp4 increased L5 BV/TV across the anabolic treatment groups as a whole and improved anabolic therapeutic thickening of the trabeculae. The observed weaker response of the rodent spine to PTH-based therapies compared with that of the femur is consistent with previous observations in C57BL/6 mice and is perhaps related to weight bearing (68).
The exaggerated recovery of Nmp4−/− trabecular bone did not come at the cost of therapeutic gains in the cortical compartment. We previously demonstrated that the Nmp4−/− osteoprogenitors express elevated levels of the PTH1R receptor and that these null cells exhibited an exaggerated response to hormone challenge (18). Calvi et al. (37) reported that constitutively active PTH1R in osteoblasts resulted in mice with increased trabecular bone volume, but decreased cortical thickness. Additionally, the elevation in PTH-induced remodeling typically leads to increased cortical porosity (69–72) with potentially detrimental effects on bone strength (7). However, our present data showed that the exaggerated response to PTH and the concurrent combination therapies in the Nmp4−/− mice did not compromise improvements in cortical area and cortical thickness nor WB BMD, which is typically 80% cortical.
“The quest will continue for the ‘holy grail’ of anabolic osteoporosis therapies, which will optimize the impact on bone formation relative to resorption” (73). This medical objective requires the use of clinical, preclinical, and basic science research. Jilka has incisively described the advantages and limitations of mouse models for investigating the pathophysiology of osteoporosis and its treatment (74), and the present model is no exception. However, the principal extraordinary feature of the Nmp4−/− phenotype is the exaggerated skeletal responses to diverse osteoanabolic therapies, whereas bone development, growth, and baseline phenotype are all largely unexceptional in the absence of provocation (18, 20, 21, 23). This demonstrates a clear and unique advantage of developing Nmp4 or one of its upstream/downstream components as a target to significantly improve efficacy of existing therapies. Moreover, because the loss of Nmp4 appears to enhance the response potency to other anabolic signals (23), we propose that abaloparatide or other PTH peptides may produce a similar heightened anabolism in these mice. Finally, this unique preclinical tool provides an opportunity for investigating the intrinsic critical barriers to pharmacologically induced bone formation.
Acknowledgments
We thank Dr. Huadong Zhao, presently at the Department of Statistics and Actuarial Science, East China Normal University, for helpful discussions on the analyses of the data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense, Indiana Clinical and Translational Science Institute (CTSI), or the National Institutes of Health.
Acknowledgments
This work was supported, in whole or in part, by Department of Defense Grant PR120563; Indiana University School of Medicine CTSI Biomedical Research Grant (to J.P.B.); and National Institutes of Health Grants AR062002 (to M.R.A.), GM049164 (to R.C.W.), and AR053237 (to A.G.R.).
Author contributions: Y.S., S.J.W., R.C.W., M.R.A., and J.P.B. designed, conceived, and coordinated the study. Y.S., Y.H., S.H.-B., K.W.C., and H.D. performed the experiments. All authors were involved in data analysis and manuscript preparation, and all authors reviewed the results and approved the final version of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Osterix | ASSLLEEEVH YGSSPLAMLT AACSKFGGSS PLRDSTTLGK AGTKKPYSVG | Anti-Sp7 | Abcam, ab94744 | Rabbit; polyclonal | 1:25 | AB_10674971 |
| CD45 | PerCP-Cy5.5 mouse; anti-mouse CD45.2 | BD Biosciences, 552950 | Mouse; monoclonal | 1:100 | AB_394528 | |
| CD105 | APC anti-mouse CD105 antibody | BioLegend, 120414 | Rat; monoclonal | 1:100 | AB_2277914 | |
| CD146 | PE rat anti-mouse CD146 | BD Biosciences, 562196 | Rat; monoclonal | 1:100 | AB_10896286 | |
| NESTIN | FITC nestin antibody (rat-401) | Santa Cruz, sc-33677 | Rat; monoclonal | 1:100 | AB_627995 |
Abbreviations: APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein; RRID, research resource identifier.
Footnotes
- micro-CT
- microcomputed tomography
- ALN
- alendronate
- BMD
- bone mineral density
- BV/TV
- trabecular bone volume per total volume
- CTX
- C-terminal telopeptide
- IHC
- immunohistochemistry
- MSPC
- mesenchymal stem/progenitor cell
- OPG
- osteoprotegerin
- PBS
- phosphate-buffered saline
- PTH
- parathyroid hormone
- RAL
- raloxifene
- RANKL
- receptor activator of nuclear factor-κB ligand
- SERM
- selective estrogen receptor modulator
- Tb.N
- trabecular number
- Tb.Sp
- trabecular spacing
- Tb.Th
- trabecular thickness
- TRAP
- tartrate-resistant acid phosphatase
- TRAP+ S/BS
- TRAP+ cell surface/bone surface
- UPR
- unfolded protein response
- VEH
- vehicle control
- WB
- whole body
- WT
- wild-type
- ZOL
- zoledronate.
References
- 1.Jilka RL. Inhibiting the inhibitor: a new route to bone anabolism. J Bone Miner Res. 2009;24(4):575–577. [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, Lindsay R. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16(5):925–931. [DOI] [PubMed] [Google Scholar]
- 3.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85(9):3069–3076. [DOI] [PubMed] [Google Scholar]
- 4.Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312–321. [DOI] [PubMed] [Google Scholar]
- 5.Cusano NE, Bilezikian JP. Combination antiresorptive and osteoanabolic therapy for osteoporosis: we are not there yet. Curr Med Res Opin. 2011;27(9):1705–1707. [DOI] [PubMed] [Google Scholar]
- 6.Seeman E, Martin TJ. Co-administration of antiresorptive and anabolic agents: a missed opportunity. J Bone Miner Res. 2015;30(5):753–764. [DOI] [PubMed] [Google Scholar]
- 7.Eriksen EF, Brown JP. Commentary: concurrent administration of PTH and antiresorptives: additive effects or DXA cosmetics. Bone. 2016;86:139–142. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Chen W, Lin Y. The efficacy of parathyroid hormone analogues in combination with bisphosphonates for the treatment of osteoporosis: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94(38):e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosman F, Eriksen EF, Recknor C, Miller PD, Guanabens N, Kasperk C, Papanastasiou P, Readie A, Rao H, Gasser JA, Bucci-Rechtweg C, Boonen S. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:503–511. [DOI] [PubMed] [Google Scholar]
- 10.Walker MD, Cusano NE, Sliney J Jr, Romano M, Zhang C, McMahon DJ, Bilezikian JP. Combination therapy with risedronate and teriparatide in male osteoporosis. Endocrine. 2013;44(1):237–246. [DOI] [PubMed] [Google Scholar]
- 11.Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005;353(6):566–575. [DOI] [PubMed] [Google Scholar]
- 12.Muschitz C, Kocijan R, Fahrleitner-Pammer A, Lung S, Resch H. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res. 2013;28:196–205. [DOI] [PubMed] [Google Scholar]
- 13.Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SA. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386(9999):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ; PaTH Study Investigators . One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–565. [DOI] [PubMed] [Google Scholar]
- 16.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ; PaTH Study Investigators . The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207–1215. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349(13):1216–1226. [DOI] [PubMed] [Google Scholar]
- 18.Childress P, Stayrook KR, Alvarez MB, Wang Z, Shao Y, Hernandez-Buquer S, Mack JK, Grese ZR, He Y, Horan D, Pavalko FM, Warden SJ, Robling AG, Yang FC, Allen MR, Krishnan V, Liu Y, Bidwell JP. Genome-wide mapping and interrogation of the Nmp4 antianabolic bone axis. Mol Endocrinol. 2015;29(9):1269–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torrungruang K, Alvarez M, Shah R, Onyia JE, Rhodes SJ, Bidwell JP. DNA binding and gene activation properties of the Nmp4 nuclear matrix transcription factors. J Biol Chem. 2002;277(18):16153–16159. [DOI] [PubMed] [Google Scholar]
- 20.Robling AG, Childress P, Yu J, Cotte J, Heller A, Philip BK, Bidwell JP. Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J Cell Physiol. 2009;219(3):734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Childress P, Philip BK, Robling AG, Bruzzaniti A, Kacena MA, Bivi N, Plotkin LI, Heller A, Bidwell JP. Nmp4/CIZ suppresses the response of bone to anabolic parathyroid hormone by regulating both osteoblasts and osteoclasts. Calcif Tissue Int. 2011;89(1):74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Childress P, Hood M Jr, Alvarez M, Kacena MA, Hanlon M, McKee B, Bidwell JP, Yang FC. Nmp4/CIZ suppresses the parathyroid hormone anabolic window by restricting mesenchymal stem cell and osteoprogenitor frequency. Stem Cells Dev. 2013;22(3):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morinobu M, Nakamoto T, Hino K, Tsuji K, Shen ZJ, Nakashima K, Nifuji A, Yamamoto H, Hirai H, Noda M. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201(6):961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamoto T, Shiratsuchi A, Oda H, Inoue K, Matsumura T, Ichikawa M, Saito T, Seo S, Maki K, Asai T, Suzuki T, Hangaishi A, Yamagata T, Aizawa S, Noda M, Nakanishi Y, Hirai H. Impaired spermatogenesis and male fertility defects in CIZ/Nmp4-disrupted mice. Genes Cells 2004;9(6):575–589. [DOI] [PubMed] [Google Scholar]
- 25.Nakamoto T, Izu Y, Kawasaki M, Notomi T, Hayata T, Noda M, Ezura Y. Mice deficient in CIZ/NMP4 develop an attenuated form of K/BxN-serum induced arthritis. J Cell Biochem. 2016;117(4):970–977. [DOI] [PubMed] [Google Scholar]
- 26.Young SK, Shao Y, Bidwell JP, Wek RC. Nuclear matrix protein 4 is a novel regulator of ribosome biogenesis and controls the unfolded protein response via repression of Gadd34 expression. J Biol Chem. 2016;291(26):13780–13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman CL, Chen NX, Smith E, Smith M, Brown D, Moe SM, Allen MR. Compromised vertebral structural and mechanical properties associated with progressive kidney disease and the effects of traditional pharmacological interventions. Bone. 2015;77:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone. 2015;71:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lui PP, Lee YW, Mok TY, Cheuk YC, Chan KM. Alendronate reduced peri-tunnel bone loss and enhanced tendon graft to bone tunnel healing in anterior cruciate ligament reconstruction. Eur Cell Mater. 2013;25:78–96. [DOI] [PubMed] [Google Scholar]
- 30.Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745–751. [DOI] [PubMed] [Google Scholar]
- 31.Cano A, Dapia S, Noguera I, Pineda B, Hermenegildo C, del Val R, Caeiro JR, Garcia-Pérez MA. Comparative effects of 17beta-estradiol, raloxifene and genistein on bone 3D microarchitecture and volumetric bone mineral density in the ovariectomized mice. Osteoporos Int. 2008;19(6):793–800. [DOI] [PubMed] [Google Scholar]
- 32.Sheng ZF, Xu K, Ma YL, Liu JH, Dai RC, Zhang YH, Jiang YB, Liao EY. Zoledronate reverses mandibular bone loss in osteoprotegerin-deficient mice. Osteoporos Int. 2009;20:151–159. [DOI] [PubMed] [Google Scholar]
- 33.Hsiao EC, Boudignon BM, Chang WC, Bencsik M, Peng J, Nguyen TD, Manalac C, Halloran BP, Conklin BR, Nissenson RA. Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci USA. 2008;105(4):1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wattanachanya L, Wang L, Millard SM, Lu WD, O’Carroll D, Hsiao EC, Conklin BR, Nissenson RA. Assessing the osteoblast transcriptome in a model of enhanced bone formation due to constitutive Gs-G protein signaling in osteoblasts. Exp Cell Res. 2015;333(2):289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erlebacher A, Derynck R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol. 1996;132(1–-2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore DS MG. Introduction to the Practice of Statistics 4th ed New York, NY: W.H. Freeman and Co. [Google Scholar]
- 37.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107(3):277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hino K, Nakamoto T, Nifuji A, Morinobu M, Yamamoto H, Ezura Y, Noda M. Deficiency of CIZ, a nucleocytoplasmic shuttling protein, prevents unloading-induced bone loss through the enhancement of osteoblastic bone formation in vivo. Bone. 2007;40(4):852–860. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. [DOI] [PubMed] [Google Scholar]
- 40.Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, Frenette PS. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29(3):340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. [DOI] [PubMed] [Google Scholar]
- 42.Tu X, Chen J, Lim J, Karner CM, Lee SY, Heisig J, Wiese C, Surendran K, Kopan R, Gessler M, Long F. Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet. 2012;8(3):e1002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, Glass EV, Myers SL, Krege JH. Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res. 2005;20:1905–1911. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Bidwell JP, Young SR, Gerard-O’Riley R, Wang H, Pavalko FM. Nmp4/CIZ inhibits mechanically induced beta-catenin signaling activity in osteoblasts. J Cell Physiol. 2010;223(2):435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10(4):301–309. [DOI] [PubMed] [Google Scholar]
- 47.Chambers JE, Marciniak SJ. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 2. Protein misfolding and ER stress. Am J Physiol Cell Physiol. 2014;307(8):C657–C670. [DOI] [PubMed] [Google Scholar]
- 48.Miki Y, Suzuki T, Nagasaki S, Hata S, Akahira J, Sasano H. Comparative effects of raloxifene, tamoxifen and estradiol on human osteoblasts in vitro: estrogen receptor dependent or independent pathways of raloxifene. J Steroid Biochem Mol Biol. 2009;113(3-5):281–289. [DOI] [PubMed] [Google Scholar]
- 49.Somjen D, Katzburg S, Sharon O, Knoll E, Hendel D, Stern N. Sex specific response of cultured human bone cells to ERα and ERβ specific agonists by modulation of cell proliferation and creatine kinase specific activity. J Steroid Biochem Mol Biol. 2011;125(3-5):226–230. [DOI] [PubMed] [Google Scholar]
- 50.Viereck V, Gründker C, Blaschke S, Niederkleine B, Siggelkow H, Frosch KH, Raddatz D, Emons G, Hofbauer LC. Raloxifene concurrently stimulates osteoprotegerin and inhibits interleukin-6 production by human trabecular osteoblasts. J Clin Endocrinol Metab. 2003;88(9):4206–4213. [DOI] [PubMed] [Google Scholar]
- 51.Taranta A, Brama M, Teti A, De luca V, Scandurra R, Spera G, Agnusdei D, Termine JD, Migliaccio S. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone. 2002;30(2):368–376. [DOI] [PubMed] [Google Scholar]
- 52.Giner M, Rios MJ, Montoya MJ, Vázquez MA, Miranda C, Pérez-Cano R. Alendronate and raloxifene affect the osteoprotegerin/RANKL system in human osteoblast primary cultures from patients with osteoporosis and osteoarthritis. Eur J Pharmacol. 2011;650(2-3):682–687. [DOI] [PubMed] [Google Scholar]
- 53.Matsumori H, Hattori K, Ohgushi H, Dohi Y, Ueda Y, Shigematsu H, Satoh N, Yajima H, Takakura Y. Raloxifene: its ossification-promoting effect on female mesenchymal stem cells. J Orthop Sci. 2009;14(5):640–645. [DOI] [PubMed] [Google Scholar]
- 54.Lin Y, Liu LJ, Murray T, Sodek J, Rao L. Effect of raloxifene and its interaction with human PTH on bone formation. J Endocrinol Invest. 2004;27(5):416–423. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Graff E, Benayahu D. Effect of raloxifene-analog (LY 117018-Hcl) on the bone marrow of ovariectomized mice. J Cell Biochem. 2000;76(3):509–517. [DOI] [PubMed] [Google Scholar]
- 56.Lezcano V, Bellido T, Plotkin LI, Boland R, Morelli S. Osteoblastic protein tyrosine phosphatases inhibition and connexin 43 phosphorylation by alendronate. Exp Cell Res. 2014;324(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104(10):1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan B, To LB, Farrugia AN, Findlay DM, Green J, Gronthos S, Evdokiou A, Lynch K, Atkins GJ, Zannettino AC. The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone. 2004;34(1):112–123. [DOI] [PubMed] [Google Scholar]
- 59.Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L, Rosen E, Ikeda H, Okawa Y, Kiziltepe T, Schoonmaker J, Xie W, Hideshima T, Weller E, Bouxsein ML, Munshi NC, Anderson KC, Raje N. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res. 2009;15(18):5829–5839. [DOI] [PubMed] [Google Scholar]
- 60.Corrado A, Neve A, Maruotti N, Gaudio A, Marucci A, Cantatore FP. Dose-dependent metabolic effect of zoledronate on primary human osteoblastic cell cultures. Clin Exp Rheumatol. 2010;28(6):873–879. [PubMed] [Google Scholar]
- 61.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. [DOI] [PubMed] [Google Scholar]
- 62.Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42(4):806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18(6):782–796. [DOI] [PubMed] [Google Scholar]
- 64.Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL, Longaker MT. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1-2):285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulkarni NH, Wei T, Kumar A, Dow ER, Stewart TR, Shou J, N’cho M, Sterchi DL, Gitter BD, Higgs RE, Halladay DL, Engler TA, Martin TJ, Bryant HU, Ma YL, Onyia JE. Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J Cell Biochem. 2007;102(6):1504–1518. [DOI] [PubMed] [Google Scholar]
- 66.Cordes C, Baum T, Dieckmeyer M, Ruschke S, Diefenbach MN, Hauner H, Kirschke JS, Karampinos DC. MR-based assessment of bone marrow fat in osteoporosis, diabetes, and obesity. Front Endocrinol (Lausanne). 2016;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Luo X, Xie X, Yan F, Chen G, Zhao W, Jiang Z, Fang C, Shen J. Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric 2016;19(3):285–291. [DOI] [PubMed] [Google Scholar]
- 68.Iida-Klein A, Zhou H, Lu SS, Levine LR, Ducayen-Knowles M, Dempster DW, Nieves J, Lindsay R. Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J Bone Miner Res. 2002;17:808–816. [DOI] [PubMed] [Google Scholar]
- 69.Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14(6):690–709. [DOI] [PubMed] [Google Scholar]
- 70.Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM. Intermittently administered human parathyroid hormone(1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2001;16:157–165. [DOI] [PubMed] [Google Scholar]
- 71.Fox J, Miller MA, Newman MK, Turner CH, Recker RR, Smith SY. Treatment of skeletally mature ovariectomized rhesus monkeys with PTH(1-84) for 16 months increases bone formation and density and improves trabecular architecture and biomechanical properties at the lumbar spine. J Bone Miner Res. 2007;22:260–273. [DOI] [PubMed] [Google Scholar]
- 72.Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28:736–745. [DOI] [PubMed] [Google Scholar]
- 73.Black DM, Schafer AL. The search for the optimal anabolic osteoporosis therapy. J Bone Miner Res. 2013;28:2263–2265. [DOI] [PubMed] [Google Scholar]
- 74.Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]