Abstract
Transgenic mice harboring high molecular weight fibroblast growth factor (FGF)2 isoforms (HMWTg) in osteoblast lineage cells phenocopy human X-linked hypophosphatemic rickets (XLH) and Hyp murine model of XLH demonstrating increased FGF23/FGF receptor signaling and hypophosphatemic rickets. Because HMWFGF2 was upregulated in bones of Hyp mice and abnormal FGF receptor (FGFR) signaling is important in XLH, HMWTg mice were used to examine the effect of the FGFR inhibitor NVP-BGJ398, now in clinical trials for cancer therapy, on hypophosphatemic rickets. Short-term treatment with NVP-BGJ398 rescued abnormal FGFR signaling and hypophosphatemia in HMWTg. Long-term treatment with NVP-BGJ398 normalized tail, tibia, and femur length. Four weeks NVP-BGJ398 treatment significantly increased total body bone mineral density (BMD) and bone mineral content (BMC) in HMWTg mice; however, at 8 weeks, total body BMD and BMC was indistinguishable among groups. Micro-computed tomography revealed decreased vertebral bone volume, trabecular number, and increased trabecular spacing, whereas femur trabecular tissue density was increased; however, NVP-BGJ398 rescued defective cortical bone mineralization, increased thickness, reduced porosity, and increased endosteal perimeter and cortical tissue density in HMWTg. NVP-BGJ398 improved femur cancellous bone, cortical bone structure, growth plate, and double labeling in cortical bone and also increased femur trabeculae double labeled surface, mineral apposition rate, bone formation rate, and osteoclast number and surface in HMWTg. The decreased NPT2a protein that is important for renal phosphate excretion was rescued by NVP-BGJ398 treatment. We conclude that NVP-BGJ398 partially rescued hypophosphatemic rickets in HMWTg. However, long-term treatment with NVP-BGJ398 further increased serum FGF23 that could exacerbate the mineralization defect.
Long-term inhibition of FGFR signaling with NVP-BGJ398 partially rescued hypophosphatemic rickets in HMWTg male mice but further increased serum FGF23 that could exacerbate the mineralization defect.
Fibroblast growth factor (FGF) ligands and their receptors (FGFRs) are important modulators of bone growth and development in mice and humans (1–5). Mutations in FGFRs cause autosomal dominant human chondrodysplasias (1–4) due to aberrant or amplified signal transduction from the tyrosine kinase domain of FGFRs (2, 3). Ligands for FGFRs have also been recently associated with human diseases (2, 3, 6). Bone-derived FGF23 is the phosphaturic factor (6) responsible for autosomal dominant hypophosphatemic rickets (7), tumor-induced osteomalacia (8), X-linked hypophosphatemic rickets (XLH) (9), and autosomal recessive hypophosphatemic rickets, caused by loss of function mutations in dentin matrix protein-1 (Dmp1) (10). Murine models of this group of disorders include the FGF23 transgenic mouse, a model of autosomal dominant hypophosphatemic rickets (11); the Hyp mouse, a homolog of XLH (12); and the Dmp1 null mouse, a model of autosomal recessive hypophosphatemic rickets (13). These murine homologs demonstrate many of the phenotypic changes associated with the respective human disorders, including elevated serum FGF23.
FGF23 plays a major role in human hypophosphatemic disorders associated with bone mineralization defect osteomalacia (1). However, the regulators of FGF23 production, the signal pathway for FGF23 induced phosphate (Pi) wasting, and the cause of bone mineralization defect in these disorders are not fully defined. Another member of the FGF family of ligands, FGF2, is mitogenic for cells including osteoblasts (OBs) and chondrocytes (2, 14, 15). The Fgf2 gene encodes multiple FGF2 high molecular weight (HMW) protein isoforms expressed from unique CUG alternative translation start sites located 5′ to the classical AUG initiation codon for the 18 kDa low molecular weight (LMW) exported isoform (2, 16). HMWFGF2 isoforms are ordinarily not released from the cells, but have nuclear localization sequences and function in an intracrine manner (17–21). In humans, there are three HMW isoforms of 22, 23, and 24 kDa and a LMW isoform of 18 kDa FGF2 protein isoform. In rodents, there are two HMW isoforms of 21 and 22 kDa and a LMW17.5 kDa FGF2 isoform that functions in an autocrine/paracrine manner (18, 19). The biological functions of the FGF2 isoforms have not been fully defined; however, studies have shown differential effects of the LMW (22) and the HMW (23) isoforms on cardiac tissue homeostasis in mice. In addition, we previously reported that global overexpression of all FGF2 isoforms caused defective bone mineralization and osteopenia in FGF2 transgenic mice (24). We also reported that global knockout of all isoforms of FGF2 in mice resulted in reduced bone mass and bone formation (25) due to lineage change resulting in increased bone marrow adipogenesis (26). We further reported that transgenic mice that express the human LMW18 kDa isoform (LMWTg) under the control of the Col3.6 promoter had increased bone mass with normal calcium, Pi homeostasis (27). Furthermore, there was no increase in FGF23 in serum of LMWTg mice relative to Vector control mice. In contrast, to the increased bone mass found in the LMWTg mice, we made the novel discovery that transgenic mice that express the human high molecular weight (HMWTg) FGF2 isoforms under the control of the Col3.6 promoter display reduced bone mineral density (BMD), rickets, defective mineralization/osteomalacia, hypophosphatemia, and increased FGF23 in serum and bone (28). Intriguingly, in the Hyp mouse, a model of human XLH, we found that Fgf2 messenger RNA (mRNA) and HMW FGF2 protein isoforms were increased in bones/OBs, and that FGF23 and HMW protein colocalized in Hyp osteocytes (28).
Although the downstream signaling pathway by which FGF23 mediates Pi wasting is not fully understood, several studies have shown that FGF23 produced by osteocytes is released into the circulation and interacts with FGF receptors and klotho on renal cells to increase mitogen activated protein kinases/ERK signaling, resulting in downregulation of the renal sodium/Pi cotransporter NPT2a, resulting in reduced tubular reabsorption of Pi (1). As in the FGF23 transgenic mice, Pi wasting in the HMWTg mice is associated with abnormal FGF23/FGFR/Klotho/ERK signaling, resulting in downregulation of the renal NPT2a (28).
FGF23 signaling via FGFR also has effects to inhibit matrix mineralization that are independent of its effects on Pi homeostasis (29). Interestingly, we previously published in vitro studies demonstrating that defective bone matrix mineralization in HMWTg mice is due in part to increased FGF23/FGFR signaling because there was partial rescue of impaired mineralized nodule formation by a neutralizing antibody to FGF23 (30). The present in vivo study examines whether bone and Pi wasting phenotypes of HMWTg mice can be fully or partially rescued by a novel FGF receptor inhibitor that was shown by Wohrle et al. (31) to ameliorate rickets in the Hyp and Dmp1ko mouse models.
Materials and Methods
Animals
All animal protocols were approved by the UConn Health Institute of Animal Care and Use Committee. As previously described in detail (28), we generated mice expressing HMW isoforms of hFGF2 in a bone-specific manner using a construct, abbreviated Col3.6-HMWFgf2 isoform-IRES-GFPsaph. Col3.6-HMWFgf2 isoform-IRES-GFPsaph was built by replacing a CAT fragment in previously made Col3.6-CAT-IRES-GFPsaph (32) with a human HMWFgf2 complementary DNA (cDNA) between AfeI and ScaI sites. This expression construct is capable of concurrently overexpressing the human HMW FGF2 isoforms (33) and GFPsaph from a single bicistronic mRNA. The construct also harbors a neomycin selection gene. Generation of the Fgf2 cDNAs was previously described (16). As control, a Col3.6-IRES/GFPsaph (Vector) construct was also generated and purified according to standard techniques. Microinjections into the pronucleus of fertilized oocytes were performed at the Gene Targeting & Transgenic Facility at UConn Health. Founder mice of the F2 (FVBN) strain were mated with wild-type mice to establish individual transgenic lines. Homozygote mice were generated by mating heterozygote male with heterozygote female. Initial characterization of the bone phenotype of the HMWTg mice was previously reported (28). Male mice were used in this study.
FGFR inhibitor treatment
NVP-BGJ398 is a novel selective pan specific FGFR inhibitor. NVP-BGJ398 (50 mg/kg body weight; Novartis, Basel, Switzerland) (31) or vehicle only (PEG-300/Glucose 5%, 2:1 mix) was administered by oral gavage. For long-term gavage treatment over 8 weeks, dosing was initiated at 5 weeks of age with a schedule of three treatments per week (31). Vector mice were treated with vehicle only. Mice were used at 5 to 6 weeks of age in the case of single-dose at 50 mg/kg body gavage administrations. We also performed additional long-term experiment that includes a Vector group subject to long-term NVP-BGJ398 treatment using a lower concentration (34). In this experiment, mice were 21 days old at treatment initiation and received daily subcutaneous (sq) administration of NVP-BGJ398 for 6 weeks (2 mg/kg body weight) or vehicle (hydrochloric acid 3.5 mM, dimethyl sulfoxide 5%) (34). Most data reported in this study are from mice that were treated with NVP-BGJ398 at 50 mg/kg orally unless otherwise specified. Mice were euthanized by CO2 for sample collection.
Faxitron X-ray
X-ray pictures of the whole mouse and excised bones were taken using a SYSTEM MX 20 from Faxitron X-ray Corp. (Wheeling, IL). X-ray images were taken under constant conditions (25 kV, 20-second exposure at 4.5 magnification).
Dual beam X-ray absorptiometry
Dual beam X-ray absorptiometry imaging was performed using the LunarPIXImus2 (GE Medical Systems, Madison, WI) densitometer to measure BMD and bone mineral content (BMC) for both in vivo and excised bones (28).
Micro-computed tomography scanning of femurs
Analysis of the middiaphysis cortical bones and metaphyseal cancellous bones of the distal femurs was performed as described previously (25) with micro-computed tomography (micro-CT) instrumentation (μCT20; Scanco Medical AG, Bassersdorf, Switzerland). Using two-dimensional data from scanned slices, three-dimensional analysis was conducted to calculate morphometric parameters defining microarchitecture, including bone volume (BV) density, BV/total volume (TV), trabecular number, trabecular thickness, trabecular spacing, connective tissue density, degree of anisotropy, subperiosteal area, cortical mask, cortical porosity, and cortical thickness.
Bone histomorphometry
Mice were labeled with calcein 7 days and xylenol orange 2 days before they were euthanized, respectively. Femurs were isolated and fixed in 10% formalin. Bones were then placed overnight in 30% sucrose dissolved in phosphate-buffered saline (PBS) and embedded in Cryomatrix. The Cryomatrix block containing each undecalcified femur was oriented in the block holder to obtain a 7-μm longitudinal central section that includes the central vein. Sections were collected on a special cold adhesive tape Cryofilm type IIC (FINETEC Co. Ltd., Japan). Unstained tapes with samples were soaked in PBS for half an hour and then mounted in 50% glycerol in PBS for dynamic parameter analysis. Additional sections were stained for tartrate-resistant acid phosphatase to visualize osteoclasts and counterstained with hematoxylin. Histomorphometric measurements were made in a blinded, nonbiased manner using the OsteoMeasure image analysis system (R & M Biometrics, Nashville, TN) interfaced with a Nikon E400 microscope (Nikon, Inc., Melville, NY). The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (35). Mineralizing surface/bone surface (BS), the bone formation rate/BS, interlabel thickness, mineral apposition rate (μm/day), BV fraction (BV/TV), percent OB surface, osteoclast surface, and osteoclast number/BS were measured. OBs were identified as cuboidal cells lining the trabecular bone. Osteoclasts were identified as multinucleated cells with more than three nuclei on the trabecular BS.
For cortical static histomorphometry, longitudinal sections were used. Osteoid volume/BV was measured along 1250 µm of middiaphysis cortical bone of femur on Masson Trichrome–stained sections (36).
Histology
For histological analysis, frozen femur sections were stained with Alcian Blue to identify cartilage matrices and with Alizarin Red to identify bone. Frozen sections were scanned to detect GFP, calcein, and xylenol orange labeling of bone cells. GFP expression in cells was visualized using an Olympus IX50 inverted system microscope equipped with an IX-FLA inverted reflected light fluorescence (Olympus America, Inc., Melville, NY). A specific excitation wavelength was obtained using filters for GFPsaph (exciter, D395/40; dichroic, 425DCLP; emitter, D510/40m) and recorded with a SPOT-camera (Diagnostic Instrument, Inc., Sterling Heights, MI). Fluorescent images were taken with equal exposure times applied to bones derived from Vector and HMWTg mice.
Frozen sections of kidneys were used for immunofluorescence staining of KLOTHO. Kidneys were isolated and fixed in 10% formalin. Kidneys were then placed overnight in 30% sucrose dissolved in PBS and embedded in Cryomatrix. The sections were washed in 1× PBS/1% fetal bovine serum (FBS) and permeabilized with 0.25% Triton X-100 in 1× PBS/1% FBS for 10 minutes. After being rinsed with 1× PBS/1% FBS, the sections were stained with anti–KLOTHO antibody [15 μg/mL; R&D Systems, Minneapolis, MN; Research Resource Identifier (RRID): AB_2296612] overnight at 4°C. After rinsing, cells were incubated with Alexa Fluor 594 rabbit antigoat lgG (Invitrogen, Grand Island, NY). After washing, coverslips were mounted on slides and nuclei were counterstained with 4’,6-diamidino-2-phenylindole.
For immunohistochemistry staining, frozen sections of kidneys were used. The primary antibodies were a monoclonal antiphospho-p44/42 mitogen activated protein kinase antibody (Cell Signaling Technology, Inc., Beverly, MA; RRID: AB_331768) used at a 1:150 dilution, anti-NPT2a antibody (Alpha Diagnostic International, Inc., San Antonio, TX; RRID: AB_1622108) used at 20 μg/mL. Briefly, the sections were blocked (0.3% H2O2/methanol) for 10 minutes, followed by blocking in 1:200 normal serum for 30 minutes, and then incubated with primary antibodies at 4°C overnight. The sections were washed three times for 5 minutes in PBS, and then a horseradish peroxidase–conjugated secondary antibody was applied for 30 minutes. Following three 5-minute PBS washes, color was developed with ABC reagent for 30 minutes. Slides were counterstained with hematoxylin. Slides were mounted, examined, and photographed using bright field microscopy (Nikon Corp., Shinagawa-ku, Tokyo, Japan).
RNA isolation and real-time polymerase chain reaction
Total RNA was extracted from whole femur using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA). For real-time quantitative reverse transcription polymerase chain reaction analysis, RNA was reverse-transcribed using the RNA to cDNA EcoDry™ Premix (Oligo dT). Quantitative polymerase chain reaction was carried out using the iTaq™ Universal SYBR® Green Supermix on a MyiQ™ instrument (Bio-Rad Laboratories, Inc., Hercules, CA). β-Actin was used as an internal reference for each sample. mRNA was normalized to the Gapdh mRNA level and expressed as the fold-change relative to the first sample for each experimental group. Relative mRNA expression was calculated using a formula reported previously (37). The primers for the genes of interest are listed in Supplemental Table 1 (82.9KB, docx) .
Biochemistry
Blood was collected from euthanized animals by cardiac puncture. After clotting, the blood was spun, and serum was collected. Serum Pi and calcium were measured using the Phosphorus Liqui-ultraviolet (StanBio Laboratory, Boerne, TX) and Calcium (CPC) Reagent Set (Eagle Diagnostics, Cedar Hill, TX), respectively. Serum intact and C-term FGF23 was measured using kits purchased from Immunotopics, Inc. (Carlsbad, CA), and serum parathyroid hormone (PTH) was determined using a mouse intact PTH enzyme-linked immunosorbent assay kit (Immunotopics, Athens, OH) according to the manufacturer’s instructions. Serum 1,25(OH)2D3 was measured using 1,25-Dihydroxy Vitamin D EIA kit from Immuno Diagnostic Systems (IDS; Gaithersburg, MD). Serum αKlotho was measured using Mouse KL/Klotho enzyme-linked immunosorbent assay kit (LifeSpan BioSciences, Inc., Seattle, WA). For short-term experiments, spot urine samples were collected during euthanasia, and phosphate excretion index (PEI) was determined. For the urine Pi assay of long-term experiments, mice were kept in metabolic cages, and 24-hour urine was collected. Urine Pi was measured using the Phosphorus Liqui-ultraviolet kit (StanBio Laboratory). For long-term experiments, serum was prepared at 24 hours after last gavage of the 8-week study.
Western blot analysis
Total protein of kidneys was extracted using 1× radioimmune precipitation buffer (Cell Signaling Technology, Inc., Danvers, MA), and protein concentration was assayed with BCA protein assay reagent (Thermo Fisher Scientific, Waltham, MA). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis on Any kD™ Mini-PROTEAN® TGX™ Gels, proteins were transferred to Immun-Blot PVD membranes (Bio-Rad Laboratories, Inc.). Membranes were blocked overnight in TBS-T containing 5% nonfat dry milk (Bio-Rad Laboratories, Inc.). Membranes were then incubated with an antiphospho Pp44/42 (Cell Signaling Technology, Inc.; RRID: AB_331768) for 1 hour, washed for 1 hour with TBS-T, and then incubated with a donkey antirabbit secondary antibody (Amersham Biosciences, Piscataway, NJ) for Pi-p44/42 in TBS-T/1% nonfat milk for 1 hour. After incubation with antibodies, membranes were washed for 1 hour with TBS-T. After further washing with TBS–T, immunoreactive bands were visualized using Amersham ECL Plus Western Blotting Detection System (GE Health Care, Buckinghamshire, UK) and Hyperfilm-ECL film (Amersham Biosciences, Europe, GMBH) in accordance with the manufacturer’s instructions. Equivalent amounts of total proteins were run on a separate gel and probed for total p44/42 antibody (Cell Signaling Technology, Inc.; RRID: AB_330744) as a loading control. For KLOTHO and NPT2a, 300 μg of total protein from kidney were diluted with 1× radioimmune precipitation buffer and incubated at 4°C overnight with Protein A/G PLUS-agarose (20 μL) (Santa Cruz Biotechnology), as well as KLOTHO (R&D Systems; RRID: AB_2296612), or NPT2a (Alpha Diagnostic International, Inc., San Antonio, TX; RRID: AB_1622108) antibody. Beads were washed three times, and precipitated proteins were followed by Western blot, using KLOTHO, Pp44/42, and NPT2a antibodies. Equivalent amounts of total proteins were run on a separate gel and probed for Actin (Santa Cruz Biotechnology, Dallas, TX; RRID: AB_630835) as a loading control. Band densities were quantified densitometrically by ImageJ64.
Statistical analysis
Experimental values are reported as mean ± standard error (SE; standard error of the mean). Analysis of variance followed by least significant difference for post hoc multiple comparisons was used. SPSS software (IBM Corp, Armonk, NY) was used for statistical analysis, and the results were considered significantly different at P < 0.05.
Results
Short-term treatment with FGFR inhibitor NVP-BGJ398 restores mineral ion homeostasis in HMWTg mice
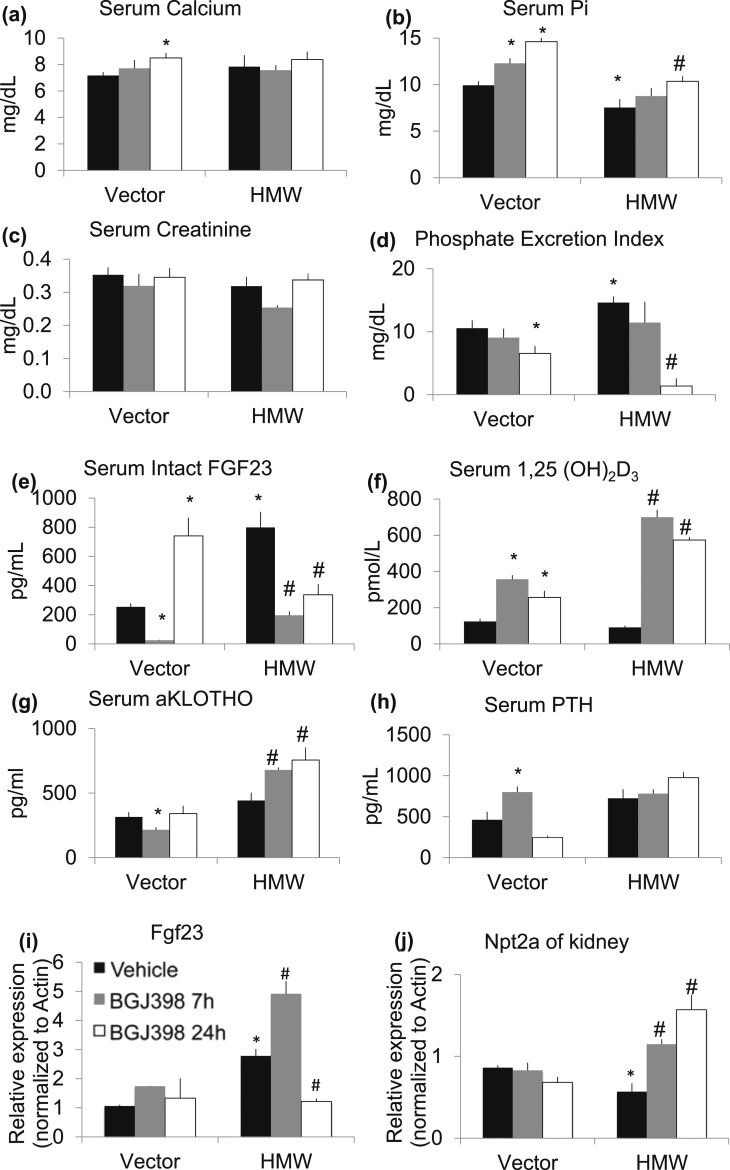
To initially assess the effect of NVP-BGJ398 on FGFR/FGF23 signaling in both Vector and HMWTg mice, we performed a single-dose, short-term treatment study in which serum biochemical markers were analyzed at 7 hours and 24 hours after gavage to determine the immediate effects of FGFR inhibition before the onset of feedback regulation. As shown in Figure 1(a), short-term FGFR inhibition at 7 hours after NVP-BGJ398 treatment did not affect serum calcium level in either Vector or HMWTg mice. However, NVP-BGJ398 significantly increased serum calcium level in Vector mice at 24 hours after NVP-BGJ398 treatment. Consistent with our previous publication (28), there was a significant decrease in serum Pi in HMWTg-Vehicle mice compared with Vector-Vehicle. NVP-BGJ398 significantly increased serum Pi level in Vector mice at 7 and 24 hours, and in HMWTg mice at 24 hours after gavage [Fig. 1(b)]. NVP-BGJ398 treatment did not affect serum creatinine level in Vector mice. However, there was a transient repression of serum creatinine at 7 hours NVP-BGJ398 treatment in HMWTg mice [Fig. 1(c)]. NVP-BGJ398 suppressed Pi excretion in kidneys in Vector mice at 24 hours after administration. Increased PEI in HMWTg mice was suppressed at 7 and 24 hours by NVP-BGJ398 administration [Fig. 1(d)].
Figure 1.
Effect of short-term NVP-BGJ398 administration on serum and urinary biochemical markers in Vector and HMWTg mice. Five- to six-week-old Vector or HMWTg mice received a single oral dose of the FGFR inhibitor NVP-BGJ398 (50 mg/kg, oral) or vehicle and were studied 7 or 24 hours after administration of NVP-BGJ398. Vector mice were treated with vehicle only. Serum was collected during euthanasia. (a) Serum calcium, (b) Pi, and (c) creatinine levels were measured. (d) Spot urine samples were collected during euthanasia and PEI was determined. (e) Serum intact FGF23, (f) serum 1,25(OH)2D3, (g) serum αKlotho, and (h) serum PTH levels were determined by enzyme-linked immunosorbent assay. (i) Whole femur Fgf23 mRNA level and (j) kidney Npt2a mRNA level were analyzed by quantitative polymerase chain reaction. Data are mean ± SE, n = 4 to 7 per group. *Compared with Vector-Vehicle group, P < 0.05; #Compared with HMWTg-Vehicle group, P < 0.05.
There was a transient repression of FGF23 serum levels at 7 hours NVP-BGJ398 treatment, but increased at 24 hours after NVP-BGJ398 treatment in Vector mice. In contrast, increased serum FGF23 level in HMWTg-Vehicle mice compared with Vector-Vehicle was significantly suppressed by NVP-BG398 at both 7 and 24 hours [Fig. 1(e)]. It is known that FGF23 regulate serum Pi in part through regulating 1,25(OH)2D3 production via 1-α-hydroxylase in the kidney (38). Although serum FGF23 was significantly increased in HMWTg-Vehicle mice, serum 1,25(OH)2D3 in HMWTg-Vehicle mice were not significantly different compared with Vector-Vehicle mice [Fig. 1(e) and 1(f)]. In both Vector and HMWTg mice, NVP-BGJ398 treatment resulted in a significant increase in serum 1,25(OH)2D3 at 7 and 24 hours after dosing [Fig. 1(f)].
We also examined the effect of FGFR inhibitor treatment on αKlotho serum levels [Fig. 1(g)]. NVP-BGJ398 treatment transiently reduced serum αKlotho in Vector mice at 7 hours after gavage. However, in HMWTg mice, NVP-BGJ398 significantly increased serum αKlotho at 7 and 24 hours after treatment.
The effect of FGFR inhibitor treatment on serum levels of PTH was also determined [Fig. 1(h)]. In Vector-Vehicle mice, PTH levels were significantly increased after 7 hours of NVP-BGJ398 treatment, whereas PTH level was reduced to Vector-Vehicle level at 24 hours after administration. In contrast, serum PTH was 56% higher in HMWTg-Vehicle compared with Vector-Vehicle mice, even though the increase did not reach significance. NVP-BGJ398 treatment did not affect PTH level in HMWTg mice. Taken together, these results suggest that pharmacological inhibition of FGFRs with NVP-BGJ398 counteracts FGF23 signaling in Vector and HMWTg mice.
We examined the effect of NVP-BGJ398 on Fgf23 mRNA expression in whole femur [Fig. 1(i)]. Fgf23 mRNA level was increased in HMWTg-Vehicle mice compared with Vector-Vehicle. At 7 hours after treatment, there was an increase in Fgf23 mRNA level in HMWTg mice. However, at 24 hours after treatment, Fgf23 mRNA was significantly decreased.
Because the type 2 sodium-dependent Pi cotransporter Npt2a plays critical roles in the reabsorption of Pi by renal proximal tubular, we examined Npt2a mRNA expression in kidney. As shown in Fig. 1(j), Npt2a expression was significantly decreased in HMWTg-Vehicle group compared with Vector-Vehicle group. NVP-BGJ398 significantly increased Npt2a expression in HMWTg mice at 7 and 24 hours after treatment.
Effects of long-term NVP-BGJ398 treatment on mineral ion homeostasis in HMWTg mice
To determine whether long-term inhibition of FGFR regulate BMD in Vector and HMWTg mice, both Vector and HMWTg mice were treated with Vehicle or NVP-BGJ398 (2 mg/kg, sq) for 6 weeks. As shown in Supplemental Figure 1(a) (47.2KB, pptx) , femur BMD was decreased in HMWTg-Vehicle mice compared with Vector-Vehicle mice. Long-term NVP-BGJ398 treatment significantly increased femur BMD in HMWTg mice but did not increase BMD in Vector mice.
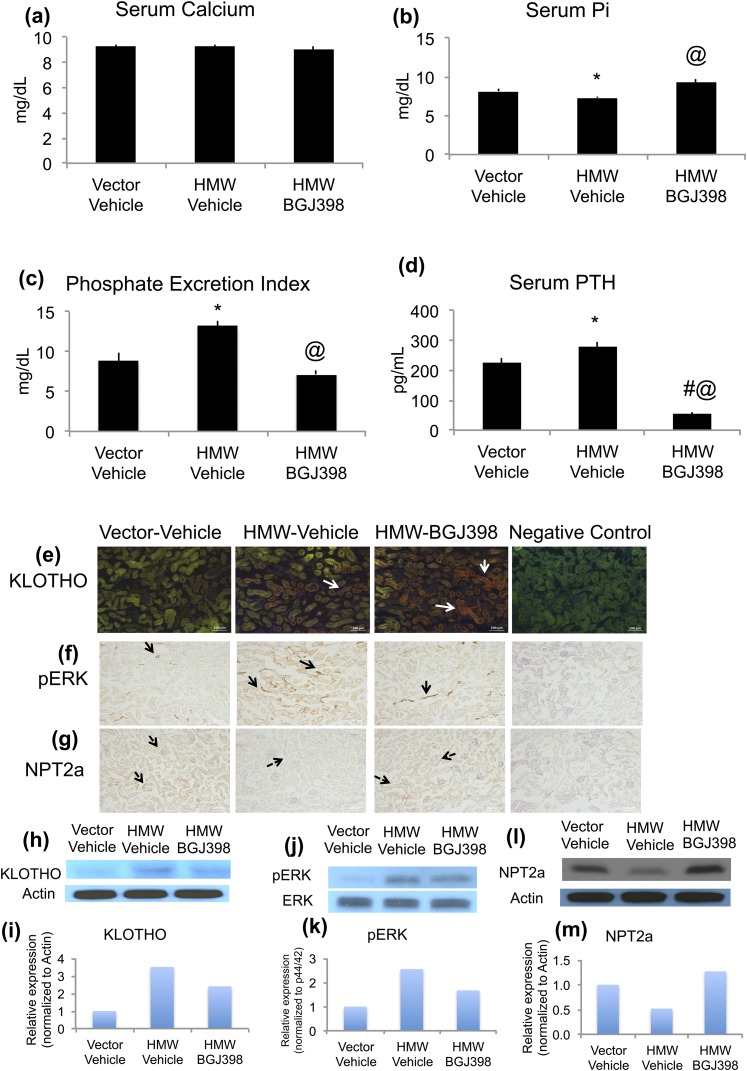
To determine whether long-term inhibition of FGFR modulated Pi and calcium homeostasis in HMWTg mice, serum calcium and Pi was measured at the end of the 8-week study (NVP-BGJ398 at 50 mg/kg, oral). As shown in Figure 2(a), there was no difference in serum calcium among groups. Decreased serum Pi and urine Pi wasting in HMWTg mice were completely rescued by NVP-BGJ398 treatment [Fig. 2(b) and 2(c)]. In contrast to the repressive effect of short-term FGFR inhibition on FGF23 expression, long-term treatment with NVP-BGJ398 (2 mg/kg, sq) resulted in a further increase of FGF23 serum concentrations in HMWTg mice [Supplemental Fig. 1(b) (47.2KB, pptx) ]. Long-term NVP-BGJ398 treatment led to normalization of PTH levels in HMWTg mice [Fig. 2(d)]. Long-term NVP-BGJ398 treatment did not affect serum 1,25(OH)2D3 levels in HMWTg mice [Supplemental Fig. 1(c) (47.2KB, pptx) ].
Figure 2.
Effects of long-term NVP-BGJ398 treatment on mineral ion homeostasis and FGF23-KLOTHO axis in kidney in HMWTg mice. Starting at 5 weeks of age, HMWTg mice were treated with the FGFR inhibitor NVP-BGJ398 (50 mg/kg, oral) or vehicle three times per week for 8 weeks. Vector mice were treated with vehicle only. Mice were euthanized 24 hours after the last NVP-BGJ398 administration. Serum and kidneys were collected during euthanasia. (a) Serum calcium was measured, n = 8 to 15 per group; (b) serum Pi was measured, n = 8 to 14 per group. (c) PEI was determined, n = 5 to 8 per group; (d) serum PTH was determined, n = 4 to 6. Data are mean ± SE. *Vector-Vehicle vs HMWTg-Vehicle, P < 0.05; #Vector-Vehicle vs HMWTg- NVP-BGJ398, P < 0.05; @HMWTg-Vehicle vs HMWTg- NVP-BGJ398, P < 0.05. (e, h, i) Immunofluorescence staining and immunoblotting for detection of KLOTHO, (f, j, k) Pp44/42, and (g, l, m) NPT2a protein expression in mouse kidneys. Note increased KLOTHO and pERK and decreased NPT2a protein expression in HMW-Vehicle kidneys. NVP-BGJ398 treatment normalized pERK and NPT2a expression in HMWTg mice. Negative control had no primary antibody during immunostaining.
Signaling molecules important in Pi homeostasis was determined after long-term inhibition of FGFR. Specifically, immunohistochemical and Western blotting analysis of KLOTHO, phospho ERK1/2 (pERK), and NPT2A were determined [Fig. 2(e)–2(m)]. Significant renal Pi wasting in HMWTg-Vehicle mice was accompanied by decreased NPT2a protein expression in the kidneys, which was normalized with NVP-BGJ398 treatment [Fig. 2(g), 2(l), and 2(m)]. Relative to Vector-Vehicle kidneys, HMW-Vehicle kidneys exhibited increased immunoreactivity for Klotho that was not changed with NVP-BGJ398 treatment [Fig. 2(e), 2(h), and 2(i)]. The HMWTg mice treated with Vehicle showed markedly increased pERK protein expression compared with Vector-Vehicle group. NVP-BGJ398 treatment decreased renal pERK immune reactivity [Fig. 2(f), 2(j), and 2(k)].
Long-term FGFR inhibition did not increase body weight, but increased tail length, total body BMD, BMC, and rescued dwarfism in HMWTg mice
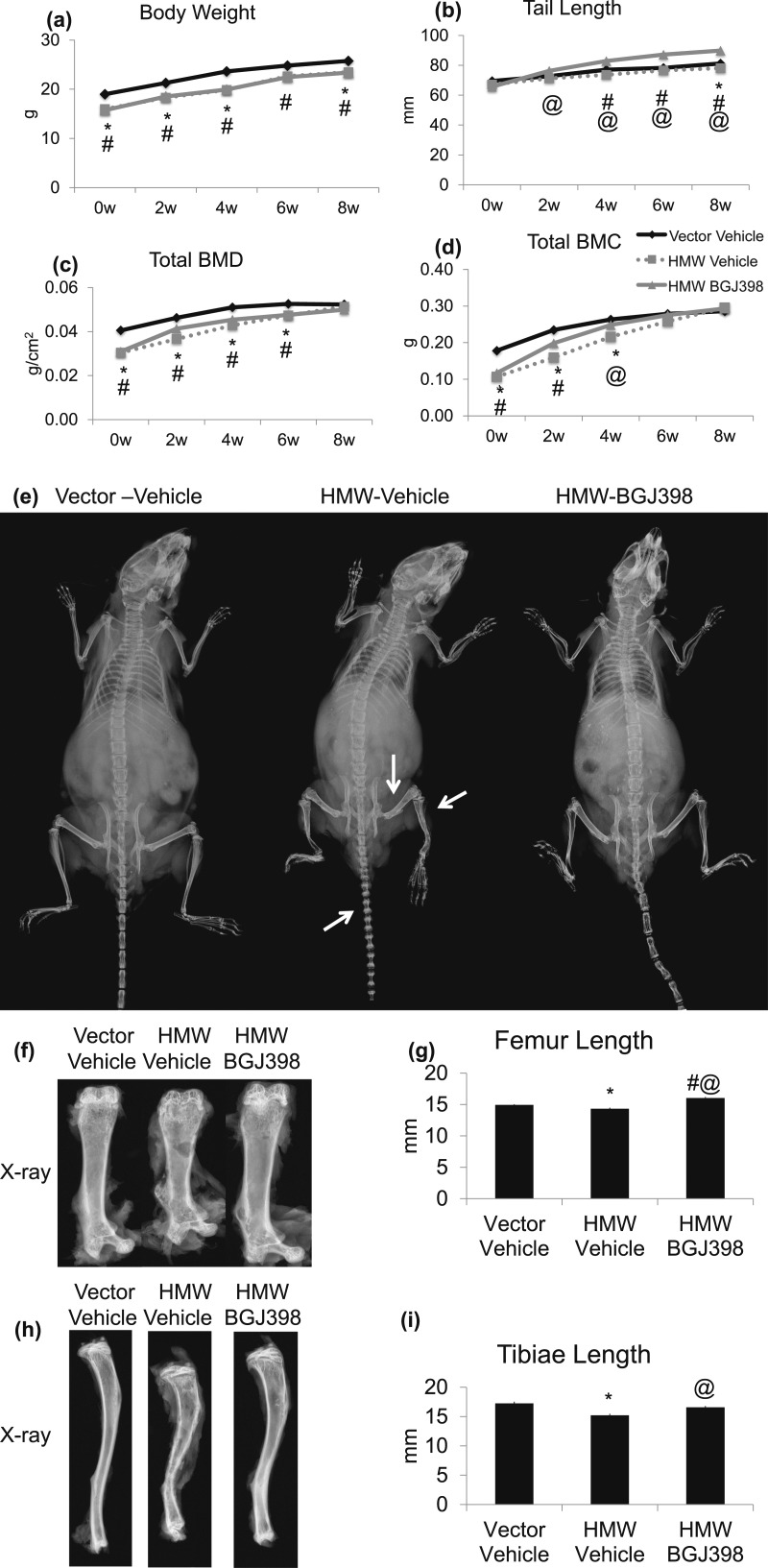
Because a single dose of NVP-BGJ-398 rescued hypophosphatemia of HMWTg mice, we assessed whether long-term inhibition of FGFR could rescue the rachitic bone phenotype in HMWTg mice. Before treatment, at 5 weeks of age, body weight of HMWTg mice was significantly lower than Vector control mice. Time course studies show that body weight of both HMWTg-Vehicle and HMWTg- NVP-BGJ398 mice remained significantly lower compared with Vector-Vehicle mice during the 8-week course of treatment [Fig. 3(a)].
Figure 3.
Effect of long-term NVP-BGJ398 administration on growth and total body BMD and BMC in HMWTg mice. Starting at 5 weeks of age, HMWTg mice were treated with the FGFR inhibitor NVP-BGJ398 (50 mg/kg, oral) or vehicle three times per week for 8 weeks. Vector mice were treated with vehicle only. (a) Body weight, (b) tail length, (c) total body BMD, and (d) BMC were monitored. (e) Representative X-ray images of mice at 8 weeks after treatment showed dwarfism phenotype that was rescued with NVP-BGJ398. (f) Radiographs of excised femur from Vector or HMWTg mice treated with vehicle or NVP-BGJ398. (g) Quantification of femoral length. (h) Radiographs of excised tibia from Vector or HMWTg mice treated with vehicle or NVP-BGJ398. (i) Quantification of tibial length. Data are mean ± SE, n = 10 to 15 per group. *Vector-Vehicle vs HMWTg-Vehicle, P < 0.05; #Vector-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05; @HMWTg-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05.
Before treatment, at 5 weeks of age, tail length was similar among all groups. However, as the mice grew, tail length was noted to be shorter in HMWTg-Vehicle mice compared with Vector-Vehicle mice. Interestingly, during the 8 weeks of treatment, HMWTg-NVP-BGJ398 mice displayed a much greater increase in tail length compared with Vector-Vehicle mice [Fig. 3(b) and 3(e)].
Prior to treatment, at 5 weeks of age, total body BMD and total body BMC was lower in HMWTg mice compared with Vector mice. As the mice grew, HMWTg mice displayed a much greater increase in total body BMD and BMC than Vector mice. At 4-week treatment, NVP-BGJ398 significantly increased total body BMD and BMC in HMWTg mice. However, at 8-week treatment, total body BMD and BMC was indistinguishable among groups [Fig. 3(c) and 3(d)].
As shown by radiography, the dwarf phenotype observed in HMWTg-Vehicle mice was rescued with NVP-BGJ398 treatment [Fig. 3(e)]. In addition, femur length that was significantly reduced by 4% in HMWTg compared with Vector mice (P < 0.05) was completely normalized with NVP-BGJ398 treatment [Fig. 3(f) and 3(g)]. Similar normalization of tibiae length was found in HMWTg mice [Fig. 3(h) and 3(i)].
In contrast to total BMD, dual beam X-ray absorptiometry analysis revealed that excised vertebrae BMD in HMWTg was further decreased after NVP-BGJ398 treatment and was also significantly decreased when compared with Vector-Vehicle (P < 0.05). Femur BMD was also significantly decreased by NVP-BJG398 compared with Vector-Vehicle. There were no significant differences in femur BMC among groups (Supplemental Table 2 (58.5KB, docx) ).
Effects of long-term BGJ398 treatment on femur metaphyseal structure in HMWTg mice
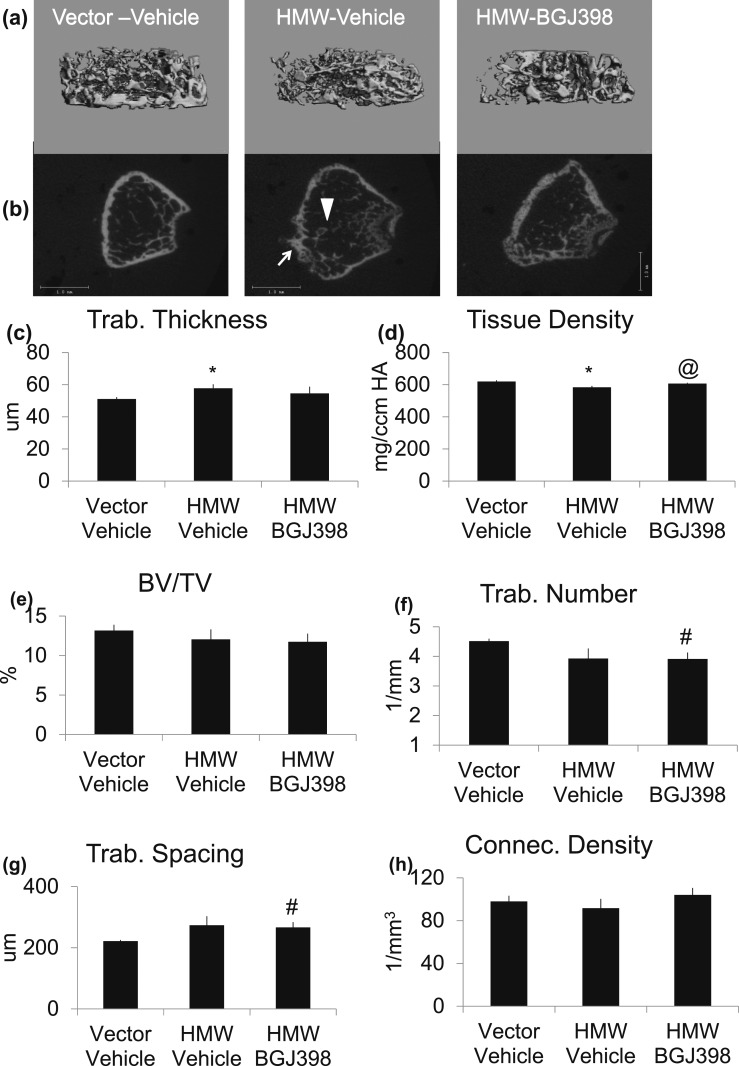
Bone structure of excised femurs was assessed in Vehicle or NVP-BGJ398-treated HMWTg mice, by micro-CT analysis at 8 weeks after treatment. Trabecular area of femur [Fig. 4(a) and 4(b)] revealed abnormal outgrowth of trabecular from cortical bone as shown by arrowhead and bubbling of cortical bone as shown by arrow in Figure 4(b). These structural abnormalities were accompanied by significantly increased trabecular thickness and significantly decreased tissue density, which were partially rescued by NVP-BGJ398 treatment [Fig. 4(c) and 4(d)]. NVP-BGJ398 did not affect BV/TV and connective tissue density and did not rescue reduced trabecular number or abnormal trabecular spacing [Fig. 4(e)–4(h)].
Figure 4.
Micro-CT analysis of metaphysis of femur after long-term administration of NVP-BJG398 in HMWTg mice. Five-week-old, HMWTg mice were treated with the FGFR inhibitor NVP-BGJ398 (50 mg/kg, oral) or vehicle three times per week for 8 weeks. Vector mice were treated with vehicle only. Mice were euthanized 24 hours after the last NVP-BGJ398 administration. Micro-CT showed FGFR inhibition improves integrity of femoral trabecular bone of HMWTg mice. (a, b) Representative micro-CT images of femur metaphysis of Vector and HMWTg treated with NVP-BGJ398. Note the abnormal outgrowth of trabecular from cortical bone as shown by arrowhead and bubbling of cortical bone as shown by arrow in HMWTg mice that was normalized with NVP-BGJ398 treatment. Quantification of (c) trabecular thickness, (d) tissue density, (e) BV/TV, (f) trabecular number, (g) trabecular spacing, and (h) connective tissue density. Data are mean ± SE, n = 6 per group. *Vector-Vehicle vs HMWTg-Vehicle, P < 0.05; #Vector-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05; @HMWTg-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05. Connec, connectivity; Trab, trabecular.
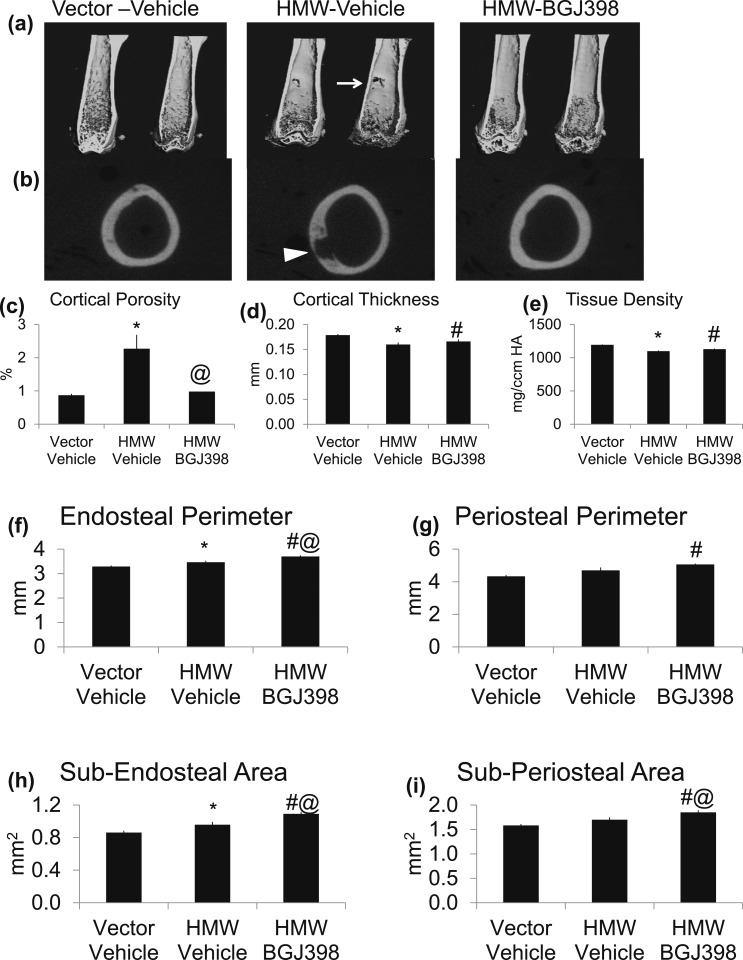
Long-term FGFR inhibition improved cortical integrity in HMWTg mice
Micro-CT scanning of femurs showed radiolucency [arrow in Fig. 5(a) and arrowhead in Fig. 5(b)] in cortical bone of HMWTg-Vehicle mice that normalized by 8 weeks of NVP-BGJ398 treatment. Micro-CT analysis revealed a significant 160% increase (P < 0.01) in cortical porosity in HMWTg-Vehicle, which was completely normalized with NVP-BGJ398 treatment [Fig. 5(c)]. There was a 10% significant decrease in cortical thickness [Fig. 5(d)], as well as an 8% significant decrease (P < 0.05) in cortical tissue density [Fig. 5(e)] in HMWTg-Vehicle mice compared with Vector-Vehicle, and both were partially normalized with NVP-BGJ398 treatment. As shown in Figure 5(f) and 5(h), endosteal perimeter and subendosteal area were increased in HMWTg-Vehicle mice compared with Vector-Vehicle mice, and was further increased with NVP-BGJ398 treatment. In addition, NVP-BGJ398 also increased periosteal perimeter and subperiosteal area in HMWTg mice [Fig. 5(g) and 5(i)].
Figure 5.
Micro-CT analysis of cortical of femur after long-term administration of NVP-BJG398 in HMWTg mice. Starting at 5 weeks of age, HMWTg mice were treated with the FGFR inhibitor NVP-BGJ398 (50 mg/kg, oral) or vehicle three times per week for 8 weeks. Vector mice were treated with vehicle only. Mice were euthanized 24 hours after the last NVP-BGJ398 administration. Micro-CT showed FGFR inhibition improves integrity of femoral cortical bone of HMWTg mice. (a, b) Representative micro-CT images of femoral cortical bone of Vector and HMWTg treated with NVP-BGJ398. Note radiolucency [arrow in (a) and arrowhead in (b)] in cortical of HMWTg-Vehicle mice, which was normalized with NVP-BGJ398 treatment. Quantification of (c) cortical porosity, (d) cortical thickness, (e) tissue density, (f) endosteal perimeter, (g) periosteal perimeter, (h) sub-endosteal area, and (i) sub-periosteal area. Data are mean ± SE, n = 6 per group. *Vector-Vehicle vs HMWTg-Vehicle, P < 0.05; #Vector-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05; @HMWTg-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05.
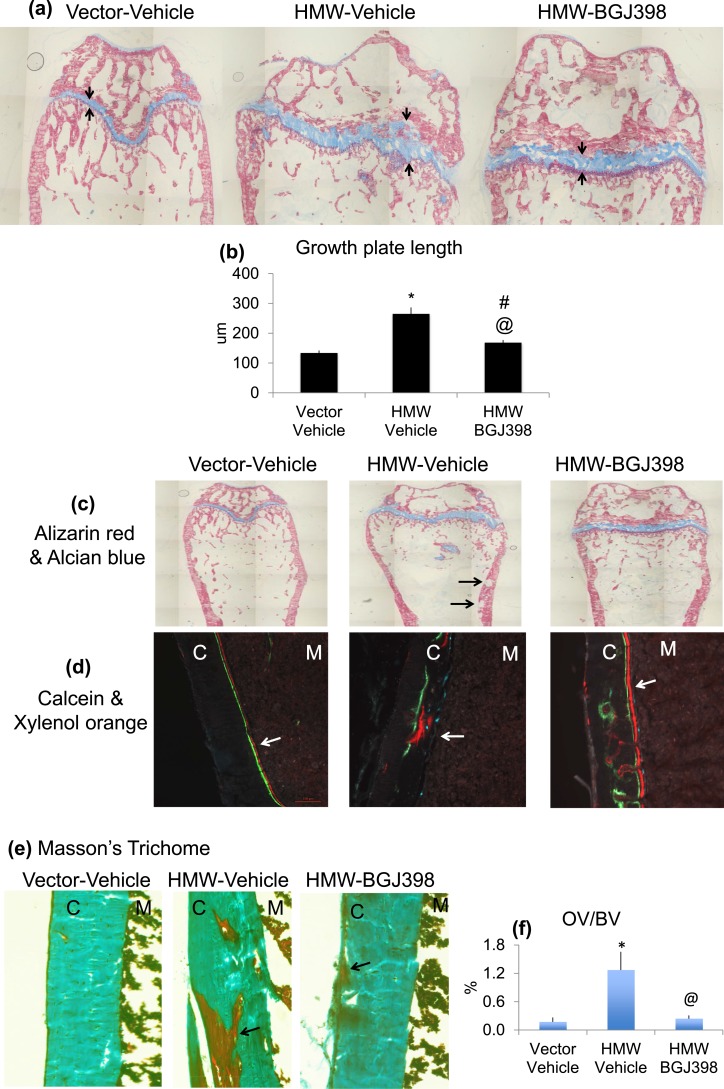
Long-term NVP-BGJ398 treatment ameliorates growth plate and mineralization defect in HMWTg mice
To examine the long-term effect of FGFR inhibition on bone structure in more detail, histologic analysis was performed on femurs harvested at the end of 8 weeks of treatment. Given the significant effects of NVP-BGJ398 on skeletal growth in HMWTg mice, we analyzed the growth plate after long-term BGJ398 treatment. As shown in Figure 6(a) and 6(b), Alcian Blue/Alizarin Red staining revealed thickening and disorganized growth plate in HMWTg mice that was partially normalized with NVP-BGJ398 treatment. NVP-BGJ398 treatment did not affect growth plate morphology of the Vector mice.
Figure 6.
Long-term treatment with NVP-BGJ398 ameliorates growth plate and mineralization defect in HMWTg mice. Starting at 5 weeks of age, HMWTg mice were treated with the FGFR inhibitor NVP-BGJ398 (50 mg/kg, oral) or vehicle three times per week for 8 weeks. Vector mice were treated with vehicle only. Mice were euthanized 24 hours after the last NVP-BGJ398 administration. (a, b) Alizarin Red and Alcian Blue staining showed thickening, and disorganized growth plate in HMWTg mice was partially normalized with NVP-BGJ398 treatment. (c) Alizarin Red and Alcian Blue staining showed impaired mineralization of the cortical bone area in HMWTg mice (black arrows). Mineralized tissue is shown in red, and cartilage is visualized by blue staining. (d) Calcein and Xylenol orange labeling showed smeared double labeling in HMWTg mice was partially rescued with NVP-BGJ398 (white arrows). (e) Masson Trichrome staining of femur sections. Mineralized tissue is shown in green, and unmineralized osteoid is visualized by red staining. (f) Osteoid volume (OV)/BV determined by histomorphometry in the femur middiaphysis. Data are mean ± SE, n = 9 per group. *Vector-Vehicle vs HMWTg-Vehicle, P < 0.05; #Vector-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05; @HMWTg-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05.
Alizarin Red and Alcian Blue staining showed impaired mineralization of the cortical bone area in HMWTg mice [Fig. 6(c), black arrows] that was normalized with NVP-BGJ398 treatment. Histomorphometric analysis further revealed a significant amelioration of the increased cortical osteoid volume/BV in HMWTg mice [Fig. 6(c), 6(e), and 6(f)]. Dynamic histomorphometric analysis of cortical bone revealed that smeared double labeling in HMWTg mice was partially rescued with NVP-BGJ398 [Fig. 6(d), white arrows].
Static histomorphometry (Table 1) revealed significant reduction in metaphyseal cancellous BV/TV and trabecular number among groups in HMWTg that were partially improved by NVP-BGJ398. It should be noted that in HMWTg mice, irrespective of treatment, the amount of metaphyseal trabecular BSs available for quantitative analysis was low and lead to less accuracy of the measurement. However, increased trabecular spacing in HMWTg was reduced by NVP-BGJ398 treatment. Osteoclast number and osteoclast surface were significantly increased in HMWTg-Vehicle and were further significantly increased by NVP-BGJ398 treatment. Dynamic histomorphometry showed that double labeled surface, bone formation rate, and MAR, a parameter for single OB activity, were significantly increased in HMWTg after NVP-BGJ398 treatment (Table 1). These data suggest that FGFR inhibitor treatment significantly reduced the mineralization defects in HMWTg mice and abnormalities in histomorphometric indices, and indicate a favorable effect of FGFR inhibition on structural integrity and mineralization of bone in HMWTg mice.
Table 1.
Bone Histomorphometry Analysis of Femoral Trabecular Bone
| Static | Vector Vehicle (n = 9) | HMW Vehicle (n = 9) | HMW NVP-BGJ398 (n = 5) |
|---|---|---|---|
| BV/TV, % | 4.98 ± 0.72 | 2.95 ± 0.56a | 3.25 ± 0.80 |
| TbTh, μm | 25.65 ± 1.67 | 24.19 ± 1.88 | 22.90 ± 1.54 |
| ObS/BS, % | 9.36 ± 1.73 | 9.96 ± 1.10 | 6.83 ± 1.13b |
| OcS/BS, % | 11.94 ± 2.14 | 19.10 ± 2.51a | 23.05 ± 3.76b |
| OcN/BS,1/mm | 3.32 ± 0.55 | 5.04 ± 0.56a | 5.38 ± 0.76b |
| TbN, 1/mm | 1.91 ± 0.18 | 1.19 ± 0.19a | 1.37 ± 0.26 |
| TbSp, μm | 534.5 ± 49.5 | 1062.4 ± 215.2a | 815.6 ± 143.8b |
| Dynamic | Vector Vehicle (n = 8) | HMW Vehicle (n = 9) | HMW NVP-BGJ398 (n = 8) |
| Interlabel thickness, u | 7.54 ± 0.47 | 7.31 ± 0.46 | 8.88 ± 0.47c |
| Mineralizing surface/BS, % | 21.64 ± 1.47 | 24.09 ± 1.25 | 25.80 ± 1.64 |
| Mineral apposition rate, μm/d | 1.51 ± 0.09 | 1.46 ± 0.09 | 1.78 ± 0.09c |
| Bone formation rate/BS, u3/u2/d | 0.32 ± 0.02 | 0.35 ± 0.02 | 0.46 ± 0.04b,c |
Abbreviations: ObS, osteoblast surface; OcN, osteoclast number; OcS, osteoclast surface; TbN, trabecular number; TbSp, trabecular separation; TbTh, trabecular thickness.
Vector-Vehicle vs HMW-Vehicle, P < 0.05.
Vector-Vehicle vs HMW-BGJ398, P < 0.05.
HMW-Vehicle vs HMW-BGJ398, P < 0.05.
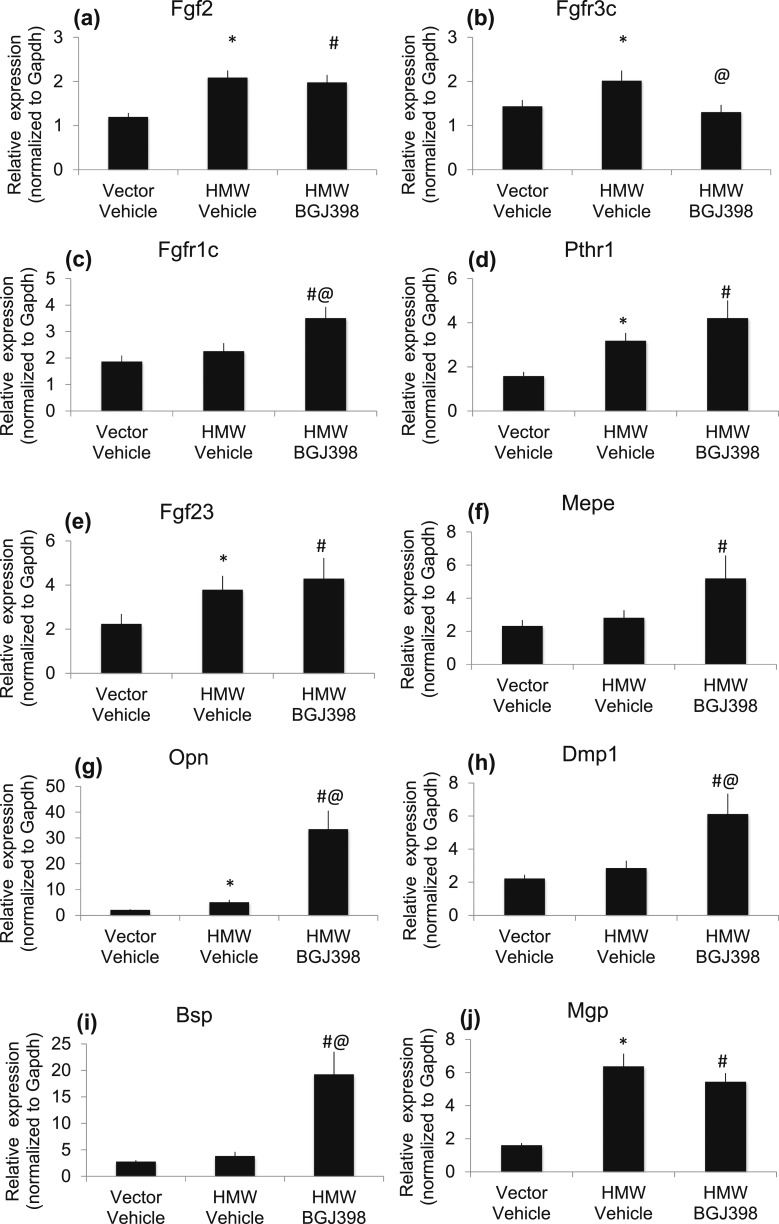
Effects of long-term NVP-BGJ398 treatment on expression of mineralization genes in HMWTg mice
To determine whether improved mineralization is associated with enhanced expression of genes involved in mineralization, RNA was extracted from the whole femur of long-term treated Vector and HMWTg mice and quantitative reverse transcription polymerase chain reaction was performed. As expected, Fgf2 gene expression was high in HMWTg mice compared with Vector mice and was not altered by NVP-BGJ398 treatment [Fig. 7(a)]. FGFR3c mRNA was increased in HMWTg-Vehicle mice compared with Vector-Vehicle mice and was normalized by NVP-BGJ398 treatment [Fig. 7(b)]. There was no difference in FGFR1c mRNA level between Vector-Vehicle and HMW-Vehicle; however, NVP-BGJ398 treatment significantly increased FGFR1c expression level [Fig. 7(c)]. PTHR1 mRNA was increased in HMWTg-Vehicle mice compared with Vector-Vehicle mice and was further increased with NVP-BGJ398 treatment [Fig. 7(d)]. Consistent with our previous finding in 2-month-old mice, Fgf23 mRNA expression was significantly increased in HMWTg mice and was not normalized by NVP-BGJ398 treatment [Fig. 7(e)].
Figure 7.
Effect of long-term NVP-BGJ398 treatment expression of mineralization genes in HMWTg mice. Starting at 5 weeks of age, HMWTg mice were treated with the FGFR inhibitor NVP-BGJ398 (50 mg/kg, oral) or vehicle three times per week for 8 weeks. Vector mice were treated with vehicle only. Mice were euthanized 24 hours after the last NVP-BGJ398 administration. Whole femurs were collected and used for RNA extraction. Quantitative polymerase chain reaction analysis was performed for the following gene expression: (a) Fgf2, (b) FGFR3c, (c) FFGFR1c, (d) PTHR1, (e) Fgf23, (f) Mepe, (g) Opn, (h) Dmp1, (i) Bsp, and (j) Mgp. Data are mean ± SE, n = 10 to 15 per group. *Vector-Vehicle vs HMWTg-Vehicle, P < 0.05; #Vector-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05; @HMWTg-Vehicle vs HMWTg-NVP-BGJ398, P < 0.05.
Because we observed partial but not complete rescue of the mineralization defect in response to long-term NVP-BGJ398, the expression of matrix-related genes including matrix extracellular phosphoglycoprotein (Mepe), Dmp1, osteopontin (Opn), and bone sialoprotein (Bsp) was determined. These genes encode for sibling proteins with an acidic serine aspartate-rich Mepe-associated motif (ASARM motif) involved in bone and teeth mineralization (39–41). Gene expression analysis showed that Mepe mRNA was similar in HMWTg-Vehicle and Vector-Vehicle mice, but NVP-BGJ398 treatment significantly increased Mepe expression in HMWTg mice [Fig. 7(f)]. Opn mRNA level was significantly increased in HMWTg mice and was further increased with NVP-BGJ398 treatment [Fig. 7(g)]. Dmp1 mRNA [Fig. 7(h)] and Bsp mRNA [Fig. 7(i)] expression were similar between Vector-Vehicle and HMW-Vehicle; however, NVP-BGJ398 significantly increased both Dmp1 and Bsp expression in HMWTg mice. The expression of matrix gla protein (Mgp), an inhibitor of mineralization, was significantly increased in HMWTg-Vehicle mice [Fig. 7(j)]. However, NVP-BGJ398 treatment did not significantly decrease Mgp expression.
Discussion
The findings of this in vivo study demonstrate that the rachitic bone and Pi wasting phenotypes of transgenic mice overexpressing FGF2 HMW isoforms in OB lineage cells is due in part to increased FGF23/FGFR signaling that can be partially rescued by a novel FGF receptor inhibitor that was shown by Wohrle et al. (31) to ameliorate rickets and hypophosphatemia in the Hyp and Dmp1ko mouse models. Consistent with the studies using Hyp mice (31) that overexpress HMWFGF2 in osteocytes (28), we observed that single dose treatment with NVP-BGJ398 increased serum Pi and reduced urine Pi excretion in both Vector and HMWTg mice. This observation is consistent with our recent studies showing that NVP-BGJ398 rescued Pi wasting via increased expression of sodium Pi transporter Npt2a in kidneys of HMWTg mice (42). However, in contrast to our findings, NVP-BGJ398 did not increase renal expression of Npt2a or fractional excretion of Pi in the Hyp mice in the studies of Whorle et al. (31) where it was postulated that NVP-BGJ398 corrected hypophosphatemia in Hyp mice via intestinal absorption of dietary Pi due to increased 1,25(OH)2D3. Because NVP-BGJ-398 also caused a significant increase in 1,25(OH)2D3 in serum of HMWTg mice, it is possible that increased intestinal absorption of Pi also contributes to the correction of the hypophosphatemia in HMWTg mice. Consistent with the effects of long-term treatment with NVP-BGJ398 in Hyp mice, long-term treatment with NVP-BGJ398 completely normalized serum and urine Pi in HMWTg mice. Similar to observations in NVP-BGJ398-treated wild type littermates of Hyp mice, our studies demonstrated a significant increase in serum calcium in Vector control with short-term but not long-term treatment with the FGFR inhibitor (31).
Our studies show that Npt2a expression in the kidney was increased in HMWTg after both single dose and long-term treatment. This suggests that the improvement in serum Pi levels was due to increased renal Pi reabsorption via Npt2a. However, FGF23 levels are low with single dose 24-hour treatment and high in long-term treatment. This is in consistent with the report by Whorle et al. (31) using Hyp mice. In their study, FGFR inhibition with single dose NVP-BGJ398 resulted in a decrease in Fgf23 mRNA level and FGF serum levels, but the reduction of FGF23 levels was transient and closely correlated with the pharmacokinetic of FGFR inhibition by NVP-BGJ398. The increased FGF23 after long-term treatment is indicative of a feedback regulation as a consequence of the correction of hypophosphatemia as observed in Hyp mice by NVP-BGJ398 treatment (31).
FGF23 also directly inhibits PTH expression and secretion in the parathyroid gland (43). Single dose NVP-BGJ398 treatment increased serum PTH level in Vector mice. Once the compound was cleared after 24 hours, serum PTH was decreased in WT mice that is coincident with increased serum calcium level in Vector mice. Consistent with our previous report, HMWTg-Vehicle have higher PTH level compared with Vector-Vehicle group (28). However, acute NVP-BGJ398 treatment did not affect PTH levels in HMWTg mice. This suggests that PTH does not directly contribute to the effects on mineral ion metabolism observed in HMWTg mice in short-term treatment. Interestingly, after long-term treatment, serum PTH level was decreased in HMWTg mice, and this was associated with increased serum FGF23 levels.
Because 1,25(OH) vitamin D is a potent regulator of bone FGF23 production, we measured the serum 1,25(OH) vitamin D levels in both single dose and after multidose long-term treatment. Previous studies showed that a single dose of the NVP-BGJ398 orally administrated at 50 mg/kg is cleared by 24 hours after administration in wild-type and Hyp mice (31). Because of the transient nature of this compound on inhibition of FGFR signaling, it could regulate Pi and/or 1,25(OH)2D3 homeostasis. In this study, after long-term treatment, serum was collected 24 hours after last dosing and serum 1,25(OH)D3 level was normal. This indicates that in contrast to the transiently increased serum 1,25(OH)2D3 level after single dose NVP-BGJ398, long-term BGJ398 does not result in an excessive increase in VD3, which has negative effects on mineral homeostasis (44).
In contrast to the increase in body weight observed in Hyp mice treated long-term with NVP-BGJ398 (31), NVP-BGJ398 was not observed to increase body weight in HMWTg mice. However, similar to its effect in Hyp mice, we observed a significant increase in longitudinal bone growth, as well as a significant reduction in osteoid due to normalization of bone mineralization in the cortical bone. Interestingly, similar to the studies in Hyp mice (31), long-term treatment with NVP-BGJ398 only partially improved metaphyseal cancellous bone in HMWTg mice. However, serum FGF23 was significantly elevated in HMWTg vehicle treated mice and remained significantly elevated after long-term NVP-BGJ398 treatment. It should also be noted that long-term treatment of Hyp mice with NVP-BGJ398 caused a further increase in serum FGF23 as reported by Wohrle et al. (31). It is interesting to speculate that FGF23, which is known to have effects on bone matrix mineralization independent of its effects on Pi (29), may be contributing to the failure of NVP-BGJ398 to fully restore cancellous bone. However, this would not explain the improved matrix mineralization observed in the cortical bone of HMWTg mice in response to the FGFR inhibitor. In support of additional effects of HMWFGF2 on matrix mineralization that are independent of FGF23/FGFR, we previously conducted in vitro studies to assess the mechanism of the inhibitory effects of HMWFGF2 on bone marrow stromal cell differentiation and matrix mineralization and reported that FGF23 neutralizing antibody and the FGFR inhibitor SU5402 only partially rescued the reduced mineralized bone formation in HMWTg bone marrow stromal cultures (30). It should also be noted that similar to the NVP-BGJ398 studies in Hyp mice that were 5 to 7 weeks old (31), we conducted our studies in HMWTg mice that were 5 to 6 weeks old. Whether earlier initiation of therapy would have caused a further rescue of the bone phenotype is worthy of consideration.
The effects of long-term treatment with NVP-BGJ398 on Fgf2 and PTHR1 mRNA in bones of HMWTg revealed that NVP-BGJ398 did not affect the expression of these genes, suggesting HMWFGF2 modulate their expression independent of cell surface FGF ligand/FGFR receptor signaling. Interestingly, however, increased FGFR3 expression was decreased in HMWTg in response to NVP-BGJ398 treatment, and NVP-BGJ398 treatment increased FGFR1 expression suggesting that HMWTg differentially modulates FGFRs. The increased PTHR1 in HMWTg that remained persistently elevated after long-term NVP-BGJ398 is interesting and may contribute to the increased FGF23 expression in bone and serum because previous studies showed that parathyroid hormone receptor signaling in osteocytes increases the expression of FGF23 in vitro and in vivo (45). Studies by Fan et al. (46) showed that bone-specific ablation of PTHR1 decreased FGF23 protein in bone and serum.
It is known that FGF23 impairs mineralization (47). However, the persistent increase in Fgf23 mRNA in the bones and serum of long-term NVP-BGJ398-treated mice in the context of improved mineralization indicates that the bone abnormalities in HMWTg mice cannot be solely attributed to local production of FGF23. In view of this, we reasoned that other matrix modulators might play a role in the bone phenotype of HMWTg mice. Several studies have demonstrated important roles for the small integrin-binding ligand N-linked glycoprotein (SIBLING) family of noncollagen proteins consisting of Opn, Bsp, Dmp1, and Mepe in bone. These proteins share many structural characteristics, are primarily located in bone and dentin, and play an important role in matrix mineralization (48). In the current study, long-term NVP-BGJ398 improved tissue density, which is accompanied with increases in the expression of Mepe, Opn, Dmp1, and Bsp mRNA suggesting that NVP-BGJ398 improves mineralization in part through modulation of the SIBLING proteins. Interestingly, although increased Mepe expression was observed during OB matrix mineralization, overexpression of Mepe in mice resulted in a growth and mineralization defect due to decrease in bone remodeling (48). Also of interest, in contrast to our observation that HMWFGF2 isoforms increase Mepe, published studies showed that addition of LMW isoform of FGF2 to OBs down regulated Mepe levels (49) supporting different biological functions of LMW and HMWFGF2 isoforms in matrix mineralization.
Another reason for the failure of NVP-BGJ398 to fully rescue impaired mineralization, in bones of HMWTg, is the increased expression of Mgp, an extracellular matrix mineralization protein and a potent inhibitor of mineralization (50). Previously, we reported increased Mgp mRNA expression level in tibiae of 2-month-old male HMWTg mice and cultured BMSCs from these mice. Consistent with these earlier studies, in the current study, Mgp was increased in HMWTg-Vehicle mice compared with Vector-Vehicle at 3-months of age. However, in contrast to the results of our in vitro studies (30) where the SU5402, another FGF receptor tyrosine kinase inhibitor, increased mineralization in HMWTg BMSC cultures associated with marked reduction in Mgp mRNA, in vivo long-term NVP-BGJ398 treatment did not significantly decrease expression of Mgp in whole femur. The differences between BMSC cultures and intact bone may be due to loss of some of the features of the in vivo HMWTg phenotype in vitro or confounding effects of greater cell heterogeneity in the in vivo assessment of whole femur.
Consistent with the studies by Wohrle et al. (31) using Hyp mice, we observed that long-term treatment with NVP-BGJ398 improved the disorganized growth plate structure in HMWTg mice that is associated with normalization of serum Pi level. However, the rescue is incomplete. This may be due to further increase in serum FGF23 after long-term treatment. It is known that FGF23 and Klotho are present in the growth plate (51). FGF23 regulates chondrocytes in a Klotho-dependent (52) and Klotho-independent manner (53). FGF23 suppresses chondrocyte proliferation in the presence of soluble a-Klotho both in vitro and in vivo (52). NVP-BGJ398 may act by inhibiting FGF23 binding to FGFR leading to inhibition of negative feedback and therefore increased FGF23 in long-term treatment. Increased FGF23 after long-term treatment could be at high enough levels to overcome the inhibitory effect on FGFR binding resulting in partial instead of complete rescue of the observed bone phenotype (54).
In summary, our studies demonstrate increased bone structure integrity in HMWTg mice with NVP-BGJ398 treatment, despite a dramatic increase in FGF23. This suggests that NVP-BGJ398 attenuates FGF23-independent consequences of HMWFGF2 overexpression that may be critical determinants of skeletal pathology in HMWTg mice.
Acknowledgments
We thank Diana Graus-Porta (Novartis, Basel, Switzerland) for supplying the FGFR inhibitor NVP-BGJ398.
Financial Support: This project was supported in part by National Institutes of Health Grant DK098566 (to M.M.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No. | Species Raised in | Dilution Used | RRID |
|---|---|---|---|---|---|
| KLOTHO | R&D Systems, AF1819 | Goat | 15 μg/mL for immunohistochemistry, 0.1 μg/mL for Western blot | AB_2296612 | |
| pERK | Cell Signaling Technology, Inc., 9106 | Mouse | at 1:150 for immunohistochemistry, 1:1000 for Western blot | AB_331768 | |
| NPT2a | Alpha Diagnostic International, Inc., NPT23-A | Rabbit | 20 μg/mL for immunohistochemistry, 1 μg/mL for Western blot | AB_1622108 | |
| ERK | Cell Signaling Technology, Inc., 9102 | Rabbit | at 1:1000 for Western blot | AB_330744 | |
| Actin | Santa Cruz Biotechnology, sc-1615 | Goat | at 1:10,000 for Western blot | AB_630835 |
Footnotes
- BMC
- bone mineral content
- BMD
- bone mineral density
- BS
- bone surface
- Bsp
- bone sialoprotein
- BV
- bone volume
- cDNA
- complementary DNA
- Dmp1
- dentin matrix protein-1
- FGF
- fibroblast growth factor
- FGFR
- fibroblast growth factor receptor
- HMW
- high molecular weight
- LMW
- low molecular weight
- Mepe
- matrix extracellular phosphoglycoprotein
- Mgp
- matrix gla protein
- micro-CT
- micro-computed tomography
- mRNA
- messenger RNA
- OB
- osteoblast
- Opn
- osteopontin
- PBS
- phosphate-buffered saline
- PEI
- phosphate excretion index
- Pi
- phosphate
- PTH
- parathyroid hormone
- RRID
- Research Resource Identifier
- SE
- standard error
- sq
- subcutaneous
- TV
- total volume
- XLH
- X-linked hypophosphatemic rickets.
References
- 1.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Ann Rev Med. 2010;61:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurley MM, Marie PJ, Florkiewicz RZ. Fibroblast growth factor (FGF) and FGF receptor families in bone In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. San Diego, CA: Academic Press; 2002:825–851. [Google Scholar]
- 3.Muenke M, Schell U. Fibroblast-growth-factor receptor mutations in human skeletal disorders. Trends Genet. 1995;11(8):308–313. [DOI] [PubMed] [Google Scholar]
- 4.De Moerlooze L, Dickson C. Skeletal disorders associated with fibroblast growth factor receptor mutations. Curr Opin Genet Dev. 1997;7(3):378–385. [DOI] [PubMed] [Google Scholar]
- 5.Coffin JD, Florkiewicz RZ, Neumann J, Mort-Hopkins T, Dorn GW II, Lightfoot P, German R, Howles PN, Kier A, O’Toole BA, Sasse J, Gonzalez AM, Baird A, Doetschman T. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol Biol Cell. 1995;6(12):1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, White KE. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 2005;16(2):221–232. [DOI] [PubMed] [Google Scholar]
- 7.ADHR Consortium Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. [DOI] [PubMed] [Google Scholar]
- 8.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98(11):6500–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11(2):130–136. [DOI] [PubMed] [Google Scholar]
- 10.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Jüppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145(7):3087–3094. [DOI] [PubMed] [Google Scholar]
- 12.Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99(6):1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38(11):1248–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Globus RK, Plouet J, Gospodarowicz D. Cultured bovine bone cells synthesize basic fibroblast growth factor and store it in their extracellular matrix. Endocrinology. 1989;124(3):1539–1547. [DOI] [PubMed] [Google Scholar]
- 15.Wang JS, Aspenberg P. Basic fibroblast growth factor infused at different times during bone graft incorporation. Titanium chamber study in rats. Acta Orthop Scand. 1996;67(3):229–236. [DOI] [PubMed] [Google Scholar]
- 16.Florkiewicz RZ, Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci USA. 1989;86(11):3978–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arese M, Chen Y, Florkiewicz RZ, Gualandris A, Shen B, Rifkin DB. Nuclear activities of basic fibroblast growth factor: potentiation of low-serum growth mediated by natural or chimeric nuclear localization signals. Mol Biol Cell. 1999;10(5):1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delrieu I. The high molecular weight isoforms of basic fibroblast growth factor (FGF-2): an insight into an intracrine mechanism. FEBS Lett. 2000;468(1):6–10. [DOI] [PubMed] [Google Scholar]
- 19.Touriol C, Bornes S, Bonnal S, Audigier S, Prats H, Prats AC, Vagner S. Generation of protein isoform diversity by alternative initiation of translation at non-AUG codons. Biol Cell. 2003;95(3-4):169–178. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Dang X, Claus P, Hirst C, Fandrich RR, Jin Y, Grothe C, Kirshenbaum LA, Cattini PA, Kardami E. Chromatin compaction and cell death by high molecular weight FGF-2 depend on its nuclear localization, intracrine ERK activation, and engagement of mitochondria. J Cell Physiol. 2007;213(3):690–698. [DOI] [PubMed] [Google Scholar]
- 21.Yu PJ, Ferrari G, Galloway AC, Mignatti P, Pintucci G. Basic fibroblast growth factor (FGF-2): the high molecular weight forms come of age. J Cell Biochem. 2007;100(5):1100–1108. [DOI] [PubMed] [Google Scholar]
- 22.Liao S, Porter D, Scott A, Newman G, Doetschman T, Schultz JelJ. The cardioprotective effect of the low molecular weight isoform of fibroblast growth factor-2: the role of JNK signaling. J Mol Cell Cardiol. 2007;42(1):106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang ZS, Jeyaraman M, Wen GB, Fandrich RR, Dixon IM, Cattini PA, Kardami E. High- but not low-molecular weight FGF-2 causes cardiac hypertrophy in vivo; possible involvement of cardiotrophin-1. J Mol Cell Cardiol. 2007;42(1):222–233. [DOI] [PubMed] [Google Scholar]
- 24.Sobue T, Naganawa T, Xiao L, Okada Y, Tanaka Y, Ito M, Okimoto N, Nakamura T, Coffin JD, Hurley MM. Over-expression of fibroblast growth factor-2 causes defective bone mineralization and osteopenia in transgenic mice. J Cell Biochem. 2005;95(1):83–94. [DOI] [PubMed] [Google Scholar]
- 25.Montero A, Okada Y, Tomita M, Ito M, Tsurukami H, Nakamura T, Doetschman T, Coffin JD, Hurley MM. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest. 2000;105(8):1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, Hurley MM. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone. 2010;47(2):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao L, Liu P, Li X, Doetschman T, Coffin JD, Drissi H, Hurley MM. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J Biol Chem. 2009;284(5):3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao L, Naganawa T, Lorenzo J, Carpenter TO, Coffin JD, Hurley MM. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J Biol Chem. 2010;285(4):2834–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89(2):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao L, Esliger A, Hurley MM. Nuclear fibroblast growth factor 2 (FGF2) isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. J Bone Miner Res. 2013;28(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wöhrle S, Henninger C, Bonny O, Thuery A, Beluch N, Hynes NE, Guagnano V, Sellers WR, Hofmann F, Kneissel M, Graus Porta D. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res. 2013;28(4):899–911. [DOI] [PubMed] [Google Scholar]
- 32.Dacic S, Kalajzic I, Visnjic D, Lichtler AC, Rowe DW. Col1a1-driven transgenic markers of osteoblast lineage progression. J Bone Miner Res. 2001;16(7):1228–1236. [DOI] [PubMed] [Google Scholar]
- 33.Xiao L, Liu P, Sobue T, Lichtler A, Coffin JD, Hurley MM. Effect of overexpressing fibroblast growth factor 2 protein isoforms in osteoblastic ROS 17/2.8 cells. J Cell Biochem. 2003;89(6):1291–1301. [DOI] [PubMed] [Google Scholar]
- 34.Komla-Ebri D, Dambroise E, Kramer I, Benoist-Lasselin C, Kaci N, Le Gall C, Martin L, Busca P, Barbault F, Graus-Porta D, Munnich A, Kneissel M, Di Rocco F, Biosse-Duplan M, Legeai-Mallet L. Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model. J Clin Invest. 2016;126(5):1871–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. [DOI] [PubMed] [Google Scholar]
- 36.Homer-Bouthiette C, Doetschman T, Xiao L, Hurley MM. Knockout of nuclear high molecular weight FGF2 isoforms in mice modulates bone and phosphate homeostasis. J Biol Chem. 2014;289(52):36303–36314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohata Y, Yamazaki M, Kawai M, Tsugawa N, Tachikawa K, Koinuma T, Miyagawa K, Kimoto A, Nakayama M, Namba N, Yamamoto H, Okano T, Ozono K, Michigami T, Michigami T. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J Bone Miner Res. 2014;29(7):1627–1638. [DOI] [PubMed] [Google Scholar]
- 39.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280(2):460–465. [DOI] [PubMed] [Google Scholar]
- 40.Rowe PSN, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67(1):54–68. [DOI] [PubMed] [Google Scholar]
- 41.Rowe PSN. The wrickkened pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med. 2004;15(5):264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du E, Xiao L, Hurley MM. FGFR inhibitor ameliorates hypophosphatemia and impaired engrailed-1/Wnt signaling in FGF2 high molecular weight isoform transgenic mice. J Cell Biochem. 2016;117(9):1991–2000; (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 43.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117(12):4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010;95(4):1846–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan Y, Bi R, Densmore MJ, Sato T, Kobayashi T, Yuan Q, Zhou X, Erben RG, Lanske B. Parathyroid hormone 1 receptor is essential to induce FGF23 production and maintain systemic mineral ion homeostasis. FASEB J. 2016;30(1):428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sitara D, Kim S, Razzaque MS, Bergwitz C, Taguchi T, Schüler C, Erben RG, Lanske B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4(8):e1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staines KA, MacRae VE, Farquharson C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling [published correction appears in J Endocrinol. 2013;219(2):X1]. J Endocrinol. 2012;214(3):241–255. [DOI] [PubMed] [Google Scholar]
- 49.Zhang GX, Mizuno M, Tsuji K, Tamura M. Regulation of mRNA expression of matrix extracellular phosphoglycoprotein (MEPE)/ osteoblast/osteocyte factor 45 (OF45) by fibroblast growth factor 2 in cultures of rat bone marrow-derived osteoblastic cells. Endocrine. 2004;24(1):15–24. [DOI] [PubMed] [Google Scholar]
- 50.Kaipatur NR, Murshed M, McKee MD. Matrix Gla protein inhibition of tooth mineralization. J Dent Res. 2008;87(9):839–844. [DOI] [PubMed] [Google Scholar]
- 51.Raimann A, Ertl DA, Helmreich M, Sagmeister S, Egerbacher M, Haeusler G. Fibroblast growth factor 23 and Klotho are present in the growth plate. Connect Tissue Res. 2013;54(2):108–117. [DOI] [PubMed] [Google Scholar]
- 52.Kawai M, Kinoshita S, Kimoto A, Hasegawa Y, Miyagawa K, Yamazaki M, Ohata Y, Ozono K, Michigami T. FGF23 suppresses chondrocyte proliferation in the presence of soluble α-Klotho both in vitro and in vivo. J Biol Chem. 2013;288(4):2414–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianchi A, Guibert M, Cailotto F, Gasser A, Presle N, Mainard D, Netter P, Kempf H, Jouzeau JY, Reboul P. Fibroblast Growth Factor 23 drives MMP13 expression in human osteoarthritic chondrocytes in a Klotho-independent manner. Osteoarthritis Cartilage. 2016;24(11):1961–1969. [DOI] [PubMed] [Google Scholar]
- 54.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]