Abstract
Introduction
Poor oral health has emerged as a risk factor for squamous cell carcinoma of the head and neck (HNSCC) but its impact on survival has not been examined. We sought to estimate the impact of oral health indicators on survival in a population-based HNSCC cohort.
Materials and Methods
Cases (n=1381) and age-, sex- and race-matched controls (n=1396) were participants in the Carolina Head and Neck Cancer Epidemiologic Study (CHANCE). Vital status was determined via linkage with the National Death Index. Survival was considered at 5 years post-diagnosis or study-enrollment for controls. Oral health was assessed using self-reported indicators including frequency of routine dental exams and tooth brushing. We used Kaplan-Meyer analyses and Cox regression to estimate adjusted hazard ratios (HR) and 95% confidence intervals (CI).
Results
Routine dental visits during the preceding 10 years were associated with decreased mortality risk (>10 visits: HR=0.6, 95% CI=0.4–0.8) after adjusting for confounders. This effect was most pronounced for oral cavity cancer—(e.g., >10 visits: HR=0.4, 95% CI=0.2–0.9). Dental visits were also positively associated with survival among controls. No other routine health screening (e.g., eye exams) was associated with survival.
Conclusion
We found significant associations between markers of oral health and survival among both HNSCC cases and controls. This association was most pronounced for sites closer to the dentition. Oral health may have a direct effect on tumor biology due to the associated immune or inflammatory response. It may also represent a proxy for wellness or unmeasured social determinants of health.
Keywords: oral health, head and neck cancer, dental hygiene, oral cancer, cancer survival, case-control studies, oropharyngeal cancer, laryngeal cancer, periodontal disease, squamous cell carcinoma
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) contributes substantially to the global burden of cancer.1–3 It is the sixth most common cancer worldwide,4 and the fifth most common cancer in the United States, affecting approximately 40,000 new patients each year.1 It encompasses cancers of the oral cavity, oropharynx, and larynx among others. While the mortality rate for HNSCC has improved recently, it still has poorer survival rates than some other common malignancies such as breast, cervical, and colorectal cancers.5,6 The traditional risk factors for HNSCC have also been shown to affect survival.7–11 These include tobacco and alcohol consumption, p16 status, demographics, and socioeconomic status. 2,7–10,12–14
Poor oral health has recently been recognized as a risk factor for HNSCC.15–18 Specifically, oral health indicators including good oral hygiene, daily tooth brushing and annual dental visits have been linked to modestly reduced HNSCC risk. Although the mechanisms underlying this postulated association have not been elucidated, oral biofilm-induced conditions, like periodontitis, can confer a substantial systemic and local (i.e., oral cavity) inflammatory burden,19–22 which can alter both the behavior of tumors and the resulting immunological response. Moreover, good dental health can also be a marker for general wellness and health-promoting behaviors that likely influence cancer risk and survival.23
In spite of the accumulating evidence regarding oral health and HNSCC risk, very little information exists on the possible influence of oral health on HCSCC survival. To address this knowledge gap, we carried out this analysis aiming to estimate the impact of oral health indicators on survival in a large population-based HNSCC cohort. We also examined the association by HNSCC site (i.e., oral, laryngeal, pharyngeal) and the relationship of routine dental exams with other behaviors-measures of wellness (e.g., routine physical exams, eye exams, colonoscopies) as predictors of HNSCC survival.
Methods
Study population
Data for this analysis was obtained from the Carolina Head and Neck Cancer Epidemiology Study (CHANCE); a population based-based case-control study in North Carolina.16,18 Cases were eligible to participate in CHANCE if they had been diagnosed with a first primary squamous cell carcinoma of the oral cavity, pharynx, or larynx between January 1, 2002, and February 28, 2006; were ages 20 to 80 years at diagnosis; and resided in a 46-county region in central North Carolina. Our inclusion criteria was squamous cell carcinoma of the head and neck; all other diagnoses were excluded (i.e. patients with benign tumors, carcinomas in situ, and papillary or adenoid cystic carcinomas). There were 1,381 cases in CHANCE. The control group (n=1,396) was identified through the North Carolina Department of Motor Vehicle records, and those individuals were frequency-matched with cases on age, race, and sex. The study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Exposure Assessment
Oral health was assessed by trained nurse-interviewers using a structured questionnaire during an in-home visit for both cases and controls.16,18 Cases were interviewed soon after cancer diagnosis (the average time between diagnosis and interview was 5.3 months) and they were specifically asked about their oral health and care before cancer diagnosis. Self-reported oral health variables collected via the interview included: 1) frequency of dental visits in the past 10 years; 2) the number of natural teeth lost, excluding third molars and teeth extracted for orthodontic reasons; 3) frequency of tooth brushing during each participant’s adult life; 4) history of tooth mobility or “teeth loose in their socket” because of disease; and 5) gum disease diagnosed by a dentist. The number of dental visits in the past 10 years was chosen as the primary oral health indicator, as it was previously the oral health variable most strongly associated with increased cancer incidence.16,18 In addition, it was likely the most reliable metric of oral health that could be collected by the trained interviewers.
Survival Assessment
CHANCE data were linked to the National Death Index (NDI) based on name, social security number, date of birth, sex, race, and state of residence to identify deaths through December 31, 2013. The NDI is a national file of identified death record information, including cause of death compiled from computer files submitted by State Vital Statistics offices. More than 75% of the CHANCE cases were perfect or near-perfect NDI matches on social security number, date of birth, and sex. The remaining near-matches were confirmed by examining the United States Social Security Death Index and obituaries on newspaper websites. We chose 5-year survival as our endpoint for this study, as after 5 years the initial tumor likely plays a diminished role in their overall survival.
Questionnaire and Clinical Assessment
Demographic, lifestyle, diet, and other data also were collected during the in-home interview. Potential confounders to be adjusted for in statistical models were selected a priori based on their potential association with survival and oral health. These included age, race, gender, education, annual income, smoking and alcohol consumption. In order to assess for HPV-associated cancer, p16 immunohistochemistry was retrospectively performed on a subset patients using a previously described protocol.18 Smoking was dichotomized at 10 pack-years and alcohol use at 1 drink per week.
Clinical information such as tumor site was abstracted from participants’ medical records and reviewed independently by a pathologist and a head neck cancer surgeon. Tumors were classified by site according to International Classification of Diseases for Oncology, third edition, topography codes for the oral cavity (C02.0–C02.3, C03.0, C03.1, C03.9, C04.0, C04.1, C04.8, C04.9, C05.0, C06.0–C06.2, C06.8, and C06.9), the larynx (C32.0–C32.3, and C32.8–C32.9), the hypopharynx (C13.0, C13.1, C13.2, C13.8, and C13.9), and the oropharynx (C01.9, C02.4, C05.1, C05.2, C09.0, C09.1, C09.8, C09.9, C10.0–C10.4, C10.8, and C10.9).
Statistical Analysis
Descriptive statistics were calculated and bivariate testing methods included t and chi-squared tests. Overall survival was calculated as time from diagnosis to either date of death due to any cause or censoring on December 31, 2013, whichever came first. Overall and stratified Kaplan-Meier survival plots were constructed. Hazard ratios (HRs) and 95% confidence intervals (CI) for the independent effects of oral health indicators on overall survival were estimated by Cox proportional hazards regression modeling adjusting for sex, age, race, education, income, insurance status, smoking, and alcohol use. Cases and controls were analyzed in both separate analyses and a pooled analysis. For cases, the Cox proportional hazard models were also adjusted for T, N and M classification. Models were run with and without treatment variables (surgery, chemotherapy, and radiation therapy). The proportional hazards assumption for the oral health indicator variables was tested and satisfied.
We tested for site-specific heterogeneity of survival effect estimates with a global Wald chi-squared test using a conservative criterion of p<0.2, and further examined post hoc differences between sites using pairwise homogeneity Z-scores and corresponding p-values.24 STATA 13 (StataCorp, College Station, TX) was used for analyses.
Results
Descriptive information of the CHANCE participants is presented by case/control status and according to receipt of a dental exam during the last 10 years in Table 1. Cases had mean age of 59 years and had mostly high school education or less; affected sites were mostly larynx/hypopharynx (n=481), followed by oropharynx (n=327) and oral cavity (n=164). Almost half (51%) of cases and three-quarters (76%) of controls had a dental exam during the preceding 10 years (p < 0.001). Notable differences in dental exam status were found according to most other examined participant characteristics including sociodemographics, behaviors and other oral health indicators; e.g., dental exams were more frequent among whites, more educated and more affluent cases and controls. Lack of dental visits was also associated with other deleterious health behaviors including smoking and alcohol use (among cases), as well as less frequent tooth brushing and increased tooth loss.
Table 1.
| Table 1a. Descriptive statistics for cases and controls by dental visit frequency
| ||||||
|---|---|---|---|---|---|---|
| Cases (1381) | Controls (1396) | |||||
|
| ||||||
| Variable | No Exam | Dental Exam | P-Value | No Exam | Dental Exam | P-Value |
| (n = 679) | (n = 702) | (n = 333) | (n = 1063) | |||
| Age (SD) | 59.4 (10.0) | 58.6 (10.8) | 0.125 | 64.6 (10.0) | 60.7 (12.8) | < 0.001 |
| Sex | ||||||
| Male (%) | 528 (77.8) | 523 (74.5) | 0.156 | 245 (73.6) | 721 (67.8) | 0.047 |
| Female (%) | 151 (22.2) | 179 (25.5) | 88 (26.4) | 342 (32.1) | ||
| Race (%) | ||||||
| White | 417 (61.4) | 589 (83.9) | < 0.001* | 229 (68.8) | 885 (83.3) | < 0.001 |
| Black | 247 (36.4) | 100 (14.3) | 101 (30.3) | 163 (15.3) | ||
| Other | 15 (2.2) | 13 (1.9) | 3 (0.9) | 15 (1.4) | ||
| Education (%) | ||||||
| Less Than High School | 347 (72.9) | 129 (27.1) | < 0.001 | 130 (39.0) | 89 (8.4) | < 0.001 |
| High School Grad | 211 (54.2) | 178 (45.8) | 109 (32.7) | 225 (21.2) | ||
| Greater than High School | 121 (23.5) | 395 (76.6) | 94 (28.2) | 749 (70.5) | ||
| Household income (%) | ||||||
| < 20k | 335 (51.9) | 154 (22.8) | < 0.001 | 132 (41.1) | 129 (12.6) | < 0.001 |
| 20k – 50k | 232 (35.9) | 233 (34.5) | 144 (44.9) | 349 (34.1) | ||
| > 50k | 79 (12.2) | 289 (42.8) | 45 (14.0) | 545 (53.3) | ||
| Insurance Status (%) | ||||||
| Private | 150 (23.0) | 325 (47.9) | < 0.001 | 70 (21.2) | 486 (46.4) | < 0.001 |
| Medicaid/Medicare | 286 (43.9) | 176 (26.0) | 162 (49.1) | 275 (26.3) | ||
| None | 122 (18.7) | 45 (6.6) | 42 (12.7) | 34 (3.3) | ||
| Other | 94 (14.4) | 132 (19.5) | 56 (17.0) | 252 (24.1) | ||
| Smoking (%) | ||||||
| < 10 PY | 74 (11.0) | 219 (31.7) | < 0.001 | 125 (37.7) | 650 (61.5) | < 0.001 |
| 10+ PY | 602 (89.1) | 473 (86.4) | 207 (62.4) | 407 (38.5) | ||
| Alcohol (%) | ||||||
| < 1 drink/week | 76 (11.6) | 116 (17.3) | 0.003 | 103 (31.8) | 357 (33.9) | 0.481 |
| >= 1 drink/week | 582 (88.5) | 555 (82.7) | 221 (68.2) | 696 (66.1) | ||
| Lost permanent tooth or have pulled (%) | ||||||
| 0–5 | 187 (27.8) | 412 (59.5) | < 0.001 | 78 (23.5) | 757 (71.4) | < 0.001 |
| 6–15 | 81 (12.1) | 145 (20.9) | 46 (13.9) | 164 (15.5) | ||
| 16–28 | 404 (60.1) | 136 (19.6) | 208 (62.7) | 139 (13.1) | ||
| Did a dentist say you had gum disease (%) | ||||||
| No | 448 (66.3) | 478 (69.0) | 0.285 | 234 (70.9) | 788 (74.5) | 0.199 |
| Yes | 228 (33.7) | 215 (31.0) | 96 (29.1) | 270 (25.5) | ||
| Have loose permanent tooth due to disease (%) | ||||||
| No | 351 (52.0) | 492 (70.9) | < 0.001 | 200 (60.2) | 871 (82.2) | < 0.001 |
| Yes | 324 (48.0) | 202 (29.1) | 132 (39.8) | 189 (17.8) | ||
| How often brush teeth (%) | ||||||
| < Once / Day | 98 (16.2) | 35 (5.1) | < 0.001 | 51 (16.2) | 23 (2.2) | < 0.001 |
| Once / Day | 336 (55.6) | 261 (37.9) | 166 (52.7) | 379 (35.8) | ||
| >= 2× / Day | 170 (28.2) | 393 (57.0) | 98 (31.1) | 657 (62.0) | ||
| Number of dental visits in last 10 years | ||||||
| 0 | 679 (100) | 0 | 333 (100) | |||
| 1–5 | 0 | 211 (33.1) | 0 | 226 (21.7) | ||
| 6–10 | 0 | 129 (20.2) | 0 | 210 (20.1) | ||
| 11–20 | 0 | 257 (40.3) | 0 | 532 (51.0) | ||
| 21–40 | 0 | 41 (6.4) | 0 | 76 (7.3) | ||
| Number of Routine Physical Exams (Excluding exams for illness) | ||||||
| One or Less | 338 (49.8) | 161 (24.8) | < 0.001 | 48 (17.0) | 77 (7.6) | < 0.001 |
| 2 to 10 | 152 (22.4) | 220 (33.9) | 120 (42.4) | 406 (40.2) | ||
| 10+ | 189 (27.8) | 269 (41.4) | 115 (40.6) | 527 (52.2) | ||
| Number of Routine Eye Exams | ||||||
| No eye exams | 216 (31.8) | 85 (13.1) | < 0.001 | 50 (15.0) | 76 (7.3) | < 0.001 |
| 1 to 5 | 356 (52.4) | 370 (56.9) | 195 (58.6) | 529 (50.6) | ||
| 5+ | 107 (15.8) | 195 (13.0) | 88 (26.4) | 441 (42.2) | ||
| Number of Colonoscopies | ||||||
| No colonoscopy | 452 (67.1) | 375 (57.9) | 0.001 | 167 (50.2) | 414 (39.6) | 0.001 |
| Colonoscopy | 222 (32.9) | 273 (42.1) | 166 (49.9) | 632 (60.4) | ||
| Table 1b: Descriptive statistics for cases and controls by dental visit frequency
| ||||||
|---|---|---|---|---|---|---|
| Tumor Characteristics | No Exam | Dental Exam | P-Value | |||
| Hypopharynx | 38 (55.9) | 30 (44.1) | < 0.001 | |||
| Larynx | 274 (57.2) | 205 (42.8) | ||||
| NOS | 103 (41.4) | 146 (58.6) | ||||
| Oral cavity | 111 (52.6) | 100 (47.4) | ||||
| Oropharynx | 151 (40.7) | 220 (59.3) | ||||
| T-stage | ||||||
| T1 | 161 (37.3) | 271 (62.7) | < 0.001* | |||
| T2 | 230 (50.7) | 224 (49.3) | ||||
| T3 | 155 (61.9) | 96 (38.3) | ||||
| T4 | 133 (54.5) | 111 (45.5) | ||||
| N-stage | ||||||
| N0 | 379 (51.2) | 361 (48.8) | 0.102 | |||
| N1+ | 300 (46.8) | 341 (53.2) | ||||
| M-stage | ||||||
| M0 | 676 (49.4) | 693 (50.6) | 0.093 | |||
| M1 | 3 (25.0) | 9 (75.0) | ||||
| Stage | ||||||
| I | 130 (41.8) | 181 (58.2) | 0.673* | |||
| II | 141 (56.9) | 107 (43.2) | ||||
| III | 122 (52.4) | 111 (47.6) | ||||
| IVA | 200 (47.7) | 219 (52.3) | ||||
| IVB | 83 (52.5) | 75 (47.5) | ||||
| IVC | 3 (25.0) | 9 (75.0) | ||||
| P16 Overexpression** | ||||||
| Negative | 160 (58.2) | 115 (41.8) | < 0.001 | |||
| Positive | 83 (38.6) | 132 (61.4) | ||||
| Chemo | ||||||
| No | 378 (50.5) | 370 (49.5) | 0.772 | |||
| Yes | 234 (49.7) | 237 (50.3) | ||||
| Radiation | ||||||
| No | 130 (46.6) | 149 (53.4) | 0.17 | |||
| Yes | 482 (51.3) | 458 (48.7) | ||||
| Surgery | ||||||
| No | 291 (54.7) | 241 (45.3) | 0.006 | |||
| Yes | 321 (46.7) | 366 (53.3) | ||||
P-value for high vs. low stage
P16 expression was determined by immunohistochemistry on a subset of patients as a marker for HPV-associated disease
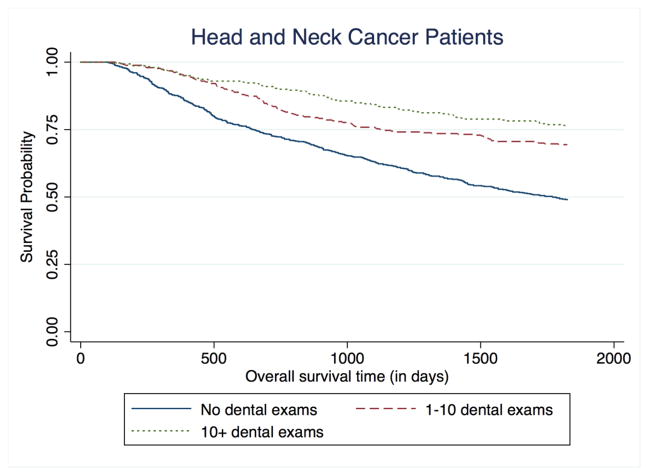
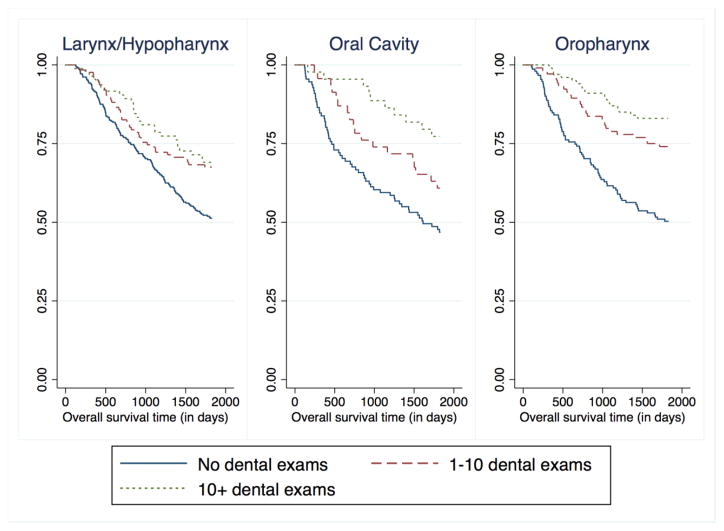
During the 5-year follow-up period, there were 578 deaths among cases (survival: 58%; 95% CI=55% – 61%) and 146 deaths among controls (survival: 91%; 95% CI=89% – 0.92%). Routine dental visits during the preceding 10 years were associated with decreased mortality risk among cancer cases (Figure 1). After adjustment for confounders, dental visits were associated with almost 40% decreased survival, although no exposure-response gradient was found. When compared with no visits, 1 to 10 visits were associated with a HR of 0.62 for mortality (95% CI=0.49–0.80) and >10 visits a HR of 0.63 (95% CI=0.46–0.89) (Table 2). Adjustment for tumor stage (T, N M) and other oral health indicators (e.g., frequency of tooth brushing, number of lost teeth, the presence of gum disease) did not result in any material change in the HR estimates. The inverse association between visits and mortality differed by cancer site (Figure 2). Specifically, the reduced HR was most pronounced for oral cavity cancer (e.g., HR=0.40; 95% CI=0.17–0.93 for 10+ visits), followed by oropharyngeal cancer and then laryngeal/hypopharyngeal cancer (Table 3). The site-heterogeneity was statistically significant (X2, p=0.10 for 1–10 visits; p<0.001 for >10 visits). An exposure-related gradient also appeared for oral cavity cancer and oropharyngeal cancer (Table 3). There were no significant interactions between oral health and alcohol or tobacco use, race, or income. To account for possible confounding by p16 status, the model was run without p16+ cases (new n = 988). There were no changes in the magnitude or significance of the hazard ratios for oral health.
Figure 1.
Kaplan-Meier curve for 5-year overall survival among head and neck cancer patients, stratified by frequency of dental exams (pre-cancer diagnosis) over the past 10 years.
Table 2.
Adjusted hazard ratios from the Cox proportional hazard regression
| Variable | Haz. Ratio | [95% Conf. | Interval] | P-Value |
|---|---|---|---|---|
| Cases N = 1185; 468 deaths | ||||
|
| ||||
| Dental Exams in past 10 years (relative to none) | ||||
| 1–10 Exams | 0.62 | 0.49 | 0.80 | < 0.01 |
| 10 + Exams | 0.63 | 0.46 | 0.86 | < 0.01 |
| Age | 1.01 | 1.00 | 1.02 | 0.03 |
| Female Sex (relative to male) | 1.03 | 0.82 | 1.30 | 0.78 |
| White Race (relative to non-white) | 0.79 | 0.63 | 0.99 | 0.04 |
| Education > high school (relative to < HS) | 0.73 | 0.58 | 0.92 | 0.01 |
| Income (relative to < 20 K) | ||||
| 20k – 50k | 1.31 | 0.98 | 1.74 | 0.06 |
| > 50k | 1.38 | 1.01 | 1.89 | 0.04 |
| Private Insurance (relative to Medicare, Medicaid, or other) | 0.57 | 0.44 | 0.73 | < 0.01 |
| Smoking > 10 PY (relative to < 10 PY) | 1.16 | 0.88 | 1.53 | 0.29 |
| Alcohol use > 1 drink / week (relative to < 1 drink / week) | 1.25 | 0.90 | 1.72 | 0.18 |
| T Stage (relative to T1) | ||||
| T2 | 1.24 | 0.96 | 1.60 | 0.10 |
| T3 | 1.41 | 1.06 | 1.89 | 0.02 |
| T4 | 1.85 | 1.39 | 2.45 | < 0.01 |
| N1 (vs. N0) | 1.52 | 1.25 | 1.84 | < 0.01 |
| M1 (vs. M0) | 8.23 | 3.79 | 17.86 | < 0.01 |
|
| ||||
| Controls N = 1192; 118 deaths | ||||
|
| ||||
| Dental Exams in past 10 years (Relative to none) | ||||
| 1–10 Exams | 0.57 | 0.34 | 0.94 | 0.03 |
| 10 + Exams | 0.60 | 0.37 | 0.98 | 0.04 |
| Age | 1.04 | 1.02 | 1.07 | 0.00 |
| Female Sex (relative to male) | 0.93 | 0.58 | 1.48 | 0.75 |
| White Race (relative to non-white) | 1.30 | 0.81 | 2.08 | 0.28 |
| Education > high school (relative to < HS) | 0.87 | 0.57 | 1.32 | 0.50 |
| Income (relative to < 20 K) | ||||
| 20k – 50k | 1.48 | 0.92 | 2.37 | 0.10 |
| > 50k | 1.08 | 0.58 | 2.00 | 0.81 |
| Private Insurance (relative to Medicare, Medicaid, or other) | 0.59 | 0.32 | 1.07 | 0.08 |
| Smoking > 10 PY (relative to < 10 PY) | 1.46 | 0.97 | 2.20 | 0.07 |
| Alcohol use > 1 drink / week (relative to < 1 drink / week) | 1.64 | 1.02 | 2.64 | 0.04 |
Figure 2.
Kaplan-Meier curve for 5-year overall survival among larynx/hypopharynx, oral cavity, and oropharynx patients, stratified by frequency of dental exams (pre-cancer diagnosis) over the past 10 years.
Table 3.
Hazard ratio by site
| Variable* | Hazard Ratio | [95% Conf. | Interval] | P-Value |
|---|---|---|---|---|
| Oral cavity Cancers (N = 164; 74 deaths) | ||||
| 1–10 Exams (Relative to none) | 0.53 | 0.28 | 1.00 | 0.05 |
| 10 + Exams (Relative to none) | 0.40 | 0.17 | 0.93 | 0.03 |
| Oropharynx Cancers (N = 327; 111 deaths) | ||||
| 1–10 Exams (Relative to none) | 0.69 | 0.42 | 1.13 | 0.14 |
| 10 + Exams (Relative to none) | 0.57 | 0.29 | 1.11 | 0.10 |
| Larynx/Hypopharynx Cancers (N = 481; 201 deaths) | ||||
| 1–10 Exams (Relative to none) | 0.64 | 0.44 | 0.94 | 0.02 |
| 10 + Exams (Relative to none) | 0.82 | 0.51 | 1.31 | 0.41 |
Controlling for age, sex, race, income, education, insurance, tobacco and alcohol use, T-stage, N-stage, and M-stage
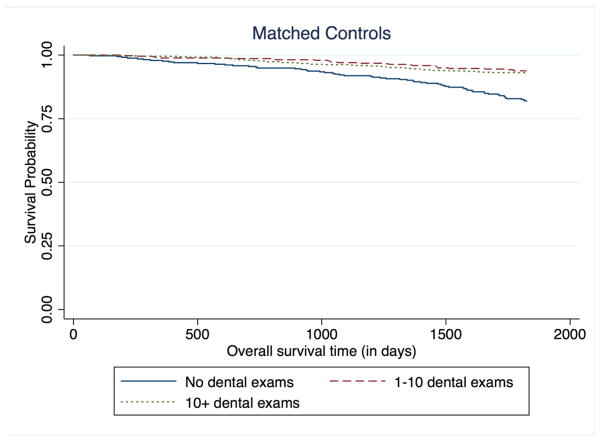
We found similar associations between routine dental visits and survival among controls, with 1 to 10 routine and >10 routine dental visits during the last 10 years associated with decreased mortality risk when compared to those with no dental visits (HR=0.57; 95% CI=0.34–0.94 and HR=0.60; 95% CI = 0.34–0.94, respectively) (Table 2, Figure 3). There was no significant interaction between case/control status and frequency of dental visits (ratio of hazard ratios 0.8; 95% CI 0.5–1.2, p = 0.3). Daily tooth brushing twice or more was another oral health indicator with similar reduced HRs among both cases (HR=0.80; 95% CI=0.64–1.00) and controls (HR=0.76; 95% CI=0.51–1.15) (Table 4). However, other dental indicators including lost teeth, gum disease, and loose teeth did not show any association with survival.
Figure 3.
Kaplan-Meier curve for 5-year overall survival among controls, stratified by frequency of dental exams over the past 10 years.
Table 4.
5-year survival with other dental indicators
| Variable* | Haz. Ratio | [95% Conf. | Interval] | P-Value |
|---|---|---|---|---|
| Cases n=1127; 443 deaths | ||||
|
| ||||
| Dental Exam in last 10 years (vs. none) | 0.76 | 0.61 | 0.95 | 0.02 |
| Amount Brush Teeth per day (once/day as comparison group) | ||||
| Twice or more | 0.80 | 0.64 | 1.00 | 0.05 |
| Less than once | 0.99 | 0.74 | 1.33 | 0.95 |
| > 5 Lost Teeth (< 5 as comparison group) | 0.94 | 0.74 | 1.18 | 0.57 |
| Gum disease | 0.83 | 0.66 | 1.04 | 0.10 |
| Loose teeth | 1.13 | 0.91 | 1.39 | 0.27 |
|
| ||||
| Controls n=1262; 115 deaths | ||||
|
| ||||
| Dental Exam in last 10 years (vs. none) | 0.64 | 0.41 | 1.01 | 0.05 |
| Amount Brush Teeth per day (once/day as comparison group) | ||||
| Twice or more | 0.76 | 0.51 | 1.15 | 0.20 |
| Less than once | 0.94 | 0.46 | 1.90 | 0.86 |
| > 5 Lost Teeth (< 5 as comparison group) | 1.39 | 0.89 | 2.16 | 0.15 |
| Gum disease | 0.81 | 0.52 | 1.26 | 0.35 |
| Loose teeth | 1.15 | 0.73 | 1.81 | 0.56 |
Controlling for age, sex, race, income, education, insurance, tobacco and alcohol use, and T, N, and M-stage for cases
Because dental visits and tooth brushing can be regarded as measures of overall wellness and health-promoting behaviors, we examined the associations between HNSCC survival and three other types of screening and wellness behaviors: routine physical exams, routine eye exams, and routine colonoscopies in the preceding 10 years. We found no significant associations between any of these screening variables and mortality in cases or controls, with negligible HRs.
Discussion
In this study among a large cohort of HNSCC patients and population-based controls, we found a significant association with strong HRs between poor oral health indicators and survival, among both cancer cases and controls. This association differed by cancer site and was most pronounced for sites closer to the dentition. Of note, no other type of routine screening such as physical or eye exams affected survival.
Friemel and colleagues produced the only other paper examining the association between oral health and survival and HNSCC.25 The German case-only study used a composite score for oral health, including information on annual dental visits, daily tooth cleaning and use of dental floss. A low score was associated with a poor prognosis; however, this association was attenuated after controlling for age, sex, tumor stage, tumor site, treatment, education, HPV status, smoking and alcohol consumption (HR=1.30; 95% CI=0.78 – 2.15). In comparison, our study found a stronger association between poor oral health and poor survival with greater precision and a larger sample size (1185 vs. 276 cases), after controlling for a similar set of variables.
There are two possible interpretations of our results: 1) that oral health is a marker of overall wellness, or 2) poor oral health directly affects the cancer progression by affecting the development of the tumor or the associated host immune response. Multiple studies have found good oral health to be a marker for favorable survival. In a study of 21,700 elderly Japanese, tooth brushing and regular dental visits were associated with survival after controlling for age, sex, education level, smoking, drinking, and medical history.26 Other studies have found an association between tooth loss and overall mortality in Danish,27 Taiwanese,28 Scottish,29 French30 and Chinese populations.31 The mechanism is unclear and it has been suggested that markers of oral health such as tooth loss, visits to the dentist, and tooth brushing may serve as a proxy for life and health stressors that can affect mortality.23 Our study supports oral health as a marker for overall wellness in that the controls, as well as the cases, had associations between oral health and survival.
Although our data suggest that oral health may be a surrogate for overall wellness, the differential impact on survival by head and neck cancer site may suggest an additional biologic mechanism. Oral health had a significantly greater impact on survival for oral and oropharyngeal cancer cases compared to other sites. Several mechanisms have been proposed by which oral health could impact cancer susceptibility and survival; these include alterations to the immunologic response to the tumor and changes in the local environment of the tumor. Periodontitis, resulting from poor oral hygiene and an aberrant inflammatory response,32 affects local inflammation and immune function,33–35 and the effect is amenable to periodontal treatment. 19,36–38 Likewise, oral bacterial dysbiosis, particularly involving Porphyromonas gingivalis and F. nucleatum, have been implicated in the development of oral cancer in human and animal studies.39–41 Possible mechanisms include the induction of chronic inflammation, 40,42,43 promotion of cellular invasion,40 and direct production of carcinogens.40,44,45 These local mechanisms that promote bacterial carcinogenesis could also affect tumor behavior and influence patient survival.
It is noteworthy that patients with poor oral health were less educated, had lower incomes, and had poor health behaviors such as alcohol and tobacco use. It has been well documented that social determinants of health have a strong correlation with prognosis in head and neck cancer.46–51 Our multivariate models showed that oral health had a significant association with survival independent of education and income. Nonetheless, the overwhelming influence of social determinants of health on oral health is well-established, and a large number of unmeasured or unknown pathways, such as a biological mechanism, could underlie the links between social factors and oral health outcomes, including oral cancer and survival.52
The chief strength of this study is the use of a large population-based sample of head and neck cancer patients with a long period of follow up. This is the largest study to examine this association. It is also the first study to examine this association in parallel with matched control patients, and the first study to examine the association of other markers of screening and wellness on survival besides oral health. The main study limitation is in the ascertainment of dental variables. These were collected via interviews conducted by trained nurses instead of examination by trained dental professionals, and they represent surrogates of oral health instead of clinical endpoints or biomarkers. A further limitation is that this study address overall survival and does not include disease-free survival.
Future studies are needed to determine whether the association between oral health and survival is due to markers of wellness, or if oral health has a direct effect on tumor progression. The best methods would involve direct examination of oral health by dental professionals, identification and quantification of the relevant oral microbiome, and examination of oral and systemic inflammatory markers. A clear delineation of the mechanism behind the effect of oral health on survival would provide an impetus for dental care and oral hygiene recommendations for patients with head and neck cancer.
Highlights for Poor Oral Health Affects Survival in Head and Neck Cancer.
We found a significant association between markers of oral health and survival
This association was most pronounced for sites closer to dentition, and present in cases as well as controls
Oral health may represent a proxy for wellness or unexamined social determinants of health
It may also have a direct effect on tumor biology or the immune response
Acknowledgments
This study was supported in part by grants R01-CA90731 from the National Cancer Institute and T32 - DC005360-12 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
Conflicts of interest: None of the authors have any conflicts of interest or financial disclosures to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clinic proceedings. 2008;83(4):489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Parfenov M, Pedamallu CS, Gehlenborg N, et al. Characterization of HPV and host genome interactions in primary head and neck cancers. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(43):15544–15549. doi: 10.1073/pnas.1416074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horowitz AM, Goodman HS, Yellowitz JA, Nourjah PA. The need for health promotion in oral cancer prevention and early detection. Journal of public health dentistry. 1996;56(6):319–330. doi: 10.1111/j.1752-7325.1996.tb02459.x. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 7.Deleyiannis FWB, Thomas DB, Vaughan TL, Davis S. Alcoholism: Independent predictor of survival in patients with head and neck cancer. Jnci-Journal of the National Cancer Institute. 1996;88(8):542–549. doi: 10.1093/jnci/88.8.542. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Zhang Q, Jordan R, et al. Tobacco Smoking and Increased Risk of Death and Progression for Patients With p16-Positive and p16-Negative Oropharyngeal Cancer. Journal of Clinical Oncology. 2012;30(17):2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncology. 2012;48(12):1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Osazuwa-Peters N, Christopher KM, Hussaini AS, Behera A, Walker RJ, Varvares MA. Predictors of stage at presentation and outcomes of head and neck cancers in a university hospital setting. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck. 2016;38:E1826–E1832. doi: 10.1002/hed.24327. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: A global perspective on epidemiology and prognosis. Anticancer Research. 1998;18(6B):4779–4786. [PubMed] [Google Scholar]
- 12.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3(1):78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayne ST, Cartmel B, Kirsh V, Goodwin WJ. Alcohol and Tobacco Use Prediagnosis and Postdiagnosis, and Survival in a Cohort of Patients with Early Stage Cancers of the Oral Cavity, Pharynx, and Larynx. Cancer Epidemiology Biomarkers & Prevention. 2009;18(12):3368–3374. doi: 10.1158/1055-9965.EPI-09-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazul AL, Rodriguez-Ormaza N, Taylor JM, et al. Prognostic significance of non-HPV16 genotypes in oropharyngeal squamous cell carcinoma. Oral Oncology. 2016;61:98–103. doi: 10.1016/j.oraloncology.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JS, Lo HI, Wong TY, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncology. 2013;49(10):1010–1017. doi: 10.1016/j.oraloncology.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Divaris K, Olshan AF, Smith J, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010;21(4):567–575. doi: 10.1007/s10552-009-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashim D, Sartori S, Brennan P, et al. The role of oral hygiene in head and neck cancer: results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27(8):1619–1625. doi: 10.1093/annonc/mdw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazul AL, Taylor JM, Divaris K, et al. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2016 doi: 10.1002/cncr.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: Control of the local infection is associated with a reduction in serum inflammatory markers. Journal of dental research. 2004;83(2):156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 20.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PME, van der Velden U. Elevation of systemic markers related to cardiovascular disease in the peripheral blood of periodontitis patients. Journal of Periodontology. 2000;71(10):1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 21.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. Journal of Periodontology. 2001;72(9):1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 22.Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the US population. Journal of dental research. 2000;79(1):49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- 23.Friedman PK, Lamster IB. Tooth loss as a predictor of shortened longevity: exploring the hypothesis. Periodontology 2000. 2016;72(1):142–152. doi: 10.1111/prd.12128. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Rothman K. Introduction to Stratified Analysis. In: Rothman K, Greenland S, Lash T, editors. Modern Epidemiology. New York: Lippincott, Williams and Wilkins; 2008. pp. 258–282. [Google Scholar]
- 25.Friemel J, Foraita R, Gunther K, et al. Pretreatment oral hygiene habits and survival of head and neck squamous cell carcinoma (HNSCC) patients. Bmc Oral Health. 2016:16. doi: 10.1186/s12903-016-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayasaka K, Tomata Y, Aida J, Watanabe T, Kakizaki M, Tsuji I. Tooth loss and mortality in elderly Japanese adults: effect of oral care. Journal of the American Geriatrics Society. 2013;61(5):815–820. doi: 10.1111/jgs.12225. [DOI] [PubMed] [Google Scholar]
- 27.Holm-Pedersen P, Schultz-Larsen K, Christiansen N, Avlund K. Tooth loss and subsequent disability and mortality in old age. Journal of the American Geriatrics Society. 2008;56(3):429–435. doi: 10.1111/j.1532-5415.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 28.Hu H-Y, Lee Y-L, Lin S-Y, et al. Association Between Tooth Loss, Body Mass Index, and All-Cause Mortality Among Elderly Patients in Taiwan. Medicine. 2015;94(39):e1543. doi: 10.1097/MD.0000000000001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu YK, Galobardes B, Smith GD, McCarron P, Jeffreys M, Gilthorpe MS. Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart. 2007;93(9):1098–1103. doi: 10.1136/hrt.2006.097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adolph M, Darnaud C, Thomas F, et al. Oral health in relation to all-cause mortality: the IPC cohort study. Scientific reports. 2017;7:44604. doi: 10.1038/srep44604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. International journal of epidemiology. 2005;34(2):467–474. doi: 10.1093/ije/dyh375. [DOI] [PubMed] [Google Scholar]
- 32.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (London, England) 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 33.You Z, Cushman M, Jenny NS, Howard G. Tooth loss, systemic inflammation, and prevalent stroke among participants in the reasons for geographic and racial difference in stroke (REGARDS) study. Atherosclerosis. 2009;203(2):615–619. doi: 10.1016/j.atherosclerosis.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 35.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. Journal of dental research. 2005;84(3):269–273. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 37.D’Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: Results from a randomized controlled clinical trial. American Heart Journal. 2006;151(5):977–984. doi: 10.1016/j.ahj.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of Periodontitis and Endothelial Function. New England Journal of Medicine. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 39.Gholizadeh P, Eslami H, Yousefi M, Asgharzadeh M, Aghazadeh M, Kafil HS. Role of oral microbiome on oral cancers, a review. Biomedicine & Pharmacotherapy. 2016;84:552–558. doi: 10.1016/j.biopha.2016.09.082. [DOI] [PubMed] [Google Scholar]
- 40.Perera M, Al-Hebshi NN, Speicher DJ, Perera I, Johnson NW. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J Oral Microbiology. 2016;8:10. doi: 10.3402/jom.v8.32762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A Review of the Relationship between Tooth Loss, Periodontal Disease, and Cancer. Cancer causes & control : CCC. 2008;19(9):895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic Inflammation and Cytokines in the Tumor Microenvironment. Journal of Immunology Research. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooper SJ, Wilson MJ, Crean SJ. Exploring the link between microorganisms and oral cancer: A systematic review of the literature. Head & Neck. 2009;31(9):1228–1239. doi: 10.1002/hed.21140. [DOI] [PubMed] [Google Scholar]
- 45.Homann N, Tillonen J, Meurman JH, et al. Increased salivary acetaldehyde levels in heavy drinkers and smokers: a microbiological approach to oral cavity cancer. Carcinogenesis. 2000;21(4):663–668. doi: 10.1093/carcin/21.4.663. [DOI] [PubMed] [Google Scholar]
- 46.Megwalu UC, Ma Y. Racial disparities in oropharyngeal cancer survival. Oral Oncol. 2017;65:33–37. doi: 10.1016/j.oraloncology.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Hagedoorn P, Vandenheede H, Vanthomme K, Willaert D, Gadeyne S. A cohort study into head and neck cancer mortality in Belgium (2001–11): Are individual socioeconomic differences conditional on area deprivation? Oral Oncology. 2016;61:76–82. doi: 10.1016/j.oraloncology.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Dourado Martins J, Oliveira Mascarenhas Andrade J, Souza Freitas V, de Araújo TM. Social determinants of health and the occurrence of oral cancer: a systematic literature review. Revista de Salud Pública. 2014;16(5):786–798. [PubMed] [Google Scholar]
- 49.Andersen ZJ, Lassen CF, Clemmensen IH. Social inequality and incidence of and survival from cancers of the mouth, pharynx and larynx in a population-based study in Denmark, 1994–2003. European Journal of Cancer. 2008;44(14):1950–1961. doi: 10.1016/j.ejca.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Rylands J, Lowe D, Rogers SN. Outcomes by area of residence deprivation in a cohort of oral cancer patients: Survival, health-related quality of life, and place of death. Oral Oncology. 2016;52:30–36. doi: 10.1016/j.oraloncology.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Reitzel LR, Nguyen N, Zafereo ME, Li GJ, Wei QY, Sturgis EM. Neighborhood deprivation and clinical outcomes among head and neck cancer patients. Health & Place. 2012;18(4):861–868. doi: 10.1016/j.healthplace.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JY, Divaris K. The ethical imperative of addressing oral health disparities: a unifying framework. Journal of dental research. 2014;93(3):224–230. doi: 10.1177/0022034513511821. [DOI] [PMC free article] [PubMed] [Google Scholar]