Abstract
Inter-male aggression is an essential component of social behavior in organisms from insects to humans. However, when expressed inappropriately, aggression poses significant threats to the mental and physical health of both the aggressor and the target. Inappropriate aggression is a common feature of numerous neuropsychiatric disorders in humans and has been hypothesized to result from the atypical activation of reward circuitry in response to social targets. The lateral habenula (LHb) has recently been identified as a major node of the classical reward circuitry and inhibits the release of dopamine from the midbrain to signal negative valence. Here, we discuss the evidence linking LHb function to aggression and its valence, arguing that strong LHb outputs to the ventral tegmental area (VTA) and the dorsal raphe nucleus (DRN) are likely to play roles in aggression and its rewarding components. Future studies should aim to elucidate how various inputs and outputs of the LHb shape motivation and reward in the context of aggression.
Keywords: Aggression, reward, bullying, mesolimbic dopamine circuit
Introduction
Aggression is an innate and evolutionarily conserved behavior that promotes the protection of food, mating partners, progeny, and territory in social organisms (Anderson 2012). While aggression in animals can be adaptive, most forms of aggression in humans are maladaptive and produce a variety of negative social, physical, and emotional consequences for individuals, victims, and their families (Leonard 1993). In fact, inappropriate aggressive behavior is a common feature of several psychiatric disorders, including drug addiction (Beck et al. 2014, Coccaro et al. 2016), autism (Fitzpatrick et al. 2016), depression (Dolenc et al. 2015), PTSD (Miles et al. 2015), antisocial personality disorder (Anderson & Kiehl 2014), and schizophrenia (Hoptman et al. 2015). While the causes of heightened aggression in psychiatric disorders are likely heterogeneous, it has been hypothesized that the subordination of social targets is highly rewarding in some patients, particularly in those experiencing symptoms of dysregulated motivation (Salmone et al. 2016). Consequently, repeated domination of social targets is positively reinforcing and can produce a persistent motivation to seek out and engage in aggressive interactions (appetitive aggression), much like how some individuals compulsively seek out palatable food, sex, or drugs (Moran et al. 2014, Golden et al. 2016a). Interestingly, the ventral striatum, a region highly implicated in drug and natural rewards, is strongly activated in some humans while viewing videos of violence or participating in behavioral tasks aimed at punishing others (Moran et al. 2014, Chester et al. 2016, Seo et al. 2008). Findings in rodent models corroborate these results and describe a particularly important role for dopamine and serotonin signaling in controlling appetitive aggression (de Almeida et al. 2005). However, much less is known about the upstream circuits that control these particular neurotransmitter dynamics to encode the valence of intermale social interactions. Notably, the lateral habenula (LHb) has been shown to tightly regulate the activity of midbrain and brainstem target regions in reward (Lammel et al. 2012, Hikosaka 2010, Pollak Dorocic et al. 2014) and becomes dysregulated in many of the aforementioned psychiatric disorders (Lecca et al. 2014, Proulx et al. 2014). Thus, at both the neurochemical, behavioral, and anatomical level, the LHb is well positioned to integrate motivation and emotion in aggression. Here, we discuss how this diverse combination of LHb inputs and outputs can function to exert parallel control over reward circuits modulating the valence of inter-male aggressive behavior.
LHb is a dynamic regulator of VTA and DRN circuitry
The habenular complex is a relatively small, morphologically distinct region of the epithalamus that can be divided into two main regions called the medial (MHb) and lateral habenulae. While there is evidence that the MHb and LHb may share some behavioral functions (Shumake et al. 2003, Kobayashi et al. 2013), they differ substantially in their connectivity and neurochemistry. The MHb uses the neurotransmitters acetylcholine, substance P, and glutamate and projects largely to the nearby interpeduncular nucleus (IPN) (Contestabile et al. 1987, Qin and Luo et al. 2009). Conversely, nearly all of the of the neurons in the LHb are glutamatergic and possess long-ranging axons that target both midbrain and brainstem regions (Wiess and Veh 2011, Li et al. 2011, Aizawa et al. 2012, Lammel et al. 2012, Stamatakis and Stuber 2012). Interestingly, only small numbers of local inhibitory neurons have been described in the LHb, and they are primarily located in the medial aspect of the LHb (Smith et al. 1987, Zhang et al 2016). For the purpose of this review, we will focus the exclusively on how LHb circuits mediate aggression and reward–related behavior.
The LHb effectively functions as a hub between forebrain structures and neuromodulatory nuclei expressing dopamine and serotonin. While the basal ganglia and limbic forebrain comprise the majority of LHb inputs, LHb outputs mainly target midbrain nuclei such as the dopaminergic ventral tegmental area/substantia nigra complex (VTA/SNc), the GABAergic rostromedial tegmental nucleus (RMTg, projects directly to VTA dopamine neurons), and serotonergic dorsal and median raphe nuclei (DRN/MRN) (Hikosaka 2010). Interestingly, the medial aspect of the LHb appears to contain predominantly DRN-projecting neurons, whereas the lateral aspect of the LHb is thought to contain predominantly VTA and RMTg-projecting neurons (Proulx et al. 2014). Since these neuronal populations do not display a great deal of overlap, the LHb may thus have the capacity to modulate downstream dopamine and serotonin circuits in relatively independent manners, although this remains to be tested.
The LHb receives mixed inputs from a variety of brain regions (see Table 1). Glutamatergic inputs to the LHb arise from the lateral hypothalamus (LH), the anterior cingulate cortex (Miczek, Maxson et al. 2008), the medial prefrontal cortex (mPFC), and the entopeduncular nucleus (EPN) (Li et al. 2011, Kim and Lee 2012, Shabel et al. 2012, Poller et al 2013). These inputs signal primarily via α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptors to depolarize LHb target neurons, although some Ν-methyl-D-aspartic acid (NMDA) receptor signaling has been described (Li et al. 2011, Maroteaux and Mameli 2012). GABAergic inputs to the LHb arise from the EPN, the diagonal band, the ventral pallidum (VP), the VTA, and the NAc (Golden et al. 2016b, Wang et al. 2006, Bianco and Wilson 2009, Shabel et al. 2012, Stamatakis et al. 2013), and many of these inputs functionally inhibit overall LHb activity (Golden et al. 2016b, Stamatakis et al. 2013). Interestingly, a recently identified population of VTA dopamine neurons seem to co-express and selectively release GABA into the LHb (Stamatakis et al. 2013). A separate population of canonical dopaminergic neurons in the VTA also project to the LHb (Herkenham and Nauta 1977, Gruber et al. 2007) and seem to signal via dopamine receptors 2 and 4 (DRD2, DRD4), as mRNA for both receptors has been detected (Gruber et al 2007, Aizawa et al. 2012). Interestingly, DA application to the LHb produces variable responses in LHb neurons, likely due in part due to the activation of heterogeneous populations of LHb neurons projecting to different downstream targets (Shen et al. 2012). Serotonin neurons from the DRN also project to the LHb and have been shown to exert divergent effects on glutamatergic signaling in medial versus lateral LHb regions (Xie et al. 2016). However, pharmacological inhibition of 5-HT2/3 receptors similarly normalized these divergent effects, indicating that other variations in LHb microcircuitry mediate the mixed outcomes of 5-HT signaling on LHb neural activity.
Table 1.
a,organization of LHb inputs.b, organization of LHb outputs. Abbreviations: GABA: gamma-amino-butyric acid, AMPA: alpha-amino-3-hydrox-5-methyl-isoxazolepionic acid.
| a: LHb inputs | ||||||
|---|---|---|---|---|---|---|
| Input region | Behavior(s) | Input cell type | Target receptor in LHb | Target cell type in LHb | Effect on LHb | Citation |
| Entopeduncular nucleus (EPN) | Learned helplessness Cocaine withdrawal |
Glutamate/GABA | AMPA receptor | Glutamate | Excitation |
Shabel et al. 2012 Shabel et al. 2014 Meye et al. 2016 |
| Basal forebrain (BF) | Aggression | GABA | GABAA receptor | Glutamate | Inhibition | Golden et al. 2016 |
| Lateral Hypothalamus | Behavioral aversion | Glutamate | AMPA receptor | Glutamate | Excitation | Stamatakis et al. 2016 |
| Ventral tegmental area (VTA) | Behavioral reward | GABA/Dopamine Glutamate/GABA | GABAA receptor AMPA receptor | Glutamate | Inhibition |
Stamatakis et al. 2013 Yoo et al. 2016 |
| Paraventricular hypothalamus | Thirst | Vasopressin/Glutamate | Not tested | GABA | Inhibition | Zhang et al. 2016 |
| Dorsal raphe nucleus (DRN) | Not tested | Serotonin | 5-HT2 receptor/5-HT3 receptor | Glutamate | Excitation/Inhibition | Xi et al. 2016 |
| b: LHb outputs | ||||||
|---|---|---|---|---|---|---|
| Input region | Behavior(s) | LHb projection cell type | Target receptor | Target cell type | Effect on target | Citation |
| Ventral tegmental area (VTA) | Behavioral aversion Behavioral reward |
Glutamate | AMPA receptor | GABA Dopamine | Inhibition Excitation/Inhibition |
Stamatakis and Stuber 2012 Lammel et al. 2012 |
| Dorsal raphe nucleus (DRN) | Not tested | Glutamate | AMPA receptor | Serotonin GABA | Excitation Excitation/Inhibition | Pollak-Dorocic et al. 2014 Varga et al. 2003 |
| Rostromedial tegmental nucleus (RMTg) | Beha aversion vioral | Glutamate | AMPA receptor | Glutamate | Excitation (inhibits VTA) |
Stamatakis and Stuber 2012 Jhou et al. 2013 |
As mentioned above, the primary output neurons of the LHb are glutamatergic. As a result, the LHb must relay its signal through indirect inhibitory projections in order for it to inhibit the neural activity of a target region (see Figure 1, Table 1). A population consisting primarily of lateral LHb neurons projects to the RMTg, a GABAergic nucleus at the tail end of the VTA that synapses on VTA dopamine neurons to inhibit them (Jhou et al 2009, Kaufling and Aston-Jones 2015). In fact, electrical stimulation of the LHb inhibits firing activity of roughly 90% of VTA dopamine neurons (Christoph et al. 1986, Matsumoto and Hikosaka 2007). Consistent with this, lesioning the LHb increases dopamine turnover in VTA terminal fields (Lisoprawsi et al 1980). More recently, optogenetic activation of LHb terminals in the midbrain was found to evoke AMPA-mediated EPSCs almost exclusively in GABAergeic neurons in the VTA and RMTg, rather than dopamine neurons themselves (Stamakis and Stuber 2012). However, others report a smaller, unique population of LHb neurons that directly targets VTA DA neurons projecting to the mPFC, suggesting that the LHb can exert mixed downstream effects in VTA target neurons (Lammel et al 2012, Brinschwitz et al. 2010).
Figure 1.
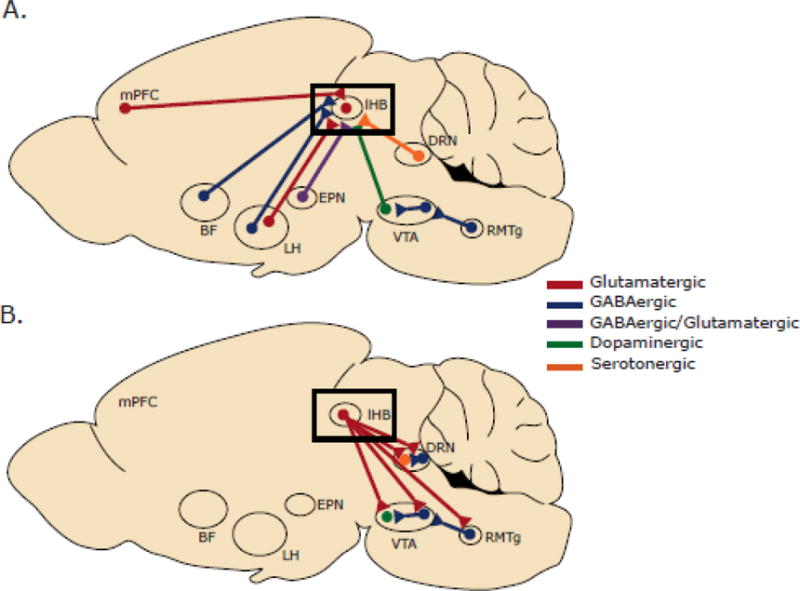
Whole brain input-output map of LHb circuitry. A. Inputs to the LHb. The LHb receives inputs from many forebrain structures, including mPFC, BF, and EPN. B. Outputs of the LHb. The majority of LHb projections target midbrain regions like the RMTg, DRN, and VTA.
Abbreviations: BF, basal forebrain; DRN, dorsal raphe nucleus; EPN, entopeduncular nucleus; LH, lateral hypothalamus; mPFC. medial prefrontal cortex; RMTg, rostromedial tegmental nucleus; VTA, ventral tegmental area.
The LHb exerts predominantly inhibitory control over DRN serotonin neurons through a similar mechanism. Electrical stimulation of the LHb transiently pauses the firing of populations of DRN neurons (Wang and Aghajannian 1977), and this appears to be mediated by a disynaptic circuit from LHb glutamate projection neurons to DRN GABA neurons that inhibit DRN serotonin neurons (Varga et al. 2003). Moreover, these LHb inputs to DRN GABA neurons have been shown to converge with mPFC inputs (Varga et al. 2003), thereby introducing another mechanism by which mPFC top-down control can influence LHb output (in addition to direct synapses between mPFC and LHb neurons in the LHb, see above). This convergence of LHb and mPFC inputs on target neurons in the VTA and DRN highlights a shared top-down inhibitory influence they exert on regions relevant to both reward and aggression. Recently, the notion that the LHb purely inhibits DRN serotonin neurons has been challenged by studies showing direct LHb inputs to serotonin neurons that provide excitatory tone (Pollak Dorocic et al. 2014). These excitatory responses were blocked by AMPA antagonist NBQX but not by GABA antagonist gabazine. The elucidation of this direct LHb glutamate input to DRN serotonin neurons emphasizes the importance of parallel circuits originating from the LHb that result in various downstream physiological effects in the DRN.
Regulation of behavioral avoidance and aversion by the LHb
Primary behavioral functions of the LHb are to promote aversive motivational states and signal unexpected reward outcomes. This behavioral function for the LHb has been primarily attributed to its downstream connections with midbrain targets, although it has not been tested if LHb-DRN projections play a role in controlling these behaviors as well. In-vivo electrophysiological recordings in primates find that LHb activity increases in response to aversive events like air puffs as well as to omitted rewards (Matsumoto and HIkosaka 2009). The LHb can also respond to cues signaling aversive or unexpected events, and this is a phenomenon observed in both rodents and primates (Matsumoto and Hikosaka 2009, Jhou et al. 2013). Optogenetic stimulation of LHb targets in the midbrain similarly promotes aversive states. A study by Stamatakis and Stuber illustrated that optogenetic stimulation of LHb terminals in the RMTg promoted active, passive, and conditioned behavioral avoidance, thus highlighting the multifaceted role that LHb outputs play in controlling aversive behavioral states (2012). Furthermore, Lammel, Kim, and colleagues (2012) showed that stimulation of LHb inputs to VTA GABAergic interneurons and dopaminergic neurons projecting to the mPFC are produce aversion in a task similar to that used by Stamakis and Stuber (2012). Inputs to the LHb appear to encode aversive outcomes as well. For example, stimulation of the EPN to LHb pathway, which is comprised of both GABAergic and glutamatergic components, promotes behavioral avoidance (Shabel et al. 2012), although this aversive state did not persist the next day as was observed in studies by Stamatakis and Stuber (2012) and Lammel and colleagues (2012). Together, these studies provide strong evidence that the LHb and its inputs and outputs function to promote behavioral avoidance and aversive motivational states.
Regulation of aggression and aggression reward by the LHb
The functional and anatomical coupling of the LHb with dopamine and serotonin systems in the midbrain combined with the relevance of these midbrain transmitter systems to aggression and reward suggests there could be an important role for the LHb in the encoding of rewarding or aversive stimuli in the context of intermale aggression. While to date there have only been a handful of studies directly investigating the role of LHb circuitry in this behavioral domain, results suggest that it indeed plays a causal role in controlling the valence of aggressive interactions. Unfortunately, it remains unknown whether the LHb interfaces with regions known to promote attack behavior such as the ventromedial hypothalamus (Lin et al. 2011).
Chou and colleagues recently described an essential role for the zebrafish ventral habenula (vHb), a homologue of the mammalian LHb, in the maintenance of loser behaviors during repeated intermale aggressive conflicts (2016, Amo et al. 2010). Like the mammalian LHb, the zebrafish vHb sends dense projections to the serotonergic median raphe (MR) and is important for regulating serotonergic neurotransmission (Amo et al. 2010). The regulation of MR serotonin neurons by the vHb appears to be mediated predominantly through direct synapses between glutamatergic vHb projection neurons and MR serotonergic targets, suggesting that the vHb depolarizes serotonin neurons in zebrafish (Amo et al. 2014, Nathan et al. 2015). Furthermore, the vHb displays tonic increases in activity as fish experience escalating levels of danger or cues signaling it, and optogenetic stimulation of the vHb promotes a real time place aversion while inhibition impairs adaptive avoidance learning (Amo et al. 2014). Therefore, like the mammalian LHb, the zebrafish vHb plays an important role in controlling negative internal valence states and driving active behavioral avoidance. When Chou and colleagues inhibited the vHb projections to the MR in a zebrafish model of the “winner effect,” whereby experimental fish are repeatedly fought against their wild-type (WT) siblings, experimental fish did not behave like WT losers, appearing to provoke and elicit attack behavior from the opposing WT fish (2016). This result suggests that without intact vHb to MR circuit function, zebrafish do not display normal behavioral adaptations to winning and losing intermale aggressive contests. While this behavioral paradigm did not directly test the role of the vHb in controlling the valence of fighting, in light of what is known about the vHb encoding negative outcomes (see above) it is likely that the experimental animals did not perceive a loss as an event with a strong negative valence and thus did not alter their subsequent fighting behavior.
Another recent study by Golden and colleagues (2016b) investigated the role of basal forebrain inputs to the LHb in mediating the rewarding aspects of inter-male aggression in mice. Using a modified CPP procedure where mice were conditioned to associate specific environmental cues with an intruder, it was found that aggressive mice that attacked and subordinated an intruder developed a preference for the intruder-paired chamber, while non-aggressive mice showed an aversion for this chamber. To investigate the mechanisms driving CPP and CPA for an intruder-paired chamber, Golden and colleagues optogenetically stimulated or inhibited basal forebrain terminal projections in the LHb. Stimulation of these terminals, which were histologically verified to be GABAergic, reduced LHb firing, while inhibition of these terminals increased LHb firing. Behaviorally, stimulation of basal forebrain-LHb GABAergic terminals promoted aggression CPP, while inhibition of these neurons promoted CPA. These results indicate that decreased LHb activity observed in aggressive mice promotes the rewarding component of aggression, which might be related to ability of the LHb to encode positive and negative emotional states.
In fact, there is early evidence to suggest that the LHb encodes the valence of precise events during aggressive social interactions in some individuals in real time to promote the escalation of aggressive behavior (Flanigan et al. 2016). By measuring fluorescence changes in the calcium indicator GCaMP6 with in-vivo fiber photometry (see Gunyadin et al. 2014), it was shown that LHb neuronal activation is sharply decreased when aggressive mice bite or pin a submissive intruder in the resident intruder task (RI). Furthermore, the overall intruder-evoked LHb activity of animals that are highly aggressive decreases with successive wins in RI, suggesting that there is a correlation between decreased LHb activity and the escalation of aggressive behavior with repeated winning, which has been shown to be rewarding. Interestingly, non-aggressive mice do not seem to significantly alter their LHb responses to intruders over successive RI tests, suggesting that aggressive experience may drive the LHb adaptation seen in highly aggressive animals. It remains unknown at this time whether these LHb neurons that are decreasing their tone as a consequence of winning represent neurons projecting to the DRN-MRN system, the VTA, or both.
Regulation of aggression and aggression reward by LHb targets
Although the role of LHb outputs to VTA and DRN nuclei have not yet been established in aggression models, there is a significant amount of evidence to implicate these downstream monoaminergic nuclei in aggression. VTA DA signaling in aggression and aggression reward was initially informed by studies correlating region-specific DA levels with repeated dominance of conspecifics. For example, VTA TH and dopamine transporter (DAT) mRNA levels are increased in mice that won contests for 20 consecutive days, and this increase persists up to 14 days following the last win (Bondar et al. 2009). In addition, there is a roughly 30% increase in NAc DA that reaches its peak 20–30 minutes following repeated aggression (Ferrari et al. 2003, van Erp and Miczek 2000). However, it remains unclear whether NAc dopamine is also increased during aggressive episodes themselves. It is essential that future studies determine the precise timing of NAc dopamine release in the context of aggression, as this will help clarify whether the function of dopamine in aggression is primarily to promote the motivation to fight, increase salience, signal the positive valence of an attack after it is committed, or some combination of these. In support of a role for VTA to NAc dopamine in processes that prepare or anticipate the biological significance of the aggressive bout, rats subjected to ten days of repeated aggression (beginning on the same time each day) display increases in NAc dopamine on day eleven at the time a confrontation would have occurred, even when no conspecific was introduced (Ferrari et al. 2003). Interestingly, dopamine release in the mPFC, which has been shown to be important in controlling working memory and attention (Xing et al. 2016), appears to reach its peak during aggression onset (van Erp and Miczek 2000). These temporal variations in dopamine signaling between NAc and mPFC circuits indicate that VTA dopamine may play a dynamic and multifaceted role in controlling various behavioral components of aggressive behavior, from encoding salience and attention to strengthening motivation. Unfortunately, while informative, the aforementioned findings are limited by their correlative nature.
The foundation for our understanding of dopamine’s causal role in aggression and aggression reward has been laid largely by behavioral pharmacology experiments in rodents, some of which are weakened by non-specific systemic drug administration. For instance, repeated systemic injections of methamphetamine, which increases dopamine release and prevents its removal from the synaptic cleft, increased aggression 15 minutes and 20 hours later (Sokolov et al. 2006). The long-lasting nature of these effects is particularly striking, as it suggests that the neuroplastic changes resulting from repeated methamphetamine is sufficient to promote aggression even after the drug has worn off. In support of this, a single injection of methamphetamine was not sufficient to alter aggression. Interestingly, there has been little consensus on which dopamine receptors (D1 or D2) are primarily mediating aggression’s rewarding effects. A seminal study utilized an operant model of aggression to investigate the precise roles of D1 and D2 NAc receptors in aggression, whereby animals were required to learn to nose-poke to gain access to a subordinate intruder (Couppis and Kennedy 2008). They found that D2R antagonists strongly reduced nose-poke behavior for access to an intruder mouse. While infusion of the D1R antagonist also decreased nose-poke behavior it also decreased locomotion at effective doses, making it difficult to interpret whether D1 receptors control the motivational aspects of aggression. Interestingly, the finding that D2Rs are playing a dominant role in controlling motivation for aggression may explain why antipsychotic medications, which largely antagonize D2Rs, are effective at decreasing aggression in human psychiatric patients. A related study showed that D2R, but not D1R, antagonist infusion into the NAc of abnormally aggressive low-anxiety bred rats decreases aggression (Beiderbeck et al. 2012). Interestingly, a more recent study found that both D1R and D2R antagonists injected systemically reduced both aggression and chances of winning a fight in experienced mice, but in this scenario the D1R antagonist had a stronger effect (Becker et al. 2015).
Notably, studies in flies corroborate the aforementioned findings in rodents describing a role for dopamine in promoting aggressive behavior, although the precise dynamics of dopamine in aggression differ slightly between rodents and flies. Using an intersectional genetics approach, Alekseyenko and colleagues altered the function of single dopaminergic neuron pairs in the fly by expressing toxins that disrupt neuronal transmission (2013). They found that both inhibition and stimulation of a dopamine neuron cluster projecting to target regions expressing a fly correlate of D1R increased aggression, whereas identical manipulation of a different dopamine neuron cluster projecting primarily to regions expressing a fly correlate of D2R increased aggression as well as sleep/wake cycle and motor output. Therefore, dopamine in the fly may play a non-linear role in controlling aggression, with either too much or too little dopamine release disrupting the expression of normal behavior (akin to a U-shaped curve). Overall, however, similar to rodents, dopamine in the fly appears to have the capacity to modulate multiple aspects of aggressive behavior.
While many questions regarding the behavioral role of mesolimbic dopamine in controlling aggression remain to be answered, a new wave of cutting-edge functional studies in rodents utilizing cell- and circuit-type specific tools have begun to more clearly elucidate the complex role of VTA circuitry in this complex social behavior. A recent study by Yu and colleagues investigated the role of monoamine oxidase A (MAOA), an enzyme responsible for the degradation of bioaminergic molecules like serotonin, dopamine, and norepinephrine, in driving aggressive behavior (2014). They found that inhibition of MAOA signaling during specific developmental periods differentially affected either serotonin or dopamine effects on aggression behavior. First, researchers found that while systemic blockade of MAOA during postnatal development (P2-21) resulted in increased depression and anxiety-like behavior in adulthood, blockade during peri-adolescence (P22-48) enhanced aggression in adulthood. To parse the relative contributions of serotonin and dopamine in these processes, researchers blocked serotonin transporter (5-HTT) during peri-adolescence, which effectively increases synaptic serotonin, and found that it reduced aggressive behavior in adulthood. Conversely, blockade of the dopamine transporter (DAT) but not the norepinephrine transporter during peri-adolescence significantly increased aggressive behavior in adulthood. Further, Yu and colleagues found that their serotonergic and dopaminergic transporter perturbations during peri-adolescence resulted in opposite effects of amphetamine on ambulatory behavior in adulthood. This finally suggested to them that dopamine was primary to the effects of peri-adolescence MAOA blockade on adult aggressive behavior. Lastly, they utilized an optogenetic approach to specifically stimulate VTA dopamine cell bodies in adult animals and found that this alone increased aggressive behavior. The results of this particular experiment represent the most convincing data to date supporting the notion that dopamine dynamics in reward circuits are causally related to aggressive behavior. Future studies should aim to determine if LHb gating of VTA dopamine neuron activity through the RMTg plays a similarly causal role in stimulating aggression and aggression reward.
There is evidence to suggest that DRN serotonin may have the ability to both promote and inhibit aggressive behavior. For example, DRN serotonin and glutamate are increased during escalated aggression, and DRN injection with l-glutamate increases both phasic DRN serotonin release as well as aggression (Takahashi et al. 2015). On the other hand, selective serotonin reuptake inhibitors (SSRIs), which elevate brain synaptic serotonin levels, decrease aggressive behavior in a manner that is dependent on serotonin signaling (Pinna et al. 2006). Interestingly, this negative regulation of aggressive behavior by serotonin signaling appears to be both sex and age specific, as agonism of serotonin receptor signaling promotes aggression in both females and adolescents (Terranova et al. 2016, Dennis et al. 2013). The developmental timing of serotonin receptor expression also appears to be important in mediating aggressive behavior. While developmental knockdown of serotonin 5HT1B receptors increased aggressive behavior, impulsivity, and promoted increased NAc dopamine signaling, knockdown only during the peri-adolescence period resulted in no change in adult aggression or impulsivity (Nautiyal et al. 2015). Rescue of 5HT1B receptor expression in adulthood reversed increases in impulsivity but not aggression. Furthermore, 5HT1B heteroreceptors on forebrain CAMKII-expressing neurons, rather than autoreceptors on midbrain serotonin neurons, were responsible for mediating this aggressive phenotype. Future studies must acutely manipulate the activity of specific serotonin neurons and their inputs and outputs during various developmental time periods will help to further elucidate the contribution of this neurotransmitter system on behaviors inextricably linked to aggression, namely reward and impulsivity. It is possible that only subsets of DRN serotonin neurons, namely those implicated in controlling reward, provide positive modulation of aggressive behavior, while a separate population of DRN serotonin neurons oppositely modulates aggression by altering impulsivity behaviors.
Very recently, a report by Niederkofler and colleagues described two small populations of molecularly defined DRN serotonin neuron subtypes that shape intermale aggressive behavior in mice (2016). Using Pet1 as a marker of serotonergic cell type they found a subtype of Pet1 neurons expressing the D2R and another expressing the D1R during peri-adolescence. They confirmed dopamine’s effects on these two neuronal subtypes to be consistent with previous reports, with dopamine inhibiting neurons expressing D2Rs and exciting neurons expressing D1Rs. Surprisingly, Optogenetic silencing of D1R Pet1-positive or D2R Pet1-positive neurons increased aggressive behaviors in adult male mice. However, only inhibition of D1R Pet1-positive neurons also increased overall social interaction time with a novel conspecific, suggesting that they play a more general role in social behavior and its rewarding components. Conversely, D2R Pet1-expressing neurons uniquely increased hyperactivity and impulsivity in novel environments. This behavioral difference between D1R and D2R-expressing Pet1-positive serotonin neurons in the DRN may reflect the differences in their downstream projection targets. While the D1R Pet1-positive neurons tended to project entirely to reward and aggression related brain regions like the VTA, D2R Pet1-positive neurons projected to these regions as well as regions important for the processing of sensory stimuli. The results of this investigation highlight the importance of utilizing cell-type and projection specific manipulations in awake behaving animals to elucidate the complex control that serotonin neurons exert over aggression and related behaviors. It would be interesting for future studies to determine whether these divergent populations of Pet1-positive neurons in the DRN receive overlapping or completely segregated inputs from upstream control regions like the LHb.
LHb dysfunction in psychiatric disorders
As mentioned above, there are a number of neuropsychiatric disorders where patients exhibit a high prevalence of aggressive behavior. Dysfunction of LHb circuitry has been implicated in many of these conditions, although the question of whether LHb dysfunction is causally tied to pathological aggression in these patients has yet to be addressed. In this section we will discuss evidence in both human and animal models linking LHb function to neuropsychiatric illness.
Patients with major depressive disorder (MDD) display LHb hyperactivity and increased LHb volume, which may be related to the persistent negative emotional state that characterizes the syndrome (Lecca et al. 2014). Furthermore, deep brain stimulation (DBS) of the LHb (which is hypothesized to reduce LHb activity) in a case study of a treatment-resistant patient with MDD markedly improved depressive symptoms (Sartorius et al. 2010). Notably, when LHb DBS was inadvertently stopped, the patient’s depressive symptoms returned. This study reveals a potential causal role for the LHb in driving MDD symptoms in humans and suggests that LHb DBS could be tolerated at least in some individuals with minimal side effects. However, more clinical evidence is needed to determine if LHb DBS is a viable, effective treatment for humans with MDD or whether it might also be an effective treatment for neuropsychiatric disorders marked by heightened aggression.
Studies in rodent models of depression similarly suggest that the LHb plays a causal role in the development of depressive behaviors. DBS stimulation of the LHb was found to suppress excitatory transmission in the LHb and prevent learned helplessness, a rodent model of depressive behavior (Li et al. 2011). In a subsequent study, Li and colleagues (2014) found that LHb CAMKII-β signaling in the LHb subsequently increased synaptic transmission onto LHb neurons and exacerbated depressive-like symptoms, while inhibition of LHb CAMKII-β signaling decreased synaptic transmission and promoted behavioral resilience. Highlighting a further layer of complexity, Shabel et al. found that inputs from the entopeduncular nucleus (EPN) to the LHb co-release GABA and glutamate and suggested that changes in the balance of release between them might be important in the expression of depressive behaviors and antidepressant responses (2014). Specifically, they found that susceptible mice in the learned helplessness model of depression exhibited a shift towards less GABA release at EPN-LHb synapses that promoted increased activation of LHb target neurons, whereas chronic treatment with standard antidepressants normalized GABA release. The apparent causal relationship between increased LHb activity and the expression of depressive symptoms in humans and animal models suggests that LHb pathophysiology may contribute to individual symptoms of MDD.
As the LHb is involved in controlling the rewarding and aversive properties of a variety of stimuli, it is perhaps unsurprising that the LHb has emerged as an important node of circuit dysfunction in drug addiction. In rodents and humans, withdrawal from drugs of abuse like cocaine have been shown to promote negative emotional states and enhance stress reactivity, often leading to relapse (Volkow et al. 2012). Repeated exposure to drugs of abuse results in multiple circuit-level adaptations within the LHb (Meye et al. 2016, Neumann et al. 2015, Meye et al. 2015, Luo et al. 2016). Interestingily, the onset of drug withdrawal behavior is accompanied by increases in LHb excitation. As in depressive states, withdrawal from cocaine has been shown to shift the balance of GABA/glutamate co-release from EPN inputs to the LHb toward glutamate, resulting in increased activity of LHb neurons projecting to GABAergicg RMTg neurons (which presumably inhibit VTA dopamine neurons) (Meye et al. 2016). Furthermore, selective overexpression of the vesicular GABA transporter (vGAT) in this EPN-LHb pathway during cocaine withdrawal rescues withdrawal-induced aversive states and suppresses stress-induced reinstatement. Neurons projecting from the LHb to the RMTg, but not those projecting to the VTA, also undergo plastic alterations in response to chronic drug exposure (Meye et al. 2015). Repeated injections of cocaine increased surface expression of GluA2-lacking AMPA receptors in LHb-RMTg neurons, which functionally increased their excitability and promoted depressive-like behaviors. Remarkably, blocking cocaine-evoked transmission at LHb-RMTg synapses produced antidepressant-like behavioral responses, further highlighting the causal role that LHb hyperexcitability plays in promoting negative emotionality in addiction. Overall, these studies suggest that the LHb undergoes surprisingly similar pre and post synaptic adaptations in response to repeated stress and drug exposure. These results highlight a significant role for the LHb in mediating affective-like disturbances in addiction and depression, however, more work is needed to define whether the LHb controls heightened aggressive symptoms often seen in these patient populations.
Conclusions and Future Directions
Aggression is an essential facet of social behavior in species from insects to mammals, yet the precise circuits controlling the many facets of aggressive behavior remain unknown. Many decades of research in humans and animal models support the notion that the domination of social targets is rewarding and heavily influenced by brain regions like the VTA and the DRN, both of which are altered by upstream LHb inputs and signal reciprocally back to the LHb. In this manner, the LHb functions as a hub for the processing of valence information about various stimuli, including but not limited to stress, drugs of abuse, and social targets. While relatively few, studies directly investigating the role of LHb circuitry on aggression and its rewarding components corroborate this idea, highlighting a negative role for LHb activity in aggression. However, researchers have not yet identified specific inputs and outputs of the LHb that contribute to these behaviors. Future studies should aim to manipulate projection-specific activity of LHb outputs to the RMTg, VTA, and DRN in aggression to determine the precise role of these circuits in controlling the valence of aggressive interactions. The elucidation of these circuit mechanisms will inform treatments for psychiatric disorders for which aggressive behavior is a symptom.
Figure 2.

Input-output map of LHb microcircuitry. Principal neurons of the LHb are vGlut2-positive and target the DRN, VTA, and RMTg. They receive a variety of inputs consisting of multiple neurotransmitters and neuropeptides. GAD65-positive neurons are also present in the LHb, but it is unknown whether these neurons synapse locally, on downstream target regions, or both. Abbreviations: BF, basal forebrain; DRN, dorsal raphe nucleus; EPN, entopeduncular nucleus; LH, lateral hypothalamus; mPFC. medial prefrontal cortex; RMTg, rostromedial tegmental nucleus; VTA, ventral tegmental area.
BOX 1. Preclinical models of appetitive aggression.
In order to appropriately study the complex neurobiological mechanisms of aggressive behavior in animals, it essential that behavioral models reflect certain components of human aggression phenotypes. The results of many preclinical aggression studies have unfortunately been limited by the use of single exposure to the resident intruder paradigm (RI), which does not clearly distinguish between aggression that is rewarding and aggression that is primarily a reaction to perceived external threats (reactive aggression) (Vitiello and Stoff 1997). Classically, RI consists of placing an intruder male conspecific in the home cage of the resident animal and observing subsequent aggressive behavior. This paradigm has been extensively used in species from fish to flies to rodents (Miczek et al. 2013). When RI is repeated over multiple days, the probability of winning a fight increases with each successive victory (Oyegbile and Marler 2005, Schwartzer et al. 2013). Termed the “winner effect,” this phenomenon is consistent with the idea that the domination of social targets is a positive experience that motivates individuals to continue expressing aggressive behavior. Therefore, while single RI exposure may be a more general model of aggression, repeated RI may more selectively model appetitive aggression than reactive aggression, although it does not necessarily fully separate the two. More recently, the adaptation of classic drug reward paradigms for the assessment of aggression reward has provided a variety of valuable tools to more precisely study motivational processes in appetitive aggression. Findings from studies utilizing these models appear to more directly corroborate the notion that fighting is rewarding for some individuals, particularly for those that repeatedly win contests. For example, dominant male mice will display conditioned-place preference (CPP) for a distinct context previously paired with a novel submissive opponent (Golden et al. 2016a, Golden et al. 2016b), a phenotype that stably persists for at least eighteen days (Golden et al. 2016a). In addition, socially dominant male mice that repeatedly win aggressive contests will learn to perform an operant task (lever press or nose-poke) that will give them access to attack a subordinate intruder (Fish et al. 2002, May and Kennedy 2009, Falkner et al. 2016), suggesting that aggression in animals can be positively reinforcing in a manner that is analogous to human instrumental aggression-seeking behavior (Elbert et al. 2010, Moran et al 2014). Moreover, up to 20% of mice trained in this type of operant paradigm will exhibit “addiction-like” behavior, expending high levels of effort to gain intruder access, demonstrating robust relapse to aggression-seeking, and displaying resistance to punishment-induced suppression of aggression-seeking behavior (Golden et al. 2017). Taken together, these findings strongly suggest that neuronal circuits controlling valence and reward are playing important roles in appetitive aggression.
Highlights.
-
-
The LHb controls valence through modulation of dopamine and serotonin neurons.
-
-
Aggressive behavior is positively reinforcing in subsets of humans and animals.
-
-
Recent studies suggest that the LHb controls the valence of aggression.
-
-
Future studies should identify precise LHb circuits that control aggression valence.
Acknowledgments
This was supported by National Institute of Mental Health (NIMH) grants R01 MH104559 (S.J.R.), MH090264 (S.J.R.), and T32 MH096678 (M.F.), and a National Center for Complementary and Integrative Health grant P50 AT008661 (S.J.R.). All authors contributed to the planning, writing, and editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, et al. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Aizawa H, et al. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520(18):4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, et al. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci U S A. 2013;110(15):6151–6156. doi: 10.1073/pnas.1303446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo R, et al. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010;30(4):1566–1574. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo R, et al. The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger. Neuron. 2014;84(1):1034–1048. doi: 10.1016/j.neuron.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Optogenetics, sex, and violence in the brain: implications for psychiatry. Biol Psychiatry. 2012;71(12):1081–1089. doi: 10.1016/j.biopsych.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NE, Kiehl KA. Psychopathy and aggression: when paralimbic dysfunction leads to violence. Curr Top Behav Neurosci. 2014;17:369–393. doi: 10.1007/7854_2013_257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres KH, et al. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407(1):130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Beck A, et al. Translational clinical neuroscience perspectives on the cognitive and neurobiological mechanisms underlying alcohol-related aggression. Curr Top Behav Neurosci. 2014;17:443–474. doi: 10.1007/7854_2013_258. [DOI] [PubMed] [Google Scholar]
- Becker EA, Marler CA. Postcontest blockade of dopamine receptors inhibits development of the winner effect in the California mouse (Peromyscus californicus) Behavioral Neuroscience. 2015;129(2):205–213. doi: 10.1037/bne0000043. [DOI] [PubMed] [Google Scholar]
- Beiderbeck DI, et al. High and abnormal forms of aggression in rats with extremes in trait anxiety–involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology. 2012;37(12):1969–1980. doi: 10.1016/j.psyneuen.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364(1519):1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Gandia MC, et al. Effect of drugs of abuse on social behaviour: a review of animal models. Behav Pharmacol. 2015;26(6):541–570. doi: 10.1097/FBP.0000000000000162. [DOI] [PubMed] [Google Scholar]
- Bondar NP, et al. Molecular implications of repeated aggression: Th, Dat1, Snca and Bdnf gene expression in the VTA of victorious male mice. Plos One. 2009;4(1):e4190. doi: 10.1371/journal.pone.0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinschwitz K, et al. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168(2):463–476. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Chang SY, Kim U. Ionic mechanism of long-lasting discharges of action potentials triggered by membrane hyperpolarization in the medial lateral habenula. J Neurosci. 2004;24(9):2172–2181. doi: 10.1523/JNEUROSCI.4891-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, et al. Looking for reward in all the wrong places: dopamine receptor gene polymorphisms indirectly affect aggression through sensation-seeking. Soc Neurosci. 2016;11(5):487–494. doi: 10.1080/17470919.2015.1119191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, et al. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science. 2016;352(6281):87–90. doi: 10.1126/science.aac9508. [DOI] [PubMed] [Google Scholar]
- Christoph GR, et al. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6(3):613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, et al. Substance use disorders: Relationship with intermittent explosive disorder and with aggression, anger, and impulsivity. J Psychiatr Res. 2016;81:127–132. doi: 10.1016/j.jpsychires.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, et al. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat, An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology (Berl) 2008;197(3):449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- DeAlmeida RM, et al. Escalated aggressive behavior: dopamine, serotonin, and GABA. Eur J Pharmacol. 2005;526(1–3):51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Dennis RL, et al. Alterations to embryonic serotonin change aggression and fearfulness. Aggress Behav. 2013;39(2):91–98. doi: 10.1002/ab.21459. [DOI] [PubMed] [Google Scholar]
- Dolenc B, et al. Relationship between affective temperaments and aggression in euthymic patients with bipolar mood disorder and major depressive disorder. J Affect Disord. 2015;174:13–18. doi: 10.1016/j.jad.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Elbert T, et al. Fascination violence: on mind and brain of man hunters. Eur Arch Psychiatry Clin Neurosci. 2010;260(Suppl 2):S100–105. doi: 10.1007/s00406-010-0144-8. [DOI] [PubMed] [Google Scholar]
- Falkner AL, et al. Hypothalamic control of male aggression-seeking behavior. Nat Neurosci. 2016;19(4):596–604. doi: 10.1038/nn.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, et al. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17(2):371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Fish EW, et al. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology (Berl) 2002;163(3–4):459–466. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SE, et al. Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat. 2016;12:1525–1538. doi: 10.2147/NDT.S84585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan ME, et al. Lateral habenula orexin receptor 2 modulates aggression reward. Poster presented at: 46th annual Society for Neuroscience meeting; 2016 Nov 12–16; San Diego, CA. 2016. [Google Scholar]
- Fonseca MS, et al. Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Curr Biol. 2015;25(3):306–315. doi: 10.1016/j.cub.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Geisler S, et al. Morphologic and cytochemical criteria for the identification and delineation of individual subnuclei within the lateral habenular complex of the rat. J Comp Neurol. 2003;458(1):78–97. doi: 10.1002/cne.10566. [DOI] [PubMed] [Google Scholar]
- Golden SA, et al. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 2016a doi: 10.1111/gbb.12310. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016b;534(7609):688–692. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, et al. Compulsive addiction-like aggressive behavior in mice. Biological Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.03.004. Epub ahead of print. Doi: http://dx.doi.org/10.1016/j.biopsych.2017.03.004. [DOI] [PMC free article] [PubMed]
- Gruber C, et al. Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rat. Neurosci Lett. 2007;427(3):165–170. doi: 10.1016/j.neulet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RJ, et al. Chronic low-dose cocaine treatment during adolescence facilitates aggression in hamsters. Physiol Behav. 2000;69(4–5):555–562. doi: 10.1016/s0031-9384(00)00220-1. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173(1):123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11(7):503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ. Impulsivity and aggression in schizophrenia: a neural circuitry perspective with implications for treatment. CNS Spectr. 2015;20(3):280–286. doi: 10.1017/S1092852915000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, et al. Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl) 2015;232(16):2889–2902. doi: 10.1007/s00213-015-3925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, et al. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61(5):786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33(17):7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Aston-Jones G. Persistent Adaptations in Afferents to Ventral Tegmental Dopamine Neurons after Opiate Withdrawal. J Neurosci. 2015;35(28):10290–10303. doi: 10.1523/JNEUROSCI.0715-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A, et al. Internal States and Behavioral Decision-Making: Toward an Integration of Emotion and Cognition. Cold Spring Harb Symp Quant Biol. 2014;79:199–210. doi: 10.1101/sqb.2014.79.024984. [DOI] [PubMed] [Google Scholar]
- Kim U, Lee T. Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur J Neurosci. 2012;35(8):1253–1269. doi: 10.1111/j.1460-9568.2012.08030.x. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, et al. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J Comp Neurol. 2004;469(2):170–184. doi: 10.1002/cne.11005. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, et al. Genetic dissection of medial-habenula interpeduncular nucleus pathway function in mice. Front Behav Neurosci. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7(4):951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Kowski AB, et al. Dopaminergic activation excites rat lateral habenular neurons in vivo. Neuroscience. 2009;161(4):1154–1165. doi: 10.1016/j.neuroscience.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, et al. Disrupted habenula function in major depression. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, et al. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39(7):1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- Lecca S, et al. Footshock-induced plasticity of GABAB signalling in the lateral habenula requires dopamine and glucocorticoid receptors. Synapse. 2016 doi: 10.1002/syn.21948. [DOI] [PubMed] [Google Scholar]
- Li B, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470(7335):535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, et al. betaCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341(6149):1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun. 2016;7:10503. doi: 10.1038/ncomms10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470(7333):221–6. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisoprawski A, et al. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183(1):229–234. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Liu Z, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81(6):1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330(6002):385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XF, et al. Lateral habenula as a link between dopaminergic and serotonergic systems contributes to depressive symptoms in Parkinson’s disease. Brain Res Bull. 2015;110:40–46. doi: 10.1016/j.brainresbull.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J Neurosci. 2012;32(36):12641–12646. doi: 10.1523/JNEUROSCI.2405-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- May ME, Kennedy CH. Aggression as positive reinforcement in mice under various ratio- and time-based reinforcement schedules. J Exp Anal Behav. 2009;91(2):185–196. doi: 10.1901/jeab.2009.91-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 2014;8(6):1857–1869. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, et al. Shifted pallidal co-release of GABA and glutamate inhabenula drives cocaine withdrawal and relapse. Nat Neurosci. 2016;19(8):1019–1024. doi: 10.1038/nn.4334. [DOI] [PubMed] [Google Scholar]
- Meye FJ, et al. Cocaine-evoked negative symptoms require AMPA receptor trafficking in the lateral habenula. Nat Neurosci. 2015;18(3):376–378. doi: 10.1038/nn.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, et al. Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res. 1998;22(8):1698–1705. [PubMed] [Google Scholar]
- Miczek KA, et al. Excessive aggression as model of violence: a critical evaluation of current preclinical methods. Psychopharmacology (Berl) 2013;226(3):445–458. doi: 10.1007/s00213-013-3008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles SR, et al. The Relationship Between Emotion Dysregulation and Impulsive Aggression in Veterans With Posttraumatic Stress Disorder Symptoms. Journal of Interpersonal Violence. 2016;31(10):1795–1816. doi: 10.1177/0886260515570746. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, et al. Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J Neurosci. 2011a;31(2):469–479. doi: 10.1523/JNEUROSCI.3714-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, et al. Activation of the central serotonergic system in response to delayed but not omitted rewards. Eur J Neurosci. 2011b;33(1):153–160. doi: 10.1111/j.1460-9568.2010.07480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, et al. Optogenetic activation of dorsal raphe serotoninneurons enhances patience for future rewards. Curr Biol. 2014;24(17):2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Moran JK, et al. Differences in brain circuitry for appetitive and reactive aggression as revealed by realistic auditory scripts. Front Behav Neurosci. 2014;8:425. doi: 10.3389/fnbeh.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. The role of the dorsal raphe nucleus in reward-seeking behavior. Front Integr Neurosci. 2013;7:60. doi: 10.3389/fnint.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan FM, et al. Neuronal connectivity between habenular glutamate-kisspeptin1 co-expressing neurons and the raphe 5-HT system. J Neurochem. 2015;135(4):814–829. doi: 10.1111/jnc.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, et al. Distinct Circuits Underlie the Effects of 5-HT1B Receptors on Aggression and Impulsivity. Neuron. 2015;86(3):813–826. doi: 10.1016/j.neuron.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PA, et al. Increased excitability of lateral habenula neurons in adolescent rats following cocaine self-administration. Int J Neuropsychopharmacol. 2014;18(6) doi: 10.1093/ijnp/pyu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkofler V, et al. Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior. Cell Rep. 2016;17(8):1934–1949. doi: 10.1016/j.celrep.2016.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile TO, Marler CA. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm Behav. 2005;48(3):259–267. doi: 10.1016/j.yhbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Petrov T, et al. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol. 1992;318(1):18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Petrov T, et al. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277(2):289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- Peyron C, et al. Lower brainstem catecholamine afferents to the rat dorsalraphe nucleus. J Comp Neurol. 1996;364(3):402–413. doi: 10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Pinna G, et al. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006;186(3):362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pollak Dorocic I, et al. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83(3):663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Poller WC, et al. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Res. 2013;1507:45–60. doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Proulx CD, et al. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17(9):1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, et al. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat Commun. 2014;5:5390. doi: 10.1038/ncomms6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161:827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- Quanty MB. Aggression: Its causes, consequences, and control, by L. Berkowitz. New York, McGraw-Hill, 1993, 485 pp. Aggressive Behavior. 1994;20(6):464–466. [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67(2):e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, et al. Prior fighting experience increases aggression in Syrian hamsters: implications for a role of dopamine in the winner effect. Aggress Behav. 2013;39(4):290–300. doi: 10.1002/ab.21476. [DOI] [PubMed] [Google Scholar]
- Seo D, et al. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and its Comorbidity with other Clinical Disorders. Aggress Violent Behav. 2008;13(5):383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, et al. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345(6203):1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, et al. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74(3):475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, et al. Stimulation of midbrain dopaminergic structures modifies firing rates of rat lateral habenula neurons. Plos One. 2012;7(4):e34323. doi: 10.1371/journal.pone.0034323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, et al. Opposite metabolic changes in habenula and ventral tegmental area of genetic model of helplessness behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, et al. Distribution of GABA-immunoreactive neurons in the thalamus of the squirrel monkey (Saimiri sciureus) Neuroscience. 1987;22(2):579–591. doi: 10.1016/0306-4522(87)90355-1. [DOI] [PubMed] [Google Scholar]
- Sokolov BP, Cadet JL. Methamphetamine causes alterations in the MAP kinase-related pathways in the brains of mice that display increased aggressiveness. Neuropsychopharmacology. 2006;31(5):956–966. doi: 10.1038/sj.npp.1300891. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80(4):1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15(8):1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, et al. Glutamate input in the dorsal raphe nucleus as a determinant of escalated aggression in male mice. J Neurosci. 2015;35(16):6452–6463. doi: 10.1523/JNEUROSCI.2450-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, et al. Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc Natl Acad Sci U S A. 2016;113(46):13233–13238. doi: 10.1073/pnas.1610446113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Heightened aggressive behavior during morphine withdrawal: effects of d-amphetamine. Psychopharmacology (Berl) 1992;107(2–3):297–302. doi: 10.1007/BF02245151. [DOI] [PubMed] [Google Scholar]
- Valentinova K, Mameli M. mGluR-LTD at Excitatory and Inhibitory Synapses in the Lateral Habenula Tunes Neuronal Output. Cell Rep. 2016;16(9):2298–2307. doi: 10.1016/j.celrep.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, et al. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci. 2003;17(2):280–286. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36(3):307–315. doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F, et al. Transcriptomic-anatomic analysis of the mouse habenula uncovers a high molecular heterogeneity among neurons in the lateral complex, while gene expression in the medial complex largely obeys subnuclear boundaries. Brain Struct Funct. 2016;221(1):39–58. doi: 10.1007/s00429-014-0891-9. [DOI] [PubMed] [Google Scholar]
- Wang DG, et al. Absence of GABA type A signaling in adult medial habenular neurons. Neuroscience. 2006;141(1):133–141. doi: 10.1016/j.neuroscience.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197(4298):89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93. doi: 10.1016/j.neuroscience.2010.10.047. [DOI] [PubMed] [Google Scholar]
- Xie G, et al. Serotonin modulates glutamatergic transmission to neurons in the lateral habenula. Sci Rep. 2016;6:23798. doi: 10.1038/srep23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, et al. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016;1641(Pt B):217–233. doi: 10.1016/j.brainres.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, et al. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry. 2014;19(6):688–698. doi: 10.1038/mp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. Thirst Is Associated with Suppression of Habenula Output and Active Stress Coping: Is there a Role for a Non-canonical Vasopressin-Glutamate Pathway? Front Neural Circuits. 2016;10:13. doi: 10.3389/fncir.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, et al. Serotonin stimulates lateral habenula via activation of the post-synaptic serotonin 2/3 receptors and transient receptor potential channels. Neuropharmacology. 2016;101:449–459. doi: 10.1016/j.neuropharm.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


