ABSTRACT
Confirmed reports of Zika virus (ZIKV) in human seminal fluid for months after the clearance of viremia suggest the ability of ZIKV to establish persistent infection in the seminiferous tubules, an immune-privileged site in the testis protected by the blood-testis barrier, also called the Sertoli cell (SC) barrier (SCB). However, cellular targets of ZIKV in human testis and mechanisms by which the virus enters seminiferous tubules remain unclear. We demonstrate that primary human SCs were highly susceptible to ZIKV compared to the closely related dengue virus and induced the expression of alpha interferon (IFN-α), key cytokines, and cell adhesion molecules (vascular cell adhesion molecule 1 [VCAM-1] and intracellular adhesion molecule 1 [ICAM-1]). Furthermore, using an in vitro SCB model, we show that ZIKV was released on the adluminal side of the SCB model with a higher efficiency than in the blood-brain barrier model. ZIKV-infected SCs exhibited enhanced adhesion of leukocytes that correlated with decreases in SCB integrity. ZIKV infection did not affect the expression of tight and adherens junction proteins such as ZO-1, claudin, and JAM-A; however, exposure of SCs to inflammatory mediators derived from ZIKV-infected macrophages led to the degradation of the ZO-1 protein, which correlated with increased SCB permeability. Taken together, our data suggest that infection of SCs may be one of the crucial steps by which ZIKV gains access to the site of spermatozoon development and identify SCs as a therapeutic target to clear testicular infections. The SCB model opens up opportunities to assess interactions of SCs with other testicular cells and to test the ability of anti-ZIKV drugs to cross the barrier.
IMPORTANCE Recent outbreaks of ZIKV, a neglected mosquito-borne flavivirus, have identified sexual transmission as a new route of disease spread, which has not been reported for other flaviviruses. To be able to sexually transmit for months after the clearance of viremia, ZIKV must establish infection in the seminiferous tubules, the site of spermatozoon development. However, little is known about the cell types that support ZIKV infection in the human testis. Currently, there are no models to study mechanisms of virus persistence in the seminiferous tubules. We provide evidence that ZIKV infection of human Sertoli cells, which are an important component of the seminiferous tubules, is robust and induces a strong antiviral response. The use of an in vitro Sertoli cell barrier to describe how ZIKV or inflammatory mediators derived from ZIKV-infected macrophages compromise barrier integrity will enable studies to explore the interactions of other testicular cells with Sertoli cells and to test novel antivirals for clearing testicular ZIKV infection.
KEYWORDS: Zika virus, blood-testis barrier, cell adhesion molecules, human Sertoli cells, in vitro Sertoli cell barrier, innate immune response, macrophages, sexual transmission, tight junction proteins
INTRODUCTION
Zika virus (ZIKV), a largely neglected arbovirus, belongs to the flavivirus genus of the Flaviviridae family, which includes other globally relevant arthropod-transmitted human pathogens such as dengue virus (DENV), West Nile virus (WNV), and Japanese encephalitis virus (JEV). The recent reemergence of ZIKV in the South Pacific and Latin America in 2015 to 2016 has been associated with more severe complications, including Guillain-Barre syndrome and severe fetal abnormalities (1). So far, 38,527 locally acquired cases have been reported in the United States, including American Samoa, the U.S. Virgin Islands, and Puerto Rico (2). However, what caught the world's attention during the recent ZIKV outbreak was the two unexpected disease transmission routes: in utero transmission, associated with a dramatic surge in microcephaly cases, and sexual transmission from infected males to their partners. In the United States alone, 45 cases of ZIKV disease transmission via the sexual route have been confirmed so far (2), and at least 12 other countries have also reported male-to-male and male-to-female transmission, leading to an urgent advisory to pregnant woman to consider all possible options to protect their pregnancy (3). Based on reports of the duration of the presence of ZIKV in the seminal fluid, it appears that the virus can be spread by males before disease symptoms start, when disease symptoms are present, and after symptoms end (4, 5). Furthermore, it is unclear if infected individuals who remain asymptomatic can also sexually transmit ZIKV and if there is any association of the level of viremia with testicular invasion by ZIKV. Although the contribution of the sexual route to disease transmission may be difficult to predict in areas where ZIKV is endemic, it certainly complicates virus epidemiology in regions of nonendemicity where the mosquito vector is absent. A recent cohort study reported that 56% of males positive for ZIKV in serum were also positive for the virus in semen, and the median time until the loss of ZIKV RNA in semen was 34 days, compared to 14 days in serum, thus suggesting a much longer infectious phase of ZIKV than of other flaviviruses traditionally transmitted via mosquitoes (6). Considering the lack of any measures approved to clear ZIKV infection and the detection of RNA of other reemerging pathogens such as Ebola virus in semen (4, 7), it has become critical to understand the mechanisms associated with testicular infection by ZIKV.
The mammalian testis is divided into two compartments, the peritubular compartment, which consists of Leydig cells and testicular macrophages, and the seminiferous tubule compartment with germ cells protected by Sertoli cells (SCs). These SCs form the blood-testis barrier, also known as the Sertoli cell barrier (SCB), which functions mainly to protect developing germ cells from systemic attack by adaptive immune cells while simultaneously providing nutritional and structural support (8, 9). As one of the tightest blood-tissue barriers, the SCB is formed by tight junction protein (TJP) complexes such as those formed by ZO-1, occludin, and claudins as well as by adherens junction proteins between connecting Sertoli cells (8). Similar to other blood-tissue barriers such as the blood-brain barrier (BBB), in addition to providing a physiologic barrier, the SCB also participates in the immune response to invading pathogens. Several viruses, including mumps virus, have been shown to infect human testes and induce inflammatory mediators associated with the disruption of blood-tissue barriers, including tumor necrosis factor alpha (TNF-α), type I interferon (IFN), matrix metalloproteinases (MMPs), and cell adhesion molecules (CAMs) (10).
The data on testicular infection by ZIKV in humans are limited; however, mouse and nonhuman primate models of ZIKV infection provide strong evidence of persistent ZIKV replication in the testes. Immunocompetent mice do not develop disease symptoms, but persistent testicular infection for up to 45 days in IFNAR1-deficient mice has been associated with proinflammatory responses, infiltration of leukocytes into the testes, and damaged architecture of the seminiferous epithelium (11, 12). Similarly, nonhuman primate studies have also reported the presence of ZIKV RNA in the testes 7 to 8 days after infection (13). In humans, some, but not all, ZIKV-infected men present with hematospermia; however, the presence of the virus in semen even after the clearance of viremia suggests that sexual transmission is not the result of hematospermia-induced blood-borne transmission and that ZIKV has the ability to establish persistent testicular infection. However, little is known about the cell types that support ZIKV infection in testes, and currently, there are no models that can be used to study mechanisms by which the virus establishes persistence in the seminiferous tubules. Here, we show that primary human SCs are highly susceptible to ZIKV infection compared to dengue virus and are capable of inducing robust antiviral immune and inflammatory responses. We further developed an in vitro SCB model to systematically investigate whether ZIKV can be released on the adluminal side of the SCB and to test how ZIKV infection of SCs and macrophages affects barrier integrity. We demonstrate that ZIKV can cross the in vitro SCB more efficiently than in BBB model without altering barrier permeability and the expression of junction proteins. However, inflammatory mediators secreted from ZIKV-infected macrophages compromised barrier integrity. Our data suggest that infection of SCs may be one of the crucial steps by which ZIKV gains access to immune-privileged seminiferous tubules and that infected SCs may serve as a reservoir for infection of other resident testicular cells, including developing spermatozoa.
RESULTS
ZIKV can infect and replicate in human Sertoli cells.
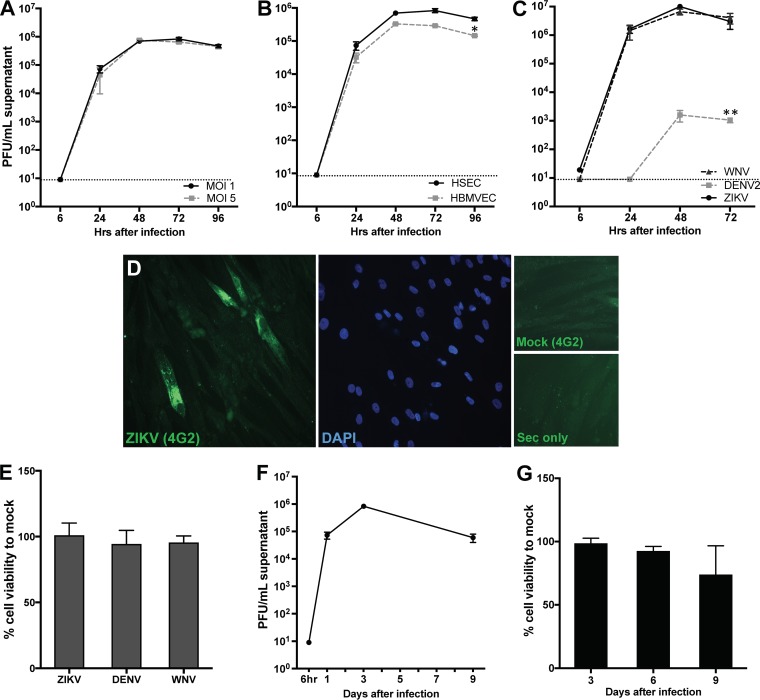
Since SCs are the primary component of the SCB that provides protection from the entry of pathogens into the seminiferous tubules, we first determined if primary human Sertoli cells (HSeCs) were susceptible to ZIKV. Low-passage-number HSeCs were infected with ZIKV strain PRVABC59 at a multiplicity of infection (MOI) of 1 or 5, and virus replication kinetics were analyzed by using multiple virologic assays. As shown in Fig. 1, while no ZIKV titers were detected 6 h after infection, titers reached about 4.5 log PFU/ml of supernatant 24 h after infection and further peaked 48 and 72 h after infection. Intracellular ZIKV replication also followed a similar trend, with peak RNA levels being seen 48 h after infection (data not shown). Since ZIKV is also a neurotropic virus (14), we next compared the infectivity of ZIKV in HSeCs to that in human brain microvascular endothelial cells (HBMVECs), a major component of the BBB. ZIKV titers in the supernatants from primary HBMVECs demonstrated replication kinetics similar to those observed in HSeCs. However, peak viral titers were almost half a log lower than those in HSeCs 72 and 96 h after infection, and this difference was statistically significant (P < 0.05) (Fig. 1B). Further confirmation of the susceptibility of HSeCs to ZIKV by immunostaining using flavivirus-specific antibody 4G2 demonstrated a robust signal of ZIKV-bound antibody in HSeCs 48 h after infection (Fig. 1D). We also compared relative infections by ZIKV, WNV, and dengue virus 2 (DENV2) in HSeCs. As shown in Fig. 1C, dengue virus replication was not detected at 24 h, and titers were almost 3 logs lower than ZIKV titers 48 and 72 h after infection (P < 0.01). Interestingly, SCs supported robust infection by WNV, virus titers were comparable to those of ZIKV at all time points (Fig. 1C), and none of these viruses induced any cytopathic effect or cell death (Fig. 1E). We also observed that HSeCs could sustain high virus titers up to day 9 after infection (Fig. 1F), after which the cell viability of both mock-infected and infected cells started to decline (Fig. 1G), suggesting that these cells can support ZIKV infection for long periods without causing significant cell death. These data collectively indicate that while ZIKV can infect both HSeCs and HBMVECs, the level of virus replication is lower in HBMVECs and that HSeCs are not a good target of dengue virus.
FIG 1.
ZIKV infection in human Sertoli cells is productive without significant cell death. (A) HSeCs were infected with ZIKV, and virus titers were quantified by a plaque assay using Vero cells and expressed as PFU per milliliter of supernatant. (B) HSeCs and HBMVECs were infected with ZIKV at an MOI of 1, and titers were measured by a plaque assay. (C) HSeCs were infected with ZIKV, WNV, and DENV2 (MOI of 1), and viral titers at different time points were measured by using a plaque assay. (D) Representative image of ZIKV immunostaining in infected HSeCs 48 h after infection at an MOI of 1. Cells were immunostained for the envelope protein (green) by using the 4G2 anti-flavivirus group antibody or secondary antibody alone (Sec only), and nuclei were stained by using DAPI (blue). (E) Cell toxicity of HSeCs at day 3 after infection with ZIKV, WNV, and DENV2 was assessed by using the CellTiter 96 AQueous One Solution cell proliferation assay kit, and percent cell viability was calculated by comparison to mock-infected cells at the corresponding time point. Error bars represent SEM of data from at least 3 to 5 independent infections. (F) HSeCs support long-term infection by ZIKV. A plaque assay shows that ZIKV titers were still high at day 9 after infection and comparable to day 1 titers. (G) Cell viability assay of mock- and ZIKV-infected HSeCs does not show a significant effect on cell death at days 6 and 9 after ZIKV infection (MOI of 1). *, P < 0.05; **, P < 0.01.
ZIKV infection induces type I IFN signaling and inflammatory cytokines in HSeCs.
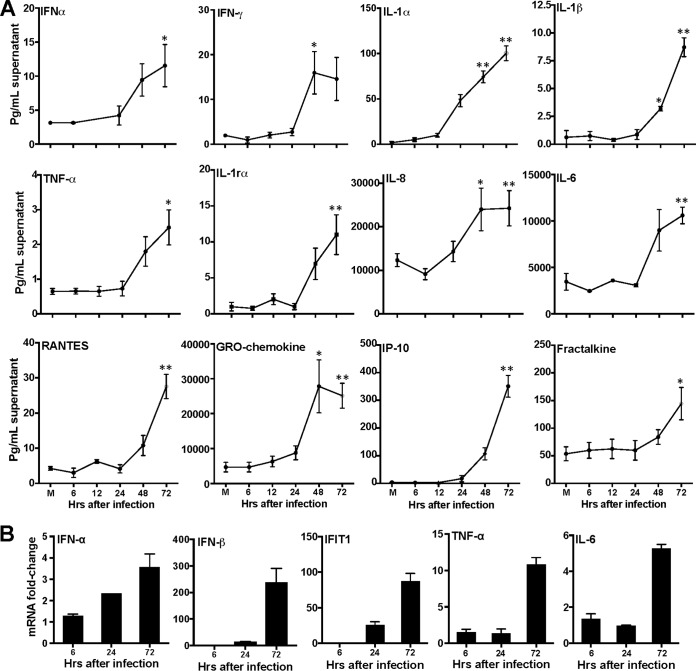
The robust production of type I IFN and inflammatory cytokines is required to restrict virus dissemination into immune-privileged tissues such as the testes. Therefore, to test whether HSeCs were capable of initiating antiviral responses to ZIKV, we next measured the levels of IFN-α and key antiviral cytokines. As shown in Fig. 2A, ZIKV infection did not induce IFN-α secretion during early time points; however, modest induction was observed in the supernatant 48 and 72 h after infection. Furthermore, levels of multiple inflammatory cytokines, such as IFN-γ, interleukin-1α (IL-1α), IL-1β, IL-6, IL-8, and TNF-α, were significantly elevated in the supernatant 48 and/or 72 h after infection, which correlated well with peak virus titers. The levels of secretion of chemokines involved in recruiting circulating leukocytes to inflammatory sites, such as RANTES (CCL5), fractalkine (CX3CL1), and IP-10 (CXCL10), were also significantly increased 72 h after infection. Interestingly, the level of the GRO chemokine (growth-related oncogene CXCL1), which is structurally related to IL-8 and has been shown to attract neutrophils and T lymphocytes to the testes during orchitis of various origins (15), was also found to be significantly elevated in the supernatant 48 and 72 h after infection (Fig. 2A). The increases in the levels of IFN-α/β, the key IFN-stimulated gene IFIT1, and selected cytokines (TNF-α and IL-6) 72 h after infection were further validated at the mRNA level by quantitative reverse transcription-PCR (qRT-PCR) (Fig. 2B). Collectively, these results demonstrate that HSeCs are capable of generating a strong innate immune response to ZIKV infection.
FIG 2.
ZIKV infection induces secretion of antiviral and proinflammatory mediators in HSeCs. (A) HSeCs were infected with ZIKV at an MOI of 1, and at different time points, IFN-α levels in the supernatant were measured by a Luminex assay. The secretion of proinflammatory cytokines (IFN-γ, IL-1β, IL-1rα, IL-1α, TNF-α, IL-8, and IL-6) and chemokines (RANTES, GRO chemokine, and IP-10) in the supernatant at different time points after infection was analyzed by using a multiplex bead-based assay. (B) Total RNA extracted from lysates was used to measure relative mRNA fold changes in IFN-α, IFN-β, IFIT1, TNF-α, and IL-6 levels compared to controls by normalizing values to the GAPDH value. Error bars represent SEM of results from at least 3 to 6 independent infections for each time point. *, P < 0.05; **, P < 0.01 (compared to mock infection).
Effect of ZIKV infection on cell adhesion molecules, MMPs, and tight junction proteins.
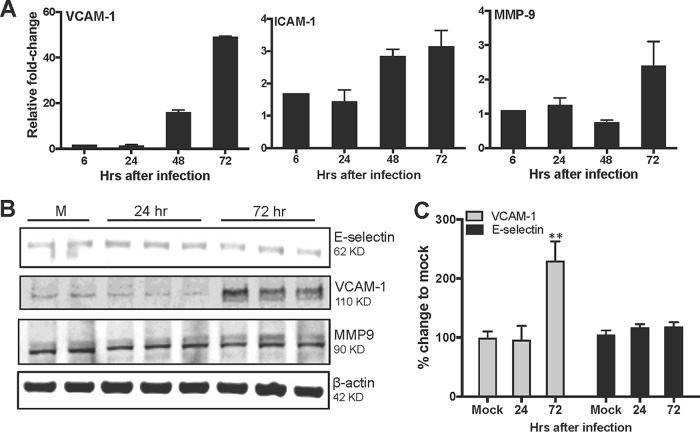
Under healthy conditions, SCs have been shown to express low levels of CAMs that participate in maintaining the adhesion of SCs to germ cells; however, inflammatory triggers or infection can further induce the expression of CAMs and MMPs associated with the degradation of TJPs (16, 17). Therefore, we next determined if ZIKV infection could alter the expression of CAMs and MMPs in HSeCs. As shown in Fig. 3A, we observed increases in the mRNA transcript levels of vascular cell adhesion molecule 1 (VCAM-1) and intracellular adhesion molecule 1 (ICAM-1) but not of E-selectin (data not shown) 72 h after infection. Further analysis of CAMs by using Western blotting demonstrated significant increases in the protein levels of VCAM-1, while the expression of E-selectin did not change significantly, thus supporting the qRT-PCR data (Fig. 3B and C). Among key MMPs, ZIKV infection did not affect the mRNA expression levels of MMP-1 and MMP-3 (data not shown). The transcription of MMP-9, however, was modestly upregulated (2- to 3-fold) 72 h after infection, but this increase could not be observed at the protein level (Fig. 3A and B).
FIG 3.
Effect of ZIKV infection on cytokines, cell adhesion molecules, and MMPs. (A) Total RNA extracted from ZIKV-infected HSeC lysates (MOI of 1) was used to measure relative mRNA fold changes in VCAM-1, ICAM-1, and MMP-9 levels compared to controls by normalizing values to the GAPDH value. (B) Whole-cell extracts were subjected to Western blotting and stained for VCAM-1, E-selectin, MMP-9, and β-actin. The Western blot is representative of results from at least three independent infections. (C) Percent changes in protein levels compared to controls, measured by densitometric analysis and normalized to the value for β-actin. *, P < 0.05 compared to mock infection.
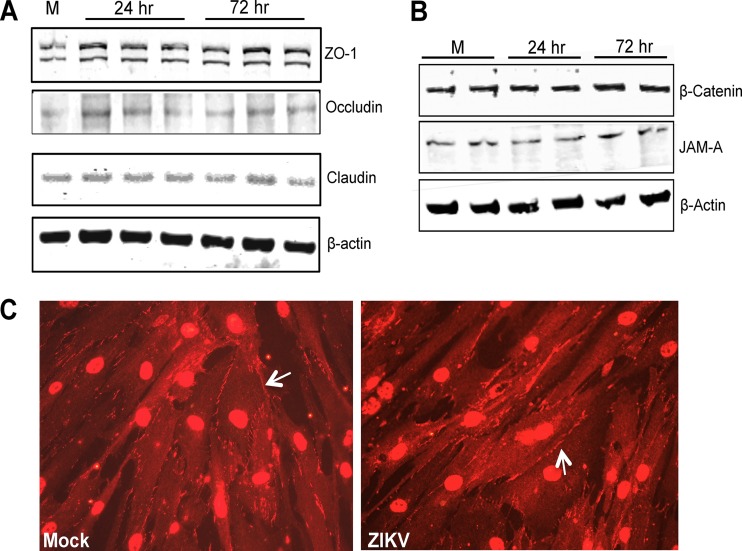
Altered gene expression or the degradation of tight and adherens junction proteins is one of the critical events associated with the leakiness of the SCB and other tissue barriers. Therefore, we next investigated if ZIKV modulates the expression of key TJPs, such as ZO-1, occludin, and claudin-1, in HSeCs. The mRNA transcript levels of ZO-1, claudin-1, and occludin were comparable to those of controls at all time points after infection (data not shown). At the protein level, we also observed no change in ZO-1, occludin, and claudin-1 expression levels in HSeCs at any time points after infection (Fig. 4A). Furthermore, immunocytochemical analysis also exhibited comparable signals of ZO-1 immunostaining in mock- and ZIKV-infected HSeC monolayers (Fig. 4C). Similarly, the expression levels of key adherens junction proteins, β-catenin and JAM-A, also did not change at any time point following ZIKV infection (Fig. 4B), thus suggesting that direct ZIKV infection of HSeCs does not result in the degradation of junction proteins.
FIG 4.
ZIKV infection does not alter the expression of tight junction and adherens junction proteins in HSeCs. (A and B) Total protein extracted from ZIKV-infected HSeCs (MOI of 1) was used to measure the levels of TJPs (ZO-1, occludin, and claudin) (A) and adherens junction proteins (β-catenin and JAM-A) and β-actin (B) by Western blotting using specific antibodies. The Western blot is representative of results from at least three independent infections. M, molecular marker. (C) Immunostaining of ZO-1 in mock- and ZIKV-infected HSeCs 48 h after infection shows comparable ZO-1 signals at the cell borders and cell-to-cell contact sites.
Cell-free ZIKV is efficiently released on the adluminal side of the in vitro SCB model.
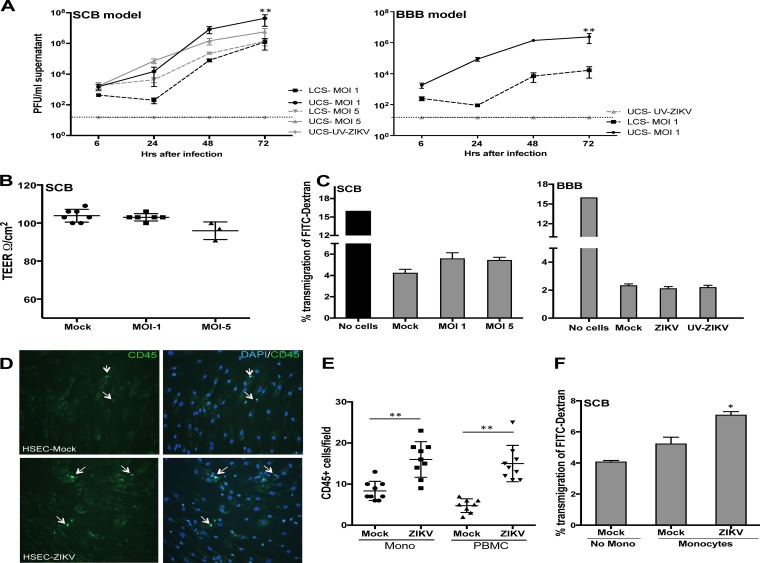
To determine if ZIKV infection of SCs represents a route for the virus to enter the seminiferous tubules, we utilized an in vitro SCB model using transwell cell culture PET (polyethylene terephthalate) inserts and quantified the transmigration of ZIKV across the inserts. Since the SCB restricts the movement of ions, well-documented methods to assess the tightness of the tissue barrier, transendothelial electrical resistance (TEER) and fluorescein isothiocyanate (FITC)-labeled dextran (FITC-dextran) transmigration assays (18, 19), were used. SCB permeability was assessed starting at day 4 after seeding and demonstrated a gradual increase of resistance from 25 to 110 to 130 Ω/cm2, which was comparable to data from other SCB studies using similar inserts with a 0.3-cm2 surface area (20). At day 8 or 10 after seeding, after ensuring a TEER of >100 Ω/cm2, the SCB model was infected with ZIKV or UV-inactivated ZIKV (UV-ZIKV) at MOIs of 1 and 5, and virus titers in the upper chamber supernatant (UCS), representing the peritubular side of the SCB, and the lower chamber supernatant (LCS), corresponding to the lumen of seminiferous tubules, were determined at different time points. Very low levels of ZIKV were detected 6 h after infection, which represented the residual inoculum after gentle washing of the inserts (Fig. 5A). The replication kinetics of ZIKV observed in the UCS was similar to that observed in ZIKV-infected HSeC monolayers in culture plates (Fig. 1A), showing a marked increase at 24 h and peaking 48 h after infection. ZIKV titers in the LCS were very low at 24 h (in the same range as those 6 h after infection), but a sharp increase to 4 to 5 log PFU/ml was observed 48 and 72 h after infection. However, virus titers in the LCS were approximately 1.5 to 2 logs lower than those in the UCS at all time points (P < 0.01), and as expected, no plaques were detected in the SCB models infected with UV-ZIKV. We further studied ZIKV transmigration across the BBB model, prepared as described in our previous studies (18, 19). As shown in Fig. 5A, ZIKV was detected both in the UCS and LCS 48 and 72 h after infection, but the level of transmigrated virus in the LCS was significantly lower in the BBB (<4 log PFU/ml) than in SCB models (6 log PFU/ml).
FIG 5.
Cell-free ZIKV is efficiently released on the adluminal side of an in vitro SCB model without affecting barrier integrity. (A) At day 8 after seeding, the upper chambers of the in vitro SCB and BBB models were mock infected or infected with UV-ZIKV and ZIKV and washed gently to remove unattached virus. The virus titers determined by a plaque assay demonstrated significant increases in both the UCS and LCS from days 1 to 3 after infection. Data represent means ± SEM of results from two independent infection experiments in triplicate. (B) Integrity of the SCB model as determined by measuring the TEER 72 h after infection. TEER values are presented as ohms per square centimeter and did not change significantly after infection compared to values for controls. The data are representative of results from at least three independent experiments. **, P < 0.01 compared to the LCS. (C) FITC-dextran permeability assay of the SCB and BBB models 72 h after infection. The percentage of FITC-dextran that crossed the BBB was determined based on the total amount of input FITC-dextran and did not vary significantly in ZIKV- or UV-ZIKV-infected inserts compared to uninfected controls. (D) HSeC monolayers on coverslips were infected with ZIKV for 72 h, and 2 × 105 monocytes or PBMCs were then added to the cells. After 2 h of incubation, nonadhered leukocytes were vigorously washed off, and adherent cells were stained with CD45 (white arrows). Shown are immunofluorescence micrographs of CD45-stained monocytes. (E) Quantitative representation of CD45+ monocytes (Mono) and PBMCs from six different areas per coverslip from at least three independent infections. **, P < 0.01 compared to mock-infected inserts. (F) The integrity of the in vitro SCB model was determined by measuring FITC-dextran transmigration after 4 h of incubation with monocytes. *, P < 0.05 compared to the control SCB without incubation with monocytes.
To assess if ZIKV infection and release in the LCS have any effect on the integrity of the SCB model, we next measured permeability before infection and on day 3 after infection. The TEER values were above 100 Ω/cm2 for all inserts before infection and remained comparable at day 3 after infection in both the controls and UV-ZIKV-infected models compared to their respective TEER values before infection. While ZIKV infection at an MOI of 1 did not affect the TEER values, we observed a slight reduction in the TEER values in inserts infected at an MOI of 5 compared to controls (Fig. 5B). Analysis of SCB permeability using the FITC-dextran assay also demonstrated that approximately 4.5% of the total amount of input FITC-dextran in the UCS transmigrated to the LCS in control inserts, which increased modestly to 5.5% in infected inserts at day 3 of infection, but this difference was not statistically significant (Fig. 5C). Similarly, FITC-dextran transmigration (Fig. 5C) and TEER values (data not shown) remained comparable in mock- and ZIKV-infected (MOI of 1) BBB models. Collectively, these results demonstrate that while cell-free ZIKV is released on the adluminal side of both the SCB and BBB models, it does not significantly affect barrier integrity. Furthermore, the ability of the virus to transmigrate across the SCB is much greater than its ability to transmigrate across the BBB.
ZIKV infection increases the adhesion of leukocytes to SCs.
Since VCAM-1 and ICAM-1 are the key CAMs involved in cell-cell interactions, the first step during leukocyte transmigration across epithelial and endothelial cells, we next utilized a cell adhesion assay to determine whether ZIKV-infected HSeCs exhibited any differences in their abilities to mediate leukocyte adhesion. As shown in Fig. 5D, while coculture of naive monocytes with mock-infected HSeC monolayers on coverslips demonstrated that only a small number of monocytes adhered to mock-infected HSeCs, the numbers of monocytes increased significantly in infected HSeCs (day 3 after infection, P < 0.01). We also observed similar results of increased adherence of CD45+ peripheral blood mononuclear cells (PBMCs) to ZIKV-infected HSeCs (Fig. 5E). To further determine the consequence of leukocyte interactions with the SCB, 250,000 naive monocytes were incubated with the inserts when the induction of CAMs by ZIKV was at its peak (day 3 after infection). SCB permeability was assessed by using FITC-dextran transmigration assays, and as shown in Fig. 5F, a significant increase in the percentage of transmigration of FITC-dextran was observed only in the ZIKV-infected inserts incubated with monocytes (P < 0.05).
ZIKV infection in human macrophages induces changes in SCB integrity.
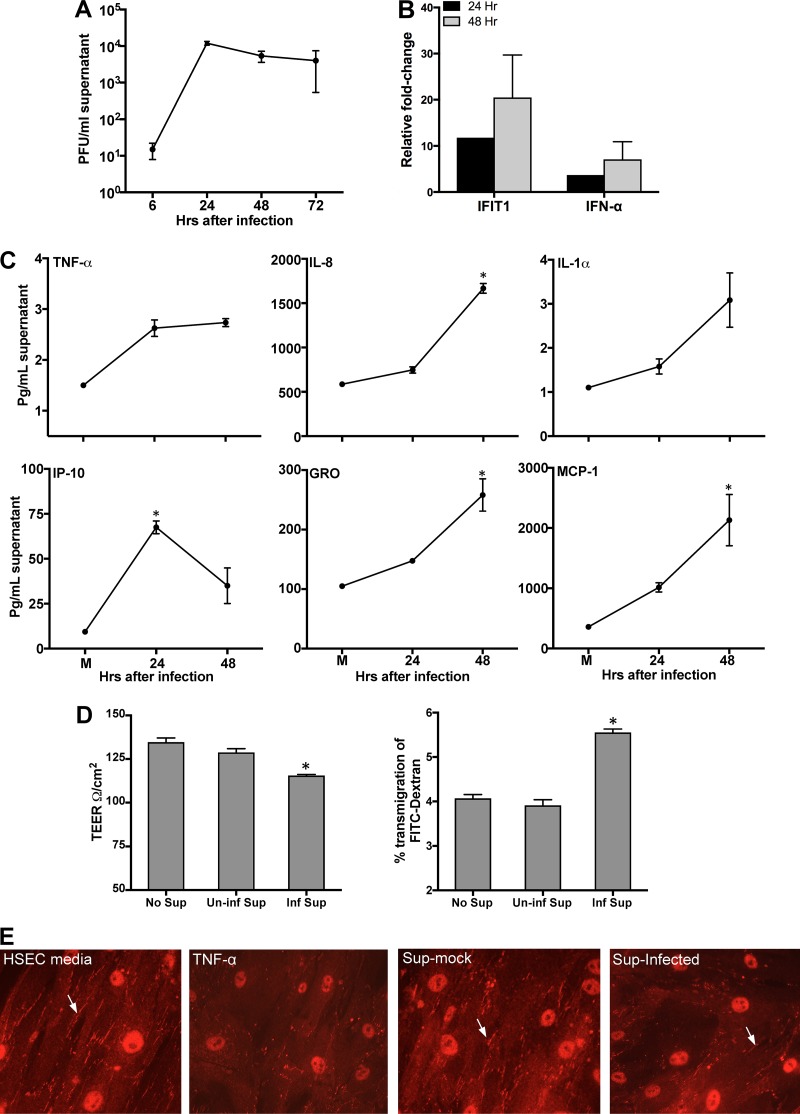
Testicular macrophages are important cell types in the peritubular compartment of the testes and have been shown to contribute to testicular inflammation (21). Therefore, we first characterized the replication kinetics of ZIKV in human macrophages. As shown in Fig. 6A, peak ZIKV replication was observed at 24 h; however, infection was not as robust as that in HSeCs. Peak virus titers were coupled with increased mRNA transcript levels of the IFN-α and IFIT1 genes (Fig. 6B) as well as the secretion of multiple inflammatory cytokines, including TNF-α, IL-1α, and IL-8, 24 and/or 48 h after infection. Additionally, ZIKV also induced the expression of important chemotactic chemokines, such as GRO, IP-10, and monocyte chemoattractant protein 1 (MCP-1), 48 h after infection (Fig. 6C). To determine whether ZIKV-induced inflammatory mediators derived from macrophages could influence SCB integrity, we conducted experiments using the UV-inactivated supernatant from either ZIKV- or mock-infected macrophages. At day 9 after seeding, when the TEER readings of the inserts were above 120 Ω/cm2, the SCB models were incubated with the ZIKV-infected macrophage supernatant collected 48 h after infection. While treatment of the inserts with the supernatant from mock-infected macrophages did not change TEER values or FITC-dextran transmigration compared to control SCB models (HSeC medium only), decreased TEER values were observed in the inserts treated with the supernatant from ZIKV-infected macrophages, which correlated with a significant increase in the percentage of FITC-dextran that transmigrated to the LCS (Fig. 6D). In parallel, we also incubated naive HSeC monolayers on coverslips with the supernatant from infected macrophages for 3 h. As shown in Fig. 6E, the intensity of ZO-1 immunostaining was reduced markedly only for coverslips treated with TNF-α (positive control) and the supernatant from ZIKV-infected macrophages 48 h after infection. These results collectively suggest that inflammatory mediators released from ZIKV-infected cells in the peritubular compartment possess the potential to disrupt the SCB.
FIG 6.
ZIKV infection in human macrophages induces mediators that alter SCB permeability. Human macrophages were infected with ZIKV at an MOI of 1. (A) Culture supernatants collected at different time points after infection were used to determine titers by using a plaque assay on Vero cells. (B) qRT-PCR analysis of IFIT1 and IFN-α in infected macrophages. The data are normalized to the values for GAPDH and expressed as relative fold increases compared to values for uninfected controls. (C) The secretion of proinflammatory cytokines and chemokines in the supernatant was analyzed by using a multiplex bead-based assay. Data represent the means ± SEM from three independent experiments. *, P < 0.05 compared to mock infection. (D) At day 9 after seeding, HSeCs and SCB models were incubated for 2 h with the supernatant from mock- and ZIKV-infected macrophages at the 48-h time point, and TEER values, presented as ohms per square centimeter, demonstrate a decrease only in the inserts incubated with supernatant from infected macrophages. An FITC-dextran permeability assay demonstrated an increase in transmigration across the inserts incubated with the supernatant from infected macrophages (Inf Sup). *, P < 0.05 compared to inserts with no treatment. (E) HSeC monolayers on coverslips were incubated with the supernatant from mock- or ZIKV-infected macrophages (48-h time point) and TNF-α as a positive control and immunostained with anti-ZO-1 (white arrows).
The antiflavivirus drug NITD008 blocks ZIKV infection in HSeCs in a dose-dependent manner.
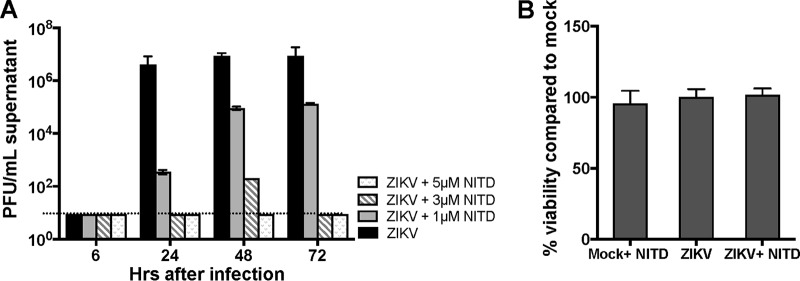
Current small-molecule drugs against flaviviruses include NITD008, a nucleoside analog targeting viral polymerases that has been shown to exhibit potent antiviral activity against DENV, WNV, hepatitis C virus, and, more recently, ZIKV in Vero cells (22, 23). Therefore, we next evaluated if this drug can block ZIKV replication in HSeCs. HSeC monolayers were treated with three different concentrations of NITD008 following ZIKV infection at an MOI of 1, and we observed a dose-dependent inhibition of ZIKV replication, as demonstrated by a plaque assay. In HSeC monolayers treated with 3 and 5 μM concentrations, NITD008 completely blocked ZIKV replication (Fig. 7A). The cell viability assay further showed that this drug at a 5 μM concentration was not cytotoxic to SCs 72 h after infection (Fig. 7B).
FIG 7.
The antiflavivirus drug NITD008 blocks ZIKV infection in HSeCs in a dose-dependent manner. (A) HSeCs were infected with ZIKV at an MOI of 1, followed by treatment with the vehicle or the indicated concentrations of NITD008 (NITD). Supernatants collected at different time points were used to measure viral titers by a plaque assay using Vero cells. The error bars represent data from three independent experiments. (B) Cell toxicity of NITD008 treatment was tested by measuring cell viability in mock-infected and infected HSeCs treated with 5 μM drug 72 h after infection, and the percent change in cell viability was calculated by comparison to mock-infected cells. The error bars represent data from 4 independent infections.
DISCUSSION
Considering the sudden increase in the number of microcephaly cases in the recent ZIKV outbreak, there is a significant interest in understanding the pathogenesis of ZIKV in the testes, specifically to identify potential targets for intervention. Based on our results using human HSeCs, macrophages, and a relevant in vitro SCB model, we propose that ZIKV infects SCs at a higher efficiency than dengue virus and induces a robust yet balanced antiviral defense response with minimal cell death. ZIKV is released on the lumen side of the in vitro SCB model with higher efficiency than in the blood-brain barrier model, without affecting barrier permeability. However, ZIKV-infected HSeCs exhibit enhanced adhesion to monocytes, most likely via increased expression levels of CAMs, leading to the disruption of the SCB. Our results also highlight the contribution of macrophages to testicular infection by ZIKV and show that inflammatory mediators derived from infected macrophages have the potential to compromise the integrity of the in vitro SCB. Finally, our study shows that SCs can be one of the potential targets of the antiflavivirus compound NITD008 and provides an experimental SCB model to test future antiviral drugs to clear testicular infections.
The SCB, comprised of SCs with unique tight and adherens junctions, is critical to preserve the integrity of immune-privileged seminiferous tubules by restricting the entry of pathogens and systemic immune cells while simultaneously providing support to differentiating germ cells. Human SCs have been shown to be the targets of other testis-tropic viruses, including mumps virus and herpes simplex virus (HSV) (24), but not human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV), which are detected mostly in testicular macrophages and T cells (25, 26). However, Govero and colleagues recently reported the presence of ZIKV RNA in mouse testicular cells, including SCs, spermatogonia, and Leydig cells, at day 7, which persisted until day 21 after infection in an immunocompromised mouse model (11). Our in vitro data using human SCs are in agreement with the mouse data and suggest that human SCs are also one of the targets of ZIKV infection. Furthermore, a direct comparison of cell tropisms demonstrating the significantly reduced replication of DENV in HSeCs leads us to speculate that the striking difference in the susceptibilities of SCs to ZIKV and DENV may be one of the reasons why only ZIKV and not DENV is able to establish persistent infection in the testes. Moreover, the comparable replication kinetics of WNV and ZIKV in SCs are not surprising, as WNV is known for a wide range of cell and tissue tropisms compared to DENV. Previous studies reported the shedding of WNV in the urine of human patients for months after recovery from disease symptoms (27); however, there are no epidemiological data so far suggesting the presence of WNV in the seminal fluid of infected humans. Therefore, it is too early to speculate the implication of these data for the sexual transmission of WNV in humans.
Our data showing a strong antiviral defense response to ZIKV, including the production of several inflammatory cytokines, agree with data from a recent in vitro mouse study that reported the production of TNF-α and IL-6 in mouse SCs but not in germ cells or peritubular myeloid cells, thus suggesting that SCs are one of the major sources of antiviral cytokines in human testes (12). Similarly, Singh and colleagues recently demonstrated that ZIKV infection of cells of the blood-retinal barrier is also associated with the production of multiple inflammatory and interferon response genes (28), suggesting that different blood-tissue barrier cells are able to mount a protective response to ZIKV. In recent years, studies have found that mammalian testicular cells, mainly SCs and Leydig cells, produce IL-6, which plays a stage-specific role in the seminiferous epithelial cycle during spermatogenesis and the maintenance of the testis immune environment (29). We speculate that this may be the reason why we observed much higher basal levels of IL-6 in SCs than in other cell types, such as Hofbauer cells, that are immunosuppressive in nature (30). Levels of IL-6 further increased ∼4-fold 72 h after infection, which correlated well with the 5- to 6-fold increases in the mRNA levels (Fig. 2B). Given that many pathogen recognition receptors, including Toll-like receptor 3 (TLR3) and RIG-I, are expressed in SCs and that the synthetic double-stranded RNA (dsRNA) analogue poly(I·C) can induce cytokine production (31), it is highly likely that the detection of ZIKV-derived dsRNA by TLR3 and/or RIG-I is one of the upstream pathways associated with the innate immune response elicited following infection. A few recent studies demonstrated that ZIKV can antagonize the type I IFN response. While Bowen and colleagues showed that ZIKV antagonized type I IFN production in human monocyte-derived dendritic cells (DCs) (32), Singh and colleagues demonstrated that ZIKV induced IFN-β in vivo in the retinas of wild-type (WT) mice following intravitreal inoculation of ZIKV (28). Our data show moderate increases in IFN-α secretion (Fig. 2A) but significant increases in downstream IFIT1 levels, thus suggesting that the IFN response to ZIKV might be cell or tissue specific.
In addition to restricting the entry of pathogens and immune cells into the seminiferous tubules, another important function of the mammalian SCB is to facilitate the movement of developing germ cells into the adluminal compartment of the seminiferous tubules. This unique mechanism for the transit of spermatocytes requires a restructuring of the SCB via modulating tight junction proteins throughout spermatogenesis without compromising the homeostasis of the seminiferous tubules (9). However, cell adhesion molecules such as ICAM-1 and VCAM-1 and MMPs induced by inflammatory triggers, including TNF-α and IL-1α, play a critical role in junction disruption and cell movement (16). The pattern of the ZIKV-induced response in the SCB is very similar to the WNV-BBB interaction. Our previous studies showed that while CAMs such as VCAM-1 and ICAM-1 were induced by WNV in BBB endothelial cells and mediated leukocyte infiltration in the central nervous system (CNS), the sources of MMPs involved in the degradation of TJPs of the BBB were resident astrocytes (18, 19). Similarly, the severity of vascular leakage induced by DENV is associated with increased levels of VCAM-1 (33), suggesting a common trend of all flaviviruses inducing CAMs to facilitate the transmigration of peripheral immune cells across the barrier.
Histological analysis revealed intensive damage of mouse seminiferous tubules accompanied by diminished populations of SCs and the infiltration of CD45+ leukocytes in the testes at days 14 and 21 after ZIKV infection of mice (11). A major question that remains is what are the mechanisms by which ZIKV and leukocytes gain access to the seminiferous tubules and cause infection of developing spermatozoa and/or testicular damage. One possible explanation based on our data is that direct infection of SCs may represent one of the routes of ZIKV entry into the immune-privileged compartment of the testis, but this process may not directly affect barrier integrity or cause cell death (Fig. 8). The direct consequence of ZIKV infection of SCs would be the avoidance of immune detection, and SCs would be a reservoir for infection of developing spermatozoa or elongated spermatids in the lumen, thereby contributing to the persistence of infection even after the clearance of viremia. However, ZIKV infection may also increase the susceptibility of SCs to interactions with other peritubular immune cells, most likely via CAMs, leading to increased infiltration across the barrier and contributing to barrier disruption as one of the mechanisms underlying ZIKV-associated damage to the seminiferous tubules.
FIG 8.
Putative interactions between ZIKV and the SCB. During the viremic stage, ZIKV reaches the peritubular compartment of the testis via peripheral immune cells and infects SCs. Infection of SCs induces a robust innate immune response with no cytopathic effects. Virus replication does not affect the levels of tight junction proteins (TJPs) or SCB integrity, but the virus is efficiently released on the adluminal side of seminiferous tubules, where elongated spermatids are present. ZIKV infection also induces the expression of cell adhesion molecules such as VCAM-1 and ICAM-1 that facilitate the adhesion of naive immune cells to SCs and compromise SCB permeability. ZIKV infection in macrophages results in the production of inflammatory cytokines and chemokines that can degrade the tight junction protein ZO-1 and directly affect the integrity of the SCB. Furthermore, the antiviral agent NITD008 is able to completely block ZIKV infection in SCs. A direct consequence of ZIKV infection in these cell types may include the establishment of persistence in the seminiferous tubules and infiltration of macrophages into the lumen.
Circulating monocytes or testicular macrophages in the peritubular compartment regulate the testicular immune environment in a manner that provides protection for developing male germ cells while permitting balanced inflammatory responses and protection against infections (21, 34). However, in murine models of experimental autoimmune orchitis and lipopolysaccharide (LPS)-induced testicular inflammation, activated macrophages were shown to contribute to inflammation, ultimately causing massive infiltration in the seminiferous tubules, the disruption of barrier junctions, and atrophy of SCs (34). There are limited data on the infection kinetics of ZIKV in human macrophages, although Quicke and colleagues recently documented productive ZIKV infection coupled with inflammatory responses in human Hofbauer cells, which are placental macrophages (30). Therefore, the demonstration that ZIKV infection in human macrophages follows the same trend involving the production of type I IFN and proinflammatory cytokines as that seen in Hofbauer cells leads to the conclusion that macrophages are capable of mounting a strong antiviral defense against ZIKV. Testicular macrophages, both “newly arrived” and “resident,” are the largest population of immune cells in the testis and play important roles in the regulation of spermatogenesis and the response to testicular infection (34). While resident macrophages are generally immunosuppressive, the increased production of chemokines such as CCL2 from SCs and Leydig cells during infection or inflammation results in an influx of large numbers of circulating monocytes that are proinflammatory in nature (34). Therefore, we believe that the macrophages used in our study may more closely resemble the newly arrived rather than the resident form of testicular macrophages. So far, the response of resident macrophages to ZIKV in humans or other animal models is not known and warrants further investigation to understand their role in testicular injury.
Since testicular macrophages are in close proximity to the seminiferous tubules, we hypothesize that they contribute to testicular infection by ZIKV in two ways. First, newly arrived macrophages could serve as a primary source of ZIKV dissemination to other resident testicular cells, including SCs and Leydig cells, during the viremic phase, and second, inflammatory mediators released from infected macrophages may alter the testicular immune environment, thus favoring the loss of TJPs and the leakiness of the SCB, as shown in Fig. 8. Our data demonstrating the loss of ZO-1 and decreased barrier integrity following treatment with the supernatant from infected macrophages support the notion that SCs are susceptible to changes in the levels of local inflammatory mediators. In an in vivo scenario, a direct consequence of ZIKV infection of both SCs and testicular macrophages could be an altered local inflammation status leading to the enhanced infiltration of macrophages and adaptive immune cells into the lumen, thereby damaging developing spermatocytes. However, the role of other cell types, e.g., Leydig cells and peritubular cells, in modulating the local inflammatory milieu and acting as a reservoir for ZIKV cannot be ruled out.
In vitro SCB models comprised of human and mouse SCs have been used to study mechanisms of SC function in spermatogenesis and effects of environmental toxins on barrier integrity leading to male infertility (35, 36). However, this study for the first time uses an in vitro model of the SCB to characterize virus transmigration kinetics and to understand the interactions of infected peritubular leukocytes with SCs. Furthermore, our data demonstrating the dose-dependent effect of a well-tested antiflavivirus compound, NITD008, on blocking ZIKV replication in SCs are encouraging and warrant future in vivo studies to test whether this drug can cross the SCB and clear testicular ZIKV infection. A recent study reported the inhibition of ZIKV by NITD008 in Vero cells and in a mouse model; however, that study did not examine the ability of this drug to clear testicular infections (22). Our in vitro model of the SCB not only can be used as a tool to characterize testicular infections by other viruses, including Ebola virus, but also will allow the rapid testing of new antiviral drugs to cross the SCB and mitigate infection in the seminiferous tubules. In summary, this study provides the much-needed evidence that ZIKV infection of human SCs is one of the mechanisms by which this virus might enter the intratubular compartment of the seminiferous tubules to establish persistent infection in testes. These data provide further insights showing that the disruption of the SCB may be one of the mechanisms by which ZIKV-infected macrophages cause testicular damage and lay a platform for future studies on how ZIKV infection may affect the interaction between SCs and other testis-resident cells, including Leydig cells. Once the specific pathways and cell types involved in the dissemination of the virus to developing spermatids are known, the system can be manipulated pharmacologically to test innovative approaches for the treatment of testicular ZIKV infection in humans.
MATERIALS AND METHODS
Ethics statement.
Human monocytes and PBMCs were isolated from fresh blood obtained from healthy donors under protocol number CHS 24091, approved by the University of Hawaii Institutional Review Board for Human Subjects. All subjects were adults and gave informed written consent.
Cells and virus.
Low-passage-number primary human Sertoli cells (HSeCs), purchased from Lonza (catalogue number MM-HSE-2305), were propagated in Dulbecco's modified Eagle's medium (DMEM) and F-12 medium at a ratio of 1:1 with HEPES, l-Gln, 100 U/ml penicillin-streptomycin, and 5% untreated fetal bovine serum (FBS) according to manufacturer's protocol. Early-passage HBMVECs, purchased from Cell Systems Corporation (catalog number CSC 2M1), were grown as previously described (37). All experiments with primary HSeCs and HBMVECs were conducted by using cells from passages 6 to 9 at 80 to 90% confluence. ZIKV strain PRVABC59 (Human/2015/Puerto Rico), acquired from the American Type Culture Collection (ATCC), was propagated once in Vero E6 cells (ATCC). The stock virus titer was measured by a plaque assay using Vero cells to calculate the MOI. Cells were infected at an MOI of 1 or 5 for 1 h at 37°C, followed by 2 washes and replacement with fresh medium. Experiments were also conducted by using a Vero cell supernatant for mock infections as a control for cell viability. UV-ZIKV was generated by exposing the virus stock to UV radiation for 10 min (Stratalinker 2400; Stratagene) as described previously for WNV and confirmed by a plaque assay (37). In some experiments, HSeCs were infected with DENV2 (New Guinea C strain) or ZIKV in the presence of NITD008, an antiflavivirus compound, at different concentrations, prepared as described previously (22).
PBMC isolation and monocyte separation.
PBMCs were isolated from 20 to 40 ml of anticoagulated blood from healthy donors by using Ficoll density gradient centrifugation, and monocytes were differentiated into macrophages by culturing adhered monocytes in 24-well plates in X-Vivo medium (hematopoietic cell medium that does not contain any exogenous growth factors of cellular proliferation) for 6 days as described previously (38). For some experiments, monocytes were separated by using the EasyStep negative-selection human monocyte enrichment kit (StemCell Technologies), as described previously (18), and used immediately for incubation with HSeC monolayers or inserts. With this method, the purity of monocytes was 99%, and viability was 97 to 99%.
Quantification of ZIKV titers using a plaque assay and qRT-PCR.
ZIKV titers in the cell culture supernatants were analyzed by a standard plaque assay using Vero cells (37). Intracellular virus replication was measured by qRT-PCR using primers and a probe specific for the ZIKV Env region, as described previously (39). The data are expressed as ZIKV PFU per milliliter of supernatant or PFU equivalents per microgram of RNA.
Analysis of the host response.
Total RNA extracted from HSeCs and macrophages collected at different time points after infection was used to measure changes in the levels of transcripts of multiple innate immune genes, TJPs, and CAMs by qRT-PCR using specific primers as described previously (18, 37). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene was utilized to normalize the values for each gene, and the fold change compared to values for the uninfected controls was calculated as described previously (37). The protein levels of TJPs (ZO-1, claudin, occludin, and JAM-A) and CAMs (VCAM-1 and E-selectin) in HSeCs were also determined by Western blotting using specific antibodies as described previously (37). For further confirmation by immunostaining, mock- and ZIKV-infected HSeCs grown on coverslips were fixed in 4% paraformaldehyde (PFA) 48 h after infection and immunostained by using biotinylated monoclonal mouse or anti-ZO-1 antibody (1:100 dilution), followed by streptavidin 488-conjugated anti-mouse secondary antibodies, and examined by using an AxioCam MR camera mounted on a Zeiss Axiovert 200 microscope as described previously (37). The levels of multiple cytokines and chemokines, including IFN-α, secreted into the cell culture supernatant from ZIKV-infected HSeCs and human macrophages were quantified by a Luminex assay using a Milliplex Map Human Cytokine/Chemokine kit (Millipore) and read on a Luminex200 LiquiChip analyzer as described previously (40). Cell viability was assessed at different days after infection by using a CellTiter 96 AQueous One Solution cell proliferation assay (Promega) as described previously (41).
Development of the SCB model, ZIKV infection, and permeability assays.
An in vitro SCB model was constructed by using Corning Biocoat human fibronectin PET inserts with a 3.0-μm pore size and a 0.33-cm2 membrane surface area in a 24-well plate. Briefly, after rehydration of the inserts according to the manufacturer's protocol, the inserts were seeded with 6 × 104 HSeCs or HBMVECs on the upper surface and incubated with 500 μl medium in both the upper and lower chambers at 37°C with 5% CO2 and 100% humidity. The integrity of the in vitro SCB model was first determined 4 days after seeding and then subsequently determined every 2 to 3 days by measuring the TEER using an epithelial volt/ohm meter (Evomx; World Precision Instrument) and an Endohm-6 chamber, and the TEER was expressed as ohms per square centimeter, as described previously (37). The medium from both the upper and lower chambers was replaced during each TEER measurement. Ten days after seeding, when the TEER readings crossed 110 Ω/cm2, the inserts were infected with ZIKV or UV-ZIKV for 1 h, after which the inserts were gently washed twice with HSeC medium to remove unbound virus. The TEER was measured 24, 48, and 72 h after infection, and the UCS and LCS were collected for quantitative virus assays. For some experiments, the upper chambers of ZIKV-infected SCB inserts were incubated with either 2.5 × 104 naive PBMCs and monocytes or 350 μl of the supernatant from ZIKV-infected human macrophages collected 48 h after infection and mixed with 150 μl of HSeC medium, and the TEER was measured after 2 h of treatment. The integrity of the inserts following PBMC/monocyte transmigration and supernatant treatment was also assessed by using the FITC-dextran (4-kDa molecular mass; Sigma) transmigration assay as described in our previous studies (37). The transmigration of FITC-dextran across the inserts was calculated as a percentage of the total amount added to the upper chamber.
Adhesion assay.
Confluent monolayers of HSeCs grown on coverslips in 24-well plates were infected with ZIKV at MOIs of 1 and 5. At day 3 of infection, 2.5 × 104 naive monocytes or PBMCs were added to the coverslips and cocultured with HSeCs for 2 h at 37°C. At the end of the incubation period, nonadherent monocytes or PBMCs were removed by washing four times to remove loosely attached cells. The cocultured monolayers were fixed in 4% PFA for 10 min and stained with FITC-conjugated anti-CD45 (a surface leukocyte marker) diluted 1:100 and with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining, as described previously (18). Controls included uninfected HSeCs cocultured with leukocytes. The number of leukocytes bound to the monolayers was determined by counting the number of adherent CD45+ cells in two central and two peripheral randomly selected fields from at least 3 coverslips from two independent infections.
Statistical analysis.
ZIKV titers, mRNA fold changes, and Luminex data are reported as means ± standard errors of the means (SEM) of data from at least triplicates from two independent experiments. An unpaired Student t test was used to compare the values determined by permeability and transmigration assays using GraphPad Prism 5.0.0 (GraphPad Software, San Diego, CA). A P value of <0.05 was considered statistically significant for all analyses.
ACKNOWLEDGMENTS
This work was partially supported by grant R21AI129465-01 and the Centers of Biomedical Research Excellence (P30GM114737), the National Institutes of Health, and University of Hawaii institutional funds.
We sincerely thank Vivek R. Nerurkar for providing HSeCs and the ZIKV stock and for critical input during scientific discussions. We also thank C.-Y. Lai and Boonya Jiyarom for technical assistance with the Luminex assays and qRT-PCRs.
REFERENCES
- 1.Musso D, Gubler DJ. 2016. Zika virus. Clin Microbiol Rev 29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. 2017. Zika virus case counts in the US. CDC, Atlanta, GA. [Google Scholar]
- 3.WHO. 2017. Zika virus, microcephaly, and Guillain-Barre syndrome situation report. WHO, Geneva, Switzerland. [Google Scholar]
- 4.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. 2016. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 65:215–216. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- 6.Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, Perez-Padilla J, Medina FA, Waterman SH, Gubern CG, Alvarado LI, Sharp TM. 14 February 2017. Persistence of Zika virus in body fluids—preliminary report. N Engl J Med doi: 10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deen GF, Knust B, Broutet N, Sesay FR, Formenty P, Ross C, Thorson AE, Massaquoi TA, Marrinan JE, Ervin E, Jambai A, McDonald SL, Bernstein K, Wurie AH, Dumbuya MS, Abad N, Idriss B, Wi T, Bennett SD, Davies T, Ebrahim FK, Meites E, Naidoo D, Smith S, Banerjee A, Erickson BR, Brault A, Durski KN, Winter J, Sealy T, Nichol ST, Lamunu M, Stroher U, Morgan O, Sahr F. 14 October 2015. Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report. N Engl J Med doi: 10.1056/NEJMoa1511410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng CY, Mruk DD. 2012. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mital P, Hinton BT, Dufour JM. 2011. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Shi L, Wang Q, Cheng L, Zhao X, Chen Q, Jiang Q, Feng M, Li Q, Han D. 2016. Mumps virus-induced innate immune responses in mouse Sertoli and Leydig cells. Sci Rep 6:19507. doi: 10.1038/srep19507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. 2016. Zika virus infection damages the testes in mice. Nature 540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, Liu M, Yan J, Li W, Qin C, Han D, Qin C, Wang N, Li X, Gao GF. 2017. Zika virus causes testis damage and leads to male infertility in mice. Cell 168:542. doi: 10.1016/j.cell.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EF, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. 2016. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med 22:1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. 2016. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubry F, Habasque C, Satie AP, Jegou B, Samson M. 2000. Expression and regulation of the CXC-chemokines, GRO/KC and IP-10/mob-1 in rat seminiferous tubules. Eur Cytokine Netw 11:690–698. [PubMed] [Google Scholar]
- 16.Lydka M, Bilinska B, Cheng CY, Mruk DD. 2012. Tumor necrosis factor alpha-mediated restructuring of the Sertoli cell barrier in vitro involves matrix metalloprotease 9 (MMP9), membrane-bound intercellular adhesion molecule-1 (ICAM-1) and the actin cytoskeleton. Spermatogenesis 2:294–303. doi: 10.4161/spmg.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riccioli A, Filippini A, De Cesaris P, Barbacci E, Stefanini M, Starace G, Ziparo E. 1995. Inflammatory mediators increase surface expression of integrin ligands, adhesion to lymphocytes, and secretion of interleukin 6 in mouse Sertoli cells. Proc Natl Acad Sci U S A 92:5808–5812. doi: 10.1073/pnas.92.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roe K, Orillo B, Verma S. 2014. West Nile virus-induced cell adhesion molecules on human brain microvascular endothelial cells regulate leukocyte adhesion and modulate permeability of the in vitro blood-brain barrier model. PLoS One 9:e102598. doi: 10.1371/journal.pone.0102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. 2010. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 397:130–138. doi: 10.1016/j.virol.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh AL, Mitchell JB, Rabinovich GA, Noble-Haeusslein LJ, John CM. 2011. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant 20:619–635. doi: 10.3727/096368910X536563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao S, Zhu W, Xue S, Han D. 2014. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol 11:428–437. doi: 10.1038/cmi.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng YQ, Zhang NN, Li CF, Tian M, Hao JN, Xie XP, Shi PY, Qin CF. 2016. Adenosine analog NITD008 is a potent inhibitor of Zika virus. Open Forum Infect Dis 3:ofw175. doi: 10.1093/ofid/ofw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson J, Roe K, Orillo B, Shi PY, Verma S. 2015. Combined treatment of adenosine nucleoside inhibitor NITD008 and histone deacetylase inhibitor vorinostat represents an immunotherapy strategy to ameliorate West Nile virus infection. Antiviral Res 122:39–45. doi: 10.1016/j.antiviral.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejucq N, Jegou B. 2001. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev 65:208–231. doi: 10.1128/MMBR.65.2.208-231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willey S, Roulet V, Reeves JD, Kergadallan ML, Thomas E, McKnight A, Jegou B, Dejucq-Rainsford N. 2003. Human Leydig cells are productively infected by some HIV-2 and SIV strains but not by HIV-1. AIDS 17:183–188. doi: 10.1097/00002030-200301240-00007. [DOI] [PubMed] [Google Scholar]
- 26.Le Tortorec A, Le Grand R, Denis H, Satie AP, Mannioui K, Roques P, Maillard A, Daniels S, Jegou B, Dejucq-Rainsford N. 2008. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS One 3:e1792. doi: 10.1371/journal.pone.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonry JH, Xiao SY, Siirin M, Chen H, da Rosa AP, Tesh RB. 2005. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am J Trop Med Hyg 72:320–324. [PubMed] [Google Scholar]
- 28.Singh PK, Guest JM, Kanwar M, Boss J, Gao N, Juzych MS, Abrams GW, Yu FS, Kumar A. 2017. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2:e92340. doi: 10.1172/jci.insight.92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedger MP, Meinhardt A. 2003. Cytokines and the immune-testicular axis. J Reprod Immunol 58:1–26. doi: 10.1016/S0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 30.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. 2016. Zika virus infects human placental macrophages. Cell Host Microbe 20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starace D, Galli R, Paone A, De Cesaris P, Filippini A, Ziparo E, Riccioli A. 2008. Toll-like receptor 3 activation induces antiviral immune responses in mouse Sertoli cells. Biol Reprod 79:766–775. doi: 10.1095/biolreprod.108.068619. [DOI] [PubMed] [Google Scholar]
- 32.Bowen JR, Quicke KM, Maddur MS, O'Neal JT, McDonald CE, Fedorova NB, Puri V, Shabman RS, Pulendran B, Suthar MS. 2017. Zika virus antagonizes type I interferon responses during infection of human dendritic cells. PLoS Pathog 13:e1006164. doi: 10.1371/journal.ppat.1006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Her Z, Kam YW, Gan VC, Lee B, Thein TL, Tan JJ, SIgN Immunomonitoring Platform, Lee LK, Fink K, Lye DC, Renia L, Leo YS, Ng LF. 2017. Severity of plasma leakage is associated with high levels of interferon gamma-inducible protein 10, hepatocyte growth factor, matrix metalloproteinase 2 (MMP-2), and MMP-9 during dengue virus infection. J Infect Dis 215:42–51. doi: 10.1093/infdis/jiw494. [DOI] [PubMed] [Google Scholar]
- 34.Winnall WR, Hedger MP. 2013. Phenotypic and functional heterogeneity of the testicular macrophage population: a new regulatory model. J Reprod Immunol 97:147–158. doi: 10.1016/j.jri.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Mruk DD, Lee WM, Wong CK, Cheng CY. 2016. Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis? Semin Cell Dev Biol 59:141–156. doi: 10.1016/j.semcdb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li MW, Mruk DD, Lee WM, Cheng CY. 2009. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, Luo H, Nakatsuka A, Nerurkar VR. 2009. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: transmigration across the in vitro blood-brain barrier. Virology 385:425–433. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiebl B, Fuhrmann R, Franke RP. 2008. Characterization of cryopreserved CD14+-human monocytes after differentiation towards macrophages and stimulation with VEGF-A(165). Clin Hemorheol Microcirc 39:221–228. [PubMed] [Google Scholar]
- 39.Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M Jr, Rajagopal L. 2016. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M Jr, Verma S. 2013. Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in West Nile virus encephalitis. J Virol 87:3655–3667. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar M, Verma S, Nerurkar VR. 2010. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J Neuroinflammation 7:73. doi: 10.1186/1742-2094-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]