Abstract
Assessment of airway secretion cells, both for research and clinical purposes, is a highly desired goal in patients with acute and chronic pulmonary diseases. However, lack of proper cell isolation and enrichment techniques hinder downstream evaluation and characterization of cells found in airway secretions. Here, we demonstrate a novel enrichment method to capture immune-related cells from clinical airway secretions using closed-loop separation of spiral inertial microfluidics (C-sep). By recirculating the output focusing stream back to the input reservoir and running continuously with a high flow processing rate, one can achieve optimal concentration, recovery and purity of airway immune cells from a large volume of diluent, which was not readily possible in the single-pass operation. Our method reproducibly recovers 94.0% of polymorphonuclear leukocytes (PMNs), with up to 105 PMNs in clear diluted buffer from 50 μL of airway secretions obtained from mechanically ventilated patients. We show that C-sep isolated PMNs show higher neutrophil elastase (NE) release following activation by phorbol 12-myristate 13-acetate (PMA) than cells isolated by conventional mucolytic method. By capturing cells without chemically disrupting their potential function, our method is expected to expand the possibility of clinical in vitro cell based biological assays for various pulmonary diseases such as acute respiratory distress syndrome, pneumonia, cystic fibrosis, and bronchiectasis.
Graphical Abstract
A number of lung conditions are characterized by acute or chronic inflammation and the influx of leukocytes as a prominent feature in the airways. However, a reliable method for the isolation and enrichment of these immune cells collected from airway secretions would be highly desirable for downstream applications. As an example, the proportion of eosinophils in sputum is an important metric that enables a more precise classification of asthma subtypes and effective tailoring of therapies to the individual.1 Neutrophilic airway inflammation is encountered in cystic fibrosis and a subset of patients with chronic obstructive pulmonary disease (COPD) with bronchitis and sputum production.2,3 Yet, surprisingly little is known regarding the function of neutrophils in specific disease processes. This is, in part, due to the fact that cells in airway secretions, or expectorated sputum, are often encapsulated in a large amount of mucin, which hinders functional assessment by in vitro assay.4 Given this barrier, there are few studies of airway neutrophil function, and most data have come from examination of neutrophils from peripheral blood, which may or may not closely reflect local pathophysiology of the lung.5
Dithiothreitol (DTT), also known as Sputalysin, is widely used for homogenization of sputum to improve cytological analysis, cell recovery, and detection of mediators.6,7 The viability of cells recovered by DTT homogenization is acceptable. However, sputum treatment with DTT can affect neutrophil function in terms of elastase and MPO release, by disrupting surface bound antigens.7,8 These impairments interfere with in vitro functional analysis using airway neutrophils, limiting the assessment of patients’ status. Therefore, a nonchemical method to isolate and enrich airway cells is preferable, in order to avoid biochemical interference. Dilution in a large volume of buffer solution enables mucin dispersion and capture of immune cells without using DTT, but the resulting low cell concentration is a challenge for diagnostic sensitivity.
Inertial microfluidics has made significant contributions in separating cells from various biofluids with high processing rates.9,10 Inertial microfluidics utilizes inertial lift forces and Dean vortices cause the particles to arrange in the channels according to their size.11 Mach et al. reported a parallel straight microchannels that passively separates bacteria from diluted blood.12 Park et al. investigated inertial focusing in a straight channel patterned with a contraction–expansion array, and Moon et al. combine this multiorifice microchannel with dielectrophoresis (DEP) to separate breast cancer cells from blood sample.13,14 Di Carlo et al. proposed an asymmetric serpentine channel to induce well-defined particle/cell focusing that balances inertial lift forces and Dean drag force.15 Our group previously demonstrated spiral inertial microfluidics to capture circulating tumor cells16,17 to detect a small amount of bacteria in blood18 and to provide continuous filtration for anchorage independent cell culture.19,20 Our group also introduced spiral microchannel with trapezoidal cross-section to separate/recover intact PMNs from diluted blood without artifactual activation encountered with the conventional RBC lysis method.21,22
Microfluidics cell separation methods have a fundamental trade-off between enrichment and recovery of separation, i.e., achieving high recovery compromises cell density, especially when there is significant overlap between separated cell/molecular streamlines.4 Martel et al., reported bioparticle concentrator that can concentrate particles more than 400 times in a single operation.10 However, the window of optimal separation parameters is narrow and difficult to identify and requires fine-tuning for each individual sample with unknown physical and chemical characteristics. Other common challenges include the need for processing large volumes of biofluid (especially for low-abundance cells) and the need to minimize molecular background in the original biofluid that may interfere with downstream assays (i.e., replacing serum with well-characterized buffer).
In this study, we introduce a novel operating method of spiral inertial microfluidics to enrich immune cells from airway secretions that overcomes the trade-off between purity and recovery, which is the limit of the previous cell separation method by inertial microfluidics. By feeding the concentrated output stream of microfluidics back to the inlet port and running continuously with a high flow processing rate, one can achieve high concentration, recovery, and purity of target cells. We recovered >95% neutrophils by physical separation using 1000-fold dilution of airway secretions from mechanically ventilated patients, despite the heterogeneous fluidic properties of the samples. In contrast, conventional DTT homogenization method showed variable recovery of PMNs. Closed-loop separation from 50 μL of the original volume of airway secretion yielded a recovery of ~105 PMNs in clear suspension in the buffer of our choice. As a nonchemical and nonlabeled separation/enrichment method, closed-loop separation can provide intact leukocytes without biochemical and mechanical disturbance, which was validated by monitoring neutrophil elastase activity of sorted cells. Our method provides a new method for harvesting delicate cells from complex biofluid sample matrixes, not only for sputum but also a variety of biofluids used in clinical diagnostics.
RESULTS AND DISCUSSION
Closed-Loop Operation of Inertial Microfluidics
Patient-derived sputum or airway secretion samples are often diverse in fluidic properties, originating from either variations in sample harvesting and/or different patient conditions. Therefore, it is a challenge to design a separation process that can handle the variability observed among patient samples. A new technique is necessary that can meet the need for achieving (1) high purity and high recovery rate of leukocytes at the same time, (2) effective removal of mucin and other backgrounds, and (3) standardized protocol for a wide variety of sample conditions.
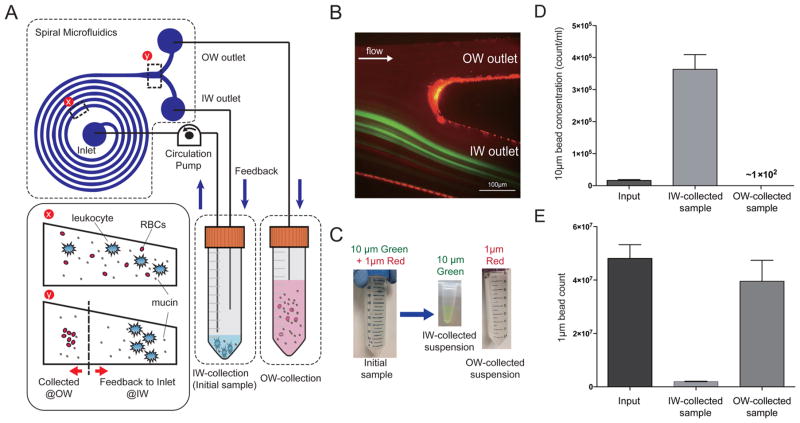
To meet the above challenges (which are commonly found in other biosample preparations), we created a new operating method of spiral microfluidics (Figure 1A and Supp. Figure S1). The sample suspension in a conical tube was connected to a circulation pump (e.g., peristaltic pump), and the sample fluid was injected into the inlet port of the spiral microchannel. While the inertial lift force primarily dictates the large particle (>10 μm) focusing on the inner-wall side (IW) of channel, the combinatory effect of Dean drag force and inertial lift force within spiral channel reduces the multiple equilibrium positions of particles into two vertically overlapping positions with the same lateral distance to IW. As a result of the size dependence of both forces, particles smaller than 6 μm occupy distinct lateral positions near the outer-wall side (OW) when flowing through the same spiral channel under a given flow rate. The outlet of the OW was connected to another conical tube (OW-collection tube), while the IW outlet (the outlet in which the target neutrophils are focused and collected) was continuously fed back to the initial sample tube. This allows unfocused small particles (<6 μm) such as mucin aggregation and background fluid to be removed through the OW outlet, whereas the IW outlet concentrated cells bigger than 10 μm in diameter. Serial connection of the platform could provide the benefit of high concentration and recovery, yet is subject to high fluidic resistance (and resulting channel deformation) as well as potential loss by manually reloading.23 The proposed system is an automated platform that can process continuously and eliminate losses due to manual processing methods. Recirculatory microfluidics was introduced previously for acoustic live cell separation.24 While it is a generic operational modality that can be used for any particle separation processes, in this work, spiral inertial microfluidics with trapezoidal cross-section was selected due to easy operation and high efficiency, throughput, and recovery, as demonstrated by our group previously.25
Figure 1.
(A) Schematic diagram of closed-loop separation using spiral inertial microfluidics (C-sep). (B) Fluorescent image of bifurcation outlet. Ten μm green fluorescent beads, emulating leukocytes, are focused at the inner wall side of outlet, while 1 μm red fluorescent beads, mimicking mucin aggregation, evenly distribute through the channel. (C) By recirculating IW outlet contents back into the IW-collection tube, 10 μm beads were concentrated in a small volume of IW-collected suspension as the background fluid containing the 1 μm beads were continuously removed through the OW outlet. (D) Concentration of 10 μm beads and (E) the amount of 1 μm beads were quantified in each suspension. After c-sep, 99.3% of 10 μm beads were concentrated in the IW-collection tube, while 96% of 1 μm beads were removed through the OW-collection tube.
As an initial proof-of-concept, phosphate buffer solution (PBS) spiked with fluorescence beads (10 μm green fluorescent beads and 1 μm red fluorescent beads) were placed through the closed-loop separation (Figure 1B,C). Green fluorescent beads with 10 μm diameter, mimicking leukocytes, appeared in the IW outlet, while 1 μm red fluorescent beads, representing mucin aggregation, distributed evenly in both IW and OW outlets. Through recirculation of the IW outlet contents, the 10 μm beads were recovered and concentrated in increasingly smaller volume of resulting suspension (achieving high concentration of cells in low recovery volume) while 1 μm beads were gradually removed from the IW-collection tube (Figure 1C). As shown in Figure 1B, the IW outlet is wide enough to achieve a high recovery rate of focused particle; however, without recirculation of IW contents, the concentration factor and purity are limited by the bifurcation ratio of IW and OW outlet dimensions (~2). In closed-loop separation, the concentration factor was defined by the volume of OW-collected background fluid. We were able to achieve 22.5 times higher concentration of suspension with 99.3% recovered cells, when compared to the initial sample (Figure 1D). At the same time, 96.0% of the 1-μm beads were removed from the initial suspension by volume reduction (Figure 1E). The sample tube was accessible during the operation, which enables one to add and isolate cells from even larger volume of diluent or buffer solution (>100 mL) in a single continuous run.
Unified Separation Protocol for Clinical Airway Secretions in Diverse Conditions
Airway secretions show varied characteristics depending upon the lung condition, in terms of cellular contents, mucin density, and other properties.26 For example, noncardiogenic pulmonary edema is the hallmark of acute respiratory distress syndrome (ARDS) that is characterized by systematic inflammation-induced injury, which is primarily caused by neutrophils.27,28 Most common risk factor for ARDS is acute infection, either arising from the lungs in the form of severe pneumonia or from an extrapulmonary source manifesting itself as sepsis.29 In cystic fibrosis (CF), thick mucus airway secretions is characterized by intense neutrophilic inflammation showing high serine protease activity.30,31 In COPD, the high number of neutrophils recruited to the lungs can also contribute to the formation of thick sputum.3,32 In all of these cases, reliable sample preparation for airway secretions must be able to handle the heterogeneity within and across specific patient populations and yielding consistent cell recovery results regardless of the condition.
In inertial microfluidics, particle/cell locate at a certain lateral position in a microchannel based on size as Reynolds number approaches 1.9,12,15,33 This has been explained by the balance of two forces: inertial lift force and Dean drag force, which gives optimal flow rate (Q) for the particle focusing in channel length of L as the function of μ and ρ are the fluid viscosity and density:
where a, W, and H is the particle diameter, channel width, and height, respectively. Lift coefficient fL varies from ~0.02–0.05 for aspect ratios of microchannel from 2 to 0.5.9,15 However, because of the variation of fluidic properties in patient samples, a single-pass operation requires fine-tuning the optimal flow rate for each sputum which limits the development of a standardized protocol for clinical sample preparations.
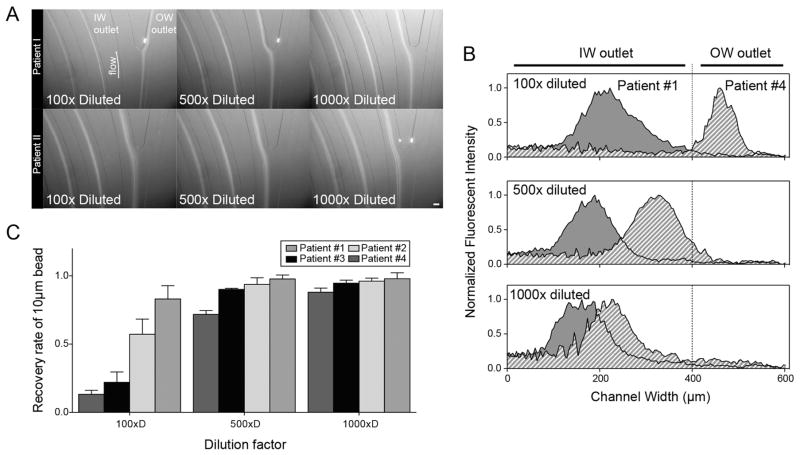
To overcome the heterogeneity of clinical airway secretions, samples were diluted to render the properties more uniform across patient samples. Four samples with different viscosity and turbidity qualities were reasonably representative. For 100-fold dilution of samples spiked with 10 μm fluorescent beads, a comparison of two patient samples showed disparate focusing behavior with 10 μm beads concentrated at the IW outlet in patient no. 1 and at OW outlet in patient no. 4 samples (Figure 2A,B). This dissimilar focusing behavior with diverse and low recovery rates of 10 μm beads (55.3% on average, ranging between 13.3 and 91.8% depending on sample) resulted from the individual fluidic properties of each sample at 1:100 dilution. However, as we increased the dilution of the input sample, focusing behavior became more uniform to the point where for a 1000-fold dilution of input samples, patient samples no. 1 and no. 4 showed the same focusing position with 94.4% recovery of 10 μm beads in a small volume of suspension (Figure 2C). Xiang et al. reported viscoelastic focusing in spiral microchannel, where 10-μm particles were focused at OW side in high Re regime (>8).33 Since elastic property of the fluid can be modified by the presence of a small amount of polymeric solutes, we suspect that different mucin content of airway secretion samples could result in dissimilar elastic force on the particle. Because of this inconsistency, it is challenging to find a separation protocol that works for all patient samples. Instead, fluidic properties can be made more even by diluting in a large volume of buffer (1000-fold dilution), to recover most of immune-related cell regardless of the specimen characteristics. As the closed-loop separation technique achieved high recovery of cellular contents as well as high concentration by volume reduction from a large initial sample volume, the proposed method enables the isolation and enrichment of leukocytes in a consistent manner despite large variations in individual characteristics of patient-derived airway secretion samples. Moreover, 1000-fold dilution of airway secretions or sputum allowed for the removal of mucin and other chemical background molecules, and resulting in a clear suspension of concentrated leukocytes.
Figure 2.
(A) Microscopic images and (B) intensity distribution of 10 μm fluorescent beads at the bifurcation outlet of spiral inertial microfluidics. Scale bar: 100 μm. (C) Recovery ratio of 10 μm beads that were spiked into different patient samples. A dilution ratio higher than 500 synchronized particle focusing site, resulting in >90% of particle recovery and achieving a standardized protocol to overcome heterogeneous fluidic properties of individual variations in airway secretion composition.
Comparison to Conventional Mucin Lysis Method
To compare the closed-loop dissociation protocol with the conventional method, DTT was used to homogenize airway secretions (Figure 3A). DTT is widely used as a mucolytic agent to separate cellular contents from sputum by severing disulfide bonds of proteoglycan aggregation.1,2,6 However, DTT was reported to interfere with leukocyte surface antigens and affect leukocyte functionality.7
Figure 3.
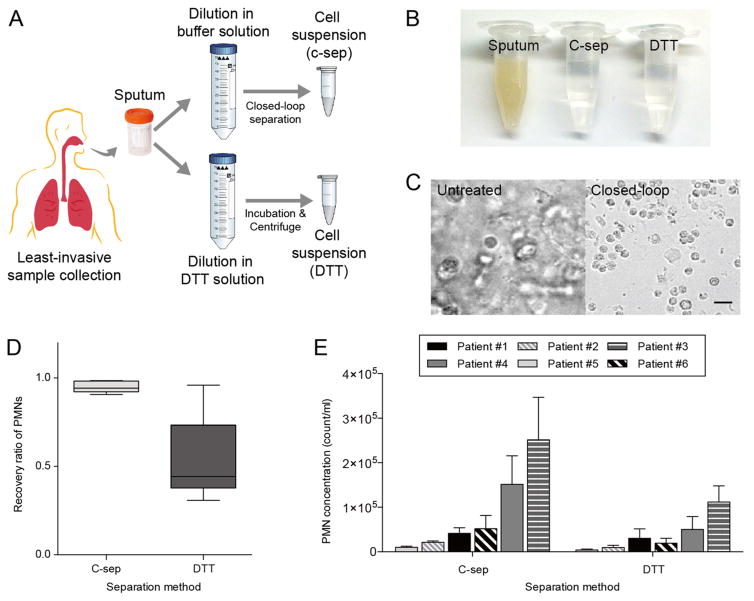
(A) Schematic diagram of airway secretion separation using C-sep and DTT dissociation methods. (B) Photograph and (C) microscopic images of original and resulting suspensions. Scale bar: 20 μm. (D) Comparison of PMN recovery rates by both methods. Closed-loop separation showed >95% recovery for all samples, while DTT protocol presented lower and greater variation in recovery ratio for samples.
Using both separation methods, we achieved clear suspensions with cellular contents (Figure 3B,C). Closed-loop separation was performed with 50 mL of 1000-fold dilution of airway secretion samples, resulting in 4.40 × 105 PMNs (0.21–5.60 × 105 PMNs, n = 6 depending on airway secretion conditions) in 5 mL of clear background fluid with 94.0% PMNs (CD66b+/CD45+) (Figure 3D,E). However, DTT separation method yielded lower recovery of 53.5% PMNs, with significant sample-to-sample variations (30.8–96.0%, n = 6). Both methods provided acceptable cell concentrations for in vitro downstream assays (generally requiring cell concentration of ~105 cell/mL). Since DTT dissociation method often requires straining the sample, heterogeneous mucolytic efficiency affects cellular recovery by potential clogging which becomes more relevant for small sample volumes (~50 μL) and for relatively thick airway secretion samples. On the other hand, closed-loop separation was initiated by diluting airway secretion samples in a large volume of buffer solution with reliable recovery of cells-of-interest regardless of samples’ original characteristics. As shown in Supp. Figure S2, we were able to enrich cellular contents of airway secretion samples in clear buffer solution, independent of the original sample state (i.e., bloody, tenacious, or watery). Operating with the spiral microchannel of trapezoidal cross-section, previously designed by our group for isolation of blood neutrophils,21,22 closed-loop separation was able to remove 80.8% of RBCs in bloody sputum sample (Supp. Figure S3). Compared to the standard mucolytic method, closed-loop separation resulted in less debris (defined as <3 μm events) in recovered samples and thus minimizing possible interference of debris with downstream biochemical assays (Supp. Figure S3).
Release of elastase was examined to assess functional integrity of PMNs captured by both enrichment methods (Figure 4). As a powerful mechanism of host defense, neutrophils phagocytize and degrade foreign organic molecules by the release of neutrophil elastase (NE).34–36 Phorbol myristate acetate (PMA) triggers protein kinase C activation and is utilized commonly to activate neutrophils in vitro.37 PMA was used to stimulate release of NE from enriched cells by both closed-loop and mucolytic (DTT) separation. We tested each method with airway secretion samples from six different patients.
Figure 4.
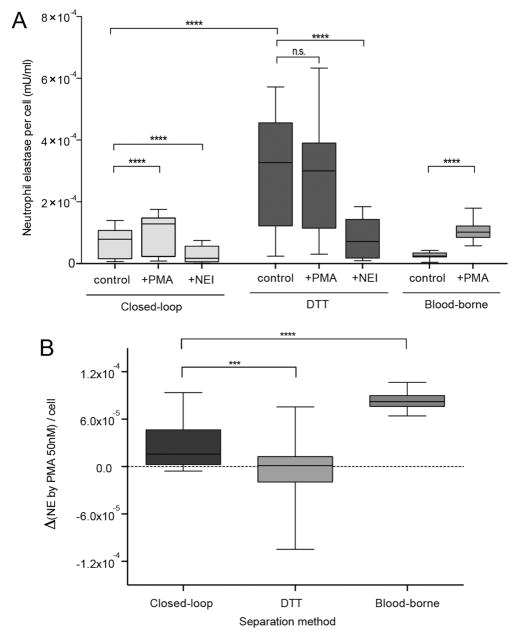
(A) Comparison of PMN functional capacity following separation by C-sep and DTT techniques. NE release was significantly increased by PMA stimulation only for PMNs by C-sep. (B) Evaluation of NE increment by PMA stimulation separated by C-sep, DTT, and blood-borne neutrophils. NE release by airway PMNs isolated by C-sep was higher than NE release by airway PMNs isolated by DTT protocol but lower when compared to blood-borne neutrophils.
Enriched samples by closed-loop separation demonstrated measurable response to PMA stimulation (as defined by increase in NE activity by PMA stimulation from baseline) for all six samples (p < 0.0001, Table 1 and Supp. Figure S5). Neutrophils isolated by closed-loop separation showed higher baseline NE activity than blood-borne neutrophils, and the NE activity increment by PMA incubation (ΔNE) is smaller than that of blood-borne neutrophils (p < 0.001) (Figure 4A,B). Neutrophils in the airway are recruited by chemoattractants such as interleukin (IL)-8 or N-formyl-methyl-leucyl-phenyl-alanine (fMLP).38,39 It was reported that preactivation of neutrophils could promote expression of cell surface elastase and degranulation without the requirement of further activators such as chemoattractants.40 It can be suggested that low ΔNE of airway-borne neutrophils are indicative of a primed activation state, as a result of migration from bloodstream to the airways.
Table 1.
Qualitative Comparisons of Clinical Samples and NE Increment of Each by PMA Enriched with Closed-Loop and DTT Separation
| patient no. 1 | patient no. 2 | patient no. 3 | patient no. 4 | patient no. 5 | patient no. 6 | |
|---|---|---|---|---|---|---|
| appearance | thick, nonbloody | thin, bloody | thick, bloody | thick, nonbloody | watery, nonbloody | thick, nonbloody |
| NE increment by PMA (C-sep) | + | + | + | + | + | + |
| NE increment by PMA (DTT) | − | + | + | − | − | + |
In contrast, neutrophils isolated by the DTT mucolytic method exhibited even higher baseline NE activity levels than airway neutrophils isolated by closed-loop separation. In addition, only three of the DTT treated samples (n = 6 total) were able to trigger increased NE activity following PMA stimulation (Figure 4). This may be due to the fact that DTT treated samples demonstrated higher NE release before and after the stimulation compared to all other cases, indicating possible chemical disturbance (and artifactual activation) of NE machinery7,8 (Supp. Figure S4A).
Suspensions resulting from both separation methods treated with NE inhibitor (N-(methoxysuccinyl)-Ala-Ala-Pro-Val-chloromethyl ketone), showed significant reductions in NE activity (Figure 4A and Supp. Figure S4B). As expected, airway PMNs separated by closed loop separation and inhibited with NE inhibitor showed comparable NE levels to those of blood-borne neutrophils in the absence of PMA stimulation. These results show that closed-loop separation method is superior to DTT homogenization, in terms of preserving neutrophil functional capacity, with negligible perturbation.
CONCLUSION
In summary, we proposed closed-loop operation of inertial microfluidics to dissociate clinical airway secretions and isolate/enrich immune-related cells for in vitro downstream assays. Recirculation of particle/cell focusing outlet achieved a high concentration factor and recovery ratio while at the same time reducing the volume of output collection sample. A high dilution ratio enabled clearance of the background fluid containing debris and mucin as well as enabling standardization of the dissociation technique regardless of patient-specific airway secretion conditions. Compared to a standard mucolytic dissociation method, closed-loop separation was not only able to consistently recover most of the cellular contents but also minimize chemical disturbances that may affect functional properties of isolated cells. By providing a standardized protocol to isolate and enrich immune cells from clinical airway secretions, this proposed method is expected to widen the in vitro clinical and translational research opportunities in pulmonary diseases, such as ARDS, pneumonia, asthma, cystic fibrosis (CF), and chronic obstructive pulmonary disease (COPD).
METHOD
Device Fabrication
Inertial spiral microfluidic devices were fabricated in polydimethylsiloxane (PDMS) using standard microfabrication soft-lithographic techniques described previously.19 The master mold with specific channel dimensions was designed using SolidWorks software and then fabricated by micromilling machine (Whits Technologies, Singapore) on aluminum for PDMS casting. The PDMS replica was fabricated by molding degassed PDMS (mixed in a 10:1 ratio of base and curing agent, Sylgard 184, Dow Corning Inc.) on the mold and baking in the oven for 1 h at 90 °C. The fluidic access holes were punched inside the device using Uni-Core Puncher (Sigma-Aldrich Co. LLC, SG) and the device was irreversibly bonded to a thick layer of plain PDMS using a plasma machine (Harrick Plasma). The assembled device was finally placed inside an oven at 70 °C for 30 min to further enhance the bonding strength. To efficiently and evenly deliver fluid from the sample tube to four spiral channels, 3D-printed (ProtoLab) guide layer with internal fluidic channel was made, which can be inserted into the PDMS device (Supp. Figure S1A).
Tracheal Secretion Collection from Mechanically Ventilated Patients
Tracheal secretions were obtained from mechanically ventilated patients within the medical intensive care unit of the University of Pittsburgh Medical Center. Tracheal aspirates were collected using a protocol modified from the original methods of Merrit et al.41 to accommodate the standard, adult ventilated patient. Briefly, when tracheal suction was clinically indicated, tracheal secretions were aspirated using a suction catheter (14F/channel, 4.67 mm diameter). The suction ballard was advanced carefully halfway into the endotracheal tube, and 5 mL of 0.9% sterile NS (10 mL prefilled syringe, BD PosiFlush, Beckton Dickinson, Franklin Lakes, New Jersey) was instilled. The ballard was then advanced fully or until resistance was met and secretions aspirated. The tracheal secretions were collected in a sterile sputum container and placed on ice. Samples were deidentified, and sent immediately for processing. Sample collection was approved by the University of Pittsburgh Institutional Review Board (IRB Nos. PRO16060443, PRO10110387).
Tracheal Secretion Sample Preparation and Experimental Setup
All airway secretion samples were mechanically dispersed in PBS before the separation. The 10-fold diluent of each sample was dispersed 10, 50, and 100 times volume of buffer, resulting diluent with 100, 500, and 1000-fold. Fluorescent polystyrene particles (1 wt %) 10 μm (10.3 μm ± 0.4 μm) (Polysciences, Inc.) were diluted in each airway secretion diluents right before being engaged with micro-fluidics. The 1000-fold diluted samples were used to perform Elastase assay comparison to DTT experiments.
For DTT comparison, airway secretion samples were processed in mucolytic protocol described previously.8 Briefly, airway secretion sample was liquefied with 4 times volume of 6.5 mM DTT in PBS. The sample was incubated on a roller at room temperature for 15 min, and 4× volumes of PBS was added. The samples were filtered through a 50-μm strain filter. Supernatant was aspirated after centrifugation at 400g for 10 min and resuspended in buffer solution.
To compare airway PMNs to blood PMNs, fresh human whole blood from healthy donors with sodium heparin as anticoagulant was purchased from Research Blood Component, LLC (Boston, MA). Leukocytes isolated using the selective RBC lysis method were obtained by treating whole blood with RBC lysis buffer (eBioscience Inc.) (1:10) for 10 min, followed by washing and resuspension in buffer solution. Whole blood was spun down at 400g for 10 min and resuspended in sample buffer in 105 count/mL concentration to perform Elastase assay.
To operate the inertial spiral microfluidics device in a closed-loop manner, the peristaltic pump (Cole-Parmer) was connected to microfluidics and the sample tube through silicone tubings (Cole-Parmer). When the sample volume reached the target volume, the operation was stopped. Our platform could not reduce the final volume of suspension below the dead volume of channels and tubing, which results in inevitable loss on recovery. The proposed method is more efficient on reducing from a large volume of diluent (>50 mL) into a microcentrifuge volume (~1 mL). To achieve optimal flow rate of 4 mL/min, the pump was set to rotate in 16 rpm (Supp. Figure S1B).
Immunofluorescence Staining and FACS Analysis
All antibodies were purchased from BD Pharmingen (BD Biosciences). To determine the separation efficiency, initial and resulting suspensions were stained with fluorescein isothiocyanate (FITC)-conjugated mouse antihuman CD66b monoclonal antibody (1:25 v/v) and allophycocyanin (APC)-conjugated mouse antihuman CD45 monoclonal antibody (1:25 v/v) for 30 min at 4 °C in the dark. Both samples before and after separation were analyzed on a BD Accuri C6 flow cytometer (BD Biosciences) to quantify PMNs in each sample. Recovery was analyzed by the ratio of input and separated particle/cell counts.
Neutrophil Elastase Assay
To compare functionality of PMNs separated by DTT homogenization and our method, a commercial neutrophil elastase assay kit (Caymanchem) was used. PMA [50 nM] was added to each resulting suspension and incubated at 37 °C for 2 h. At the end of treatment, each suspension was centrifuged at 1 200 rpm for 10 min. A volume of 10 μL of each supernatant was transferred to a 96-well plate, followed by addition of 90 μL of diluted assay buffer to match the volume of standard wells. A volume of 10 μL of the elastase substrate (Z-Ala-Ala-Ala-Ala 2Rh110) was added and incubated for 1.5 h at 37 °C. Plate was read using a fluorometer (Varioskan plate reader) at an excitation wavelength of 485 nm and an emission of 525 nm. Activity of NE was normalized by the number of PMNs measured by flow cytometry analysis. Statistical comparisons were performed by using nonparametric t test. Statistical significance was considered at p < 0.05.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIAID (Grant R21AI119042) as well as the NIH U24 Sample Sparing assay program (Grant U24-AI118656).
Footnotes
Notes
The authors declare the following competing financial interest(s): The authors have filed a patent application on the technology described here.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.anal-chem.7b00610.
Photograph images of 4-parallel spiral microfluidics with fluidic adaptor and experimental setup for closed-loop operation; photograph and microscopic images of patient-derived airway secretions before and after the closed-loop separation; flow cytometric comparison of resulting suspension by closed-loop separation and mucolytic (DTT) method; comparison of closed-loop and mucolytic (DTT) separated PMNs with blood-borne neutrophils without external stimulation and with neutrophil elastase inhibito; and photograph images of patient airway secretion samples used in NE release functional assays (PDF)
References
- 1.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF, Jr, Wardlaw AJ, Wenzel SE, Greenberger PA. J Allergy Clin Immunol. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Houston N, Stewart N, Smith DS, Bell SC, Champion AC, Reid DW. J Cystic Fibrosis. 2013;12:352–362. doi: 10.1016/j.jcf.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Gernez Y, Tirouvanziam R, Chanez P. Eur Respir J. 2010;35:467–469. doi: 10.1183/09031936.00186109. [DOI] [PubMed] [Google Scholar]
- 4.Mach AJ, Adeyiga OB, Di Carlo D. Lab Chip. 2013;13:1011–1026. doi: 10.1039/c2lc41104k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MKB. J Immunol. 2002;168:1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- 6.Hamid Q, Kelly MM, Linden M, Louis R, Pizzichini MMM, Pizzichini E, Ronchi C, Van Overveld F, Djukanovic R. Eur Respir J. 2002;20:19S–23s. doi: 10.1183/09031936.02.00001902. [DOI] [PubMed] [Google Scholar]
- 7.van Overveld FJ, Demkow U, Gorecka D, Skopinska-Rozewska E, de Backer WA, Zielinski J. J Physiol Pharmacol. 2005;56(Suppl 4):143–154. [PubMed] [Google Scholar]
- 8.Qiu D, Tan WC. J Allergy Clin Immunol. 1999;103:873–876. doi: 10.1016/s0091-6749(99)70432-x. [DOI] [PubMed] [Google Scholar]
- 9.Di Carlo D. Lab Chip. 2009;9:3038–3046. doi: 10.1039/b912547g. [DOI] [PubMed] [Google Scholar]
- 10.Martel JM, Smith KC, Dlamini M, Pletcher K, Yang J, Karabacak M, Haber DA, Kapur R, Toner M. Sci Rep. 2015;5:11300. doi: 10.1038/srep11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Yan S, Yuan D, Alici G, Nguyen NT, Ebrahimi Warkiani M, Li W. Lab Chip. 2016;16:10–34. doi: 10.1039/c5lc01159k. [DOI] [PubMed] [Google Scholar]
- 12.Mach AJ, Di Carlo D. Biotechnol Bioeng. 2010;107:302–311. doi: 10.1002/bit.22833. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Song SH, Jung HI. Lab Chip. 2009;9:939–948. doi: 10.1039/b813952k. [DOI] [PubMed] [Google Scholar]
- 14.Moon HS, Kwon K, Kim SI, Han H, Sohn J, Lee S, Jung HI. Lab Chip. 2011;11:1118–1125. doi: 10.1039/c0lc00345j. [DOI] [PubMed] [Google Scholar]
- 15.Di Carlo D, Irimia D, Tompkins RG, Toner M. Proc Natl Acad Sci U S A. 2007;104:18892–18897. doi: 10.1073/pnas.0704958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS, Lim WT, Han J, Bhagat AA, Lim CT. Sci Rep. 2013;3:1259. doi: 10.1038/srep01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warkiani ME, Khoo BL, Wu L, Tay AK, Bhagat AA, Han J, Lim CT. Nat Protoc. 2016;11:134–148. doi: 10.1038/nprot.2016.003. [DOI] [PubMed] [Google Scholar]
- 18.Hou HW, Bhattacharyya RP, Hung DT, Han J. Lab Chip. 2015;15:2297–2307. doi: 10.1039/c5lc00311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warkiani ME, Tay AK, Guan G, Han J. Sci Rep. 2015;5:11018. doi: 10.1038/srep11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warkiani ME, Wu L, Tay AK, Han J. Annu Rev Biomed Eng. 2015;17:1–34. doi: 10.1146/annurev-bioeng-071114-040818. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Guan G, Hou HW, Bhagat AA, Han J. Anal Chem. 2012;84:9324–9331. doi: 10.1021/ac302085y. [DOI] [PubMed] [Google Scholar]
- 22.Guan G, Wu L, Bhagat AA, Li Z, Chen PC, Chao S, Ong CJ, Han J. Sci Rep. 2013;3:1475. doi: 10.1038/srep01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reece AE, Kaastrup K, Sikes HD, Oakey J. RSC Adv. 2015;5:53857–53864. doi: 10.1039/c5ra10634f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsson O, Oh SS, Antfolk M, Eisenstein M, Laurell T, Soh HT. Anal Chem. 2015;87:8497–8502. doi: 10.1021/acs.analchem.5b01944. [DOI] [PubMed] [Google Scholar]
- 25.Seo J, Lean MH, Kole A. J Chromatogr A. 2007;1162:126–131. doi: 10.1016/j.chroma.2007.05.110. [DOI] [PubMed] [Google Scholar]
- 26.Rubin BK. Transl Respir Med. 2014;2:6. doi: 10.1186/2213-0802-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 28.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 29.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 30.Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. Am J Respir Cell Mol Biol. 2004;31:86–91. doi: 10.1165/rcmb.2003-0345OC. [DOI] [PubMed] [Google Scholar]
- 31.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 32.Saetta M, Turato G, Facchini FM, Corbino L, Lucchini RE, Casoni G, Maestrelli P, Mapp CE, Ciaccia A, Fabbri LM. Am J Respir Crit Care Med. 1997;156:1633–1639. doi: 10.1164/ajrccm.156.5.9701081. [DOI] [PubMed] [Google Scholar]
- 33.Xiang N, Zhang X, Dai Q, Cheng J, Chen K, Ni Z. Lab Chip. 2016;16:2626–2635. doi: 10.1039/c6lc00376a. [DOI] [PubMed] [Google Scholar]
- 34.Janoff A, Scherer J. J Exp Med. 1968;128:1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawabata K, Hagio T, Matsuoka S. Eur J Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 36.Rubin BK. Clin Chest Med. 2016;37:405–408. doi: 10.1016/j.ccm.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Kokot K, Teschner M, Schaefer RM, Heidland A. Miner Electrolyte Metab. 1987;13:133–140. [PubMed] [Google Scholar]
- 38.Keatings VM, Collins PD, Scott DM, Barnes PJ. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 39.Nocker RE, Schoonbrood DF, van de Graaf EA, Hack CE, Lutter R, Jansen HM, Out TA. Int Arch Allergy Immunol. 2004;109:183–191. doi: 10.1159/000237218. [DOI] [PubMed] [Google Scholar]
- 40.Nadel JA. Chest. 2000;117:386S–389S. doi: 10.1378/chest.117.5_suppl_2.386s. [DOI] [PubMed] [Google Scholar]
- 41.Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DK, 3rd, Gluck L. J Clin Invest. 1983;72:656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.