Abstract
Brain-machine interfaces (BMIs) define new ways to interact with our environment and hold great promise for clinical therapies. Motor BMIs, for instance, re-route neural activity to control movements of a new effector and could restore movement to people with paralysis. Increasing experience shows that interfacing with the brain inevitably changes the brain. BMIs engage and depend on a wide array of innate learning mechanisms to produce meaningful behavior. BMIs precisely define the information streams into and out of the brain, but engage wide-spread learning. We take a network perspective and review existing observations of learning in motor BMIs to show that BMIs engage multiple learning mechanisms distributed across neural networks. Recent studies highlight the advantages of BMI for parsing this learning and its underlying neural mechanisms. BMIs therefore provide a powerful tool for studying the neural mechanisms of learning that highlights the critical role of learning in engineered neural therapies.
Introduction
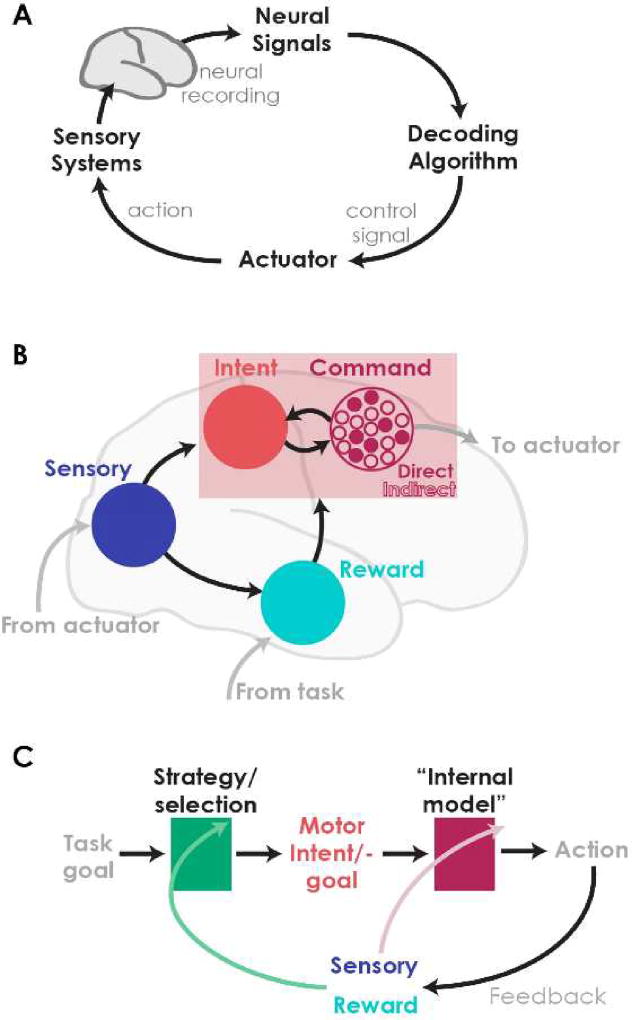
Brain-machine interfaces (BMIs) are behavioral interfaces that fundamentally alter how we control and receive feedback from our environment. BMIs restore, replace and can even augment nervous system functions by reading-out and writing-in neural information. Originally, BMIs were conceived as mimicking existing neural computations without alteration. Increasingly, however, experience shows that successful BMIs depend on the innate flexibility of the brain to perform new computations. Motor BMIs, which repurpose neural activity to control the movement of a device (Fig 1a) and promise to restore movements to people with paralysis, offer a case in point. Rather than “reading out” thoughts about movement, motor BMIs give the brain a new tool it learns to control by constructing a new neural representation. Interfacing with the brain inevitably changes the brain. Just as neural plasticity allows us to learn new abilities, neural plasticity in response to a novel interface yields BMI functionality. Harnessing BMIs’ full clinical potential, therefore, requires understanding BMI-related neural plasticity to avoid reading a book that’s re-writing itself [1].
Figure 1.
(a) Motor brain-machine interfaces (BMIs) map neural activity into a command signal to move an actuator via a “decoder”. Feedback, such as vision of the device movement closes the control loop and facilitates learning. (b) Controlling a motor BMI requires processing feedback (sensory information about movement and reward information from task context) and using this information to guide formation of a motor output (pink box). Motor output is generated through the formation of an intended action and a command. BMIs specify which nodes within this network form the command sent to the actuator (direct versus indirect nodes). (c) Learning in BMI can occur in multiple sites within this network. Existing studies provide evidence for learning specifically changing activity of the command nodes (shifting the mapping from sensory inputs to action outcome; red) and shifts in the selection of motor plans (green).
In addition to clinical benefits, BMIs also offer powerful tools for studying the neuroscience of learning. This is because BMIs support behavior through the precise specification of the relationships between the brain and environment [2–7]. Motor BMIs designate which parts of the brain control movement and how. As a result, the BMI creates a “simpler” motor apparatus that can be observed and manipulated in experiments. We contend, however, that, simplifying the system inputs and outputs does not simplify learning within the system. Instead, like all behaviors, BMIs engage a wide array of innate learning mechanisms [8]. By making explicit the system inputs and outputs, BMIs allow us to resolve the neural computations that drive learning and test how network structure influences learning.
Learning in BMI is well-established, but the underlying neural mechanisms are poorly understood and studies report conflicting results. Here, we revisit observations of learning in BMI that emphasize how BMI learning is distributed across the brain and engages multiple learning mechanisms. We then highlight recent studies that illustrate specific advantages of BMI for dissecting learning in such distributed networks. BMIs take many different forms, with variations in the brain functions being replaced, the neural signals used for control, and the control interface [9]. We focus on motor BMIs where neural activity is used to control movements of a device via a “decoder” (Fig. 1a). We will emphasize BMIs using invasively recorded neural signals, though learning is also critical in non-invasive systems (e.g. [10]). Understanding BMI learning in these systems can help inform our basic understanding of learning mechanisms in the brain, and is likely to be essential for building therapies that interface with an ever-changing brain.
BMIs define learned sensory-motor mappings
Motor BMIs create closed-loop control systems in which sensory and reward information streams can guide action (Fig. 1b). These actions involve both the formulation of a motor plan or intent, and movement execution. In BMI, movement execution is mediated directly by neural activity. BMIs allow specification of the “command” nodes1, the form of this command (e.g. controlling the velocity of a cursor versus the discrete selection of an action), and the mapping from neural activity to the command (the decoder). Feedback about the state of the effector and reward inputs from the environment allows these commands to be goal-directed.
Consider, for example, the information streams when neural activity in the motor cortex is used to drive a cursor on a screen in order to acquire a visually-presented target. Vision provides direct feedback about the relationship between neural activity and cursor movement. Vision also provides the subject information about progress towards task goals, which is supplemented by task-level reward information when the target is reached. Successful BMI control requires a sensory-motor mapping. The user must generate a particular motor command (pattern of activity in the command nodes) based on visual information about the current effector state (cursor position, velocity) and task goals (target position). This feedback can guide both the formation of motor plans and commands.
Many studies demonstrate that the sensory-motor mappings required for goal-directed BMI control are learned. Subjects’ performance can improve with practice in BMI, and these improvements coincide with changes in neural activity [11–17]. This learning is facilitated by the closed-loop nature of BMIs [2] and depends on the presence of feedback [18]. Much like in natural behaviors, the presence of feedback drives neural plasticity and adaptation (see Box 1).
Box 1. How does motor BMI learning relate to learning in natural sensorimotor learning?
BMIs are inherently artificial, raising concern that BMI learning may not share features with learning in more natural systems.
One way in which BMIs are artificial is that they are removed from the natural sensory-motor apparatus [5], and therefore provide different sensory feedback information. The majority of BMIs provide a single form of sensory feedback—most commonly visual in primate and human studies, and auditory in rodent studies. Congruent proprioceptive and tactile feedback is not provided. BMI subjects with intact motor systems will therefore experience conflicting sensory feedback streams. While this has clear impacts on performance and control [9], visual information is highly dominant over proprioceptive information (e.g. [44]), especially in goal-directed visual behaviors. In these cases, vision will therefore drive learning. More broadly, real-world tasks always involve streams of information that are relevant and irrelevant to the task.
In motor BMI, BMIs also differ in their control implementation: how they use neural activity to control effectors compared with natural movement. BMIs differ in the physical properties of the effector and the neural activity (number of neurons, their locations within the brain) driving movement. These differences will inherently change the representations of control and how learning is instantiated within the network. That is, moving the command nodes will shift where within the brain different learning mechanisms occur. This, however, does not mean that learning sensory-motor mappings in BMI must employ fundamentally different mechanisms. The same principles and innate learning mechanisms can be brought to bear on the problem.
Several reviews illustrate this point and highlight how learning patterns in motor BMIs show many striking similarities to natural sensorimotor learning [3–5]. Thus, BMI learning can leverage existing neural mechanisms and circuits for learning.
Moreover, the sensory-motor mapping to be learned is governed by the BMI’s structure. The mapping depends on the selected command nodes, the decoder, and forms of feedback, all of which are under experimenter control. Motor BMIs provide explicit control and knowledge of how neural activity drives behavioral output, effectively “simplifying” the natural sensorimotor apparatus [7]. BMIs therefore create readily manipulated, precisely defined behavioral systems that engage learning [5].
BMIs simplify the motor execution and feedback information streams, but sensory-motor mapping computations are not limited to the feedback and command nodes. Growing research shows that learning to control movements and achieve task goals engages and depends on wide-spread networks. Studies using large-scale ECoG recordings from human subjects revealed engagement of cortical areas well beyond the control nodes, and changes across the cortex with task proficiency [16]. A follow-up study also showed evidence of long-range cortico-cortical communication throughout BMI learning [19]. BMI skill learning also involves, and may require, plasticity in cortico-striatal circuits [18].
Parsing BMI Learning
Mechanisms to learn the sensory-motor mapping
Understanding BMI learning requires parsing how changes distributed across the brain shape the formation of a sensory-motor map. Recent work emphasizes two major alternatives. Neurofeedback proposes learning occurs in the command nodes driven by feedback of their activity. Repurposing existing neural repertoires, in contrast, posits that learning alters how subjects activate the command nodes for a given goal. Each alternative reflects different views on how learning influences motor planning and execution.
The neurofeedback hypothesis states that reward feedback directly conditions neural activity in the command nodes according to behavioral outcomes [2,20]. This hypothesis predicts that learning is specific to the command nodes undergoing conditioning. As a result, according to this view, BMI learning drives the formation of new functional networks that are not fundamentally constrained by existing network structure or neural representations. Several observations are consistent with a neurofeedback model of BMI learning. Subjects can learn to control BMIs even when they impose fundamentally new associations between neural activity and movement. Activity of corticomotor-neuronal cells can be divorced from muscle activity with feedback training [21] and subjects can learn decoders with arbitrary relationships between neural activity and movement [15]. BMI learning is also associated with differential modulation of command nodes relative to nearby nodes [14,18,22–25] and cortico-striatal interactions specific to command nodes [26]. While the precise neural mechanisms of neurofeedback learning are not fully understood (e.g. see [20]), this hypothesis has largely been considered in the context of error-driven adaptation and spike timing dependent plasticity mechanisms and builds on the concept of operant conditioning. Models based on Hebbian plasticity (e.g. [27]) and feedback learning (e.g. [28]) can reproduce many of the above-described experimental observations.
The repertoire repurposing hypothesis states that rather than forming entirely new associations between neural activity and movement commands, BMI learning involves repurposing existing motor repertoires in new ways. That is, subjects associate a new goal with an existing motor program. This hypothesis offers several predictions. First, learning is not necessarily specific to the direct command nodes, and is shaped or constrained by existing motor representations. Second, learning occurs “upstream” of the command nodes, and involves forming new associations between a desired goal and a motor plan or intent. This could involve more explicit strategy-based learning (e.g. [29]) or error-driven adaptation in intent/planning nodes. Consistent with these predictions, Hwang and colleagues demonstrated that neural activity in the command nodes after learning a decoder perturbation was fully consistent with a re-aiming strategy [30]*. Others have similarly noted evidence for re-aiming strategies [14,31] and non-perturbation-specific changes in neural activity [32]. Sadtler and colleagues also suggest learning strategies are constrained by natural motor repertoires. They found that subjects more readily learn BMIs when decoders respect statistical correlations of neural activity present during natural movements [33].
Multiple learning mechanisms, distributed
Experiments to test these BMI learning hypotheses have yielded seemingly inconsistent results. Evidence for re-aiming strategies, for instance, find that learning-related neural changes in the command network are not specific to which nodes directly contribute to movement, in contradiction to predictions of the neurofeedback hypothesis [30]*. While they reflect different views on BMI learning, neurofeedback and repurposing mechanisms are not mutually exclusive. Several BMI studies find evidence for co-occurrence of different learning mechanisms during adaptation to decoder perturbations [14,31]. Learning has been observed at the “intent” level of the circuit (e.g. re-aiming) and as well as at the command level (e.g. modifying the output of perturbed command nodes specifically), wholly consistent with simultaneous engagement of learning at multiple nodes of the network. These different learning mechanisms may also be related to offline sleep-dependent learning and online practice-dependent learning observed in BMI (Box 2).
Box 2. Linking BMI learning to established learning mechanisms—sleep-dependent versus practice-dependent learning.
There is evidence for both online learning (e.g. [15]) and offline improvements in performance after sleep in BMIs [42]**. This suggests the existence of multiple learning mechanisms, some sleep-dependent and others practice-dependent [45,46]. How might existing observations of learning in BMIs relate to offline and online improvements?
Command node neurofeedback and repertoire repurposing learning mechanisms differ in where within the network they occur, and may also recruit fundamentally different forms of learning. These mechanisms may share deep analogies across motor learning. Neurofeedback can be viewed as updating an “internal model” of how a given command drives movement, which in natural motor learning is thought to be a form of practice-dependent implicit procedural learning [45,47]. Repertoire repurposing, on the other hand, is a process of learning the actions to select, which may involve both explicit cognitive strategies (e.g. [29]) and sleep-dependent implicit sequence learning mechanisms (e.g. [45]). Offline improvements after sleep, therefore, may be driven primarily by changes in intent networks while online, practice-dependent performance improvements are tied to local changes within the command nodes.
By defining the command nodes in different ways, BMIs allow us to more closely examine the analogy. We could, for instance, place command nodes in primary motor cortex (M1), and then monitor practice- and sleep-related plasticity across the local command network (both direct and indirect nodes) and areas that project to those command nodes, such as the posterior parietal cortex (PPC). The above hypotheses predict that changes in PPC intent networks will be sleep-dependent (e.g. tied to occurrence of sleep spindles [42]), while local changes in M1 command nodes would only relate to practice. Interpreting changes in the command nodes will require distinguishing changes due to local learning and those driven by changing communication with the intent networks [39]**. BMIs are ideally suited to tease apart different forms of learning in the command networks.
Since multiple, distinct learning mechanisms can co-occur, determining which mechanism is driving neural plasticity is the source of substantial interpretational difficulty. Consideration of the full network engaged in BMI learning discussed above highlights that the key difference in these proposed BMI learning mechanisms is where learning takes place (Fig. 1c). According to the neurofeedback hypothesis, feedback drives learning in the command nodes in order to generate desired movements. This is akin to learning driving updates to an “internal model”—a mapping from motor commands to their resulting actions—in motor control [34,35]. In repertoire repurposing, learning occurs “upstream” of the command nodes. Rather than learning to generate new command signals, feedback drives changes in how subjects select commands for a given task goal.
BMIs define key nodes in the learning network, providing a powerful tool to probe learning. By specifying the input and output, you can change the relationship between the BMI and existing networks, and hence alter the learning. This can be seen by comparing a BMI where the command nodes are located in motor cortex with a BMI where the command nodes are located in the posterior parietal cortex. Conditioning at the command nodes and “intent”-level repertoire adaptation (Fig. 1c) may occur in both kinds of BMIs, but in different ways. Shifting the location of the command nodes may lead to changes in “intent”-level learning that inputs to the command nodes. How BMIs are constructed—how we build this new network—will influence learning. Taking a network perspective, therefore, reveals how the BMI approach can help resolve which learning mechanisms are contributing to which changes in activity.
Manipulations to probe BMI learning
BMIs are partially removed from the natural sensorimotor apparatus [5]. While BMIs are therefore artificial (Box 1), they offer advantages for learning because they can be manipulated in ways the natural system cannot.
Feedback manipulations
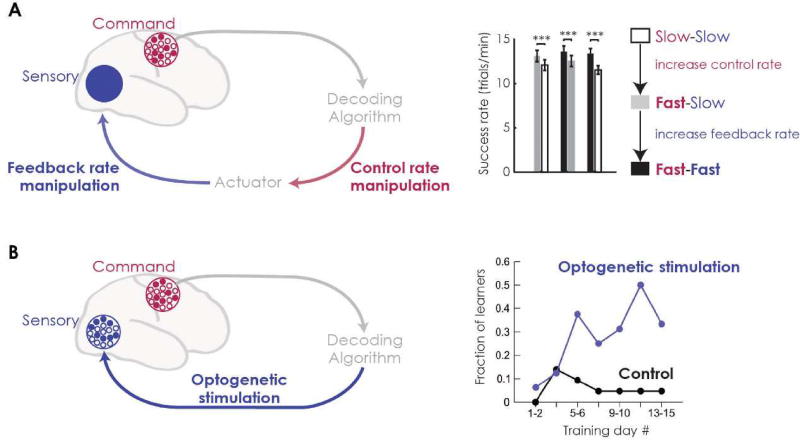
Sensory and reward inputs provide the feedback that is essential for learning (Fig 1b, c). In the majority of motor BMIs, where command nodes are located in motor-related areas, sensory and reward information is first processed by other neural circuits. Error-driven learning, then, relies on communication between circuits associated with processing the reward/sensory information and those forming the command. Understanding how reward guides learning, and how sensory networks, motor networks, and the connections between them change with learning is a critical challenge. Recent work shows how manipulations using the BMI approach can provide new insight into how these sources of information affect the motor networks and guide learning (Fig. 2).
Figure 2.
Recent BMI studies probe learning and control by manipulating the information streams in BMI. (a) Shanechi et al. [36]** used BMIs to manipulate the rates of the sensory-motor loop (left). BMIs allowed them to independently manipulate both the rate at which motor commands moved the actuator (“control rate”, red) and the rate of feedback (blue). They showed that BMI performance depends on both rates separately (right). Performance improved with faster control rates, even when subjects received slower feedback. Increasing the feedback rate then further improved performance. These results suggest that BMI may involve multiple control strategies—both predictive feed-forward control and feedback-based control. (b) Prsa et al. [25]** developed a BMI where decoder output drove optogenetic stimulation (channelrhodopsin, ChR2). This creates a system where both the command and feedback nodes can be precisely defined (left). They show that, with training, mice can learn to modulate command node activity to achieve rewards with optogenetic stimulation as their only form of sensory feedback (right). Control mice lacking ChR2 were unable to learn the task, demonstrating the necessity of this sensory feedback for learning the BMI task.
A recent study by Shanechi and colleagues used BMIs to probe how the rate of feedback influences performance in BMIs [36]*. The authors used a rate-independent decoder, which allowed them to isolate the influence of both control and feedback rates on performance (Fig. 2a). They show that both feedback and control rates influence performance, suggestive of combined feedback and feed-forward control strategies. BMIs have also been used to explore how the information available in visual feedback (e.g. seeing an end-point or a full arm) influences control [37]. These studies show how the detailed control of the visual feedback allowed by BMIs can help understand how sensory errors drive learning.
BMIs can also control the site of feedback where information is provided to guide learning. A recent study by Prsa and colleagues showed that feedback via optogenetic neural stimulation is sufficient to drive BMI learning [25]**. This demonstrates a BMI where sensory feedback is delivered to specific, experimenter-defined nodes within the brain, paired with experimenter- defined command nodes (Fig. 2b). Emerging work similarly explores delivering reward information via direct optogenetic stimulation [38]. By defining the network precisely, these BMIs can allow us to study the neural mechanisms of feedback-driven learning. Specifically, does learning depend on anatomical connectivity between feedback and command nodes, and does learning drive the formation of new functional connections that communicate feedback to the command nodes?
These studies illustrate how, because of their artificial nature, BMIs allow full control and knowledge of feedback thereby providing powerful new ways to study how feedback drives learning.
Command manipulations
The objective of learning is to shape neural networks to produce a particular command signal for a given behavioral goal. This can be driven by changes distributed across the sensorimotor network both at the command nodes themselves, as well as in the inputs to the command nodes (Fig. 1b, c). Insights into learning require understanding both changes in the command nodes, and their interactions with other parts of the network. By fully defining the control system, BMIs provide a lens through which to interpret learning-related changes in both the command nodes and the full network.
Experimenter-defined command nodes and neural activity-behavior mappings have clear advantages when studying learning (recently reviewed in [5,7]). BMIs can be used to study neural adaptation in response to decoder perturbations (e.g. [14,30–33]). Knowledge about the decoder can be used to interpret learning-related changes in command nodes [14,17,30–33,35,39]. Moreover, by defining the command nodes, BMI studies can explore the specificity of learning changes within command areas (direct versus indirect nodes) [22–25,30,40]. Functional connectivity measures like spike-field coherence [41] between command nodes and other parts of the brain can allow identification of functional networks participating in learning (e.g. [19,26]).
Gulati and colleagues recently used BMIs to explore the specificity of sleep-dependent consolidation [42]**. In this study, they first obtain evidence for sleep-dependent offline learning mechanisms in BMI that are linked to slow-wave sleep periods. Since the BMI allowed them to define the command nodes, they then showed that neural coherence with slow-wave activity was specific to neurons directly contributing to behavioral output. This suggests sleep-dependent consolidation may be critical for shaping activity specifically in the command nodes. Moreover, it points to the task-specificity of sleep consolidation learning mechanisms and paves the way to further identify the networks participating in this learning.
A hallmark of both BMI and motor skill learning more generally is gradual reduction in motor circuit variability as behavior stabilizes (e.g. [15,24,43]). Recent BMI work suggests this reduction in variability may be driven by multiple learning mechanisms, one acting locally at the level of each command node and another that shapes the command network (e.g. through changes in network inputs; Fig. 1c). Athayle and colleagues used factor analysis to analyze how the command network changes with learning [39]**. Factor analysis allowed them to separate neural variability into components unique to each neuron (individual nodes) and those shared across the population of neurons contributing to movement. They found that private variability was initially high but reduced with learning, while shared variability increased with learning. Using knowledge of the decoder, they further showed that shared variability changes contributed most to behavioral improvements. This experiment nicely demonstrates the BMI advantage when adopting a network perspective, discussed above in the context of learning sensory-motor mappings. Command manipulations that alter how the command population relates to the rest of the network—inputs to the command nodes coming from intent, feedback and reward nodes—can provide critical insights into the neural mechanisms of each form of learning.
Conclusions
BMIs create novel input-output mappings and require learning to produce meaningful behavior. As BMIs precisely define both input and output streams in the network, they provide new ways to probe learning within these networks. The result is a powerful tool for studying the neural mechanisms of learning that highlights the role of learning in engineered neural therapies.
Recent work demonstrates the many tools for studying learning within BMI, focusing on manipulations of the output or input. Future work aimed towards monitoring learning across larger networks will allow further parsing of learning mechanisms and neural implementations. Linking observed learning to well-studied learning mechanisms like sleep-dependent versus practice dependent learning can provide further insight and grounding for studies of BMI learning.
Since BMIs tap into our brains innate ability to learn, understanding learning in BMIs will ultimately advance clinical applications of this technology. In the search for effective treatments, we may find that interfaces which harness learning can improve the patient experience compared with those which simply accommodate learning.
Highlights.
Brain-machine interfaces (BMIs) engage an array of innate learning mechanisms
BMIs allow definition and manipulation of learning networks
Parsing learning across the network can resolve mechanisms of BMI learning
Acknowledgments
This work was supported by the L’Oreal USA for Women in Science program (A.L.O.), and NIH R01-EY024067 and DARPA contract N66001-11-1-4205 as part of the RENET program (BP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We use the term nodes for generality, emphasizing the network/circuit structure in the brain without specifying the resolution of computations. This also allows more general consideration of BMIs that operate at different measurement resolutions (e.g. controlled with action potentials of single units versus controlled with electrocorticographic activity).
References
- 1.Shenoy K, Carmena J. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron. 2014;84:665–680. doi: 10.1016/j.neuron.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Fetz EE. Volitional control of neural activity: implications for brain-computer interfaces. J. Physiol. 2007;579:571–579. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green AM, Kalaska JF. Learning to move machines with the mind. Trends Neurosci. 2011;34:61–75. doi: 10.1016/j.tins.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Jackson A, Fetz EE. Interfacing with the computational brain. IEEE Trans. Neural Syst. Rehabil. Eng. 2011;19:534–541. doi: 10.1109/TNSRE.2011.2158586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orsborn AL, Carmena JM. Creating new functional circuits for action via brain-machine interfaces. Front. Comput. Neurosci. 2013 doi: 10.3389/fncom.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wander JD, Rao RPN. Brain-computer interfaces: A powerful tool for scientific inquiry [Internet] Curr. Opin. Neurobiol. 2014;25:70–75. doi: 10.1016/j.conb.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golub MD, Chase SM, Batista AP, Yu BM. Brain-computer interfaces for dissecting cognitive processes underlying sensorimotor control. Curr. Opin. Neurobiol. 2016;37:53–58. doi: 10.1016/j.conb.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D. Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron. 2017;93:480–490. doi: 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Bensmaia SJ, Miller LE. Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nat. Rev. Neurosci. 2014;15:313–325. doi: 10.1038/nrn3724. [DOI] [PubMed] [Google Scholar]
- 10.Wolpaw JR. Brain-computer interfaces as new brain output pathways. J. Physiol. 2007;579:613–619. doi: 10.1113/jphysiol.2006.125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gage GJ, Ludwig KA, Otto KJ, Ionides EL, Kipke DR. Naive coadaptive cortical control. J. Neural Eng. 2005;2:52–63. doi: 10.1088/1741-2560/2/2/006. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DM, Helms-Tilery SI, Schwartz AB. Direct cortical control of 3D neuroprostheticdevices. Science. 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 13.Carmena JM, Lebedev MA, Crist RE, O’Doherty JE, Santucci DM, Dimitrov DF, Patil PG, Henriquez CS, Nicolelis MAL. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003;1:193–208. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarosiewicz B, Chase SM, Fraser GW, Velliste M, Kass RE, Schwartz AB. Functional network reorganization during learning in a brain-computer interface paradigm. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19486–91. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wander JD, Blakely T, Miller KJ, Weaver KE, Johnson LA, Olson JD, Fetz EE, Rao RPN, Ojemann JG. Distributed cortical adaptation during learning of a brain-computer interface task. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10818–23. doi: 10.1073/pnas.1221127110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orsborn AL, Moorman HG, Overduin SA, Shanechi MM, Dimitrov DF, Carmena JM. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron. 2014;82:1380–1393. doi: 10.1016/j.neuron.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Koralek AC, Jin X, Long JD, II, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012;483:331–335. doi: 10.1038/nature10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wander JD, Sarma D, Johnson LA, Fetz EE, Rao RPN, Ojemann JG, Darvas F. Cortico-Cortical Interactions during Acquisition and Use of a Neuroprosthetic Skill. PLoS Comput. Biol. 2016;12:1–20. doi: 10.1371/journal.pcbi.1004931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, Weiskopf N, Blefari ML, Rana M, Oblak E, et al. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 2016;18:86–100. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- 21.Fetz EE, Finocchio D V. Operant Conditioning of Specific Patterns of Neural and Muscular Activity. Science. 1971;174(4007):431–435. doi: 10.1126/science.174.4007.431. [DOI] [PubMed] [Google Scholar]
- 22.Ganguly K, Dimitrov DF, Wallis JD, Carmena JM. Reversible large-scale modification of cortical networks during neuroprosthetic control. Nat. Neurosci. 2011;14:662–7. doi: 10.1038/nn.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clancy KB, Koralek AC, Costa RM, Feldman DE, Carmena JM. Volitional modulation of optically recorded calcium signals during neuroprosthetic learning. Nat. Neurosci. 2014;17:807–809. doi: 10.1038/nn.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arduin P, Fre Y, Shulz DE. “Master” Neurons Induced by Operant Conditioning in Rat Motor Cortex during a Brain-Machine Interface Task. 2013;33:8308–8320. doi: 10.1523/JNEUROSCI.2744-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **25.Prsa M, Galinñanes GL, Huber D. Rapid Integration of Artificial Sensory Feedback during Operant Conditioning of Motor Cortex Neurons. Neuron. 2017;93:929–939.e6. doi: 10.1016/j.neuron.2017.01.023. This study presents an all-optical BMI system where command signals were derived from 2-photon calcium imaging and sensory feedback was delivered through optogenetic stimulation. Mice learned to control activity in a small number of neurons in motor cortex to a specified target activity level to achieve a reward. Continuous feedback of motor cortex activity was delivered to sensory cortex via optogenetic stimulation. Subjects were able to learn to control this interface, relying on this continuous feedback. Such techniques offer the opportunity to precisely define both the command and input nodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koralek A, Costa R, Carmena J. Temporally Precise Cell-Specific Coherence Develops in Corticostriatal Networks during Learning. Neuron. 2013;79:865–872. doi: 10.1016/j.neuron.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 27.Legenstein R, Chase SM, Schwartz AB, Maass W. A Reward-Modulated Hebbian Learning Rule Can Explain Experimentally Observed Network Reorganization in a Brain Control Task. J. Neurosci. 2010;30:8400–8410. doi: 10.1523/JNEUROSCI.4284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Héliot R, Ganguly K, Jimenez J, Carmena JM. Learning in closed-loop brainmachine interfaces: Modeling and experimental validation. IEEE Trans. Syst. Man Cybern. Part B Cybern. 2010;40:1387–1397. doi: 10.1109/TSMCB.2009.2036931. [DOI] [PubMed] [Google Scholar]
- 29.McDougle SD, Ivry RB, Taylor JA. Taking Aim at the Cognitive Side of Learning in Sensorimotor Adaptation Tasks. Trends Cogn. Sci. 2016;20:535–544. doi: 10.1016/j.tics.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Hwang EJ, Bailey PM, Andersen RA. Volitional control of neural activity relies on the natural motor repertoire. Curr. Biol. 2013;23:353–361. doi: 10.1016/j.cub.2013.01.027. This study explores BMI learning to test predictions of the command node conditioning and repertoire adaptation hypotheses. Monkeys were trained to use a BMI where activity from a sub-set of recorded neurons in posterior parietal cortex controlled the selection of a discrete action selection. Subjects were trained with a decoder based on neural representations of arm movement, and then learned decoders that imposed novel sensory-motor mappings. Learning-related changes in PPC activity were not specific to the direct command nodes, conflicting with the command node conditioning hypothesis. By cleverly designing the perturbed decoders, they instead find evidence that subjects learned through a re-aiming strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chase SM, Kass RE, Schwartz a. B: Behavioral and neural correlates of visuomotor adaptation observed through a brain-computer interface in primary motor cortex. J. Neurophysiol. 2012;108:624–644. doi: 10.1152/jn.00371.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armenta Salas M, Helms Tillery SI. Uniform and Non-uniform Perturbations in Brain-Machine Interface Task Elicit Similar Neural Strategies. Front. Syst. Neurosci. 2016;10:1–12. doi: 10.3389/fnsys.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, Yu BM, Batista AP. Neural constraints on learning. Nature. 2014;512:423–426. doi: 10.1038/nature13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat. Rev. Neurosci. 2011;12:739–51. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 35.Golub MD, Yu BM, Chase SM, Golub MD, Yu BM, Chase SM. Internal models for interpreting neural population activity during sensorimotor control. 2015 doi: 10.7554/eLife.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Shanechi MM, Orsborn AL, Moorman HG, Gowda S, Dangi S, Carmena JM. Rapid control and feedback rates enhance neuroprosthetic control. Nat. Commun. 2017;8:13825. doi: 10.1038/ncomms13825. The authors used a rate-independent point process filter decoder to dissect the importance of sensorimotor rates in BMI. Making the decoder rate-independent allowed them to independently manipulate the rate of visual feedback and the rate of control without changing the decoder. Their results show that both control and feedback rates influence BMI performance, suggesting roles for both feed-forward (“internal model”-based) and feedback control strategies. This study highlights the precision of manipulations available in BMI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moorman HG, Gowda S, Carmena JM. Control of redundant kinematic degrees of freedom in a closed-loop brain-machine interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2016;4320:1–1. doi: 10.1109/TNSRE.2016.2593696. [DOI] [PubMed] [Google Scholar]
- 38.Athalye VR, Santos FJ, Carmena JM, Costa RM. Closed-loop pairing of motor cortex activity and phasic VTA activation reinforces specific spatiotemporal activity patterns. Neuroscience Meeting Planner. Program Number 334.06. [Google Scholar]
- **39.Athalye VR, Ganguly K, Costa RM, Carmena JM. Emergence of Coordinated Neural Dynamics Underlies Neuroprosthetic Learning and Skillful Control. Neuron. 2017;93:955–970.e5. doi: 10.1016/j.neuron.2017.01.016. This study examines changes in population dynamics during long-term (weeks) BMI learning. They use factor analysis to explore whether task proficiency is related to learning at the level of individual command neurons (changes in “private” variability) or to changes shared across the full command network (changes in “shared” variability) putatively reflecting changes in common input to the command nodes. Their results show that both shared and private variability change with learning, and highlight the importance of shared variability for behavioral refinement. This study provides further evidence for multiple learning mechanisms in BMI, highlight the power of BMI to resolve such co-occurring learning mechanisms. [DOI] [PubMed] [Google Scholar]
- 40.So K, Koralek AC, Ganguly K, Gastpar MC, Carmena JM. Assessing functional connectivity of neural ensembles using directed information. J. Neural Eng. 2012;9:26004. doi: 10.1088/1741-2560/9/2/026004. [DOI] [PubMed] [Google Scholar]
- 41.Wong YT, Fabiszak MM, Novikov Y, Daw ND, Pesaran B. Coherent neuronal ensembles are rapidly recruited when making a look-reach decision. Nat. Neurosci. 2016;19:327–334. doi: 10.1038/nn.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Gulati T, Ramanathan DS, Wong CC, Ganguly K. Reactivation of emergent task-related ensembles during slow-wave sleep after neuroprosthetic learning. Nat. Neurosci. 2014;17:1107–1113. doi: 10.1038/nn.3759. Rodents were trained to control a BMI with firing of small populations of neurons recorded from motor cortex. The authors monitored motor cortex activity (both direct and indirect command nodes) in an initial practice session, during subsequent sleep, and a post-sleep practice session. They show that BMI learning involves both online and offline performance improvements, and that offline performance improvements are sleep-dependent. They then show that sleep consolidation learning—measured through coherence with slow-wave activity—was specific to direct command nodes. This demonstrates how BMIs can be used to identify functional networks participating in learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- 44.Rock I, Victor J. Vision and touch: an experimentally created conflict between the two senses. Science. 1964;143:594–596. doi: 10.1126/science.143.3606.594. [DOI] [PubMed] [Google Scholar]
- 45.Stickgold R. Sleep-dependent memory consolidation [Internet] Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 46.Dayan E, Cohen LG. Neuroplasticity Subserving Motor Skill Learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krakauer JW, Mazzoni P. Human sensorimotor learning: Adaptation, skill, and beyond. Curr. Opin. Neurobiol. 2011;21:636–644. doi: 10.1016/j.conb.2011.06.012. [DOI] [PubMed] [Google Scholar]