Abstract
Bronchi are exposed daily to irritants, microbes and allergens as well as extremes of temperature and acid. The airway mucosal epithelium plays a pivotal role as a sentinel, releasing alarmins when danger is encountered. To maintain homeostasis, an elaborate counter-regulatory network of signals and cellular effector mechanisms are needed. Specialized pro-resolving mediators (SPMs) are chemical mediators that enact resolution programs in response to injury, infection or allergy. SPMs are enzymatically derived from essential polyunsaturated fatty acids with potent cell-type specific immunoresolvent properties. SPMs signal by engaging cell-based receptors to turn off acute inflammatory responses and restore tissue homeostasis. Several common lung diseases involving the airways, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF), are characterized by unresolved bronchial inflammation. In preclinical murine models of lung disease, SPMs carry potent bronchoprotective actions. Here, we review cellular and molecular effects for SPM-initiated catabasis in the lung and their human translation.
Keywords: Lipoxins, Resolvins, Protectins, Maresins, Catabasis, Efferocytosis, Lung, Leukocytes, Airway, Mucosa
1. Introduction
Inflammation is the body’s response to injury or infection and manifests clinically as fever and in the lung as cough, sputum production, dyspnea and edema. Biologically, the initiation of acute inflammation results from a highly coordinated network of cellular and molecular events. Pro-inflammatory cytokines and chemokines and eicosanoids including leukotrienes and prostaglandins create a beacon of chemoattractants that results in leukocyte trafficking to sites of lung infection or injury. Endothelial and epithelial barriers become compromised by the inflammatory response creating tissue edema and purulent exudate (expectorated as sputum). Recruited granulocytes and lymphocytes then augment innate tissue-resident leukocytes and macrophages to contain and rid the body of the insult or invading pathogen. Initiating acute inflammation is vital for host protection and survival with many examples of immunosuppression increasing susceptibility to excess morbidity and mortality from infection. Equally important to health is the timely resolution of acute lung inflammation.
To counter the complexity and amplitude of pro-phlogistic mechanisms there exists endogenous resolution programs that are spatio-temporally regulated in the lung. Resolution is an active process designed to restore host tissues to a baseline non-inflamed state, a process termed catabasis. Inflammation resolution is orchestrated by several classes of mediators, including peptides, gases and lipids. Of particular relevance to this review is a superfamily of lipid mediators that are enzymatically-derived from dietary essential polyunsaturated fatty acids (PUFA). These specialized pro-resolving mediators (SPMs) include the arachidonic acid-derived lipoxins and the omega-3 fatty acid-derived resolvins, protectins, and maresins. (reviewed in (Serhan, 2014) and in Chapter XX/Serhan review for this special article). Each of the SPMs are stereoselective with structure activity relationships consistent with agonist properties at cognate receptors. During acute inflammation, lipid mediator class switching occurs as PUFA metabolism switches from pro-inflammatory mediators (e.g., prostaglandins, leukotrienes) to pro-resolving mediators (i.e., SPMs) (Levy et al., 2001). SPMs have cell-type specific and potent immunoresolvent actions to quench pro-inflammatory cytokine production by airway epithelial cells, increase epithelial production of anti-microbial peptides, halt leukocyte trafficking, and promote clearance of the inflammatory leukocytes through natural killer cell-mediated leukocyte apoptosis and macrophage phagocytosis of apoptotic leukocytes (Levy and Serhan, 2014). Thus, in health, SPMs promote the timely resolution of inflammation.
Chronic inflammation results from a failure of the body’s resolution pathways to adequately turn off acute inflammatory responses and switch on mechanisms to restore homeostasis. Chronic unresolved inflammation underlies the pathophysiology of several common human lung diseases including asthma, chronic obstructive pulmonary syndrome (COPD), and cystic fibrosis (CF). Active research in humans and pre-clinical animal models of these diseases has uncovered a deficiency of SPMs and their tissue protective pro-resolving actions. Here, we review SPMs molecular, cellular, and biochemical bronchoprotective actions in response to acute inflammation of injury, infection and allergy with human translation in health and, when defective, in airway diseases.
2. SPM Bronchial Epithelial Actions
The airway mucosa is a first line of host defense against inhaled irritants, allergens, and pathogens. The epithelium provides a physical barrier against inhaled pathogens and toxins and initiates host immune responses through production of alarmins, inflammatory chemokines and cytokines that signal to cellular members of the innate and adaptive immune system. Epithelial cells are targets for regulation by SPMs that act to attenuate pro-inflammatory responses and promote epithelial restitution. In this section, we review SPM receptor expression on bronchial mucosal epithelial cells and SPM effects on the epithelium to resolve injury and promote host defense.
2.1. SPM Receptor Expression in the Human Airway
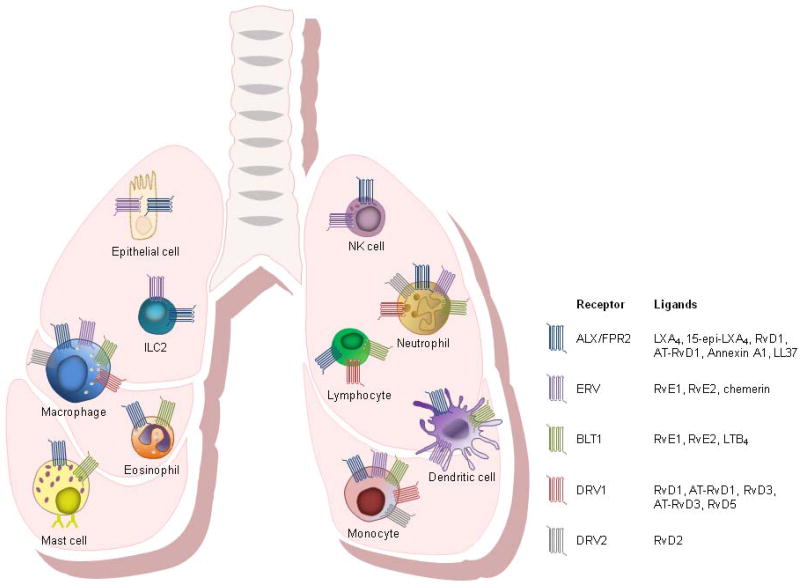
The pro-resolving and anti-inflammatory bioactions of SPMs stem from their signaling as agonists at specific receptors. To date, the molecular identify of five SPM receptors have been elucidated (Fig. 1).
Fig. 1. Specialized Pro-Resolving Mediator Receptor Expression on Airway Epithelial Cells and Leukocytes.
SPM receptors are expressed on human and mouse airway epithelial cells as well as other cells directly related to inflammation at mucosal epithelial borders, including neutrophils, eosinophils, mast cells, monocytes, macrophages, lymphocytes, dendritic cells, innate lymphoid cells (ILCs), and natural killer (NK) cells.
2.1.1 ALX/FPR2
The lipoxin A4/formyl peptide receptor 2 (ALX/FPR2) is a high affinity receptor for lipoxin A4 (LXA4) and a number of additional SPMs including 15-epi-LXA4, resolvin D1 (RvD1) and aspirin-triggered-resolvin D1 (AT-RvD1) (Chiang et al., 2006; Fiore et al., 1994; Krishnamoorthy et al., 2012; Krishnamoorthy et al., 2010; Perretti et al., 2002). LXA4 binding to ALX/FPR2 is stereoselective, specific, and reversible with a Kd of ~0.5 nM (Fiore et al., 1994; Fiore et al., 1992). ALX/FPR2 receptors can also engage and transmit signals from non-lipid ligands including annexin A1 (Chiang et al., 2006) and cathelicidin/LL-37 (Wan et al., 2011). Ligand recognition sites for lipids and peptides differ on ALX/FPR2 receptors and the ligands can trigger distinct downstream events that dramatically change the signaling properties of the receptor (Cooray et al., 2013). For example, 15-epi-LXA4 is an allosteric inhibitor of serum amyloid A at ALX/FPR2 receptors and decreases cytokine production from airway epithelial cells (Bozinovski et al., 2012).
ALX/FPR2 is expressed on human and mouse airway epithelial cells as well as other cells directly related to inflammation at mucosal epithelial borders, including neutrophils, eosinophils, mast cells, monocytes, macrophages, lymphocytes, dendritic cells, innate lymphoid cells (ILCs), and natural killer (NK) cells (Barnig et al., 2013; Bonnans et al., 2006; Chiang et al., 2006; Fiore et al., 1994; Hua et al., 2014; Maddox et al., 1997; Miyazaki et al., 2014). ALX/FPR2 cellular expression is regulated locally by cytokines, transcription factors, and epigenetic mechanisms. LXA4 itself binds to the ALX/FPR2 promoter and increases ALX/FPR2 expression in a positive feedback loop (Simiele et al., 2012). ALX/FPR2 expression is altered in several diseases of chronic inflammation. In severe asthma, ALX/FRP2 expression on granulocytes is decreased (Planaguma et al., 2008). In COPD lungs, epithelial ALX/FPR2 expression is increased in proximity to extrahepatic, submucosal serum amyloid A, and plasma pro-inflammatory ALX/FPR2 ligand serum amyloid A is produced in approximately 2-log order higher amounts than pro-resolving LXA4, creating an imbalance towards a more pro-inflammatory state in COPD despite increased ALX/FPR2 expression (Bozinovski et al., 2012).
2.1.2 ERV
The E-series resolvin (ERV) receptor (also known as chemokine receptor-like 1 (CMKLR1) and chemerin receptor 23 (ChemR23)) is a high affinity receptor for resolvin E1 (RvE1) and likely resolvin E2 (RvE2) (Oh et al., 2012). Like ALX/FPR2, ERV can also engage non-lipid ligands, in particular chemerin, a chemoattractant peptide (Wittamer et al., 2003). ERV is expressed in the lung on airway epithelial cells and leukocytes, in particular those of the innate immune system, including neutrophils, monocytes, macrophages, dendritic cells, ILCs, and NK cells (Barnig et al., 2013; Campbell et al., 2007; Cash et al., 2013; Cash et al., 2008; Du and Leung, 2009; Herova et al., 2015; Parolini et al., 2007; Samson et al., 1998). ERV expression, in particular on NK cells, is highly regulated by cytokine expression in early phases of inflammation (Parolini et al., 2007). ERV signaling pathways may play an important role in protection against viral pathogens, as ERV knockout mice are particularly susceptible to viral respiratory pathogens with compromised viral clearance, increased lung leukocyte infiltration, compromised lung function, and increased mortality (Bondue et al., 2011).
2.1.3 BLT1
The leukotriene B4 receptor 1 (BLT1) is expressed on inflammatory leukocytes including neutrophils, eosinophils, monocytes, macrophages, mast cells, dendritic cells, and lymphocytes (Yokomizo et al., 1997). Both RvE1 and RvE2 engage the BLT1 receptor as antagonists, competing with leukotriene B4 (LTB4) to counterregulate neutrophil chemotaxis, calcium mobilization, and NF-κB activation (Arita et al., 2007a). RvE1 blocks LTB4 binding at BLT1 to promote apoptosis of neutrophils and macrophage efferocytosis (El Kebir et al., 2012).
2.1.4 DRV1 and DRV2
The RvD1 receptor (DRV1; formerly GPR32) engages a number of activating lipid ligands including RvD1, AT-RvD1, RvD3, AT-RvD3, and RvD5 (Chiang et al., 2012; Dalli et al., 2013; Krishnamoorthy et al., 2012; Krishnamoorthy et al., 2010; Sun et al., 2007). DRV1 is expressed on human neutrophils, lymphocytes, macrophages, monocytes, and vascular tissues (Krishnamoorthy et al., 2010). Of note, RvD1 binds and engages DRV1 during periods of homeostasis whereas RvD1 interacts and signals through ALX/FPR2 during periods of resolving inflammation highlighting that SPMs can engage different receptors on different cell types in different physiologic states to exert spatiotemporally distinct effects.
The RvD2 receptor (DRV2; formerly GPR18) engages RvD2 to promote tissue resolution in a self-limited murine model of acute inflammation by promoting macrophage efferocytosis, enhancing phagocytosis of bacteria, and preventing neutrophil transmigration (Chiang et al., 2015). DRV2 is expressed mouse and human neutrophils, monocytes, and macrophages (Chiang et al., 2015).
2.1.5 Other SPM Receptors
Pharmacological structure activity relationships support that protectin D1 (PD1) and maresin 1 (MaR1) also exert their effects via receptor-dependent signaling mechanisms. PD1 binds sites on epithelial cells and neutrophils that are distinct from LXA4 and RvE1 (Arita et al., 2007a; Marcheselli et al., 2010) and MaR1 can block the transient receptor potential V1 signaling to reduce neuropathic inflammation in mice (Serhan et al., 2012). The molecular identify of the receptors through which PD1 and MaR1 signal are still to be determined.
2.2. Epithelial Inflammation
The airway mucosal epithelium is one of the first points of host contact for invading pathogens and inhaled irritants and provides a physical barrier against such insults. The epithelial mucosa also plays a major role in initiating the inflammatory host immune response by producing alarmins, chemokines and cytokines that attract leukocytes to the site of epithelial injury or inflammation. Epithelial cells are also equipped with surface receptors for SPMs (i.e. ALX/FPR2 and ERV), the expression of which can be regulated by cytokines, in particular IL-13 and IFN-γ (Gronert et al., 1998). SPMs can signal through receptors on epithelial cells to deactivate inflammatory pathways and activate pro-resolution pathways. LXA4 inhibits epithelial cell interactions with neutrophils and also inhibits the trans-epithelial migration of neutrophils (Bonnans et al., 2006; Colgan et al., 1993; Serhan et al., 1995). Of interest, the inhibition of 15-epi-LXA4 production by a Pseudomonas aeruginosa-derived epoxide hydrolase cif in a murine model of cystic fibrosis (CF) promotes trans-epithelial migration of neutrophils (Flitter et al., 2017). LXA4 treatment also protects against P. aeruginosa invasion in CF bronchial epithelial cells by preventing tight junction disruption (Higgins et al., 2016). In acute exacerbations of COPD, there is an imbalance in levels of serum amyloid A and LXA4. Human bronchial epithelial cells exposed to serum amyloid A release inflammatory mediators, notably IL-8, a neutrophil chemo-attractant (Bozinovski et al., 2012). LXA4 interacts with ALX/FPR2 to allosterically inhibit serum amyloid A-mediated IL-8 production by epithelial cells (Bozinovski et al., 2012). In a murine model, 15-epi-LXA4 opposes the actions of serum amyloid A at ALX/FPR2 and significantly blocks epithelial cell-derived chemokines and reduces trans-epithelial cell migration of neutrophils (Bozinovski et al., 2012). Like LXA4, RvD1 (Eickmeier et al., 2013; Wang et al., 2011), RvE1 (Campbell et al., 2007; Seki et al., 2010), and MaR1 (Krishnamoorthy et al., 2014) can also inhibit trans-epithelial migration of neutrophils in murine models of lung infection and injury. In addition to direct effects on inhibiting leukocyte transmigration and inhibiting leukocyte chemoattractant production, SPMs can also exert effects that have downstream effects on inflammation. In particular, RvD1 and AT-RvD1 downregulate the NF-κB signaling pathway in bronchial epithelial cells (de Oliveira et al., 2015; Hsiao et al., 2014) that can have significant effects on modulating lymphocyte differentiation and function.
2.3. Epithelial Injury
SPMs are also active in promoting repair of the mucosal epithelial barrier after injury. For example, aspiration of gastric acid evokes inflammatory responses by injured mucosal epithelial cells and infiltrating neutrophils and SPMs are pivotal mediators in vivo for promoting resolution of acid-induced acute lung injury in murine models. LXA4 is produced in vivo in response to acid-initiated lung injury and blocks acid-triggered IL-6 release from bronchial epithelial cells and inhibits neutrophil transmigration across bronchial epithelial cells (Bonnans et al., 2006). LXA4 also stimulates epithelial repair after acid injury by increasing basal epithelial cell proliferation (Bonnans et al., 2006). Injured bronchial epithelial cells upregulate ALX/FPR2 to promote LXA4-mediated effects on epithelial repair after acid injury (Bonnans et al., 2006). AT-RvD1 and AT-RvD3 both act to improve epithelial barrier integrity after acid-initiated lung injury and promote catabasis by decreasing alveolar edema and epithelial permeability, neutrophil infiltration and pro-inflammatory cytokines, and inhibiting NF-κB activation (Colby et al., 2016; Eickmeier et al., 2013). AT-RvD3 also promotes re-epithelialization after acid injury by increasing levels of keratinocyte growth factor and epithelial cell proliferation (Colby et al., 2016). In addition to macrophages, MaR1 is biosynthesized in the lung by neutrophil-platelet aggregates within hours after acid lung injury and protects against neutrophil infiltration, tissue edema, and pro-inflammatory cytokine production (Krishnamoorthy et al., 2014). MaR1 also modulates pro-inflammatory cytokine production from bronchial epithelial cells exposed to organic dust through mechanisms independent of the NF-κB signaling pathway (Nordgren et al., 2013).
2.4. Epithelial Host Defense
In addition to their effects on coordinating host cellular immune responses through chemokine and cytokine secretion, airway epithelial cells also express a number of antimicrobial factors that mediate additional host protective effects and also stimulate re-epithelialization to restore epithelial barrier integrity. Mucosal epithelial cells express bactericidal/permeability-increasing protein (BPI), a molecule that neutralizes bacterial endotoxin and kills gram negative bacteria (Canny et al., 2002). Lipoxins upregulate BPI expression in epithelial cells and increase mucosal bacterial killing (Canny et al., 2002). RvE1 enhances gastrointestinal mucosal epithelial cell alkaline phosphatase, which detoxifies bacterial lipopolysaccharide and impedes bacterial cell growth (Campbell et al., 2010). RvE1 also promotes epithelial cell expression of CD55, an anti-adhesive molecule that augments apical neutrophil clearance to resolve mucosal inflammation (Campbell et al., 2007). Finally, a number of SPMs reduce epithelial barrier disruption and protect against epithelial cell apoptosis (de Paiva et al., 2012; Erdinest et al., 2014; Mukherjee et al., 2004; Wang et al., 2012). These distinct actions of SPMs on mucosal epithelial cells provide another layer of antimicrobial host defense and regulation of pathogen-mediated airway inflammation.
2.5 Other Structural Cells and Innate Leukocytes in the Bronchi
SPMs can also exert effects on structural cells in the bronchi including pulmonary bronchi, airway smooth muscle, and fibroblasts. In vitro, LXA4 mediates the relaxation of pulmonary bronchi and arterioles in isolated lung strips that are pre-contracted with leukotrienes (Dahlen et al., 1988) and inhibits leukotriene-mediated airway smooth muscle migration (Parameswaran et al., 2007). LXA4 also regulates human lung fibroblast proliferation in response to connective tissue growth factor, a leading mediator of lung fibrosis (Wu et al., 2006). Together these studies demonstrate that SPMs actions on airway structural cells can counter-regulate pathological bronchial responses, including bronchoconstriction, airway smooth muscle hypertrophy, and fibrosis.
A number of innate leukocytes express SPM receptors and are targets for regulation by SPMs. Natural killer (NK) cells express ALX/FPR2 receptors and LXA4 exposure increases NK cell mediated apoptosis of neutrophils and eosinophils (Barnig et al., 2013) thus promoting granulocyte removal for resolution. NK cells also express the SPM receptor ERV and depleting NK cells inhibits RvE1-mediated resolution of eosinophilic allergic lung inflammation in vivo (Haworth et al., 2011). Type 2 innate lymphoid cells (ILC2) express ALX/FPR2 and ERV receptors and LXA4 and MaR1 exposure both potently inhibit type 2 cytokine release from ILC2s (Barnig et al., 2013; Krishnamoorthy et al., 2014). Further, MaR1 promotes ILC2 amphiregulin production, a molecule with potent tissue restorative properties for restitution of inflammed or injured bronchial mucosal tissues (Krishnamoorthy et al., 2014). Mast cells are important producers of a variety of pro-phlogistic proteins and lipid mediators, including histamine, prostaglandins, and leukotrienes. Mast cells express SPM receptors, including ALX/FPR2 and BLT1 (Gastardelo et al., 2014; Lundeen et al., 2006). Notably, the lipoxins and D-series resolvins have mast cell stabilizing effects to block mast cell degranulation and histamine release (Karra et al., 2015; Martin et al., 2012).
3. Pre-clinical Models of Lung Disease
Pre-clinical models of lung diseases have consistently uncovered potent immunomodulatory roles for SPMs. Essential PUFA regulate the host inflammatory response to acute infection and injury through their conversion to bioactive lipid-derived mediators (Samuelsson et al., 1987). In response to tissue injury or infection, arachidonic acid (AA; C20:4n-6), docosahexaenoic acid (DHA; C22:6n-3) and eicosapentaenoic acid (EPA; C20:5n-3) are rapidly released from cellular phospholipids via phospholipase A2 enzyme activity (Murakami et al., 2011) or are carried via plasma exudate into inflammatory sites (Serhan, 2011). PUFAs can be detected within minutes of tissue injury in inflammatory exudates (Kasuga et al., 2008) where they are available for rapid enzymatic conversion to SPMs, including lipoxins, resolvins, protectins, and maresins (Serhan, 2014). In this section we focus on the role of SPMs in controlling inflammation in several examples of pre-clinical murine models of lung disease (Table 1).
Table 1.
Specialized Pro-Resolving Mediator Actions in Murine Models of Lung Diseases
| Mediator | Actions | Reference |
|---|---|---|
|
Allergic Airway Disease (Asthma and Rhinitis)
| ||
| LXA4 | Prevents airway hyperreactivity, Inhibits eosinophils and lymphocyte infiltration, reduces levels of IL-4, IL-5, IL-13, prostaglandins, and cysteinyl leukotrienes | Levy et al. 2002; Levy et al. 2007 |
| Prevents antigen-specific antibody production | Ramon et al. 2014 | |
| Reduces BAL leukocyte numbers and IL-17 levels | Haworth et al. 2008 | |
|
| ||
| LXB4 | Prevents airway hyperreactivity, inhibits BAL eosinophil, macrophages and lymphocyte infiltration | Karra et al. 2015 |
| Reduces nasal mucosal and lower airway inflammation, IgE, type 2 cytokines, leukocyte infiltration, eosinophil and mast cell degranulation and mucin secretion | Karra et al. 2015 | |
|
| ||
| RvD1 | Prevents airway hyperreactivity, inhibits BAL eosinophils and leukocyte infiltration, reduces IL-5 levels and mucus metaplasia, enhances macrophages phagocytosis and clearance of allergen; Accelerates resolution of lung eosinophils, reduces airway hyperresponsiveness | Rogerio et al. 2012 |
|
| ||
| RvE1 | Prevents airway hyperreactivity, reduces eosinophils and lymphocyte recruitment, IL-13 levels, antigen- specific antibody production and mucus metaplasia | Aoki et al. 2008 |
| Increases lipoxin formation and inhibits IL-17 and IL-23 production | Haworth et al. 2008 | |
| Promotes NK cell cytotoxicity | Haworth et al. 2011 | |
|
| ||
| PD1 | Prevents eosinophil and lymphocyte recruitment, reduces pro-inflammatory cytokines and mucus metaplasia and accelerates resolution of inflammation | Levy et al. 2007 |
|
| ||
| MaR1 | Promotes de novo generation of regulatory T cells, reduces type 2 cytokine and amphiregulin production by type 2 innate lymphoid cells | Krishnamoorthy et al. 2014 |
|
| ||
|
Acute Lung Injury
| ||
| LXA4 | Reduces neutrophil recruitment | Planaguma et al. 2010 |
|
| ||
| RvD1 | Reduces neutrophil infiltration, inhibits neutrophil-platelet interaction, improves barrier integrity and decreases airway resistance; Promotes epithelial repair | Eickmeier et al. 2013 |
|
| ||
| RvD2 | Regulates systemic inflammatory responses and local tissue leukocyte trafficking in sepsis | Spite et al. 2009 |
|
| ||
| RvD3 | Reduces neutrophil infiltration and alveolar edema and promotes epithelial repair | Colby et al. 2016 |
|
| ||
| RvE1 | Reduces BAL leukocytes, enhances bacterial clearance, decreases pro-inflammatory cytokines, improves survival | Seki et al. 2010 |
|
| ||
| MaR1 | Reduces lung edema, neutrophil infiltration, and pro-inflammatory cytokine production, improves lung function | Abdulnour et al. 2014 |
|
| ||
|
Bacterial Lung Infection
| ||
| LXA4 | Promotes neutrophil apoptosis, accelerates resolution of pulmonary inflammation, enhances survival | El Kebir et al. 2009 |
|
| ||
| RvD1 | Decreases lung leukocyte trafficking, inhibits pro-inflammatory cytokines and decreases mortality | Wang et al. 2011 |
| Enhances macrophage phagocytosis of bacteria and efferocytosis of neutrophils | Abdulnour et al. 2015 | |
|
| ||
| RvE1 | Reduces neutrophil recruitment, bacterial burden, pro-inflammatory cytokine production and enhances survival; Accelerates resolution of pulmonary inflammation by promoting neutrophil apoptosis | Seki et al. 2010; El Kebir et al. 2012 |
|
| ||
|
Viral Lung Infection
| ||
| LXA4 | Promotes alternatively activated macrophage phenotype for clearance of infection, accelerates inflammation resolution | Shirey et al. 2014 |
|
| ||
| RvE1 | Administered prior to RSV infection promotes pro-resolving alternatively activated macrophage for clearance of infection and accelerates resolution | Shirey et al. 2014 |
|
| ||
| PD1 | Inhibits influenza virus replication; Improves pulmonary function and survival | Morita et al. 2013 |
Abbreviations: LXA4, lipoxin A4; LXB4, lipoxin B4; RvD1, resolvin D1; RvD2, resolvin D2; RvD3, resolvin D3; RvE1, resolvin E1; PD1, protectin D1; MaR1, maresin 1.
3.1. Allergic Airway Disease
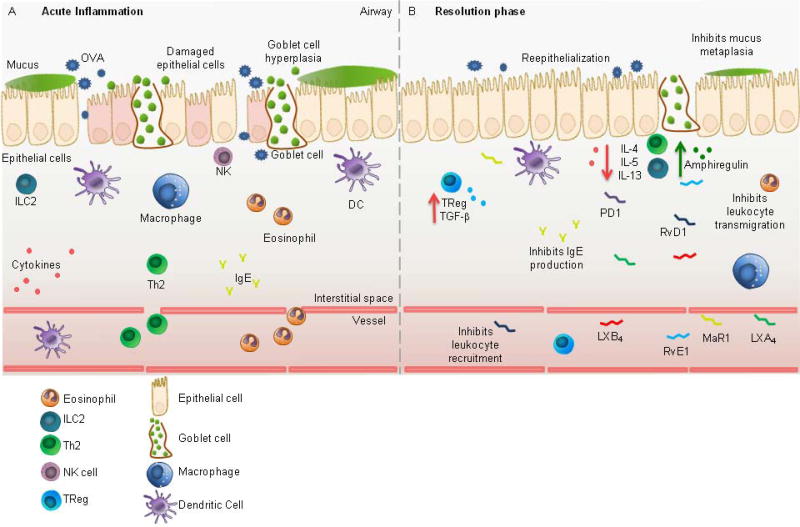
Asthma is a prevalent and complex disease of airway hyperresponsiveness, reversible airflow obstruction, inflammation and mucus metaplasia with several distinct phenotypes, including an allergic asthma phenotype characterized by type 2 inflammation and eosinophilia (Wenzel, 2012). In murine models of allergic airway inflammation, animals are first sensitized to an allergen (e.g. chicken ovalbumin (OVA)) for several weeks followed by an aerosol or direct airway challenge to simulate an allergen-induced asthma exacerbation. In allergen-sensitized mice, neutrophils, eosinophils, and lymphocytes are rapidly recruited to the lung within 12 hours of airway challenge and type 2 cytokines become elevated (Fig. 2A) (Flesher et al., 2014; Levy et al., 2002). The SPMs LXA4 (Levy et al., 2002) and PD1 (Levy et al., 2007a) are also detected in the airways of allergen-sensitized mice, but at much lower levels than pro-inflammatory cysteinyl leukotrienes and prostaglandins (Levy et al., 2002). Murine models of allergic airway disease have been critical in unraveling the effects of SPMs on modulating allergen-induced lung inflammation and in promoting the resolution of host immune responses to allergen challenge.
Fig. 2. Anti-Inflammatory and Pro-Resolving Bronchial Effects of SPMs in Allergic Asthma.
(A) Allergic asthma is characterized histologically by inflammatory leukocyte infiltration and inflammatory cytokine release, goblet cell hyperplasia and mucus metaplasia, epithelial barrier disruption, and airway edema. (B) In the resolution phase, SPMs act via cell-specific mechanisms to halt inflammation and promote tissue homeostasis including restitution of epithelial barrier integrity, inhibition of mucus metaplasia, halting leukocyte trafficking, inhibiting type 2 cytokine release and promoting ILC2 amphiregulin production.
3.1.1. SPM Effects on Prevention of Disease
LXA4 and its stable analogs have significant prophylactic effects on preventing allergic pulmonary inflammation in murine models of allergic asthma (Levy et al., 2002; Levy et al., 2007b). Administration of a stable LXA4 analog to OVA-sensitized animals prior to allergen challenge significantly inhibits bronchoconstriction to methacholine, reduces eosinophil and lymphocyte lung infiltration, and reduces lung levels of type 2 cytokines IL-5 and IL-13 and pro-phlogistic mediators prostaglandins and cysteinyl leukotrienes (Levy et al., 2002). LXA4 analogs can be designed to be active even when administered enterally and inhibit leukocyte trafficking and type 2 cytokine release in a mechanism distinct from cysteinyl leukotriene receptor antagonists such as montelukast (Levy et al., 2007b). LXA4 analogs are also effective at blunting airway inflammation and hyperreactivity when administered before allergen challenge in a cockroach allergen (CRA) model of allergic airway inflammation (Levy et al., 2007b). Further, LXA4 prevents antigen-specific antibody production in OVA-sensitized mice, which hinders memory antibody responses after rechallenge, highlighting an additional mechanism by which LXA4 regulates the host immune response (Ramon et al., 2014b).
Additional SPMs have also been shown to have effects on preventing allergic lung inflammation in animal models. Lipoxin B4 (LXB4), a structurally distinct member of the lipoxin family, decreases the number of infiltrating BAL eosinophils and lymphocytes in a dose-dependent manner when administered prior to allergen challenge in OVA-sensitized mice (Karra et al., 2015). RvD1 (Rogerio et al., 2012), RvE1 (Aoki et al., 2008; Arita et al., 2007b; Flesher et al., 2014), and PD1 (Levy et al., 2007a) all markedly abrogate the development of allergic airway responses in particular eosinophilia and lymphocyte recruitment, mucus metaplasia, and type 2 cytokine production when administered prophylactically to OVA-sensitized mice prior to allergen challenge. Maresin 1 (MaR1), a macrophage derived SPM, has potent counter-regulatory actions on the innate immune system (Krishnamoorthy et al., 2014). Prophylactic intravenous MaR1 administered to OVA-sensitized mice prior to allergen challenge reduces lung inflammation by inhibiting type 2 cytokine production by ILC2s (Krishnamoorthy et al., 2014).
3.1.2. Pro-resolving Actions of SPMs
SPMs also have potent actions to promote the resolution of inflammation when administered in a therapeutic manner after allergen challenge when the host immune response has already been established (Fig. 2B). Treatment of OVA aerosol challenged mice in the post-challenge phase with a stable 15-epi-LXA4 analog significantly reduces airway inflammation by reducing BAL leukocyte numbers and IL-17 levels (Haworth et al., 2008). Lipoxin B4 (LXB4) promotes the resolution of allergic inflammation in both the upper and lower airways. LXB4 significantly reduces nasal mucosal inflammation, IgE and type 2 cytokine levels, leukocyte infiltration, eosinophil and mast cell degranulation, and mucin secretion in a murine model of allergic rhinitis (Karra et al., 2015). In the lower airways, LXB4 treatment of allergen-challenged mice significantly accelerates the resolution of allergic inflammation by approximately 30% by decreasing BAL eosinophils, macrophages, and lymphocyte numbers and decreases airway hyper-responsiveness (Karra et al., 2015). RvD1 and AT-RvD1 treatment of allergen-challenged mice similarly accelerates the resolution interval for lung eosinophilia and blunts airway hyperresponsiveness to methacholine while also enhancing macrophage phagocytosis and clearance of allergen (Rogerio et al., 2012). Similarly, PD1 treatment after allergen challenge accelerates the resolution phase of lung inflammation and dampens type 2 cytokine production (Levy et al., 2007a). In acute and chronic allergic inflammation models, RvE1 treatment promotes the resolution of host lung inflammation similarly to other SPMs by restraining eosinophil and lymphocyte recruitment and suppressing inflammatory cytokine production (Aoki et al., 2008; Flesher et al., 2014; Haworth et al., 2008). In addition, RvE1 treatment in allergic inflammation increases lipoxin formation and directly inhibits IL-23 and IL-17 production in the lung (Haworth et al., 2008) and promotes natural killer cell cytotoxicity (Haworth et al., 2011), supporting a role for RvE1 in modulating additional inflammatory pathways to promote resolution of allergic lung inflammation. MaR1 administered therapeutically after allergen challenge exhibits anti-inflammatory and pro-resolving actions to regulate innate immune responses (Krishnamoorthy et al., 2014, 2015). MaR1 treatment increases Foxp3+ regulatory T cell numbers and suppressive actions in a TGF-β- dependent manner that restrains type 2 cytokine production by ILC2 and increases amphiregulin production, which together promotes the resolution of allergic lung inflammation (Krishnamoorthy et al., 2014, 2015).
3.2. Acute Lung Injury
Acute lung injury results in leukocyte trafficking to the lung to protect the host and restore tissue homeostasis. If excessive, lung leukocyte infiltration from the acute inflammatory response can result in the overwhelming and deleterious lung inflammation seen in the acute respiratory distress syndrome (ARDS), a pathologic condition in which the leukocyte-rich exudate fills alveoli and prevents adequate oxygenation with high rates of morbidity and mortality (Baron and Levy, 2016). Human clinical trials have failed to demonstrate effective treatments for ARDS that target the overabundant host inflammatory response. Therapies harnessing SPMs to promote inflammation resolution represent a new treatment strategy and in murine models of acute lung injury SPMs have shown promise in controlling inflammation from lung injury.
3.2.1. SPM Effects on Prevention of Disease
Pre-injury administration of 15-epi-LXA4 decreases lung neutrophil recruitment in a murine model of aspiration pneumonia (Planaguma et al., 2010). Lovastatin triggers 15-epi-LXA4 formation and when present can lessen the severity of lung injury (Planaguma et al., 2010). AT-RvD1 prophylaxis decreases peak BAL inflammatory neutrophil infiltration by ~75%, inhibits platelet-neutrophil interactions, improves barrier integrity, and decreases airway resistance in a murine acid aspiration model (Eickmeier et al., 2013). Similarly, RvE1 prophylaxis significantly decrease peak BALF inflammatory leukocyte infiltration in a murine model of acid injury while also enhancing bacterial clearance and decreasing lung levels of pro-inflammatory cytokines, resulting in marked improvement in survival (Seki et al., 2010).
3.2.2. Pro-resolving Actions of SPMs
Resolution of acute acid-induced lung injury requires intact signaling of LXA4 and 15-epi LXA4 through ALX/FPR2 receptors and is impaired when COX-2 activity is inhibited (Fukunaga et al., 2005). Exogenous treatment with AT-RvD1 or AT-RvD3 after acid injury in murine models reduces neutrophil tissue infiltration and alveolar edema and promotes bronchial epithelial repair (Colby et al., 2016; Eickmeier et al., 2013). RvD2 potently regulates systemic inflammatory responses and local tissue leukocyte trafficking in sepsis (Spite et al., 2009), a common cause of acute lung injury and ARDS in humans. Finally, MaR1 is organ protective in a murine model of acid-induced acute lung injury (Abdulnour et al., 2014). In addition to macrophages, early MaR1 production is dependent on platelet-neutrophil interaction and when given exogenously 1 hour after acid-induced lung injury, MaR1 significantly reduces lung edema, neutrophil infiltration, and pro-inflammatory cytokine production leading to improved lung function (Abdulnour et al., 2014).
3.3. Bacterial Lung Infection
Bacterial pneumonia is a common disease in humans and for the majority of individuals is a self-limited and mild disease. With some particularly virulent microbes or in a subset of susceptible patients, bacterial pneumonia evolves into respiratory failure and ARDS characterized by overwhelming inflammation that compromises oxygenation and ventilation and leads to high rates of morbidity and mortality (Baron and Levy, 2016). Studies of SPMs in murine models of bacterial pneumonia have highlighted beneficial preventative and treatment effects of SPMs on host lung defense and inflammation.
3.3.1. SPM Effects on Prevention of Disease
In a murine model of lipopolysaccharide (LPS)-induced acute lung injury, RvD1 administered prior to LPS exposure was protective resulting in decreased lung leukocyte trafficking, inhibition of pro-inflammatory cytokine secretion, and decreased mortality (Wang et al., 2011). Prophylactic RvE1 also blunts host inflammation in a murine model of aspiration pneumonia (Seki et al., 2010). Animals pretreated with intravenous RvE1 prior to intratracheal instillation of hydrochloric acid followed by Escherichia coli had 50% reduced recruitment of neutrophils to the lung, reduced bacterial burden, decreased pro-inflammatory cytokines, and enhanced survival (Seki et al., 2010).
3.3.2. Pro-resolving Actions of SPMs
SPM treatment of animals with bacterial pneumonia also promotes the timely resolution of the host inflammatory response. LXA4 and RvE1 both promote neutrophil phagocytosis-induced apoptosis to accelerate resolution of pulmonary inflammation and improve survival in a murine model of E. coli pneumonia (El Kebir et al., 2012; El Kebir et al., 2009). Early treatment with RvD1 within one hour of infection with E. coli or Pseudomonas aeruginosa enhances lung macrophage phagocytosis of bacteria and efferocytosis of neutrophils in vivo and promotes macrophage phagocytosis of E. coli particles in ex vivo lung sections (Abdulnour et al., 2015). Moreover, the combination of RvD1 and antibiotics has additive effects on enhancing bacterial clearance (Abdulnour et al., 2015). These studies provide evidence that SPMs could provide a potent augmentative therapeutic approach to treating bacterial pneumonia that harnesses mechanisms targeting both the host and the microbe to enhance pathogen clearance, mitigate leukocyte activation and trafficking to the lung, and promote efferocytosis of apoptotic leukocytes to restore the lung to a homeostatic state.
3.4. Viral Lung Infection
Viral pulmonary infections are a dominant cause of respiratory illnesses in particular in the pediatric and elderly populations and treatment is largely limited to supportive care. Most common viral pathogens generate self-limited and mild infections but highly virulent strains can result in overwhelming pulmonary inflammation that can lead to critical illness and even death (Thompson et al., 2003). Influenza is a seasonal pathogen with notable severely pathogenic strains that have resulted in global pandemics with excess mortality (i.e. the 1918 and 2009 H1N1 pandemics). In murine models of influenza, SPMs are inversely correlated with the virulence of the influenza strain. The more virulent strains of virus (i.e. H5N1) are associated with decreased levels of lipoxins (Cilloniz et al., 2010) and protectin D1 (Morita et al., 2013) with increased lung leukocyte recruitment, and lethal viral dissemination.
3.4.1. SPM Effects on Prevention of Disease
When administered prophylactically intravenously 12 hours prior to infection, PD1 markedly inhibits influenza virus replication by interfering with the virus RNA nuclear export machinery, thereby inhibiting nuclear export of viral transcripts and resulting in improved survival in animals with severe disease (Morita et al., 2013). Further, the SPM 17-HDHA enhances virus-specific humoral immunity in a pre-clinical influenza vaccination model where animals vaccinated with both 17-HDHA and influenza proteins have superior protection against live viral challenge compared to animals immunized with viral antigens alone (Ramon et al., 2014a). In a pre-clinical model of respiratory syncytial virus (RSV) infection, pre-treatment of macrophages with LXA4 or RvE1 prior to RSV infection promoted a pro-resolving macrophage phenotype (i.e. alternatively activated) for clearance of infection and inflammation and resolution of lung pathology (Shirey et al., 2014).
3.4.2. Pro-resolving Actions of SPMs
PD1 has therapeutic benefit even when administered after influenza infection has been established. Animals treated with PD1 48 hours after influenza infection exhibited improved pulmonary function and markedly improved survival compared to animals treated with the antiviral medication peramivir (Morita et al., 2013). Thus, PD1 had marked beneficial effects, both prophylactically and therapeutically, in an in vivo model of influenza infection. Of interest, engaging toll-like receptor 7 (TLR7), a receptor for viral single stranded RNA, triggers the generation of PD1 and RvD1 and enhances resolution of type 2 airway inflammation in mice (Koltsida et al., 2013). Together these studies suggest that SPMs are powerful agents to affect virus-host interactions and modify host- and pathogen-mediated lung pathology. Further studies, in particular in humans, are warranted.
4. Human Translation
SPMs are detectable in numerous human body fluids and tissues including peripheral blood, plasma and serum, bronchoalveolar lavage fluid and sputum, cerebrospinal fluid, breast milk, synovial joint fluid, and tears (Serhan, 2014). The levels of lipoxins, resolvins, and protectins in healthy human serum are in the picomolar to nanomolar range (Psychogios et al., 2011) and dietary supplementation with omega-3 fatty acids can increase serum SPM levels (Colas et al., 2014; Mas et al., 2012; Psychogios et al., 2011). Common airway diseases such as asthma and COPD are characterized by chronic inflammation that fails to resolve. In this section, we review studies that have identified deficiencies in SPMs in these common human lung diseases (Table 2). Together, this body of evidence suggests that insufficient levels and activity of SPMs in the resolution phase of inflammation may prolong acute inflammatory responses and contribute to the persistent inflammation in the lungs of patients with these chronic diseases.
Table 2.
Deficient Specialized Pro-Resolving Mediator Biosynthesis in Human Lung Diseases
| Mediator | Finding | Reference |
|---|---|---|
|
Asthma
| ||
| LXA4 | Decreased in blood, bronchoalveolar lavage fluid, sputum and exhaled breath condensates in patients with severe asthma | Bhavsar et al. 2010; Celik et al. 2007; Fritscher et al. 2012; Kazani et al. 2013;Levy et al. 2005; Planaguma et al. 2008; Vachier et al. 2005 |
| Reduced in exhaled breath condensates of children with status asthmaticus | Hasan et al. 2012 | |
| Decreased in children with exercise-induced asthma | Tahan et al. 2008 | |
| Decreased in aspirin-intolerant asthma | Celik et al. 2007; Sanak et al. 2000; Yamaguchi et al. 2011 | |
|
| ||
| PD1 | Decreased in exhaled breath condensates in uncontrolled asthma | Levy et al. 2007 |
| Decreased eosinophil production in in severe asthma | Miyata et al. 2013 | |
|
| ||
|
Chronic Obstructive Pulmonary Disease (COPD)
| ||
| LXA4 | Reduced in exhaled breath condensates in moderate to severe COPD | Fritscher et al. 2012 |
| Reduced relative to serum amyloid A (SAA) during acute exacerbations of COPD | Bonzinovski et al. 2012 | |
|
| ||
| LXB4 | Reduced in exhaled breath condensates in moderate to severe COPD patients | Croasdell et al. 2015 |
|
| ||
| RvD1 | Reduced in BALF and serum in COPD | Croasdell et al. 2015 |
|
| ||
|
Cystic Fibrosis (CF)
| ||
| LXA4 | Decreased in airway secretions | Karp et al. 2004; Yang et al. 2012 |
| Production is compromised by Pseudomonas aeruginosa | Flitter et al. 2017 | |
|
| ||
| RvE1 | Undetectable levels are related to lower lung function | Yang et al. 2012 |
Abbreviations: LXA4, lipoxin A4; LXB4, lipoxin B4; RvD1, resolvin D1; RvE1, resolvin E1; PD1, protectin D1.
4.1. Asthma
Asthma is a chronic disease of unrestrained airway inflammation and hyperresponsiveness that is triggered by irritant, allergic, and infectious stimuli resulting in an influx of inflammatory leukocytes (in particular eosinophils, neutrophils, and lymphocytes) and pro-inflammatory mediators (e.g., type 2 cytokines) to the airway (Fahy, 2015). Approximately 10% of patients have severe asthma characterized by daily symptoms and uncontrolled disease despite high dose inhaled and/or systemic corticosteroids (Wenzel, 2012).
4.1.1 Deficiency of SPMs in Asthma
Severe asthma patients have decreased levels of LXA4 in blood, BALF, induced sputum, and exhaled breath condensates that correlates with disease severity and compromised lung function (Table 2) (Bhavsar et al., 2010; Celik et al., 2007; Fritscher et al., 2012; Kazani et al., 2013; Levy et al., 2005; Planaguma et al., 2008; Vachier et al., 2005). Decreased lipoxin generation in uncontrolled asthma is at least partially attributable to dysregulated lipoxin biosynthetic genes (Planaguma et al., 2008). Further, lipoxin levels are also decreased in severe asthma as a consequence of oxidative stress (Ono et al., 2014). Patients with aspirin-exacerbated respiratory disease also have decreased LXA4 biosynthesis and elevated leukotriene levels suggesting that an imbalance in pro-inflammatory and pro-resolving mediators underlies the pathogenesis of this asthma phenotype (Celik et al., 2007; Sanak et al., 2000; Yamaguchi et al., 2011). In health, the airway mucosa is highly enriched with DHA; however, mucosal levels of DHA are reduced in asthma (Freedman et al., 2004) decreasing the amount of DHA locally available in the airway for conversion to DHA-derived SPMs, including D-series resolvins, protectins, and maresins. Indeed, levels of PD1 are decreased in exhaled breath condensates of uncontrolled asthmatic patients during acute exacerbations (Levy et al., 2007a) and eosinophil production of PD1 is reduced in severe asthma (Miyata et al., 2013).
4.1.2 Biologic Actions of SPMs in Asthma
Importantly, LXA4 actions in blunting bronchoconstriction have been confirmed in vivo in humans (Table 3). In a human clinical trial, inhaled LXA4 protects asthmatic patients from leukotriene-mediated bronchoprovocation (Christie et al., 1992). Recent studies have demonstrated that LXA4 can also modulate cellular immune responses in asthma. LXA4 promotes pro-resolving functions of natural killer (NK) cells by enhancing NK cell-mediated apoptosis of neutrophils and eosinophils to terminate acute inflammation (Barnig et al., 2013). Further, IL-13 production by ILC2s is inhibited by LXA4 (Barnig et al., 2013), supporting the potential therapeutic role for LXA4 in dampening type 2 inflammation in asthma. Supplementing pregnant women with high doses of the n-3 PUFAs DHA and EPA reduces the incidence of persistent wheeze and asthma in their children by one-third (Bisgaard et al., 2016) lending further support to results in pre-clinical experimental models demonstrating the effectiveness of SPMs in mitigating the development and symptoms of asthma.
Table 3.
Biological Actions of Specialized Pro-Resolving Mediators in Human Lung Diseases
| Mediator | Actions | Reference |
|---|---|---|
|
Asthma
| ||
| LXA4 | Relaxes of bronchoconstricted airways in vitro and in vivo | Dahlen et al. 1988; Christie et al. 1992 |
| Enhances natural killer cell-mediated apoptosis of granulocytes | Barnig et al. 2013 | |
| Inhibits IL-13 production by type 2 innate lymphoid cells | Barnig et al. 2013 | |
| Topical 15-epi-LXA4 relieves dermal inflammation in infants with eczema | Wu et al.2013 | |
|
| ||
| n-3 PUFA | High dose supplementation of pregnant women reduces the incidence of persistent wheeze and asthma in their children by one-third | Bisgaard et al. 2016 |
|
| ||
|
Chronic Obstructive Pulmonary Disease (COPD)
| ||
| RvD1 | Suppresses cytokine production from human lung fibroblasts and airway epithelial cells | Hsiao et al. 2013 Hsiao et al. 2014 |
| Inhibits NF-κB signaling in human airway epithelial cells | ||
|
| ||
| RvD2 | Reduces pro-inflammatory cytokine release from cigarette smoke-exposed human alveolar macrophages | Croasdell et al. 2015 |
|
| ||
| RvE1 | Attenuates cigarette smoke-induced superoxide production and death in human macrophages | Takamiya et al. 2012 |
|
| ||
|
Cystic Fibrosis
| ||
| LXA4 | Inhibits IL-8 production by P. aeruginosa-exposed human bronchial epithelial cells | Karp et al. 2004 |
| Levels increase in patients after antibiotic treatment | Chiron et al. 2008 | |
| 15-epi-LXA4 | BALF levels are positively correlated with lung function | Flitter et al. 2017 |
Abbreviations: LXA4, lipoxin A4; n-3 LCPUFA, n-3 long-chain polyunsaturated fatty acids; RvD1, resolvin D1; RvD2, resolvin D2; RvE1, resolvin E1; 15-epi-LXA4, 15-epimer of lipoxin A4.
4.2. Chronic Obstructive Pulmonary Disease (COPD)
COPD is a chronic inflammatory lung disease triggered by cigarette smoke exposure that results in a pro-inflammatory environment in the lung with excess cellular and soluble mediators predisposing patients to recurrent exacerbations and progressive lung dysfunction (Pauwels and Rabe, 2004). Like asthma, COPD is a disease of unrestrained chronic lung inflammation that can be refractory to the anti-inflammatory actions of corticosteroids and fails to resolve. The role of SPMs in regulating COPD pathogenesis is an area of active investigation.
4.2.1 Deficiency of SPMs in COPD
Moderate to severe COPD patients have reduced levels of LXA4 (Fritscher et al., 2012) and LXB4 (Croasdell et al., 2015) in exhaled breath condensates and elevated levels of pro-inflammatory leukotrienes (Table 2) (Vachier et al., 2005). During acute exacerbations in patients with COPD, LXA4 levels are 2–3 log orders lower than serum amyloid A, which can pirate ALX/FPR2 receptors to promote inflammation and overwhelm LXA4-mediated protective effects (Bozinovski et al., 2012). DHA-derived RvD1 levels in BALF and serum of COPD patients are also lower than in healthy individuals (Croasdell et al., 2015).
4.2.2 Biologic Actions of SPMs in COPD
SPMs have anti-inflammatory and pro-resolving effects on COPD pathophysiology in vitro (Table 3). Pharmacologic dosing of 15-epi-LXA4 abrogates neutrophilic inflammation induced by intranasal SAA challenge in mice by reducing BALF neutrophil chemokine secretion and neutrophil recruitment by more than 50% (Bozinovski S, 2012). Of note, 15-epi-LXA4 actions are distinct from the anti-inflammatory corticosteroid dexamethasone, which does not significantly alter airway neutrophilia after SAA challenge (Bozinovski S, 2012). RvD1 and AT-RvD1 are effective in vitro at dampening pro-inflammatory cytokine release from human alveolar macrophages isolated from COPD patients (Croasdell et al., 2015). RvD1 significantly inhibits neutrophilic lung inflammation and pro-inflammatory cytokine production elicited by smoke exposure and promotes an alternatively activated macrophage phenotype with enhanced capacity for neutrophil efferocytosis (Hsiao et al., 2013). Further, RvD1 also upregulates production of the anti-inflammatory cytokine IL-10 and accelerates the resolution of lung inflammation when administered after cessation of smoke exposure (Hsiao et al., 2013). RvE1 is also active at attenuating cigarette-smoke induced superoxide production and cell death in macrophages (Takamiya et al., 2012). Together, these studies highlight important mechanisms by which SPMs can influence macrophage phenotype and function to promote inflammation resolution and halt pro-inflammatory immune signals in this disease of chronic lung inflammation.
4.3. Cystic Fibrosis (CF)
CF is a genetic disease of dysfunctional chloride channels that affects multiple organs including the lungs, pancreas, GI tract and liver and leads to significant lifelong morbidities and early mortality. The lung pathology in CF is significant with tenacious mucus secretions that obstruct airways leading to hypoxemia and providing a nidus for bacterial infection. Patients with CF have recurrent airway infections and chronic airway inflammation that lead to destruction of airways and compromised lung function over time and result in frequent hospitalizations and eventually lung transplantation or death.
4.2.1 Deficiency of SPMs in CF
As in asthma and COPD, levels of LXA4 in airway secretions are reduced in patients with CF (Table 2) (Karp et al., 2004; Yang et al., 2012). Moreover, Pseudomonas aeruginosa, a common pathogen amongst CF patients, inhibits 15-epi-LXA4 generation and function through the actions of a secreted epoxide hydrolase, thus promoting airway neutrophilia and impairing lung function (Flitter et al., 2017). Sputum LXA4 levels can be increased in CF patients by antibiotic treatment correlating with decreased sputum neutrophils and IL-8 levels (Chiron et al., 2008). CF patients with detectable RvE1 have better lung function compared to patients with undetectable RvE1 levels who have the lowest lung function (Yang et al., 2012). Finally, mucosal DHA levels are also reduced in CF patients (Freedman et al., 2004) underscoring the broad deficiency of both precursor PUFAs as well as lipid-derived SPMs in CF.
4.2.2 Biologic Actions of SPMs in CF
In a murine model of CF, lipoxin treatment suppresses neutrophilic lung inflammation and decreases bacterial burden resulting in a less severe disease course (Table 3) (Karp et al., 2004). This study provides further evidence that leveraging lipoxins and other SPMs to target diseases of chronic lung inflammation may provide alternate therapeutic avenues for these morbid diseases.
4.4. Pediatric Considerations
Asthma is the most common chronic disease of childhood affecting nearly 10% of children in the United States, a current total of 7 million children (Akinbami et al., 2009). As in adult asthma, an imbalance in pro-inflammatory and pro-resolving mediators may contribute to the pathophysiology of pediatric asthma. LXA4 levels are reduced in exhaled breath condensates of asthmatic children admitted to a pediatric intensive care unit with status asthmaticus relative to healthy children (Hasan et al., 2012). Similarly, children with exercise-induced asthma have lower circulating plasma LXA4 levels after exercise challenge relative to those children with asthma not triggered by exercise and LXA4 levels are inversely correlated with lung function in these children (Tahan et al., 2008). Children whose mothers were supplemented with high doses of n-3 PUFA during pregnancy are one-third less likely to develop persistent wheeze and asthma compared to their peers whose mothers did not received fish oil supplementation (Bisgaard et al., 2016). This sentinel study underscores the importance of lipid-derived mediators in not only modulating the disease course of asthma but in preventing its development in children.
Mother-to-child transmission of SPMs through breast milk is a likely contributor to maternal-derived immune defense mechanisms for infants as breast milk is enriched in the n-3 PUFAs DHA and EPA (Peng et al., 2009). Human breast milk contains a biochemical profile rich in PUFA-derived SPMs including lipoxins, resolvins, protectins, and maresins at physiologically relevant levels (Arnardottir et al., 2016; Weiss et al., 2013). Human breast milk lipid mediator isolates are effective at stimulating the resolution of acute infectious peritonitis by regulating neutrophil trafficking, enhancing bacterial clearance, and shortening the resolution interval of acute inflammation as well as by enhancing human macrophage efferocytosis (Arnardottir et al., 2016). Taken together, these findings provide additional support for the health benefits of breastfeeding by highlighting a program of SPM-driven resolution of inflammation and infection that may provide another layer of immune protection for vulnerable infants.
Human clinical trials evaluating the efficacy of SPM compounds on ameliorating inflammation are beginning to be conducted. Recently, topical 15-epi-LXA4 was shown to be as effective as topical steroids at relieving dermal inflammation in infants with eczema (Wu et al., 2013) supporting the therapeutic potential of SPM-based therapies in human disease. Topical RvE1 and LXA4 analogs are currently being investigated in clinical trials focused on diseases of eye and periodontal inflammation (e.g., NCT02329743, NCT02342691).
5. Conclusions
In health, the host airway immune response to invading pathogens, injury, allergen or other noxious stimuli is a spatiotemporally regulated network of cellular and molecular pathways whereby acute inflammatory responses are activated and once the threat is contained, an equally sophisticated resolution pathway is engaged to turn off inflammation for catabasis. With their anti-inflammatory and pro-resolving actions, the PUFA-derived endogenous SPMs are central to the body’s counter-regulatory inflammation resolution program and help restore the host tissues to homeostasis. When the natural resolution pathways are interrupted or deficient, chronic inflammation ensues. Here, we have reviewed that the pathophysiology of several common and morbid diseases of bronchial inflammation, notably asthma, COPD, and CF, are linked to insufficient levels or activity of SPMs. Current therapies targeting lung inflammation rely heavily on medications that are anti-inflammatory in nature but are non-specific in their mechanism of action. For example, corticosteroids, used commonly as anti-inflammatory therapies in the treatment of asthma and COPD, have the unintended side effect of immunosuppression, which leaves the host at risk for infection and poor wound healing. Therapies that target lung inflammation and promote inflammation resolution without causing undesirable immunosuppression are greatly needed. Murine models of human inflammatory and infectious lung diseases have clearly demonstrated the efficacy of SPMs in promoting key pathways of inflammation resolution including inhibiting trans-epithelial leukocyte transmigration, reducing pro-inflammatory cytokine production, promoting macrophage efferocytosis, restoring epithelial barrier integrity, and improving lung function and survival. Human clinical trials of SPMs and the precursor PUFAs have demonstrated encouraging success in inhibiting bronchoprovocation in patients with asthma, preventing the development of asthma in children, and alleviating dermal irritation in infants with eczema (Bisgaard et al., 2016; Christie et al., 1992; Wu et al., 2013). In addition to the insights into lung physiology and disease pathogenesis provided by mapping of endogenous resolution pathways, leveraging the potent bronchial protective actions of SPMs to pharmacologically promote inflammation resolution and antimicrobial host defense without the untoward side effects (i.e. immunosuppression) of traditional anti-inflammatory medications would enhance our arsenal of therapies for common airway diseases of chronic lung inflammation, such as asthma, COPD, and CF.
Acknowledgments
This work was funded in part by National Institutes of Health Grants R01-HL122531, U01-HL108712, U10-HL109172, U24-AI118656, P01-GM095467 (BDL), and K12-HD047349 (MGD) and by the Sao Paulo Research Foundation (FAPESP) grant 2015/26048-5 (TRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
BDL is a co-inventor on patents assigned to Brigham and Women’s Hospital. The interests of BDL were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. MGD and TRB have no pertinent interests to disclose.
References
- Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(46):16526–16531. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, Ai X, Serhan CN, Levy BD. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal immunology. 2015 doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochemical and biophysical research communications. 2008;367(2):509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of immunology. 2007a;178(6):3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 Selectively Interacts with Leukotriene B4 Receptor BLT1 and ChemR23 to Regulate Inflammation. The Journal of Immunology. 2007b;178(6):3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arnardottir H, Orr SK, Dalli J, Serhan CN. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal immunology. 2016;9(3):757–766. doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science translational medicine. 2013;5(174):174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Levy BD. Recent advances in understanding and treating ARDS. F1000Res. 2016:5. doi: 10.12688/f1000research.7646.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar PK, Levy BD, Hew MJ, Pfeffer MA, Kazani S, Israel E, Chung KF. Corticosteroid suppression of lipoxin A4 and leukotriene B4 from alveolar macrophages in severe asthma. Respiratory research. 2010;11:71. doi: 10.1186/1465-9921-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdottir S, Folsgaard NV, Fink NR, Thorsen J, Pedersen AG, Waage J, Rasmussen MA, Stark KD, Olsen SF, Bonnelykke K. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med. 2016;375(26):2530–2539. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S, Van Gool F, Communi D, De Vuyst P, Desmecht D, Parmentier M. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS pathogens. 2011;7(11):e1002358. doi: 10.1371/journal.ppat.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. The American journal of pathology. 2006;168(4):1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski SAG. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. 2012 doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, Anderson GP. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(3):935–940fff. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(12):3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, Scully M, Bruyninckx WJ, Colgan SP. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, Colgan SP. Lipid mediator-induced expression of bactericidal/ permeability-increasing protein (BPI) in human mucosal epithelia. Proceedings of the National Academy of Sciences. 2002;99(6):3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JL, Bena S, Headland SE, McArthur S, Brancaleone V, Perretti M. Chemerin15 inhibits neutrophil-mediated vascular inflammation and myocardial ischemia-reperfusion injury through ChemR23. EMBO Rep. 2013;14(11):999–1007. doi: 10.1038/embor.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. The Journal of experimental medicine. 2008;205(4):767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik GE, Erkekol FO, Misirligil Z, Melli M. Lipoxin A4 levels in asthma: relation with disease severity and aspirin sensitivity. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007;37(10):1494–1501. doi: 10.1111/j.1365-2222.2007.02806.x. [DOI] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. The Journal of experimental medicine. 2015;212(8):1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484(7395):524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacological reviews. 2006;58(3):463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Chiron R, Grumbach YY, Quynh NV, Verriere V, Urbach V. Lipoxin A(4) and interleukin-8 levels in cystic fibrosis sputum after antibiotherapy. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2008;7(6):463–468. doi: 10.1016/j.jcf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. The American review of respiratory disease. 1992;145(6):1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- Cilloniz C, Pantin-Jackwood MJ, Ni C, Goodman AG, Peng X, Proll SC, Carter VS, Rosenzweig ER, Szretter KJ, Katz JM, Korth MJ, Swayne DE, Tumpey TM, Katze MG. Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection. J Virol. 2010;84(15):7613–7624. doi: 10.1128/JVI.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. American journal of physiology. Cell physiology. 2014;307(1):C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby JK, Abdulnour RE, Sham HP, Dalli J, Colas RA, Winkler JW, Hellmann J, Wong B, Cui Y, El-Chemaly S, Petasis NA, Spite M, Serhan CN, Levy BD. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Protective for Injured Epithelia. The American journal of pathology. 2016;186(7):1801–1813. doi: 10.1016/j.ajpath.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. The Journal of clinical investigation. 1993;92(1):75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(45):18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L888–901. doi: 10.1152/ajplung.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen SE, Franzen L, Raud J, Serhan CN, Westlund P, Wikstrom E, Bjorck T, Matsuda H, Webber SE, Veale CA, et al. Actions of lipoxin A4 and related compounds in smooth muscle preparations and on the microcirculation in vivo. Adv Exp Med Biol. 1988;229:107–130. doi: 10.1007/978-1-4757-0937-7_9. [DOI] [PubMed] [Google Scholar]
- Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chemistry & biology. 2013;20(2):188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira JR, Favarin DC, Tanaka SC, Balarin MA, Teixeira DN, Levy BD, Rogerio Ade P. AT-RvD1 modulates CCL-2 and CXCL-8 production and NF-kappaB, STAT-6, SOCS1, and SOCS3 expression on bronchial epithelial cells stimulated with IL-4. Biomed Res Int. 2015;2015:178369. doi: 10.1155/2015/178369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Schwartz CE, Gjorstrup P, Pflugfelder SC. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31(11):1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- Du XY, Leung LL. Proteolytic regulatory mechanism of chemerin bioactivity. Acta biochimica et biophysica Sinica. 2009;41(12):973–979. doi: 10.1093/abbs/gmp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal immunology. 2013;6(2):256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(37):14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, Jozsef L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. American journal of respiratory and critical care medicine. 2009;180(4):311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdinest N, Ovadia H, Kormas R, Solomon A. Anti-inflammatory effects of resolvin-D1 on human corneal epithelial cells: in vitro study. J Inflamm (Lond) 2014;11(1):6. doi: 10.1186/1476-9255-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. The Journal of experimental medicine. 1994;180(1):253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Ryeom SW, Weller PF, Serhan CN. Lipoxin recognition sites. Specific binding of labeled lipoxin A4 with human neutrophils. The Journal of biological chemistry. 1992;267(23):16168–16176. [PubMed] [Google Scholar]
- Flesher RP, Herbert C, Kumar RK. Resolvin E1 promotes resolution of inflammation in a mouse model of an acute exacerbation of allergic asthma. Clin Sci (Lond) 2014;126(11):805–814. doi: 10.1042/CS20130623. [DOI] [PubMed] [Google Scholar]
- Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, Kwak DH, Bahl CD, Hampton TH, Morisseau C, Hammock BD, Liu X, Lee JS, Kolls JK, Levy BD, Madden DR, Bomberger JM. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(1):136–141. doi: 10.1073/pnas.1610242114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O'Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350(6):560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- Fritscher LG, Post M, Rodrigues MT, Silverman F, Balter M, Chapman KR, Zamel N. Profile of eicosanoids in breath condensate in asthma and COPD. Journal of breath research. 2012;6(2):026001. doi: 10.1088/1752-7155/6/2/026001. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 Plays a Pivotal Role in the Resolution of Acute Lung Injury. The Journal of Immunology. 2005;174(8):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- Gastardelo TS, Cunha BR, Raposo LS, Maniglia JV, Cury PM, Lisoni FC, Tajara EH, Oliani SM. Inflammation and cancer: role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma. PloS one. 2014;9(12):e111317. doi: 10.1371/journal.pone.0111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. The Journal of experimental medicine. 1998;187(8):1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan RA, O'Brien E, Mancuso P. Lipoxin A(4) and 8-isoprostane in the exhaled breath condensate of children hospitalized for status asthmaticus. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13(2):141–145. doi: 10.1097/PCC.0b013e3182231644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. Journal of immunology. 2011;186(11):6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nature immunology. 2008;9(8):873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herova M, Schmid M, Gemperle C, Hersberger M. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. Journal of immunology. 2015;194(5):2330–2337. doi: 10.4049/jimmunol.1402166. [DOI] [PubMed] [Google Scholar]
- Higgins G, Fustero Torre C, Tyrrell J, McNally P, Harvey BJ, Urbach V. Lipoxin A4 prevents tight junction disruption and delays the colonization of cystic fibrosis bronchial epithelial cells by Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2016;310(11):L1053–1061. doi: 10.1152/ajplung.00368.2015. [DOI] [PubMed] [Google Scholar]
- Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PloS one. 2013;8(3):e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao HM, Thatcher TH, Levy EP, Fulton RA, Owens KM, Phipps RP, Sime PJ. Resolvin D1 attenuates polyinosinic-polycytidylic acid-induced inflammatory signaling in human airway epithelial cells via TAK1. Journal of immunology. 2014;193(10):4980–4987. doi: 10.4049/jimmunol.1400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Jin Y, Chen Y, Inomata T, Lee H, Chauhan SK, Petasis NA, Serhan CN, Dana R. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Invest Ophthalmol Vis Sci. 2014;55(9):5944–5951. doi: 10.1167/iovs.14-14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nature immunology. 2004;5(4):388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- Karra L, Haworth O, Priluck R, Levy BD, Levi-Schaffer F. Lipoxin B(4) promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal immunology. 2015;8(4):852–862. doi: 10.1038/mi.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. Journal of immunology. 2008;181(12):8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazani S, Planaguma A, Ono E, Bonini M, Zahid M, Marigowda G, Wechsler ME, Levy BD, Israel E. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. The Journal of allergy and clinical immunology. 2013;132(3):547–553. doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltsida O, Karamnov S, Pyrillou K, Vickery T, Chairakaki AD, Tamvakopoulos C, Sideras P, Serhan CN, Andreakos E. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med. 2013;5(5):762–775. doi: 10.1002/emmm.201201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD. Cutting Edge: Maresin-1 Engages Regulatory T Cells To Limit Type 2 Innate Lymphoid Cell Activation and Promote Resolution of Lung Inflammation. Journal of immunology. 2014 doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015;194(3):863–867. doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. The American journal of pathology. 2012;180(5):2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(4):1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E, Severe Asthma Research Program, N.H.L., Blood, I Diminished lipoxin biosynthesis in severe asthma. American journal of respiratory and critical care medicine. 2005;172(7):824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature immunology. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nature medicine. 2002;8(9):1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. Journal of immunology. 2007a;178(1):496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, Parkinson JF. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007b;21(14):3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annual review of physiology. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen KA, Sun B, Karlsson L, Fourie AM. Leukotriene B4 Receptors BLT1 and BLT2: Expression and Function in Human and Murine Mast Cells. The Journal of Immunology. 2006;177(5):3439–3447. doi: 10.4049/jimmunol.177.5.3439. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. The Journal of biological chemistry. 1997;272(11):6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Winkler JW, Petasis NA, Serhan CN, Bazan NG. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins, leukotrienes, and essential fatty acids. 2010;82(1):27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Ruddick A, Arthur GK, Wan H, Woodman L, Brightling CE, Jones DJ, Pavord ID, Bradding P. Primary human airway epithelial cell-dependent inhibition of human lung mast cell degranulation. PloS one. 2012;7(8):e43545. doi: 10.1371/journal.pone.0043545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clinical chemistry. 2012;58(10):1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, Sayama K, Arai H, Betsuyaku T, Arita M, Asano K. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. The Journal of allergy and clinical immunology. 2013;131(2):353–360. e351–352. doi: 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Gastardelo TS, Cunha BR, Raposo LS, Maniglia JV, Cury PM, Lisoni FCR, Tajara EH, Oliani SM. Inflammation and Cancer: Role of Annexin A1 and FPR2/ALX in Proliferation and Metastasis in Human Laryngeal Squamous Cell Carcinoma. PLoS ONE. 2014;9(12):e111317. doi: 10.1371/journal.pone.0111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153(1):112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(22):8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150(3):233–255. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- Nordgren TM, Heires AJ, Wyatt TA, Poole JA, LeVan TD, Cerutis DR, Romberger DJ. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respiratory research. 2013;14:51. doi: 10.1186/1465-9921-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. Journal of immunology. 2012;188(9):4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD, Douda DN, Tabet Y, Khaddaj-Mallat R, Sirois M, Sirois C, Rizcallah E, Rousseau E, Martin R, Sutherland ER, Castro M, Jarjour NN, Israel E, Levy BD, National Heart, L., Blood Institute's Asthma Clinical Research, N Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. American journal of respiratory and critical care medicine. 2014;190(8):886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran K, Radford K, Fanat A, Stephen J, Bonnans C, Levy BD, Janssen LJ, Cox PG. Modulation of human airway smooth muscle migration by lipid mediators and Th-2 cytokines. American journal of respiratory cell and molecular biology. 2007;37(2):240–247. doi: 10.1165/rcmb.2006-0172OC. [DOI] [PubMed] [Google Scholar]
- Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, Moretta A, Sozzani S. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109(9):3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364(9434):613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhou T, Wang Q, Liu P, Zhang T, Zetterstrom R, Strandvik B. Fatty acid composition of diet, cord blood and breast milk in Chinese mothers with different dietary habits. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81(5–6):325–330. doi: 10.1016/j.plefa.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nature medicine. 2002;8(11):1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. American journal of respiratory and critical care medicine. 2008;178(6):574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma A, Pfeffer MA, Rubin G, Croze R, Uddin M, Serhan CN, Levy BD. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal immunology. 2010;3(3):270–279. doi: 10.1038/mi.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PloS one. 2011;6(2):e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martinez-Sobrido L, Topham DJ, Phipps RP. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? Journal of immunology. 2014a;193(12):6031–6040. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S, Bancos S, Serhan CN, Phipps RP. Lipoxin A(4) modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. European journal of immunology. 2014b;44(2):357–369. doi: 10.1002/eji.201343316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. Journal of immunology. 2012;189(4):1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M, Edinger AL, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms RW, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. European journal of immunology. 1998;28(5):1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, Serhan CN, Szczeklik A. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. The European respiratory journal. 2000;16(1):44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. Journal of immunology. 2010;184(2):836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(5):1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(4):1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34(44):14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- Shirey KA, Lai W, Pletneva LM, Karp CL, Divanovic S, Blanco JC, Vogel SN. Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology. Mucosal immunology. 2014;7(3):549–557. doi: 10.1038/mi.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiele F, Recchiuti A, Mattoscio D, De Luca A, Cianci E, Franchi S, Gatta V, Parolari A, Werba JP, Camera M, Favaloro B, Romano M. Transcriptional regulation of the human FPR2/ALX gene: evidence of a heritable genetic variant that impairs promoter activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(3):1323–1333. doi: 10.1096/fj.11-198069. [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. The Journal of biological chemistry. 2007;282(13):9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Tahan F, Saraymen R, Gumus H. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2008;45(2):161–164. doi: 10.1080/02770900701847068. [DOI] [PubMed] [Google Scholar]
- Takamiya R, Fukunaga K, Arita M, Miyata J, Seki H, Minematsu N, Suematsu M, Asano K. Resolvin E1 maintains macrophage function under cigarette smoke-induced oxidative stress. FEBS Open Bio. 2012;2:328–333. doi: 10.1016/j.fob.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, Chanez P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. The Journal of allergy and clinical immunology. 2005;115(1):55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Wan M, Godson C, Guiry PJ, Agerberth B, Haeggstrom JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(5):1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulmonary pharmacology & therapeutics. 2011;24(4):434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wang SB, Hu KM, Seamon KJ, Mani V, Chen Y, Gronert K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(4):1506–1516. doi: 10.1096/fj.11-198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GA, Troxler H, Klinke G, Rogler D, Braegger C, Hersberger M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids in health and disease. 2013;12:89. doi: 10.1186/1476-511X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. The Journal of experimental medicine. 2003;198(7):977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol. 2013;168(1):172–178. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A4 inhibits proliferation of human lung fibroblasts induced by connective tissue growth factor. American journal of respiratory cell and molecular biology. 2006;34(1):65–72. doi: 10.1165/rcmb.2005-0184OC. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Higashi N, Mita H, Ono E, Komase Y, Nakagawa T, Miyazawa T, Akiyama K, Taniguchi M. Urinary concentrations of 15-epimer of lipoxin A(4) are lower in patients with aspirin-intolerant compared with aspirin-tolerant asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41(12):1711–1718. doi: 10.1111/j.1365-2222.2011.03839.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free radical biology & medicine. 2012;53(1):160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387(6633):620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]