Abstract
Rationale
Borderline personality disorder (BPD) is highly associated with alcohol use disorder, but little is known about how BPD individuals consume alcohol, or the immediate effects of their consumption. There is therefore a need for research investigating drinking behavior in BPD.
Objectives
The current study examined rate of alcohol consumption in BPD (N = 54) and community individuals (COM; N = 59) within ecologically valid drinking episodes. We hypothesized that rate of consumption would be elevated in BPD individuals. We further hypothesized that rate of consumption would be positively associated with subjective stimulation, but not sedation, and that stimulation would be associated with increased positive affect (PA) and reduced negative affect (NA).
Methods
Ambulatory Assessment was used to assess rate of consumption, subjective alcohol response, and affect in the moment (Nobservations = 3,444). Rate of consumption was defined as change in estimated blood alcohol concentration (eBAC) relative to drinking episode start. Multilevel modeling was used to test hypotheses.
Results
As hypothesized, BPD individuals demonstrated a faster increase in eBAC than COM individuals. Rate of consumption was associated with subjective stimulation, but not sedation, in both groups. Stimulation was associated with increased PA in both groups and reduced NA in the BPD group.
Conclusions
BPD individuals consumed alcohol more rapidly than COM individuals. Faster consumption may serve as a means for BPD individuals to maximize the rewarding pharmacological effects of alcohol and to increase positive and reduce negative affect.
Keywords: alcohol consumption, affect regulation, borderline personality disorder, rate of consumption, estimated blood alcohol concentration, ambulatory assessment
Borderline personality disorder (BPD) is a serious mental disorder affecting 1–3% of the general population (Lenzenweger et al., 2007; Trull et al., 2010). BPD individuals have a propensity to engage in maladaptive behavior in order to regulate negative affect (NA; Crowell et al., 2009). One particularly common maladaptive behavior is alcohol use: approximately half of BPD individuals meet criteria for alcohol use disorder (AUD; Tomko et al., 2014; Trull et al., 2016; Trull et al., 2000), and approximately 15% of individuals with AUD meet criteria for BPD (Trull et al., 2016; Trull et al., 2000).
Despite the link between BPD and AUD, little is known about how BPD individuals consume alcohol. Most of those with BPD experience drinking-related problems, but it is unclear what aspects of their drinking habits (e.g., too much, too often, too quickly) distinguish them from groups less likely to experience problems. Previous research has also not examined how their consumption relates to their reasons for use (e.g., affect regulation; Trull et al., 2000), or the immediate effects of use. Thus, the current study sought to shed light on alcohol use in BPD.
BPD is also a disorder of emotion dysregulation (Carpenter & Trull, 2013; Crowell et al., 2009) and is comorbid with mood disorders (Tomko et al., 2014). Reinforcement models of alcohol use posit that individuals drink to heighten positive affect (PA) and reduce NA (e.g., Baker et al., 2004; Cooper et al., 1995). Knowing more about consumption in BPD and how it differs from consumption in non-BPD individuals would increase our knowledge of the link between alcohol use and emotion dysregulation more broadly.
Quantity-Frequency
Alcohol consumption is often measured in terms of quantity (the volume consumed) and frequency (the number of drinking episodes; Q-F; Greenfield & Kerr, 2008), which together provide an estimate of the total volume of alcohol consumed in a time period, typically consisting of several weeks or longer. Existing research, while limited, does not find consistent evidence of elevated Q-F in BPD. In an investigation of alcohol craving, Lane et al. (2016), working from the same dataset as the present study, found that BPD individuals, compared to community comparisons, drank a similar number of drinks per drinking episode (quantity) and had fewer drinking episodes (frequency) over three weeks of ambulatory assessment (AA; also referred to as ecological momentary assessment). Rolland et al. (2015) found that BPD participants with comorbid alcohol dependence did not differ from participants with only alcohol dependence in average weekly Q-F prior to beginning an alcohol treatment program. Using a nationally representative sample, Maclean and French (2014) found that BPD was positively associated with Q-F indicators. However, findings were largely restricted to men, and many of the associations became nonsignificant when evidence of impairment was required for each BPD criterion (cf., Trull et al., 2010). Thus, the available evidence does not suggest a strong association between BPD and Q-F.
In part, this may result from the fact that, despite the strengths of Q-F measures, total volumes derived from such measures may be equal, yet result from different underlying drinking patterns. Differences in drinking patterns may be of clinical and research interest. For example, seven episodes of one drink each will have different subjective and pharmacological effects than one episode of seven drinks. BPD individuals, then, may tend to engage in drinking patterns that are more maladaptive than those of non-BPD individuals, but indistinguishable in terms of Q-F.
Rate of Consumption
Q-F, however, are not the only measures of alcohol consumption. Others exist, one being the rate of consumption, defined as the amount of alcohol consumed per unit time within a drinking episode. By incorporating a more granular measure of time, rate of consumption can more readily distinguish different drinking patterns than Q-F. Therefore, rate of consumption may be more sensitive than Q-F to the drinking behavior of BPD individuals. Rate of consumption may also be more closely related to the pharmacological effects of consumption.
Rate has long been recognized as a risk factor for problematic drinking and is inherent in the idea of drinking “too much too fast” (Leeman et al., 2010; Li et al., 2007). It is also inherent in binge drinking or the consumption of five or more drinks (four for females) in a period of about 2 hours (Dietary Guidelines Advisory Committee, 2010). Binge drinking is associated with numerous adverse health effects (e.g., Wechsler et al., 1994) and is also associated with AUD criteria (Saha et al., 2007). However, rate of consumption is a broader construct than binge drinking, as individuals can consume alcohol at elevated rates without bingeing.
While not frequently studied, rate of consumption has been associated with alcohol-related problems, specifically cognitive impairment (Jones & Vega, 1972; Moskowitz & Burns, 1976) and self-reported anxiety and hostility (Connors & Maisto, 1979). “Gulping” of drinks is associated with an increased probability of blackout (Goodwin et al., 1969; Perry et al., 2006; Ryback, 1970). Using AA, Trela et al. (2016) found that lower alcohol sensitivity, a well-documented risk factor for AUD, was associated with elevated rate of consumption, as measured by a steeper estimated blood alcohol concentration (eBAC) slope after drinking initiation.
Elevated rate of consumption is also related to greater subjective intoxication (Martin et al., 2006; Martin & Earleywine, 1990) and, in men with family histories of alcoholism, greater stimulant response, as measured by heart rate change (Conrod et al., 1997; Conrod et al., 2001). These studies agree with experimental findings that rising BAC is positively associated with subjective stimulation (Hendler et al., 2013; Martin et al., 1993). Individuals may increase consumption rate, then, in order to maximize the hedonically positive effects of subjective stimulant and intoxication effects of alcohol (e.g., Martin et al., 1993). However, despite the evidence of the importance of rate of consumption, no previous study has examined rate in BPD.
The Present Study
The goal of the present study was to examine the association of BPD and rate of consumption. We were specifically interested in rate of consumption within the drinking episode. Therefore, we used AA (Shiffman et al., 2008; Trull & Ebner-Priemer, 2013) to track drinking in daily life. We computed momentary eBAC values using a validated formula well-suited for ad libitum drinking episodes (Hustad & Carey, 2005; Mathews & Miller, 1979). By incorporating information about the individual and their drinking, eBAC offers a measure of consumption informed by the pharmacokinetics of alcohol. In order to assess rate of consumption, we assessed change in eBAC over time within drinking episodes.
Our primary hypothesis was that BPD would be associated with greater rate of consumption, evidenced by a faster positive change in eBAC relative to the initial drink. In contrast, we did not predict group differences in terms of mean level of eBAC, which we expected to closely correspond to the number of drinks consumed in an episode (a measure of Q-F). As noted above, Lane et al., (2016) previously reported that BPD individuals in the current dataset did not differ from community (COM) comparisons in terms of number of drinks consumed per episode. We hypothesized that rate of consumption would be related to BPD, when Q-F is not, because of rate’s ability to distinguish between patterns of use.
Second, we predicted that BPD individuals would consume alcohol at elevated rates in order to maximize the rewarding pharmacological effects of alcohol. Therefore, we additionally expected that rate of consumption would be associated with subjective stimulant, but not (hedonically negative) sedative, response to alcohol, consistent with past research (Conrod et al., 1997; Conrod et al., 2001; Martin et al., 2006; Martin & Earleywine, 1990). We did not expect this relationship to differ by group, as we expected this to be a pharmacological effect that is relatively consistent across individuals. This finding, though, would be particularly meaningful for the BPD group, if BPD individuals, as predicted above, consume alcohol at elevated rates.
Third, we were interested in the implications of increased subjective stimulation for affective state. We predicted that subjective stimulation would be associated with increased PA and decreased NA. Following reinforcement models of substance use (e.g., Baker et al., 2004; Copper et al., 1995), Trull et al. (2000) theorize that BPD individuals consume alcohol in order to regulate mood, in particular to reduce NA. Given the positive hedonic nature of subjective stimulation, we predicted that greater stimulation would lead to increased PA and reduced NA in the moment. Again, we predicted that these effects would not differ by group. We did not make hypotheses regarding the relationship of subjective sedation and affect, as sedation effects are unpleasant and our focus was on the rewarding effects of alcohol consumption.
Method
Participants
The initial sample was the same used by Lane et al. (2016) and consisted of 116 participants. Of these, 56 were in the BPD group, while the remaining 60 were in the COM group. One COM participant was removed because of 3 drinking episodes with eBAC estimates exceeding .300g%, including the only estimate in the sample above .500g%.1 Another 2 BPD participants did not report any alcohol consumption over the course of the study. This left 54 in the BPD group and 59 in the COM group, with 113 participants overall. BPD participants were recruited from local psychiatric clinics via flyers and the general community via advertisements. COM participants were recruited through separate advertisements to the general community. Potential participants completed a phone screening, which, if they appeared eligible, was followed by an in-depth, face-to-face diagnostic interview.
Participants were required to meet the following eligibility requirements: a) age between 18 and 45 years old, b) report at least one drinking episode per week on average over the past month, c) report no current psychosis, intellectual disability, or neurological dysfunction, d) report no significant head trauma history, e) report no past-year unsuccessful attempts to cut down or stop drinking or physiological withdrawal symptoms (indicators of severe alcohol use problems), and f) not currently in treatment or interested in treatment for alcohol use disorder. Women were excluded if they reported being pregnant or planning to become pregnant. Individuals in the BPD group had to meet criteria for DSM-IV BPD and endorse the affective instability criterion for BPD (APA, 2013). COM participants could not meet criteria for BPD or endorse the affective instability criterion.
DSM-IV Axis I and II disorders were determined via two semi-structured interviews: the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbons, & Williams, 1995) and the Structured Interview for DSM-IV Personality (SIDP-IV; Pfohl, Blum, & Zimmerman, 1994). Inter-rater agreement was computed for 20 randomly selected participants by having a second interviewer rate video recordings of the original interviews. Agreement was excellent for the diagnosis of BPD (κ = .88) and for presence of affective instability (κ = .89). Mean age for the BPD group was 26.02 (SD = 7.16) and for the COM group was 26.78 (SD = 7.16). All BPD and two COM participants were currently in mental health treatment. See Table 1 for more detail on demographics and comorbidity.
Table 1.
Demographics and Comorbid Conditions of the Study Sample (N=113)
| BPD (n=54)
|
COM (n=59)
|
||||
|---|---|---|---|---|---|
| n | % | n | % | χ2 | |
| Gender-Female | 44 | 81.5 | 44 | 74.6 | 0.78 |
| Ethnicity | 2.84 | ||||
| African-American | 4 | 7.4 | 3 | 5.1 | |
| Hispanic | 1 | 1.9 | 2 | 3.4 | |
| Caucasian | 45 | 83.3 | 51 | 86.4 | |
| Asian/Asian-American | 2 | 3.7 | 3 | 5.1 | |
| Other | 2 | 3.7 | 0 | 0.0 | |
| Marital Status | 7.44 | ||||
| Single, Never Married | 39 | 72.2 | 37 | 63.8 | |
| Married | 7 | 13.0 | 16 | 27.6 | |
| Cohabitating | 1 | 1.9 | 3 | 5.2 | |
| Divorced or Separated | 7 | 13.0 | 2 | 3.5 | |
| Annual Income | 17.65** | ||||
| $0 to $25,000 | 42 | 77.8 | 23 | 39.0 | |
| $25,001 to $50,000 | 6 | 11.1 | 17 | 28.8 | |
| $50,001 to $75,000 | 2 | 3.7 | 5 | 8.5 | |
| $75,001 to $100,000 | 2 | 3.7 | 5 | 8.5 | |
| Above $100,000 | 2 | 3.7 | 9 | 15.3 | |
| Currently Employed | 42 | 77.8 | 47 | 79.7 | 0.06 |
| Current Psychotropic Medication | 40 | 74.1 | 5 | 8.5 | 50.63*** |
| Current Diagnoses | |||||
| Any anxiety disorder | 36 | 66.7 | 12 | 20.3 | 24.77*** |
| Any mood disorder | 23 | 42.8 | 1 | 1.7 | 28.19*** |
| Any eating disorder | 4 | 7.4 | 0 | 0.0 | 4.53* |
| Any drug use disorder | 11 | 20.4 | 1 | 1.7 | 10.36** |
| Any alcohol use disorder | 17 | 32.1 | 7 | 12.3 | 6.31* |
| Any PD (other than BPD) | 28 | 51.9 | 1 | 1.7 | 37.18*** |
| Narcissistic PD | 5 | 9.3 | 0 | 0.0 | 5.72* |
| Histrionic PD | 1 | 1.9 | 0 | 0.0 | 1.10 |
| Antisocial PD | 11 | 20.4 | 0 | 0.0 | 13.31*** |
| Schizotypal PD | 1 | 1.9 | 0 | 0.0 | 1.10 |
| Schizoid PD | 0 | 0.0 | 0 | 0.0 | 0.00 |
| Paranoid PD | 6 | 11.1 | 0 | 0.0 | 6.92** |
| Avoidant PD | 9 | 16.1 | 1 | 1.7 | 7.83** |
| Dependent PD | 2 | 3.7 | 0 | 0.0 | 2.22 |
| Obsessive Compulsive PD | 8 | 14.8 | 0 | 0.0 | 9.41** |
Note. N = 113. BPD = Borderline Personality Disorder; COM = Community; PD = Personality Disorder.
p < .05,
p < .01,
p < .001.
Electronic Diary Protocol
Participants were trained to use an electronic diary (ED; Palm Tungsten E2© handheld computer) at an orientation session, during which they also completed self-report questionnaires. Participants were instructed on how to report alcohol drinks and given the definition of a standard drink. Participants carried the ED for approximately 21 days (M = 21.7; SD = 2.1, Range = 7–25) and were paid $50 each week of the study, provided compliance was above 80% (payment was reduced $10 for every 10% below 80%). Participants were additionally paid $20 for the diagnostic interview, and $10 each for the orientation and completing a self-report battery at study end (maximum compensation = $190). Two participants dropped out of the study early, but are included in analyses as they provided at least 7 days of data. The remaining 111 participants provided at least 18 days of data. Compliance with the diary protocol was high, with participants completing 90.26% of random prompts and 92.95% of all follow-up prompts.
The ED protocol consisted of seven types of reports. Morning reports were made daily at wake-up. Random prompts were scheduled to occur 6 times throughout each day. Random prompts could not occur during a drinking episode sequence. User-initiated cigarette reports and initial self-harm reports were made by participants following cigarette use or a self-harm event. Self-harm follow-ups occurred at intervals following an initial self-harm report.
Participants made user-initiated initial drink reports at the completion of their first drink of alcohol in an episode. Additionally, participants were asked at all non-alcohol centric reports whether they had consumed alcohol since their prior report. This was built into the ED protocol as a precautionary measure, and does not necessarily constitute non-compliance. For example, a random prompt may occur before a participant had the opportunity to complete an initial drink report. Drinking follow-ups occurred at set intervals (30, 60, 120, and 180 minutes) following any report of an initial drink. At each follow-up, participants reported the number of drinks, if any, consumed since the previous prompt. Beginning at 180 minutes, an additional follow-up was scheduled 60 minutes following each time a new drink was reported. Thus, a drinking episode was considered to begin when an initial drink was reported and end 1) 3 hours after the last reported drink 2) when the participant indicated they were going to sleep, or 3) when the participant failed to complete the last scheduled follow-up. The present analyses examined drinking follow-up reports and any report where alcohol consumption was reported.
Measures
Subjective Alcohol Effects
At each assessment, participants were asked to rate, on a five point Likert scale, “In the PAST 15 MINUTES, did you feel/have” in regard to six items: sluggish, difficulty concentrating, talkative, energized, dizzy, and buzzed. The first four items were taken from the Biphasic Alcohol Effects Scale (Martin et al., 1993), and the last two have been used previously to assess momentary subjective intoxication (Piasecki et al., 2011). We combined dizzy, sluggish, and difficulty concentrating to create a sedative subjective effects indicator, and buzzed, talkative, and energized to create a stimulant subjective effects indicator. As reported in the Supplementary Material, both indicators exhibited good reliability.
Affect
PA and NA were assessed using items from the Positive and Negative Affect Schedule-Extended version (PANAS-X; Watson & Clark, 1999). At each prompt, participants were asked to rate the extent to which they felt each affective state on a five point Likert scale “in the PAST 15 MINUTES.” Overall positive (10 items) and negative (21 items) affect were created by taking the average of each item set.
eBAC
Estimated BAC has long been found to be valid and reliable, with the correlation between eBAC and BAC measured through venous or breath samples ranging from r = .500 to .600 (Hustad & Carey, 2005). Momentary eBACs were computed for each ED report using the Mathews and Miller (1979) formula, which has shown superior accuracy for ad lib drinking episodes (Hustad & Carey, 2005). The formula utilizes gender, weight, average population rate for metabolizing alcohol, time elapsed, and drink number to estimate BAC. In the case of the initial drink, the ED did not assess the length of consumption and we assumed this drink was always consumed over a 20-minute period. While arbitrary, a non-zero value is more reasonable than assuming instantaneous absorption of the first drink at the moment of report. The use of a constant value across participants and episodes affects the scaling of momentary eBAC, but not the rank ordering of eBAC data or correlations between eBAC and other variables.
Participants could record multiple drinking episodes in a given day, provided the initial episode ended as described above (nepisodes = 91). These episodes were included in the original episodes if eBAC had not yet returned to .000g% (nepisodes = 52). Secondary episodes where eBAC had returned to .000g% (nepisodes = 39) were not included as we were less confident that these episodes truly began at .000g%. Finally, instances where the eBAC formula produced an extreme, and unlikely, value (e.g., values associated with coma and death; nobservations = 55), we Winsorized to .250g%.2 Results did not differ when eBAC scores were not Winsorized.
Analytic Strategy
Data preparation
Across all data, time of observations ranged from 20 (the initial drink) to 689 minutes (M = 106.02, SD = 99.37). Few assessments occurred after the sixth follow-up (320 minutes; nobservations = 111) and we therefore censored data after 350 minutes. Thus, the final data included 3,444 assessments ranging from 20 to 349 minutes (M = 94.61, SD = 75.87). The majority (82.4%) were completed within 5 minutes of the programmed follow-up time window. Therefore, for MLM analyses, we binned observations according to the follow-up to which they were closest in time.3 The follow-up schedule presented a natural choice for binning the data as participants could only report additional consumption at these times. This yielded seven time points centered around 20 min., 50 min., 80 min., 140 min., 200 min., 260 min., and 320 min. (Time 0 – Time 6). Table 2 presents a breakdown of assessments.
Table 2.
Frequency of completed prompts and average estimated blood alcohol concentration (eBAC) by time bin.
| Time Bin | Minutes elapsed (range) | Total | BPD | COM | |||
|---|---|---|---|---|---|---|---|
| N (%) | eBAC M (SE) | N (%) | eBAC M (SE) | N (%) | eBAC M (SE) | ||
| 0 | 20 (20 – 35) | 909 (26.39) | .022 (.003) | 372 (27.72) | .021 (.004) | 537 (25.55) | .023 (.003) |
| 1 | 50 (36 – 65) | 725 (21.05) | .037 (.003) | 284 (21.16) | .038 (.004) | 441 (20.98) | .036 (.004) |
| 2 | 80 (66 – 110) | 675 (19.6) | .043 (.003) | 263 (19.6) | .046 (.004) | 412 (19.6) | .039 (.004) |
| 3 | 140 (111 – 170) | 526 (15.27) | .046 (.003) | 196 (14.61) | .050 (.004) | 330 (15.7) | .042 (.004) |
| 4 | 200 (171 – 230) | 420 (12.20) | .052 (.003) | 158 (11.77) | .058 (.004) | 262 (12.46) | .046 (.004) |
| 5 | 260 (231 – 290) | 123 (3.57) | .089 (.004) | 46 (3.43) | .098 (.006) | 77 (3.66) | .082 (.005) |
| 6 | 320 (291 – 350) | 66 (1.92) | .103 (.005) | 23 (1.71) | .104 (.008) | 43 (2.05) | .102 (.006) |
Note: BPD = Borderline Personality Disorder; COM = Community. Minutes elapsed includes the additional 20 minutes added to the initial drink report to provide time for absorption. Reported means were least squares means.
Modeling approach
We used multilevel modeling (MLM) using restricted maximum likelihood estimation to evaluate our hypotheses. This was done because drinking reports occurred in the moment and were nested within drinking episodes, which were nested within person. Additionally, observations were unevenly spaced across episodes and persons. Unless noted otherwise, models had three levels (moment, episode, and person) and included random intercepts at the episode and person level (see Supplementary Material for more detail). Models also included age (sample centered), day of study, day of week, and hour after wakeup as covariates. We used the PROC MIXED procedure in SAS® 9.4 (SAS, 2014) for these analyses.
Our primary goal was to examine group differences in rate of consumption, taking into account the nonlinear rise and fall of eBAC estimates over time (Jones et al., 2006). We therefore sought to develop an MLM model that closely approximated actual changes in eBAC over time with the fewest assumptions possible. Accomplishing this was complicated by the fact that, in the current data, participants’ drinking episodes differed in terms of length and consisted of reports corresponding to different points in time. Thus, each drinking episode had a unique eBAC curve. For this reason, we fit a free-curve MLM with eBAC as the outcome and group, Time 1 – Time 6 (Time 0 was the reference), and two-way interactions of group with Time 1 – Time 6 as independent variables (IVs; see Supplementary Material for the model specification). This allowed for the estimation of six different slopes, or regression splines, each representing change in eBAC relative to Time 0. Thus, the first slope corresponded to change in eBAC from Time 0 (20 min.) to Time 1 (50 min.), the second from Time 0 to Time 2 (80 min.) and so forth. As each slope was estimated separately, they captured the nonlinearity in eBAC change over the drinking episode (i.e., they allowed for relative increase or decrease in eBAC at each time point for each episode).4 The two-way interactions of group and Time 1 – Time 6 were the primary effects of interest, representing group differences in change in eBAC at each time point.
To ensure that the MLM model testing change in eBAC accurately represented the data, we also conducted a comparable nonparametric loess regression with a smoothing window corresponding to 10% overlap in the adjacent windows (Cleveland et al., 1988). Loess regression makes fewer assumptions than MLM about the form of data and, as a result, has advantages in characterizing raw data. However, it is substantially more cumbersome to test for group differences and therefore was used only to provide a visual comparison for the MLM model. The fact that our MLM model closely matched the loess regression is taken as evidence that our model accurately represented the data, strengthening our confidence that the findings reflected actual differences and not artifacts of our modeling approach.
For the analyses regarding subjective alcohol response, we were interested in whether change in eBAC was associated with subjective response. To test this, we calculated difference scores (subtracting the eBAC value at the previous time point from the current time point) to create a change in eBAC variable (cf. Laird & Weems, 2011). We then conducted two MLMs, with stimulant and sedative effects as the dependent variable (DV), respectively, with Time 1 – Time 6 (Time 0 was the reference), change in eBAC, and covariates as IVs. To test that effects were specifically due to change in eBAC, we also included eBAC level. We did not predict group differences for these models, but included interactions for change in eBAC and group.
To examine the relationship of subjective stimulation and mood, we conducted two additional MLMs with PA and NA as the DV, respectively, and subjective stimulation included as an IV. We separately modeled momentary, episode, and person effects for stimulation (Curran & Bauer, 2011), and included interactions with group. Additional IVs were Time 1 – Time 6 (Time 0 was the reference), change in eBAC, eBAC level, and covariates.
Results
Preliminary Analyses
There were 903 drinking episodes, of which 436 were initiated by a report other than an initial drink report (90.6% random prompts, 8.8% cigarette reports, 0.6% self-harm reports).5 There were 2,489 completed drinking follow-ups and 52 secondary episode reports that were treated as drinking follow-ups. The cumulative number of drinks reported in the study was 2,858.6 As the eBAC formula standardizes based on weight and gender, we tested for group differences on these variables. There were no significant differences between groups on weight (BPD: M = 174.3 lbs., SD = 50.82 lbs.; COM: M = 160.8 lbs., SD = 33.11 lbs.; t(90.09) = 1.65, p = .102) or gender (Table 1). Average length of a drinking episode was 155.71 minutes (SD = 85.30). Groups did not differ in length of episodes, t(107) = 1.05, p = .295. Mean eBAC for the entire sample was .038 (SE = .003). Mean eBAC for the BPD group was .040 (SE = .004) and for the COM group was .037 (SE = .004). As expected, and consistent with Lane et al., (2016), groups did not differ on overall episodic eBAC level (t(107) = −0.62, p = .535).
Rate of Consumption
We next examined change in eBAC (rate of consumption). Results of the MLM are presented in Table 3. Figure 1 depicts the eBAC estimates plotted as a function of time since the first reported drink of an episode. Also included are the MLM and loess regression fits by group. We first note that the MLM fits closely parallel those from the loess regression, although they diverge somewhat at Times 5 and 6.7 This suggests that the free-curve MLM was able to accurately capture variations across time, giving us confidence that our model adequately characterized the nonlinearity in each group’s average eBAC curve.
Table 3.
Parameter estimates for multi-level model of change in estimated blood alcohol concentration (eBAC) over time by group (N = 3,444).
| Est. | SE | βa | dfb | t | |
|---|---|---|---|---|---|
|
|
|||||
| Intercept | .007 | .005 | 521 | 1.36 | |
| Group | −.002 | .005 | −0.05 | 135 | −0.45 |
| Time 1 | .011 | .002 | 0.23 | 2,764 | 5.85*** |
| Time 2 | .014 | .002 | 0.29 | 2,871 | 7.21*** |
| Time 3 | .015 | .002 | 0.31 | 3,125 | 6.96*** |
| Time 4 | .017 | .002 | 0.34 | 3,319 | 6.87*** |
| Time 5 | .051 | .004 | 1.03 | 3,234 | 12.65*** |
| Time 6 | .070 | .005 | 1.40 | 3,211 | 13.27*** |
| Time 1 × Group | .006 | .003 | 0.12 | 2,761 | 1.90 |
| Time 2 × Group | .009 | .003 | 0.18 | 2,789 | 2.84** |
| Time 3 × Group | .010 | .003 | 0.21 | 2,820 | 2.99** |
| Time 4 × Group | .015 | .004 | 0.30 | 2,836 | 3.93*** |
| Time 5 × Group | .018 | .006 | 0.36 | 2,886 | 2.83** |
| Time 6 × Group | .003 | .009 | 0.06 | 2,872 | 0.35 |
| Study Day | −.0003 | .0002 | −0.01 | 885 | −1.95 |
| Weekday (Saturday is reference) | |||||
| Sunday | −.003 | .003 | −0.06 | 862 | −0.86 |
| Monday | −.005 | .004 | −0.10 | 896 | −1.34 |
| Tuesday | −.008 | .004 | −0.15 | 885 | −2.07* |
| Wednesday | −.001 | .004 | −0.03 | 899 | −0.38 |
| Thursday | −.002 | .003 | −0.03 | 887 | −0.49 |
| Friday | −.001 | .003 | −0.02 | 868 | −0.28 |
| Hour after Wakeup | .002 | .000 | 0.09 | 1,024 | 6.28*** |
| Age | −.001 | .000 | −0.15 | 110 | −3.08** |
Note. N = 113 individuals, 3,444 observations used. df = degrees of freedom.
Effect sizes were calculated by scaling eBAC values by the overall SD across occasions, days, and persons. Values are interpreted as the SD change in eBAC corresponding to unit change of each predictor (age and hour after wakeup were standardized using the between-person SD and SD of between-person averages, respectively).
Degrees of freedom were calculated using the Kenward-Roger approximation. Covariates in the model consisted of age (centered), day of study, weekday, and hour after wakeup.
p < .05.
p < .01.
p < .001.
Figure 1.
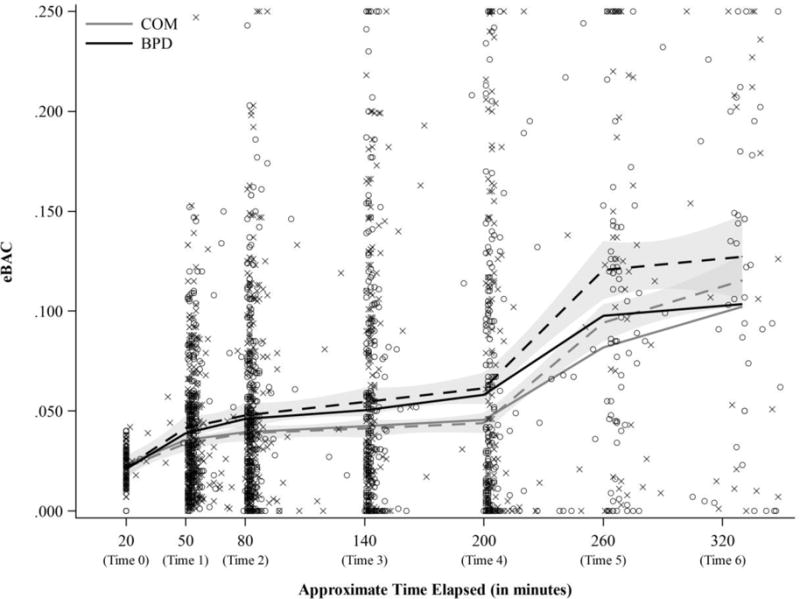
Estimated blood alcohol concentration (eBAC) over time by group. Dotted lines represent loess regression and solid lines represent model fits (‘X’s indicate BPD eBAC estimates and ‘O’s indicate COM estimates).
There were main effects for all six time points, indicating that eBAC increased, on average, in both groups across the drinking episode. The six two-way interactions of group and each time point tested group differences in the change of eBAC, relative to Time 0. There were significant two-way interactions for group with Time 2 (b = .009, SE = .003, β = 0.18), Time 3 (b = .010, SE = .003, β = 0.21), Time 4 (b = .015, SE = .004, β = 0.30), and Time 5 (b = .018, SE = .006, β = 0.36). These interactions indicated that the BPD group, compared to the COM group, was associated with significantly elevated change in eBAC at these time points.
Rate and subjective stimulation
We examined whether change in eBAC, relative to the previous time point, was associated with self-reported sedative and stimulant effects.8 For sedation, there was a main effect for group (b = 0.43, SE = 0.10, t(108) = 4.49, p < .001, β = 0.64), indicating BPD individuals reported more sedation. There was also a significant effect for eBAC level (b = 0.02, SE = 0.003, t(3,320) = 6.24 p < .001, β = 0.12), indicating that higher eBAC level was associated with greater sedation. There was no effect for change in eBAC (b = −0.01, SE = 0.01, t(2,904) = −1.35, p = .176, β = −0.03), indicating that change in eBAC was not significantly related to sedative effects. There was also no interaction of change in eBAC with group (b = 0.01, SE = 0.01, t(3,147) = 1.01, p = .310, β = 0.02).
For stimulation, there was a main effect for group (b = 0.19, SE = 0.09, t(111) = 2.04, p = .044, β = 0.22), indicating BPD individuals reported more stimulation. There was also a significant effect for eBAC level (b = 0.05, SE = 0.004, t(3,227) = 12.20, p < .001, β = 0.24), indicating that being at a higher eBAC level was associated with greater stimulation. Finally, there was a significant effect for change in eBAC (b = 0.05, SE = 0.01, t(2,815) = 6.26, p < .001, β = 0.12), indicating that greater positive change in eBAC was related to greater stimulation. As predicted, there was no interaction of change in eBAC with group (b = 0.01, SE = 0.01, t(3,038) = 1.29, p = .200, β = 0.03).
Subjective stimulation and affect
We next tested the relationship of subjective stimulation and PA and NA. For PA, there were main effects for momentary (b = 0.32, SE = 0.02, t(2,624) = 16.83, p < .001, β = 0.10), episode (b = 0.43, SE = 0.03, t(805) = 13.48, p < .001, β = 0.15), and person-level (b = 0.79, SE = 0.13, t(103) = 6.18, p < .001, β = 0.61) stimulation, such that greater stimulation corresponded with greater PA. There were no significant interactions for stimulation with group.
For NA, there were no main effects, but there were interactions with group for momentary (b = −0.03, SE = 0.01, t(2,20) = −2.16, p = .031, β = −0.02), and episode-level (b = −0.08, SE = 0.03, t(764) = −2.95, p = .003, β = −0.05) stimulation, such that stimulation was associated with reduced NA in the BPD group. There was also a main effect for group (b = 0.36, SE = 0.07, t(105) = 5.11, p < .001, β = 0.82), such that the BPD group reported more NA overall.
Supplemental Analyses
We conducted several supplementary analyses, details of which may be found in the Supplementary Materials, but are also summarized here for the reader’s convenience. First, we tested for group differences in the propensity to engage in binge-like behavior. BPD patients engaged in binge drinking (i.e., achieving an eBAC of .080 in the first two hours of an episode) during 23.71% of episodes, while COM individuals did so in 16.04% of episodes. Using a generalized logistic model, we determined that BPD patients were 1.53 times the odds more likely to engage in binge drinking (OR = 1.53; 95% Confidence Interval [CI] = [1.09 – 2.14]).
As many initial drinks were not self-initiated by participants, we tested whether results for rate of consumption would change if we limited data to episodes initiated by participants. The pattern of results did not differ, except that the interaction for group at Time 6 became significant (Table S2). We were also interested in whether group differences in consumption rate were due, at least in part, to diagnoses highly comorbid with BPD. We conducted a model that included current AUD and its interactions with group and time as predictors (Table S3). There were significant interactions for BPD, independent of current AUD, at Times 2, 3, 4, and 5, and significant interactions for current AUD at Times 3, 4, 5, and 6. We also examined whether the presence of current mood and/or anxiety disorder influenced rate above and beyond BPD (Table S4). Current mood/anxiety was largely not associated with rate of consumption, and BPD continued to be associated with elevated rate of consumption. Finally, in light of the associations between rate and stimulation, and stimulation and affect, it seemed plausible to expect that rate of consumption might be positively associated with PA and negatively with NA. Therefore, we conducted two MLMs with PA and NA as the DV. Change in eBAC was associated with greater PA (b = 0.02, SE = 0.01, t(2,805) = 4.08, p < .001), but not NA (b = 0.002, SE = 0.003, t(2,675) = 0.57, p = .567). There were no group interactions. We also tested whether subjective stimulation mediated the relationship of consumption rate and affect (Supplementary Material). For PA, we found an indirect effect of stimulation (b = 0.01, 95% CI = [0.01, 0.02], p < .001), but no equivalent effect for NA beyond a non-significant trend in the BPD group (p = .095).
Discussion
Results supported hypotheses regarding rate of consumption: the BPD group displayed greater change in eBAC compared to the COM group across the majority of the episode. The size of the difference, as indicated by the interaction of group and time point, was about .010g% at Time 2 and nearly .020g% at Time 5, a meaningful difference in terms of intoxication. The BPD group spent more time (potentially hours more) at elevated eBAC than the COM group. BPD individuals were also more likely to binge drink, a complementary indicator of elevated rate of consumption in this group. It should be noted, however, that group differences were apparent early in the drinking episode, before they would have been detected by an analysis of binge drinking. These results provide important information about the way in which BPD individuals consume alcohol, a topic that has received relatively little attention.
In turn, change in eBAC was positively related to momentary subjective stimulation, but not sedation, independent of the effect for eBAC level. This fits with previous findings that rate of consumption is associated with subjective intoxication and stimulation (Conrod et al. 1997; Conrod et al., 2001; Martin et al., 2006; Martin & Earleywine, 1990), and that rising BAC is related to stimulation (Hendler et al., 2013; Martin et al., 1993). The findings suggest, as expected, that drinking at an elevated rate may serve to maximize the rewarding pharmacological effects of alcohol. Although this association occurred across groups, the implications are more pronounced for the BPD group, given their elevated rate of consumption. To our knowledge, this is the first study to examine the relationship of BPD and subjective alcohol effects.
BPD individuals may seek to maximize pleasurable effects of alcohol as a result of impulsivity (APA, 2013), to remove aversive states (e.g., NA), to enhance positive ones (e.g., PA), or for other reasons (see Trull et al., 2000). The finding that subjective stimulation was associated with increased PA in both groups and, in the BPD group, reduced NA, supports the idea that BPD individuals elevate rate of consumption in order to regulate mood. The finding that NA was only reduced in the BPD group may have been due to a floor effect, as the COM group reported little NA across the study (MBPD = 1.42, SEBPD = 0.05; MCOM = 1.06, SECOM = 0.05). This was further supported by supplementary analyses indicating that rate of consumption was positively associated with PA, though not NA, and mediation analyses that found an indirect effect of stimulation on PA and, in the BPD group, a trend on NA (see Supplementary Material).9 In sum, although the evidence was stronger for PA than NA, the results support the idea that alcohol use is a maladaptive behavior that BPD individuals use for affective self-regulation (Crowell et al., 2009; Trull et al., 2000).
In contrast to findings for rate of consumption, groups did not differ in overall episodic eBAC level, paralleling Q-F comparisons in this sample reported by Lane et al. (2016). Although this is congruent with the existing, albeit limited, literature, this absence remains somewhat surprising. It may be that Q-F is not a sensitive measure of the drinking habits of BPD individuals. As already noted, rate of consumption is better able to distinguish patterns of use, especially within drinking episode. Given that factors hypothesized to influence drinking in BPD individuals, such as NA, are often transient and prone to change, the pattern of consumption, as opposed to total volume, may be particularly important in this population.
It is additionally possible that several environmental factors may have contributed to reduced Q-F in the BPD group. For example, all BPD participants were in treatment and most were taking psychotropic medication. Many such medications discourage alcohol use and, although treatment could not focus on alcohol, therapies may have encouraged moderation secondarily. BPD participants were also more likely to have an annual income under $25,000, and, thus, may have been less able to afford alcohol. However, if these or other factors led to a suppression of Q-F in the BPD group, then the findings suggest that consumption rate is resistant to such effects, or may serve as a compensatory mechanism for suppressed Q-F. If this is the case, clinicians treating BPD and AUD should pay particular attention to rate of consumption.
Strengths, Limitations, and Conclusion
There were multiple strengths to the present work. First, the intensive sampling strategy provided good temporal resolution for assessing rate of consumption in the natural environment. Second, eBAC scores allowed us to more closely account for the pharmacokinetics of alcohol consumption. Third, our analytic strategy allowed us to accommodate a complicated data structure and develop a statistical model that efficiently characterized individuals’ eBAC trajectories. Fourth, this was the first study to examine the relationship of subjective stimulation and affect. The findings suggest that subjective response to alcohol has a predictable and meaningful effect on affective experience.
There were also limitations. First, as this was a quasi-experimental study employing a non-representative sample, all effects are correlational and should be replicated in future work. Second, we relied upon participants to report their initial drinks. Approximately half of all initial drinks were not reported through the initial drink report, but detected in other surveys. Importantly, results remained consistent when examining only drinking episodes begun by an initial drink report (Table S2). Third, while calculated using a reliable and valid formula (Hustad & Carey, 2005), eBAC was based on self-report. Future studies should examine the reliability of self-reported alcohol use using wearable devices capable of objectively measuring BAC. There is also the possibility of reactivity, or changes in consumption, as a result of monitoring. The available evidence, while limited, does not find strong evidence for reactivity in AA (e.g., Shiffman, Stone, & Hufford, 2008). The main concern would be if reactivity differed systematically across groups, a possibility we have no evidence for but cannot rule out. Additionally, despite adjusting for several variables relevant to absorption, the eBAC formula did not account for others (e.g., food intake, daily weight fluctuations, metabolic tolerance), and the formula assumed the population average for alcohol elimination rate. However, with the exception of the supplemental binge drinking analyses, we were not interested in participants’ absolute BAC. The primary concern would be if groups differed on a variable not accounted for by the eBAC formula (e.g., greater food intake). One potential concern is medication, as BPD individuals were more likely to be taking psychotropic medications (Table 1). However, while many medications may interact with alcohol to increase sedation, few have clear effects on BAC or subjective stimulation (Weathermon & Crabb, 1999).
Fourth, a majority of our sample was female, leaving it unclear how well the results generalize to males. Fifth, comorbidity, a hallmark of BPD (Tomko et al., 2014), was much greater in the BPD group (Table 1), particularly for AUD and emotion dysregulation disorders (e.g., mood and anxiety disorders). It is possible that findings for rate of consumption were not due to BPD in particular, but driven by AUD or factors common to many disorders (e.g., emotion dysregulation). However, effects remained for BPD when AUD was included in the model (Table S3), and we did not find an effect for current mood and/or anxiety disorders above that for BPD (Table S4). These findings suggest that effects may be specific to BPD, but should be interpreted with caution, as it is not possible to truly disentangle BPD from comorbid disorders in the present study. Furthermore, the comorbidity analyses were underpowered, as relatively few COM individuals had comorbid disorders, while few BPD individuals did not. Future work should examine the relationship of rate of consumption with BPD symptoms, AUD, and general indicators of emotion dysregulation.
The present study employed a novel approach to assessing alcohol consumption as it occurs in the real world. We applied this approach to better understanding consumption in BPD, a disorder associated with problematic drinking. The findings suggest that it may be important to consider in what way (e.g., rate of consumption) individuals consume alcohol. We found that rate of consumption distinguished BPD and COM participants, such that BPD participants consumed more in a shorter period of time. Elevated rate of consumption was associated with greater stimulant, but not sedative, subjective intoxication effects in the moment. Subjective stimulation was associated with increased PA and, in the BPD group, reduced NA. Rate of consumption, then, may serve as a means for BPD individuals to maximize the rewarding pharmacological effects of alcohol and, thereby, regulate affect. Future work should further investigate rate of consumption, both in BPD and more broadly. In particular, the effect of individual differences (e.g., affective instability, impulsivity, drinking to cope) on rate of consumption should be explored, as well as the negative consequences of drinking at an elevated rate.
Supplementary Material
Acknowledgments
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants P60 AA11998 (Trull/Andrew C. Heath), F31 AA023447 (Carpenter), and T32 AA013526 (Kenneth J. Sher).
Footnotes
Including this participant in the analyses did not significantly alter the results.
Possible reasons for a discontinuity between extreme eBAC values and the actual BAC achieved include participant error and emesis.
Results did not significantly differ depending on whether observations were binned by time point or not, but results from the binned model are substantially easier to interpret.
Our approach, consisting of binning assessments and then fitting a model beginning with a free-curve, follows recent methodological recommendations for characterizing longitudinal trajectories (Grimm, Ram, & Estabrook, 2016; Wang, Wood, & Heath, 2015; Wood, Steinley, & Jackson, 2015). We considered whether a simpler model might be preferred to the free-curve model in terms of parsimony. However, simpler models did not fit the data well (see Supplementary Material and Table S1).
The proportion of initial drink reports and other initial reports did not differ across groups (χ2(1) = 0.01, p = .914).
Mirroring effects reported in Lane et al. (2016), COM participants reported significantly more drinking episodes than BPD participants (MBPD = 6.80, SDBPD = 4.44; MCOM = 9.08, SDCOM = 4.31, t(111) = 2.78, p = .007), but not more overall drinks (MBPD = 21.72, SDBPD = 15.99; MCOM = 28.56, SDCOM = 22.62, t(111) = 1.65, p = .101).
This was a result of the fact that loess regression considers local observations within a moving window, while linear regression takes every observation into account. Given the relatively fewer observations at Times 5 and 6, linear regression produces lower estimates of eBAC at these time points than loess regression.
To increase interpretability, effects for eBAC level and change were scaled to represent the amount of change in the dependent variable for an increase in eBAC of .010%.
Note that we had no a priori hypotheses regarding rate and affect and that these analyses were post hoc. Additionally, the mediation models we tested are only two of many possible models through which effects may operate and should be interpreted with caution.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) Am Psychiatr Publ 2013 [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Carpenter RW, Trull TJ. Components of emotion dysregulation in borderline personality disorder: A review. Curr Psychiatry Rep. 2013;15:335–342. doi: 10.1007/s11920-012-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS, Devlin SJ, Grosse E. Regression by local fitting. J Econom. 1988;37:87–114. [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA. Effects of alcohol, instructions, and consumption rate on affect and physiological sensations. Psychopharmacology. 1979;62:261–266. doi: 10.1007/BF00431957. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacol. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcohol Clin Exp Res. 1997;21:140–149. [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality: Elaborating and extending Linehan’s theory. Psychol Bull. 2009;135:495–510. doi: 10.1037/a0015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. 2011;62:583–619. doi: 10.1146/annurev.psych.093008.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietary Guidelines Advisory Committee. U.S Department of Agriculture, U.S. Department of Health and Human Services. Dietary guidelines for Americans. U.S. Government Printing Office; 2010. [Google Scholar]
- Goodwin DW, Crane JB, Guze SB. Alcoholic “blackouts”: A review and clinical study of 100 alcoholics. Am J Psychiatry. 1969;126:191–198. doi: 10.1176/ajp.126.2.191. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Ed. (SCID-I/P, version 2) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Greenfield TK, Kerr WC. Alcohol measurement methodology in epidemiology: recent advances and opportunities. Addiction. 2008;103:1082–1099. doi: 10.1111/j.1360-0443.2008.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Estabrook R. Growth modeling: Structural equation and multilevel modeling approaches. New York: Guilford; 2016. [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Dommer DW. Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci. 2013;13:489–509. doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- Hustad JT, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: a validity study. J Stud Alcohol. 2005;66:130–138. doi: 10.15288/jsa.2005.66.130. [DOI] [PubMed] [Google Scholar]
- Jones AW, Wigmore JG, House CJ. The course of the blood-alcohol curve after consumption of large amounts of alcohol under realistic conditions. Can Soc Forensic Sci J. 2006;39(3):125–140. [Google Scholar]
- Jones BM, Vega A. Cognitive performance measured on the ascending and descending limb of the blood alcohol curve. Psychopharmacologia. 1972;23:99–114. doi: 10.1007/BF00401185. [DOI] [PubMed] [Google Scholar]
- Laird RD, Weems CF. The equivalence of regression models using difference scores and models using separate scores for each informant: implications for the study of informant discrepancies. Psychol Assess. 2011;23:388–397. doi: 10.1037/a0021926. [DOI] [PubMed] [Google Scholar]
- Lane SP, Carpenter RW, Sher KJ, Trull TJ. Alcohol craving and consumption in Borderline Personality Disorder: When, where, and with whom. Clin Psychol Sci. 2016;4:775–792. doi: 10.1177/2167702615616132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS. Ethanol consumption: How should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey replication. Biol Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Hewitt BG, Grant BF. The Alcohol Dependence Syndrome, 30 years later: A commentary. Addiction. 2007;102:1522–1530. doi: 10.1111/j.1360-0443.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- Maclean JC, French MT. Personality disorders, alcohol use, and alcohol misuse. Soc Sci Med. 2014;120:286–300. doi: 10.1016/j.socscimed.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Martin CS, Balaban CD, McBurney DH. Tonic and phasic processes in the acute effects of alcohol. Exp Clin Psychopharmacol. 2006;14:209–218. doi: 10.1037/1064-1297.14.2.209. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M. Ascending and descending rates of change in blood alcohol concentrations and subjective intoxication ratings. J Subst Abuse. 1990;2:345–352. doi: 10.1016/s0899-3289(10)80006-9. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research. Addict Behav. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Burns M. Effects of rate of drinking on human performance. J Stud Alcohol. 1976;37:598–605. doi: 10.15288/jsa.1976.37.598. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Argo TR, Barnett MJ, Liesveld JL, Liskow B, Hernan JM, Brabson MA. The association of alcohol-induced blackouts and grayouts to Blood Alcohol Concentrations. J Forensic Sci. 2006;51:896–899. doi: 10.1111/j.1556-4029.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured interview for DSM-IV personality disorders Iowa City. University of Iowa Hospitals and Clinics 1994 [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, Sher KJ. The subjective effects of alcohol–tobacco co-use: An ecological momentary assessment investigation. J Abnormal Psychol. 2011;120:557–571. doi: 10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland B, Valin T, Langlois C, Auffret M, Gautier S, Deheul S, Cottencin O. Safety and drinking outcomes among patients with comorbid alcohol dependence and borderline personality disorder treated with high-dose baclofen: A comparative cohort study. Int Clin Psychopharmacol. 2015;30:49–53. doi: 10.1097/YIC.0000000000000054. [DOI] [PubMed] [Google Scholar]
- Ryback RS. Alcohol amnesia: Observations in seven drinking inpatient alcoholics. Q J Stud Alcohol. 1970;31:616–632. [PubMed] [Google Scholar]
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82–92. doi: 10.1016/j.drugalcdep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Ann Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: Comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734–750. doi: 10.1521/pedi_2012_26_093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela CJ, Piasecki TM, Bartholow BD, Heath AC, Sher KJ. The natural expression of individual differences in self-reported level of response to alcohol during ecologically assessed drinking episodes. Psychopharmacology. 2016;233:2185–2195. doi: 10.1007/s00213-016-4270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Ebner-Priemer U. Ambulatory assessment. Annu Rev Clin Psychol. 2013;9:151–176. doi: 10.1146/annurev-clinpsy-050212-185510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clin Psychol Rev. 2000;20:235–253. doi: 10.1016/S0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Brown WC, Tomko RL, Schaefer L, Mclaughlin KD, Jahng S. Substance use disorders and personality disorders. In: Sher K, editor. Oxford Handbook of Substance Use Disorders: Volume 2. New York, NY: Oxford University Press; 2016. pp. 116–148. [Google Scholar]
- Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. J Pers Disord. 2010;24:412–426. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wood PK, Heath AC. Dependent Data in Social Sciences Research. Springer; Cham: 2015. Can psychometric measurement models inform behavior genetic models? A Bayesian model comparison approach; pp. 231–259. [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. University of Iowa; Iowa City, IA: 1999. Unpublished manuscript. [Google Scholar]
- Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23:40–54. [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college: A national survey of students at 140 campuses. J Am Med Assoc. 1994;272:1672–1677. [PubMed] [Google Scholar]
- Wood PK, Steinley D, Jackson KM. Right-sizing statistical models for longitudinal data. Psychological Methods. 2015;20(4):470–488. doi: 10.1037/met0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


