Abstract
Three different sources of human stem cells—bone marrow-derived mesenchymal stem cells (BM-MSCs), neural progenitors (NPs) derived from immortalized spinal fetal cell line (SPC-01), and induced pluripotent stem cells (iPSCs)—were compared in the treatment of a balloon-induced spinal cord compression lesion in rats. One week after lesioning, the rats received either BM-MSCs (intrathecally) or NPs (SPC-01 cells or iPSC-NPs, both intraspinally), or saline. The rats were assessed for their locomotor skills (BBB, flat beam test, and rotarod). Morphometric analyses of spared white and gray matter, axonal sprouting, and glial scar formation, as well as qPCR and Luminex assay, were conducted to detect endogenous gene expression, while inflammatory cytokine levels were performed to evaluate the host tissue response to stem cell therapy. The highest locomotor recovery was observed in iPSC-NP-grafted animals, which also displayed the highest amount of preserved white and gray matter. Grafted iPSC-NPs and SPC-01 cells significantly increased the number of growth-associated protein 43 (GAP43+) axons, reduced astrogliosis, downregulated Casp3 expression, and increased IL-6 and IL-12 levels. hMSCs transiently decreased levels of inflammatory IL-2 and TNF-α. These findings correlate with the short survival of hMSCs, while NPs survived for 2 months and matured slowly into glia- and tissue-specific neuronal precursors. SPC-01 cells differentiated more in astroglial phenotypes with a dense structure of the implant, whereas iPSC-NPs displayed a more neuronal phenotype with a loose structure of the graft. We concluded that the BBB scores of iPSC-NP- and hMSC-injected rats were superior to the SPC-01-treated group. The iPSC-NP treatment of spinal cord injury (SCI) provided the highest recovery of locomotor function due to robust graft survival and its effect on tissue sparing, reduction of glial scarring, and increased axonal sprouting.
Keywords: Spinal cord injury (SCI), iPSC-derived human neural progenitors, Inflammatory response, Human fetal neural stem cells, Human mesenchymal stem cells (hMSCs)
Introduction
The spinal cord is prone to injuries that result in damage to the existing neural structures, leading to the destruction of locomotor and sensory pathways. This process is followed by secondary damage processes, including a local immune response, cavitation, glial scar formation, and tissue atrophy, which enlarge the primary insult leading to impaired regeneration1,2. In recent years, stem cell application after spinal cord injury (SCI) has generated promising results. A variety of stem cells have shown their potential for neurotransplantation. Transplanted stem cells can either repair damaged tissue by replacing cells destroyed during the primary insult or rescue cells in the injured spinal cord by producing cytokines (interleukins) and neurotrophic factors that facilitate regeneration or revascularization, thus reducing glial scar formation.
Mesenchymal stem cells (MSCs) possess self-renewal properties and are highly multipotent, giving rise to different cells of mesodermal origin such as adipocytes, chondrocytes, and osteocytes3,4. Although MSCs are often considered for stem cell therapy in SCI due to their anti-inflammatory5, immunomodulatory6, and neuroprotective properties7,8, their ability to functionally replace neurons and glial cells remains highly debatable9. Furthermore, MSCs can secrete a whole set of neurotrophic, growth, and angiogenic factors10,13, which exert a paracrine effect that can increase neuronal survival, stimulate axonal regeneration and endogenous angiogenesis, and thus enhance functional recovery. The possibility of autologous transplantation using MSCs has drawn the interest of many scientific groups14,16. The majority of clinical studies using MSC transplantation to treat SCI are in phase 1 or 2, and their number is increasing, suggesting that despite several drawbacks that still need to be addressed at basic and preclinical levels, MSCs are considered potentially beneficial for translational studies. The cells used in clinical trials are usually autologous to minimize the risk of rejection and are often administered intrathecally (IT) to avoid invasive surgical interventions.
Neural stem cells (NSCs) or neural progenitors (NPs) are multipotent cells with the ability to differentiate into neurons, oligodendrocytes, and astrocytes. NSCs are present in the adult as well as developing central nervous system (CNS) and can be isolated and expanded in vitro17,20 or prepared as differentiated derivatives from embryonic21,22 or induced pluripotent stem cells (iPSCs)23,24. The use of NPs in repairing the damaged spinal cord is generally focused on the replacement of loss as well as on trophic support of the remaining host nervous tissue25. Functional improvement assessed by the Basso, Beattie, and Bresnahan (BBB) locomotor scale has been reported by several authors after the transplantation of NSCs in SCI animals26,30. The source of implanted NSCs seems to play an important role in defining their impact and fate after SCI17,24,26,31. Since it is difficult to obtain fetal tissue in the required quantity and quality, immortalized cell lines have been developed using various techniques worldwide. One of these approaches is conditionally immortalized neuroepithelial stem cell lines, in which the immortalizing gene is downregulated upon transplantation into the host tissue. The technology (c-mycERTAM) employed to achieve conditional growth control is a fusion protein composed of a growth-promoting gene (c-myc) and a hormone receptor that is regulated by a synthetic drug, 4-hydroxy-tamoxifen (4-OHT)32. One of these cell lines, CTX0E03, has recently been approved in the UK for a clinical phase II trial in stroke patients (http://clinicaltrials.gov/show/NCT02117635).
iPSCs, originally described by Takahashi and Yamanaka33, are derived from differentiated cells by various reprogramming techniques by introducing specific transcription factors responsible for pluripotency. Currently, a variety of methods have been developed to derive iPSCs34,35. Human (h)NSCs derived from iPSCs have already shown strong potential in the experimental treatment of SCI24,28,31,36. Despite some unresolved issues raising concern (such as teratoma formation, the immunogenicity of iPSC-derived cells37, and epigenetic factors), the first clinical trial using iPSCs in the damaged retina has started in Japan38. Recently, the possibility of clinical application of allogenic precursor cells derived from iPSC lines is under investigation in nonhuman primates39,40.
In this investigation, we compared human bone marrow-derived mesenchymal stem cells (BM-MSCs) manufactured under good manufacturing practices (GMPs) and two types of NPs, including cells from a human conditional fetal spinal line (SPC-01, c-mycERTAM technology), and human induced pluripotent stem cell-derived neural progenitors (iPSC-NPs) in the treatment of a clinically relevant SCI model of balloon-induced spinal cord compression in rats. The route of stem cell delivery was also chosen according to the most appropriate clinical application of each stem cell type for SCI to provide robust preclinical data for cell therapy translation. We evaluated the effect of the application of the three cell types on locomotor recovery (BBB, flat beam test, and rotarod). Histological and immunohistochemical analyses were performed to assess white and gray matter (WM/GM) sparing, axonal sprouting, and astrogliosis, while the immunomodulatory effect of the implanted cells was evaluated by an analysis of cytokines released after SCI (Luminex assay). Quantitative polymerase chain reaction (qPCR) was used to compare and evaluate the expression of selected endogenous growth factors, caspase 3, and M1- and M2- related macrophage genes after cell treatment in SCI.
Materials and Methods
Cell Cultures
Human (h)MSCs were obtained based on the informed consent from patients enrolled in the clinical trial AMSC-DSD-001 and were provided by Bioinova Ltd. (Prague, Czech Republic). Cells were prepared under GMP conditions and supplied in 1.5 ml of cell suspension in Nunc (suspension of autologous MSC 3P). The mononuclear fraction containing hMSCs was separated from the bone marrow of healthy donors by gradient separation using 25% Gelofusine (B. Braun, Melsungen, Germany). The cells were expanded in minimal essential medium (MEM) Eagle alpha media (Lonza, Basel, Switzerland), supplemented with 5% mixed allogeneic thrombocyte lysate (Bioinova Ltd.) and 10 μg/ml gentamicin (Lek Pharmaceuticals, Ljublanja, Slovenia). Cells (third passage) were analyzed using fluorescence-activated cell sorting (FACS) (LSR II; BD Biosciences, San Diego, CA, USA) and used for transplantation. The cells were positive for CD73, CD105, CD90, and major histocompatibility complex (MHC) class I, and negative for CD45, CD14, CD34, CD16, CD3, CD19, CD80, and MHC class II surface molecules. Control differentiation protocol (adipogenic, osteogenic, and chondrogenic) was performed to confirm the multipotent potential of the cells. Cell phenotyping was performed by Bioinova Ltd.
The human neural conditionally immortalized cell line (SPC-01) was prepared according to the protocol described in previous publications17,41. A retroviral vector encoding the immortalizing construct (c-mycERTAM) was used to immortalize the cells from 10-week-old human fetal spinal cords. Additionally, the SPC-01 cells were transduced by green fluorescent protein (GFP). SPC-01 GFP+ cells were cultured in laminin-coated tissue culture flasks in growth media, composed of Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA)/F12 supplemented with human serum albumin (HAS; 0.03%; Baxter Healthcare Ltd., Norfolk, UK), human transferrin (100 μg/ml), L-glutamine (2 mM), putrescine dihydrochloride (16.2 μg/ml), sodium selenite (selenium; 40 ng/ml), human insulin (5 μg/ml), epidermal growth factor (EGF; 20 ng/ml), progesterone (60 ng/ml), and 4-OHT (100 nM) (all from Sigma-Aldrich), and fibroblast growth factor (FGF; 10 ng/ml; PeproTech, London, UK). The medium was changed three times per week. SPC-01 GFP+ cells from passages 26-29 were used in all experiments.
iPSC-NPs were prepared according to Polentes et al.42. In order to derive the human iPSC line, a lentivirus-mediated combination of octamer-binding transcription factor 4 (OCT4), sex-determining region Y-box 2 (SOX2), NANOG, and LIN28 human cDNA33 (prepared plasmid from Addgene, Cambridge, MA, USA) was used for transduction of female (IMR90) human fetal lung fibroblasts (ATCC; Manassas, VA, USA). Clone selection, validation of the iPSC line, and derivation of neuronal progenitors are described in detail42. Cells were routinely cultured in tissue culture flasks coated with laminin (10 μg/ml in DMEM/F12) and poly-L-ornithine (0.002% in distilled water), both from Sigma-Aldrich. Growth medium comprising DMEM/F12 and Neurobasal medium (1:1), N2 supplement (1:100), B27 supplement (1:50; GIBCO, Life Technologies, Grand Island, NY, USA), L-glutamine (2 mM; Sigma-Aldrich), penicillin and streptomycin (50 U/ml; GIBCO), EGF (10 ng/ml), FGF (10 ng/ml), and brain-derived neurotrophic factor (BDNF; 20 ng/ml; PeproTech) was changed three times per week. A 7-day process of predifferentiation in the same medium, except for the omission of FGF and EGF, was used prior to transplantation of iPSC-NPs.
Animals
Ten-week-old male Wistar rats (Anlab, Prague, Czech Republic) (n = 225) with body weights of 300 ± 15 g were used. The rats were housed in pairs and maintained in environmentally controlled rooms (22°C-24°C) with a 12-h light/dark cycle. Seven days after injury, all rats were implanted either IT with MSCs (n = 47) or saline (n = 38), or intraspinally (IS) at the level of SCI with SPC-01 cells (n = 50), iPSC-NPs (n = 49), or saline (n = 41). Animals used for cytokine assay (sacrificed 10, 14, and 28 days after injury) and gene expression assay (sacrificed 10 and 28 days after injury) were not used for behavioral evaluation. At each time point, five rats per group were allocated for protein expression studies (Luminex, Austin, TX, USA) and for qPCR. The second group of rats (saline, n = 16; MSCs, n = 22; SPC-01, n = 25; iPSC-NPs, n = 24) was used for behavioral, histological, immunohistochemical, and 2-month qPCR studies. Histological and immunohistochemical evaluation was performed either on longitudinal spinal sections [saline (IS + IT), n = 7 + 6; MSCs, n = 11; SPC-01, n = 14; iPSC-NPs, n = 11] or on cross-sections [saline (IS + IT), n = 8 + 7; MSCs, n = 7; SPC-01, n = 8; iPSC-NPs, n = 10] 2 months after grafting. Preliminary experiments were performed to determine the cell survival of the transplanted cells (MSCs, n = 4; SPC-01, n = 3; iPSC-NPs, n = 3) 2 weeks following their administration. All experiments were performed in accordance with the European Communities Council Directive on September 22, 2010 (2010/63/EU) regarding the use of animals in research and were approved by the ethics committee of the Institute of Experimental Medicine, Academy of Sciences of the Czech Republic.
SCI and Cell Transplantation
A balloon-induced spinal cord compression lesion was used as the model of SCI in rats43. The surgical procedures were performed under sterile conditions. After the induction of anesthesia (3.5 vol%; Forane; AbbVie, Prague, Czech Republic) and an intramuscular injection of analgesic (Rimadyl 50 μl; Zoetis, Prague, Czech Republic), 1 cm of 2-French Fogarty catheter was inserted into the epidural space through a laminectomy at T10. Body temperature was maintained at 37°C during the entire surgical procedure. Spinal cord compression was induced by inflation of the balloon with 15 μl of saline for 5 min at the T8 spinal level and then the catheter was emptied, removed, and the wound was sutured in anatomical layers. Gentamicin (5 mg/kg; Sandoz, Prague, Czech Republic) was given intramuscularly within 10 days to prevent postsurgical infection. Retention of urine was prevented by manual bladder expression performed twice per day. Seven days after SCI, hMSCs (5 × 105/50 μl saline) were injected into the subdural space through the L5-L6 intervertebral space according to De la Calle and Paino44. NPs, SPC-01 cells (5 × 105/5 μl saline), or iPSC-NPs (5 × 105/5 μl saline) were implanted in situ into the lesioned tissue at the area of Th8-9 according to Amemori et al.26. Control rats for the MSC group were injected with 50 μl of saline into the L5-L6 intervertebral space. Control animals for the SPC and iPSC-NP groups were injected with 5 μl of saline into the lesioned tissue. A daily injection of immunosupressants [cyclosporine A (Sandimmune; 10 mg/kg; Novartis, Prague, Czech Republic) and azathioprine sodium (Imuran; 2 mg/kg; GlaxoSmithKline, Prague, Czech Republic)] was used to prevent the rejection of the cell transplants and was performed in all groups of animals (including controls).
Behavioral Assessment
BBB Test
Basic locomotor functions were evaluated using the BBB open field test45. Two independent examiners scored the locomotor performance of experimental rats placed into a circular arena on a scale of 0-21 for approximately 4 min once a week, starting from the first week after SCI.
Flat Beam Test
The flat beam test was used to assess motor function and forelimb-hindlimb coordination. The central part of the beam (1 m long) was used to evaluate the walking distance and velocity. The latency and the trajectory were recorded for a period of 60 s using a video-tracking system (TSE-Systems Inc., Bad Homburg, Germany). After pretraining, testing was performed twice per day for 3 consecutive days. The rats were examined before surgery and then every week from the second week after lesion induction. The rats were scored on a 5-point scale modified from Goldstein46, reflecting their ability to cross the beam.
Rotarod Test
Motor function and the coordination of the hindlimbs were examined in a four-lane rotarod unit (Ugo Basile, Comerio, Italy). Rats underwent 5 consecutive days of training prior to the injury. In training mode, the rod was accelerated from 5 to 10 rpm over a period of 5 min. Lesioned rats were allowed to stay on the rod at a fixed speed of 5 rpm during a 1-min observation period. The latency to fall from the rotating rod was automatically recorded. The test was performed before surgery and 2, 4, 6, and 8 weeks after the injection of stem cells (MSCs, SPC-01, or iPSC-NPs) or saline.
Plantar Test
The plantar test was performed using an Ugo Basile test apparatus (Ugo Basile). A radiant thermal stimulus was applied to the plantar surface of the paws, and the latency of the paw withdrawal response was measured. Each paw was stimulated five times. The test was performed before SCI and then weekly after SCI throughout the whole survival period. Hyperalgesia, as a response to the thermal stimulus, was defined as a significant decrease in the withdrawal latency.
Immunohistochemical and Histological Staining
To investigate the presence of viable stem cells at the site of injury, MSCs were labeled with the long-term tracer carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) before transplantation. Two (MSCs, n = 4; SPC-01, n = 3; and iPSC-NPs, n = 3) and 8 (MSCs, n = 18; SPC-01, n = 22; and iPSC-NPs, n = 21) weeks after transplantation, the rats were transcardially perfused with 4% paraformaldehyde (PFA) (Penta s.r.o., Prague, Czech Republic) in phosphate-buffered saline (PBS), and their spinal cords were removed. A 2-cm-long segment of the spinal cord was dissected between 1 cm cranial and 1 cm caudal to the injury epicenter. To monitor the survival, migration, and differentiation of the transplanted cells, serial longitudinal sections of the spinal cord (14 μm) were cut through the areas of interest, using a Leica CM1850 cryostat (Leica Microsystems GmbH, Vienna, Austria). For assessing glial scar formation, axonal sprouting, and the extent of spared WM/GM, tissue samples from the 9-week survival period (e.g., 8 weeks after cell transplantation) were embedded in paraffin and cut into cross sections (5-μm thickness).
To identify human cells transplanted into the rat spinal cord, antibodies directed against human nuclei [HuNu (Millipore, Billerica, MA, USA), Ku80, and human mitochondrial marker MTC02 (both Abcam, Cambridge, UK)] were used for all cells in addition to GFP labeling of the SPC-01 cells. To follow the fate of the transplanted stem cells and their interaction with the host tissue, antibodies directed against nestin (Millipore), glial fibrillary acidic protein (GFAP; Sigma-Aldrich), βIII-tubulin (both Sigma-Aldrich), Nkx6.1 (DSHB, Iowa City, IA, USA), oligodendrocyte transcription factor 2 (Olig2), microtubule-associated protein 2 (MAP2; both Abcam), and doublecortin (DCX; Santa Cruz Biotechnology, Heidelberg, Germany) were used.
To estimate the number of implanted NPs (iPSC-NPs and SPC-01) 2 months after implantation, every sixth longitudinal spinal cord section (14-μm thickness) was chosen. Imaging of the whole graft area per section was performed (Observer D1 microscope; Carl Zeiss, Weimar, Germany). The surviving human cells were recognized using HuNu staining, and the analysis was performed using ImageJ software [National Institutes of Health (NIH), Bethesda, MA, USA]. The total number of human cells that survived was estimated according to the spinal cord volume in which the cells were found (number of HuNu+ sections multiplied by six). The percentage of surviving transplanted cells was calculated by dividing the estimated total number of surviving cells by the total number of transplanted cells (5 × 105). Data are presented as an average per transplanted group.
To visualize primary antibody reactivity, appropriate secondary antibodies were used: goat anti-mouse immunoglobulin G (IgG) conjugated with Alexa Fluor 488, as well as goat anti-rabbit IgG conjugated with Alexa Fluor 594 (Molecular Probes, Eugene, OR, USA). The histological and immunohistochemical evaluations were carried out using a ZEISS AXIO Observer D1 microscope (Carl Zeiss). Either Wizzard (Carl Zeiss), or ImageJ programs were used for image analysis of the histological and immunohistochemical staining. Excel 2010 (Microsoft, Redmond, WA, USA) and CorelDRAW ×5 (Corel Corporation, Ontario, Canada) were used to create the graphics.
Cresyl Violet-Luxol Staining
To distinguish the spinal cord WM/GM, cresyl violet (CV) and Luxol fast blue (both Sigma-Aldrich) staining were used on serial cross sections. For each sample, 15 sections were selected at 1-mm intervals along the craniocaudal axis, including the lesion center on the eighth slide. Images of the spinal cord were taken with an Axioskop 2 plus microscope (Zeiss, Oberkochen, Germany). The amounts of spared gray and white matter were analyzed using ImageJ software.
GAP43 Staining
For immunohistochemical analysis of axonal sprouting, a primary antibody against GAP43 (Millipore) was used on serial cross sections around the central lesion cavity 9 weeks after SCI. For each sample, 15 sections were selected at 1-mm intervals along the craniocaudal axis, including the lesion center on the eighth slide. Images of the spinal cord were taken with an Axioskop 2 plus microscope. The number of GAP43+ fibers per slide was manually counted. The graphical presentation of these results shows the average number of GAP43+ fibers per slide in percentage, when compared with the saline group, which was set to 100%.
GFAP Staining
For immunohistochemical analysis of glial scaring, a CY3-conjugated primary antibody against GFAP was used on serial cross sections around the central lesion cavity 9 weeks after SCI. For each sample, 15 sections were selected at 1-mm intervals along the craniocaudal axis including the lesion center on the eighth slide. Images of the spinal cord were taken with an Axioskop 2 plus microscope. The number of protoplasmic astrocytes per slice and the area of GFAP positivity surrounding the main lesion cavity were analyzed using ImageJ.
qPCR
The expression of rat target genes [neurotrophin-3 (NT-3); sortilin 1 (Sort1)], FGF2, mannose receptor C type 1 (MRC1), interferon regulatory factor 5 (IRF5), OLIG2, GAP43, CASP3, GFAP, vascular endothelial growth factor A (VEGFA), and ciliary neurotrophic factor (CNTF)] was studied using quantitative real-time polymerase chain reaction (qRT-PCR) at 10 and 28 days and at 2 months after stem cell transplantation [MSCs, n = 5 per time point; SPC-01, n = 5 per time point; iPSC-NPs, n = 5 per time point; saline (IT + IS), n = 5 + 5 per time point]. The paraffin sections of injured spinal cord from the region of the lesion epicenter (center of the lesion + 2 cranial and 2 caudal sections) were taken for the study. RNA was isolated from tissue cross sections using the High Pure RNA Paraffin Kit (Roche, Penzberg, Germany) following the manufacturer's recommendations. RNA amounts were quantified using a spectrophotometer (NanoPhotometerTM P-Class, Munchen, Germany). The isolated RNA was reverse transcribed into cDNA using Transcriptor Universal cDNA Master (Roche) and a thermal cycler (T100™ Thermal Cycler; Bio-Rad, Hercules, CA, USA). The qRT-PCRs were performed using cDNA solution, FastStart Universal Probe Master (Roche), and the following TagMan Gene Expression Assays (Life Technologies, Carlsbad, CA, USA): Gapdh/Rn01775763_g1, Olig2/Rn01767116_m1, SORT1 (NT-3)/Rn01521847_m1, Fgf2/Rn00570809_m1, Casp3/Rn00563902_m1, Gfap/Rn00566603_m1, Gap43/Rn01474579_m1, Vegfa/Rn01511601_m1, Bdnf/Rn0253 1967_s1, Cntf/Rn00755092_m1, nerve growth factor (Ngf)/Rn01533872_m1, Irf5/Rn01500522_m1, CD86/Rn00571654_m1, Mrc1/Rn01487342_m1, and CD163/Rn01492519_m1.
The qRT-PCR was carried out in a final volume of 10 μl containing 25 ng of extracted RNA. Amplification was performed on a StepOnePlus™ real-time PCR cycler (Life Technologies). All amplifications were run under the same cycling conditions: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. All samples were run in duplicate, and a negative control was included in each array. Relative quantification of gene expression was determined using the ΔΔCt method. The results were analyzed with StepOnePlus® software. The gene expression levels were normalized based on glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference gene; control samples with vehicle (PBS) transplantation were used as a calibrator. The statistical significance of the differences between the transplanted and control (saline) groups was determined on ΔCt values level using a one-way analysis of variance (ANOVA) test with a post hoc pair-to-pair test. Differences were considered statistically significant if p < 0.05. Data are expressed as the mean ± the standard error of mean (SEM).
Cytokine Evaluation
The levels of cytokines were measured to analyze the effects of the transplantation of various stem cells (MSCs, SPC-01, and iPSC-NPs) on inflammation in the region of the lesion 10, 14, and 28 days after SCI (n = 5 per time point in all groups). A piece of the spinal cord at the site of the lesion was dissected out and incubated in cell media [DMEM supplemented with 10% fetal bovine serum (FBS); Gibco, Thermo Fisher Scientific, Waltham, MA, USA] and 0.2% primocin (Life Technologies, Thermo Fisher Scientific)47-49. To measure the levels of inflammatory cytokines, the medium was collected after a 24-h incubation period. Cytokine levels were determined using a customized Milliplex inflammatory cytokine kit (Millipore) and Magpix instrumentation software (Thermo Fisher Scientific). Rat cytokine Luminex custom 8-plex kits [for IL-1β, IL-4, IL-2, IL-6, IL-12p70, macrophage inflammatory protein 1α (MIP-1α), tumor necrosis factor-α (TNF-α), and regulated on activation, normal T-cell expressed and secreted (RANTES)] were used for customized bead assays. The assays were performed in 96-well filter bottom plates according to the manufacturer's protocol (Thermo Fisher Scientific). Antibody-conjugated beads were used at a concentration of 5,000 beads per marker in accordance with the manufacturer's protocol. Biotinylated detection antibodies were added together with streptavidin-RPE (streptavidin-R-phycoerythrin) (both from Thermo Fisher Scientific), and then the levels of cytokines were measured on a Luminex xMAP 200 system and analyzed using the Magpix instrumentation software. To calculate the concentration of each cytokine, the raw data, consisting of mean fluorescence intensity (MFI), were used. A four- or five-parameter logistic fit curve was generated for each cytokine from seven standards. The lowest standard, which was at least three times above the background, was used to determine the lower limit of quantification (LLOQ). The calculation of the LLOQ was performed using a specific calculation by subtracting the MFI of the background (diluent) from the MFI of the lowest standard concentration, and then back calculating the concentration from the standard curve. The results are presented as the percentage change from nonlesioned tissue for each time point.
Statistical Analyses
All data are presented in graphs as the mean ± SEM. The statistical significance of differences between the MSC-, SPC-01-, iPSC-NP-, and saline-injected groups was determined using either one-way ANOVA or two-way ANOVA in the case of a second factor (time). In the case of repeated measurements (behavioral tests) or the spatial distribution of the treatment effect (gray/white matter sparing, glial scar distribution), two-way repeated-measures (RM) ANOVA was used. The Student-Newman-Keuls (SNK) post hoc pair-to-pair test was used to specify for which groups and at which time points were the changes significant (all in Sigmastat 3.1; Systat Software Inc., San Jose, CA, USA). Differences were considered statistically significant if p < 0.05.
Results
Cell Fate and the Pattern of Discrete Differentiation Following Stem Cell Implantation
The survival of IT (MSCs) and IS (SPC-01 and iPSC-NPs) grafted cells was evaluated 2 weeks and 2 months postimplantation, respectively. Only a few MSCs were observed partially attached to the dorsal spinal cord surface (Fig. 1A) 2 weeks after application, and none were detected 2 months postgrafting. In contrast, noticeably high numbers of neural precursors derived from either SPC-01 cells (16 ± 3%) or iPSC-NPs (11 ± 3%) were detected around the injection site at 2 months after implantation (Fig. 1B and C). Both types of human NPs 2 months after implantation remained rather immature. SPC-01 cells were predominantly positive for GFAP (Fig. 1D) or for early neural markers such as nestin (Fig. 1E), olig2 (Fig. 1F), and Nkx6-1. Most of the iPSC-NPs were positive for βIII-tubulin (Fig. 1G), Map2 (Fig. 1H), and DCX (Fig. 1I), with only some of the grafted cells being GFAP+ (Fig. 1J) (for more details, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html).
Figure 1.
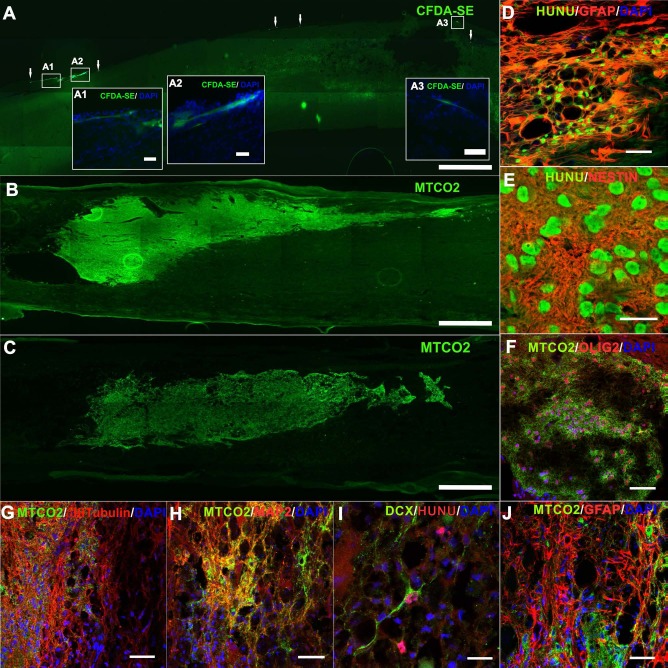
Survival of implanted stem cells and differentiation pattern of SPC-01 and iPSC-NP cells following their implantation after SCI. Only a few MSCs were detected on the spinal cord surface 2 weeks after application (A), while SPC-01 cells (B) and iPSC-NPs (C) survived robustly for at least 2 months after implantation. Arrows mark the attached MSCs. Insets (A1-A3) show the MSC morphology. Staining for MTCO2 (B, C) or CFDA-SE (A) was used to detect the implanted cells in the injured spinal cord. The differentiation patterns of SPCs (D-F) and iPSC-NPs (G-J) demonstrated that the majority of SPCs differentiated into astrocytes (D) or remained immature (E, F), while on the other hand transplanted iPSC-NPs expressed mainly neuronal markers (G-I) and less astrocytic markers (J). (D), (F)-(H), and (J) were taken from the edge of the graft, (E) is from the graft center, and (I) shows cell migration into the host tissue (Orto images are online in additional files http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html). Scale bars: 300 μm (A), 500 μm (B, C), 20 μm (A1, A2, A3, D-J). CFDA-SE, carboxyfluorescein diacetate succinimidyl ester; MTC02, anti-mitochondria antibody; HuNu, human nuclei (all green); DAPI, 4′,6-diamidin-2-fenylindol (blue); MSC, mesenchymal stem cell; iPSC-NPs, human induced pluripotent stem cell-derived neural precursors; OLIG2, oligodendrocyte transcription factor 2; DCX, doublecortin; SPC, spinal fetal cell; SCI, spinal cord injury.
Functional Recovery From SCI Following Stem Cell Implantation
The BBB test was performed once a week after lesion induction to evaluate hindlimb locomotor recovery. All stem cell-treated rats performed significantly better when compared with saline-treated animals. Additionally, iPSC-NP- and MSC-injected rats were superior to the SPC-01-treated group (two-way RM ANOVA, treatment; p < 0.001; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 2A).
Figure 2.
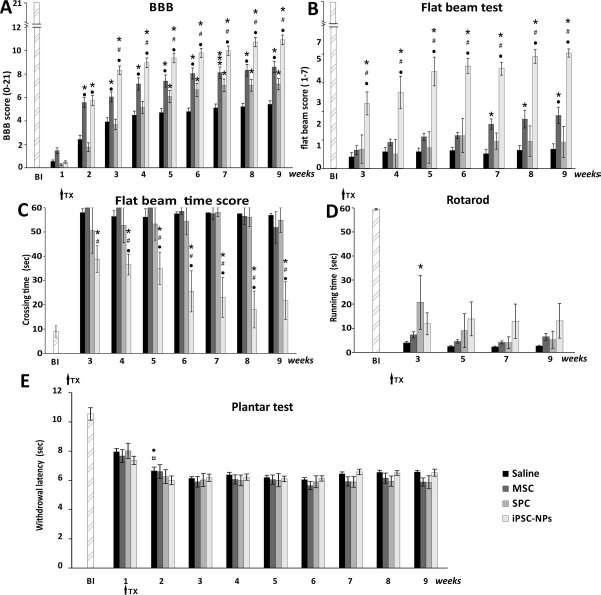
Locomotor and sensory recovery following stem cell transplantation after SCI. Recovery from SCI after the administration of MSCs, SPC-01 cells, or iPSC-NPs. The locomotor ability of saline- or stem cell-treated rats was assessed using the BBB (A), flat beam (B), time score (C), and rotarod tests (D). All treated animals showed significantly higher performances in the open-field BBB test in comparison with saline controls (A). Additionally, the iPSC-NP and MSC groups demonstrated significantly higher performances compared to the other treatment group. In tests requiring more advanced locomotor skills, such as the flat beam test (B), all treated groups showed a significant difference when compared with the saline group, but with a dominance of iPSC-NP. For the rotarod test (D), for which body weight-supported stepping is essential, a stable but insignificant trend of iPSC-NPs in higher performance was observed. Thermal nociception was assessed using the plantar test (E) after stem cell or saline transplantation, and no additional hyperalgesia after grafting of stem cells was observed. *p < 0.05 versus saline; #p < 0.05 versus MSCs; •p < 0.05 versus SPC-01; ¤p < 0.05 versus iPSC-NPs. For p values, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html. BBB, Basso, Beattie, and Bresnahan test; BI, before injury; MSC, mesenchymal stem cell; iPSC-NPs, human induced pluripotent stem cell-derived neural precursors; SPC, spinal fetal cell; SCI, spinal cord injury; TX, stem cell transplantation.
The recovery of advanced locomotor function was evaluated using the flat beam test. In this test, rats grafted with iPSC-NPs showed the greatest degree of improvement when compared to the saline-treated animals as well as other stem cell groups. However, probably because of the severe injury and higher level of coordination and muscle strength that is needed for the flat beam test, not all cell-treated groups showed significant improvement when compared to saline-injected rats (two-way RM ANOVA, treatment; p < 0.001; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 2B).
The time score in the flat beam test reflected the time it took to cross the beam within a 60-s test period; the test was performed starting from the third week onward after treatment. The iPSC-NP-treated rats showed a marked ability to cross the beam in a shorter time interval than any other group and displayed progressive improvement over the experimental period; no other group showed a significant improvement during the measurement period (two-way RM ANOVA, treatment; p < 0.001; for post hoc pair-to-pair test p values, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 2C).
To determine long-term weight support and endurance and footstep coordination, the rotarod test was used. As expected, because of the difficulty of the test, no significant differences in stem cell-treated rats were observed when compared with saline-treated animals (two-way RM ANOVA, treatment; p > 0.05; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 2D).
Changes in the sensory function of the hindlimb after SCI (increased sensitivity to a thermal stimulus) were determined using the plantar test apparatus (Ugo Basile). Hyperalgesia is generally correlated to the pathological state induced by SCI as well as to sensory function adaptation over time. All stem cell-treated animals were similar to the saline-treated group, which implies that no additional hyperalgesia appeared following stem cell transplantation (two-way RM ANOVA, treatment; p > 0.1; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 2E).
Estimation of White and Gray Matter Sparing Following Stem Cell Treatment After SCI
The area of spared WM/GM in the spinal cord (μm2 per cross section) was measured on 15 CV-Luxol-stained spinal cross sections 2 months after injury. The values presented in Figure 3A are given in relation to the areas measured in saline-treated control animals, which were set as 100%. Despite the observed impact of all stem cell types on WM/GM sparing, only the implantation of iPSC-NPs showed a statistically significant preservation of WM/GM, in comparison with saline-treated as well as other stem cell-treated animals (two-way RM ANOVA, treatment; p < 0.001; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (the statistical significance within the link) (Fig. 3A, a1-a3).
Figure 3.
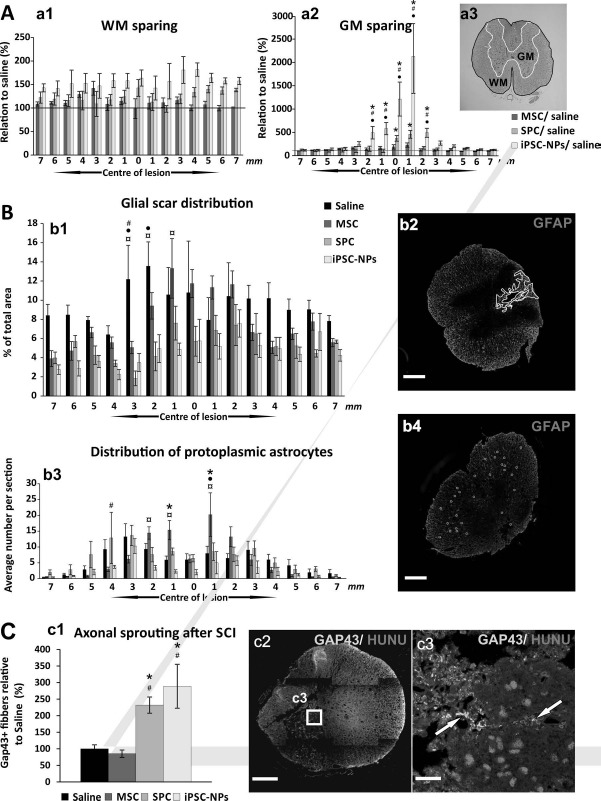
Histological and immunohistochemical analysis 2 months after SCI. (A) The extent of spared white (a1) and gray (a2) matter was determined 2 months after SCI in rats treated with saline or stem cells (MSC, SPC-01, and iPSC-NP). CV (a3 and GM) was used for gray matter staining, while Luxol blue was used to stain the white matter (a3 and WM). Both the WM/GM surface areas were calculated according to the example on an illustrative image (a3). All implanted cells had a positive impact on tissue sparing in the WM/GM of the injured spinal cord. However, only the iPSC-NP group showed a statistically significant preservation of spinal tissue. (B) The effect of implanted MSCs, SPC-01, and iPSC-NP cells on glial scar formation, showing the area of astrogliosis surrounding the main cavity as a percentage of the total tissue area (b1, b2) and the average number of protoplasmic astrocytes per section (b3, b4) around the central lesion cavity 9 weeks after SCI. Illustrative images show GFAP-CY3 staining of the glial scar (b2) and protoplasmic astrocytes (b4). Scale bars: 500 μm (b2, b4). Both neural precursors reduced glial scaring and the number of protoplasmic astrocytes, whereas MSCs modulated the distribution of glial scarring and only mildly reduced it. (C) The effect of implanted MSC, SPC-01, and iPSC-NP cells on axonal sprouting (c1). The numbers of GAP43+ fibers (15 sections per rat) are presented as percentages relative to the number of positive fibers following saline treatment (set to 100%). Illustrative image of a GAP43+-labeled section (c2) with grafted cells. Illustrative image of positive nuclear staining for Ku80, indicating cells of human origin (c3); arrows show GAP43+ fibers. Scale bars: 500 μm (c2), 20 μm (c3). *p < 0.05 versus saline; #p < 0.05 versus MSCs; •p < 0.05 versus SPC-01; ¤p < 0.05 versus iPSC-NPs. For p values of (A)-(C), see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html. MSC, mesenchymal stem cell; iPSC-NPs, human induced pluripotent stem cell-derived neural precursors; SPC, spinal fetal cell; SCI, spinal cord injury; GM, gray matter; WM, white matter; GFAP-CY3, glial fibrillary acidic protein cyanine 3; GAP43, growth-associated protein 43; HuNu, human nuclei.
Astrogliosis and Morphological Changes in GFAP+ Cells Following Stem Cell Transplantation
The area of the glial scar surrounding the central lesion was measured on 15 GFAP-cyanine 3 (CY3)-stained spinal cord cross section slices 2 months after injury. The values shown in Figure 3B are presented as the percentage of the GFAP+ area compared to the total tissue area. All stem cell-treated groups had significantly smaller GFAP+ volume compared to saline-treated animals, when calculated as a sum of area of single slices. Additionally, rats grafted with SPC-01 cells or iPSC-NPs had significantly smaller GFAP+ volume in comparison with rats receiving MSCs (two-way RM ANOVA, treatment; p < 0.001; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 3B, b1, b2). Moreover, the effect of stem cells on GFAP phenotype after SCI was shown. Rats injected with saline, MSCs, or SPC-01 cells had a significantly higher number of protoplasmic astrocytes per slice in comparison with the iPSC-NP group (two-way RM ANOVA, treatment; p < 0.05; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 3B, b3, b4).
Determination of Axonal Sprouting Following Stem Cell Implantation After SCI
The number of GAP43+ fibers was calculated in cross sections of injured spinal cords 2 months after injury. The values presented in Figure 3C are given in relation to the value found in saline-treated animals, which was set as 100%. A higher number of GAP43+ fibers in the spinal cord cross sections of both types of NP-transplanted rats were observed, when compared to both saline-and MSC-treated animals (two-way RM ANOVA, treatment; p < 0.05; for p values of post hoc pair-to-pair test, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 3C, c1-c3).
M1 and M2 Macrophage-Related Gene Expression in Response to Stem Cell Transplantation After SCI
The expression of genes related to the macrophage phenotypes M1 (IRF5 and CD86) and M2 (MRC1 and CD163) was determined by qRT-PCR from spinal cord samples taken from the lesion site 10 and 28 days after cell transplantation. Ten days after SCI, spinal cord tissue treated with MSCs revealed the upregulation of IRF5 (p < 0.05) and MRC1 when compared to the control tissue. Gene expression remained unaltered in samples taken from the SPC-01-treated spinal cords. On the other hand, iPSC-NP-treated rats showed a downregulation of CD86 and CD163, markers of M1 and M2 macrophages, respectively (Fig. 4A, a1). Twenty-eight days after grafting, all of the studied genes related to M1 and M2 macrophages were upregulated; however, this upregulation remained statistically insignificant (post hoc pair-to-pair test; for p values, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 4A, a2).
Figure 4.
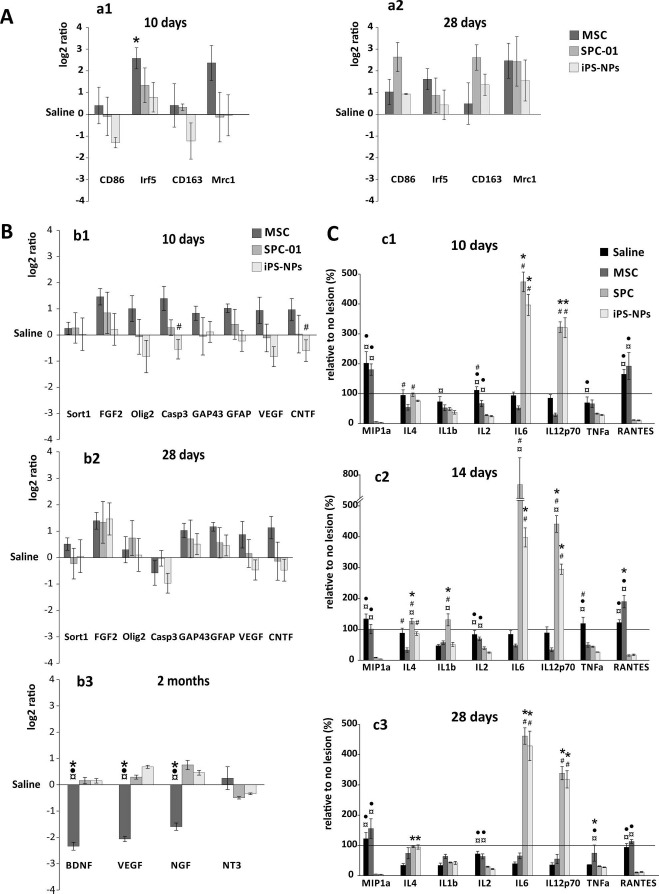
Effect of stem cell implantation on the inflammatory response and the gene expression of factors related to recovery after SCI. (A) Relative expression of macrophage-related genes (M1-CD86, IRF5; M2-CD163, MRC1) in response to grafted MSCs, SPC-01, and iPSC-NP cells 10 and 28 days after SCI, demonstrating that the most significant changes were evident following MSC treatment, when IRF5 upregulation was observed 10 days after SCI. The expression levels measured in saline-treated rats were set to 0. (B) Relative gene expression of factors related to the recovery process in response to grafted MSCs, SPC-01, or iPSC-NP cells 10 days (b1), 28 days (b2), and 2 months (b3) after SCI. The expression levels in saline-treated rats were set to 0. (C) The levels of cytokines and chemokines related to inflammation are presented following stem cell treatment 10 (c1), 14 (c2), and 28 (c3) days after SCI, which correspond to 3, 7, and 21 days after implantation. The levels measured in animals with no lesion were set to 100% as indicated by a horizontal line in the graphs. *p < 0.05 versus saline; #p < 0.05 versus MSCs;? •p < 0.05 versus SPC-01; ¤p < 0.05 versus iPSC-NPs. For p values of (A)-(C), see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html. MSC, mesenchymal stem cell; iPSC-NP, human induced pluripotent stem cell-derived neural precursors; SPC, spinal fetal cell; SCI, spinal cord injury; IRF5, interferon regulatory factor 5; MRC1, mannose receptor C type 1; SORT1, sortilin 1; FGF, fibroblast growth factor; OLIG2, oligodendrocyte transcription factor 2; GAP43, growth-associated protein 43; GFAP, glial fibrillary acidic protein; VEGF, vascular endothelial growth factor; CNTF, ciliary neurotrophic factor; BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; NT-3, neurotrophin-3; MIP, macrophage inflammatory protein; TNF-α, tumor necrosis factor α; RANTES, regulated on activation, normal T-cell expressed and secreted.
Expression of Intrinsic Genes After Stem Cell Treatment of SCI
The relative expression of rat genes related to growth factors [SORT1 (NT-3), FGF2, CNTF, and BDNF], apoptosis (Casp3), vascularization (VEGFA), axonal sprouting (GAP43), astrogliosis (GFAP), and oligodendrocytes (OLIG2) following stem cell transplantation was determined 10 (Fig. 4B, b1) and 28 (Fig. 4B, b2) days and 2 months (Fig. 4B, b3) after SCI. Ten days after SCI (3 days after grafting), animals with implanted MSCs displayed an elevated expression of all genes of interest as a strong but insignificant trend. Similar levels of gene expression in the MSC-treated group continued after 28 days, apart from the downregulated expression of Casp3. Two months after SCI, the MSC group showed a significant downregulation of BDNF, VEGF, and NGF in comparison with the saline- and both NP-treated groups. The transplantation of SPC-01 cells and iPSC-NPs did not cause any notable changes in gene expression at any of the time points; however, a gradual alteration in gene expression, mostly pertaining to genes related to recovery processes, was observed at 28 days as well as 2 months after SCI (Fig. 4B, b2, b3). The greatest upregulation of gene expression was found for FGF2; however, this change was not significant (post hoc pair-to-pair test; for p values, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 4B, b1 and b2).
Changes in Cytokine Profiles After Stem Cell Treatment Following SCI
To determine the inflammatory response following treatment with MSCs, SPC-01 cells, or iPSC-NPs, the levels of cytokines (MIP-1α, IL-4, IL-1β, IL-2, IL-6, IL-12p70, TNF-α, and RANTES) were assayed at 10, 14, and 28 days after SCI. The levels of MIP-1α, IL-2, and TNF-α were decreased relatively to those of the saline-treated controls at 10 days post-SCI in all three stem cell groups and persisted through day 14. At day 28 in the MSC group, the MIP-1α, IL-2, and TNF-α levels increased in comparison with those of other groups. The levels of RANTES remained significantly depressed below saline control levels in the SPC-01 and iPSC-NP groups, while its concentration was above control levels in the MSC group. IL-4 levels were reduced in MSC-treated animals at day 10 and increased insignificantly at day 28. In contrast, levels of IL-4 in both NP groups gradually increased when compared to that of the saline group. The levels of IL-1β were insignificantly lowered compared to that of the controls at day 10 in MSC and SPC-01 groups, and significantly in the case of the iPSC-NP group. At 14 and 28 days after SCI, levels of IL-1β remained similar to those of the saline-treated group, apart from an increase in the SPC-01 group on day 14 after SCI. IL-6 and IL-12p70 levels were noticeably higher in both NP groups compared to the control and MSC groups. This pattern continued throughout the whole experimental period (post hoc pair-to-pair test; for p values, see http://www.iem.cas.cz/en/research/departments/tissue_culture/supplementary_documents.html) (Fig. 4C, c1-c3).
Discussion
This investigation demonstrates the effectiveness of MSCs and two NPs derived from either human fetal spinal cord precursors (in our study SPC-01) or from iPSCs (iPSC-NPs) in the cell therapy of SCI. In detail, it provides a description of the different mechanisms underlying their support of SCI recovery, in particular the survival and differentiation patterns, as well as the anti-inflammatory action of these cells, which together govern the repair and recovery process.
The intrathecal application of MSCs and the intraspinal application of SPC-01s and iPSC-NPs were chosen as the routes of delivery. Stem cell properties in vivo and their impact on behavior recovery and the physiological changes related to SCI are closely associated with the mode of their administration, that is, local or systemic18,50,51. Nevertheless, meta-analyses of behavioral recovery after MSC application in experimental rat models of SCI failed to detect any statistically significant differences in locomotor recovery between intraparenchymal, intrathecal, or intravenous administration14. Intrathecal administration of MSCs has been proposed to be a less invasive method52, and this route is not only well suited to multiple injections over an extended time period, but also by using an intrathecal catheter, the treatment can be administered closer to the injury site, thereby increasing the local concentration of MSCs. In our study, we applied MSCs IT since our previous study using intraparenchymal injections of rat MSCs into a balloon compression model of SCI resulted in a similar recovery as that seen in animals following intrathecal application5,7. It is also widely accepted that the effect of MSCs is mainly paracrine, and the cells do not differentiate into neural cells9, so there is no necessity to have them grafted into the parenchyma from the point of functional integration. In addition, the intrathecal application of MSCs has often been used in recent clinical trials53,55 and is less invasive for patients. Moreover, the intrathecal application of MSCs in an animal model of amyotrophic lateral sclerosis (ALS) prolonged life span, reduced apoptosis, and preserved the number of motoneurons and their perineural nets56. On the other hand, the administration of iPSC-NPs into the intrathecal space of lesioned rats had much less effect when compared with intraspinal injection57. Moreover, NPs can differentiate into neural cells, which can contribute to spinal cord repair. Therefore, an injection into the spinal parenchyma was chosen for both NPs.
In correspondence with our results, several studies have shown that the subacute grafting of MSCs5,7,58,59, iPSC-NPs23,28, or NPs derived from either fetal tissue or ESCs17,18,26,60,61 leads to the improvement of locomotor recovery. However, the mechanisms underlying the cells' effects on locomotor recovery may vary depending on the methods and approaches utilized25. In our study, the highest behavioral scores were observed in the iPSC-NP-treated animals when compared not only to saline-treated animals but also to other cell-treated groups. This outcome was observable in the basic and advanced locomotor settings. In contrast to the other treatment groups, the iPSC-NP-treated rats extended occasional weight support at 9 weeks after SCI. Surprisingly, the high effect on locomotor recovery was also observed in the MSC group. Since the rapid recovery in all stem cell-treated animals was observed within weeks after grafting, the impact of stem cell treatment was caused by a paracrine effect in all three types of cells (Fig. 2A-C).
The significant paracrine effect following the intraspinal grafting of both types of NP cells was most likely due to their ability to robustly survive for the entire experimental period in conjunction with the maturation and differentiation of these cells, which provided a continuous supply of new cells to the injured host tissue. These findings are in agreement with those of other authors19,23,24,26,28,36. Furthermore, the ability of iPSC-NPs to differentiate into a neuronal phenotype was clearly evidenced (Fig. 1); yet on the other hand, clusters of grafted SPC-01 cells formed structures resembling dense clouds, with only a few host neurofilaments penetrating the graft. These cells differentiated mainly into astrocytes and displayed less communication with the tissue, as observed previously19,26. It is important to note that at the end of this study, the implanted NPs were only partially matured, since the full maturation of human cells requires a longer period.
In our study, in a time period matching the MSC survival period after implantation, elevated levels of endogenous VEGF, CNTF, and FGF2 were found. Increased levels of VEGF are tightly connected with angiogenesis, which is highly desirable for vitalizing lesioned tissue62,63, and CNTF is a promising factor in the treatment of SCI, with a strong neuroprotective effect in both the CNS and also the peripheral nervous system (PNS)64. The presence of CNTF promotes the differentiation and maturation of oligodendrocyte precursor cells into oligodendrocytes. Additionally, CNTF promotes mature oligondendrocyte survival. Increased CNTF levels may thus serve to improve remyelination after SCI65,66. In our study, the intrinsic CNTF expression was significantly lower in the iPSC-NP-implanted group when compared to MSC-treated animals. Together with other elevated growth factor levels found in the rats treated with MSCs, this could be one of the reasons why, despite worse results in other analyses, MSC-treated animals exhibited some of the improvement in locomotor function seen in the iPSC-NP-treated group.
Recently, the strategies using MSCs in experimental treatment of CNS injuries and degenerative diseases have focused on MSC-produced secretomes, instead of cell therapy itself 67. These secretomes are defined by specific conditions of cultivation, and the effect of their application could be different than the use of MSCs68,70. This opens up new possibilities for combinatory therapies based on MSC secretomes as a conditional pretreatment for NP implantation71. Despite these tendencies, the majority of preclinical investigations and clinical trials are still using a cellular approach.
In most cases, the improvements in locomotor or sensory deficits can be correlated with the preservation of WM/GM after SCI72,74. MSCs have been described as immunomodulatory and proregenerative. Their paracrine effect (secretome) has proven to be beneficial in CNS regeneration67,75. In our study, the weaker effect on tissue sparing observed in MSC-treated animals was perhaps due to the absence of MSCs in the tissue parenchyma, thus reflecting a purely paracrine effect as the MSCs never made direct contact with the host tissue. On the other hand, both types of NPs, which were directly injected into the lesion, displayed robust survival in conjunction with the maturation and differentiation of these cells, suggesting their involvement in the partial reconstruction of damaged tissue, which was also reflected by the extent of spared WM/GM, more effective glial scar modulation, and robust axonal sprouting, when compared to saline- or MSC-treated animals (Fig. 3). These findings are in agreement with those of other authors19,23,24,26,28,36.
The tissue response to the implantation of both NPs and MSCs in terms of up- or downregulated expression levels of genes of interest correlates with the observed differences in glial scar remodulation (GFAP expression vs. GFAP-CY3 staining) and tissue sparing (Casp3) after SCI, where the most contradictory effects were observed between the iPSC-NP and MSC groups. The intrinsic growth factor production in the NP-treated rats 2 months after SCI (BDNF, VEGF, and NGF), even though not significantly higher than saline-treated animals, showed a long-term stable trend toward upregulation, which might explain the superior impact of both types of NPs on tissue modulation.
The macrophage response reflects the type of immune response after SCI. The ratio of M1/M2 macrophages after injury is thought to reflect the balance of the pro-/anti-inflammatory response. This balance may result in the increased integration of implanted cells, influence their migratory abilities, and lead to enhanced recovery after SCI76. The attenuation of this balance by the application of stem cells77,78 or immunomodulatory molecules79,80 is currently of great interest. In our study, the M1/M2 macrophage gene expression profile after SCI in the cell-treated animals was slightly shifted toward M2 specific, which might be associated with the observed differences in tissue sparing as well as glial scar formation.
In this study we also evaluated the levels of cytokines in response to stem cell grafting. The immune response of the spinal tissue can differ from the systemic response and can provide relevant answers to questions regarding cell survival and the route of application. The most profound changes in the immunologic response to implanted stem cells were seen in the levels of MIP-1α, TNF-α, RANTES, IL-1 β, IL-4, IL6, and IL12.
The reduction of MIP-1α and RANTES was most pronounced in the SPC-01 and iPSC-NP groups when compared to both control and MSC-treated rats. The MIP-1α has been implicated in the phagocytosis of myelin after SCI and is a reliable marker of inflammation81, and the reduction of RANTES using the anti-inflammatory compound curcumin has been demonstrated to decrease the size of the lesion cavity82, increase neuronal viability, and decrease cell death83. The response of both NPs nicely reflects their impact on tissue preservation and axonal sprouting. TNF-α has long been used as a target for anti-inflammatory therapy. In SCI, TNF-α induces apoptosis via an NF-κB-dependent mechanism as well as a mitochondrial pathway regulated by the B-cell lymphoma 2 (BCL-2) family protein, Bax84. Inhibitors of TNF-α in the rat model of SCI have been proven to be an important cofactor for successful therapy85. In our study, the strongest effect on TNF-α was observed in the iPSC-NP group. Therefore, our results show that iPSC-NPs may be the most potent stem cell population, given that they most strongly suppressed TNF-α. MSCs have been described to suppress TNF-α levels after neuronal damage78. In our study, this effect was also transiently achieved in the MSC-treated animals.
The levels of IL-1β distinguish the two neural precursor populations from each other, since the levels in the SPC-01-treated group were significantly higher at day 14 when compared to the control groups, while these levels remained unchanged and below control values in the iPSC-NP group. The IL-1β levels in the MSC group were unchanged throughout the study. IL-1β has been implicated as a proinflammatory cytokine, whose inhibition may prove to be beneficial in SCI86. The levels of IL-1β in the MSC- and saline-treated animals remained undistinguished throughout the study. On the other hand, the increased IL-1β level in the SPC-01-treated group (day 14), and the initial decreased levels in iPSC-NP group when compared to the saline-treated animals, may partially reflect the impact of their origin and correlate to recovery after SCI.
IL-4 has been proposed to play an anti-inflammatory role. Therefore, the increase in IL-4 levels at day 14 in both the SPC-01 and iPSC-NP groups may be beneficial and promote recovery. Specifically, IL-4-mediated arginase may promote recovery and reduce inflammation87. When IL-4 was neutralized in a rat model of SCI, more extensive cavitation was noted 4 weeks after injury88. Therefore, increased levels of IL-4 in the SPC-01 and iPSC-NP groups, as observed at days 14 and 28 in our study, may contribute to neuroprotection, leading to the reduction of secondary injury or gliosis. In MSC-treated animals, 10 and 14 days after SCI, a decrease in IL-4 was observed when compared with both saline- and NP-treated rats. This is in agreement with elevated IRF5 and MRC1 RNA levels following macrophage stimulation.
IL-6 remains a cytokine with dual functions. On the one hand, it has been demonstrated to modulate the immune response by increasing inflammation after SCI. High levels of IL-6 can shift NSC differentiation toward astrocytes rather than neurons, thus promoting glial scar formation89. Additionally, suppression of IL-6 levels reduces secondary injury by reducing inflammatory cell infiltration90 and may result in improved recovery after SCI91. On the other hand, a mild level of IL-6 is important for preserving neurogenesis and neuroprotection following CNS injuries92,93. Moreover, IL-6 has been shown to inhibit voltage-gated sodium channel currents, indicating a potentially neuroprotective role against excitotoxicity94. In our study, MSCs partially decreased IL-6 levels, while high levels of IL-6 were observed in the SPC-01 and iPSC-NP groups. Nevertheless, based on our observations, we believe that IL-6 remains a cytokine with dual functions. IL-12p70 may play an anti-inflammatory role, as its levels have been shown to decrease after SCI95 (Fig. 4). Targeted administration of IL-12 has been shown to induce remyelination and de novo neurogenesis96 and partially prevents T-mediated demyelination97 after neuronal damage and neurodegeneration. This evidence suggests that increased IL-12p70 levels may play an anti-inflammatory role and could be desirable after SCI, as seen in the SPC-01 and iPSC-NP groups.
Conclusion
In our experimental setting, and taking into consideration two different routes of application, the use of iPSC-NPs to treat a balloon compression SCI positively affected the majority of studied factors involved in spinal cord regeneration, such as glial scar formation, axonal sprouting, tissue sparing, and cytokine levels. The cumulative effect resulted in better performance in advanced locomotor tests (flat beam test and rotarod) requiring better movement coordination. The application of SPC-01 cells, despite their long-term survival and effect on axonal sprouting, has not shown the potential of iPSC-NPs on behavioral recovery. On the other hand, the implantation of MSCs, despite their limited survival period, led to a significant improvement in locomotor abilities, which might be connected with their initial effect on intrinsic growth factor production and suppression of the immune response. Strategies to prolong MSC survival and to extend their effect on injured tissues by repeated application should be taken into account in future studies. To fully support the potential of iPSC-NPs for SCI treatment, the cells should be prepared by techniques that do not require viral integration.
Acknowledgments
This study was supported by grant GAČR (Grant Agency of the Czech Republic) 13-00939S from the Norwegian Financial Mechanism 2009-2014 and the Ministry of Education, Youth and Sports under Project Contract No. MSMT-28477/2014; project 7F14057; and EATRIS-CZ (LM2015064). Author contributions: Jiri Ruzicka: data analysis and interpretation, collection and/or assembly of data, and manuscript writing; Lucia Machova-Urdzikova: data analysis and interpretation, collection and/or assembly of data, and manuscript writing; John Gillick: collection and/or assembly of data; Takashi Amemori: data analysis and interpretation and collection and/or assembly of data; Nataliya Romanyuk: data analysis and interpretation and collection and/or assembly of data; Kristyna Karova: collection and/or assembly of data; Kristyna Zaviskova: collection and/or assembly of data; Jana Dubisova: collection and/or assembly of data; Sarka Kubinova: data analysis and interpretation; Raj Murali: financial support, data analysis, and interpretation; Eva Sykova: financial support, conception, and design; Meena Jhanwar-Uniyal: data analysis and interpretation, conception and design, and manuscript writing; Pavla Jendelova: financial support, data analysis and interpretation, conception and design, manuscript writing, and final approval of manuscript. The authors declare no conflicts of interest.
References
- 1.Anthony D.C., Couch Y. The systemic response to CNS injury. Exp Neurol. 2014; 258: 105–11. [DOI] [PubMed] [Google Scholar]
- 2.LaPlaca M.C., Simon C.M., Prado G.R., Cullen D.K. CNS injury biomechanics and experimental models. Prog Brain Res. 2007; 161: 13–26. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P., Riminucci M., Gronthos S., Robey P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001; 19(3): 180–92. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411): 143–7. [DOI] [PubMed] [Google Scholar]
- 5.Urdzikova L.M., Ruzicka J., LaBagnara M., Karova K., Kubinova S., Jirakova K., Murali R., Sykova E., Jhanwar-Uniyal M., Jendelova P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014; 15(7): 11275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai L., Lennon D.P., Eaton V., Maier K., Caplan A.I., Miller S.D., Miller R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009; 57(11): 1192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amemori T., Jendelova P., Ruzickova K., Arboleda D., Sykova E. Co-transplantation of olfactory ensheathing glia and mesenchymal stromal cells does not have synergistic effects after spinal cord injury in the rat. Cytotherapy 2010; 12(2): 212–25. [DOI] [PubMed] [Google Scholar]
- 8.Torres-Espin A., Corona-Quintanilla D.L., Fores J., Allodi I., Gonzalez F., Udina E., Navarro X. Neuroprotection and axonal regeneration after lumbar ventral root avulsion by re-implantation and mesenchymal stem cells transplant combined therapy. Neurotherapeutics 2013; 10(2): 354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu P., Blesch A., Tuszynski M.H. Induction of bone marrow stromal cells to neurons: Differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004; 77(2): 174–91. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Chen J., Chen X.G., Wang L., Gautam S.C., Xu Y.X., Katakowski M., Zhang L.J., Lu M., Janakiraman N., Chopp M. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology 2002; 59(4): 514–23. [DOI] [PubMed] [Google Scholar]
- 11.Uccelli A., Benvenuto F., Laroni A., Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011; 24(1): 59–64. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J., Li Y., Chen J., Yang M., Katakowski M., Lu M., Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004; 1030(1): 19–27. [DOI] [PubMed] [Google Scholar]
- 13.Bao X., Wei J., Feng M., Lu S., Li G., Dou W., Ma W., Ma S., An Y., Qin C., Zhao R.C., Wang R. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011; 1367: 103–13. [DOI] [PubMed] [Google Scholar]
- 14.Oliveri R.S., Bello S., Biering-Sorensen F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol Dis. 2014; 62: 338–53. [DOI] [PubMed] [Google Scholar]
- 15.Saito F., Nakatani T., Iwase M., Maeda Y., Murao Y., Suzuki Y., Fukushima M., Ide C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor Neurol Neurosci. 2012; 30(2): 127–36. [DOI] [PubMed] [Google Scholar]
- 16.Forostyak S., Jendelova P., Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 2013; 95(12): 2257–70. [DOI] [PubMed] [Google Scholar]
- 17.Cocks G., Romanyuk N., Amemori T., Jendelova P., Forostyak O., Jeffries A.R., Perfect L., Thuret S., Dayanithi G., Sykova E., Price J. Conditionally immortalized stem cell lines from human spinal cord retain regional identity and generate functional V2a interneurons and motorneurons. Stem Cell Res Ther. 2013; 4(3): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng I., Mayle R.E., Cox C.A., Park D.Y., Smith R.L., Corcoran-Schwartz I., Ponnusamy K.E., Oshtory R., Smuck M.W., Mitra R., Kharazi A.I., Carragee E.J. Functional assessment of the acute local and distal transplantation of human neural stem cells after spinal cord injury. Spine J. 2012; 12(11): 1040–4. [DOI] [PubMed] [Google Scholar]
- 19.Ruzicka J., Romanyuk N., Hejcl A., Vetrik M., Hruby M., Cocks G., Cihlar J., Pradny M., Price J., Sykova E., Jendelová P. Treating spinal cord injury in rats with a combination of human fetal neural stem cells and hydrogels modified with serotonin. Acta Neurobiol Exp (Wars) 2013; 73(1): 102–15. [DOI] [PubMed] [Google Scholar]
- 20.Parr A.M., Kulbatski I., Zahir T., Wang X., Yue C., Keating A., Tator C.H. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 2008; 155(3): 760–70. [DOI] [PubMed] [Google Scholar]
- 21.Yang J.R., Liao C.H., Pang C.Y., Huang L.L., Chen Y.L., Shiue Y.L., Chen L.R. Transplantation of porcine embryonic stem cells and their derived neuronal progenitors in a spinal cord injury rat model. Cytotherapy 2013; 15(2): 201–8. [DOI] [PubMed] [Google Scholar]
- 22.Salewski R.P., Mitchell R.A., Shen C., Fehlings M.G. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015; 24(1): 36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romanyuk N., Amemori T., Turnovcova K., Prochazka P., Onteniente B., Sykova E., Jendelova P. Beneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repair. Cell Transplant. 2015; 24(9): 1781–97. [DOI] [PubMed] [Google Scholar]
- 24.Sareen D., Gowing G., Sahabian A., Staggenborg K., Paradis R., Avalos P., Latter J., Ornelas L., Garcia L., Svendsen C.N. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J Comp Neurol. 2014; 522(12): 2707–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawryluk G.W., Mothe A., Wang J., Wang S., Tator C., Fehlings M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012; 21(12): 2222–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amemori T., Romanyuk N., Jendelova P., Herynek V., Turnovcova K., Prochazka P., Kapcalova M., Cocks G., Price J., Sykova E. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res Ther. 2013; 4(3): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cusimano M., Biziato D., Brambilla E., Donega M., Alfaro-Cervello C., Snider S., Salani G., Pucci F., Comi G., Garcia-Verdugo J.M., De Palma M., Martino G., Pluchino S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 2012; 135(Pt 2): 447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi Y., Okada Y., Itakura G., Iwai H., Nishimura S., Yasuda A., Nori S., Hikishima K., Konomi T., Fujiyoshi K., Tsuji O., Toyama Y., Yamanaka S., Nakamura M., Okano H. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One 2012; 7(12): e52787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., Brock J., Blesch A., Rosenzweig E.S., Havton L.A., Zheng B., Conner J.M., Marsala M., Tuszynski M.H. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012; 150(6): 1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuszynski M.H., Wang Y., Graham L., Gao M., Wu D., Brock J., Blesch A., Rosenzweig E.S., Havton L.A., Zheng B., Conner J.M., Marsala M., Lu P. Neural stem cell dissemination after grafting to CNS injury sites. Cell 2014; 156(3): 388–9. [DOI] [PubMed] [Google Scholar]
- 31.Nutt S.E., Chang E.A., Suhr S.T., Schlosser L.O., Mondello S.E., Moritz C.T., Cibelli J.B., Horner P.J. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp Neurol. 2013; 248: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littlewood T.D., Hancock D.C., Danielian P.S., Parker M.G., Evan G.I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995; 23(10): 1686–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126(4): 663–76. [DOI] [PubMed] [Google Scholar]
- 34.Seki T., Fukuda K. Methods of induced pluripotent stem cells for clinical application. World J Stem Cells 2015; 7(1): 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Fang H., Dai J., Liu G., Xu Z.J. Induced pluripotent stem cells for spinal cord injury therapy: Current status and perspective. Neurol Sci. 2013; 34(1): 11–7. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji O., Miura K., Fujiyoshi K., Momoshima S., Nakamura M., Okano H. Cell therapy for spinal cord injury by neural stem/progenitor cells derived from iPS/ES cells. Neurotherapeutics 2011; 8(4): 668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu X. The immunogenicity of cells derived from induced pluripotent stem cells. Cell Mol Immunol. 2014; 11(1): 14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cyranoski D. Japanese woman is first recipient of next-generation stem cells. NATURE | NEWS. 2014. Available from http://www.nature.com/news/japanese-woman-is-first-recipient-of-next-generation-stem-cells-1.15915 [Google Scholar]
- 39.Kawamura T., Miyagawa S., Fukushima S., Maeda A., Kashiyama N., Kawamura A., Miki K., Okita K., Yoshida Y., Shiina T., Ogasawara K., Miyagawa S., Toda K., Okuyama H., Sawa Y. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Reports 2016; 6(3): 312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morizane A., Doi D., Kikuchi T., Okita K., Hotta A., Kawasaki T., Hayashi T., Onoe H., Shiina T., Yamanaka S., Takahashi J. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a nonhuman primate. Stem Cell Reports 2013; 1(4): 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollock K., Stroemer P., Patel S., Stevanato L., Hope A., Miljan E., Dong Z., Hodges H., Price J., Sinden J.D. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006; 199(1): 143–55. [DOI] [PubMed] [Google Scholar]
- 42.Polentes J., Jendelova P., Cailleret M., Braun H., Romanyuk N., Tropel P., Brenot M., Itier V., Seminatore C., Baldauf K., Turnovcova K., Jirak D., Teletin M., Côme J., Tournois J., Reymann K., Sykova E., Viville S., Onteniente B. Human induced pluripotent stem cells improve stroke outcome and reduce secondary degeneration in the recipient brain. Cell Transplant. 2012; 21(12): 2587–602. [DOI] [PubMed] [Google Scholar]
- 43.Urdzikova L., Jendelova P., Glogarova K., Burian M., Hajek M., Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma 2006; 23(9): 1379–91. [DOI] [PubMed] [Google Scholar]
- 44.De la Calle J.L., Paino C.L. A procedure for direct lumbar puncture in rats. Brain Res Bull. 2002; 59(3): 245–50. [DOI] [PubMed] [Google Scholar]
- 45.Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12(1): 1–21. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein L.B. Effects of bilateral and unilateral locus coeruleus lesions on beam-walking recovery after subsequent unilateral sensorimotor cortex suction-ablation in the rat. Restor Neurol Neurosci. 1997; 11(1): 55–63. [DOI] [PubMed] [Google Scholar]
- 47.Pan J.Z., Ni L., Sodhi A., Aguanno A., Young W., Hart R.P. Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J Neurosci Res. 2002; 68(3): 315–22. [DOI] [PubMed] [Google Scholar]
- 48.Su Y., Fan W., Ma Z., Wen X., Wang W., Wu Q., Huang H. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 2014; 266: 56–65. [DOI] [PubMed] [Google Scholar]
- 49.Machova Urdzikova L., Karova K., Ruzicka J., Kloudova A., Shannon C., Dubisova J., Murali R., Kubinova S., Sykova E., Jhanwar-Uniyal M., Jendelova P. The anti-inflammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int J Mol Sci. 2015; 17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul C., Samdani A.F., Betz R.R., Fischer I., Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: A comparison of delivery methods. Spine (Phila Pa 1976) 2009; 34(4): 328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker P.A., Letourneau P.A., Bedi S., Shah S.K., Jimenez F., Cox C.S., Jr Progenitor cells as remote “bioreactors”: Neuroprotection via modulation of the systemic inflammatory response. World J Stem Cells 2011; 3(2): 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakshi A., Hunter C., Swanger S., Lepore A., Fischer I. Minimally invasive delivery of stem cells for spinal cord injury: Advantages of the lumbar puncture technique. J Neurosurg Spine 2004; 1(3): 330–7. [DOI] [PubMed] [Google Scholar]
- 53.Geffner L.F., Santacruz P., Izurieta M., Flor L., Maldonado B., Auad A.H., Montenegro X., Gonzalez R., Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transplant. 2008; 17(12): 1277–93. [DOI] [PubMed] [Google Scholar]
- 54.Kishk N.A., Gabr H., Hamdy S., Afifi L., Abokresha N., Mahmoud H., Wafaie A., Bilal D. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair 2010; 24(8): 702–8. [DOI] [PubMed] [Google Scholar]
- 55.Kuroda S., Shichinohe H., Houkin K., Iwasaki Y. Autologous bone marrow stromal cell transplantation for central nervous system disorders—Recent progress and perspective for clinical application. J Stem Cells Regen Med. 2011; 7(1): 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forostyak S., Homola A., Turnovcova K., Svitil P., Jendelova P., Sykova E. Intrathecal delivery of mesenchymal stromal cells protects the structure of altered perineuronal nets in SOD1 rats and amends the course of ALS. Stem Cells 2014; 32(12): 3163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amemori T., Ruzicka J., Romanyuk N., Jhanwar-Uniyal M., Sykova E., Jendelova P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther. 2015; 6: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexanian A.R., Fehlings M.G., Zhang Z., Maiman D.J. Transplanted neurally modified bone marrow-derived mesenchymal stem cells promote tissue protection and loco-motor recovery in spinal cord injured rats. Neurorehabil Neural Repair 2011; 25(9): 873–80. [DOI] [PubMed] [Google Scholar]
- 59.Arboleda D., Forostyak S., Jendelova P., Marekova D., Amemori T., Pivonkova H., Masinova K., Sykova E. Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cell Mol Neurobiol. 2011; 31(7): 1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erceg S., Ronaghi M., Oria M., Rosello M.G., Arago M.A., Lopez M.G., Radojevic I., Moreno-Manzano V., Rodriguez-Jimenez F.J., Bhattacharya S.S., Cordoba J., Stojkovic M. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells 2010; 28(9): 1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niapour A., Karamali F., Nemati S., Taghipour Z., Mardani M., Nasr-Esfahani M.H., Baharvand H. Cotransplantation of human embryonic stem cell-derived neural progenitors and Schwann cells in a rat spinal cord contusion injury model elicits a distinct neurogenesis and functional recovery. Cell Transplant. 2012; 21(5): 827–43. [DOI] [PubMed] [Google Scholar]
- 62.Gianni-Barrera R., Bartolomeo M., Vollmar B., Djonov V., Banfi A. Split for the cure: VEGF, PDGF-BB and intussusception in therapeutic angiogenesis. Biochem Soc Trans. 2014; 42(6): 1637–42. [DOI] [PubMed] [Google Scholar]
- 63.Wietecha M.S., DiPietro L.A. Therapeutic approaches to the regulation of wound angiogenesis. Adv Wound Care (New Rochelle) 2013; 2(3): 81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naumann T., Schnell O., Zhi Q., Kirsch M., Schubert K.O., Sendtner M., Hofmann H.D. Endogenous ciliary neurotrophic factor protects GABAergic, but not cholinergic, septohippocampal neurons following fimbria-fornix transection. Brain Pathol. 2003; 13(3): 309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linker R.A., Maurer M., Gaupp S., Martini R., Holtmann B., Giess R., Rieckmann P., Lassmann H., Toyka K.V., Sendtner M., Gold R. CNTF is a major protective factor in demyelinating CNS disease: A neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002; 8(6): 620–4. [DOI] [PubMed] [Google Scholar]
- 66.Stankoff B., Aigrot M.S., Noel F., Wattilliaux A., Zalc B., Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: A novel role for CNTF and CNTF-related molecules. J Neurosci. 2002; 22(21): 9221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teixeira F.G., Carvalho M.M., Sousa N., Salgado A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013; 70(20): 3871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carvalho M.M., Teixeira F.G., Reis R.L., Sousa N., Salgado A.J. Mesenchymal stem cells in the umbilical cord: Phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Curr Stem Cell Res Ther. 2011; 6(3): 221–8. [DOI] [PubMed] [Google Scholar]
- 69.Teixeira F.G., Carvalho M.M., Panchalingam K.M., Rodrigues A.J., Mendes-Pinheiro B., Anjo S., Manadas B., Behie L.A., Sousa N., Salgado A.J. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson's disease. Stem Cells Transl Med. 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teixeira F.G., Panchalingam K.M., Anjo S.I., Manadas B., Pereira R., Sousa N., Salgado A.J., Behie L.A. Do hypoxia/normoxia culturing conditions change the neuroregulatory profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell Res Ther. 2015; 6: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao Y., Huang C., Gu P., Wen T. Combined MSC-secreted factors and neural stem cell transplantation promote functional recovery of PD rats. Cell Transplant. 2016; 25(6): 1101–13. [DOI] [PubMed] [Google Scholar]
- 72.Menezes K., Nascimento M.A., Goncalves J.P., Cruz A.S., Lopes D.V., Curzio B., Bonamino M., de Menezes J.R., Borojevic R., Rossi M.I., Coelho-Sampaio T. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS One 2014; 9(5): e96020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritfeld G.J., Nandoe Tewarie R.D., Vajn K., Rahiem S.T., Hurtado A., Wendell D.F., Roos R.A., Oudega M. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. 2012; 21(7): 1561–75. [DOI] [PubMed] [Google Scholar]
- 74.Wilcox J.T., Satkunendrarajah K., Zuccato J.A., Nassiri F., Fehlings M.G. Neural precursor cell transplantation enhances functional recovery and reduces astrogliosis in bilateral compressive/contusive cervical spinal cord injury. Stem Cells Transl Med. 2014; 3(10): 1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cantinieaux D., Quertainmont R., Blacher S., Rossi L., Wanet T., Noel A., Brook G., Schoenen J., Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS One 2013; 8(8): e69515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang K., Zheng J., Bian G., Liu L., Xue Q., Liu F., Yu C., Zhang H., Song B., Chung S.K., Ju G., Wang J. Polarized macrophages have distinct roles in the differentiation and migration of embryonic spinal-cord-derived neural stem cells after grafting to injured sites of spinal cord. Mol Ther. 2015; 23(6): 1077–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Busch S.A., Hamilton J.A., Horn K.P., Cuascut F.X., Cutrone R., Lehman N., Deans R.J., Ting A.E., Mays R.W., Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci. 2011; 31(3): 944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakajima H., Uchida K., Guerrero A.R., Watanabe S., Sugita D., Takeura N., Yoshida A., Long G., Wright K.T., Johnson W.E., Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma 2012; 29(8): 1614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang M.H., Chung E., Chi G.F., Ahn W., Lim J.E., Hong H.S., Kim D.W., Choi H., Kim J., Son Y. Substance P induces M2-type macrophages after spinal cord injury. Neuroreport 2012; 23(13): 786–92. [DOI] [PubMed] [Google Scholar]
- 80.Matsubara K., Matsushita Y., Sakai K., Kano F., Kondo M., Noda M., Hashimoto N., Imagama S., Ishiguro N., Suzumura A., Ueda M., Furukawa K., Yamamoto A. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity. J Neurosci. 2015; 35(6): 2452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ousman S.S., David S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J Neurosci. 2001; 21(13): 4649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Machova Urdzikova L., Karova K., Ruzicka J., Kloudova A., Shannon C., Dubisova J., Murali R., Kubinova S., Sykova E., Jhanwar-Uniyal M., Jendelova P. The anti-inflammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int J Mol Sci. 2015; 17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin M.S., Sun Y.Y., Chiu W.T., Hung C.C., Chang C.Y., Shie F.S., Tsai S.H., Lin J.W., Hung K.S., Lee Y.H. Curcumin attenuates the expression and secretion of RANTES after spinal cord injury in vivo and lipopolysaccharide-induced astrocyte reactivation in vitro. J Neurotrauma 2011; 28(7): 1259–69. [DOI] [PubMed] [Google Scholar]
- 84.Guadagno J., Xu X., Karajgikar M., Brown A., Cregan S.P. Microglia-derived TNFalpha induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis. 2013; 4: e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Wei F.X., Cen J.S., Ping S.N., Li Z.Q., Chen N.N., Cui S.B., Wan Y., Liu S.Y. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res. 2014; 1575: 87–100. [DOI] [PubMed] [Google Scholar]
- 86.Boato F., Rosenberger K., Nelissen S., Geboes L., Peters E.M., Nitsch R., Hendrix S. Absence of IL-1beta positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J Neuroinflammation 2013; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fenn A.M., Hall J.C., Gensel J.C., Popovich P.G., Godbout J.P. IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: Consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J Neurosci. 2014; 34(26): 8904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee S.I., Jeong S.R., Kang Y.M., Han D.H., Jin B.K., Namgung U., Kim B.G. Endogenous expression of interleukin-4 regulates macrophage activation and confines cavity formation after traumatic spinal cord injury. J Neurosci Res. 2010; 88(11): 2409–19. [DOI] [PubMed] [Google Scholar]
- 89.Mukaino M., Nakamura M., Okada S., Toyama Y., Liu M., Okano H. [Role of IL-6 in regulation of inflammation and stem cell differentiation in CNS trauma]. Nihon Rinsho Meneki Gakkai Kaishi 2008; 31(2): 93–8. [DOI] [PubMed] [Google Scholar]
- 90.Nakamura M., Okada S., Toyama Y., Okano H. Role of IL-6 in spinal cord injury in a mouse model. Clin Rev Allergy Immunol. 2005; 28(3): 197–204. [DOI] [PubMed] [Google Scholar]
- 91.Arima H., Hanada M., Hayasaka T., Masaki N., Omura T., Xu D., Hasegawa T., Togawa D., Yamato Y., Kobayashi S., Yasuda T., Matsuyama Y., Setou M. Blockade of IL-6 signaling by MR16-1 inhibits reduction of docosahexaenoic acid-containing phosphatidylcholine levels in a mouse model of spinal cord injury. Neuroscience 2014; 269: 1–10. [DOI] [PubMed] [Google Scholar]
- 92.Herrmann O., Tarabin V., Suzuki S., Attigah N., Coserea I., Schneider A., Vogel J., Prinz S., Schwab S., Monyer H., Brombacher F., Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003; 23(4): 406–15. [DOI] [PubMed] [Google Scholar]
- 93.Bowen K.K., Dempsey R.J., Vemuganti R. Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport 2011; 22(3): 126–30. [DOI] [PubMed] [Google Scholar]
- 94.Li X., Chen W., Sheng J., Cao D., Wang W. Interleukin-6 inhibits voltage-gated sodium channel activity of cultured rat spinal cord neurons. Acta Neuropsychiatr. 2014; 26(3): 170–7. [DOI] [PubMed] [Google Scholar]
- 95.Stammers A.T., Liu J., Kwon B.K. Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J Neurosci Res. 2012; 90(4): 782–90. [DOI] [PubMed] [Google Scholar]
- 96.Yaguchi M., Ohta S., Toyama Y., Kawakami Y., Toda M. Functional recovery after spinal cord injury in mice through activation of microglia and dendritic cells after IL-12 administration. J Neurosci Res. 2008; 86(9): 1972–80. [DOI] [PubMed] [Google Scholar]
- 97.Zandian M., Mott K.R., Allen S.J., Chen S., Arditi M., Ghiasi H. IL-2 suppression of IL-12p70 by a recombinant HSV-1 expressing IL-2 induces T-cell auto-reactivity and CNS demyelination. PLoS One 2011; 6(2): e16820. [DOI] [PMC free article] [PubMed] [Google Scholar]


