Abstract
AKI is a frequent condition that involves renal microcirculation impairment, infiltration of inflammatory cells with local production of proinflammatory cytokines, and subsequent epithelial disorders and mitochondrial dysfunction. Peroxisome proliferator-activated receptor γ coactivator 1-α (PPARGC1A), a coactivator of the transcription factor PPAR-γ that controls mitochondrial biogenesis and function, has a pivotal role in the early dysfunction of the proximal tubule and the subsequent renal repair. Here, we evaluated the potential role of hepatocyte nuclear factor–1β (HNF-1β) in regulating PPARGC1A expression in AKI. In mice, endotoxin injection to induce AKI also induced early and transient inflammation and PPARGC1A inhibition, which overlapped with downregulation of the HNF-1β transcriptional network. In vitro, exposure of proximal tubule cells to the inflammatory cytokines IFN-γ and TNF-α led to inhibition of HNF-1β transcriptional activity. Moreover, inhibition of HNF-1β significantly reduced PPARGC1A expression and altered mitochondrial morphology and respiration in proximal tubule cells. Chromatin immunoprecipitation assays and PCR analysis confirmed HNF-1β binding to the Ppargc1a promoter in mouse kidneys. We also demonstrated downregulation of renal PPARGC1A expression in a patient with an HNF1B germinal mutation. Thus, we propose that HNF-1β links extracellular inflammatory signals to mitochondrial dysfunction during AKI partly via PPARGC1A signaling. Our findings further strengthen the view of HNF1B-related nephropathy as a mitochondrial disorder in adulthood.
Keywords: HNF1B, acute kidney injury, mitochondria, PPARGC1A
AKI, a life-threatening condition with a worldwide incidence of 13.5 million patients per year, contributes to about 1.7 million deaths every year.1 For survivors, there is a nine times higher risk of CKD and a two times higher risk of premature death than for matched patients without AKI.2,3 Although some renal lesions or cell responses are specific to the underlying cause of AKI most, if not all, AKI include various degrees of endothelial dysfunction, renal inflammation including inflammatory cells infiltration and local cytokines (IFN-γ, TNF-α, and IL-6) or chemokines (Ccl2 and Ccl5) production, and severe mitochondrial dysfunction in epithelial cells.4–7
Mitochondrial dysfunction is a hallmark of AKI.5,8–9 In endotoxin-induced AKI, alterations in mitochondrial biogenesis and function in proximal tubule cells are now considered an adaptive response to the injury, associated with metabolic reprogramming favoring cell survival at the expense of kidney function.6 In this setting, the pivotal role of the transient cytokine-mediated inhibition of PPARGC1A (PGC-1α), a coactivator of the PPAR-γ transcription factor involved in the mitochondria biogenesis or function, has been emphasized,10 but how PPARGC1A expression and activity are modulated at the early stages of AKI remains largely unknown.
In a recent study, we reported early and transient inhibition of the transcriptional activity of the hepatocyte nuclear factor–1β (HNF-1β) after hemorrhagic shock-related AKI that might be involved in renal epithelial injury or repair.11 HNF-1β is a transcription factor encoded by the HNF1B gene and expressed in various organs with tubular epithelium structure, like kidney, pancreas, biliary tree, or gut.12 HNF1B-related nephropathy is a dominantly inherited, highly heterogeneous disorder characterized by hyperechogenic kidneys of normal size and microcysts during the antenatal period,13 hypoplastic kidneys with cortical cysts in childhood,14 and chronic tubulointerstitial nephritis with few or no cysts in adulthood.15 Other findings include renal wasting of magnesium and/or potassium in >50% of patients, and more rarely a proximal tubulopathy. One striking feature in HNF1B-related nephropathy is the progressive renal function decline accompanied by interstitial fibrosis observed in most patients, which is not correlated to the extent of cystic changes. On top of this progressive renal function decline, in some patients with HNF1B alteration, sudden, nonexplained, rapidly progressive renal failure is observed.15
The pivotal role of HNF-1β during renal morphogenesis (planar cell polarity, tubulogenesis, and epithelial differentiation) has been elucidated in animal studies and generated insight in the human renal phenotype observed in the antenatal period or in childhood (renal cysts and various developmental disorders) in HNF1B-mutated individuals.16–20 In contrast, the role of HNF-1β in adult normal (quiescent) or injured kidney is less clear.21 Data obtained in vitro or in animal models suggest that HNF-1β may also regulate mitochondrial oxidative phosphorylation.22–24 Furthermore, the renal phenotype of HNF1B mutated individuals overlaps with that of patients with renal mitochondrial disorders (i.e., interstitial fibrosis, electrolyte disorders, and rarely, proximal tubular dysfunction).15,25,26 We therefore hypothesized that (1) HNF-1β may directly control PPARGC1A expression and subsequent mitochondrial biogenesis or function in the postembryonic kidney, thus shifting the paradigm of the HNF1B-related nephropathy in adulthood from a developmental nephropathy to a mitochondrial disorder; and (2) transient HNF-1β inhibition may control the mitochondrial dysfunction observed at the early phases of AKI.
Results
AKI Is Followed by HNF-1β Inhibition and Mitochondrial Dysfunction
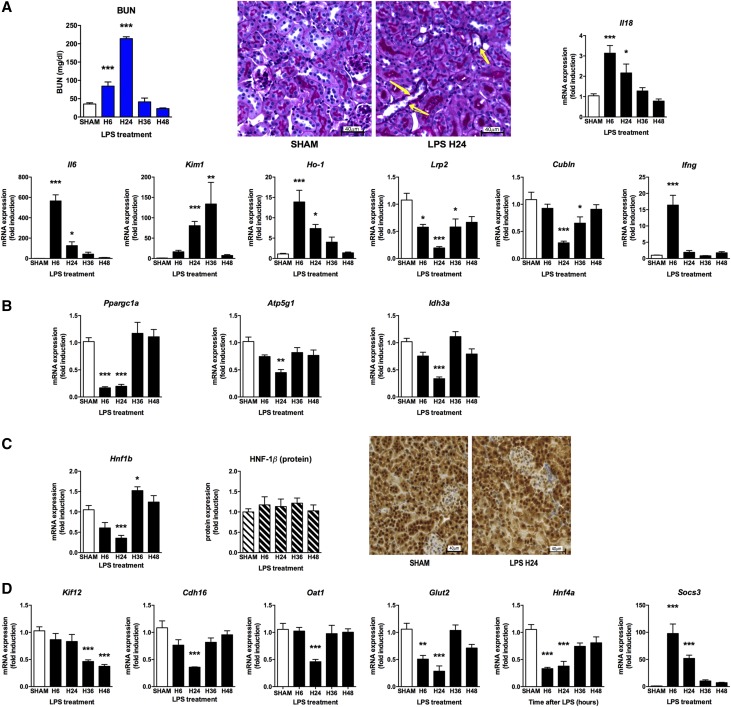
In an attempt to better understand the role of HNF-1β during AKI, we first assessed its expression as well as the expression of its target genes in a mouse model of sepsis-induced AKI. For induction of endotoxic AKI, C57Bl6 mice received an intraperitoneal injection of LPS (10 mg/kg) that within 6 hours induced AKI characterized by oliguria or anuria, BUN increase, and a dramatic upregulation of renal AKI biomarker genes, including IL-18, IL-6, Kim1, and Ho1, although with different expression profiles over time (Figure 1A). Forty-eight hours after LPS injection, renal function recovered and most of the transcriptional changes were normalized. As previously reported,10 only minor histologic changes were observed (minor tubular cell detachment) (Figure 1A). Intrarenal mRNA synthesis of the proinflammatory cytokines IFN-γ and TNF-α were observed early after the injection of LPS (Figure 1A, Supplemental Figure 1A). Concomitantly, a dramatic downregulation of the proximal tubule markers of the megalin-cubilin complex (Lrp2, Cubln) was observed (Figure 1A). The expression of Ppargc1a and some of its target genes involved in mitochondrial biogenesis and function (Atp5g1 and Idh3a) were also downregulated in the early phases of endotoxin-induced AKI (Figure 1B).
Figure 1.
Endotoxic-related AKI is followed by renal inflammation and down-regulation of HNF-1β target genes and Ppargc1a. C57Bl6 mice (males 10–12 weeks old) were injected with either saline or LPS intraperitoneally (LPS 10 μg/g) and euthanized at 6, 24, 36, and 48 hours. (A) BUN was increased 6–24 hours after the LPS injection. Periodic acid–Schiff staining of a kidney section (×20) revealed few epithelial lesions in LPS-injected mice (right panel; arrow, some tubular cells within the lumen; arrows: tubular cells slightly swallowed) compared with control mice (left panel). Markers of inflammation and renal injury were dramatically increased after LPS injection. (B) mRNA expression of Ppargc1a and its target genes Atp5g1 and Idh3a was dramatically decreased after LPS injection. (C) Western blotting and immunostaining revealed normal HNF-1β protein expression and localization after LPS injection. (D) Downregulation of the HNF-1β transcriptional network after LPS injection. *P<0.05; **P<0.01; ***P<0.001.
Six hours after LPS injection, we observed a significant downregulation of the renal Hnf1b mRNA expression whereas the quantity and the localization of the HNF-1β protein were not changed (Figure 1C). In parallel to renal inflammation, we observed a dramatic downregulation of known HNF-1β target genes (i.e., Kif12, Cdh16, Oat1, Glut2, and Hnf4a) (Figure 1C). In contrast, the expression of the suppressor of cytokine signaling 3 gene (Socs3), a gene negatively regulated by HNF-1β in epithelial renal cells,27 displayed a significant increase in abundance at that time.
These results suggest early and concomitant dysregulation of HNF-1β (inhibition of its transcriptional activity without decrease of its protein expression) and its target genes, as well as genes involved in mitochondrial biogenesis and function including Ppargc1a.
Proinflammatory Cytokines Control the Transcriptional Activity of HNF-1β
AKI is a condition characterized by infiltration of inflammatory cells (IFN-γ secreting NKT cells, neutrophils, and monocytes) through local production of proinflammatory cytokines, including IFN-γ and TNF-α.28,29 Epithelial dysfunction characterized by transcriptional changes and mitochondrial dysfunction is also commonly observed.10,30 This data prompted us to test whether we could confirm these findings in vitro using IFN-γ or TNF-α stimulation.
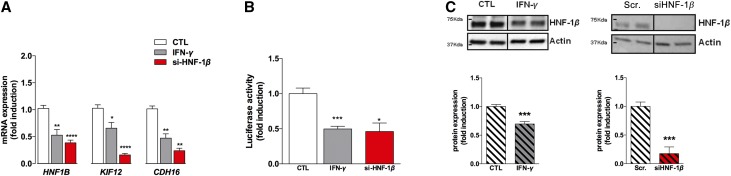
IFN-γ treatment in HK-2 cells, a cell line derived from human proximal tubules that has basal expression of HNF1B,31 led to an approximately two-fold decrease of the expression of HNF-1β mRNA and its direct target genes, KIF12 and CDH16, a finding recapitulating what is observed after siRNA-mediated HNF1B inhibition (Figure 2A). To confirm these observations, HK-2 cells were transfected with a Kif12 promoter-reporter plasmid, whose expression is dependent on HNF-1β,32 and luciferase activity was measured at basal state or after IFN-γ exposure. As shown in Figure 2B, transfection of this plasmid in HK-2 cells was followed by a significant expression of the luciferase activity, consistent with the basal expression of HNF-1β in these cells. In contrast, IFN-γ exposure significantly inhibited the luciferase activity. However, in contrast to what observed in the in vivo AKI model, IFN-γ treatment induced a moderate but significant decrease in HNF-1β protein expression (Figure 2C), whereas HNF1B protein levels were drastically decreased (six- to seven-fold) when HNF1B gene expression was inhibited with an siRNA, suggesting that these in vitro conditions did not fully recapitulate the in vivo condition where HNF-1β protein abundance is probably regulated by multiple cytokines.
Figure 2.
The inflammatory cytokine IFN-γ regulates the transcriptional activity of HNF-1β. (A) Exposure to inflammatory cytokines downregulated the target genes of HNF-1β, such as KIF12 and CDH16, recapitulating what is observed after siRNA-mediated inhibition of HNF-1β. (B) Western blot analysis of HNF-1β revealed that IFN-γ downregulated the HNF-1β protein, recapitulating what is observed after siRNA-mediated inhibition of HNF-1β. (C) HK-2 cells were transfected with a Kif12 promoter-reporter plasmid, whose expression is dependent on HNF-1β.32 Measurement of the luciferase assay revealed that IFN-γ downregulates the transcriptional activity of HNF-1β, recapitulating what is observed after siRNA-mediated HNF-1β invalidation. *P<0.05; **P<0.01; ***P<0.001.
To test this hypothesis, HK-2 cells were also submitted to TNF-α. Again, the expression of the HNF-1β target genes KIF12 and CDH16 was significantly decreased whereas HNF-1β expression was not dramatically modified (Supplemental Figure 1B). TNF-α significantly downregulated the luciferase activity of a HNF-1β–dependent Kif12 promoter-reporter plasmid, thus confirming that TNF-α also participates in the inflammation-controlled inhibition of the transcriptional activity of HNF-1β (Supplemental Figure 1C).
HNF-1β Controls the Expression of PPARGC1A
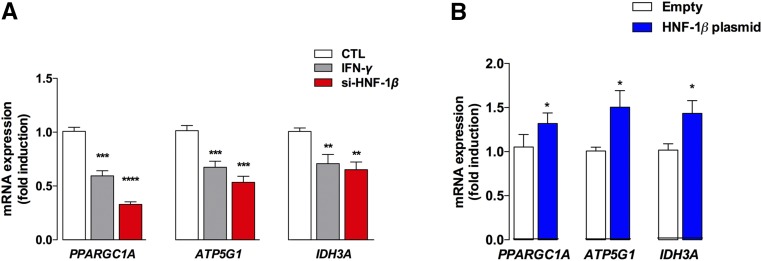
siRNA-mediated inhibition of HNF1B in HK-2 cells significantly inhibited the expression of PPARGC1A and its target genes, ATP5G1 and IDH3A, recapitulating what is observed after IFN-γ exposure (Figure 3A). Furthermore, overexpression of HNF1B in HEK-293 cells, using a plasmid expressing the human form of HNF-1β, led to significant upregulation of PPARGC1A, ATP5G1, and IDH3A, suggesting that HNF-1β indeed at least partly controls their expression (Figure 3B).
Figure 3.
HNF-1β controls the expression of PPARGC1A and its target genes. (A and B) siRNA-mediated inhibition and plasmid-related overexpression of HNF-1β in HK-2 cells leads to dramatic down- or upregulation of PPARGC1A and its target genes, ATPG1A and IDH3A, respectively. *P<0.05; **P<0.01; ***P<0.001.
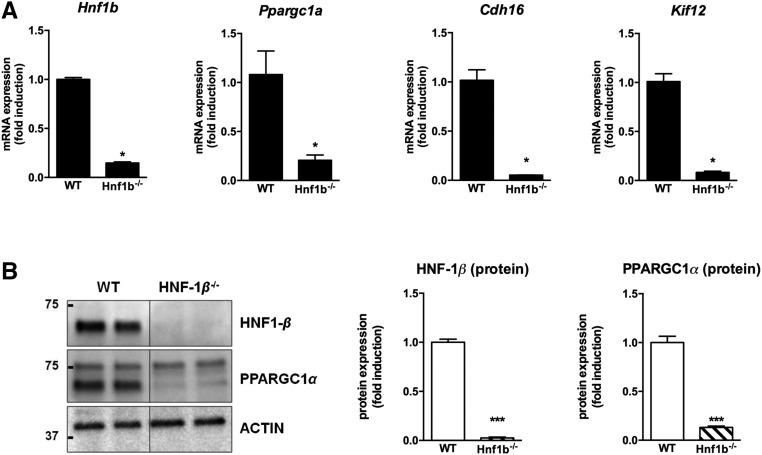
We next deleted the Hnf1b gene in the mouse MCT cell line using the CRISPR/Cas9 approach (subsequently named Hnf1b−/− cells). mRNA and protein expression of Ppargc1a was significantly decreased in the Hnf1b−/− cells (Figure 4, A and B), as well as HNF-1β target genes Cdh16 and Kif12.
Figure 4.
PPARGC1A is dramatically downregulated in mouse proximal tubule cells with HNF-1β invalidation. (A and B) In MCT cells with CRISPR/Cas9-mediated Hnf1b invalidation, target genes of HNF-1β and Ppargc1a are significantly downregulated at both mRNA and protein levels. *P<0.05; **P<0.01; ***P<0.001.
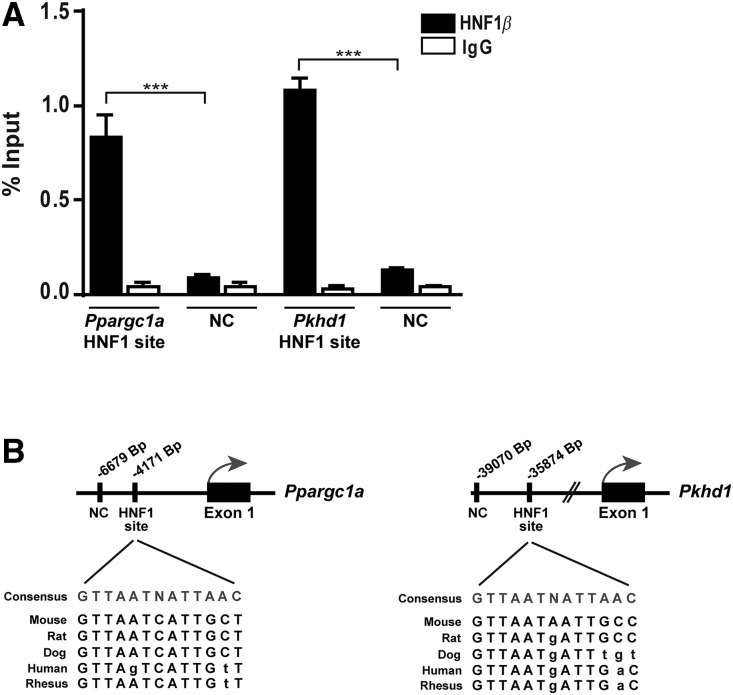
To further confirm these findings of HNF-1β controlling PPARGC1A, we performed immunoprecipitation of chromatin from mouse kidney with an anti–HNF-1β antibody, and showed that HNF-1β can bind to a specific DNA sequence within the promoter of Ppargc1a as well as Pkhd1, a previously recognized target gene of HNF-1β (Figure 5A). This sequence, localized 4171-bp upstream of the transcription site start of Ppargc1a, is highly conserved among species (Figure 5B). Thus, we identified Ppargc1a as a target gene directly regulated by HNF-1β.
Figure 5.
PPARGC1A is a target gene of HNF-1β. (A) ChIP-qPCR analysis performed on mouse kidney chromatin using HNF-1β antibodies or control IgG. There is a significant enrichment on an HNF1-binding site identified in the Ppargc1a promoter compared with a region with no predicted HNF1 consensus sequence (NC, negative control). A known binding site in Pkhd1 promoter17 was used as a positive control. (B) The position of HNF1-binding sites and negative regions are indicated relative to the transcription start site TSS of the corresponding gene. Sequence conservation of HNF1-binding sites among different species is shown below the HNF1-binding sites. Results are expressed as percentage of immunoprecipitated DNA compared with input DNA and shown as mean±SEM. Statistical significance was determined by ANOVA analysis followed by Bonferroni post-test. ***P<0.001.
HNF-1β Modulates the Production of Reactive Oxygen Species
Given the roles of HNF-1β11 and PPARGC1A10 in AKI and their overlapping expression profiles and transcriptional activity, we put forward the hypothesis that HNF-1β may modulate the expression of PPARGC1A and subsequently mitochondrial function.
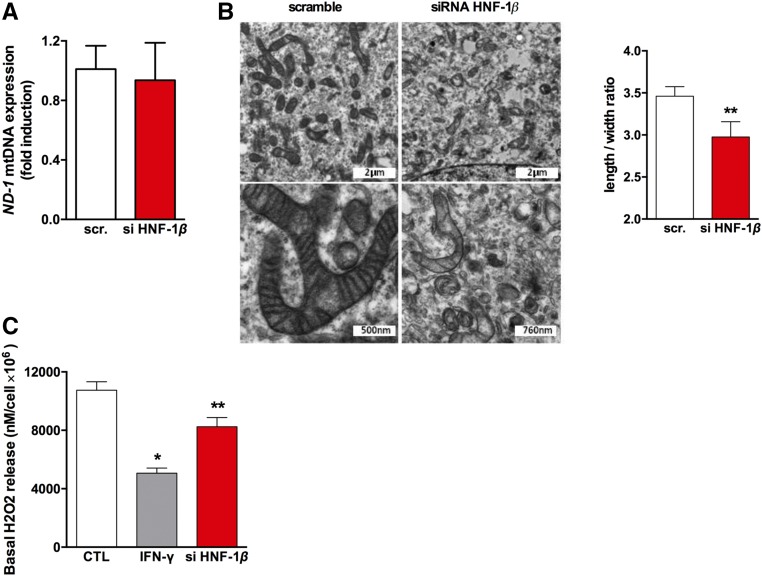
We therefore assessed whether HNF-1β inhibition could alter mitochondrial biogenesis and structure. Although the mitochondrial DNA content (mtRNA ND1 expression), a surrogate marker of mitochondria count, was not modified by siRNA-mediated downregulation of HNF1B (Figure 6A), electron microscopy showed segmented and swollen mitochondria, confirmed by the reduced form ratio (i.e., the ratio between the major and the minor axis of the equivalent ellipse of the mitochondrion), a partial loss of cristae, and an increase of autophagolysosomes in siRNA-HNF1B–treated cells (Figure 6B). This suggests that HNF-1β can be involved in cellular metabolism and mitochondrial function. This was further evaluated by measuring the effect of HNF-1β inhibition on the production of reactive oxygen species and on mitochondrial respiration. We compared the production of hydrogen peroxide (H2O2), a derivative of mitochondrial reactive oxygen species, in wild-type (wt) and HNF-1β–deficient HK2 cells. As shown in Figure 6C, inhibition of HNF-1β led to a decrease in H2O2 production. This finding was also observed when epithelial HK-2 cells were exposed to IFN-γ and TNF-α (Figure 6C, Supplemental Figure 1D).
Figure 6.
Inhibition of HNF-1β leads to mitochondrial disorders including a lower production of reactive oxygen species. (A) Relative RNA expression of a mitochondrial gene (ND-1 gene) and a nuclear gene (ACTNB) in HK-2 cells transfected with scrambled siRNA or an siRNA targeting HNF1B mRNA showed that the mitochondrial content was similar in both conditions. (B) Electron microscopy showed that inhibition of HNF-1β leads to mitochondrial swelling, partial cristae disappearance, and autophagolysosomes increase. Mitochondrion form ratio measurement was significantly reduced in Hnf1b−/− MCT cells compared with controls (n=282). (C) Real-time H2O2 measurement in the supernatant of HK-2 cells after IFN-γ exposure and HNF-1β inhibition. *P<0.05; **P<0.01; ***P<0.001.
HNF-1β Controls Mitochondrial Respiration
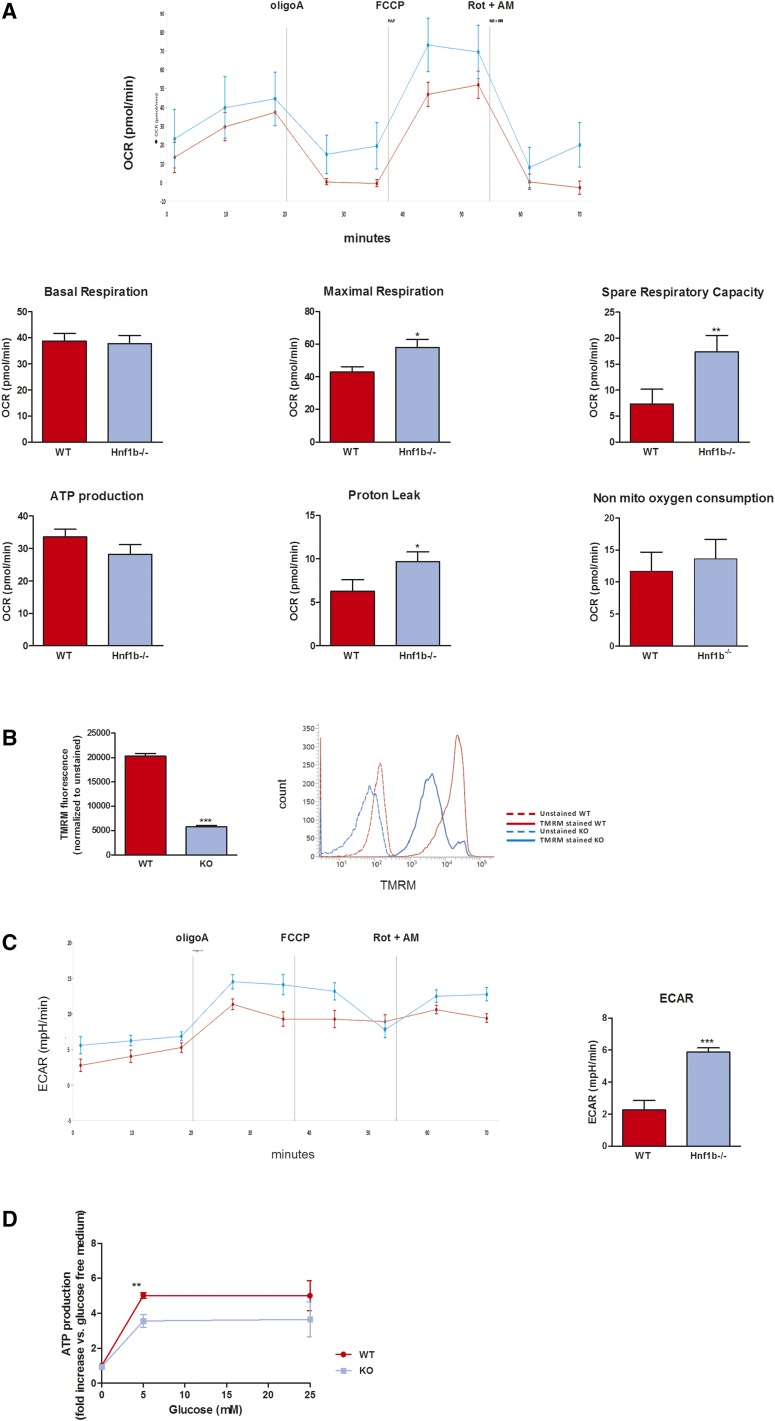
To better characterize the metabolic and mitochondrial consequences of HNF-1β inhibition, we directly measured the oxygen consumption rate in wt and Hnf1b−/− MCT cells. Using the Seahorse approach, we were able to assess the various aspects of mitochondrial and nonmitochondrial respiration. As shown in Figure 7A, whereas the basal respiration, nonmitochondrial respiration (i.e., O2 consumption after exposure to antimycin A and rotenone), and the ATP production–dependent respiration (i.e., oxygen [O2] consumption after exposure to oligomycin) were similar in wt and Hnf1b−/− cells, maximal and spare respiratory capacities were significantly increased in Hnf1b−/− cells. ATP-independent proton leak was also increased in Hnf1b−/− cells. To better understand the mitochondrial changes that lead to reduced H2O2 production despite altered mitochondrial respiration, we measured the mitochondrial membrane potential (Δψm) in both cell lines using tetramethylrhodamine methylester staining, and observed a significant decrease of Δψm in Hnf1b−/− cells compared with wt cells (Figure 7B), a finding confirming the Seahorse data and suggestive of uncoupling between the proton conductance of the inner mitochondrial membrane and the oxidative respiration.
Figure 7.
Hnf1b deletion leads to changes of the oxidative phosphorylation/glycolysis ratio. (A) The O2 consumption rate was measured using the Seahorse analyzer at basal state (three measurements over 20 minutes) and after sequential injection of oligomycin (respiration linked to proton leakage), FCCP (maximal respiration), and rotenone + antimycin A (nonmitochondrial respiration). Two measurements per drug were performed. Basal respiration was similar in both cell lines but maximal and spare respiration capacities were increased in Hnf1b−/− MCT cells, as well as proton leak. (B) The mitochondrial membrane potential (Δψm) was measured using tetramethylrhodamine methylester staining. A significant decrease of Δψm was observed in Hnf1b−/− cells, confirming Seahorse data that suggested mitochondrial uncoupling. (C) Extracellular acidification rate (ECAR) was measured concomitantly to the O2 consumption rate (Seahorse analyzer). In Hnf1b−/− MCT cells, a significant higher ECAR was observed, suggesting a metabolic shift from oxidative respiration to glycolysis. (D) ATP synthesis was reduced in Hnf1b−/− MCT cells grown at a limited glucose concentration (5 mM), compared with wt cells. *P<0.05; **P<0.01; ***P<0.001.
Concomitantly, extracellular acidification rate was increased in Hnf1b−/− compared with wt cells at basal state and after blocking of mitochondrial respiration (i.e., after exposure to oligomycin; Figure 7C), suggesting activation of the glycolysis pathway. To confirm the latter, we measured the intracellular ATP concentration of both cell lines exposed to various glucose concentrations. ATP was measured in cells grown in a glucose-free, glucose-limited (5 mM), or glucose-saturated (25 mM) medium. Indeed, the increase of ATP production was slightly reduced in Hnf1b−/− cells grown at a limited glucose concentration (glucose 5 mM; Figure 7D).
Of note, deletion of Hnf1b in MCT cells was also accompanied by a lower proliferation rate (Δ −13%; n=36; P=0.001) (Supplemental Figure 2A) and cell viability (FACS analysis using the Live/Dead Fixable dead cell stain kits; Supplemental Figure 2B).
In summary, the Seahorse based analysis suggested that Hnf1b−/− MCT cells harbored mitochondrial injuries, mild uncoupling between the proton gradient and oxidative respiration, and an increase of ATP demand requiring an activation of the glycolysis pathway. The combined evidence suggests that in vitro HNF-1β controls the expression of PPARGC1A and the bioenergetics metabolism and morphology of mitochondrion in epithelial proximal tubule cells, partly recapitulating what was observed in the mouse model of AKI.
PPARGC1A Is Downregulated in the Kidney of an HNF1B Patient
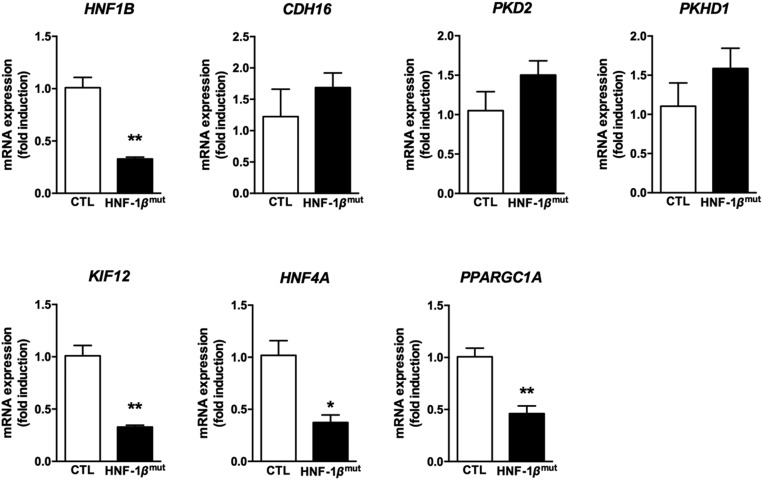
In a 32-year-old patient harboring a point-mutation in the HNF1B gene (p.Leu286Val) and presenting with progressive renal failure, we identified a generalized defect of proximal and distal tubular function, including tubular proteinuria, generalized aminoaciduria, renal glycosuria, phosphaturia, hypomagnesemia, hypokalemia, and incomplete renal tubular acidosis associated with hypercalciuria and recurrent nephrolithiasis (Table 1; patient 20.1 from Faguer et al.15). In this patient, concomitant mutation of HNF1A and CLCN5 (two genes whose mutations may cause proximal tubule dysfunction33,34) were ruled out. To address whether HNF-1β mutation is accompanied by abnormal mitochondrial function or mitochondrial gene expression within kidney in humans, and that mitochondrial dysfunction may contribute to the renal phenotype of the human HNF1B-related disease, we quantified the mRNA expression of HNF1B, its target genes, and mitochondrial genes including PPARGC1A in a kidney sample of this patient who underwent unilateral nephrectomy because of suspected, but not confirmed, renal neoplasm. At that time, his GFR was 23 ml/min per 1.73 m2. Optical examination showed few oxalate crystals within the interstitium and mild renal fibrosis. We observed decreased renal HNF1B expression in this patient compared with nonmutated (control) renal tissue obtained in a patient with normal renal function who benefited from unilateral nephrectomy because of confirmed renal neoplasm (Figure 8). As previously described,21 a number of target genes of HNF-1β were not downregulated in the postembryonic kidney, including CDH16, PKD2, and PKHD1. However, the expression of HNF-1β target genes HNF4A and KIF12 was significantly reduced. Finally, downregulation of HNF1B expression was accompanied by a significant reduction in renal PPARGC1A expression in this patient (Figure 8).
Table 1.
Clinical characteristics of an HNF1B patient who benefited from surgical nephrectomy
| Characteristic | Presentation |
|---|---|
| Sex | Male |
| HNF1B mutation | p.Leu286Val |
| Age at presentation, yr | 5 |
| Renal involvement | Urolithiasis, nephrocalcinosis, no renal cyst |
| CKD with tubulointerstitial presentation and progressive renal failure | |
| Low serum potassium | |
| Renal glycosuria | |
| Phosphaturia | |
| Incomplete distal tubular acidosis | |
| Generalized aminoaciduria, tubular proteinuria | |
| Extrarenal involvement | |
| Pancreas | Maturity onset diabetes of the young (age 23 yr) |
| Liver | Tail hypoplasia and calcifications |
| Genital tract | Liver tests abnormalities |
| Other | No cyst |
| Not assessed | |
| Epilepsy | |
| Renal presentation at nephrectomy | |
| eGFR | 23 ml/min |
| Serum potassium | 3 mmol/L |
Figure 8.
Renal expression of HNF1B and its target genes KIF12, HNF4A and PPARGC1A is decreased in patients with HNF1B mutation. *P<0.05; **P<0.01; ***P<0.001.
Thus, the downregulation of HNF1B expression in this patient with suspected mitochondrial dysfunction (i.e., electrolyte disorders, renal fibrosis) was accompanied by downregulation of PPARGC1A expression, potentially supporting the role of the HNF-1β cascade involving PPARGC1A toward mitochondrial dysfunction in patients in vivo.
Discussion
Proximal tubule cells are highly specialized epithelial cells with high metabolism activity and energy demand, thus requiring a very high number of mitochondria, almost equal to cardiac myocytes. Mitochondria also drive the epithelial differentiation, function, and cell cycle.35 Fatty acid oxidation by the mitochondrial respiration is now recognized as the main energy source of proximal tubule cells.36 Acute or chronic kidney injuries are both characterized by inflammation and metabolism reprograming, including mitochondrial respiration inhibition.37 This latter was mostly related to the downregulation of the key transcription factor PPARGC1A. In our study, we identified transcription factor HNF-1β as a direct regulator of PPARGC1A expression and mitochondrial respiration, and we show that inflammatory cytokines IFN-γ and TNF-α inhibit the transcriptional activity of HNF-1β, thus positioning HNF-1β as a regulator of the inflammation-mediated metabolism reprograming of proximal tubule cells. Moreover, luciferase assays showed that IFN-γ and TNF-α mainly regulate the transcriptional activity of HNF-1β rather than its protein expression, thus providing a molecular mechanism by which changes in the microenvironment (i.e., intrarenal inflammation) may induce transcriptional changes in epithelial cells.
HNF-1β is a transcription factor expressed in various organs with epithelia characterized by a tubular shape (for instance, kidney, liver, and pancreas).12 The nephropathy associated with mutations of HNF1B in human is usually considered as a developmental disorder given its frequent recognition in the antenatal period or in childhood,13,14 the increased rate of associated renal malformations, and the role of HNF-1β at various stages of renal developmental (for instance, epithelial differentiation, tubulogenesis, and planar cell polarity).16–18 Data concerning the role of HNF-1β in the adult normal or injured kidney are far scarcer. A mouse model of kidney-limited conditional invalidation of Hnf1b after the embryonic period (i.e., in the mature kidney) was not associated with an overt renal phenotype.21 However, in this model, the invalidation of Hnf1b was limited to the loop of Henle and the distal tubules, thus preventing an accurate assessment of its role in the proximal tubule, a pivotal tubular segment in the pathophysiology of AKI and CKD.38
In proximal tubule cells with HNF1B inhibition, we observed significant changes of the mitochondrial respiration and a decrease in the production of H2O2, a derivative of the radical oxygen species mainly produced by mitochondria. We did not observe a marked change of mitochondrial copy number, but we identified a significant downregulation of PPARGC1A and genes involved in mitochondrial biogenesis and respiration (i.e., IDH3A and ATP5G1), as well as mitochondrial morphologic changes. In addition, despite a similar mitochondrial basal respiration in Hnf1b−/− cells, we observed uncoupling between the proton conductance of the inner mitochondrial membrane and the oxidative respiration, a finding that suggests a lower dependency of the ATP synthesis on oxidative respiration. Hence, the concomitant increase of the extracellular acidification rate and the dependency of ATP synthesis to the availability of the extracellular glucose is suggestive of a shift from oxidative phosphorylation to glycolysis. Accordingly, metabolic disturbances and mitochondrial dysfunction are also observed in ovarian clear cell carcinoma, a subtype of ovarian carcinoma characterized by an overexpression of HNF-1β.24 In these cancer cells, HNF-1β promotes metabolic reprogramming in favor of the aerobic glycolysis (known as the Warburg effect). Although aerobic conditions should favor oxidative phosphorylation, aerobic glycolysis and the Warburg effect are characterized by a shift from pyruvate to lactate and a lactate efflux out of the cells. Interestingly, during ischemic AKI, H2O2 production is significantly downregulated and a robust depletion of pyruvate and lactate is observed in the renal cortex after ischemia reperfusion.39 Thus, abnormal expression of HNF-1β in renal proximal tubule cells may lead to a transcriptional reprograming of key metabolic genes, including downregulation of PPARGC1A, mitochondrial uncoupling, and a metabolic shift from oxidative phosphorylation to glycolysis, thus highlighting a new role of HNF-1β in renal physiology and during AKI and repair. This finding is also reminiscent of the role of HNF-1β in cholesterol metabolism,40 and establishes HNF-1β as a crucial regulator at cross-section of mitochondrial energy metabolism pathways in proximal tubular cells. Because metabolic changes observed in Hnf1b−/− MCT cells did not fully overlap with findings previously observed after PPARGC1A inhibition,36 a multilevel approach combining transcriptomic, proteomic, and functional analyses of bioenergetics changes after HNF-1β inhibition should be performed in further studies.
Our results could have several important implications. First, we showed that HNF-1β deletion leads to mitochondrial disorders (an increase of the maximal and spare respiratory capacities and a lower mitochondrial membrane potential). One may hypothesize that the transient HNF-1β inhibition observed after AKI is an adaptive bioenergetic response to inflammatory injury that gives a protective phenotype to epithelial cells (i.e., lower H2O2 production). However, these metabolic changes and the identification of a lower mitochondrial membrane potential in Hnf1b−/− cells suggestive of mitochondrial injuries and/or respiratory uncoupling, may subsequently favor epithelial injury if the downregulation of HNF-1β persists over time. Second, to date, the molecular mechanisms that underlie the development of a progressive renal fibrosis and CKD in HNF1B patients remain largely unknown. In a recent study, Kang et al. reported that defective fatty acid oxidation in renal tubular cells promotes kidney fibrosis, and that TGF-β–induced fatty acid oxidation blockade is probably mediated by PPARGC1A.36 They also showed that favoring glycolysis worsened the development of fibrosis, whereas promoting PPAR-α activity using fenofibrate prevents the TGF-β–induced effects. Our in vitro and in vivo findings suggest that the progressive renal decline observed in HNF1B patients, which is not correlated with the cystic involvement,15 may be partly mediated by a downregulation of PPARGC1A in proximal tubule cells. Indeed, it is noteworthy that the expression of CDH16 was not downregulated in the kidney of the HNF1B patient we tested. Because CDH16 inhibition is a hallmark of renal fibrosis, we strongly suspect here that other transcriptional changes observed in this HNF1B patient (including the downregulation of PPARGC1A) are related to the mutation of HNF1B, rather than a nonspecific association with renal fibrosis.
Although further confirmatory studies including the development of a mouse model of conditional inactivation of Hnf1b limited to the proximal tubule cells are also required, our findings suggest that HNF1B-related nephropathy should be considered as a mitochondrial disorder in adulthood. Whether mitochondrial dysfunction in the distal tubule cells is involved in the renal presentation of most HNF1B patients (i.e., renal wasting of magnesium and potassium), similarly to patients with inherited mitochondrial dysfunction,37 also remains to be addressed.
Potentially related to this hypothesis, about 50% of HNF1B patients develop diabetes mellitus, characterized by a combination of β cell dysfunction and insulin resistance, as well as pancreas hypoplasia.41 Mitochondrial dysfunction and uncoupling of mitochondrial oxidative phosphorylation were also observed in islets cells from mice with complete invalidation of HNF-1α, a transcription factor with 90% homology, and a common target DNA sequence with HNF-1β.42 Diabetes mellitus is also a common finding in patients with inherited mitochondrial disorders. Whether mitochondrial dysfunction in pancreatic β cells also promotes diabetes mellitus in HNF1B patients needs to be addressed.
Moreover, the identification that inflammation, especially IFN-γ and TNF-α cytokines, inhibits the transcriptional activity of HNF-1β, combined with our in vivo findings in a AKI model, also suggests that the epithelial dysfunction observed in various inflammatory conditions (for instance endotoxin-induced shock) and culminating in the multiorgan failure syndrome may be at least partly related to a HNF-1β downregulation with subsequent PPARGC1A inhibition and mitochondrial dysfunction. The role of PPARGC1A in shock-related pancreas and liver failure has already been reported.43,44 Targeting HNF-1β or its upstream regulators may thus be a valuable treatment approach in these severe conditions.
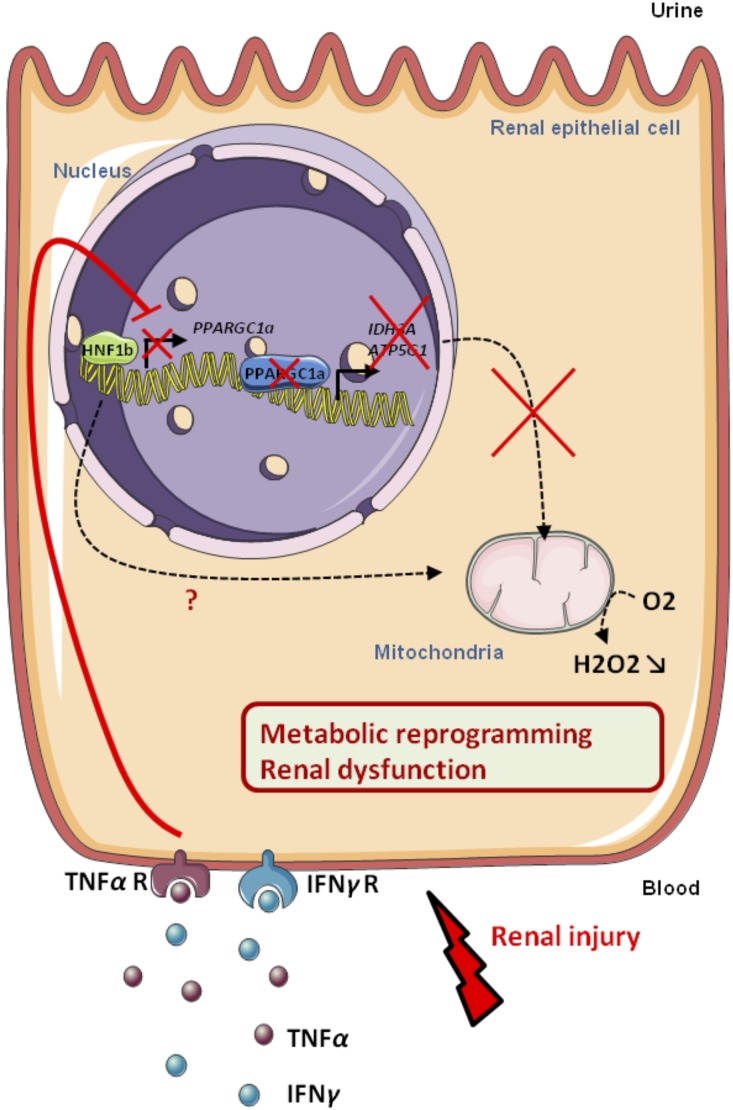
In summary, our data prompt us to propose a scheme (Figure 9), whereby HNF-1β activity tightly modulates the metabolic and transcriptional response of renal epithelial cells to the IFN-γ and TNF-α inflammatory signals.
Figure 9.
Pro-inflammatory cytokines IFN-γ and TNF-α inhibit the transcriptional activity of HNF-1β in epithelial tubular cells with subsequent PPARGC1A down-regulation and mitochondrial dysfunction.
Concise Methods
Animals
Male C57Bl6 mice (8–12 weeks old) were obtained from Janvier (Le Genest Saint Isle, France) and kept in our animal facility (INSERM U1048, Toulouse, France) under standard laboratory conditions. Mice were injected intraperitoneally with 10 μg/g LPS (InvivoGen, Toulouse, France). After general anesthesia (ketamine/xylazine), mice were euthanized at 0, 6, 24, 36, and 48 hours after the injection of LPS (6–10 mice per group at each time point). Kidneys were collected and immediately frozen or fixed. Blood was collected and centrifuged, and plasma was isolated and frozen at −20°. All experiments reported were conducted as stated in the National Institutes of Health, USPHS, Guide for the Care and Use of Laboratory Animals and were approved by our Institution’s Animal Care and Use Committee.
Cell Culture and Transfection
Human HK-2 cells were grown in a DMEM/F-12 Nut Mix medium supplemented with 10% heat-inactivated FCS (GIBCO, Grand Island, NY), 10 µg/ml EGF, 5 µg/ml insulin, 4 pg/ml triiodothyronine, 36 ng/ml hydrocortisone, and a combination of penicillin G and streptomycin. After 24 hours of FCS starvation, cells were treated with 100 ng/ml IFN-γ (Millipore, Bedford, MA) for 24 hours with subsequent protein or mRNA extraction. For all transfections, cells were seeded in 12-well plates and transfected using appropriate transfection reagents. For siRNA transfection, HK-2 cells were transfected for 48 hours with siRNA oligos targeting the HNF1B mRNA, or with scrambled siRNA (Ambion, Carlsbad, CA), using the DharmaFECT Duo transfection reagent (Dharmacon, Lafayette, CO).
For HNF1B overexpression, HEK-293 cells were transfected for 48 hours with empty vector or QE-TriSystem-6 vector carrying the HNF1B gene (QIAGEN, Hilden, Germany), using the Fugene HD transfection reagent (Promega, Madison, WI).
For luciferase activity, HK-2 cells were cotransfected with Kif12 firefly luciferase vector (pGL3 basic vector; Promega) and Renilla luciferase (control vector, pRL-TK plasmid; Promega) using the Fugene HD transfection reagent (Promega). After 24 hours, cells were treated or not with IFN-γ (100 ng/ml) for 24 hours. Luminescence was measured on Mithras LB940 (Berthold, Thoiry, France). Results are expressed as fold induction, which is the ratio of luciferase activity induced in Kif12-transfected cells treated by IFN-γ, relative to basal luciferase activity in Kif12-transfected HK-2 cells.
Mouse MCT cells were grown in DMEM with Glutamax-1 (Life Technologies, Gaithersburg, MD) containing 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Hnf1b deletion was obtained using the CRISPR/Cas9 method, according to the manufacturer’s protocol (Santa Cruz Biotechnology, Santa Cruz, CA). Cell viability was determined using fluorescence labeling followed by FACS analysis (LIVE/DEAD assay kit L34955; Thermo Fisher Scientific).
Western Blotting
HK-2 cells or total mouse kidneys were lysed in RIPA buffer containing protease inhibitors (Sigma, St Louis, MO). Protein concentrations were determined (Bio-Rad assay reagent; BioRad, Hercules, CA) and equal amounts of protein were separated by SDS-PAGE and transferred to PVDF membranes (GE Healthcare, Uppsala, Sweden). Blots were blocked in 5% nonfat milk TBST and incubated overnight at 4°C with the primary antibody (anti–HNF-1β H-85; Santa Cruz Biotechnology and anti–β-actin AC-15; Sigma). Then, blots were incubated with HRP-conjugated species-specific secondary antibodies (Bethyl, Montgomery, AL), followed by enhanced chemiluminescence reaction (BioRad). Results were normalized to β-actin.
mRNA Extraction, Reverse Transcription, and Quantitative PCR
RNA were prepared from whole kidney lysates, HK-2 cells, or human renal biopsy specimens using Qiagen RNeasy mini columns, and 500 ng of mRNA were reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative amplification of the cDNA was performed using a StepOne thermocycler. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene and the relative mRNA fold changes between groups were calculated using the 2−ΔΔCt method (Supplemental Table 1).
Renal Pathology
Formalin-fixed, paraffin-embedded sections (4 µm) of bisected kidneys were stained with periodic acid–Schiff using standard protocols. Immunostaining of HNF-1β was performed as follows: paraffin-embedded sections (4 µm) of bisected kidneys were deparaffined, rehydrated, permeabilized with 0.2% Triton-PBS, blocked with 3% BSA-PBS, and then stained for 1 hour with a rabbit anti–HNF-1β antibody (H-85; Santa Cruz Biotechnology). After several washes, the antibody reactions were visualized with the EnVision Plus kit detection system (Vector Laboratories, Burlington, CA). After staining, the sections were washed in distilled water and dehydrated in graded alcohol. Finally, the sections were mounted with coverslips.
Chromatin Immunoprecipitation
Before chromatin extraction, kidneys were treated with a protocol optimized for HNF-1β binding to chromatin (unpublished data; M. Pontoglio). Chromatin was crosslinked with 1% formaldehyde (Sigma) at room temperature for 10 minutes. Crosslinking reaction was stopped by the addition of glycine to a final concentration of 125 mM. Chromatin was sonicated with a Bioruptor Pico (Diagenode) and ChIP was performed as described in Lerner et al. in 201645 using antibodies to HNF-1β (sc22840×; Santa Cruz Biotechnology) or rabbit IgG (sc-2027×; Santa Cruz Biotechnology). HNF1 binding sites were identified using a previously described in silico approach.46 ChIP assay was repeated on four independent chromatin preparations.
Transmission Electron Microscopy
Cells were fixed with 2% glutaraldehyde in 0.1 M Sorensen phosphate buffer (pH 7.4) for 1 hour and washed with the Sorensen phosphate buffer (0.1 M) for 12 hours. Then they were postfixed with 1% OsO4 in Sorensen phosphate buffer (Sorensen phosphate 0.05 M, glucose 0.25 M, OsO4 1%) for 1 hour, washed twice with distilled water, and stained with 2% of uranyl acetate aqueous solution for 12 hours. Samples were dehydrated in an ascending ethanol series and embedded in epoxy resin (Epon 812; Electron Microscopy Sciences). After 24 hours of polymerization at 60°C, ultrathin sections (70-nm thick) were mounted on 150-mesh collodion-coated copper grids and stained with UranyLess and 8.5% lead citrate before being examined on a HT7700 Hitachi electron microscope at an accelerating voltage of 80 KV.
H202 Production
After a 10-minute recovery period, the spontaneous H2O2 release was measured at 37°C for 10 minutes by using a H2O2-specific amperometric probe (ISO-HPO, 100 µm diameter, 5 mm length; World Precision Instruments, Stevenage, UK) directly placed into the well.47 The H2O2-specific amperometric probe was calibrated as per manufacturer’s instructions (ISO-HPO-100; World Precision Instruments) as previously described.47 Briefly, the probe was left in 20 ml of 0.1 M PBS buffer. After the sensor had stabilized, solution of H2O2 (from 100 to 800 nM) was added to the PBS solution. The current observed was directly proportional to H2O2 concentration. The sensitivity of a fresh probe was at least 1 pA/nM. Probes were tested before each experiment to validate their sensitivity. The concentration of H2O2 in the tissue was measured in real time with data acquisition (Apollo1000; World Precision Instruments) at a sampling rate of 10 values/sec. The computer-interfaced DataTrax2 software (World Precision Instruments) performed data acquisition. Data are expressed as Δ variation of H2O2 release from basal.
Mitochondrial Respiration and Membrane Potential Measurements
O2 consumption was determined using the Seahorse XF24 extracellular Flux equipment (Agilent Technologies). To determine O2 consumption, 2000 cells were seeded in the 24-well XF24 cell culture plate in the respective culture media and allowed to grow for 24 hours. After 24 hours, culture media was replaced by DMEM and plates were incubated at 37°C for 1 hour for pH and temperature stabilization. Next the plates were placed in the Seahorse equipment and basal O2 consumption was measured at three points in time during 20 minutes. In the following 50 minutes, three mitochondrial inhibitors (i.e., 1 µM oligomycin A, 300 mM FCCP, and 1 µM rotenone + antimycin A) were sequentially injected and two O2 consumption measurements were performed after each injection. Oligomycin A was used to determine the level of O2 consumption attributable to proton leak. FCCP allowed for the estimation of the maximum rate of respiration, and nonmitochondrial respiration was estimated after injection of rotenone + antimycin A.
The mitochondrial membrane potential was measured according to the manufacturer’s instructions. Briefly, MCT cells (0.1×106 per well) were cultured and allowed to attach for 24 hours in medium culture with 25 mM of glucose. Then, cells were stained with tetramethylrhodamine methylester (100 mM, reference no. I34361; Thermo Fisher Scientific) for 20 minutes at 37°C in the dark. Next, cells were suspended by trypsin treatment and washed with PBS. The red fluorescence intensity of 10,000 suspended cells was then analyzed by flow cytometer (BS FACS Serve) in the FL2 channel.
Disclosures
None.
Supplementary Material
Acknowledgments
S.F. received a grant from the Fondation du Rein (Fondation pour la recherche Médicale) and from the AIRG (Association pour l'information et la recherche sur les maladies rénales génétiques). This work was also supported by the Association pour l’information et la recherche sur les maladies rénales génétiques.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016050508/-/DCSupplemental.
References
- 1.Lewington AJP, Cerdá J, Mehta RL: Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84: 457–467, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Emlet DR, Shaw AD, Kellum JA: Sepsis-associated AKI: Epithelial cell dysfunction. Semin Nephrol 35: 85–95, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA: A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41: 3–11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang HR, Rabb H: Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH: The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24: 1250–1261, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, Novelli R, Remuzzi G, Benigni A: Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 125: 715–726, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM: PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faguer S, Mayeur N, Casemayou A, Pageaud A-L, Courtellemont C, Cartery C, Fournie GJ, Schanstra JP, Tack I, Bascands J-L, Chauveau D: Hnf-1β transcription factor is an early hif-1α-independent marker of epithelial hypoxia and controls renal repair. PLoS One 8: e63585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffinier C, Barra J, Babinet C, Yaniv M: Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev 89: 211–213, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, Aziza J, Bandin F, Schanstra JP, Bellanné-Chantelot C: Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol 18: 923–933, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschênes G, Bouissou F, Bensman A, Bellanné-Chantelot C: Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol 17: 497–503, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Faguer S, Decramer S, Chassaing N, Bellanné-Chantelot C, Calvas P, Beaufils S, Bessenay L, Lengelé J-P, Dahan K, Ronco P, Devuyst O, Chauveau D: Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int 80: 768–776, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M: A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massa F, Garbay S, Bouvier R, Sugitani Y, Noda T, Gubler M-C, Heidet L, Pontoglio M, Fischer E: Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140: 886–896, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S: vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 137: 347–357, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Heliot C, Desgrange A, Buisson I, Prunskaite-Hyyryläinen R, Shan J, Vainio S, Umbhauer M, Cereghini S: HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development 140: 873–885, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Verdeguer F, Le Corre S, Fischer E, Callens C, Garbay S, Doyen A, Igarashi P, Terzi F, Pontoglio M: A mitotic transcriptional switch in polycystic kidney disease. Nat Med 16: 106–110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dmitrieva RI, Hinojos CA, Boerwinkle E, Braun MC, Fornage M, Doris PA: Hepatocyte nuclear factor 1 and hypertensive nephropathy. Hypertension 51: 1583–1589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui S, Eauclaire SF, Matthews RP: Interferon-gamma directly mediates developmental biliary defects. Zebrafish 10: 177–183, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandai M, Amano Y, Yamaguchi K, Matsumura N, Baba T, Konishi I: Ovarian clear cell carcinoma meets metabolism; HNF-1β confers survival benefits through the Warburg effect and ROS reduction. Oncotarget 6: 30704–30714, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Aco KE, Manno M, Clarke C, Ganesh J, Meyers KEC, Sondheimer N: Mitochondrial tRNA(Phe) mutation as a cause of end-stage renal disease in childhood. Pediatr Nephrol 28: 515–519, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JJ, Tripi LM, Erbe RW, Garimella-Krovi S, Springate JE: A mitochondrial DNA deletion presenting with corneal clouding and severe Fanconi syndrome. Pediatr Nephrol 27: 869–872, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Ma Z, Gong Y, Patel V, Karner CM, Fischer E, Hiesberger T, Carroll TJ, Pontoglio M, Igarashi P: Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci U S A 104: 20386–20391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarjou A, Agarwal A: Sepsis and acute kidney injury. J Am Soc Nephrol 22: 999–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Morrell ED, Kellum JA, Hallows KR, Pastor-Soler NM: Epithelial transport during septic acute kidney injury. Nephrol Dial Transplant 29: 1312–1319, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B: HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8127021. Accessed November 10, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Gong Y, Ma Z, Patel V, Fischer E, Hiesberger T, Pontoglio M, Igarashi P: HNF-1beta regulates transcription of the PKD modifier gene Kif12. J Am Soc Nephrol 20: 41–47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansour-Hendili L, Blanchard A, Le Pottier N, Roncelin I, Lourdel S, Treard C, González W, Vergara-Jaque A, Morin G, Colin E, Holder-Espinasse M, Bacchetta J, Baudouin V, Benoit S, Bérard E, Bourdat-Michel G, Bouchireb K, Burtey S, Cailliez M, Cardon G, Cartery C, Champion G, Chauveau D, Cochat P, Dahan K, De la Faille R, Debray F-G, Dehoux L, Deschenes G, Desport E, Devuyst O, Dieguez S, Emma F, Fischbach M, Fouque D, Fourcade J, François H, Gilbert-Dussardier B, Hannedouche T, Houillier P, Izzedine H, Janner M, Karras A, Knebelmann B, Lavocat M-P, Lemoine S, Leroy V, Loirat C, Macher M-A, Martin-Coignard D, Morin D, Niaudet P, Nivet H, Nobili F, Novo R, Faivre L, Rigothier C, Roussey-Kesler G, Salomon R, Schleich A, Sellier-Leclerc A-L, Soulami K, Tiple A, Ulinski T, Vanhille P, Van Regemorter N, Jeunemaître X, Vargas-Poussou R: Mutation update of the CLCN5 gene responsible for dent disease 1. Hum Mutat 36: 743–752, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach JP, Babinet C, Yaniv M: Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 84: 575–585, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Teslaa T, Teitell MA: Pluripotent stem cell energy metabolism: An update. EMBO J 34: 138–153, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang HM, Ahn SH, Choi P, Ko Y-A, Han SH, Chinga F, Park ASD, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emma F, Montini G, Parikh SM, Salviati L: Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol 12: 267–280, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV: Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zager RA, Johnson AC, Becker K: Renal cortical pyruvate depletion during AKI. J Am Soc Nephrol 25: 998–1012, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aboudehen K, Kim MS, Mitsche M, Garland K, Anderson N, Noureddine L, Pontoglio M, Patel V, Xie Y, DeBose-Boyd R, Igarashi P: Transcription factor hepatocyte nuclear factor-1β regulates renal cholesterol metabolism. J Am Soc Nephrol 27:2408–2421, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haldorsen IS, Vesterhus M, Raeder H, Jensen DK, Søvik O, Molven A, Njølstad PR: Lack of pancreatic body and tail in HNF1B mutation carriers. Diabet Med 25: 782–787, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Pongratz RL, Kibbey RG, Kirkpatrick CL, Zhao X, Pontoglio M, Yaniv M, Wollheim CB, Shulman GI, Cline GW: Mitochondrial dysfunction contributes to impaired insulin secretion in INS-1 cells with dominant-negative mutations of HNF-1alpha and in HNF-1alpha-deficient islets. J Biol Chem 284: 16808–16821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diao L, Marshall AH, Dai X, Bogdanovic E, Abdullahi A, Amini-Nik S, Jeschke MG: Burn plus lipopolysaccharide augments endoplasmic reticulum stress and NLRP3 inflammasome activation and reduces PGC-1α in liver. Shock 41: 138–144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li N, Brun T, Cnop M, Cunha DA, Eizirik DL, Maechler P: Transient oxidative stress damages mitochondrial machinery inducing persistent beta-cell dysfunction. J Biol Chem 284: 23602–23612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerner J, Bagattin A, Verdeguer F, Makinistoglu MP, Garbay S, Felix T, Heidet L, Pontoglio M: Human mutations affect the epigenetic/bookmarking function of HNF1B. Nucleic Acids Res 44: 8097–8111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallania F, Schiavone D, Dewilde S, Pupo E, Garbay S, Calogero R, Pontoglio M, Provero P, Poli V: Genome-wide discovery of functional transcription factor binding sites by comparative genomics: The case of Stat3. Proc Natl Acad Sci U S A 106: 5117–5122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drougard A, Duparc T, Brenachot X, Carneiro L, Gouazé A, Fournel A, Geurts L, Cadoudal T, Prats A-C, Pénicaud L, Vieau D, Lesage J, Leloup C, Benani A, Cani PD, Valet P, Knauf C: Hypothalamic apelin/reactive oxygen species signaling controls hepatic glucose metabolism in the onset of diabetes. Antioxid Redox Signal 20: 557–573, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.