Abstract
Patients with CKD suffer from food aversion, anorexia, and malnutrition. Although olfaction has a significant role in determining food flavor, our understanding of olfactory impairment and of the olfaction-nutrition axis in patients with kidney disease is limited. We quantified odor identification, odor threshold, and subjective odor perception in a cohort (n=161) comprising 36 participants with CKD, 100 participants with ESRD, and 25 controls. We investigated olfaction-nutrition associations in these participants and examined a novel intervention to improve olfaction in ESRD. The mean odor identification score was lower in patients with CKD (75.6%±13.1%; P=0.02) and ESRD (66.8%±15.1%; P<0.001) than in controls (83.6%±11.4%). Patients with ESRD exhibited higher odor threshold than the remaining participants exhibited. All groups had similar scores for subjective smell assessment. In multivariable adjusted analyses, kidney disease associated with increased odds of odor identification deficits (odds ratio, 4.80; 95% confidence interval, 1.94 to 11.89). A reduction in odor identification score was associated with higher subjective global assessment score and lower serum total cholesterol, LDL cholesterol, and albumin concentrations. We found no associations between odor threshold and nutritional parameters. In a proof of concept, 6-week, open-label clinical trial, intranasal theophylline (an epithelial membrane transport and proton secretion activator) increased odor identification score in five out of seven (71%) patients with ESRD. In conclusion, patients with kidney disease have olfactory deficits that may influence their nutritional status. Our preliminary results regarding olfactory improvement using intranasal theophylline warrant confirmation in a randomized controlled trial.
Keywords: chronic kidney disease, end-stage renal disease, olfaction, malnutrition, theophylline
Olfaction plays a critical role in determining the flavor of food. Olfactory deficits have been shown to predispose patients to food aversion, anorexia, and malnutrition.1 Patients with CKD and ESRD suffer from altered taste sensation, food aversion, and anorexia.2–4 These symptoms contribute to malnutrition, one of the major determinants of morbidity and mortality in patients with kidney disease.5,6 However, our understanding of olfactory function and its relationship to nutritional status in patients with kidney disease is limited.7 Comprehensive characterization of the olfactory deficits and their influence on nutrition in patients with kidney disease is important to develop novel interventions to address nutritional complications in this population.
Consequently, we set out (1) to characterize odor identification (ability to identify various odor stimuli at the suprathreshold level) and odor threshold (the lowest concentration of an odorant that one can reliably detect) in patients with CKD and ESRD; (2) to determine the association of olfactory deficits and nutritional markers in patients with CKD and ESRD; and (3) to examine the safety and efficacy of nasal theophylline (an epithelial membrane transport and proton secretion activator) for alleviating olfactory deficits in patients with ESRD.
Results
Study Cohort
The study participants (n=161) included controls with normal kidney function (n=25), patients with CKD (n=36), and patients with ESRD (n=100). Patients with CKD were recruited from an outpatient nephrology clinic, patients with ESRD were recruited from outpatient dialysis units, and the controls were recruited from our institution’s volunteer database.
The baseline characteristics of study participants are presented in Table 1. The control, CKD, and ESRD groups did not differ with respect to age, sex, education (high school graduation rate), and current tobacco use. No participants in the control, CKD, or ESRD groups had medical/surgical conditions directly linked with defects in olfaction, such as allergic rhinitis, trauma to olfactory apparatus, neurodegenerative diseases, or cancer.8 All patients with ESRD were dialysis-dependent; 94 were on hemodialysis and six were on peritoneal dialysis. The median dialysis vintage for patients with ESRD was 14.7 months (interquartile range, 5.5–36.5 months). The mean Kt/V for patients with ESRD was 1.44±0.34. All except two patients on hemodialysis were on three times a week, in-center hemodialysis (one patient was receiving two times a week, in-center hemodialysis and the other was receiving four times a week, in-center hemodialysis).
Table 1.
Baseline characteristics of study participants
| Characteristic | Control Participants (n=25) | CKD Participants (n=36) | ESRD Participants (n=100) | P Value |
|---|---|---|---|---|
| Age, yr | 58±6 | 60±12 | 63±15 | 0.27 |
| Sex, % men | 44.0 | 44.4 | 61.0 | 0.12 |
| Race, % white | 92.0 | 63.9 | 89.0 | 0.001 |
| Creatinine, mg/dl | 0.8±0.2 | 2.6±1.9 | 7.1±3.2 | <0.001 |
| BUN, mg/dl | 14.8±4.1 | 36.9±15.5 | 51.5±19.4 | <0.001 |
| eGFR, ml/min per 1.73 m2 | 90.4±11.8 | 31.3±16.1 | 9.0±7.4 | <0.001 |
| Diabetes mellitus, % | 8.0 | 50.0 | 50.0 | <0.001 |
| Current tobacco smokers, % | 4.0 | 8.6 | 6.0 | 0.76 |
| Education ≥high school, % | 100.0 | 77.8 | 84.0 | 0.05 |
| Systolic BP, mm Hg | 126.2±16.0 | 131.2±15.9 | 135.0±21.9 | 0.12 |
| Diastolic BP, mm Hg | 78.6±8.5 | 75.2±11.4 | 69.6±14.4 | 0.003 |
| Body mass index, kg/m2 | 27.1±5.1 | 29.5±7.2 | 30.0±7.1 | 0.17 |
| Waist circumference, inches | 37.3±5.9 | 39.2±4.8 | 37.3±4.4 | 0.20 |
| SGA score | 0.8±1.8 | 4.8±4.6 | 6.2±3.8 | <0.001 |
| Total cholesterol, mg/dl | 203.5±53.9 | 170.2±53.0 | 141.8±38.5 | <0.001 |
| LDL cholesterol, mg/dl | 106.2±50.5 | 87.4±42.5 | 67.6±32.1 | 0.001 |
| Albumin, g/dl | 4.5±0.3 | 4.2±0.4 | 3.8±0.4 | <0.001 |
| Prealbumin, mg/dl | 26.3±5.1 | 28.0±8.7 | 27.2±8.0 | 0.71 |
| Normalized protein catabolic rate, g/kg per d | N/A | N/A | 0.97±0.31 | N/A |
| Aspirin, % | 0.0 | 41.7 | 48.0 | <0.001 |
| Statin, % | 20.0 | 58.3 | 69.0 | <0.001 |
| β Blocker, % | 4.0 | 52.8 | 74.0 | <0.001 |
| Angiotensin inhibitor, % | 12.0 | 41.7 | 29.0 | 0.043 |
| Nutritional and/or active vitamin D, % | 4.0 | 38.9 | 32.0 | 0.01 |
| Cinacalcet, % | 0.0 | 2.8 | 14.0 | 0.030 |
Angiotensin inhibitor includes angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Mean±SD values are reported for continuous variables. N/A, not applicable.
The control, CKD, and ESRD groups did not differ in terms of body mass index or waist circumference. However, subjective global assessment (SGA) score, total cholesterol, LDL cholesterol, and albumin demonstrated deterioration of nutritional status among patients with kidney disease.
Olfactory Defects in Patients with Kidney Disease
Odor Identification
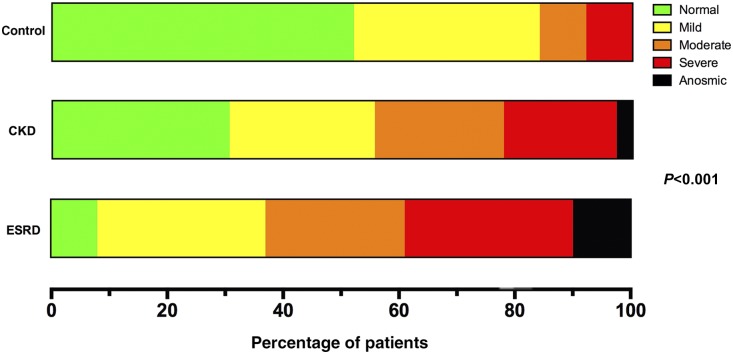
Odor identification was assessed using the University of Pennsylvania Smell Identification Test (UPSIT), a validated 40-item smell identification test.9 We computed odor identification score for each participant as the percentage of correctly identified odors. As shown in Table 2, the mean odor identification score was lower in patients with CKD (75.6%±13.1%) and ESRD (66.8%±15.1%) compared with controls (83.6%±11.4%). On the basis of a previously established classification pattern,10 we categorized olfactory function as follows: normal olfaction (at least 34 correct answers for men or at least 35 correct answers for women), mild hyposmia (30–33 correct answers for men or 31–34 correct answers for women), moderate hyposmia (26–29 correct answers for men or 26–30 correct answers for women), severe hyposmia (19–25 correct answers for both sexes), and anosmia (≤18 correct answers for both sexes). The majority of patients with CKD (69%) and ESRD (92%) had abnormal odor identification (Figure 1). Mild hyposmia was present in 32% of controls, 25% of patients with CKD, and 29% of patients with ESRD; moderate hyposmia was present in 8% of controls, 22% of patients with CKD, and 24% of patients with ESRD; severe hyposmia was present in 8% of controls, 19% of patients with CKD, and 29% of patients with ESRD; and anosmia was present in 0% of controls, 3% of patients with CKD, and 10% of patients with ESRD (P<0.001 for differences in olfactory function categories across control, CKD, and ESRD groups).
Table 2.
Results of olfactory assessments in study participants
| Olfactory Assessment | Control Participants (n=25) | CKD Participants (n=36) | ESRD Participants (n=100) | P Value |
|---|---|---|---|---|
| Odor identification | ||||
| UPSIT, number of correct answers | 33.4±4.5 | 30.3±5.3 | 26.7±6.0 | <0.001 |
| Odor identification score, % | 83.6±11.4 | 75.6±13.1 | 66.8±15.1 | <0.001 |
| UPSIT time, minutes | 14.2±4.6 | 18.1±6.3 | 16.9±6.1 | 0.02 |
| Odor threshold | ||||
| Threshold, −log (concentration, M) | 4.3±1.3 | 4.5±1.4 | 3.9±1.3 | 0.04 |
| No. of attempts | 23.4±5.3 | 24.3±5.4 | 21.1±6.1 | 0.01 |
| Subjective assessment | ||||
| Subjective assessment score, % | 83.9±14.3 | 78.2±19.4 | 83.4±16.3 | 0.38 |
Mean±SD values are reported for all variables.
Figure 1.
Distribution of study participants among olfactory identification functional categories. On the basis of the results of the UPSIT, controls with normal kidney function and patients with CKD and ESRD are categorized as normosmic (Normal, green), mildly hyposmic (Mild, yellow), moderately hyposmic (Moderate, orange), severely hyposmic (Severe, red), or anosmic (black). Shown are the percentages of study participants from each of these cohorts that were assigned to the five functional olfactory categories.
We also measured the time taken by study participants to complete the UPSIT, as a measure of the patients’ difficulty in identifying the odorants.11,12 As shown in Table 2, the mean UPSIT completion time was higher in patients with kidney disease, 18.1±6.3 minutes in patients with CKD and 16.9±6.1 minutes in patients with ESRD compared with 14.2±4.6 minutes in controls (P=0.02 for the differences across control, CKD, and ESRD groups).
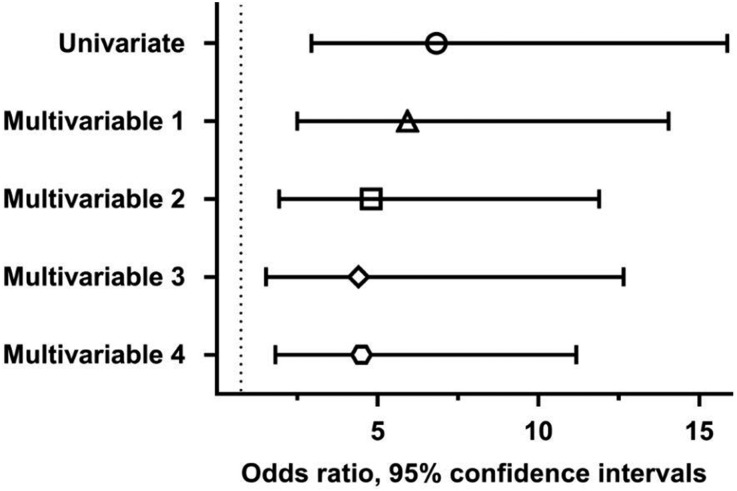
Presence of kidney disease was associated with increased odds of odor identification deficits in univariate analysis (odds ratio, 6.83; 95% confidence interval, 2.94 to 15.88) (Figure 2). Presence of kidney disease remained a significant predictor of odor identification deficits in a multivariable logistic regression model adjusted for age, race, and sex (odds ratio, 5.93; 95% confidence interval, 2.50 to 14.05); in a model adjusted for age, race, sex, education, presence of diabetes, and current tobacco use (odds ratio, 4.80; 95% confidence interval, 1.94 to 11.89); in a model adjusted for age, race, sex, education, presence of diabetes, current tobacco use, and for the use of the following medications: aspirin, statin, β blocker, angiotensin inhibitor, vitamin D (nutritional and/or active vitamin D), and cinacalcet (odds ratio, 4.41; 95% confidence interval, 1.53 to 12.65); and in a model adjusted for age, race, sex, education, presence of diabetes, current tobacco use, and prior stroke (odds ratio, 4.50; 95% confidence interval, 1.82 to 11.18). Dialysis vintage and Kt/V were not associated with odor identification scores.
Figure 2.
Kidney disease is a predictor of odor identification defects in univariate and multivariable ordinal logistic regression models. Multivariable 1 denotes a model adjusted for age, race, and sex; multivariable 2 denotes a model adjusted for age, race, sex, current tobacco use, education, and presence of diabetes; multivariable 3 denotes a model adjusted for age, race, sex, education, presence of diabetes, current tobacco use, and for the use of the following medications: aspirin, statin, β blocker, angiotensin inhibitor, vitamin D (nutritional and/or active vitamin D), and cinacalcet; and multivariable 4 denotes a model adjusted for age, race, sex, education, presence of diabetes, current tobacco use, and prior stroke. Shown are the odds ratios (circle for univariate model, triangle for multivariable 1, square for multivariable 2, diamond for multivariable 3, and hexagon for multivariable 4) and 95% confidence intervals. Dotted line marks an odds ratio of 1.
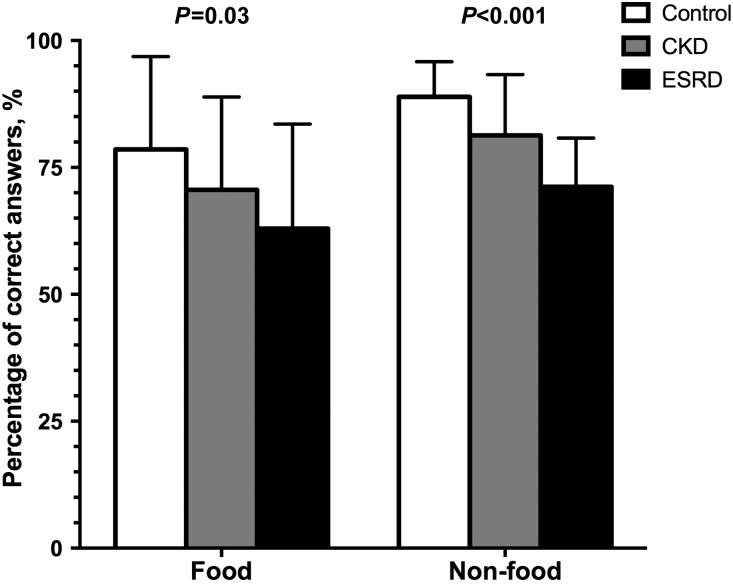
The control participants recorded a higher percentage of correct answers than patients with CKD or ESRD for both food-related (P=0.03) and nonfood-related (P<0.001) odorants on the UPSIT (Figure 3). The largest differences in the percentage of correct answers between control and ESRD groups were found for: pineapple (identified correctly by 31% more controls than patients with ESRD); pine (30% difference); cheddar cheese and rose (28% difference for both); and menthol, strawberry, and turpentine (26% difference for all three).
Figure 3.
Percentages of correct answers for food-related and nonfood-related odorants on the odor identification test are higher in the control than in the CKD and ESRD groups.
Odor Threshold
We used the Snap & Sniff Threshold Test to determine the lowest 2-phenylethanol concentration that a study participant can reliably detect.13 As shown in Table 2, the mean odor threshold was similar between controls (−4.3±1.3 log vol/vol) and patients with CKD (−4.5±1.4 log vol/vol). However, patients with ESRD (−3.9±1.3 log vol/vol) exhibited a higher odor detection threshold compared with patients with CKD (P=0.04 versus patients with CKD; P=0.01 versus remaining cohort). The mean threshold value in patients with ESRD corresponds to an approximately four-fold higher odorant concentration (126 μM) compared with the mean concentration that patients with CKD are able to detect (approximately 32 μM). The number of attempts (odorant presentations) during the administration of the threshold test was lower in patients with ESRD compared to controls and patients with CKD.
Subjective Odor Perception
On average, participants rated their sense of smell at approximately 80% on a scale of 0%–100% (0, complete impairment of olfaction and 100, no impairment in olfaction). Scores on subjective assessment of smell did not differ among control, CKD, and ESRD groups (Table 2). We observed a correlation between odor identification and subjective olfactory assessment in the control (r=0.41; P=0.04) and CKD groups (r=0.41; P=0.02); however, a similar correlation was not observed for the ESRD group (r=0.12; P=0.23).
Olfaction-Nutrition Association
We assessed the association between olfaction and nutrition in the study participants by determining the SGA score and serum levels of nutritional markers. The SGA score, a validated nutritional assessment tool that addresses food intake, weight changes, and gastrointestinal symptoms such as nausea and anorexia,14 increased as odor identification score worsened (r=−0.22; P<0.01). A reduction in odor identification score was also associated with lower serum total cholesterol (r=0.36; P<0.001), LDL cholesterol (r=0.34; P<0.001), and albumin (r=0.32; P<0.001). No association was noted between odor identification score and prealbumin (r=0.11; P=0.18). In patients with ESRD, no association was noted between odor identification score and normalized protein catabolic rate (r=−0.04; P=0.70).
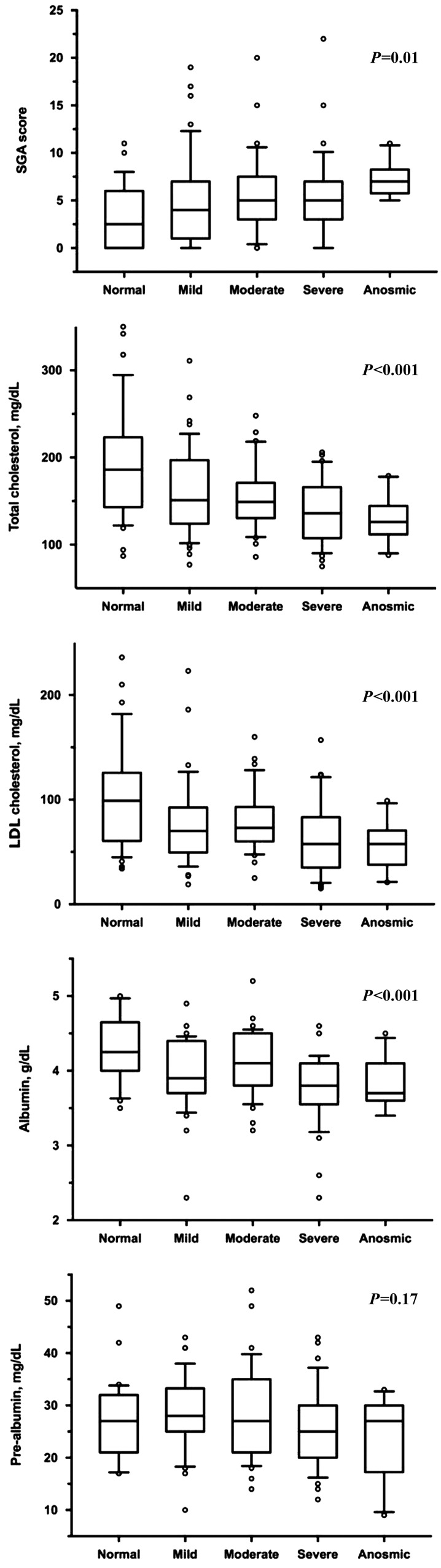
The SGA score increased (P=0.01), whereas total cholesterol, LDL cholesterol, and albumin demonstrated reductions (P<0.001 for all three nutritional parameters) across the graded odor identification categories (Figure 4). In analyses restricted to patients with kidney disease (CKD and ESRD) or ESRD alone, trends suggesting similar olfaction-nutrition association were noted.
Figure 4.
Box plots of nutritional markers across the olfactory functional categories in all study participants indicate a worsening of the nutritional status associated with olfactory identification impairment.
No associations were noted between odor threshold and SGA score (r=−0.14; P=0.08), total cholesterol (r=0.11; P=0.17), LDL cholesterol (r=0.15; P=0.08), albumin (r=0.07; P=0.36), and prealbumin (r=0.04; P=0.66). In patients with ESRD, no association was noted between odor threshold and normalized protein catabolic rate (r=0.01; P=0.95).
In multivariable analyses adjusted for age, race, sex, current tobacco use, education, and presence of diabetes, the associations between odor identification deficits and the SGA score and the associations between odor identification deficits and nutritional markers (total cholesterol, LDL cholesterol, and albumin) remained significant (Table 3). In multivariable analyses restricted to patients with ESRD, similar associations were noted for total cholesterol and LDL cholesterol. The magnitude of association statistics for SGA score and albumin levels were attenuated; however, trends in a similar direction were noted for patients with ESRD.
Table 3.
Results from univariate and multivariable linear regression analyses showing association between olfactory functional categories and nutritional markers
| Analysis | SGA Score | Total Cholesterol | LDL Cholesterol | Albumin | Prealbumin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | β | P Value | β | P Value | |
| All study participants | ||||||||||
| Univariate | 0.678 | 0.01 | −15.423 | <0.001 | −11.470 | <0.001 | −0.116 | <0.001 | −0.702 | 0.17 |
| Multivariable 1 | 0.733 | 0.01 | −14.082 | <0.001 | −10.663 | <0.001 | −0.103 | 0.001 | −0.055 | 0.92 |
| Multivariable 2 | 0.633 | 0.04 | −13.470 | <0.001 | −10.066 | 0.001 | −0.097 | 0.004 | −0.261 | 0.63 |
| ESRD participants | ||||||||||
| Univariate | 0.448 | 0.18 | −7.265 | 0.03 | −7.278 | 0.01 | −0.027 | 0.47 | −1.420 | 0.05 |
| Multivariable 1 | 0.451 | 0.21 | −8.772 | 0.02 | −7.919 | 0.01 | −0.015 | 0.72 | −0.494 | 0.50 |
| Multivariable 2 | 0.450 | 0.23 | −8.795 | 0.02 | −7.118 | 0.03 | −0.024 | 0.57 | −0.626 | 0.39 |
Multivariable 1 model adjusted for age, race, and sex; multivariable 2 model adjusted for age, race, sex, current tobacco use, educational level and presence of diabetes.
Pilot Clinical Trial
In a proof of concept clinical trial (Clinicaltrials.gov identifier NCT02479451), we enrolled seven patients with ESRD with at least mild odor identification deficit detected by the UPSIT, and examined the safety and efficacy of intranasal theophylline for mitigating olfactory deficits. Before treatment, we tested for and confirmed that there was no learning effect by retesting odor identification in trial participants. This is in agreement with previous reports that concluded that the UPSIT has high test-retest reliability (r=0.91).9,15 Intranasal theophylline has been previously reported to improve olfactory function in hyposmic patients without kidney disease.16,17 In our trial, 20 μg intranasal theophylline was self-administered by study patients once daily into each naris for 6 weeks. The trial participants were followed at 2-week intervals for 6 weeks. The trial participants tolerated intranasal theophylline well and no adverse events were reported during follow-up visits. Serum theophylline levels assessed at every follow-up were undetectable throughout the study period in all participants. Theophylline treatment caused an increase in mean odor identification score in five out of the seven (71%) participants (mean improvement of 5.0%±3.5% compared with baseline). In two patients, the mean odor identification score deteriorated during treatment by 1.5% and 2.7%, respectively. The maximum increase in odor identification score during the trial period was 10.7%±4.2% compared with baseline. For three trial patients the increase in odor identification score translated into improvement by one functional category (e.g., from severe to moderate hyposmia, or from moderate to mild hyposmia). The mean odor detection threshold improved in four patients after theophylline treatment by 10.5%±14.1%, but the average value within the group was not different from the baseline threshold.
Discussion
We performed a large cross-sectional study to examine olfactory deficits in patients with kidney disease. Our data demonstrate that odor identification is impaired in a majority of patients with CKD (approximately 70%) and ESRD (approximately 90%). In our study, olfactory threshold values were comparable between patients with kidney disease and controls, but patients with ESRD exhibited a higher threshold (i.e., worse odor detection) compared with patients with CKD. In a pilot clinical trial, olfactory deficits appear to be correctable with intranasal theophylline administration. Our results highlight olfactory deficit as a potential novel mechanism to comprehend malnutrition, as patients with odor identification deficits in our study were noted to have a higher SGA score and lower levels of biochemical measures of nutritional status. Our findings of patients with CKD demonstrating defects in odor identification and patients with ESRD demonstrating defects in odor identification and odor threshold may facilitate the development of interventions tailored to the severity of underlying kidney disease.
Possible explanations for the occurrence of olfactory deficits in patients with CKD and ESRD include reduced capacity and impaired regeneration of olfactory epithelial cells in the presence of uremic toxins.7,18–20 Olfactory function has also been reported as a marker of uremia associated neurologic dysfunction.7 Our data validate previous smaller reports suggesting that kidney disease affects odor identification capacity,21,22 and that olfactory dysfunction in patients with kidney disease correlates with the severity of the underlying kidney disease.18,23 As far as odor threshold is concerned, prior studies have yielded mixed results, from no change in odor detection threshold in patients with kidney disease24,25 to a significant impairment in odor threshold in patients with kidney disease compared with controls.18 This study is notable as it provides the largest sample size compared with any of the aforementioned investigations of olfaction in patients with kidney disease. Because a large part of a meal’s flavor is attributed to olfactory input,26 loss or alteration of smell could indeed lead to loss or alteration of taste.27 This may underline the association between poor olfaction and poor nutritional status.28,29
Odor identification is thought to reflect central olfactory processing pathways, whereas detection thresholds may reflect peripheral events, i.e., processes occurring at the level of the epithelial olfactory sensory neurons.22 The question regarding which aspect of olfaction—odor threshold or identification—is affected in kidney disease can inform the development of future targeted therapeutics that modulate central or peripheral pathways. Landis et al. previously reported that a given kidney patient cohort might detect one odor normally and yet exhibit an elevated detection threshold for another odorant.22 This allowed them to hypothesize that the olfactory deficit in patients with ESRD is underlined by mixed mechanisms, i.e., they are due to defects in both central and peripheral processing pathways.7,22 Results from our pilot clinical trial suggest that the kidney disease-associated odor identification deficit may be corrected by nasal theophylline administration, a phenomenon that needs further mechanistic evaluation considering that effects of theophylline are likely mediated via peripheral olfactory processing pathways.
Data from our pilot clinical trial are consistent with findings regarding reversibility of olfactory deficits in patients without kidney disease. In an open-label study of 312 patients with hyposmia caused by influenza, chronic sinusitis, and head injury, 60% of participants reported improvement in olfactory function after receiving oral theophylline.16 A subsequent study by the same group reported an 80% rate of response to intranasal theophylline.17 Theophylline, a nonselective phosphodiesterase inhibitor, activates epithelial ion channels and other membrane transporters by increasing intracellular cAMP and cGMP levels. One possible novel mechanism for theophylline action involves the vacuolar proton-pumping ATPase (V-ATPase), which we found to be highly expressed in rodent30,31 and human olfactory epithelium (T.G. Păunescu, D. Brown, and E.H. Holbrook, unpublished data). We and others have independently demonstrated the functional importance of V-ATPase–mediated proton secretion for odor detection in mice,31,32 and showed that elevation of intracellular cAMP and cGMP levels markedly activates V-ATPases in renal proton-secreting cells.33,34 The role of V-ATPase as a target for theophylline action on olfaction needs future investigation.
Despite the clear separation seen among the three cohorts in the odor identification test, subjective self-assessments of smell were similar in the control, CKD, and ESRD groups. In addition, in the ESRD group there was no correlation between subjective and objective measures of olfaction, indicating that these patients are unaware of their disease-associated olfactory decline. Taken together with previously published data from smaller populations,21,22,35 our findings emphasize the importance of objective olfactory assessment in future studies. This occurrence has also been described in patients with diabetes36 and Alzheimer disease.37
Although the associations between odor identification score and nutritional markers demonstrated consistent directionality and retained significance in multivariable analyses, the moderate strength of these associations suggests that olfactory impairment is an important but not comprehensive explanator of nutrition. Our findings of differences in SGA score among control, CKD, and ESRD groups despite similar body mass index and waist circumference emphasize the advantage gained from a multidimensional dynamic assessment that incorporates food intake, weight changes, and gastrointestinal symptoms.
Our study has limitations. Residual confounding cannot be ruled out in an association study of this nature and causality cannot be attributed to the described associations. A future longitudinal CKD progression study will corroborate the severity of olfactory impairment and malnutrition as kidney function deteriorates. We did not investigate olfactory hedonics (specific assessments of pleasant versus unpleasant stimuli); however, such assessments should be included in a longitudinal study because it was suggested that patients exhibit changes in food preferences with development of kidney disease.38 A longitudinal study in patients with kidney disease is also needed to examine whether the association between olfactory impairment and mortality observed in the general population holds true in patients with CKD and ESRD.39 Although none of our patients had known neurodegenerative diseases, a formal assessment of cognition was not conducted, limiting our ability to exclude the influence of nonuremic etiologies of cognitive impairment on olfactory function. The majority of our ESRD group were receiving in-center hemodialysis three times a week; thus, our current findings may not be generalizable to patients receiving peritoneal dialysis or hemodialysis prescription different than in this study. The olfactory impairment has been suggested to improve with hemodialysis treatment, and future studies that compare effects of different dialysis prescriptions on olfaction are needed.7,22
In conclusion, our study demonstrates that patients with CKD and ESRD have olfactory deficits that correlate with markers of malnutrition. Our findings from a pilot clinical trial support further testing of nasal theophylline to alleviate olfactory deficits and malnutrition in patients with kidney disease.
Concise Methods
Our studies (cross-sectional study and clinical trial) were approved by the Massachusetts General Hospital (MGH) Institutional Review Board. We adhered to the Declaration of Helsinki protocol.
Research Setting and Study Participants
Any adult (18–85 years of age) without kidney disease or with CKD or ESRD who was capable of providing informed consent was eligible to participate. Between March of 2015 and May of 2016, 161 patients signed informed consent forms to enroll in the cross-sectional study. Participants included controls with normal kidney function (n=25), patients with CKD (n=36), and patients with ESRD (n=100).
Participants were recruited from outpatient settings including a CKD clinic, peritoneal dialysis center, and outpatient dialysis units affiliated with the Fresenius Medical Care North America and MGH. Patients’ demographic information, medical history, SGA score, and subjective rating of olfaction were recorded and 10 ml of serum were collected from each patient.
Assessment of Olfaction
Olfactory function was assessed using the UPSIT and the Snap & Sniff Threshold Test (Sensonics, Inc., Haddon Heights, NJ). The UPSIT15 includes 40 multiple choice questions, each referring to an encapsulated scratch-and-sniff odorant. The odorants on the UPSIT include 18 food-related odorants (e.g., pineapple, dill pickle, and chocolate) and 22 nonfood-related odorants (e.g., pine, motor oil, and leather).
The Snap & Sniff Threshold Test uses the pure olfactory nerve stimulant 2-phenylethanol40 at half-log dilutions ranging from 10−2 to 10−9 M in a protocol based on a single staircase forced-choice paradigm.13,41
For subjective self-assessments of olfaction, patients were asked to rate their own sense of smell and taste on a scale of 0–100, with 100 being the best.
Assessment of Nutritional Status
Nutritional status was assessed using the SGA score, by measurements of serum total cholesterol, LDL cholesterol, albumin, and prealbumin, and by computing normalized catabolic rate (for patients with ESRD).
Serum collected from each patient was aliquoted, and the aliquots were frozen at −80°C. Samples were further processed by LabCorp (Burlington, NC). Patients’ serum was assessed for concentrations of creatinine, BUN, albumin, prealbumin, total cholesterol, and LDL cholesterol.
Pilot Clinical Trial
We recruited hemodialysis-dependent patients with ESRD from the cross-sectional study described above for a pilot, open-label clinical study to examine the safety and efficacy of intranasal theophylline for mitigating olfactory deficits (Clinicaltrials.gov identifier NCT02479451). Inclusion criteria were as described above for patients with ESRD. Seven patients provided written informed consent.
Nasal theophylline (20 μg theophylline methylpropyl paraben in a 0.4-ml saline solution) was self-administered by study patients once daily (in the morning) into each naris for a total of 6 weeks. The study medication is consistent with what was used in a previously published study in the non-ESRD setting and was provided by Foundation Care Pharmacy (Earth City, MO).16,17 Follow-up visits occurred at 2, 4, and 6 weeks, when study procedures including objective assessment of olfaction, subjective rating of smell, SGA score determination, and blood collection were performed as described above. Serum theophylline levels were assessed with an immunoassay using THEO2 in vitro tests (Roche Diagnostics, Indianapolis, IN) on a Roche/Hitachi cobas c analyzer. A physical examination for study participants was also performed, as well as an assessment of adverse events.
Statistical Analyses
Baseline characteristics were compared using one-way ANOVA tests for Gaussian continuous measures, Kruskal–Wallis test for non-Gaussian continuous variables, and chi-squared test for categorical variables. Olfactory assessments among control, CKD, and ESRD groups are summarized as the mean and SD and compared using one-way ANOVAs. For statistically significant ANOVAs, all pairwise comparisons among the three groups are tested using the Tukey studentized range adjustment.
The association between the presence of kidney disease and olfaction deficits, where olfactory defects are categorized on an ordinal scale (normal, mild, moderate, severe, or anosmia), was analyzed using univariate and multivariable ordinal logistic regression models. Four sets of covariates were adjusted: multivariable 1, adjusted for age, race, and sex; multivariable 2, adjusted for age, race, sex, current tobacco use, educational level, and presence of diabetes; multivariable 3, adjusted for age, race, sex, current tobacco use, educational level, presence of diabetes, and for the use of the following medications: aspirin, statin, β blocker, angiotensin inhibitor, vitamin D (nutritional and/or active vitamin D), and cinacalcet; and multivariable 4, adjusted for age, race, sex, current tobacco use, educational level, presence of diabetes, and prior stroke. Odds ratios and 95% confidence intervals were reported.
We applied both visual and analytical approaches to study the association between olfactory defects and nutritional markers. The distribution of each nutritional marker across the olfactory functional categories was visualized by box and whisker plots (boxes demonstrate median and 25%–75% range; whiskers demonstrate the 10%–90% range). Unadjusted and adjusted linear regression models were performed. For adjusted analyses, two multivariable models were constructed: multivariable model 1, adjusted for age, race, and sex; and multivariable model 2, adjusted for age, race, sex, current tobacco use, educational level, and presence of diabetes. In addition, subgroup analyses were performed among patients with ESRD.
The statistical significance was set at a two-sided P<0.05. All analyses were performed using the SAS program version 9.4 (SAS Institute, Inc., Cary, NC) or R version 3.2.2.
Disclosures
S.U.N. reports receiving speaker honorarium from Sanofi-Aventis and has served as a consultant to Ardelyx. R.I.T. is a consultant to Fresenius Medical Care North America and Celgene, and has received a research grant from Abbott Laboratories.
Acknowledgments
The authors would like to thank Fresenius Medical Care North America and Foundation Care Pharmacy.
This study was funded by the Massachusetts General Hospital Division of Nephrology through a Leslie Fang Translational Research Award, and received support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic health care centers.
Preliminary findings of this manuscript were presented as an oral abstract at the American Society of Nephrology Kidney Week 2016 on November 17, 2016 in Chicago, IL.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Malaty J, Malaty IA: Smell and taste disorders in primary care. Am Fam Physician 88: 852–859, 2013 [PubMed] [Google Scholar]
- 2.Bossola M, Luciani G, Rosa F, Tazza L: Appetite and gastrointestinal symptoms in chronic hemodialysis patients. J Ren Nutr 21: 448–454, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Atkin-Thor E, Goddard BW, O’Nion J, Stephen RL, Kolff WJ: Hypogeusia and zinc depletion in chronic dialysis patients. Am J Clin Nutr 31: 1948–1951, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Lopes AA, Elder SJ, Ginsberg N, Andreucci VE, Cruz JM, Fukuhara S, Mapes DL, Saito A, Pisoni RL, Saran R, Port FK: Lack of appetite in haemodialysis patients--Associations with patient characteristics, indicators of nutritional status and outcomes in the international DOPPS. Nephrol Dial Transplant 22: 3538–3546, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hakim RM, Levin N: Malnutrition in hemodialysis patients. Am J Kidney Dis 21: 125–137, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bomback AS, Raff AC: Olfactory function in dialysis patients: a potential key to understanding the uremic state. Kidney Int 80: 803–805, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Boyce JM, Shone GR: Effects of ageing on smell and taste. Postgrad Med J 82: 239–241, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doty RL, Newhouse MG, Azzalina JD: Internal consistency and short-term test-retest reliability of the University of Pennsylvania Smell Identification Test. Chem Senses 10: 297–300, 1985 [Google Scholar]
- 10.Doty RL: The Smell Identification testTM Administration Manual, 3rd Ed., Haddon Heights, NJ, Sensonics, Inc., 2008 [Google Scholar]
- 11.Warner MD, Peabody CA, Flattery JJ, Tinklenberg JR: Olfactory deficits and Alzheimer’s disease. Biol Psychiatry 21: 116–118, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Velayudhan L, Pritchard M, Powell JF, Proitsi P, Lovestone S: Smell identification function as a severity and progression marker in Alzheimer’s disease. Int Psychogeriatr 25: 1157–1166, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Deems DA, Doty RL: Age-related changes in the phenyl ethyl alcohol odor detection threshold. Trans Pa Acad Ophthalmol Otolaryngol 39: 646–650, 1987 [PubMed] [Google Scholar]
- 14.Steiber AL, Kalantar-Zadeh K, Secker D, McCarthy M, Sehgal A, McCann L: Subjective global assessment in chronic kidney disease: A review. J Ren Nutr 14: 191–200, 2004 [PubMed] [Google Scholar]
- 15.Doty RL, Shaman P, Kimmelman CP, Dann MS: University of Pennsylvania smell identification test: A rapid quantitative olfactory function test for the clinic. Laryngoscope 94: 176–178, 1984 [DOI] [PubMed] [Google Scholar]
- 16.Henkin RI, Velicu I, Schmidt L: An open-label controlled trial of theophylline for treatment of patients with hyposmia. Am J Med Sci 337: 396–406, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Henkin RI, Schultz M, Minnick-Poppe L: Intranasal theophylline treatment of hyposmia and hypogeusia: A pilot study. Arch Otolaryngol Head Neck Surg 138: 1064–1070, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Griep MI, Van der Niepen P, Sennesael JJ, Mets TF, Massart DL, Verbeelen DL: Odour perception in chronic renal disease. Nephrol Dial Transplant 12: 2093–2098, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Owen WF Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Schiffman SS, Nash ML, Dackis C: Reduced olfactory discrimination in patients on chronic hemodialysis. Physiol Behav 21: 239–242, 1978 [DOI] [PubMed] [Google Scholar]
- 21.Frasnelli JA, Temmel AF, Quint C, Oberbauer R, Hummel T: Olfactory function in chronic renal failure. Am J Rhinol 16: 275–279, 2002 [PubMed] [Google Scholar]
- 22.Landis BN, Marangon N, Saudan P, Hugentobler M, Giger R, Martin PY, Lacroix JS: Olfactory function improves following hemodialysis. Kidney Int 80: 886–893, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Armstrong JE, Laing DG, Wilkes FJ, Kainer G: Smell and taste function in children with chronic kidney disease. Pediatr Nephrol 25: 1497–1504, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Vreman HJ, Venter C, Leegwater J, Oliver C, Weiner MW: Taste, smell and zinc metabolism in patients with chronic renal failure. Nephron 26: 163–170, 1980 [DOI] [PubMed] [Google Scholar]
- 25.Korytowska A, Szmeja Z: [Smell and taste in patients with chronic renal failure treated by hemodialysis]. Otolaryngol Pol 47: 144–152, 1993 [PubMed] [Google Scholar]
- 26.Stevenson RJ: An initial evaluation of the functions of human olfaction. Chem Senses 35: 3–20, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Wrobel BB, Leopold DA: Clinical assessment of patients with smell and taste disorders. Otolaryngol Clin North Am 37: 1127–1142, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raff AC, Lieu S, Melamed ML, Quan Z, Ponda M, Meyer TW, Hostetter TH: Relationship of impaired olfactory function in ESRD to malnutrition and retained uremic molecules. Am J Kidney Dis 52: 102–110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griep MI, Mets TF, Vercruysse A, Cromphout I, Ponjaert I, Toft J, Massart DL: Food odor thresholds in relation to age, nutritional, and health status. J Gerontol A Biol Sci Med Sci 50: B407–B414, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Păunescu TG, Jones AC, Tyszkowski R, Brown D: V-ATPase expression in the mouse olfactory epithelium. Am J Physiol Cell Physiol 295: C923–C930, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Păunescu TG, Rodriguez S, Benz E, McKee M, Tyszkowski R, Albers MW, Brown D: Loss of the V-ATPase B1 subunit isoform expressed in non-neuronal cells of the mouse olfactory epithelium impairs olfactory function. PLoS One 7: e45395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norgett EE, Golder ZJ, Lorente-Cánovas B, Ingham N, Steel KP, Karet Frankl FE: Atp6v0a4 knockout mouse is a model of distal renal tubular acidosis with hearing loss, with additional extrarenal phenotype. Proc Natl Acad Sci U S A 109: 13775–13780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Păunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D: cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winter C, Kampik NB, Vedovelli L, Rothenberger F, Păunescu TG, Stehberger PA, Brown D, John H, Wagner CA: Aldosterone stimulates vacuolar H(+)-ATPase activity in renal acid-secretory intercalated cells mainly via a protein kinase C-dependent pathway. Am J Physiol Cell Physiol 301: C1251–C1261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reaich D: Odour perception in chronic renal disease. Lancet 350: 1191, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen MB, Buch NH: Studies on the sense of smell and taste in diabetics. Acta Otolaryngol 53: 539–545, 1961 [DOI] [PubMed] [Google Scholar]
- 37.Nordin S, Monsch AU, Murphy C: Unawareness of smell loss in normal aging and Alzheimer’s disease: Discrepancy between self-reported and diagnosed smell sensitivity. J Gerontol B Psychol Sci Soc Sci 50: 187–192, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Dobell E, Chan M, Williams P, Allman M: Food preferences and food habits of patients with chronic renal failure undergoing dialysis. J Am Diet Assoc 93: 1129–1135, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK: Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One 9: e107541, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD: Intranasal trigeminal stimulation from odorous volatiles: Psychometric responses from anosmic and normal humans. Physiol Behav 20: 175–185, 1978 [DOI] [PubMed] [Google Scholar]
- 41.Betchen SA, Doty RL: Bilateral detection thresholds in dextrals and sinistrals reflect the more sensitive side of the nose, which is not lateralized. Chem Senses 23: 453–457, 1998 [DOI] [PubMed] [Google Scholar]