Abstract
Studies using Dahl salt-sensitive (SS) rats identified specific quantitative trait loci that predispose animals to hypertension-associated albuminuria and kidney injury. We explored the hypothesis that kidney-specific expression of the transcription factor Ets-1, located within one of these loci on chromosome 8, mediates glomerular injury in SS hypertension. During the first week on a high-salt diet, SS rats and SS rats with only one functioning Ets-1 gene (ES rats) demonstrated similar increases in BP. However, serum creatinine concentration, albuminuria, and glomerular expression of ETS-1 and two ETS-1 targets, MCP-1 and MMP2, did not increase as substantially in ES rats as in SS rats. Mean BP subsequently increased further in SS rats and remained higher than that of ES rats for the rest of the study. After 4 weeks of high-salt intake, ES rats still showed a lower mean serum creatinine concentration and less albuminuria, as well as less histologic evidence of glomerular injury and kidney fibrosis, than SS rats did. To investigate the specific contribution of renal Ets-1, we transplanted kidneys from ES or SS rats into salt-resistant SS-Chr 13BN/McwiCrl (SS-13BN) rats. Within 10 days on a high-salt diet, BP increased similarly in ES and SS allograft recipients, becoming significantly higher than the BP of control isograft recipients. However, mean serum creatinine concentration and albuminuria remained lower in ES allograft recipients than in SS allograft recipients at 2 weeks, and ES allografts showed less glomerular injury and interstitial fibrosis. In conclusion, reduced renal expression of ETS-1 prevented hypertension-associated kidney injury in SS rats.
Keywords: hypertension, Ets-1, albuminuria, kidney disease, kidney transplantation
Hypertension afflicts approximately 30% of the United States adult population and remains the second most common cause of kidney disease. In 2014, the adjusted prevalence of ESRD with hypertension listed as the primary cause was about 505 per million.1 Thus, about 0.7% of hypertensive patients ultimately develop ESRD from hypertension. Although numerous interpretations might explain why only some hypertensive patients develop ESRD, several studies have suggested a genetic predisposition to hypertension-induced kidney disease. Genome-wide association studies have identified multiple loci associated with eGFR and CKD in both European2,3 and non-European populations.4,5 A recent meta-analysis of genome-wide association studies further identified loci associated with the phenotype of kidney function decline.6 These and other observations suggest a genetic predisposition to hypertension-associated kidney failure.
The Dahl salt-sensitive (SS) rat is a well recognized genetic model of salt-sensitive hypertension; these animals also serve as a model of hypertension-induced end-organ injury.7–10 SS rats develop severe hypertension when fed a diet high in salt content.11 Hypertension in SS rats results in progressive loss of GFR, and morphologic changes consistent with CKD. Along with prominent vascular remodeling, SS rats develop albuminuria and glomerular injury with mesangial expansion and glomerulosclerosis.12,13 Genetic analysis of animals obtained from backcrossing an F1, which was crossed between spontaneously hypertensive rats (SHR) and SS rats, to the SS strain have shown that urinary albumin excretion rate is a polygenic trait and most of the quantitative trait loci (QTL) that modify albumin excretion do not associate with QTLs for BP.14 Importantly, in a follow-up study that evaluated the temporal effect of increased salt intake and the associated severity of kidney damage, only the QTL on chromosome 8 demonstrated significant linkage with albumin excretion and kidney damage.15 These studies suggested that there is an independent genetic predisposition to hypertension-associated albuminuria and kidney injury.
Avian Erythroblastosis Virus E26 Oncogen Homolog-1 (abbreviated ETS-1) is a widely expressed transcription factor that is involved in multiple biologic processes including normal development and differentiation.16 ETS-1 plays a critical role in kidney development.17,18 Glomerular expression of ETS-1 is increased after induction of GN in two different rat models.19,20 However, these studies did not demonstrate a direct role of ETS-1 in either exacerbating or limiting glomerular injury. The gene encoding ETS-1 is located among the genes within the chromosome 8 QTL, which has been associated with albuminuria and kidney injury. Further, our recent study using a systemic pharmacologic inhibitor demonstrated involvement of ETS-1 in kidney injury in response to angiotensin II and in salt-sensitive hypertension.21,22 Using SS rats and a novel strain of SS rats that had a single inactivating mutation of ETS-1, this study explored the hypothesis that kidney expression of ETS-1 is a critical mediator of glomerular injury in salt-sensitive hypertension and hypertensive nephrosclerosis.
Results
SS Rats with a Single Inactivating Mutation of Ets-1 Had Less Kidney Damage and Associated Hypertension than SS Rats
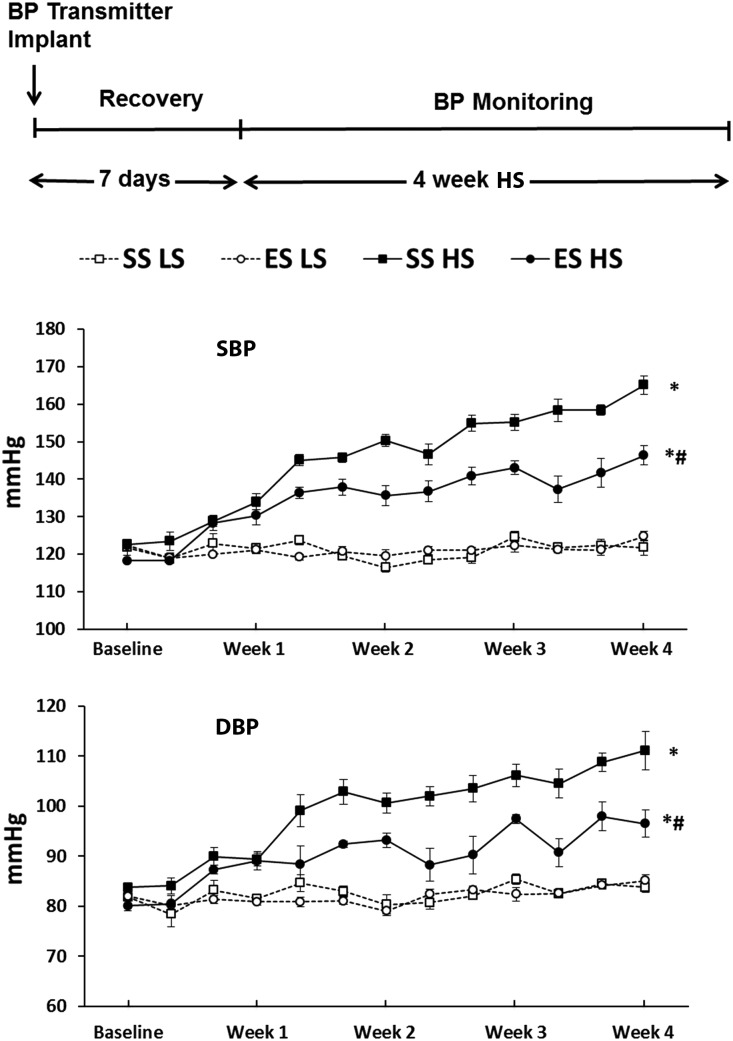
The Ets-1 mutation in ETS-1em1mcwi (ES) rats was confirmed (Figure 1). Mutant rats appeared phenotypically normal and their growth rate did not differ from SS rats while maintained on a normal salt diet. BP was monitored by radio-telemetry in the four groups of rats during a 4-week period on 0.3% low-salt (LS) and 4% high-salt (HS) diets: SS rats on LS (SS LS), ES rats on LS (ES LS), SS rats on HS (SS HS), and ES rats on HS (ES HS), n=6. At baseline, mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) did not differ among the four groups of rats. When compared with the two groups on the LS diet, both SS HS and ES HS rats showed a similarly rapid and progressive increase in mean SBP and mean DBP (Figure 2). In the first week of the study, the mean SBP and mean DBP, as well as the rate of increase in BP, did not differ between SS HS and ES HS groups. After the first week, when compared with the SS HS group, mean SBP and mean DBP were lower in the ES HS group and remained different for the remainder of the study (SBP: SS HS, 164.8±3.4 versus ES HS, 146.3±2.6; DBP: SS HS, 110.9±5.3 versus ES HS, 97.2±3.2; P value <0.05). Heart rates did not differ between SS and ES rats, during either HS or LS intake (Supplemental Figure 1).
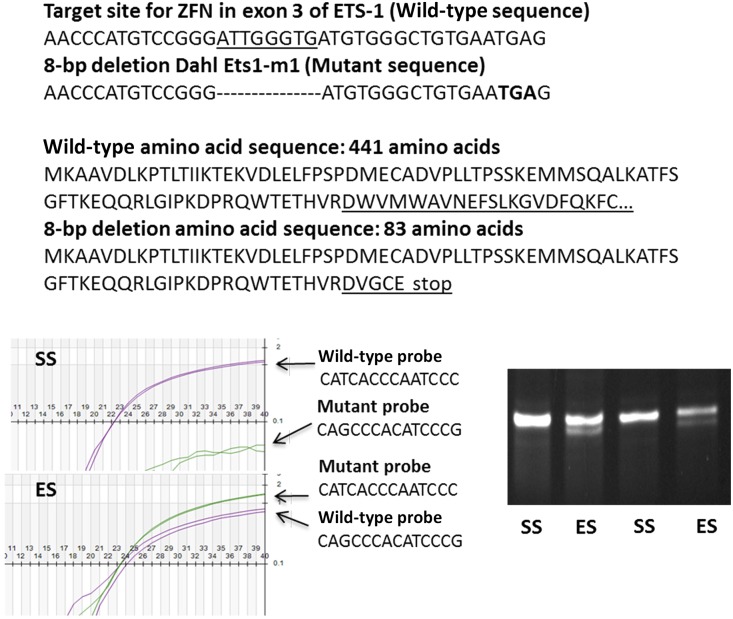
Figure 1.
Genotyping confirmed the presence of a single mutated Ets-1 in ES rats. As shown, the 8-bp deletion was predicted to create a frameshift mutation, generating a premature stop codon (TGA) and a subsequent truncated, nonfunctional ETS-1 protein of only 83 amino acids. This deletion produced a nonfunctional mutant ETS-1 protein that lacked a critical transcription domain (amino acid residues 131–331) and DNA-binding domain (amino acid residues 331–440). Genotyping was confirmed using Taqman probes that recognized wild-type Ets-1 and the mutation (bottom left panel). The genotypes were determined as wild-type (homozygous SS) (upper left), heterozygous (ES) (lower left), or homozygous for the mutation, depending upon whether the samples were positive with only wild-type probes, positive with both wild-type and mutant probes, or positive with only mutant probes, respectively. During breeding, we did not find any rat pups homozygous for the mutation. To confirm the mutation in Ets-1 mRNA, total RNA was extracted from the rat kidney and the cDNA was amplified with PCR primers covering the target cDNA site; the resulting amplicons were separated on a 5%–10% polyacrylamide gel (bottom right). Wild-type (SS) had one band (100-bp, as predicted) and heterozygotes (ES) had two bands (100-bp and 92-bp, as predicted).
Figure 2.
HS intake elevated BP, which was reduced by Ets-1 mutation. Four groups of rats followed the protocol outlined at the top of the figure. Baseline BP did not differ among the groups during LS intake. When placed on the HS (4% NaCl) diet, both ES (termed ES HS) and SS (termed SS HS) groups demonstrated sharp increases in SBP and DBP during the first week of study. For the remainder of the study, the elevation of BP was significantly reduced in rats with the Ets-1 mutation (ES HS), compared with SS HS. BP did not significantly increase in both SS and ES rats that remained on the LS diet (groups SS LS and ES LS). *P value <0.05 versus SS LS; #P value <0.05 versus SS HS; n=6 animals in each group.
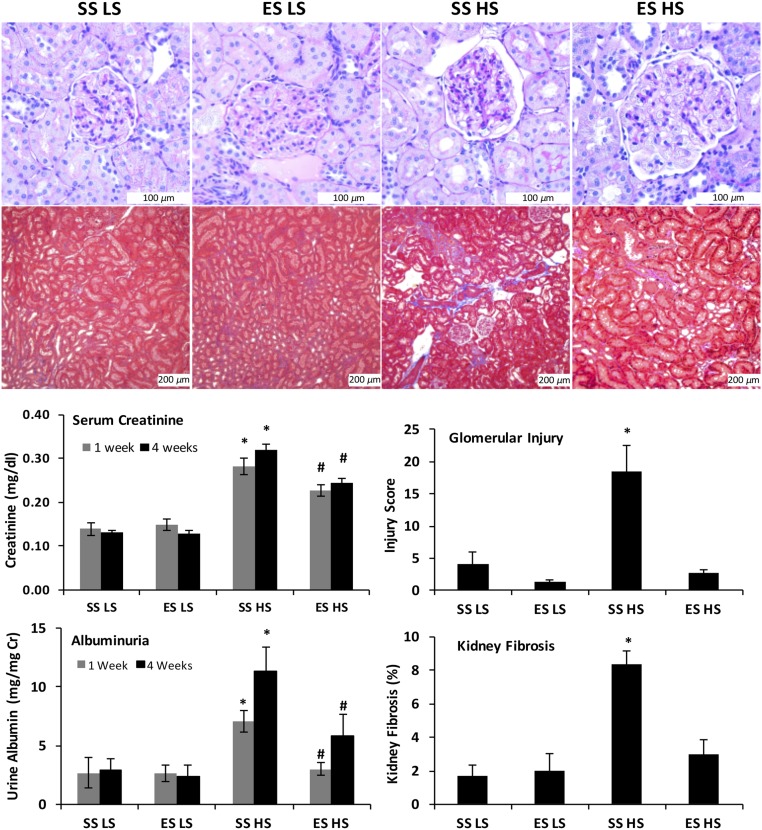
Mean serum creatinine concentrations and mean albumin excretion rates did not differ between SS and ES rats maintained on the LS diet (Figure 3). After 1 week of HS intake, mean serum creatinine concentration and urine albumin excretion rate increased in SS rats, and was significantly higher than that in ES rats. At this early time point, the BP of SS HS and ES HS did not differ. After 4 weeks of HS intake, SS HS and ES HS rats had a significant increase in mean serum creatinine concentration and mean urinary albumin excretion rates. When compared with SS HS rats, ES HS rats showed lower mean serum creatinine concentration (SS HS, 0.32±0.01 versus ES HS, 0.25±0.01 mg/dl; P value <0.05) and lower mean urinary albumin excretion rates (SS HS, 11.39±1.98 versus ES HS, 5.83±1.82 mg/mg Cr; P value <0.05). To assess the effect of Ets-1 mutation on renal injury, glomerular injury score (GIS) was assessed by a renal pathologist (H.F.) in periodic acid–Schiff (PAS)–stained sections and interstitial fibrosis in trichrome-stained sections. Compared with the SS HS rats, the presence of the Ets-1 mutation resulted in significantly lower GIS and kidney fibrosis (P value <0.05) (Figure 3). Mean GIS and amount of fibrosis observed in ES HS rats did not differ from the SS LS and ES LS groups (Figure 3).
Figure 3.
Ets-1 mutation significantly reduced kidney injury and improved kidney function during salt-induced hypertension in Dahl SS rat. Representative PAS and trichrome sections (top) showed distinct expansion of the mesangium and increased interstitial matrix in HS SS rats. Semiquantitative analyses (bottom right graphs) confirmed that SS rats with the single Ets-1 mutation showed less histologic evidence of glomerular injury (P value <0.05, SS HS versus ES HS) and increased interstitial fibrosis (P value <0.05, SS HS versus ES HS). After 1 or 4 weeks of HS intake, mean serum creatinine concentrations of the SS HS and ES HS groups were higher than the groups maintained on the LS diet, whereas the mean serum creatinine levels were less in the ES HS group, compared with SS HS at both time points (P value <0.05, SS HS versus ES HS). After 1 week of HS intake, SS HS rats also showed a significant increase in the urinary excretion of albumin as compared with the other three groups of rats. After 4 weeks on HS diet, SS HS rats had a significant increase in the urinary excretion of albumin as compared with SS LS rats, and the Ets-1 mutation resulted in persistent reductions in albuminuria. *P value <0.05 versus SS LS; #P value <0.05 versus SS HS; n=6 animals in each group. Cr, creatinine.
SS Rats with a Single Inactivating Ets-1 Mutation Produced Less MCP-1 and MMP-2 in Glomeruli than SS Rats
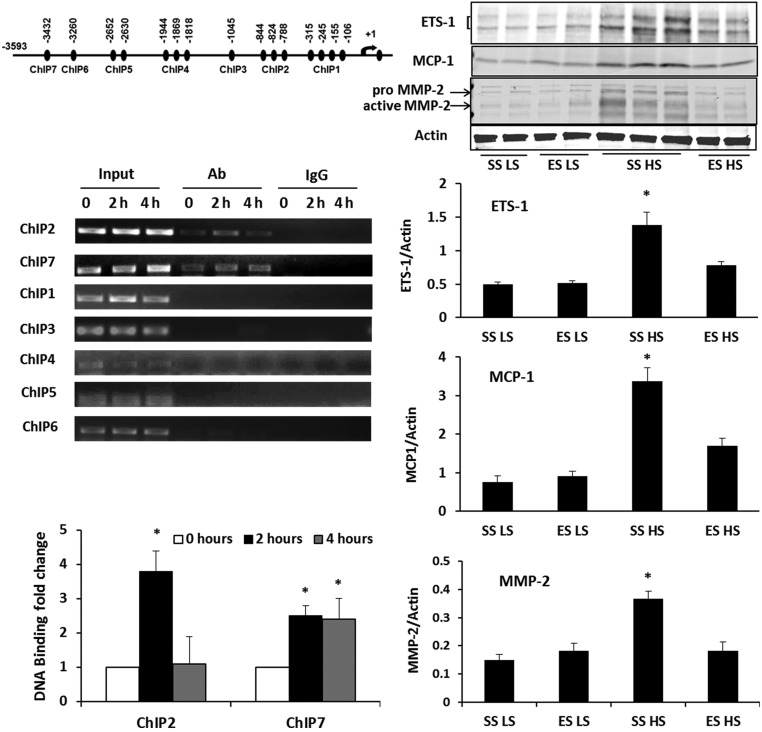
Because prior studies demonstrated that angiotensin II promoted ETS-1 binding to the promoter region of the proinflammatory chemokine, Monocyte Chemoattractant Protein-1 (MCP-1), in smooth muscle cells and resulted in increased MCP-1 expression,23 in this study we used angiotensin II to determine ETS-1 activity in mesangial cells in culture. A chromatin immunoprecipitation (ChIP) assay tested whether the transcription factor ETS-1 directly bound to any of the seven cis-acting elements that are present in the rat MCP-1 promoter and predicted to bind ETS-1. Nuclear extracts from cultured rat mesangial cells stimulated with 1 µM angiotensin II for different amounts of time (0, 2, and 4 hours) were generated and ETS-1 in each sample was immunoprecipitated using an ETS-1 antibody; nonspecific IgG was used as a control. Angiotensin II did not increase binding of ETS-1 to ChIP1, ChIP3, ChIP4, ChIP5, and ChIP6. However, two areas—ChIP2 (−788 to −844) and ChIP7 (−3432)—showed increased binding of ETS-1 at 2 and 4 hours after angiotensin II treatment (Figure 4).
Figure 4.
ETS-1 was involved in glomerular expression of MCP-1 and MMP2. In initial studies, rat mesangial cells were incubated in medium containing angiotensin II, 1 µM, for 0, 2, and 4 hours and then subjected to ChIP assays (left panel). Two regions, ChIP2 (−788 to –844) and ChIP7 (−3432) in the MCP-1 promoter, showed increased binding of ETS-1. *P value <0.05 versus angiotensin II for 0 hours. Western analyses were performed on glomerular lysates obtained from the SS or ES rats on the HS or LS diet for 7 days (right panel). Basal levels of ETS-1 expression were low in both SS and ES rats maintained on the LS diet. Increased dietary salt intake increased ETS-1 expression in the glomerular lysates in SS HS group, but was attenuated in the ES HS group. MCP-1 and MMP-2 expression were also increased with 7 days of high salt intake in the SS group, and were attenuated in the ES group. *P value <0.05 versus SS LS, ES LS, and ES HS groups; n=6 animals in each group. Ab, antibody.
To demonstrate if a single Ets-1 mutation promoted allelic insufficiency, SS and ES rats were fed for 1 week diets containing either LS or HS. The 1-week time point was chosen because radio-telemetry–recorded BP did not differ between SS and ES rats at that time (Figure 2). Compared with samples obtained from SS rats on the LS diet, levels of ETS-1, MCP-1, and, Matrix Metalloproteinase-2 (MMP-2), another target of ETS-1,24 were increased in isolated glomeruli of SS rats after 1 week on the HS diet (Figure 4). In contrast, these effects were attenuated in ES rats, indicating the single mutation produced haploinsufficiency of Ets-1.
Kidneys from SS Rats That Have a Single Inactivating Mutation of Ets-1 Were Protected from the Effects of Hypertension
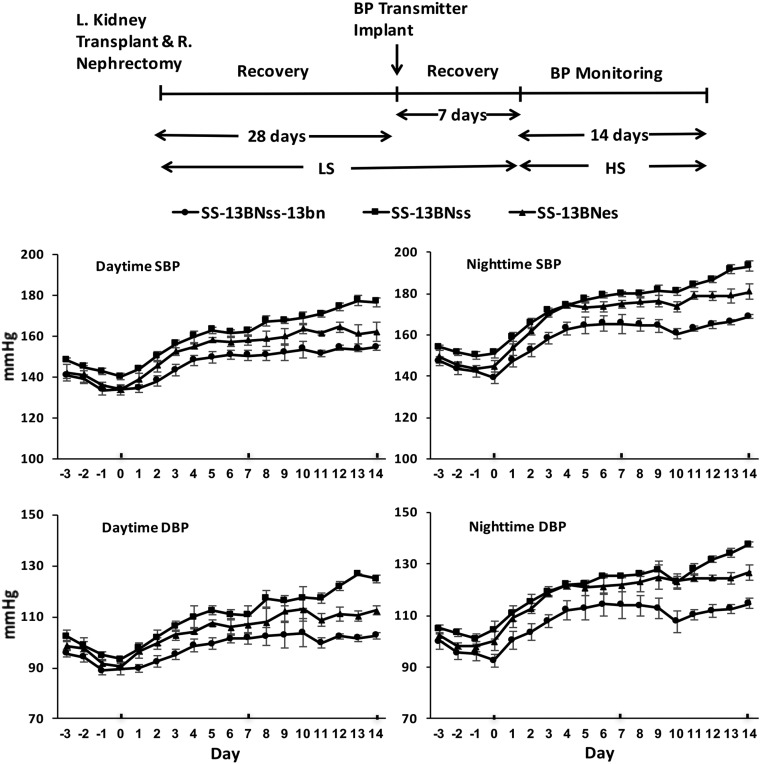
To evaluate further the role of kidney and specifically renal expression of Ets-1 in salt-sensitive hypertension, we transplanted kidneys from either SS or ES rats into salt-resistant SS-Chr 13BN/McwiCrl (termed SS-13BN) rats, using the protocol outlined in Figure 5.
Figure 5.
Transplantation of either ES or SS kidneys into SS-13BN rats increased BP during HS intake. The experimental protocol is outlined at the top of the figure. The three groups of rats, SS-13BNss, SS-13BNes, and SS-13BNss-13bn, were switched from the 0.3% NaCl diet to the 4% NaCl diet after kidney transplantation and implantation of radio-transmitters. Mean daytime and nighttime SBP and DBP of the three groups of rats increased with HS intake. BP of SS-13BNss and SS-13BNes rats did not differ in the early course of HS diet, but was less in SS-13BNes rats during the final 4 days of study. By the end of the study, mean daytime and nighttime SBP and DBP of the SS-13BNss group were greater (P value <0.05) than the other two groups. L, left; R, right.
Three groups of rats (n=7 in each group) were studied: SS-13BNss (SS-13BN rats with SS kidney allografts), SS-13BNes (SS-13BN rats with ES kidney allografts), and SS-13BNss-13bn (SS-13BN rats with SS-13BN kidney isografts). After recovery from the surgical interventions, rats were fed the HS diet for 14 days, with continuous monitoring of BP using radio-telemetry. At the start of the monitoring (day 0), mean daytime SBP of the SS-13BNss-13bn, SS-13BNss, and SS-13BNes did not differ; mean nighttime SBP of the SS-13BNss group was greater (P value <0.05) than mean nighttime SBP of the BNss-13bn group, but did not differ from mean nighttime SBP of the SS-13BNes group (Figure 5, Table 1). Mean daytime and nighttime SBP and DBP of the three groups of rats progressively increased with HS intake. After 1 week on the diet, mean SBP of the SS-13BNss-13bn group was less (P value <0.05) than mean SBP of the SS-13BNss group, but did not differ between the SS-13BNss and SS-13BNes groups. After day 11 of the study, mean BP of the SS-13BNss group increased further and separated from the mean BP of the SS-13BNes. By the end of the study, mean daytime and nighttime SBP and DBP of the SS-13BNss group were greater (P value <0.05) than the other two groups. Mean daytime and nighttime heart rates did not differ among the three groups of kidney-transplanted rats during either HS or LS intake (Supplemental Figure 2).
Table 1.
BP recordings of the three groups of kidney transplant recipients at various time points during the study
| Days | Daytime SBP (mmHg) | Nighttime SBP (mmHg) | Daytime DBP (mmHg) | Nighttime DBP (mmHg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS-13BNss-13bn | SS-13BNss | SS-13BNes | SS-13BNss-13bn | SS-13BNss | SS-13BNes | SS-13BNss-13bn | SS-13BNss | SS-13BNes | SS-13BNss-13bn | SS-13BNss | SS-13BNes | |
| LS | 134.0±2.5 | 140.2±1.6 | 133.8±1.6 | 139.2±2.8 | 151.1±2.3a | 144.7±3.0 | 90.4±2.0 | 93.9±1.1 | 90.4±1.0 | 94.1±2.4 | 103.6±2.7a | 99.6±3.0 |
| HS 7 | 150.5±2.6 | 162.3±2.0a | 158.1±2.5a | 165.2±4.5 | 179.9±1.2a | 174.8±1.8 | 100.5±2.0 | 109.4±2.9a | 106.2±3.0 | 114.0±4.1 | 124.4±0.8a | 121.5±2.2 |
| HS 10 | 153.5±3.9 | 169.0±2.8a | 163.5±2.1a | 160.5±2.6 | 180.8±1.6a | 173.7±2.5a | 103.3±4.1 | 115.2±3.9a | 112.3±3.9a | 108.6±3.3 | 123.2±1.8a | 123.3±2.4a |
| HS 11 | 151.3±1.5 | 170.8±1.7a | 161.3±0.9a,b | 162.9±1.3 | 184.2±1.5a | 178.9±1.7a | 100.5±1.6 | 115.4±2.2a | 108.4±2.0a | 110.6±1.4 | 127.2±1.7a | 124.3±1.2a |
| HS 12 | 154.3±1.1 | 174.1±1.8a | 164.4±2.6a,b | 165.0±1.9 | 186.6±1.5a | 179.1±2.1a | 102.7±0.9 | 119.9±2.4a | 110.4±2.1a,b | 112.1±1.9 | 130.2±1.7a | 124.1±0.9a,b |
| HS 13 | 153.5±0.9 | 177.4±2.6a | 161.0±4.6b | 166.1±1.0 | 191.6±2.2a | 178.9±3.3a,b | 101.5±0.9 | 124.3±2.2a | 108.0±2.9b | 112.7±1.4 | 132.8±1.9a | 123.9±2.0a,b |
| HS 14 | 154.4±1.1 | 176.7±2.2a | 162.1±4.9b | 168.8±1.0 | 193.3±2.3a | 181.0±3.8a,b | 102.2±1.1 | 124.9±1.4a | 109.6±3.1b | 114.8±1.4 | 136.4±1.5a | 125.8±2.5a,b |
Data presented as mean±SEM.
P value <0.05 versus SS-13BNss-13bn.
P value <0.05 versus SS-13BNss; n=7 rats in each group.
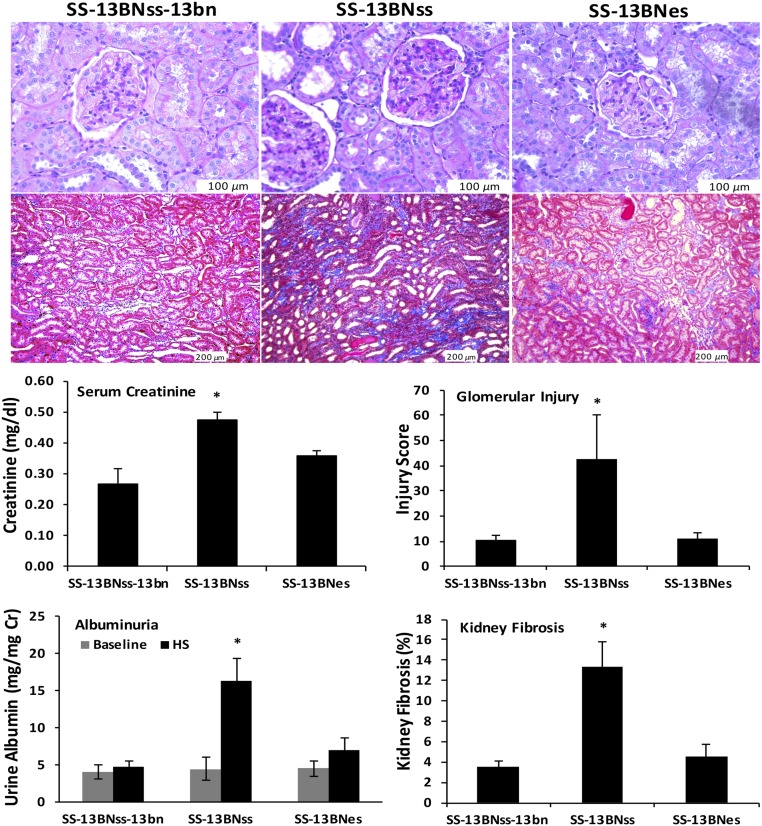
Urine obtained before the institution of the HS diet showed that mean albumin excretion rates did not differ among the three groups of rats (SS-13BNss-13bn, 4.14±0.95; SS-13BNss, 4.46±1.52; and SS-13BNes, 4.54±1.07 mg/mg Cr), suggesting that the kidney transplant surgery did not significantly affect renal function among the three groups of rats. After 2 weeks on the HS diet, mean urinary excretion rates of albumin were greater in SS-13BNss rats than values observed in the SS-13BNss-13bn and SS-13BNes rats (Figure 6). The Ets-1 mutation in the kidney resulted in significant reductions in albuminuria in SS-13BNes rats (SS-13BNss, 16.39±2.97 versus SS-13BNes, 6.99±1.59 mg/mg Cr; P value <0.05). Mean serum creatinine concentrations were greater in SS-13BNss compared with the other two groups (SS-13BNss-13bn, 0.27±0.05; SS-13BNss, 0.47±0.03; and SS-13BNes, 0.36±0.02 mg/dl; P value <0.05).
Figure 6.
Kidney allografts with a single Ets-1 mutation showed significantly reduced glomerular injury and renal fibrosis, and improved kidney function in SS-13BNes, comparing to that in SS-13BNss during HS intake. Representative PAS-stained and trichrome-stained kidney sections were included in the top panel. Semiquantitative analyses showed that, compared with the SS-13BNss-13bn and SS-13BNes groups, glomerular injury and kidney fibrosis were increased in the SS-13BNss group (bottom right graphs). After 2 weeks on the HS diet, mean serum creatinine concentrations (left) were greater in SS-13BNss, compared with the other two groups (SS-13BNss-13bn, 0.27±0.05; SS-13BNss, 0.47±0.03; and SS-13BNes, 0.36±0.02 mg/dl; P value <0.05). Albuminuria (bottom left) did not differ among the three groups of rats during the LS intake (BNbn, 4.14±0.95; SS-13BNss, 4.46±1.52; and SS-13BNes, 4.54±1.07 mg/mg Cr). Mean urinary excretion rates of albumin were greater in SS-13BNss rats than values observed in the SS-13BNss-13bn and SS-13BNes rats during HS intake. The Ets-1 mutation in the kidney resulted in significant reductions in albuminuria in SS-13BNes rats (SS-13BNss, 16.39±2.97 versus SS-13BNes, 6.99±1.59 mg/mg Cr; P value <0.05); n=7 animals in each group. Cr, creatinine.
To determine the effect of the Ets-1 mutation in kidney on renal injury in SS hypertension, we evaluated glomerular injury in PAS-stained kidney sections and interstitial fibrosis in trichrome-stained sections of the three groups of rats after 2 weeks of HS intake. GIS was significantly increased in SS-13BNss rats as compared with that in SS-13BNss-13bn rats (Figure 6). We also observed significant increases in interstitial fibrosis in SS-13BNss rats as compared with SS-13BNss-13bn rats (Figure 6). Ets-1 mutation in kidney resulted in significant improvements in GIS and reduced interstitial fibrosis in SS-13BNes (P value <0.05).
Discussion
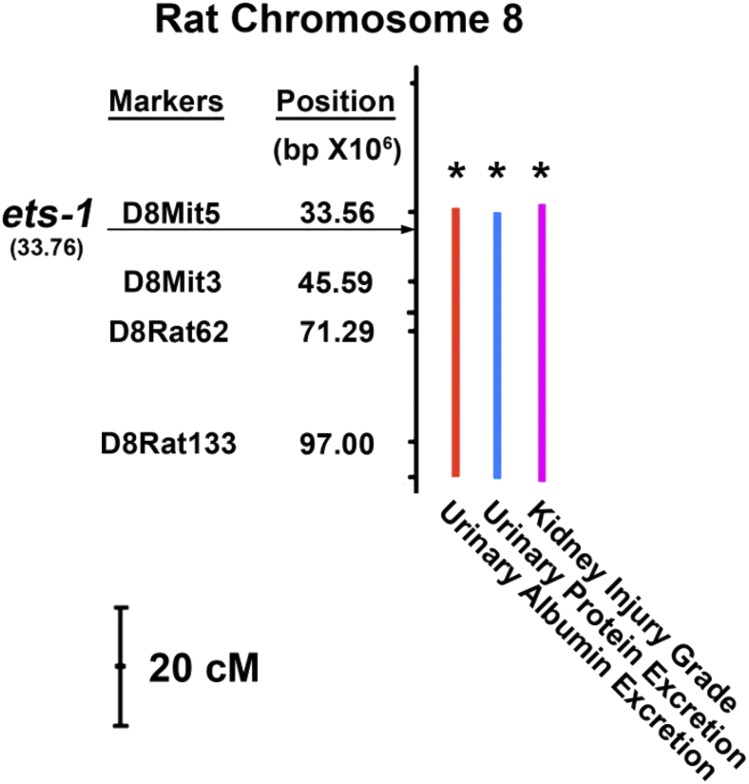
This study demonstrated that a functional mutation of a single Ets-1 allele was sufficient to reduce glomerular expression of ETS-1 and ETS-1–inducible targets that included MCP-1 and MMP-2 in ES rats treated with an HS diet for 1 week. Further, haploinsufficiency of Ets-1 specifically in the kidney abrogated hypertension-associated increases in serum creatinine concentration and albuminuria and prevented glomerular injury and tubulointerstitial fibrosis in the SS rat. Experiments performed in the first week on the HS diet, when BP did not differ between ES and SS rats, demonstrated lower mean serum creatinine concentration and less albuminuria in ES rats when compared with SS rats. These data, along with the kidney crosstransplantation studies, showed that kidney protection occurred without preventing salt-induced increases in BP, which was documented using radio-telemetry (Figure 5). The findings also suggested that worsening kidney function promoted a positive feedback loop that accelerated the effect of salt intake on BP. These findings were further strengthened by previous studies that demonstrated a beneficial effect of pharmacologic blockade of ETS-1 in SS rats during HS intake21 or in mice with angiotensin II infusion.22 Prior breeding studies using SS rats and SHR, a strain that is resistant to the detrimental effects of hypertension on the kidney, observed that hypertension-associated albuminuria was a polygenic trait. In particular, a QTL on chromosome 8 demonstrated significant linkage with an index of the severity of kidney damage and albumin excretion, but not with BP.15 The gene encoding ETS-1 has been shown to reside within this QTL on rat chromosome 8 (Figure 7). Thus, the combined observations supported the novel finding that Ets-1 is a critical candidate gene involved in hypertension-associated glomerular injury in the SS rat.
Figure 7.
Ets-1 is localized within a QTL on chromosome 8 that associates with kidney injury. Genome scan (at chromosome 8) for SBP, UPE, UAE, KLG, HW, and TKW at 8, 12, and 16 weeks of age for the backcross population (n=228) raised on a 2% NaCl diet. Significant increases of UPE, UAE, and KLG (right) are correlative to the QTLs of chromosome 8 (left). ETS-1 is at this critical area, according to its chromosome position. HW, heart weight; UPE, urinary protein excretion; UAE, urinary albumin excretion; TKW, total kidney weight; KLG, kidney lesion grade. The bar represented 20 centiMorgan (cM). Modified from reference 15, with permission.
ETS-1 is the founding member of a growing family of transcription factors that contain in the carboxy-terminal portion of the molecule a DNA-binding site characterized by a winged helix-turn-helix motif.16 ETS-1 is regulated by cell-signaling events involving serine phosphorylation,25 which activates an autoinhibitory domain that inhibits binding to DNA, and threonine phosphorylation,26 which activates ETS-1 to promote DNA transcription. Activity is further regulated through direct interactions with other transcription factors.27 The biologic importance of ETS-1 in development has been suggested by observations that include activation in multiple cell types during organogenesis28 and mice that lacked Ets-1 had a high neonatal (50%) mortality.29 In this study, genome engineering of Ets-1 with Zinc-Finger Nucleases (ZFN) produced an 8-bp mutation that produced a premature stop codon, which deleted the DNA-binding domain, producing a truncated nonfunctional ETS-1 protein (Figure 1). The importance of ETS-1 in organogenesis was also confirmed, because breeding did not produce any rats that were homozygous for the mutated Ets-1. However, ES rats, which were haploinsufficient for ETS-1, grew normally and were not hypertensive at baseline. When stressed with the development of salt-sensitive hypertension, levels of ETS-1 and two ETS-1–driven products—MCP-123 and MMP-224—were reduced in the glomeruli of ES rats, which subsequently developed less glomerular injury, albuminuria, and kidney failure when compared with SS rats.
Infiltrating immune cells and macrophages/monocytes have been found to play an important inflammatory role in SS hypertension and kidney injury.30,31 In mice infused with angiotensin II, pharmacologic blockade of ETS-1 did not prevent the development of hypertension, but reduced renal oxidative stress, macrophage infiltration, and kidney fibrosis.22 The findings suggested that ETS-1–mediated macrophage infiltration was integrally involved in angiotensin-II–dependent hypertensive kidney injury. Because of the critical involvement of ETS-1 in T and B cell development and function,32–35 a role of ETS-1 in immune cells in kidney injury was considered. In this study, however, crosstransplantation experiments confirmed an intrinsic Ets-1–dependent alteration in glomerular function in the development of kidney injury, because ETS-1 function in the immune system of the host was not affected in these experiments.
Studies using a mesangial cell line found that stimulation of cells with angiotensin II increased ETS-1 binding in the promoter region of MCP-1 and further that MCP-1 increased in glomeruli of SS rats in vivo after 1 week on the HS diet. These findings were consistent with a previous report that ETS-1 transcriptionally mediated MCP-1 expression in smooth muscle cells.23 This study also demonstrated that 1 week on the HS diet increased MMP-2, but the increase was also blunted in the ES rats. Both MCP-1 and MMP-2 have been shown to be important mediators of inflammatory injury and remodeling.36–38 Although other pathways may be involved in glomerular injury in this model, taken together, the findings suggested that expression of ETS-1 in the glomerulus promoted a proinflammatory milieu during the development of salt-sensitive hypertension.
In summary, the data showed that ETS-1 was involved in the production of a proinflammatory state in the glomerulus and was a critical mediator of hypertension-associated kidney injury. The observation that Ets-1 haploinsufficiency did not diminish the BP responses early in the course of HS intake comports well with prior linkage analyses that suggested different QTL independently associated with albuminuria and BP, respectively, in SS rats and with our prior study that used a pharmacologic agent that systemically inhibited ETS-1.21 In the first week on the HS diet, average BP did not differ between SS and ES rats, but the increases in serum creatinine and albumin excretion rates were lower in the ES rats. Subsequent monitoring in the first study did show significant differences in BP of ES versus SS rats when maintained on the HS diet. Therefore, the crosstransplantation studies were pursued. The kidney transplant recipient in these experiments was a consomic strain (SS-13BN) in which chromosome 13 from normotensive inbred Brown Norway rats had been introgressed into the background of SS rats. These animals were resistant to the hypertensive effects of HS intake.39,40 BP did not differ between the transplant recipients that received SS and ES kidneys until the final 4 days of the study (Figure 5). Therefore, a caveat to these experiments is that these changes in BP of this short duration were unlikely to have produced the changes in observed renal function and more likely the result of ongoing and progressive kidney injury in the SS allografts. The combined findings suggested that ETS-1 is a potential target for inhibition to limit progression of hypertension-associated kidney disease.
Concise Methods
Animals
This study was performed in accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at the University of Alabama at Birmingham approved the project. Studies were conducted using Dahl SS rats (SS/JrHsd/Mcw, abbreviated SS),8,9,11,41 Dahl SS/JrHsd/Mcw rats that have a deletion mutation in Ets-1 (ETS-1em1mcwi, abbreviated ES), and consomic SS-Chr 13BN/McwiCrl rats (abbreviated SS-13BN).39,40 SS and SS-13BN rats were obtained commercially (Charles River, Wilmington, MA). The ES strain of rats was a generous gift from Dr. Aron Geurts, Medical College of Wisconsin. ES rats were genetically identical to SS rats, except for a heterozygotic gene mutation of Ets-1 introduced using genome engineering with ZFN,42 which were designed to target exon 3 of rat Ets-1 gene, resulting in an 8-bp (ATTGGGTG) deletion mutation (Figure 1). Rat breeders and weanlings were fed purified AIN-76A rodent diet (Dyets, Inc., Bethlehem, PA) containing 0.3% NaCl and tap water ad libitum until study.
Genotyping and Confirmation of Ets-1 Mutation
For genotyping, Taqman probes that recognized the 8-bp deletion sequence (mutant probe) or wild-type sequence (wild-type probe) were designed. The wild-type Taqman probe was 5′-CATCACCCAATCCC-3′ and the Taqman probe to detect the mutation in Ets-1 was 5′-CAGCCCACATCCCG-3′. The genotypes were determined as wild-type (homozygous SS), heterozygous (ES), or homozygous for the mutation, depending upon whether the samples were positive with only wild-type probes, positive with both wild-type and mutant probes, or positive with only mutant probes, respectively. Samples of DNA of rats in the study were amplified with real-time PCR and used to determine genotype (Transnetyx, Cordova, TN) (Figure 1). The forward primer sequence was 5′-GCAGTGGACAGAAACCCATGT-3′ and the reverse sequence was 5′-TGCTGCTCCATTCATACAGAACTTC-3′. To confirm the presence of the mutation of Ets-1, RNA was isolated from SS or ES rats, reverse-transcribed, and PCR-amplified using the same primers. The resulting amplicons were loaded and separated on a 5%–10% polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA). Ethidium bromide staining revealed either a single 100-bp band or a double band (100- and 92-bp), if the mutation was present (Figure 1). Genotyping confirmed the presence of a single mutation (ES rats) or absence of the mutation (SS rats), which served as littermate controls.
Study 1
Experiments were performed using age-matched male ES rats and littermates (SS) (Figure 2). Rats were divided into four groups (n=6 rats per group) and fed diets (AIN-76A Rodent Diet; Dyets, Inc.) that contained either 0.3% NaCl (LS) or 4% NaCl (HS) for 4 weeks. The four groups were named as follows: SS LS (SS rats fed the LS diet), SS HS (SS rats fed the HS diet), ES LS (ES rats fed the LS diet), and ES HS (ES rats fed the HS diet). Implantable transmitters (HD-S10; DSI, St. Paul, MN) were used to record awake, unrestrained BP continuously by radio-telemetry.43 The rats were anesthetized using 2% isoflurane and left femoral arteries were isolated with a ventral incision. The catheter of the telemetry probe was inserted into the femoral artery. A pocket along the left flank was formed through the same ventral incision and the body of the transmitter slipped into the pocket, and secured with tissue adhesive. The ventral incision was then closed. Rats were recovered for 7 days before initiating the study. BP was then monitored for 2 hours (11 a.m. to 1 p.m.) every other day for 4 weeks. The BP was recorded as the average of the reading obtained on each day.
After 4 weeks on the diets, the rats were placed in metabolic cages for 24 hours to collect urine for determination of concentrations of albumin and creatinine. Urinary albumin concentrations were determined using a rat albumin ELISA kit (Bethyl, Montgomery, TX) and adjusted by the urinary creatinine concentration, which was determined using a kit (BioAssay System, Hayward, CA). Rats were then euthanized and the kidneys were harvested for histology and molecular analyses and blood was collected for determination of serum creatinine using liquid chromatography tandem mass spectrometry (Waters 2795 LC-MS/MS; Conquer Scientific, San Diego, CA).44
Study 2
Experiments were performed using age-matched male ES rats and littermates (SS). Rats were divided into four groups (n=6 rats per group) and fed diets (AIN-76A Rodent Diet; Dyets, Inc.) that contained either 0.3% NaCl (LS) or 4% NaCl (HS) for 7 days. The rats were euthanized and the kidneys were perfused in situ through the aorta with cold normal saline until blanched (50–60 ml saline over 2 minutes). Glomeruli were isolated using a graded sieving technique as described.45 The renal cortices from each rat were individually dissected and minced to a paste-like consistency. The homogenate was passed through a 106-μm metal sieve that excluded blood vessels and a 75-μm nylon sieve that retained the glomeruli and allowed cells and small tubular segments to pass through. Glomeruli were washed three times with ice-cold PBS and centrifuged at 120 × g for 5 minutes. All glomerular preparations consisted of >95% glomeruli with minimal tubular contamination, as assessed visually at 40× magnification. Lysates of the pelleted glomeruli were prepared for western blotting analyses using T-Per Tissue Protein Extraction Reagent and protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL). The glomerular protein concentration was determined using a kit (Bio-Rad Laboratories, Hercules, CA), and the samples were processed for western blotting. Protein extracts (40–60 µg) were boiled for 5 minutes in Laemmli buffer and separated in 5%–15% SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA) before electrophoretic transfer onto polyvinylidene difluoride membranes. The membranes were blocked in 5% nonfat milk and then probed with ETS-1 monoclonal antibody (C4, diluted 1:200), and polyclonal antibodies to MCP-1 (R-17, diluted 1:200), MMP-2 (H-76, diluted 1:200), and β-actin (C-11, diluted 1:1000) overnight in 4°C. All antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX). After washes, the appropriate fluorescence-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were applied to the membranes at room temperature for 1 hour. The membranes were scanned and density of the bands was quantified using Odyssey CLx Imager system and Image Studio 4 software (Li-COR Biotechnology, Lincoln, NE).
Study 3
Kidney transplantation studies were performed in three groups of rats: SS-13BNss-13bn (kidneys from SS-13BN rats transplanted into SS-13BN rats), SS-13BNss (kidneys from SS rats transplanted into SS-13BN rats), and SS-13BNes (kidneys from ES rats transplanted to SS-13BN rats). The study protocol is schematized in Figure 5. These studies were feasible in part because the strains used in these experiments shared an identical MHC, which resides on rat chromosome 20.
Kidney transplantation was performed as described previously,46 with modifications. In brief, after induction of anesthesia in donor rats, laparotomy was performed. An intravenous catheter was secured in the aorta. The kidneys were perfused at 90 mmHg with 2 ml of normal saline containing heparin, 30 U/ml. The kidneys were rapidly divided from the renal bed and then placed in ice-cold saline, dissected, and prepared for transplantation. Left renal artery (LRA) and left renal vein (LRV) were retained to facilitate subsequent anastomosis to recipient LRA and LRV. The ureter was also dissected and prepared for the anastomosis. After the recipient rats were anesthetized, a left nephrectomy was performed and orthotopic kidney transplantation was performed. An end-to-end anastomosis of recipient and donor LRAs, and the anastomosis of LRVs were accomplished using 10–0 nylon with eight interrupted sutures and continuous stitches, respectively. The ureter was anastomosed end-to-end to the recipient ureter over a 3-mm PE-10 polyethylene stent. Warm ischemia time, which was the time required to complete both arterial and venous anastomoses, was maintained consistently at approximately 25–30 minutes. The right kidney of the recipient was removed before suturing the abdominal incision. The overall mortality of kidney transplantation was about 10%. Three rats (two SS-13BNss and one SS-13BNes) died in the 2 weeks after the kidney transplantation surgery. The daytime SBP in four rats (one SS-13BNss-13bn, one SS-13BNss and two SS-13BNes) did not return to <145 mmHg during LS intake and were therefore excluded from the remainder of the study. Seven rats (n=7) in each group completed the protocol and were included in the subsequent analyses.
After a 28-day recovery period, the rats were again anesthetized using 2% isoflurane and a radio-transmitter (HD-S10; DSI, St. Paul, MN) was inserted into the left femoral artery to permit continuous recording of awake, unrestrained BP. After another 7-day recovery period, rats were fed the 4% NaCl diet (AIN-76A Rodent Diet; Dyets, Inc.) for 14 days. BP was monitored for 24 hours each day throughout the two weeks. Daytime BP was recorded as the average of BP from 6 a.m. to 6 p.m.; nighttime BP was recorded as the average of BP from 6 p.m. to 6 a.m. At the end of the study, the rats were placed in metabolic cages for 24 hours to collect urine for determination of concentrations of albumin and creatinine. Rats were then euthanized and the kidneys were harvested for histology and molecular analyses and blood was collected for determination of serum creatinine.
Morphometric Analysis for GIS and Renal Fibrosis
Kidney sections at 5-µm thickness from the different experimental groups were stained using PAS and trichrome, and were used for morphometric analyses. GIS was evaluated in PAS-stained kidney sections by an experienced renal pathologist (H.F.) purposely blinded to the different experimental conditions and utilizing a 0+ to 4+ scale as previously described.21,47 Briefly, the severity of the lesion was graded from 0 to 4+ according to the percentage of glomerular involvement. Thus, a 1+ lesion represented an involvement of 25% of the glomerulus whereas a 4+ lesion indicated that 100% of the glomerulus was involved. All glomeruli (160–230) available in each section were analyzed. Renal fibrosis was evaluated by the same pathologist in trichrome-stained kidney sections and expressed as percentage of fibrosis in the interstitium.21
ChIP Assays
Rat mesangial cells (CRL-2573; America Type Culture Collection, Rockville, MD) grown to 70% confluence on 150-mm dishes were treated with angiotensin II, 1 µM, for 0, 2, or 4 hours. Angiotensin II has been shown to increase steady-state mRNA levels of Ets-1 in vascular smooth muscle cells.23 After treatment, cells were fixed in 1% formaldehyde for 5 minutes at room temperature. Cells were collected and incubated in ice-cold lysis buffer for 30 minutes. Cells were then homogenized gently in a dounce homogenizer to release the nuclei. After centrifugation, the nuclei pellets were saved and enzymatically sheared for 8 minutes. ChIP assays were performed using a kit (ChIP-IT Express Enzymatic kit from Active Motif, Carlsbad, CA), following the manufacturer’s instructions. The sheared DNA was purified, quantitated, and immunoprecipitated with ETS-1 antibody using magnetic beads. A nonspecific rabbit IgG antibody (sc-2027×; Santa Cruz Biotechnology, Dallas, Texas) was used as control. After reverse crosslinking, the immunoprecipitated DNA and input DNA were assayed by SYBR Green real-time PCR with the primers in Supplemental Table 1, which covered the potential ETS-1 binding sites in MCP-1 promoter.
Statistical Analyses
Data were expressed as the mean±SEM. Two-way ANOVA was performed with a Holm–Sidak post hoc test for Study 1. One-way ANOVA was performed with a Holm–Sidak post hoc test for Study 2 and Study 3. A P value <0.05 assigned statistical significance.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful for the support of Core Facilities at the O’Brien Kidney Center at the University of Alabama at Birmingham; Dr. Shanrun Liu helped in analysis of ETS-1 DNA and amino acid structures.
A grant from the American Heart Association (15SDG25760063), grant (1 IP1 BX001595) from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, the National Institutes of Health George M. O’Brien Kidney and Urological Research Centers Program (P30 DK079337) and R56 (HL 128285), and an Anderson Innovation Award, supported this research.
W.F., B.C., D.X., and X.L. performed the experiments; W.F., H.F., and P.W.S. analyzed the data and interpreted results of the experiments; W.F. and P.W.S. prepared the figures; W.F. and P.W.S. drafted the manuscript; W.F., E.A.J., and P.W.S. edited and revised the manuscript; W.F., B.C., D.X., X.L., H.F., E.A.J., and P.W.S. approved the final version of the manuscript; W.F. and P.W.S. conceived and designed the research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Ets in the Kidney—Unraveling the Molecular Mechanism Underlying Renal Damage in Salt-Sensitive Hypertension,” on pages 3131–3133.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017010085/-/DCSupplemental.
References
- 1.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, and Hirth RA: US renal data system 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 67: Svii, S1–305, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Köttgen A, Pattaro C, Böger CA, Fuchsberger C, Olden M, Glazer NL, Parsa A, Gao X, Yang Q, Smith AV, O’Connell JR, Li M, Schmidt H, Tanaka T, Isaacs A, Ketkar S, Hwang SJ, Johnson AD, Dehghan A, Teumer A, Paré G, Atkinson EJ, Zeller T, Lohman K, Cornelis MC, Probst-Hensch NM, Kronenberg F, Tönjes A, Hayward C, Aspelund T, Eiriksdottir G, Launer LJ, Harris TB, Rampersaud E, Mitchell BD, Arking DE, Boerwinkle E, Struchalin M, Cavalieri M, Singleton A, Giallauria F, Metter J, de Boer IH, Haritunians T, Lumley T, Siscovick D, Psaty BM, Zillikens MC, Oostra BA, Feitosa M, Province M, de Andrade M, Turner ST, Schillert A, Ziegler A, Wild PS, Schnabel RB, Wilde S, Munzel TF, Leak TS, Illig T, Klopp N, Meisinger C, Wichmann HE, Koenig W, Zgaga L, Zemunik T, Kolcic I, Minelli C, Hu FB, Johansson A, Igl W, Zaboli G, Wild SH, Wright AF, Campbell H, Ellinghaus D, Schreiber S, Aulchenko YS, Felix JF, Rivadeneira F, Uitterlinden AG, Hofman A, Imboden M, Nitsch D, Brandstätter A, Kollerits B, Kedenko L, Mägi R, Stumvoll M, Kovacs P, Boban M, Campbell S, Endlich K, Völzke H, Kroemer HK, Nauck M, Völker U, Polasek O, Vitart V, Badola S, Parker AN, Ridker PM, Kardia SL, Blankenberg S, Liu Y, Curhan GC, Franke A, Rochat T, Paulweber B, Prokopenko I, Wang W, Gudnason V, Shuldiner AR, Coresh J, Schmidt R, Ferrucci L, Shlipak MG, van Duijn CM, Borecki I, Krämer BK, Rudan I, Gyllensten U, Wilson JF, Witteman JC, Pramstaller PP, Rettig R, Hastie N, Chasman DI, Kao WH, Heid IM, Fox CS: New loci associated with kidney function and chronic kidney disease. Nat Genet 42: 376–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pattaro C, Köttgen A, Teumer A, Garnaas M, Böger CA, Fuchsberger C, Olden M, Chen MH, Tin A, Taliun D, Li M, Gao X, Gorski M, Yang Q, Hundertmark C, Foster MC, O’Seaghdha CM, Glazer N, Isaacs A, Liu CT, Smith AV, O’Connell JR, Struchalin M, Tanaka T, Li G, Johnson AD, Gierman HJ, Feitosa M, Hwang SJ, Atkinson EJ, Lohman K, Cornelis MC, Johansson Å, Tönjes A, Dehghan A, Chouraki V, Holliday EG, Sorice R, Kutalik Z, Lehtimäki T, Esko T, Deshmukh H, Ulivi S, Chu AY, Murgia F, Trompet S, Imboden M, Kollerits B, Pistis G, Harris TB, Launer LJ, Aspelund T, Eiriksdottir G, Mitchell BD, Boerwinkle E, Schmidt H, Cavalieri M, Rao M, Hu FB, Demirkan A, Oostra BA, de Andrade M, Turner ST, Ding J, Andrews JS, Freedman BI, Koenig W, Illig T, Döring A, Wichmann HE, Kolcic I, Zemunik T, Boban M, Minelli C, Wheeler HE, Igl W, Zaboli G, Wild SH, Wright AF, Campbell H, Ellinghaus D, Nöthlings U, Jacobs G, Biffar R, Endlich K, Ernst F, Homuth G, Kroemer HK, Nauck M, Stracke S, Völker U, Völzke H, Kovacs P, Stumvoll M, Mägi R, Hofman A, Uitterlinden AG, Rivadeneira F, Aulchenko YS, Polasek O, Hastie N, Vitart V, Helmer C, Wang JJ, Ruggiero D, Bergmann S, Kähönen M, Viikari J, Nikopensius T, Province M, Ketkar S, Colhoun H, Doney A, Robino A, Giulianini F, Krämer BK, Portas L, Ford I, Buckley BM, Adam M, Thun GA, Paulweber B, Haun M, Sala C, Metzger M, Mitchell P, Ciullo M, Kim SK, Vollenweider P, Raitakari O, Metspalu A, Palmer C, Gasparini P, Pirastu M, Jukema JW, Probst-Hensch NM, Kronenberg F, Toniolo D, Gudnason V, Shuldiner AR, Coresh J, Schmidt R, Ferrucci L, Siscovick DS, van Duijn CM, Borecki I, Kardia SL, Liu Y, Curhan GC, Rudan I, Gyllensten U, Wilson JF, Franke A, Pramstaller PP, Rettig R, Prokopenko I, Witteman JC, Hayward C, Ridker P, Parsa A, Bochud M, Heid IM, Goessling W, Chasman DI, Kao WH, Fox CS; CARDIoGRAM Consortium, ICBP Consortium, CARe Consortium, Wellcome Trust Case Control Consortium 2 (WTCCC2) : Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet 8: e1002584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu CT, Garnaas MK, Tin A, Kottgen A, Franceschini N, Peralta CA, de Boer IH, Lu X, Atkinson E, Ding J, Nalls M, Shriner D, Coresh J, Kutlar A, Bibbins-Domingo K, Siscovick D, Akylbekova E, Wyatt S, Astor B, Mychaleckjy J, Li M, Reilly MP, Townsend RR, Adeyemo A, Zonderman AB, de Andrade M, Turner ST, Mosley TH, Harris TB, Rotimi CN, Liu Y, Kardia SL, Evans MK, Shlipak MG, Kramer H, Flessner MF, Dreisbach AW, Goessling W, Cupples LA, Kao WL, Fox CS; CKDGen Consortium : Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS Genet 7: e1002264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada Y, Sim X, Go MJ, Wu JY, Gu D, Takeuchi F, Takahashi A, Maeda S, Tsunoda T, Chen P, Lim SC, Wong TY, Liu J, Young TL, Aung T, Seielstad M, Teo YY, Kim YJ, Lee JY, Han BG, Kang D, Chen CH, Tsai FJ, Chang LC, Fann SJ, Mei H, Rao DC, Hixson JE, Chen S, Katsuya T, Isono M, Ogihara T, Chambers JC, Zhang W, Kooner JS, Albrecht E, Yamamoto K, Kubo M, Nakamura Y, Kamatani N, Kato N, He J, Chen YT, Cho YS, Tai ES, Tanaka T; KidneyGen Consortium, CKDGen Consortium, GUGC consortium : Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat Genet 44: 904–909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorski M, Tin A, Garnaas M, McMahon GM, Chu AY, Tayo BO, Pattaro C, Teumer A, Chasman DI, Chalmers J, Hamet P, Tremblay J, Woodward M, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Smith AV, Mitchell BD, O’Connell JR, Shuldiner AR, Coresh J, Li M, Freudenberger P, Hofer E, Schmidt H, Schmidt R, Holliday EG, Mitchell P, Wang JJ, de Boer IH, Li G, Siscovick DS, Kutalik Z, Corre T, Vollenweider P, Waeber G, Gupta J, Kanetsky PA, Hwang SJ, Olden M, Yang Q, de Andrade M, Atkinson EJ, Kardia SL, Turner ST, Stafford JM, Ding J, Liu Y, Barlassina C, Cusi D, Salvi E, Staessen JA, Ridker PM, Grallert H, Meisinger C, Müller-Nurasyid M, Krämer BK, Kramer H, Rosas SE, Nolte IM, Penninx BW, Snieder H, Fabiola Del Greco M, Franke A, Nöthlings U, Lieb W, Bakker SJ, Gansevoort RT, van der Harst P, Dehghan A, Franco OH, Hofman A, Rivadeneira F, Sedaghat S, Uitterlinden AG, Coassin S, Haun M, Kollerits B, Kronenberg F, Paulweber B, Aumann N, Endlich K, Pietzner M, Völker U, Rettig R, Chouraki V, Helmer C, Lambert JC, Metzger M, Stengel B, Lehtimäki T, Lyytikäinen LP, Raitakari O, Johnson A, Parsa A, Bochud M, Heid IM, Goessling W, Köttgen A, Kao WH, Fox CS, Böger CA: Genome-wide association study of kidney function decline in individuals of European descent. Kidney Int 87: 1017–1029, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahl LK, Heine M, Tassinari L: Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature 194: 480–482, 1962 [DOI] [PubMed] [Google Scholar]
- 8.Zicha J, Dobešová Z, Vokurková M, Rauchová H, Hojná S, Kadlecová M, Behuliak M, Vaněčková I, Kuneš J: Age-dependent salt hypertension in Dahl rats: Fifty years of research. Physiol Res 61[Suppl 1]: S35–S87, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council : Salt sensitivity of blood pressure: A scientific statement from the American Heart Association. Hypertension 68: e7–e46, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Dahl LK, Heine M, Thompson K: Genetic influence of renal homografts on the blood pressure of rats from different strains. Proc Soc Exp Biol Med 140: 852–856, 1972 [DOI] [PubMed] [Google Scholar]
- 11.Cowley AW Jr, Stoll M, Greene AS, Kaldunski ML, Roman RJ, Tonellato PJ, Schork NJ, Dumas P, Jacob HJ: Genetically defined risk of salt sensitivity in an intercross of Brown Norway and Dahl S rats. Physiol Genomics 2: 107–115, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Chen PY, Sanders PW: Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension 22: 812–818, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Chen PY, St John PL, Kirk KA, Abrahamson DR, Sanders PW: Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary L-arginine supplementation. Lab Invest 68: 174–184, 1993 [PubMed] [Google Scholar]
- 14.Garrett MR, Dene H, Rapp JP: Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol 14: 1175–1187, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Garrett MR, Joe B, Yerga-Woolwine S: Genetic linkage of urinary albumin excretion in Dahl salt-sensitive rats: Influence of dietary salt and confirmation using congenic strains. Physiol Genomics 25: 39–49, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Slupsky CM, Gentile LN, Donaldson LW, Mackereth CD, Seidel JJ, Graves BJ, McIntosh LP: Structure of the Ets-1 pointed domain and mitogen-activated protein kinase phosphorylation site. Proc Natl Acad Sci USA 95: 12129–12134, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cederberg A, Hulander M, Carlsson P, Enerbäck S: The kidney-expressed winged helix transcription factor FREAC-4 is regulated by Ets-1. A possible role in kidney development. J Biol Chem 274: 165–169, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Gomez RA, Norwood VF: Recent advances in renal development. Curr Opin Pediatr 11: 135–140, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Naito T, Razzaque MS, Nazneen A, Liu D, Nihei H, Koji T, Taguchi T: Renal expression of the Ets-1 proto-oncogene during progression of rat crescentic glomerulonephritis. J Am Soc Nephrol 11: 2243–2255, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Raffetseder U, Wernert N, Ostendorf T, van Roeyen C, Rauen T, Behrens P, Floege J, Mertens PR: Mesangial cell expression of proto-oncogene Ets-1 during progression of mesangioproliferative glomerulonephritis. Kidney Int 66: 622–632, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Feng W, Chumley P, Prieto MC, Miyada K, Seth DM, Fatima H, Hua P, Rezonzew G, Sanders PW, Jaimes EA: Transcription factor avian erythroblastosis virus E26 oncogen homolog-1 is a novel mediator of renal injury in salt-sensitive hypertension. Hypertension 65: 813–820, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng W, Chumley P, Hua P, Rezonzew G, Jaimes D, Duckworth MW, Xing D, Jaimes EA: Role of the transcription factor erythroblastosis virus E26 oncogen homolog-1 (ETS-1) as mediator of the renal proinflammatory and profibrotic effects of angiotensin II. Hypertension 60: 1226–1233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P: Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest 115: 2508–2516, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemoto T, Kubota S: Ornithine decarboxylase overexpression enhances ERK and p38 phosphorylation and matrix metalloproteinase-2 expression. Cell Biol Int 31: 141–147, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Rabault B, Ghysdael J: Calcium-induced phosphorylation of ETS1 inhibits its specific DNA binding activity. J Biol Chem 269: 28143–28151, 1994 [PubMed] [Google Scholar]
- 26.Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, Hume DA, Maki RA, Ostrowski MC: Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol 16: 538–547, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yordy JS, Muise-Helmericks RC: Signal transduction and the Ets family of transcription factors. Oncogene 19: 6503–6513, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Kola I, Brookes S, Green AR, Garber R, Tymms M, Papas TS, Seth A: The Ets1 transcription factor is widely expressed during murine embryo development and is associated with mesodermal cells involved in morphogenetic processes such as organ formation. Proc Natl Acad Sci USA 90: 7588–7592, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, Lanier LL, Leiden JM: The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity 9: 555–563, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG: Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H: Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bories JC, Willerford DM, Grévin D, Davidson L, Camus A, Martin P, Stéhelin D, Alt FW: Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature 377: 635–638, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Anderson MK, Hernandez-Hoyos G, Diamond RA, Rothenberg EV: Precise developmental regulation of Ets family transcription factors during specification and commitment to the T cell lineage. Development 126: 3131–3148, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Bhat NK, Komschlies KL, Fujiwara S, Fisher RJ, Mathieson BJ, Gregorio TA, Young HA, Kasik JW, Ozato K, Papas TS: Expression of ets genes in mouse thymocyte subsets and T cells. J Immunol 142: 672–678, 1989 [PubMed] [Google Scholar]
- 35.Muthusamy N, Barton K, Leiden JM: Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature 377: 639–642, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Deshmane SL, Kremlev S, Amini S, Sawaya BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res 29: 313–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tveitarås MK, Skogstrand T, Leh S, Helle F, Iversen BM, Chatziantoniou C, Reed RK, Hultström M: Matrix metalloproteinase-2 knockout and heterozygote mice are protected from hydronephrosis and kidney fibrosis after unilateral ureteral Obstruction. PLoS One 10: e0143390, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Chen K, Lei H, Sun Z: Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26: 121–132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ: Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Drenjancevic-Peric I, Lombard JH: Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol 287: H957–H962, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Dahl M, Kasanen A, Peltonen T: The effect of Segontin, Persantin and nitroglycerin on the coronary artery. Experimental studies with cinecoronarography. Ann Med Exp Biol Fenn 40: 423–426, 1962 [PubMed] [Google Scholar]
- 42.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R: Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325: 433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng W, Ying WZ, Aaron KJ, Sanders PW: Transforming growth factor-β mediates endothelial dysfunction in rats during high salt intake. Am J Physiol Renal Physiol 309: F1018–F1025, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying WZ, Allen CE, Curtis LM, Aaron KJ, Sanders PW: Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J Clin Invest 122: 1777–1785, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ying WZ, Sanders PW: Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-beta1. Am J Physiol 275: F18–F24, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Sanders PW, Gibbs CL, Akhi KM, MacMillan-Crow LA, Zinn KR, Chen YF, Young CJ, Thompson JA: Increased dietary salt accelerates chronic allograft nephropathy in rats. Kidney Int 59: 1149–1157, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Raij L, Azar S, Keane W: Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.