Abstract
Purpose of review
Non-alcoholic fatty liver disease (NAFLD) is a liver disease with high prevalence in western countries. Progression from NAFLD to non-alcoholic steatohepatitis (NASH) occurs in 10–20%. NASH pathogenesis is multifactorial including genetic and environmental factors. The gut microbiota is involved in disease progression and its role is complex.
Recent findings
NASH is associated with changes in the intestinal microbiota, although findings in recent studies are inconsistent. Dysbiosis can trigger intestinal inflammation and impair the gut barrier. Microbial products can now reach the liver, induce hepatic inflammation and contribute to NAFLD and NASH progression. As the gut microbiota is also involved in the regulation of metabolic pathways, metabolomic approaches identified unique metabolomic profiles in patients with NASH. Altered metabolite patterns can serve as biomarkers, while specific metabolites (such as ethanol) have been linked with disease progression. Modifying metabolic profiles might serve as new microbiome-based approaches.
Summary
In this review, we will highlight findings from the recent literature important to the gut-liver axis. We will predominantly focus on human studies with NASH.
Keywords: intestinal microbiome, metabolites, bacterial translocation, dysbiosis, intestinal inflammation
Introduction
The intestine harbors a large quantity of microbes including bacteria, archaea, viruses and fungi. It is estimated that the numbers of bacteria in the human body equals the number of human cells [1]. Hepatologists have appreciated the existence of a gut – liver axis for quite some time. Reports in the 1950’s showed that non-absorbable antibiotics prevent cirrhosis in an animal model of NASH [2]. More direct evidence for the contribution of intestinal bacteria to progression of liver disease was observed with the advent of next generation sequencing technologies for microbiota analysis and with new experimental approaches. Such novel approaches included the use of germfree mice that were resistant to high fat diet-induced obesity and hepatic steatosis [3]. Seminal findings showed that the obesity phenotype is transmissible. Germfree mice transplanted with feces from an obese donor accumulated more fat as compared to germfree mice receiving feces from a lean donor [4]. Similarly, microbial transfer can result in development of exacerbated NASH in mice [5]. In recent years, compositional changes in the intestinal microbiota have been proposed to mechanistically contribute to obesity, NAFLD and NASH. Mechanisms include increased energy harvest by the microbiota from an obese individual, higher short chain fatty acid production, dysbiosis-induced intestinal inflammation and gut barrier dysfunction, regulation of appetite and affecting the host immune system [6, 7]. Some of these changes appear to be relevant for mice, but not for humans [8].
This review summarizes ties between intestinal microbiota changes and development of NASH that have been discovered over the last two years. We will predominantly focus on NASH rather than on obesity and NAFLD. We will also preferentially focus on human studies, although mechanistic studies require rodent experiments.
1. Intestinal microbiota changes in patients with NASH
Various liver diseases including NASH are associated with intestinal dysbiosis [9]. Gut microbiota affects digestion and absorption of nutrients, the host immune system and the production of gut hormones [10]. Human microbiota studies in NASH are sparse with only very few reports demonstrating an association between gut dysbiosis and NASH. In a recent study, two genera, Bacteroides and Prevotella were significantly different in fecal samples of NASH patients compared to healthy controls [11]. Whereas Bacteroides had a higher abundance and was independently associated with NASH, proportions of Prevotella were lower in NASH patients. Bacteroides and Prevotella act as competitors and have an inverse relationship [12]. Dietary composition can influence this balance and western diets rich in fat, animal proteins and sugar favor Bacteroides [13] and have been associated with NASH [14]. Besides diet, several other factors could explain increased proportions of Bacteroides in NASH. Abundance of Bacteroides correlated with increased levels of oligosaccharides (that contain glucose and fructose), D-pinitol, deoxycholic acid and decreased levels of short chain fatty acids (SCFA) and amino acids [15]. Whereas e.g. deoxycholic acid can induce apoptosis in rat livers [16] and is found in higher levels in human livers with NASH [17], fructose promotes liver inflammation and fibrosis [18].
A subanalysis looking at microbiota changes associated with fibrosis severity demonstrated an independent association of Ruminococcus abundance with fibrosis F≥2. Ruminococci populations can be affected by diet [19]. Since the Rumiococcus genus is very heterogeneous including both, beneficial and deleterious bacteria, a mechanism linking Ruminococcus abundance with fibrosis is not yet clear and requires further studies. In contrast to the above cited report in adults with NASH, two recent studies in children with NASH demonstrated different results [20, 21]. In contrast to the adult samples detecting differences only at the genera level, one study demonstrated differences at the phylum, family and genera level in fecal samples of children with NASH. Proportions of Proteobacteria/Enterobacteriacieae/Escherichia were higher in pediatric NASH patients compared to healthy controls and obese patients [21]. Other genera that showed significant differences between healthy controls and pediatric NASH patients included decreased levels of Alistipes, Blautia, Coprococcus, Eubacterium, Oscillospira and Bifidobacterium. In contrast to adult NASH patients that showed decreased levels of Prevotella, samples from children with NASH demonstrated a significant increase in Prevotella [21]. Another study using pediatric NASH patients found decreased levels of Oscillispira and, in line with the study using adult patients, increased levels of Ruminococcus. Other genera that were significantly different in pediatric NASH patients versus healthy controls included increased levels of Dorea and Blautia [20]. Differences in these various studies may in part be explained by the small sample size, differences in age (adult versus children), diet and diagnostic criteria.
Patients with NAFLD and NASH do not only show compositional changes in the gut microbiota, but also bacterial overgrowth in the small intestine. The prevalence of small intestinal bacterial overgrowth is 56% in patients with NAFLD, which is increased when compared with healthy controls (21%). However, there was no significant correlation between small intestinal bacterial overgrowth and presence of NASH, lobular inflammation, or fibrosis score within the NAFLD patient cohort [22]. Using cultures of duodenal aspirates, small intestinal bacterial overgrowth (defined as colony count above 105 cfu/ml) was present in 38% of patients with NAFLD [23]. Patients with small intestinal bacterial overgrowth had significantly higher endotoxin levels, but there was again no association with NASH [23]. A recent preclinical trial emphasized the importance of bacterial overgrowth mediated by a prolonged orofecal transit time in patients with NAFLD and NASH. The promotility agent mosapride improved NASH in mice [24]. Surprisingly, numbers of fecal bacteria were not reduced with mosapride treatment, but compositional changes were observed. This was associated with increased systemic glucagon-like peptide 1 (GLP1) levels, reduced colonic inflammation and lower serum endotoxin levels [24].
2. How does the intestinal microbiota contribute to NASH?
2.1. Intestinal inflammation and gut barrier dysfunction
Several studies have linked gut barrier dysfunction to increased bacterial translocation and hepatic inflammation. One proposed mechanism involves an increased susceptibility to intestinal permeability in patients with NASH [25]. As a consequence, serum endotoxin levels were significantly higher and may be responsible for liver injury in these patients. Presumably, lipopolysaccharide (LPS or endotoxin), derived from the gut microflora translocates via a dysfunctional gut barrier to the portal vein and liver, thereby inducing an inflammatory response through activation of inflammatory cells in the liver. As a result, mice deficient in Toll-like receptor (TLR)-4 and myeloid-differentiation factor-2 (MD2) are protected from methionine-choline deficient diet induced liver inflammation and fat deposition [26]. Other microbial products might also cause a progression of liver disease. Plasma from mice and patients with NASH contain high levels of mitochondrial DNA, a potent TLR9 activator [27]. A complete deletion of TLR9 and mice deficient in TLR9 on lysosome-producing cells protects from high fat diet-induced hepatic steatosis and inflammation. Furthermore, a TLR9 antagonist blocked the development of NASH when given prophylactically and therapeutically in mice [27]. Exposure of microbial products to the liver is not necessarily detrimental; some studies also demonstrate a beneficial role of bacterial products. TLR5 recognizes bacterial flagellin. Mice lacking TLR5 on hepatocytes showed exacerbated disease upon methionine-, choline-deficient diet and high fat diet. TLR5 expressed on hepatocytes plays therefore an important role in the protection of the liver against diet-induced liver disease [28] (Table 1).
Table 1.
Summary of key pathways involving the gut-liver axis important for NASH
| Receptor | Type of deficiency | Mechanism | Model used | Effect | Ref |
|---|---|---|---|---|---|
| Pattern recognition receptors | |||||
| TLR4 | Global deficiency | TLR4/MD2 mediated signals contribute to liver pathology via NADPH-dependent lipid-peroxidation and oxidative stress | MCD diet | Detrimental | [26] |
| TLR5 | TLR5 deficiency in hepatocytes | Loss of hepatocyte TLR5 potentiates high-fat diet induced pro-inflammatory gene expression via Nod-like receptor C4 inflammasome | MCD diet; HFD | Protective | [28] |
| TLR9 | TLR9 deficiency in lysosome producing cells (neutrophils and KCs) | Pro-inflammatory response via TLR9 activation | HFD | Detrimental | [27] |
| Bile acid receptors | |||||
| FXR | Intestine specific deficiency | Reduced triglyceride accumulation due to low ceramide synthesis genes[29]; Gut-restricted FXR activation reduces diet-induced weight gain, inflammation, hepatic glucose production and enhances thermogenesis and browning of white adipose tissue [30] | HFD | Controversial | [29, 30] |
| TGR5 | Global deficiency | Antilipogenic effects of intestinal FXR agonist are TGR5 dependent | HFD | Part of the protective effect of the intestinal specific FXR agonist is reduced in TGR5 deficient mice | [30] |
HFD, high-fat diet; TLR, Toll-like Receptor; MCD, methionine and choline deficient diet; NASH, nonalcoholic steatohepatitis; FXR, farnesoid X receptor: TGR, Takeda-G-protein coupled receptor 5; KC, Kupffer cell;
It is not clear, however, if altered permeability is cause or consequence of endotoxin exposure. A recent study, using mice deficient in the tight junction molecule Junctional adhesion molecule (JAM)-A, showed increased intestinal permeability and bacterial translocation to the liver driving hepatic inflammation and NASH. Interestingly, administration of antibiotics abrogated hepatic inflammation and development of NASH. This clearly demonstrates a role for microbiota in driving hepatic inflammation. Using colonic biopsies from patients with NASH the authors further showed decreased levels of JAM-A and increased mucosal inflammation in the colon. Genetic disposition leading to defects in the integrity of the epithelial barrier may therefore predispose patients to hepatic inflammation and NASH progression [31].
Besides genetic predisposition, the inciting event responsible for increased intestinal permeability has not been clearly identified. Intestinal inflammation and the resulting production of several cytokines could play an underlying role in permeability changes [32, 33]. Recent evidence suggests diet-induced obesity as a trigger of intestinal inflammation and insulin resistance [34]. A pro-inflammatory shift in gut immune cell populations was demonstrated in mice upon high fat diet feeding and obese humans. Treatment with 5-aminosalicylic acid (5-ASA) that acts as a gut anti-inflammatory agent reduced bowel inflammation and insulin resistance in mice. Interestingly, 5-ASA treatment also significantly improved intestinal permeability and reduced liver steatosis [34] further providing evidence for a link between intestinal inflammation, changes in gut permeability and progression of liver disease. Increased intestinal inflammation is also present in patients with NAFLD as exemplified by intestinal tight junction disruption, changes in immune cell populations and increased intestinal cytokine levels [35].
2.2. Bacterial metabolites
As diagnosis of NASH requires a liver biopsy, extensive efforts have been made to find non-invasive, sensitive methods to detect early stages of disease and to stage progressive NASH. Bacterial metabolites might serve as biomarkers, but they might also be linked mechanistically to NASH progression. A recent study using pediatric NASH patients demonstrated a unique profile with increased levels of fecal 2-butanone and 4-methyl-2-pentanone. Together with the observed metagenomics data (lower levels of Oscillospira and high abundances of Blautia, Dorea and Ruminococcus), this unique signature could serve as potential biomarker [20].
Another important metabolite that has been associated with progression of NAFLD is ethanol. Both, pediatric NASH [21] and NAFLD [35] patients exhibited significantly increased blood ethanol levels. Of note, serum ethanol levels in pediatric NASH patients (average ~0.00016 g/dL, calculated based on Fig. 4 [21]) were 1000 times lower than plasma ethanol levels in children with NAFLD (average ~0.115g/dL, calculated based on Fig. 1 [35]). While such low ethanol levels are likely not important contributors to liver disease progression, reported levels in NAFLD are above the legal limit for driving in the US. Technical problems in measuring blood ethanol levels might account for these differences. Different mechanisms have been proposed for elevated blood ethanol levels. One hypothesis to explain the differences in blood ethanol is the increased abundance of ethanol producing bacteria that have been detected in NASH microbiomes [21]. In contrast, another study suggests that alterations in insulin signaling followed by decreased ADH activity in the liver are responsible for an impaired ethanol metabolism [35]. Future studies are required to determine the role of ethanol for progression of NAFLD and NASH.
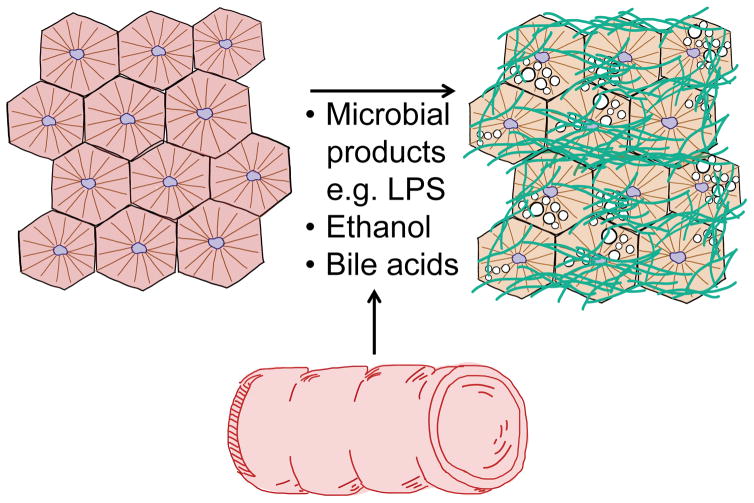
Figure 1. Gut-derived products that contribute to NASH progression.
Intestinal products reaching the liver can have multi-faceted effects on liver physiology. Increased levels of microbial products, ethanol and an altered bile acid profile have been detected in patients with NASH.
2.3. Bile acids
Bile acids are derived from cholesterol and synthesized in the liver through a series of reactions involving cytochrome P450 (CYP) enzymes. After these oxidative processes, bile acids are conjugated with the amino acids taurine or glycine and secreted into the intestine after a meal. In the intestine they have major functions in lipid solubilization and digestion. The majority (90%) of luminal conjugated primary bile acids are actively reabsorbed in the terminal ileum. The remaining luminal bile acids are deconjugated and dehydroxylated by the intestinal microbiota to unconjugated secondary bile acids, which are passively reabsorbed and return to the liver via the portal vein. Bile acids can have direct effects on intestinal microbiota by causing membrane disruption through their amphipathic-detergent like nature. It is therefore not surprising that patients with NASH have changes in their bile acid profiles. Patients with non-cirrhotic NASH have higher total serum bile acid concentrations, in particular increased taurine- and glycine-conjugated primary and secondary bile acids than healthy volunteers [36]. Total unconjugated, primary unconjugated bile acids and tauro-conjugated lithocholic acid were higher in feces of patients with NASH [37]. In addition to their digestive functions, they regulate their own synthesis by stimulating the nuclear receptor farnesoid x receptor (FXR) in ileal enterocytes and releasing fibroblast growth factor (FGF)-19 into the portal circulation. FGF19 reaches hepatocytes and suppresses the rate-limiting enzyme in the bile acid synthesis pathway, CYP7A1 [38]. Serum FGF19 was not different between NASH patients and healthy controls [37] indicating that changes in intestinal bile acid composition did not alter FXR activity in the ileum. Despite unchanged FGF19 levels, serum C4 (a bile acid intermediate and indicator of de novo biosynthesis of bile acids in the liver) was elevated in NASH compared with healthy controls [37] indicating that increased bile acid synthesis is unlikely driven by intestinal dysbiosis (Table 1).
Bile acids are ligands not only for the nuclear receptor FXR, but also for several other receptors including the cell membrane G-protein-coupled bile acid receptor 1 (GPBAR1; also called TGR5 for Takeda G-protein-coupled receptor 5). Bile acids are important regulators of glucose and lipid metabolism, thermogenesis and inflammation (recent reviews [38, 39]). The bile acid derivative 6-ethylchenodeoxycholic acid (obeticholic acid) is a potent activator of FXR. A double-blind, placebo-controlled, randomized clinical trial showed improved histological features in patients with non-cirrhotic NASH patients with obeticholic acid given orally (25 mg daily) for 72 weeks [40]. A higher number of patients treated with obeticholic acid had improvement in fibrosis, hepatocellular ballooning, steatosis, and lobular inflammation [40]. Treatment with obeticholic acid was associated with higher concentrations of total serum cholesterol and LDL cholesterol, and a decrease in HDL cholesterol. The significance of these changes on cardiovascular outcome is not known. In addition, patients treated with obeticholic acid had more pruritus [40]. Newer, non-bile acid based, synthetics FXR agonists are currently being tested that might have fewer side effects [38]. Thus, bile acids are important communicating molecules between the liver and the intestine and can serve as target for therapy. An experimental, intestine restricted FXR agonist, fexaramine, improved hepatic steatosis in mice [30], although there is controversy about the role of intestinal FXR for lipid metabolism [29].
Conclusions and future directions
NASH is a multifactorial disease and over nutrition might be the most important target for therapy. The intestinal microbiota is one contributing pathogenic factor (Figure 1). NASH is associated with changes in the intestinal microbiota composition and metabolome, intestinal and systemic inflammatory response, and bile acid profiles. Altered microbial metabolites might serve as excellent targets for NASH therapy. However, a better characterization of the intestinal microbiota and metabolome in larger and better characterized human cohorts is required. This might identify subgroups of patients for which a tailored microbiome-based therapy might be beneficial. Precision microbiome therapy could include microbiota transplantation with more defined microbial communities, engineered bacterial strains, or drugs that target bacterial metabolic pathways. In addition, targeting the inflammatory response or the altered bile acid profile provide other novel therapeutic strategies currently under investigation.
Key Points.
NASH is associated with an altered composition of the intestinal microbiota.
Intestinal inflammation is an important cause for increased intestinal permeability.
Translocation of microbial products contributes to NASH.
Microbial metabolites are altered in humans with NASH and might serve as excellent targets for NASH therapy.
Acknowledgments
The manuscript was supported in part by NIH grants R01 AA020703, U01 AA021856, and by Award Number I01BX002213 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (to BS).
Abbreviations
- ASA
aminosalicylic acid
- cfu
colony forming unit
- CYP
cytochrome P450
- FGF
fibroblast growth factor
- FXR
farnesoid X receptor
- GPBAR1
G-protein-coupled bile acid receptor 1
- JAM
Junctional adhesion molecule
- LPS
lipopolysaccharide
- MD2
myeloid-differentiation factor-2
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- SCFA
short-chain fatty acids
- TGR5
Takeda G-protein-coupled receptor 5
- TLR
toll-like receptor
Footnotes
Conflict of interest: The author declares the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Rutenburg AM, Sonnenblick E, Koven I, et al. The role of intestinal bacteria in the development of dietary cirrhosis in rats. J Exp Med. 1957;106:1–14. doi: 10.1084/jem.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnabl B, Brenner DA. Interactions Between the Intestinal Microbiome and Liver Diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1018–G1036. doi: 10.1152/ajpgi.00245.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llorente C, Schnabl B. The gut microbiota and liver disease. Cell Mol Gastroenterol Hepatol. 2015;1:275–284. doi: 10.1016/j.jcmgh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caligiuri A, Gentilini A, Marra F. Molecular Pathogenesis of NASH. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17091575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jegatheesan P, Beutheu S, Freese K, et al. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br J Nutr. 2016;116:191–203. doi: 10.1017/S0007114516001793. [DOI] [PubMed] [Google Scholar]
- 14.Xie G, Wang X, Liu P, et al. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget. 2016;7:19355–19366. doi: 10.18632/oncotarget.8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Wu J, Li JV, et al. Gut microbiota composition modifies fecal metabolic profiles in mice. J Proteome Res. 2013;12:2987–2999. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira DM, Afonso MB, Rodrigues PM, et al. c-Jun N-terminal kinase 1/c-Jun activation of the p53/microRNA 34a/sirtuin 1 pathway contributes to apoptosis induced by deoxycholic acid in rat liver. Mol Cell Biol. 2014;34:1100–1120. doi: 10.1128/MCB.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aranha MM, Cortez-Pinto H, Costa A, et al. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur J Gastroenterol Hepatol. 2008;20:519–525. doi: 10.1097/MEG.0b013e3282f4710a. [DOI] [PubMed] [Google Scholar]
- 18.Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Chierico F, Nobili V, Vernocchi P, et al. Gut microbiota profiling of pediatric NAFLD and obese patients unveiled by an integrated meta-omics based approach. Hepatology. 2016 doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Baker SS, Gill C, et al. Characterization of the gut microbiome in non-alcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 22.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 23.Kapil S, Duseja A, Sharma BK, et al. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:213–221. doi: 10.1111/jgh.13058. [DOI] [PubMed] [Google Scholar]
- 24.Okubo H, Nakatsu Y, Sakoda H, et al. Mosapride citrate improves nonalcoholic steatohepatitis with increased fecal lactic acid bacteria and plasma glucagon-like peptide-1 level in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2015;308:G151–158. doi: 10.1152/ajpgi.00198.2014. [DOI] [PubMed] [Google Scholar]
- 25.Farhadi A, Gundlapalli S, Shaikh M, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csak T, Velayudham A, Hritz I, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Martinez I, Santoro N, Chen Y, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–864. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etienne-Mesmin L, Vijay-Kumar M, Gewirtz AT, Chassaing B. Hepatocyte Toll-Like Receptor 5 Promotes Bacterial Clearance and Protects Mice Against High-Fat Diet-Induced Liver Disease. Cell Mol Gastroenterol Hepatol. 2016;2:584–604. doi: 10.1016/j.jcmgh.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang C, Xie C, Li F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman K, Desai C, Iyer SS, et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luther J, Garber JJ, Khalili H, et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. 2015;1:222–232. doi: 10.1016/j.jcmgh.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P, Stärkel P, Turner JR, et al. Dysbiosis-induced intestinal inflammation activates TNFRI and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luck H, Tsai S, Chung J, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–542. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Engstler AJ, Aumiller T, Degen C, et al. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut. 2016;65:1564–1571. doi: 10.1136/gutjnl-2014-308379. [DOI] [PubMed] [Google Scholar]
- 36.Ferslew BC, Xie G, Johnston CK, et al. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3318–3328. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouzaki M, Wang AY, Bandsma R, et al. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11:e0151829. doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arab JP, Karpen SJ, Dawson PA, et al. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology. 2016 doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez FJ, Jiang C, Patterson AD. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology. 2016;151:845–859. doi: 10.1053/j.gastro.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]