Abstract
Ongoing clinical trials are evaluating the benefits of systemic blockade of lymphocyte activation gene-3 (LAG-3) signals to improve immunity to tumors. Those studies are founded on the well-established inhibitory role of LAG-3 in regulating CD8+ T cells during chronic virus infection and anti-tumor responses. However, the T cell response in LAG-3 deficient mice is similar in size and function to that in wild type animals, suggesting LAG-3 has nuanced immune-regulatory functions. We performed a series of adoptive transfer experiments in mice to better understand the T cell-intrinsic functions of LAG-3 in the regulation of CD8+ T cell responses. Our results indicate that LAG-3 expression by CD8+ T cells inhibits their competitive fitness and results in a slightly reduced rate of cell division in comparison to LAG-3 deficient cells. This cell-intrinsic effect of LAG-3 was consistent across both acute and chronic virus infections. These data show that LAG-3 directly modulates the size of the T cell response and support the use of LAG-3 blockade regimens to enhance CD8+ T cell responses.
Introduction
Chronic virus infections are a major health issue worldwide, with millions of people currently living with diseases caused by viruses such as HIV, HCV, and HBV. During these infections, T cell responses become functionally inactive through a process known as T cell exhaustion and fail to clear the infection (1). Exhausted CD8+ T cells show diminished cytokine production, weakened cytolytic activity, and lower antigen-induced proliferation in large part due to their co-expression of a series of surface inhibitory receptors, including programmed cell death protein 1 (PD-1), lymphocyte activation gene-3 (LAG-3, CD223), 2B4 (CD244), CD160, and T cell immunoglobulin mucin-3 (Tim-3) (2). Recent data in mouse models indicates that T cell dysfunction can be reversed by combined antibody blockade of these receptors, especially PD-1 and LAG-3, leading to improved clearance of viral and bacterial pathogens (3, 4). Similar results have also been seen following blockade of LAG-3 in tumor models, resulting in the elimination of tumors (5–7). Therefore, it is important to understand the specific effects of LAG-3 on CD8+ T cells to improve vaccines and the treatment of persistent infections and tumors.
LAG-3 is a surface receptor expressed by activated CD8+ T cells that binds to MHC-II with a higher affinity than the T cell co-receptor CD4 (8–11). LAG-3 associates with the TCR at the immunological synapse following TCR stimulation and inhibits signaling (12, 13). During an acute infection LAG-3 is expressed early upon activation, perhaps due to its rapid translocation to the surface from a recycling endosomal compartment (14). Surface expression of LAG-3 can be efficiently reduced by two transmembrane metalloproteases that cleave LAG-3 from the cell surface (2, 15). Early studies highlighted the ability of LAG-3 to act as an inhibitory receptor for CD8+ T cell expansion and homeostasis (16, 17). Further studies showed that surface LAG-3 expression is maintained for an extended period of time during persistent virus infections, typically for as long as the antigen is present (2). The inhibitory function of LAG-3 was confirmed by antibody blockade studies in which LAG-3 interference led to enhanced T cell responses and improved virus clearance (3). However, in some circumstances the absence of LAG-3 appears to have no effect on either T cell expansion or clearance of a persistent virus infection (18).
The discrepancies between these results could be partially due to the expression of LAG-3 by other cell types. Activated CD4+ T cells express LAG-3, which negatively regulates CD4+ T cell responses and homeostatic proliferation (16, 17, 19). CD4+ regulatory T cells (Treg) also express LAG-3 and lose functionality in the absence of LAG-3 (20). LAG-3 deficiency leads to altered homeostatic regulation of plasmacytoid dendritic cells (pDC), a key cell type in the production of type I interferon (21, 22). NK cells express LAG-3, and LAG-3 deficient mice have reduced NK cell activity (11, 23). There has also been a report documenting LAG-3 expression on B cells (24), though the functional significance of LAG-3 in B cell responses is not known. Thus, many cell types express LAG-3 and any or all of these cells could impact CD8+ T cell responses. Because of this, the interpretation of data involving systemic antibody-mediated blockade of LAG-3 must also take into account other LAG-3+ targets and their regulatory effects.
Herein, we addressed the cell-intrinsic role for LAG-3 expression on CD8+ T cell responses following acute and chronic virus infection. We found that LAG-3 expression by CD8+ T cells decreases the size of the virus-specific CD8+ T cell response without inducing functional changes. We used competition experiments to reveal that LAG-3 deficient T cells outcompeted their LAG-3 sufficient counterparts. Thus, LAG-3 has a cell-intrinsic negative effect on CD8+ T cell responses that results in reduced fitness.
Materials and Methods
Mice and Virus
C57BL/6 mice were purchased from Jackson ImmunoResearch Laboratory. LAG-3−/− mice were originally generated by Drs. Diane Mathis and Christophe Benoist at the Institut Génétique Biologie Moléculaire Cellulaire (Illkirch-Graffenstaden, France) and subsequently backcrossed to C57BL/6 by Dr. Dario Vignali at St. Jude Children's Research Hospital (Memphis, TN, USA) and are a gift from Dr Vignali and St. Jude Children's Hospital (16, 23, 25). P14 TCR-transgenic mice that have T cells specific for the LCMV epitope GP33–41 were crossed to C57BL/6.Thy1.1 or C57BL/6.Ly5a mice to generate P14 Thy1.1+ and P14 Ly5a+ mice. The P14 Ly5a+ mice were crossed to LAG-3−/− mice to generate the P14 LAG-3 deficient TCR-transgenic mice. Armstrong and Clone13 strains of LCMV were prepared and quantitated as described previously (26). Mice were infected by intravenous administration of 2 × 106 plaque forming units of Clone13 or intraperitoneal administration of 2 × 105 plaque forming units of Armstrong. Infectious LCMV in serum, liver, lung, and kidney was quantified by plaque assay on Vero cell monolayers (26). All mouse experiments were approved by the University of North Carolina-Chapel Hill Institutional Animal Care and Use Committee. Mice were sacrificed by cervical dislocation after anesthetization with isoflurane.
Flow cytometry
Spleen cells were stained directly ex vivo with antibodies against CD8 (53–6.7), CD44 (IM7), CD62L (MEL-14), CD127 (LG.3A10), CD160 (7H1), CD244 (m2B4(B6)458.1), KLRG-1 (2F1/KLRG1), Ly5a (A20), LAG-3 (C97BW), PD-1 (RMP1-30), and Thy1.1 (eBioscience, HIS5.1). Cells were stained for intracellular Ki-67 (16A8), Bcl2 (3F11, BD Biosciences), and Bim (C34C5, Cell Signaling Technology) following fixation and permeabilization using buffers from Biolegend. Cytokine production was assessed by the intracellular cytokine staining (ICS) assay as described previously (27). In brief, splenocytes were stimulated ex vivo with LCMV peptide GP33-41 for 5-6 hours in the presence of brefeldin A, followed by surface staining, fixation, permeabilization, and staining for IFN-γ (XMG1.2), TNF-α (MP6-XT22), IL-2 (JES6-5H4). All antibodies were from Biolegend, except where indicated. Tetramer staining was done using strepavidin-APC-labeled tetrameric-complexes of Db complexed with LCMVGP33-41 peptide; the tetramers were produced from the NIH core facility. Cell staining was analyzed by four-color flow cytometry using a BD Biosciences FACSCALIBUR and analyzed by FlowJo software (www.treestar.com).
Adoptive transfers
Spleen cells were isolated from WT or LAG-3 deficient P14 mice and the frequency of CD8+ T cells was determined by flow cytometry. The spleen cells were injected directly into WT or LAG-3 deficient mice via tail vein injection; in the dual adoptive transfer experiments the WT and LAG-3−/− spleen cells were mixed to contain equivalent numbers of CD8+ T cells prior to injection. The cells were allowed to engraft for 4 days before the recipient mice were infected. The donor cell populations were identified due to their differing expression of the Thy1.1, Thy1.2, Ly5a, or Ly5b antigens, as indicated.
Statistics
Statistical analyses were performed using Prism software (www.graphpad.com), employing an unpaired two-tailed Student's t-test to evaluate the significance of differences between groups.
Results
Antiviral CD8+ T cell responses are normal in LAG-3 deficient mice
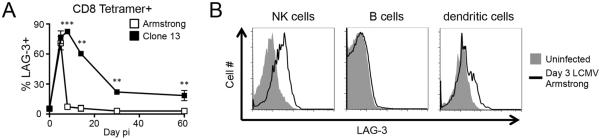
LAG-3 is a T cell activation marker whose prolonged expression on virus-specific CD8+ T cells during chronic infection identifies cells that are functionally exhausted (2). There is significant interest in LAG-3 because antibody-mediated blockade of LAG-3, particularly in combination with PD-1 blockade, restores functionality to exhausted T cells in models of chronic infection and cancer therapy (3–7). However, multiple reports have seen little effect on antiviral T cell responses in mice deficient for LAG-3, implying that the functional consequences of LAG-3 are limited in time or context (18, 23). To better understand the role of LAG3 in T cell responses, we quantified the expression of LAG-3 on LCMV-specific tetramer+ CD8 T cells in response to different variants of LCMV that result in acute (Armstrong) or persistent (Clone13) infection. LAG-3 was induced to high levels at day 5 with both strains. The percentage of LAG-3 expressing T cells returned to baseline by day 8 for Armstrong (Fig. 1A), however, following Clone13 infection, LAG-3 expression was maintained for at least 60 days on a population of LCMV-specific CD8+ T cells (Fig. 1A). The surface level of LAG-3 closely tracked with the amount of virus in the animals, consistent with previous studies (2). LAG-3 is typically regarded as a negative regulator of T cell responses, but multiple cell types can express LAG-3, including CD4+ and CD8+ T cells, Tregs, B cells, pDCs, and NK cells (11, 20, 21, 24). LAG-3 was induced on NK cells and dendritic cells early after LCMV-Armstrong infection, though expression on B cells was undetectable (Fig. 1B); LAG-3 expression on non-T cells (i.e., cells lacking CD4 & CD8) was transient, as we could not detect it during the memory phase (data not shown).
Figure 1. LAG-3 expression on CD8+ T cells, NK cells, B cells, and dendritic cells.
(A)WT B6 mice were infected with LCMV-Armstrong or LCMV-Clone13. The frequency of splenic LCMV-specific CD8+Db/GP33+ cells expressing LAG-3 at various times post-infection is shown as mean +/− SEM, n=3–6 per group over 3 experiments. (B) WT B6 mice were infected with LCMV-Armstrong. The histograms depict LAG-3 expression on NK1.1+DX5+ NK cells, CD19+ B cells, and CD11c+CD3− dendritic cells in the spleen at day 3. One mouse, representative of 3, is shown for each.
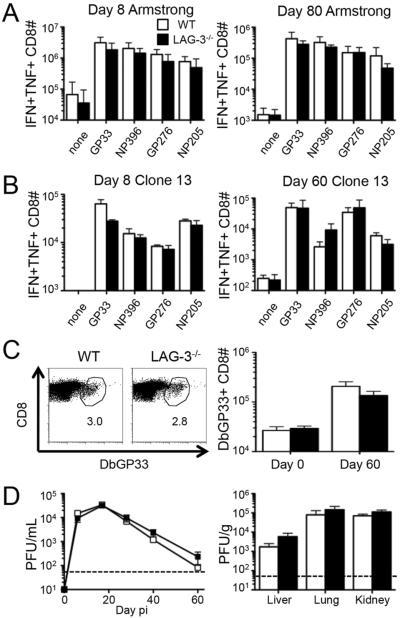
To evaluate the function of LAG-3, we measured antiviral T cell responses following LCMV infection of C57BL/6 (WT) and LAG-3 deficient (LAG3−/−) mice. In response to Armstrong infection, WT and LAG-3 deficient mice had a massive expansion of CD8+ T cells, with equivalent numbers of double positive IFN-γ / TNF-α producing cells at the peak of the primary response (day 8) (Fig. 2A). Furthermore, infectious virus was undetectable in WT and LAG-3 deficient mice, indicating efficient CTL-mediated viral clearance of the acute infection (data not shown). There was also no defect in the formation of memory CD8+ T cells, as at day 80 there was no difference in the number of LCMV-specific cytokine producing cells (Fig. 2A).
Figure 2. The complete absence of LAG-3 does not affect the number, function, or longevity of antiviral CD8+ T cell responses.
T cell responses and viral loads were measured in WT or LAG-3−/− mice following acute or chronic infection. (A) WT B6 and LAG-3−/− mice were infected with LCMV-Armstrong and the total number of splenic IFN-γ/TNF-α double positive CD8+ T cells following ex vivo stimulation with various LCMV peptides was measured by intracellular cytokine staining at days 8 (left) and 80 (right) post-infection (pi). (B–D) WT B6 and LAG-3−/− mice were infected with LCMV-Clone 13. (B) The total number of splenic IFN-γ/TNF-α double positive CD8+ T cells following ex vivo stimulation with various LCMV peptides was measured by ICS at days 8 (left) and 60 (right) pi. (C) Examples of Db/GP33 tetramer staining of CD8+ T cells in the spleens of either WT or LAG-3−/− mice at day 60 (left); the numbers indicate the percentage of tetramer+ cells among CD8+ T cells. The total number of splenic LCMV-specific CD8+Db/GP33+ cells in either WT or LAG-3−/− mice at day 60 pi (right). (D) The level of infectious virus was determined by plaque assay from serum samples over time (left) and liver, lung, and kidney tissues at day 60 (right). The dashed line represents the limit of detection. The data in panel A represent 9 mice from 3 independent experiments. All other panels represent 6 mice from 2 independent experiments.
In response to Clone13 infection, the CD8+ T cell responses at early (day 8) and late (day 60) time points were predictably reduced compared to Armstrong; however, the numbers of LCMV-specific cytokine-producing T cells were equivalent between WT and LAG-3 deficient mice (Fig. 2B). Tetramer staining also revealed equivalent numbers of virus-specific T cells in WT and LAG-3 deficient mice (Fig. 2C). Consistent with the equivalent T cell responses, there was no difference between LAG-3 sufficient and LAG-3 deficient mice in the amount of virus detected over time in the blood and at day 60 in various tissues (Fig. 2D). These data indicate that the complete loss of LAG-3 does not impact antiviral T cell responses and clearance of acute or chronic infections, confirming previous studies (18).
T cell-intrinsic LAG-3 expression inhibits the accumulation and maintenance of CD8+ T cells
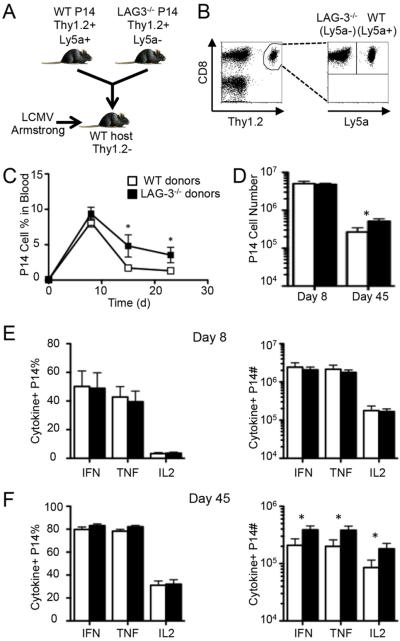
We considered that LAG-3 could affect T cell responses through direct or indirect mechanisms. To determine if there is a negative role for LAG-3 that is intrinsic to CD8+ T cells, we crossed the LCMVGP33-41-specific TCR-transgenic P14 mice onto the LAG-3 knockout background to generate LAG-3 deficient CD8+ T cells that specifically recognize LCMV. These LAG-3−/− P14 cells were adoptively transferred in conjunction with equal numbers of WT P14 cells into congenic WT mice prior to Armstrong infection (Fig. 3, A & B). This dual-adoptive transfer approach allows us to directly compare responses between WT and LAG-3−/− T cells within identical inflammatory environments. In uninfected mice there was a 1:1 ratio between the two cell types, signifying that there was no difference in engraftment or homeostatic proliferation (Supplemental Fig. S1A–C). Additionally, there was no difference in the in vitro proliferative ability of the WT and LAG-3 deficient P14 cells to GP33-41 peptide (Supplemental Fig. S1D). At day 8 post infection there was a massive expansion of both WT and LAG-3−/− T cells with little difference in the WT:KO ratio or the total number of each population (Fig. 3, C and D). However, by day 15 there was an increase in the frequency and total number of LAG-3 deficient P14 cells (Fig. 3C). The difference between WT and LAG-3−/− P14 cells that was observed in the blood as early as day 15 was maintained in the spleen through day 45 (Fig. 3D). These data show that the primary expansion of CD8+ T cells during an acute infection is independent of LAG-3, but the formation or maintenance of memory cells is inhibited by LAG-3 in a cell-intrinsic manner.
Figure 3. Direct LAG-3 signaling reduces the abundance of memory CD8+ T cells following acute viral infection.
A dual adoptive transfer approach was used to directly compare the effect of LAG-3 expression on CD8+ T cells following acute infection. (A) A mix containing 2×103 Thy1.2+Ly5a+ WT P14 cells and 2×103 Thy1.2+Ly5a− LAG-3−/− P14 cells mice was adoptively transferred into Thy1.1+ B6.PL mice; 4 days later the mice were infected with LCMV-Armstrong. (B) An example of the gating strategy used to identify the WT and LAG-3−/− P14 cells. After gating on CD8+Thy1.2+ cells, the transferred cells were distinguished by their expression of Ly5a. (C) The frequency of each population of P14 cells among blood leukocytes in mice that were bled repeatedly following infection. (D) The total number of splenic WT and LAG-3−/− P14 cells at days 8 and 45 pi. (E–F) The frequency (left) and total number (right) of cytokine+ splenic WT and LAG-3−/− P14 cells at day 8 (E) and day 40 (F) as measured by ICS after ex vivo stimulation with GP33-41 peptide. The day 8 data represent 5 mice from 2 independent experiments while the day 40 data represent 9 mice from 3 independent experiments.
To determine the functional consequences of the absence of LAG-3 on CD8+ T cells, we measured cytokine production by the WT and LAG-3−/− P14 cells at day 8 and 45 post infection. At day 8 equal percentages of WT and LAG-3−/− cells produced IFN-γ, TNF-α, and IL-2, resulting in equivalent numbers of cytokine-positive cells (Fig. 3E). There was also no difference in the percentage of cells making each cytokine at day 45, but the total number of LAG-3−/− cytokine-positive cells was increased because there were more LAG-3−/− cells in these animals (Fig. 3F). Taken together, these data indicate that LAG-3 reduces the total number of memory CD8+ T cells but does not perturb memory cell expression of cytokines.
Because T cell expression of LAG-3 led to fewer memory cells, we considered that LAG-3 might affect memory precursor cells that develop early during an acute infection. CD8+ T cells can be divided into memory-precursor effector cell (MPEC) and short-lived effector cell (SLEC) subsets (28, 29). These subsets are distinguished by their expression of KLRG-1 and IL7Ra. At day 8, a majority of the WT and LAG-3 knockout P14 cells expressed KLRG-1 and not IL-7Ra (SLEC), while at day 45 similar proportions of cells expressed IL-7Ra and KLRG-1 (MPEC) (Supplemental Fig. S2). The cells uniformly expressed the activation marker CD44 at both time points, while CD62L was higher at day 45 compared to day 8 (Supplemental Fig. S2). Interestingly, the frequencies of P14 cells expressing these markers were not changed by the absence of LAG-3 (Supplemental Fig. S2). These data suggest that LAG-3 on T cells does not shift the differentiation of CD8+ T cells towards the MPEC subset.
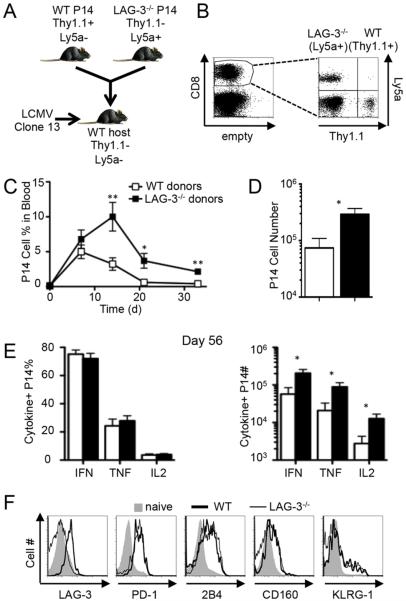
Since transient LAG-3 expression after acute infection had long-term negative effects on the number of memory CD8+ T cells, we next assessed whether sustained LAG-3 expression during persistent infection would reduce T cell responses (2). We followed the same procedure as above by adoptively transferring congenically marked WT and LAG-3−/− P14 cells into WT B6 recipient mice (Fig. 4, A and B). The frequency of each cell type was followed in the blood over time after infection with the Clone13 strain of LCMV. At day 7 the WT and LAG-3−/− P14 cells were found at equivalent frequencies, indicating that the initial expansion is independent of LAG-3 expression (Fig. 4C). At later time-points the LAG-3 deficient cells were much more abundant than their WT counterparts in the blood (Fig. 4C). This culminated in a 5-fold increase in the number of LAG-3−/− splenic P14 cells at day 56 when compared to the number of WT cells (Fig. 4D). Therefore, intrinsic LAG-3 signaling restricts the magnitude of the CD8+ T cell response during persistent and acute viral infections.
Figure 4. Direct LAG-3 signaling inhibits the long-term maintenance of virus-specific CD8+ T cells during chronic infection.
As illustrated in (A), 2×103 Thy1.1+Ly5a− WT P14 cells and 2×103 Thy1.1−Ly5a+ LAG-3−/− P14 cells were co-adoptively transferred into WT B6 mice (Thy1.1−Ly5a−); 4 days later the mice were infected with LCMV-Clone 13 and T cell responses were measured at multiple times after infection. (B) An example of the gating strategy used to identify the transferred cells. After gating on CD8+ cells, the WT and LAG-3−/− P14 cells were distinguished by their expression of Thy1.1 and Ly5a, respectively. (C) The frequency of each population of P14 cells among blood leukocytes in mice that were bled repeatedly following infection. (D) The total number of splenic WT and LAG-3−/− P14 cells at day 56 pi. (E) The frequency (left) and total number (right) of cytokine+ splenic WT and LAG-3−/− P14 cells at day 56, as measured by ICS following GP33-41 peptide stimulation. (F) Examples of surface staining for LAG-3, PD-1, 2B4, CD160, and KLRG-1 on splenic WT (thick line) and LAG-3−/− (thin line) P14 cells and naive CD8+ T cells (shaded) at day 56. These data represent 6 mice from 2 independent experiments.
We measured the production of cytokines and surface expression of exhaustion and activation markers to determine if T cell function is altered by LAG-3 signaling. The frequency of P14 cells that produced IFN-γ, TNF-α, and IL-2 was equivalent between WT and LAG-3−/− cells, though the total numbers of LAG-3−/− cytokine-positive cells were higher than WT due to the increased number of virus-specific LAG-3−/− cells (Fig. 4E). None of the cell surface proteins that correlate with functional exhaustion (PD-1, 2B4, and CD160) or T cell activation (KLRG-1) were differentially expressed by WT and LAG-3 knockout CD8+ T cells (Fig. 4F). Thus, the absence of LAG-3 on T cells results in more virus-specific CD8+ T cells but does not prevent CD8+ T cell exhaustion during disseminated infection.
LAG-3 deficient CD8+ T cells outcompete cells that express LAG-3
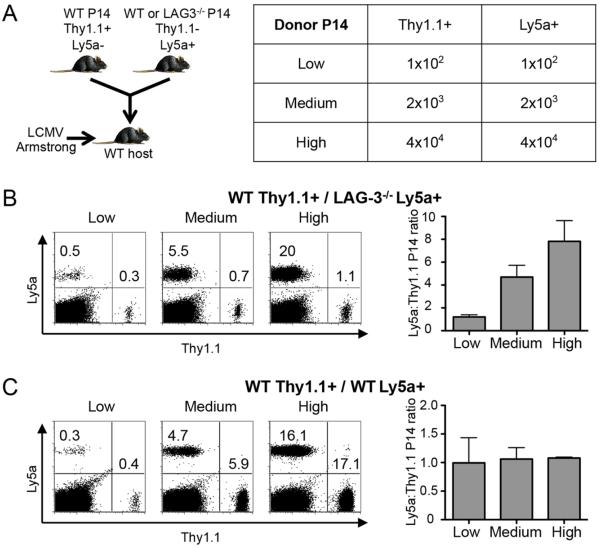
T cells compete for limited resources (antigen, cytokines, etc.) as they respond to infection (30, 31). We hypothesized that the LAG-3−/− P14 cells better competed for these resources than WT P14 cells and were better able to accumulate after infection. To address this possibility we performed the adoptive transfer approach with different numbers of transferred cells. At low cell transfer numbers there should be ample resources for all cells (limited competition), while increasing the number of transferred cells should limit the available resources and reveal whether LAG3−/− cells have a competitive advantage over WT cells. Thus, we co-transferred 102, 2×103, or 4×104 each of WT and LAG3−/− P14 cells to WT host mice and infected with Armstrong (Fig. 5A). At the lowest cell transfer dose, there was equivalent expansion of WT and LAG-3 deficient cells, with a 1:1 ratio between the two at day 16 (Fig. 5B). However, the LAG-3−/−:WT cell ratio increased to 4:1 at the medium cell dose and 8:1 at the highest transfer dose (Fig. 5B). Therefore, as the number of transferred cells increased, the competitive advantage of the LAG-3−/− P14 cells became more apparent. The difference between WT and LAG-3 deficient cells was not due to selective rejection of the WT cells, as we performed a similar experiment with two separate populations of congenically marked WT cells (Fig. 5C). As expected, the WT:WT cell ratio was 1:1 at all cell transfer doses, indicating that when there is no competitive advantage of one population over the other, the donor cells expand equivalently (Fig. 5C). In summary, these data indicate that LAG-3 deficient CD8+ T cells are able to outcompete WT CD8+ T cells following acute infection.
Figure 5. LAG-3 deficient CD8+ T cells outcompete WT CD8+ T cells.
As illustrated in (A), 1×102, 2×103, or 4×104 Thy1.1+Ly5a− WT P14 cells with 1×102, 2×103, or 4×104 Thy1.1−Ly5a+ LAG-3−/− (in B) or control WT (in C) P14 cells mice were co-transferred into WT B6 mice (Thy1.1−Ly5a−); 4 days later the mice were infected with LCMV-Armstrong and T cell responses were measured at day 16 post infection. (B) Examples of staining for Ly5a and Thy1.1 on gated CD8+ T cells (left) and the ratio of Ly5a+:Thy1.1+ (LAG-3−/−:WT) P14 cells (right) in the spleen. (C) Examples of staining for Ly5a and Thy1.1 on gated CD8+ T cells (left) and the ratio of Ly5a+:Thy1.1+ (WT:WT) P14 cells (right) in the spleen. These data depict 3 mice from 1 experiment.
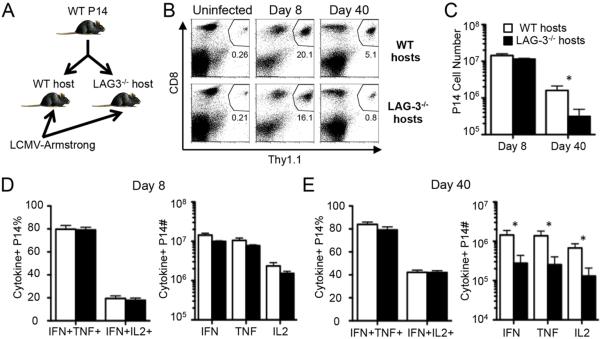
To further explore the role of competition between WT and LAG-3−/− CD8+ T cells during antiviral immune responses, we adoptively transferred WT LCMVGP33-41-specific TCR-transgenic P14 cells into WT or LAG-3−/− host mice (Fig. 6A). Thus, the same LAG-3 sufficient CD8+ T cells are compared in mice that have competing populations of endogenous WT or LAG-3 deficient CD8+ T cells. Following Armstrong infection, the P14 cells expanded equivalently in the WT and LAG-3 deficient hosts at day 8 (Fig. 6, B and C). In contrast, there was a significant decrease in the frequency of memory P14 cells in the LAG-3−/− mice at day 40 (Fig. 6, B and C). To determine the functional impact of competition with endogenous T cells, we measured cytokine production by the WT P14 cells after their expansion in WT and LAG-3 deficient environments. The percentages of P14 CD8 T cells producing IFN-γ & TNF or IFN-γ & IL-2 were equivalent between the WT and LAG-3−/− hosts at day 8 and 40 (Fig. 6, D and E). However, there was a significant decrease in the overall number of cytokine-producing P14 cells at day 40 when the cells were in the LAG-3 deficient hosts (Fig. 6E). These data further show that WT CD8+ T cells are outcompeted by LAG-3 deficient T cells without changing the functional capacity of the cells.
Figure 6. WT CD8+ T cells establish lower levels of memory in the presence of endogenous LAG-3−/− CD8+ T cells.
Cell transfer experiments were performed to determine whether LAG-3 expression by the host affects T cell responses to acute infection. (A) 2×104 Thy1.1+ WT P14 cells mice were adoptively transferred into Thy1.1− WT B6 or Thy1.1− LAG-3−/− mice; 4 days later the mice were infected with LCMV-Armstrong. (B) Examples of staining for the CD8+Thy1.1+ WT P14 cells in the spleens of WT or LAG-3−/− host mice at days 0 (uninfected), 8, and 40 after infection; the numbers indicate the percentage of P14 cells among all spleen cells. (C) The total number of splenic WT P14 cells in either WT or LAG-3−/− host mice at days 8 and 40 pi. (D–E) The frequency (left) and total number (right) of cytokine+ splenic WT P14 cells in either WT or LAG-3−/− host mice at day 8 (D) and day 40 (E) as measured by ICS after ex vivo stimulation with GP33-41 peptide. These data represent 5-6 mice from 2 independent experiments.
Minor influence of LAG-3 on CD8+ T cell division and apoptosis
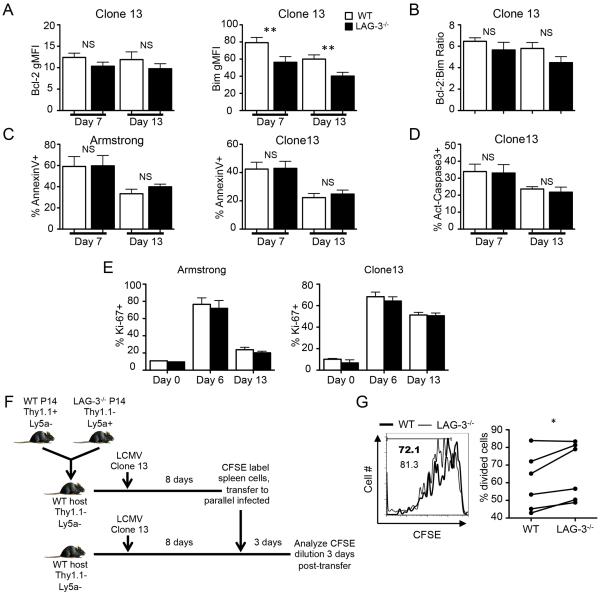
The competitive advantage of the LAG-3−/− T cell response is likely due to either enhanced proliferation or reduced apoptosis. The apoptosis of naïve and activated T cells is tightly regulated by proteins of the Bcl-2 superfamily (32–34). We measured the expression of the anti-apoptotic family member Bcl-2 and the pro-apoptotic family member Bim in WT and LAG-3 deficient P14 cells at day 7 and 13 post-infection. The level of Bim was significantly lower in the absence of LAG-3; this coincided with a slightly, but not statistically significant, lower level of Bcl-2 in LAG-3−/− CD8+ T cells (Fig. 7A). The ratio between Bim and Bcl-2 can be used to evaluate whether T cells will likely undergo apoptosis (32). There was a trend towards a higher Bim:Bcl-2 ratio in the WT P14 cells, suggesting that WT cells are more prone to apoptosis than LAG-3−/− cells, but the difference in the ratio was not statistically significant (Fig. 7B).
Figure 7. LAG-3 expression has a minor impact on cell proliferation and apoptotic pathways.
2×103 Thy1.1+Ly5a− WT P14 cells and 2×103 Thy1.1−Ly5a+ LAG-3−/− P14 cells mice were adoptively transferred into the same WT B6 mice (Thy1.1−Ly5a−); 4 days later the mice were infected with LCMV-Armstrong or Clone13. (A) The gMFI of Bcl-2 and Bim on WT and LAG-3−/− P14 cells at days 7 and 13 during Clone13 infection. (B) The Bim:Bcl-2 ratio within each cell type at day 13 of Clone13 infection. (C and D) Splenocytes were isolated on days 7 or 13 and cultured in vitro for 6 hours. The frequency of AnnexinV+ (C) and activated Caspase 3 (D) P14 cells after the in vitro culture. (E) The frequency of Ki-67+ splenic WT and LAG-3−/− P14 cells at various times after infection. (F) Illustration showing the design of the in vivo proliferation assay. At 8 days post-infection, CD8+ T cells were isolated by MACS negative selection, CFSE labeled, and re-transferred to day 8 Clone13 infected mice that had not previously received P14 cells; CFSE dilution was analyzed 3 days later. (G) Examples of CFSE dilution by WT and LAG3−/− P14 cells (left) and the total frequency of cells that had divided (right), with the lines indicating the paired analyses. The data in panels A and B represent 11 mice from 4 independent experiments. The data in panel C, D, and G represent 6 mice from 2 independent experiments. The data in panel E represent 9 mice from 3 independent experiments.
For a functional readout of the effects of LAG-3 on T cell survival, we cultured WT and LAG-3−/− P14 cells ex vivo and stained for surface Annexin-V, which is indicative of cells undergoing apoptosis. On day 7 or 13 post-Armstrong or -Clone13 infection, WT and LAG-3−/− T cells showed no difference in Annexin-V binding (Fig. 7C). Additionally, the percentage of T cells expressing activated Caspase 3, a key protein in the apoptotic cascade, was not different for WT and LAG-3 deficient P14 cells during Clone13 infection (Fig. 7D). These data indicate that despite lower levels of the pro-apoptotic protein Bim, the increased expansion of LAG-3−/− CD8+ T cells does not appear to be due to enhanced survival.
Next we stained WT and LAG-3−/− P14 cells with Ki-67, which specifically detects dividing cells (35), to determine if LAG-3 signaling impacts cell division. Following Armstrong infection, 70–80% of P14 cells were Ki-67 positive at day 6 when there is replicating virus, but by day 13, when the virus was eliminated, the frequency of dividing cells declined to ~20% (Fig. 7E). In response to Clone13, 60–70% of WT and LAG-3−/− P14 cells proliferated at day 6 and 50% of them continued to divide at day 13 (Fig. 7E), likely due to the prolonged virus infection. In all cases, there was no difference in the frequency of Ki-67-positive cells between the WT and LAG-3 deficient populations (Fig. 7E). These data indicate that the frequency of WT and LAG3−/− T cells that are within the cell cycle is not changed; however Ki-67 staining does not provide a sensitive analysis of how rapidly cells divide. To more definitively measure the rate of proliferation, we performed a cell transfer experiment using CFSE dilution as a measurement of cell division (Fig. 7F). WT and LAG-3−/− P14 cells were co-transferred to WT B6 mice and infected with Clone13. At day 8 post-infection splenic CD8+ T cell were labeled with CFSE and re-transferred to new WT B6 mice that had been infected in parallel; CFSE dilution was measured 3 days later. There was a slightly higher frequency of divided LAG-3 deficient P14 cells compared to WT P14 cells in the same host (Fig. 7G). In 5 of the 6 recipient mice there was a greater percentage of divided LAG3−/− CD8+ T cells in comparison to the WT cells. These data suggest that the LAG-3 deficient T cells have a slightly higher rate of proliferation than WT cells.
Discussion
The data presented here show that LAG-3 directly inhibits the accumulation but not function of virus-specific T cells following acute or chronic infections. We find that when LAG-3−/− CD8+ T cells are compared “side-to-side” with LAG3-sufficient T cells, the LAG-3 deficient T cells outcompete WT T cells as they establish memory to acute infection. LAG-3 deficient CD8+ T cells also are sustained at higher levels than WT T cells during chronic virus infection. Thus, LAG-3 regulates CD8+ T cell responses through a cell-intrinsic manner. Our data support a model wherein virus-specific T cell numbers are constrained by available resources (antigen, cytokines, etc.) and inhibited by LAG-3.
That LAG-3 reduces the abundance of virus-specific T cells after infection has not been noted before, most likely because LAG-3 deficient mice generate T cell responses that are equivalent to those in infected WT mice (Figure 2; 18). These findings have partly contributed to the perspective that reversal of T cell exhaustion should rely on simultaneous blockade of LAG-3 and PD-1. The competitive advantage of the LAG-3 deficient T cells is apparent only when WT and LAG-3−/− CD8+ T cells are compared in the same animals. Thus, when LAG-3 deficient mice are infected, T cells within that mouse compete vigorously against each other, yielding a response that is equivalent to that seen in LAG-3 sufficient mice, whose T cells develop under less stringent conditions. The lack of additional inflammatory resources in the LAG-3 knockout mice prevents the T cell response in these animals from being any larger, regardless of the enhanced fitness of the T cells.
The data in Figure 6 show that WT memory is reduced when WT cells are in a LAG-3−/− recipient. We interpret this as being due to strong competition from endogenous LAG-3−/− CD8+ T cells in the recipients. Nevertheless, there are other possible explanations for a CD8+ T cell-extrinsic effect of LAG-3 that promotes T cell responses. Multiple cell types can express LAG-3, including pDCs, Tregs, and NK cells (Figure 1; 11, 20, (21, 22). For example, it could be that LAG-3 deficient Treg cells are more suppressive than those in WT mice, though previous studies in other models have shown that LAG-3 deficient Treg cells have reduced suppressive activity (20). Another possibility is that cDCs, which we show in Figure 1 express low levels of LAG-3 in infected mice, could be important in this process. It is plausible that DC-intrinsic LAG-3 signaling improves APC function and T cell responses; thus, cDCs in LAG-3 deficient mice could be weaker at stimulating T cells. In addition to intrinsic LAG-3 signaling, cDCs also express MHC-II, which interacts with LAG-3. It has been noted that a LAG-3-Ig fusion protein enhances cDC activity by binding to MHC-II (15, 36, 37), and surface-bound LAG-3 on human T cells activates cDCs in an in vitro culture system (38). IMP321, a soluble fusion protein consisting of LAG-3, has been evaluated for its ability to stimulate cDCs and improve T cell responses to several tumors. These studies raise the possibility that WT CD8+ T cells poorly establish memory in the absence of LAG-3 expression on non-CD8+ T cells.
Figure 1 shows that LAG-3 on T cells is dynamic and varies with infection. LAG-3 is transient during acute infection but sustained during chronic infection. Correspondingly, the LAG-3 KO memory T cells are roughly 2-fold increased over WT T cells after acute infection (Figure 3C–D) but 3-4-fold increased during chronic infection (Figure 4C–D). Thus the strength of the response is linked to the duration of LAG3-expression and inhibition of WT T cells. Interestingly, when LAG-3 is expressed, T cells show a distribution of expression with some cells LAG-3hi and others LAG-3lo. Given the direct inhibitory effect of LAG-3, it may be that the LAG-3lo T cells better survive to memory whereas the LAG-3hi T cells are selected out of memory, thus impacting immunodominance hierarchies. It is noteworthy that the expression of LAG-3 was brief following acute infection, yet the effect on T cell number manifested during the contraction phase and on into memory. This implies that early LAG-3 signaling during the expansion phase programs T cell contraction at a later time when LAG-3 expression has ceased. These data suggest that pharmacological compounds that interfere with this pathway early on, such as during vaccination, might improve vaccine-induced T cell memory.
As T cell numbers increase (and intraclonal competition becomes more severe), the advantage of not expressing LAG-3 for generating large populations of virus-specific T cells becomes more apparent (Figure 5A–B). We are unsure how LAG-3 deficiency improves the ability of T cells to establish greater numbers of memory cells. It could be that enhanced T cell competition for cytokines gives LAG-3−/− T cells an advantage, however, we did not detect differences in the expression levels of IL-2, IL-7, and IL-15 cytokine receptors (Supplemental Fig. S2 & data not shown) that promote memory T cell maintenance. Perhaps LAG-3-MHC-II interactions impact the movement of CD8+ T cells within lymphoid environments and affect their ability to access these cytokines without varying the expression level of the cytokine receptors.
We considered that LAG-3 signals affect either T cell apoptosis or proliferation. We found that direct LAG-3 signaling had no effect on Ki-67 staining and a marginal negative effect on CD8+ T cell proliferation (Fig. 7). These data are consistent with results showing that LAG-3 blockade during chronic LCMV infection enhances cell division (3). Additionally, one study used antibodies to cross-link LAG-3, and another study used metalloprotease inhibitors to prevent LAG-3 cleavage from cells; the resulting data were consistent with LAG-3 signals decreasing cellular proliferation (12, 15). We considered that LAG-3 may promote T cell apoptosis. The expression level of the pro-apoptotic protein Bim was significantly higher in LAG-3-expressing T cells (Supplemental Figure S3). Though we did not identify changes in apoptosis after culturing the cells in vitro, the in vivo survival of T cells is likely subject to requirements that are not replicated in the in vitro experiments. The enhanced Bim levels in WT cells may reflect greater apoptosis in vivo. While the differences in apoptosis potential or cell proliferation appear minor, slight changes in cell division that are compounded across many cycles can exponentially increase overall responses.
Interference with LAG-3 and PD-1 improves tumor-infiltrating lymphocyte responses to tumors (6, 39). New clinical trials are evaluating the safety and efficacy of anti-LAG-3 to improve immune responses to chronic lymphocytic leukemia, Hodgkin lymphoma, non-Hodgkin lymphoma, and solid tumors (NCT01968109; NCT02061761) (40). Our results show that LAG-3 expression by T cells restricts the competitive fitness of antigen-specific CD8+ T cells and directly reduces their number during acute and chronic virus infections. These data support the use of LAG-3 antibody-blockade as a method of enhancing T cell responses.
Supplementary Material
2Abbreviations
- ICS
intracellular cytokine staining
- LAG-3
lymphocyte activation antigen-3
- LCMV
lymphocytic choriomeningitis virus
- PFU
plaque forming unit
- pi
post-infection
- pDC
plasmacytoid dendritic cell
- Treg
regulatory T cell
Footnotes
This research was supported in part by funds from NIH grants R01-AI074862, R56-AI110682, and R21-AI117575 to J.K.W. Additional support included start-up funds from The University of North Carolina at Chapel Hill.
References
- 1.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol. 1994;24:3216–3221. doi: 10.1002/eji.1830241246. [DOI] [PubMed] [Google Scholar]
- 9.Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–337. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, Maigret B, Dreano M, Triebel F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U S A. 1997;94:5744–5749. doi: 10.1073/pnas.94.11.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 13.Hannier S, Triebel F. The MHC class II ligand lymphocyte activation gene-3 is co-distributed with CD8 and CD3-TCR molecules after their engagement by mAb or peptide-MHC class I complexes. Int Immunol. 1999;11:1745–1752. doi: 10.1093/intimm/11.11.1745. [DOI] [PubMed] [Google Scholar]
- 14.Woo SR, Li N, Bruno TC, Forbes K, Brown S, Workman C, Drake CG, Vignali DA. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. Eur J Immunol. 2010;40:1768–1777. doi: 10.1002/eji.200939874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, Hartmann D, Black RA, Rossi JJ, Blobel CP, Dempsey PJ, Workman CJ, Vignali DA. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007;26:494–504. doi: 10.1038/sj.emboj.7601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 17.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 18.Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2009;22:13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- 19.Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33:970–979. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 20.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nature immunology. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki T, Dierich A, Benoist C, Mathis D. Independent modes of natural killing distinguished in mice lacking Lag3. Science. 1996;272:405–408. doi: 10.1126/science.272.5260.405. [DOI] [PubMed] [Google Scholar]
- 24.Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005;35:2081–2088. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- 25.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32:2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook KD, Whitmire JK. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol. 2013;190:641–649. doi: 10.4049/jimmunol.1202448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitmire JK, Benning N, Eam B, Whitton JL. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J Immunol. 2008;180:6777–6785. doi: 10.4049/jimmunol.180.10.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grayson JM, Weant AE, Holbrook BC, Hildeman D. Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J Virol. 2006;80:8627–8638. doi: 10.1128/JVI.00855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, Hildeman DA. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol. 2011;186:5729–5737. doi: 10.4049/jimmunol.1100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 36.Andreae S, Piras F, Burdin N, Triebel F. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223) J Immunol. 2002;168:3874–3880. doi: 10.4049/jimmunol.168.8.3874. [DOI] [PubMed] [Google Scholar]
- 37.Avice MN, Sarfati M, Triebel F, Delespesse G, Demeure CE. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-alpha and IL-12 production by monocytes and dendritic cells. J Immunol. 1999;162:2748–2753. [PubMed] [Google Scholar]
- 38.Casati C, Camisaschi C, Novellino L, Mazzocchi A, Triebel F, Rivoltini L, Parmiani G, Castelli C. Human lymphocyte activation gene-3 molecules expressed by activated T cells deliver costimulation signal for dendritic cell activation. J Immunol. 2008;180:3782–3788. doi: 10.4049/jimmunol.180.6.3782. [DOI] [PubMed] [Google Scholar]
- 39.Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, Elliott S, Gill D, Marlton P, Seymour J, Khanna R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108:2280–2289. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 40.Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer. 2014;85:101–109. doi: 10.1016/j.lungcan.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.