Abstract
Detergents are amphiphilic molecules widely used to solubilize biological membranes and/or extract their components. Nevertheless, because of the complex composition of biomembranes, their solubilization by detergents has not been systematically studied. In this review, we address the solubilization of erythrocytes, which provide a relatively simple, robust and easy to handle biomembrane, and of biomimetic models, to stress the role of the lipid composition on the solubilization process. First, results of a systematic study on the solubilization of human erythrocyte membranes by different series of non-ionic (Triton, CxEy, Brij, Renex, Tween), anionic (bile salts) and zwitterionic (ASB, CHAPS) detergents are shown. Such quantitative approach allowed us to propose Re sat—the effective detergent/lipid molar ratio in the membrane for the onset of hemolysis as a new parameter to classify the solubilization efficiency of detergents. Second, detergent-resistant membranes (DRMs) obtained as a result of the partial solubilization of erythrocytes by TX-100, C12E8 and Brij detergents are examined. DRMs were characterized by their cholesterol, sphingolipid and specific proteins content, as well as lipid packing. Finally, lipid bilayers of tuned lipid composition forming liposomes were used to investigate the solubilization process of membranes of different compositions/phases induced by Triton X-100. Optical microscopy of giant unilamellar vesicles revealed that pure phospholipid membranes are fully solubilized, whereas the presence of cholesterol renders the mixture partially or even fully insoluble, depending on the composition. Additionally, Triton X-100 induced phase separation in raft-like mixtures, and selective solubilization of the fluid phase only.
Keywords: Solubilization, Detergents, Model/biological membranes, Giant unilamellar vesicles, Phase separation, Erythrocyte membrane domains
Introduction
Detergents, beyond their physiological roles (e.g., pulmonary and tear film surfactants, bile salts in the digestive system), have a wide range of applications in biochemistry, including cell rupture, DNA and protein extraction, protein reconstitution, solubilization of fatty materials, etc (Jones 1999; Seddon et al. 2004; Linke 2009). Detergents are amphiphilic molecules and, therefore, are able to intercalate in the membrane, rearrange membrane components, and ultimately extract lipids and proteins into micellar structures. Different mechanisms have been proposed to explain the membrane solubilization process by detergent, but all of them can be explained as a bilayer-to-micelle transition. Membrane solubilization can be described as a three-step process: (1) intercalation of the detergent in the membrane—at low detergent:lipid molar ratios—until a limiting saturation, Re sat is reached; (2) coexistence between detergent-containing (mixed) vesicles and lipid-containing (mixed) micelles, since lipids are extracted from the bilayer by already formed micelles; and (3) complete bilayer solubilization (at a particular detergent/lipid ratio, Re sol), leading to a decrease in particle size, as the detergent concentration increases further (Helenius and Simons 1975; Lasch 1995; Lichtenberg et al. 2000; Le Maire et al. 2000). Biophysicists are particularly interested in the physicochemical and molecular aspects of detergent–membrane interaction, such as the interplay between bilayer and micellar structure as a function of lipid/detergent molar ratio (e.g., Kragh-Hansen et al. 1998; Heerklotz and Seelig 2000; Preté et al. 2011; Lichtenberg et al. 2013).
Because of the complex nature of biomembranes, their solubilization by detergents has not been systematically studied. In this review, we will discuss detergent–membrane interactions based on different approaches. On the one hand, the solubilization of erythrocytes, a relatively simple, robust and easy to handle biological membrane, will be addressed. On the other hand, studies with biomimetic models will be discussed to help clarify the role of lipid composition on the solubilization process.
In the first part of this review, we will address quantitative aspects of the hemolytic effects promoted by detergents from seven different series (Fig. 1), as well as the structures formed during the bilayer–micelle transition.
Fig. 1.
Chemical Structures of detergents
However, the solubilization of biological membranes by detergents is not always complete, giving rise to insoluble fragments rich in sphingolipids and cholesterol (Brown and Rose 1992). Such detergent-resistant (DRM) fractions, due to similarities of composition and characteristics, have been associated with lipid rafts and the liquid-ordered (Lo) phase of model membranes (Sonino and Prinetti 2013).
Yet, in the fluid mosaic model (Singer and Nicolson 1972), lipids were viewed as the simple structural components of biological membranes, with minor functional importance. However, the contemporary view of cell membranes includes lateral heterogeneity, which is created by lipids organization into functional domains rich in sphingolipids and cholesterol (Simons and Ikonen 1997; Goñi 2014). These highly dynamic domains, also called lipid rafts, with sizes in the nanometer order are thought to be involved in membrane phenomena such as signal transduction, parasite infection, and intracellular trafficking of lipids and protein (Simons and Toomre 2000; Simons and Ehehalt 2002; Regen 2002; Kusumi et al. 2011). The rafts theory appeared in the nineties and became as popular as controversial (Munro 2003; Lichtenberg et al. 2005; Hancock 2006; Levental and Veatch 2016) by the way it addresses the well-known and biophysically-based concept of lipid heterogeneity. Also, the study of rafts in natural biological membranes has been a challenge due to the complexity of the system composition and dynamic characteristic. Despite that, the physicochemical nature of rafts is closely related to those found in DRMs (Brown and Rose 1992; Heerklotz 2002; Sot et al. 2002). And although DRMs cannot be directly associated with lipid rafts (Lichtenberg et al. 2005), because they are detergent- and protocol-dependent, they can provide interesting information about the lateral inhomogeneity of membranes. Then, in the second part of this review, the composition and organization of detergent-resistant membrane fractions obtained as result of a partial solubilization of human erythrocyte membranes by TX-100, C12E8 and Brij detergents will be addressed. Different from the literature, such DRMs were obtained not only at low temperature (4 °C) but also in physiological condition (37 °C).
Finally, considering that the solubilization process of lipid membranes by detergents is largely influenced by the membrane composition and phase, in the last part, we will focus on the effect of Triton X-100 on membranes composed of lipid mixtures of different complexity. Such vesicles of tuned lipid composition provide a very robust biomimetic model to help unravel mechanistic details of intricate processes involving biological membranes. Therefore, the use of detergents as a tool to explore phase separation in both biological and mimetic membranes will be discussed. We hope this review can provide new insights into the understanding of organization and architecture of biological membranes.
Quantitative assessment of biomembranes solubilization
Erythrocytes are good models for studying the interaction between amphiphiles and membranes, and providing information about their partitioning and solubilization effect on lipid and protein components, enzyme activity, etc. The information obtained from the perturbation and disruption of erythrocyte membranes is especially important when the amphiphiles under study have their site of action at the membrane level, as for local anesthetics, phenothiazines and detergents (Schreier et al. 2000).
Hypotonic hemolysis: biphasic (protective vs. lytic) effect of detergents
Amphiphiles can stabilize erythrocytes against hypotonic hemolysis at low concentration (Seeman 1966, 1972; Duwe and Sackmann 1990; Hägerstrand and Isomaa 1991), while at higher concentration (normally at/above their cmc), they solubilize the membrane. The biphasic hemolytic effect is easily observed under hypotonic condition, being directly related to the partition of monomers into the bilayer (Isomaa et al. 1986; Miseta et al. 1995; Malheiros et al. 2000).
As expected, the length/size of the hydrophobic portion of the detergent plays an important role in membrane interaction, but there is no simple relationship between the protective (or antihemolytic) activity and the alkyl chain length within a detergent series (Hägerstrand and Isomaa 1991; Miseta et al. 1995). For example, in his earlier studies on hypotonic condition, Seeman has shown that the concentration of lipid-soluble molecules and amphiphiles in the membrane phase for the maximum protection (Cprot) is about the same, irrespective of their different oil/water partition coefficients (Seeman 1972). However, in series of C10-C16 derivatives of the ionic detergents alkyl sulfates, alkyltrimethylammonium bromides and alkyldimethylammonium propanesulfonates (Isomaa et al. 1986) and C10-C16 analogs of non-ionic detergents (octa-ethyleneglycol-alkyl ethers) (Isomaa and Hägerstrand 1988), all the compounds showed anti-hemolytic effect, as seen by the inorganic phosphate release from human erythrocytes, with lower Cprot values for the C16 analogs and higher concentrations for the shorter chain ones.
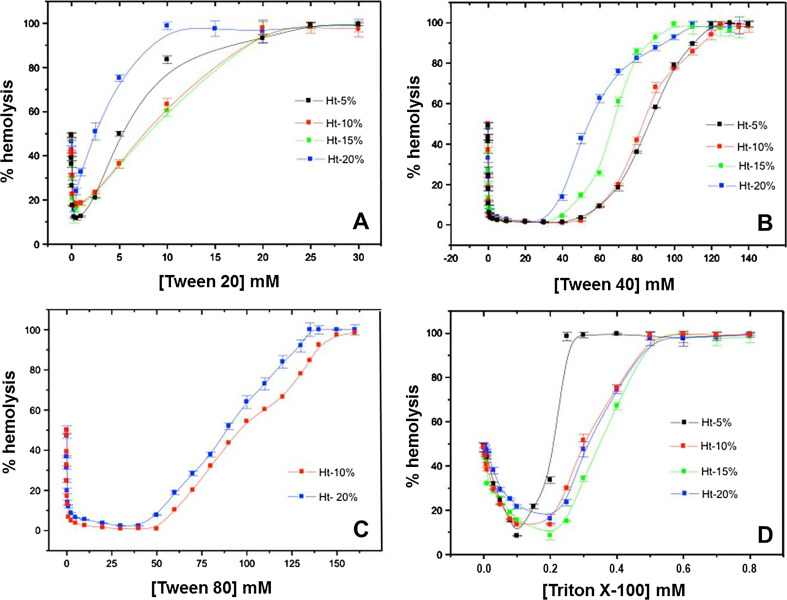
Figure 2 shows the protective effect (inward curve) induced by detergents from the Tween series (Fig. 1) and Triton X-100 (TX-100) against hypotonic lysis of human erythrocytes at different hematocrits (Ht), while at higher concentrations, their action is lytic (upward curves). Typically, the maximal protective concentration (Cprot) values are lower than the cmc of the detergents, as for TX-100 (Cprot = 0.2 mM; Fig. 2) and C12E8 (Isomaa and Hägerstrand 1988), i.e., membrane stabilization being caused by the monomeric detergent form. However, in the case of Tween detergents, with three polyethylene heads and one single acyl chain (hydrophilic-lipophilic balance, HLB > 15), Cprot values are two orders of magnitudes higher than their cmc, ratifying their weak membrane interaction (Fig. 2). Moreover, the increased hydrophobic character of Tween 80 over Tween 40 and Tween 20 (lower HLB and cmc values; see Table 1) was not enough to guarantee a strong partition into the erythrocyte membrane; on the contrary, Cprot increased in the order Tween 20 < Tween 40 < Tween 80, in agreement with the decreasing binding constants of these analogs (see Table 1). The non-lytic profile of Tween 80 explains its use (but not Tween 20) as the adjuvant of choice for the solubilization of natural products in pharmacy, or lyophilized protein formulations (Passot et al. 2005 and data mining from patient information leaflets), reflecting its low affinity for biomembranes.
Fig. 2.
Protective effect of Tween 20 (a), Tween 40 (b), Tween 80 and TX-100 (d) against hypotonic hemolysis. Human erythrocytes (Ht = 5–20%) in 5 mM PBS buffer (75 mM NaCl), pH 7.4 were incubated in the presence of detergents for 30 min, at 37 °C prior to monitoring hemoglobin release
Table 1.
Classification of detergents from different series, taking into account the binding constant (K) and critical micelle concentration (cmc) (Heerklotz and Seelig 2000) and Re sat
| Detergent | HLBa | cmc (mM)b | K (M)c | K × cmc | Classificationd | Re sat | Classificatione |
|---|---|---|---|---|---|---|---|
| C10E8 | 14.5 | 0.970 | 2400 | 2.3 | Weak | 2.23 | Weak |
| C12E8 | 13.7 | 0.088 | 6000 | 0.5 | Strong | 0.21 | Strong |
| C14E8 | 13.0 | 0.008 | 25,500 | 0.2 | Strong | 0.13 | Strong |
| C16E8 | 12.4 | 0.00073 | 31,500 | 0.0 | Strong | 0.05 | Strong |
| C18E8 | 11.9 | 0.000059 | 47,700 | 0.0 | Strong | 0.03 | Strong |
| C12E4 | 9.8 | 0.065 | 11,600 | 0.8 | Strong | 0.42 | Strong |
| C12E5 | 10.9 | 0.062 | 11,400 | 0.7 | Strong | 0.44 | Strong |
| C12E6 | 11.8 | 0.067 | 10,700 | 0.7 | Strong | 0.43 | Strong |
| C12E10 | 14.1 | 0.09 | 20,400 | 1.8 | Weak | 0.39 | Strong |
| Brij 58 | 15.7 | 0.077 | 23,957 | 1.8 | Weak | 0.37 | Strong |
| Brij 97 | 12.4 | 0.022 | 65,660 | 1.4 | Weak | 0.13 | Strong |
| Brij 98 | 15.3 | 0.025 | 22,302 | 0.5 | Strong | 0.18 | Strong |
| C | 1.6 | 6.5 | 630 | 4.1 | Weak | 44.2 | Weak |
| DC | 1.6 | 2.5 | 1310 | 3.3 | Weak | 14.3 | Weak |
| UD | 0.8 | 7.0 | nd | - | Weak | ||
| LC | 1.7 | <0.03f | 69,310 | 2.1 | Weak | 1.60 | Weak |
| GC | 4.4 | 7.0 | nd | - | Weak | ||
| GD | 3.8 | 1.1 | 1560 | 1.7 | Weak | 14.3 | Weak |
| TC | 5.8 | 4.5 | nd | - | Weak | ||
| TD | 5.4 | 1.5 | 650 | 1.0 | mild | 22.3 | Weak |
| Renex 60 | 10.9 | <0.04g | 13,300 | 0.5 | Strong | 0.37 | Strong |
| Renex 95 | 13.0 | 0.078–0.092 | 7700 | 0.7 | Strong | 0.42 | Strong |
| Renex 100 | 13.3 | 0.075–0.09 | 11,400 | 0.9 | Strong | 0.36 | Strong |
| Renex 150 | 15.0 | 0.12 | 23,400 | 2.8 | Weak | 1.15 | Weak |
| Tween 20 | 16.7 | 0.059 | 522 | 0.03 | Strong | 33.4 | Weak |
| Tween 40 | 15.6 | 0.027 | 16.4 | 0.0 | Strong | 1170 | Weak |
| Tween 80 | 15.0 | 0.012 | 9.0 | 0.0 | Strong | 2350 | Weak |
| Triton X-100 | 13.5 | 0.25 | 5900 | 1.5 | Weak | 1.60 | Weak |
| ASB 14 | 11.6 | 0.11 | 7047 | 0.8 | Strong | 0.22 | Strong |
| ASB 16 | 10.9 | 0.014 | 15,609 | 0.2 | Strong | 0.08 | Strong |
| CHAPS | 8.2 | 6–10 | 236 | 1.9 | Weak | 40.6 | Weak |
nd not determined
aCalculated as described in Griffin (1949)
bFrom the literature (Isomaa and Hägerstrand 1988; Heerklotz and Seelig 2000; Hait and Moulik 2001; Preté et al. 2002b)
c Values determined from the hemolytic curves (e.g. Fig. 2)
dAccording to K.cmc parameter (Heerklotz and Seelig 2000) e
According to Re sat
fRoda et al. 1983
gCampos et al. 2012
As for the Triton series, similar protective effects under hyposmotic condition were observed for TX-45, TX-100, TX-102 and TX-114 (with the same terc-octyl-phenoxy tail), but TX-45, with the shortest polar headgroup (4–5 PEG units), exhibited no hemolysis at all (Tragner and Csordas 1987).
Studying the bile salts, Hägerstrand and Isomaa (1991) reported that the presence of an OH group in the hydrophobic polycyclic nucleus of cholate (C) determined a considerable decrease in its anti-hemolytic effect in comparison to deoxycholate (DC). In fact, our experiments with a series of bile salts and human erythrocytes at hyposmotic condition confirmed that the Cprot ratio between pairs of oxy-deoxy analogs, C/DC (3), GC/GD (7) and TC/TD (2), was always remarkable (unpublished results).
Isosmotic hemolysis and parameters to describe detergent solubilization power
Although hemolytic assays have been widely used to access the cytotoxicity of xenobiotics (Gandhi and Cherian 2000; Youssef et al. 2004), the lack of a standard agreed methodology is the rule. In 1985, Lichtenberg proposed a quantitative approach to analyze the lipid-bilayer disruption induced by detergents (Lichtenberg 1985). The approach was further employed to describe detergent-induced hemolysis by the group of Goñi (Partearroyo et al. 1992, 1996; Requero et al. 1995) and ours (Malheiros et al. 2000; Preté et al. 2002a, b; Domingues et al. 2008).
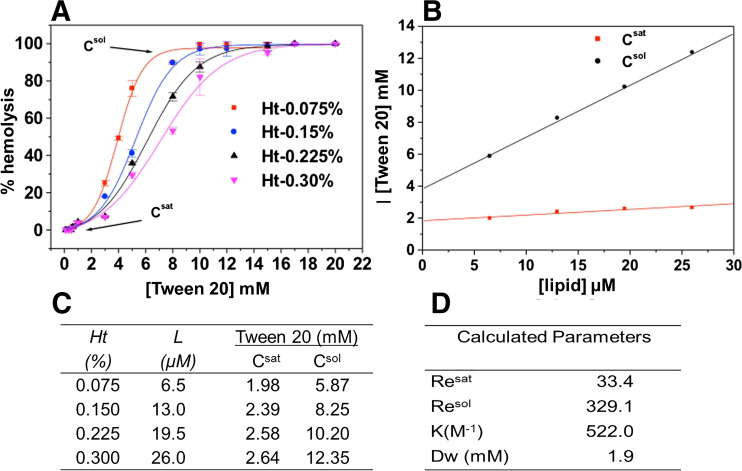
First of all, the hemolytic curves at increasing detergent concentrations are determined with at least four different erythrocyte suspensions, as shown in Fig. 3 for Tween 20. The easiest way to monitor erythrocyte membrane disruption is through quantification of the amount of hemoglobin released in the supernatant after incubation of the red cells with the detergents; the data are expressed in percent hemolysis, as previously described (Preté et al. 2002a, b). Then, the values of Csat and Csol (indicated in Fig. 3a), denoting the detergent concentration required for membrane saturation (onset of hemolysis) and complete membrane solubilization (100% lysis), are determined for each erythrocyte suspension.
Fig. 3.
Hemolytic effect elicited by Tween 20 on human erythrocytes under isotonic condition: a Hemoglobin-release curves determined at Ht = 0.075, (■). 0.15% (●). 0.225% (▴) and 0.30% (▾), in 5 mM PBS buffer, pH 7.4, 37 °C; b Tween 20 concentration (Csat, Csol) for the onset (saturation) and total solubilization vs membrane lipid concentration (L); c Csat and Csol values, determined from the hemolytic curves in (a); d effective detergent/lipid ratios for the onset (Re sat) and 100% solubilization (Re sol), binding constant (K) and concentration of free detergent in water (Dw)
After that, plots of Csat and Csol as a function of membrane lipid concentration (Fig. 3b) are used to determine the effective detergent/lipid molar ratio, both for the onset (Re sat) and completion (Re sol) of hemolysis (Fig. 3d), as described by Eqs. 1 and 2 (Partearroyo et al. 1992; Requero et al. 1995):
| 1 |
where Dt is the total surfactant concentration at the onset/complete solubilization (Csat, Csol) and L refers to the lipid concentration in the membrane (Malheiros et al. 2000). Re values and Dw, the free-surfactant concentration in water, were calculated from the slope and y-intercept of the resulting straight lines, respectively. K, the detergent–erythrocyte molar binding constant, can be derived from the values of Re sat and Dw sat, according to Eq. (2): R e = K . D W/(1 − K . D w) (2)
Considering the bilayer-to-micelle transition that occurs during membrane solubilization, Re sat and Re sol set the coexistence limit for mixed-membranes and mixed-micelles, respectively. The Re sat and Re sol values determined from the curves for Tween 20 were 33.4 and 329.1, respectively, while the binding constant (K) to the erythrocytes was 522 M−1 (Fig. 3d). The Re values higher than unit confirm that Tween 20 is not a stronger solubilizer when compared to other polyoxyethylene ether detergents such as C12E8 of equivalent acyl chain (Re sat = 0.21) or TX-100 (Re sat = 1.58). Accordingly, the binding constant of Tween 20 is not as high as that of C12E8 and TX-100 (~ 6000 M−1) (Preté et al. 2002a, b).
Table 1 summarizes the quantitative parameters calculated from hemolytic curves such as those in Fig. 3 where K and Re sat values obtained for different detergent series can be seen, including Tween. The smaller the Re sat value found, the higher the detergent capacity to solubilize the erythrocyte membranes. For erythrocyte membranes, the differences in Re sat and Re sol values observed within a homolog series of CxE8 polyoxyethylene alkyl ethers detergents have been reported (Preté et al. 2002b), showing that the effective detergent/lipid ratio decreased with the longer hydrocarbon chains analogs. The small Re values reported indicate that the solubilization process is strongly dependent on hydrophobic interactions between the acyl chains of the detergents and the erythrocyte membrane components. Moreover, as for any physicochemical determination, Re values depend on factors such as the composition of the aqueous phase and temperature (Lichtenberg et al. 2005).
The contribution of the polar head for membrane solubilization is clearly seen in the Brij and Tween series (Table 1). Brij 97 and Brij 98 have the same (oleic acid) hydrophobic tail but Brij 98 has twice the PEG units of Brij 97 (10), which decreases its membrane binding and Re value. Additionally, taking the data for the Tween series in Table 1, the Re sat values are considerably higher than those reported for the CXE8 series (Preté et al. 2002b), probably reflecting their greater HLB, as discussed before. Nevertheless, the profile of lytic effect and binding constant: Tween 20 > Tween 40 > Tween 80, agreed with the results obtained at hypotonic condition (Fig. 2), revealing that the misbalance (3 PEG crowns for a single acyl chain) restrain the partition of the polyoxyethylene sorbates into the membrane, determining a weak solubilizing performance even for long-chain analogs.
Data in Table 1 also uncover the correlation between the solubilization and the aggregative (cmc values) properties of the detergents. Although the listed cmc were taken from the literature (for the sake of discussion of the K.cmc parameter proposed by Heerklotz and Seelig 2000, see below), it is worth noticing that an estimative of the cmc of the detergents can also be obtained from the quantitative hemolytic assays, such as those in Fig. 3. Dw, the concentrations of free detergent in water taken from the intercept of the straight curves (Eq. 1), are related but always smaller than the cmc values (Lichtenberg 1985), once the membrane lipids offer an additional driving force for detergent aggregation, decreasing the concentration for micelle formation (Domingues et al. 2008). For instance, the Dw value found for TX-100 (51 μM, Preté et al. 2002a) is, as expected, smaller than its cmc (Table 1).
According to Heerklotz and Seelig (2000), the membrane disruption potency of detergents can be measured by the relationship between their cmc and binding constants, whereby strong detergents (K.cmc <1) are able to solubilize lipid membranes at low detergent-to-lipid molar ratios. For instance, over palmitoyl phosphatidylcholine (POPC) vesicles, TX-100 was found to be a strong (K.cmc = 0.7), while zwitterionic bile-salt analog CHAPS was a weak (K.cmc = 6) detergent. Thus, by applying the K.cmc product (Heerklotz and Seelig 2000) to classify detergents with respect to their membrane disruption potency, all the studied Tweens would be assigned as strong detergents (Table 1, antepenultimate column), which is certainly not the case.
We here propose that the values of Re sat (Table 1) determined from the quantitative hemolytic experiments provide an alternative parameter for the determination of the strength of the detergents, as follows: Re sat < 1 for weak and Re sat > 1 for strong solubilizers. Table 1 confronts K.cmc versus Re sat classification for 31 detergents from seven different families of non-ionic (alkyl and aryl polyoxyethylene ethers: CxEy/Brij, Triton, Renex and Tween), anionic (bile salts) and zwitterionic (ASB, CHAPS). Resat values allowed us to confirm the categorization of at least two agents: Lithocholate and Renex 60, to which the K.cmc classification is doubtful since their cmc values have not so far been precisely determined (Schönfeldt 1969; Roda et al. 1983; Campos et al. 2012). The majority of the detergents achieved the same classification from both parameters (K.cmc and Re sat), including the non-hemolytic detergents, e.g., UD, GC, TC in Table 1, plus C12E2 (HLB 6.5) and Renex 230 (Galembeck et al. 1998), for which the lack of Re sat or K values put them among the weakest membrane solubilizer detergents. However, different classifications were obtained for the Tween series, C12E10, Brij 57 and 97 and TD, (as pointed out above), probably because of inaccurate cmc values.
Yet the great advantage of using quantitative hemolysis instead of K.cmc is that one single parameter, the effective detergent/lipid ratio in the membrane (Re sat values), is taken in exactly the same experimental condition, avoiding errors related to determination of cmc and binding constants (e.g., methodology, temperature, water phase composition). Then, Re sat values appear as a precise and easy parameter to determine the strength of solubilization of detergents, mainly for those which whose cmc values are extremely low and difficult to determine, not allowing for K compensation, and exposing the limitation of the K.cmc parameter. Of course, Re sat values can change from membrane to membrane (depending on their composition, amount of cholesterol, etc.) as well as the detergent binding constant. But, for a specific membrane, regardless of its complexity (e.g., erythrocyte membranes), Re sat is a useful tool to determine the strength of solubilization of non-ionic and ionic detergents.
Among the studied families of detergents, Re sat values smaller than unit were found for Renex analogs of small polar head groups (< 10 PEG units), and all those with acyl chains longer than 12 carbons, reflecting their strong solubilization power. Re sat values higher than 1 were detected for bile salts, CxEy of short acyl chain, Tweens (with a sorbitol ring and 3 PEG crowns), Renex of big polar heads (> 15 PEG) and TX-100, classifying them as weak solubilizers, also in accordance with the K.cmc parameter (Table 1).
The quantitative analysis of the erythrocyte lysis provided very interesting results and they encouraged us to try to understand what defines the solubilizing power of a detergent. In the next section, we show results that reveal the different steps of the solubilization process, induced by TX-100. This detergent was chosen because it is a weak solubilizer of erythrocyte membranes at 37 °C (Table 1), but a stronger one for POPC vesicles at room temperature (Heerklotz and Seelig 2000). In that sense, TX-100 could be considered a mild detergent, i.e. those for which K.cmc and Re sat values are never far from 1.
Measuring the bilayer/micelle transition during detergent-induced hemolysis
Among the spectroscopic techniques used in the study of detergent–membrane interactions, electron paramagnetic resonance (EPR) has been one of the most important, since one can monitor molecular order and dynamics in both bilayers and micelles (Preté et al. 2011). EPR spectra taken from such membrane systems—doped with spin labels—are sensitive to changes in molecular organization caused by the partitioning of amphiphiles, phase separation, bilayer/micelle transition, etc. For instance, detergent incorporation into erythrocytes decreases the molecular order and increases the mobility of the phospholipid hydrocarbon chains (Galembeck et al. 1998; Preté et al. 2011; Rodi et al. 2006, 2014) and induces phase separation (Crepaldi Domingues et al. 2009; Domingues et al. 2010; Preté et al. 2011; Rodi et al. 2006, 2014; Casadei et al. 2014a).
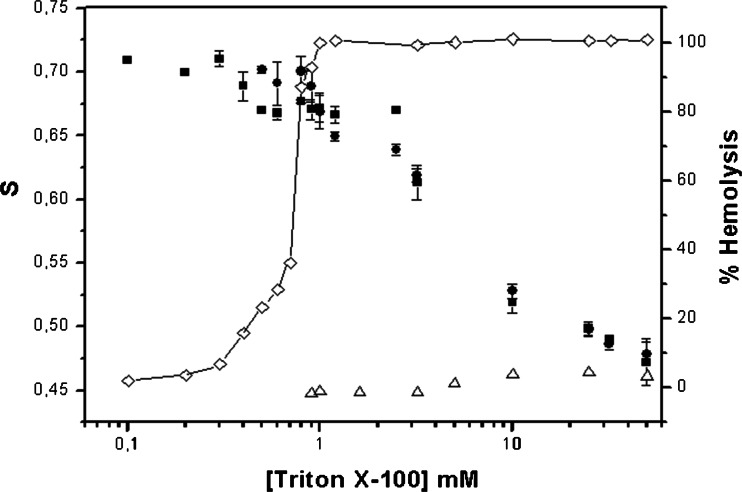
Using 5-doxyl stearic acid spin label (5-SASL), we conducted some EPR experiments with the attempt to unravel the events involved in the membrane solubilization process induced by detergents. First, EPR has been used to determine the order parameter of TX-100 micelles (S = 0.46). Then, the hemolytic effect of Triton X-100—followed either by inorganic phosphate or Hb release—was determined at high lipid contents (Ht 10–50%), and Csat and Csol values were found in the range of 0.5–0.9 mM TX-100 (Preté et al. 2011). Finally, EPR spectra of whole blood, supernatant and pellet fractions of the hemolytic experiment were obtained, revealing important details of the hemolytic effect: (1) the order parameter of erythrocyte membranes (S = 0.710) slightly decreased at TX-100 concentrations higher than Csat, and an anisotropic spectrum was detected in the supernatant, which S values were comparable to those of erythrocyte membranes; (2) at TX-100 concentrations higher than Csol the S values in the supernatant steadily decreased, and (3) they reach micelle-like values (S < 0.5) at excess (10 times Csol) TX-100. Figure 4 shows the EPR results combined with the hemoglobin release from erythrocytes.
Fig. 4.
Relative hemolysis and order parameters (S values) for whole human erythrocyte suspension: 40% Ht in 5 mM PBS buffer, pH 7.4, 22 ± 2 °C (■), and supernatants (●) as a function of TX-100 concentration. Error bars represent the standard deviation (n = 3). S values for 5-SASL spectra in pure TX-100 solutions (Δ) are also plotted. Figure originally published in Biochim. Biophys. Acta 1808:164–170, 2011; http://www.sciencedirect.com/science/article/pii/S0005273610003706
The results prove that a bilayer to micelle transition characterizes erythrocyte membrane solubilization by TX-100: pure membrane is replaced by mixed-membrane (above Csat), while mixed-micelle (above Csol) converges to (almost pure) micelle at higher (10 times Csol) TX-100 concentrations. Such transition is in accordance with the solubilization effect of TX-100 on model phospholipid membranes (Ruiz et al. 1988) as well as with the phase-boundary model proposed by Lichtenberg (Lichtenberg et al. 2000).
Figure 4 also shows that hemoglobin release and membrane order (S values) do not display a parallel behavior, since, at concentrations where hemolysis is almost complete (0.9 mM), S values are still high. We have proposed that hemoglobin is released from erythrocytes through pores created by the partition of TX-100 (Preté et al. 2011). Likewise, Gennaro and colleagues. have also reported that hemoglobin release and solubilization of phospholipids/cholesterol evoked by TX-100 were processes not directly correlated, once hemoglobin release preceded solubilization. They confirmed our hypothesis that hemoglobin is released from erythrocytes through holes created by the partition and fast segregation of TX-100 to cholesterol poor (liquid disordered, Ld) regions of the membrane, while most of the lipids remained insoluble (Rodi et al. 2014). Those authors have found different results with the zwitterionic and weak solubilizer CHAPS, to which hemoglobin release and lipid solubilization occurred at the same detergent concentration range.
Detergent-resistant erythrocyte membranes
The partial membrane solubilization promoted by mild detergents such as TX-100 has been extensively used to obtain the so-called detergent-resistant membranes (DRMs). Besides helping to understand details on the detergent–lipid interaction that determine solubilization, the isolation of detergent-insoluble membrane fractions is also a valuable approach to study the lateral order of biological membranes. Moreover, since DRMs exhibit remarkable features, such as enrichment in cholesterol and sphingolipids and high membrane lipid order, they might reflect to some extent the properties of rafts in living cells (Sonino and Prinetti 2013).
Different protocols have been suggested to isolate DRMs, and therefore these membrane fractions can present different lipid and protein compositions depending on the conditions and, particularly, on the detergent used (Heerklotz 2002; Lichtenberg et al. 2005). Considering that DRMs are not identical to membrane microdomains (rafts), the composition of these structures must be careful analyzed according to each cell membrane and protocol of isolation. Interestingly, a study conducted on a model membrane composed of POPC, egg sphingomyelin (SM) and cholesterol (chol) (1:1:1) showed that TX-100 promotes coalescence of preexisting nanodomains (Pathak and London 2011). The latter results contradict the idea that detergent induces the formation of domains by remodeling the plasma membrane. Therefore, a complete understanding of the action of detergents upon cell membrane is still a matter of debate.
Despite the fact that lipid rafts and DRMs have been intensively discussed in the literature, there is a lack of studies reporting these structures on erythrocyte membrane. Although details of composition and structure of erythrocyte membrane have been described in detail (Yawata 2003), the lateral inhomogeneities and corresponding function in this cell type remain elusive. The resistance of erythrocyte membrane to nonionic detergents was actually first reported in the 1970s (Yu et al. 1973; Steck 1974; Sheetz 1979), even before the concept of lipid rafts had been introduced (Simons and van Meer 1988; Simons and Ikonen 1997; Brown and London 1998). In the past decades, only a few DRM studies have been conducted on erythrocytes, in comparison to several studies on other cell types. Most of these studies were based on the protocol reported by Brown and Rose (1992). Therefore, partial solubilization of erythrocyte membranes has been mostly conducted by treating the membrane with TX-100 at 4 °C without major care (Salzer and Prohaska 2001; Samuel et al. 2001; Rivas and Gennaro 2003; Koumanov et al. 2005; Kamata et al. 2008). Differently, Ciana et al. (2005, 2011, 2014) have reported the necessity of a special protocol, including leukodepletion and the use of specific protease inhibitors in addition to sodium carbonate, as crucial steps to isolate DRMs from erythrocytes (as light density fractions in sucrose gradients). For detailed information regarding the historical aspects involving DRMs in erythrocyte, see a comprehensive review recently published by Ciana et al. (2014).
Our group has explored the use of TX-100 to study DRMs from erythrocytes in different conditions, as described below. Since TX-100 is the most traditional detergent used to solubilize cell membranes, we have also investigated the structure and properties of DRMs obtained by treating erythrocytes with other nonionic detergents: C12E8, which has similar binding constant to erythrocyte membranes as TX-100 (Table 1); and detergents of the Brij series, Brij 58 and Brij 98, which are strong hemolytic agents (Re sat values, Table 1) (Crepaldi Domingues et al. 2009; Domingues et al. 2010; Casadei et al. 2014b). All detergents were used above their cmc and at 16 mM (equivalent to 1% w/v TX-100), and DRMs were isolated as a floating fraction in a non-linear sucrose density gradient at the 5–30% sucrose interface (Fig. 5). Furthermore, the crucial steps described above (Ciana et al. 2005, 2011) were followed irrespective of the detergent used, to minimize and avoid artifacts. In our hands, sodium carbonate was always necessary to obtain light density DRMs in sucrose gradient. This fact, therefore, corroborates the hypothesis of existence of electrostatic interactions between DRMs and membrane-skeleton, the disruption of which is facilitated by carbonate (Crepaldi Domingues 2009; Domingues et al. 2010; Casadei et al. 2014b). However, the components responsible for this association (i.e. pH and ionic strength dependent) remain unclear.
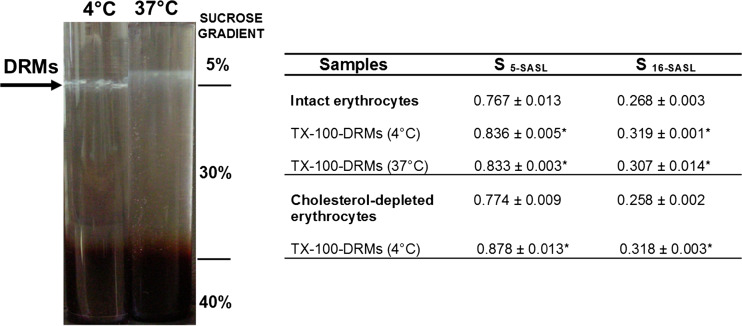
Fig. 5.
Representative image of DRMs obtained in the sucrose density gradients after centrifugation of intact erythrocytes previously treated with TX-100 at 4 °C and 37 °C. The table shows the order parameter (S) values calculated from EPR spectra of the spin labels 5 and 16-SASL in intact and depleted-cholesterol erythrocytes and their respective DRMs. Statistical differences for S values between all DRMs and their corresponding controls (intact cells) were observed: *P < 0.001, unpaired Students’s t test; n = 8–10 for intact erythrocytes, n = 4–5 for cholesterol-depleted erythrocytes, n = 3–5 for DRMs. S values were extracted from Domingues et al. (2010) in which details are discussed
Order parameter of membrane lipids resistant to detergents
The erythrocyte membranes as well as other plasma membranes exhibit a complex mixture of lipids and, as a result, distinct coexisting phases. The Ld phase is formed mostly by unsaturated phospholipids, while the Lo phase is enriched in saturated lipids and cholesterol (Ipsen et al. 1987; Veatch and Keller 2003). Therefore, raft-like domains formed by the favorable association between (glyco)sphingolipids and cholesterol are in the Lo phase. TX-100-DRMs isolated from erythrocytes were also described as structures enriched in cholesterol (54–57% of total lipids) and sphingomyelin (45% in mass, related to phosphatidylcholine and phosphatidylethanolamine) (Crepaldi Domingues et al. 2009; Casadei et al. 2014b). Interestingly, our EPR results with the use of doxyl-stearate probes (5- and 16-SASL) showed that these erythrocytes membrane fractions (TX-100-DRMs) are less fluid than the intact membrane and, consequently, the acyl chain packing is compatible with the Lo domains (Crepaldi Domingues et al. 2009). Curiously, when cholesterol-depleted erythrocytes were incubated with TX-100, the obtained DRMs were even less fluid (higher S values, determined with 5-SASL probe) than the corresponding DRMs isolated from intact erythrocytes at 4 °C. These results indicate that the increased SM/cholesterol ratio favors the lipid packing, leading to a more organized structure.
In contrast to the traditional protocols used to isolate DRMs, we showed for the first time that temperature might not be as critical as previously reported for other cell types. Detergent-resistant membrane fractions were obtained by treating erythrocytes with TX-100 at physiological temperature (37 °C) (Domingues et al. 2010) (Fig. 5). Moreover, the cholesterol content and acyl chain packing measured for DRMs obtained at 37 °C were very similar to those obtained at 4 °C (see Fig. 5). These results confirm that DRMs can be obtained in a Lo-like phase, not only at low temperature but also at physiological temperature.
DRMs obtained with C12E8 displayed comparable results to those described above for TX-100-DRMs, at both 4 °C and 37 °C (Domingues et al. 2010). The increased order parameter measured from the 5-SASL spectra in DRMs confirmed the existence of a Lo-like phase, enriched in cholesterol. Although cholesterol may have a key role to keep the stability of DRMs, high lipid packing can still be obtained after cholesterol depletion, probably because of sphingolipids (i.e. sphingomyelin) enrichment. Despite the reports saying that sphingomyelin is more easily solubilized than phosphatidylcholine (Helenius and Simons 1975; Sot et al. 2002) and that self-aggregation of sphingolipids is not favorable (Uragami et al. 2001; Maggio et al. 2006), TX-100-DRMs obtained from intact erythrocytes are enriched in sphingomyelin (Casadei et al. 2014b). Curiously, Brij 98- and Brij 58-DRMs were also enriched in cholesterol but they did not show increased order parameters, as observed for TX-100 and - C12E8 DRMs. Brij-DRMs were not enriched in sphingomyelin and exhibited a phospholipid composition very similar to that observed in erythrocyte membrane ghosts (Casadei et al. 2014b). Therefore, it seems that lipids from the outer leaflet such as sphingomyelin, associated with cholesterol (TX-100 insoluble fraction) play a pivot role in the formation of the Lo phase. Considering that Lo microdomains do exist in cell membranes and that they are detergent-resistant, it would be reasonable to think that DRMs are formed by those domains. However, our experiments with erythrocyte membrane and different detergents showed that the structures and properties of DRMs might not reflect the structures pre-existing in the cell membrane.
DRMs-associated proteins
An ordered lipid environment (i.e. cholesterol- and sphingolipids-enriched domains) can be crucial for the activity of certain membrane proteins. Therefore, protein–lipid interactions play an important role for the activity of membrane domains. Particularly for erythrocytes, the existence of a connection between their complex membrane-skeleton and membrane domains might be important for the biomechanical properties (i.e. deformability) and other cell functions.
New insights into these Lo-like phase membrane structures (DRMs) and their association with membrane-skeleton arises from studies of DRMs obtained from erythrocyte suspension not subjected to a previous purification step (contaminated by neutrophils) and in the absence of carbonate (Ciana et al. 2011). In that scenario, a direct interaction between spectrin and membrane-rafts was proposed and phosphatidylserine would be the candidate to play a role as an anchor (Ciana et al. 2011; Ciana et al. 2014).
We have shown that lipid raft markers such as flotillin-2 and stomatin are present in TX-100-DRMs, but they are partially solubilized in DRMs obtained at 37 °C (Domingues et al. 2010). Also, membrane-skeleton proteins were found only in the soluble fractions. These results indicate that, at physiological temperature, these proteins are less resistant to TX-100, in contrast to that observed for cholesterol. Moreover, the reduction of flotillin-2 detected when DRMs were isolated from cholesterol-depleted erythrocytes suggests an association between this protein and cholesterol-enriched membrane domains.
As for DRMs prepared with Brij detergents, the temperature of the solubilization process did not affect flotillin-2 and stomatin content (Casadei et al. 2014b). Interestingly, band 3 (the most abundant integral protein of the erythrocytes and barely detected in TX-100-DRMs) was remarkably present in Brij-DRMs obtained at 4 °C and partially reduced in Brij-DRMs obtained at 37 °C (Ciana et al. 2005; Domingues et al. 2010). These results clearly demonstrate that not only detergent but also temperature and lipid packing affect the distribution and preference of proteins to such domains.
Considering the intrinsic structure and physicochemical properties of each detergent, one can expect different interactions with a particular membrane. As we have previously reported, TX-100 and Brij detergents disrupt the erythrocyte membrane in diverse ways (Casadei et al. 2014b). TX-100 is known to preferentially solubilize phospholipids from the inner leaflet of the cell membrane (Ingelmo-Torres et al. 2009; Koumanov et al. 2005; Lichtenberg et al. 2005) while Brij detergents are not as selective as TX-100 (Schuck et al. 2003). Rodi et al. (2014) have also demonstrated that TX-100 incorporates and solubilize lipid components from the erythrocyte membranes in a different way compared with CHAPS (a zwitterionic detergent). Those results also indicate that TX-100 is able to flip to the inner leaflet and incorporate in poor cholesterol regions of the membrane, while CHAPS probably due to its charges is prevented from flip-flop and thus acts on the surface of the erythrocyte membrane (Rodi et al. 2014). Given that Brij detergents do not selectively solubilize components from the inner and outer leaflets, Brij-DRMs can reflect important aspects regarding membrane domains. It can be speculated that band 3 plays a role in the structure of those microdomains and should be considered as a candidate involved in the association of membrane domains (i.e. rafts) and membrane-skeleton.
Altogether, the data described above indicate that partial and differential detergent solubilization of the erythrocyte membrane can provide a powerful tool to explore the multiple levels of lateral order existing in biological membranes. Since TX-100 produced resistant membranes with raft-like features, such as high cholesterol/protein ratio and raft-marker proteins (flotillin-2 and stomatin), we have investigated its association with model membranes that mimic the erythrocyte membrane composition.
Using biomimetic model systems to understand the membrane solubilization process by TX-100
Biological membranes are inherently complex and highly dynamic. Lipid vesicles with tuned lipid composition provide a very robust biomimetic model that allows the understanding of the mechanistic details of intricate processes involving biological membranes. In particular, the solubilization of lipid vesicles by detergents has been extensively studied in recent decades (Partearroyo et al. 1996; Patra et al. 1998; Heerklotz and Seelig 2000; Sot et al. 2002; Heerklotz 2008; Ahyayauch et al. 2010; Lichtenberg et al. 2013), mainly using large unilamellar vesicles (LUVs). Among the detergents studied, TX-100 has attracted special attention because of its ubiquitous use in solubilization protocols (Hjelmeland 1990). The solubilization of membranes by TX-100 has been shown to be highly dependent on the lipid bilayer composition (Sot et al. 2002; Heerklotz 2002; Heerklotz et al. 2003; Tsamaloukas et al. 2006; Arnulphi et al. 2007; Ahyayauch et al. 2012). Especially, the presence of cholesterol has a great impact on the membrane resistance to effective and complete solubilization (Patra et al. 1999; Li et al. 2001; Schnitzer et al. 2005; El Kirat and Morandat 2007; Ahyayauch et al. 2009).
More recently, optical microscopy of giant unilamellar vesicles (GUVs), which are cell-sized lipid vesicles, started contributing significantly to this field (Nomura et al. 2001; Sudbrack et al. 2011). GUVs can be individually observed and tracked in time in the presence of detergents, revealing important aspects of the interaction between detergents and membranes, such as morphological effects, that remain hidden in bulk experiments performed on a large population of small vesicles. We have used this approach, in combination with conventional studies with LUVs, to investigate the effects of TX-100 on membranes of different lipid compositions mimicking compositions/phases that are biologically relevant. Compositions of up to three types of lipids found in abundance in the external leaflet of erythrocyte membranes were used: POPC, SM and chol. The phospholipid POPC has one unsaturated tail and is therefore in the Ld phase at room temperature, whereas egg SM comprises a high fraction of 16-carbon saturated chains and it is found in the gel phase at room temperature. Depending on the fraction of cholesterol in binary mixtures with these phospholipids, the Lo phase can be reached. Interestingly, ternary raft-like mixtures, such as POPC/SM/chol, can exhibit Ld/Lo phase coexistence depending on composition and temperature (Veatch and Keller 2003; Baumgart et al. 2003). Raft-like ternary mixtures have been widely used to investigate phase coexistence in model systems and to help elucidate the formation of membrane rafts (Almeida et al. 2003; Veatch and Keller 2005; Heberle et al. 2013). The results presented here will be divided into two sets of compositions: First, the solubilization of pure POPC, pure SM and mixtures of these phospholipids with 30 mol% cholesterol will be compared and discussed. Then, results obtained with a complex biological membrane (erythrocyte) extract and biomimetic ternary mixtures of POPC/SM/chol will be shown.
Solubilization of binary lipid mixtures containing cholesterol
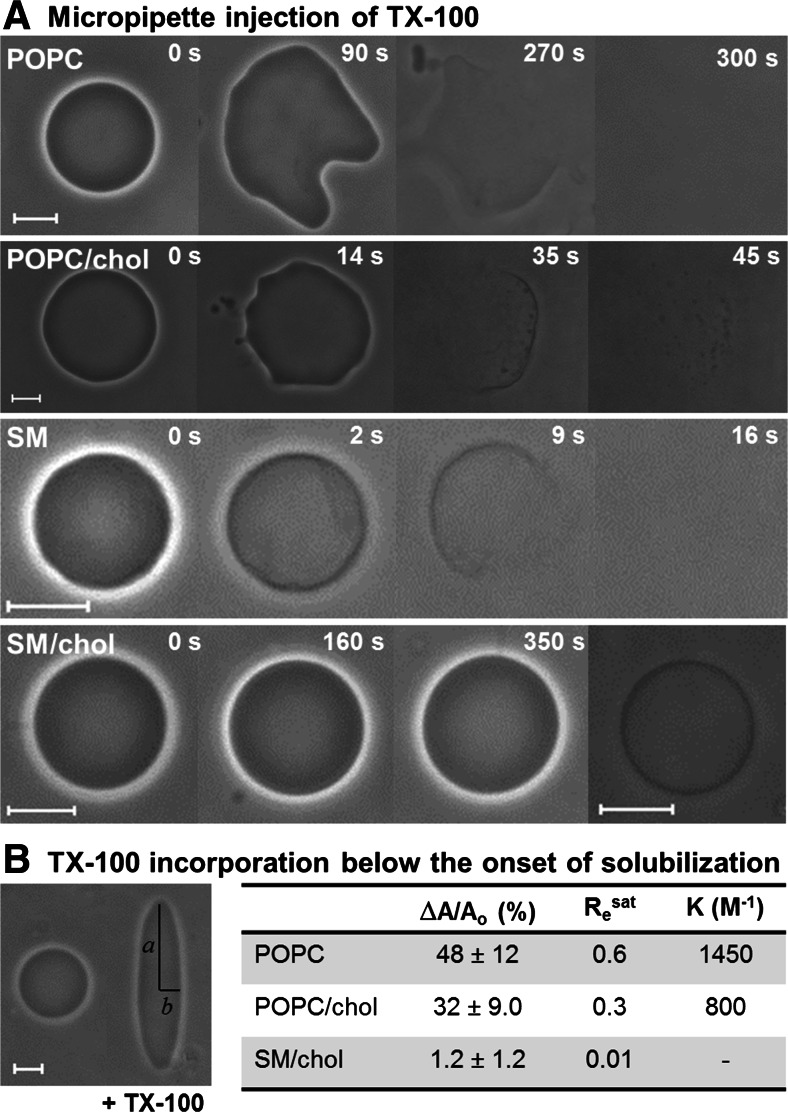
The action of TX-100 on membranes can be nicely followed with optical microscopy of GUVs (Mattei et al. 2015). Figure 6a shows representative image sequences of individual GUVs of POPC, SM, POPC/chol 7:3 and SM/chol 7:3, subjected to injection of TX-100 through a glass micropipette, at room temperature. The experiments were performed with phase contrast microscopy and the GUVs were prepared with an asymmetry in the sugar distribution (0.2 M sucrose inside/0.2 M glucose outside). Because of the different refractive index of the two sugar solutions, the GUVs exhibit a high optical contrast. The concentration of TX-100 loaded in the micropipette was around 1–5 mM, thus sufficiently above its cmc (0.25 mM; see Table 1). In this way, the whole solubilization process could be followed in single GUVs. The images in the first row show a POPC GUV (Ld phase) during its solubilization process. The first effect caused by TX-100 is a substantial increase in the vesicle surface area (90 s), which clearly shows that TX-100 is first incorporated in the external leaflet, but rapidly equilibrates between the two layers. The onset of solubilization is then reached (270 s), the optical contrast is lost, a large hole opens and eventually the membrane is completely solubilized (300 s) into submicroscopic micellar structures. During the solubilization, extensive formation of macroscopic pores was detected (Sudbrack et al. 2011). A similar course of events was observed for GUVs of POPC/chol, most probably in the Ld phase, as exemplified with a sequence in the second row of Fig. 6a. The area increase here also resulted in the expulsion of several buds (14 s). Importantly, solubilization was not complete, as insoluble membrane fragments remained (45 s). The solubilization of SM GUVs (gel phase) was complete, as shown in the sequence in the third row of Fig. 6a. No clear increase in vesicle surface area was detected initially (2 s), but it should be noted that membranes in the gel phase exhibit high resistance to shearing, and thus local area increase would most probably cause local deformations, which were observed in some GUVs. Very differently from the other compositions, GUVs of SM/chol 7:3, in the Lo phase, were virtually resistant to TX-100 (see image sequence in the fourth row of Fig. 6a). The only detectable effect was increased membrane permeability in several GUVs, which resulted in loss of the high optical contrast due to equilibration of the sugar solutions (last image, obtained from another SM/chol GUV).
Fig. 6.
a Time sequences of phase contrast microscopy images of GUVs during injection of 5 mM TX-100 through a glass micropipette. The timestamps are relative to the moment when the micropipette was brought close to each vesicle. Scale bars 10 μm. b Quantification of the area increase due to TX-100 incorporation. The image shows a POPC/chol GUV before (left) and at the maximum prolate deformation (right) after the TX-100 diffused through the chamber and reached the vesicle. The GUV is subjected to an AC field (200 V/cm; 200 kHz) throughout the whole experiment. The prolate semi-axes a and b are shown. The GUV contained 0.2 mM NaCl inside to ensure that the conductivity inside the vesicle was higher than outside, and therefore the AC field would cause a prolate deformation (Aranda et al. 2008). The table shows the maximum area increase ∆A/Ao (measured directly from a/b for 20–50 vesicles/system), the TX-100 to lipid molar ratio at the saturating condition Re sat and the binding constant K. The details are discussed in Mattei et al. (2015). Figure adapted from Mattei et al. (2015)
The experiments with micropipette injection allow following individual GUVs throughout the whole solubilization process, including before contact with TX-100. However, the effective detergent concentration reaching the vesicle along the process cannot be determined. This was done in another setup, in which GUVs were added to increasing concentrations of TX-100 and observed right after (Mattei et al. 2015). Such experiments showed that GUVs of POPC and POPC/chol became permeable and gained area at 0.3 mM TX-100 and were solubilized at 0.4 mM, thus in a concentration range close to the cmc of TX-100. This was expected based on the very low lipid concentration of GUVs (~1–10 μM). For SM GUVs, permeabilization occurred at 0.2 mM TX-100 and solubilization at 0.3 mM. The detergent-induced permeabilization will be discussed below.
The increase in vesicle surface area below the onset of solubilization shown in Fig. 6a is directly related to the amount of TX-100 molecules that are incorporated into the membrane. Therefore, if the increase in surface area can be quantified, and knowing the molecular area of each membrane component, the saturating TX-100 to lipid molar ratio at the onset of solubilization (Re sat) can be directly extracted from the microscopy data. To that purpose, a different experimental setup was used. GUVs were placed in a special chamber with two electrodes and addition of TX-100 was carried out in the presence of an AC field (Mattei et al. 2015), which is known to induce prolate deformation in GUVs with excess area (Aranda et al. 2008; Riske et al. 2009). The extent of deformation, quantified though the ratio between the two semi-axes a/b (see Fig. 6b), can be directly used to measure the relative increase in area (∆A/Ao) from simple geometrical relations (see details in Riske et al. 2009; Mattei et al. 2015). Initially spherical or quasi-spherical GUVs were chosen and an aliquot of concentrated TX-100 was added to the chamber. After diffusion, TX-100 reached the GUVs in the presence of the AC field and substantial prolate deformation was observed as TX-100 was incorporated in the membrane (see Fig. 6b). Eventually, the onset of solubilization was reached. The maximum a/b attained was measured for several GUVs composed of POPC, POPC/chol and SM/chol; this procedure could not be carried out for gel phase vesicles because they do not deform in the same way. The maximum area increase ∆A/Ao is listed in the table shown in Fig. 6b. Clearly, incorporation of TX-100 is substantial in the POPC membrane, decreases when cholesterol is added and becomes negligible in vesicles of SM/chol (Lo phase). The area increase can be translated into Re sat (maximum TX-100/lipid ratio in the membrane) by using the known molecular areas of the membrane constituents in each case (see details in Mattei et al. 2015). The values extracted are shown in the table in Fig. 6b, and the values agree with results previously obtained from other techniques (de la Maza and Parra 1994; Partearroyo et al. 1996; Heerklotz 2002; Sudbrack et al. 2011). From a simple partition model, in which Re sat = K.Dw (Heerklotz and Seelig 2000), and assuming that the concentration of free TX-100 at the onset of solubilization is Dw = 0.4 mM, the binding constant K could be obtained for POPC and POPC/chol, as listed in Fig. 6b. The results are similar to values obtained from isothermal titration calorimetry experiments (Tsamaloukas et al. 2006; Caritá et al. 2017). It is clear from the data that cholesterol significantly reduces the partition of TX-100 into the bilayer, and only a small amount of TX-100 is able to insert into the Lo phase.
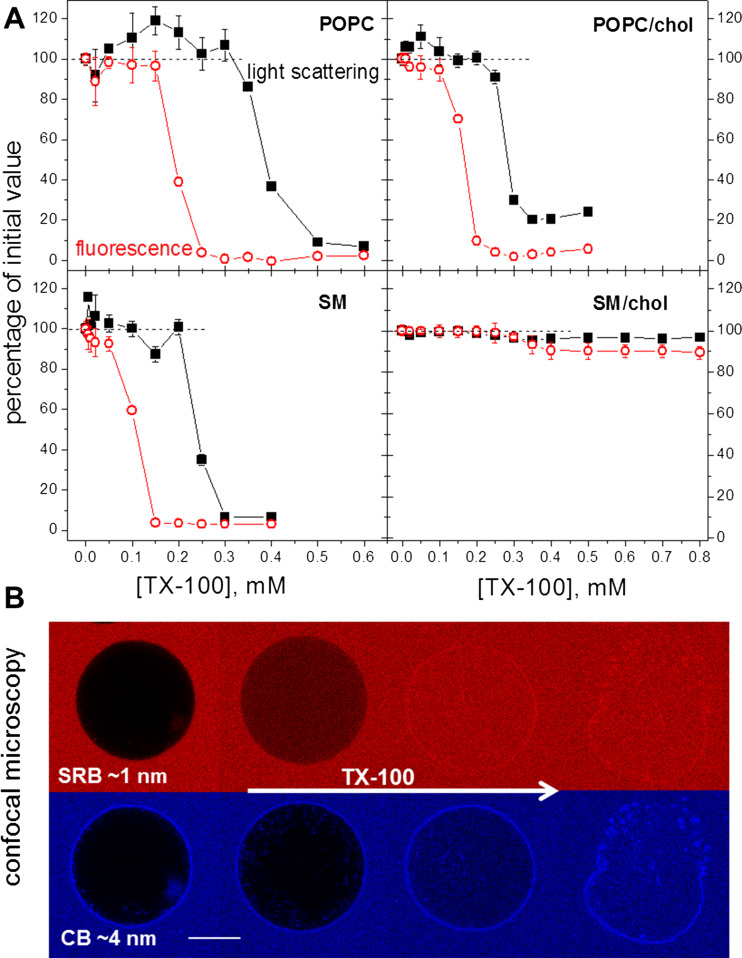
TX-100 was shown to induce membrane permeabilization below the onset of solubilization (see Fig. 6a; Ahyayauch et al. 2010; Mattei et al. 2015). To study that in detail, we used a fluorescence-based assay to quantify the leakage induced by TX-100 on LUVs of the same compositions explored in Fig. 6a (Mattei et al. 2017). The LUVs were prepared in a solution containing the aqueous soluble fluorescent dye pyrenetetrasulphonic acid (PTS) and were then diluted in a solution containing increasing concentrations of TX-100 and methyl viologen (MV), a fluorescence quencher. Membrane permeabilization by TX-100 was detected by a decrease in the fluorescence intensity of the initially encapsulated PTS, since the fluorescence of the external PTS was quenched by MV. To correlate the extent of permeabilization with the process of solubilization, the light scattering of the same samples was measured after the fluorescence intensity kinetic runs. Sample turbidity or light scattering are among the most used methods to assess the solubilization profile of lipid vesicles by detergents, because turbidity/light scattering decreases as lipid vesicles get solubilized into micellar structures (de la Maza and Parra 1994; Partearroyo et al. 1996; Lichtenberg et al. 2013). Figure 7a shows the results of the permeabilization (red circles) and solubilization (black squares) profiles for the different compositions. The data are depicted as fluorescence and light scattering percentage values relative to the values measured for the dispersion of LUVs before contact with TX-100. The results show that POPC, POPC/chol and SM become completely permeable (0% fluorescence intensity) below the onset of solubilization, which is detected when sample light scattering starts to decrease. POPC and SM are completely solubilized by TX-100, although more TX-100 is required to fully solubilize the Ld phase, as reported previously (Ahyayauch et al. 2012). On the other hand, the solubilization of POPC/chol is not complete, as seen from the residual light scattering value (~20%) at high detergent concentration. SM/chol vesicles exhibit a very mild permeabilization and are virtually insoluble. Importantly, the onsets of permeabilization and solubilization occur at the same TX-100 concentrations for LUVs and GUVs of the same composition, and these concentrations are all close to the cmc. This was expected since the lipid concentrations used in both experiments were low.
Fig. 7.
a Profiles of membrane permeabilization and of the solubilization extent. The graphs show percentages of 90° light scattering (λ = 500 nm) (closed black symbols) and of PTS fluorescence intensity (λex = 355 nm; λem = 405 nm) (open red symbols) of LUVs as a function of the TX-100 concentration, in respect to LUVs without TX-100. LUVs were prepared in 10 mM HEPES pH 7.4 with 1 mM PTS and diluted 100× in 10 mM HEPES pH 7.4 with 2 mM MV and the given concentration of TX-100, to yield a final 0.1 mM lipid concentration. Each point represents the values obtained ~5 min after dilution of the LUV dispersion. b Confocal microscopy image sequences obtained from one POPC GUV in two channels (red for SRB: λex = 552 nm, λem = 560–630 nm and blue for CB: λex = 405 nm, λem = 410–480 nm) as TX-100 diffused through the chamber and reached the vesicle. Scale bar 20 μm. Figure adapted from Mattei et al. (2017)
To further explore membrane permeabilization, confocal microscopy experiments were performed with GUVs of the same lipid compositions dispersed in a medium containing two fluorescent dyes of different sizes: sulforhodamine B (SRB; 0.6 kDa and ca. 1 nm size) and cascade blue – Dextran (CB; 10 kDa and ca. 4 nm size) (Mattei et al. 2017). Figure 7b shows a sequence of images of a POPC GUV experiencing a flux of TX-100 detected with both channels (red for SRB and blue for CB). Clearly, permeabilization occurs in at least two sequential events: First, the membrane becomes selectively permeable to SRB, showing that the pores opened are smaller than 4 nm. Later on, the pores grow and/or coalesce allowing the passage of CB, and after that, visible macropores open and the solubilization ensues. The same chain of events was observed for POPC/chol. For gel phase SM, both dyes entered simultaneously, indicating that most probably cracks of irregular shape opened. Very differently, vesicles of SM/chol exhibited gradual entrance of both dyes, and no well-defined permeabilization event was triggered by TX-100.
Solubilization of raft-like mixtures
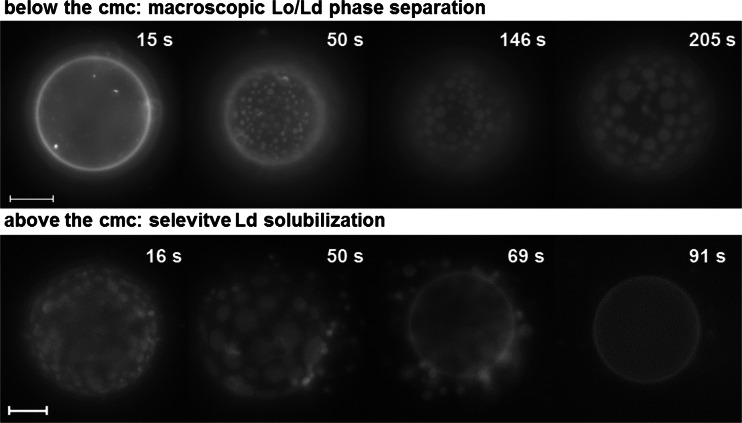
In order to investigate the effects of TX-100 on biomimetic models of compositions better representing real membranes, GUVs of the lipid extract of erythrocyte membranes (erythro-GUVs) were prepared and exposed to TX-100 (Casadei et al. 2014a). These experiments were performed with fluorescence microscopy and the GUVs contained traces of the fluorescent probe Rh-DPPE, which is known to preferentially partition into the Ld phase (Baumgart et al. 2003; Bagatolli 2003). Figure 8 shows representative images of erythro-GUVs dispersed in solutions containing TX-100 below (0.2 mM) and above (0.4 mM) its cmc. The erythro-GUVs exhibited a homogeneous fluorescence in the absence of TX-100 (see first snapshot in the first row), showing that no macroscopic (i.e. above the optical resolution limit of ~0.3 μm) phase separation occurred in this system. However, after contact with TX-100, domains enriched in the fluorescent probe, thus in the Ld phase, appeared (50 s; the vesicle pole is in focus). The domains formed were round and could freely diffuse and coalesce (50–205 s), indicating that the non-fluorescent matrix was also liquid, and therefore in the Lo phase. When the erythro-GUVs were dispersed in 0.4 mM TX-100 (above its cmc), macroscopic Lo/Ld phase separation was readily induced by TX-100 (16 s, in the second row) and coalesced (50 s). Then, the Ld domains were extracted (69 s) and completely solubilized, leaving an insoluble smaller Lo vesicle (91 s).
Fig. 8.
Fluorescence microscopy time sequences of erythro-GUVs added to a TX-100 solution below (0.2 mM; top) and above (0.4 mM; bottom) its cmc. The timestamps are relative to the moment when the erythro-GUVs were added to the observation chamber containing the TX-100 solution. Scale bars (top) 20 μm, (bottom) 10 μm. Figure adapted from Casadei et al. (2014a)
The insoluble surface area fraction can be easily quantified from the vesicle diameter before (Dbefore) and after (Dafter) contact with TX-100 above its cmc as Xinsol = (Dafter/Dbefore)2. This was carried out by exposing several erythro-GUVs to a flux of 5 mM TX-100, from a micropipette. The results are given in Table 2 and show that roughly 2/3 of the initial vesicle surface area is insoluble to TX-100 at room temperature.
Table 2.
Fraction of insoluble surface area measured from the ratio between the GUV diameter before and after contact with TX-100: Xinsol = (Dafter/Dbefore)2 at room temperature (~23 °C) and at physiological temperature (~37 °C); results shown in Casadei et al. (2014a)
| Xinsol (~23 °C) | Xinsol (~37 °C) | |
|---|---|---|
| erythro-GUVs | 0.66 ± 0.09 (n = 124) | 0.55 ± 0.07 (n = 51) |
| 2:1:2 POPC/SM/chol | 0.69 ± 0.11 (n = 116) | 0.53 ± 0.10 (n = 19) |
| 0.8:1:2 POPC/SM/chol | 1.0 (n = 6) | - |
Based on the lipid distribution from the lipid extract of erythrocyte membranes (Leidl et al. 2008), a simple biomimetic ternary lipid composition was chosen, namely 2:1:2 POPC/SM/chol. GUVs of that composition were grown and exposed to micropipette injection of 5 mM TX-100 (Casadei et al. 2014a). Interestingly, the same qualitative and quantitative behavior was observed: The GUVs were initially homogenous, exhibited macroscopic Lo/Ld phase separation after contact with TX-100 and the Ld domains were selectively solubilized, leaving around 2/3 of the initial vesicle surface area insoluble to TX-100 (see results in Table 2). If we assume that mainly POPC is selectively solubilized, and knowing the molecular area occupied by each component (see details in Casadei et al. 2014a), then the expected insoluble vesicle should be composed of 0.8:1:2 POPC/SM/chol. In fact, GUVs of that composition were exposed to TX-100 and were virtually insoluble (see Table 2). Therefore, this composition is a good biomimetic model for DRMs at room temperature.
The experiments above were conducted at room temperature. As the temperature increases, the Lo/Ld fractions are expected to change, and, ultimately, above the miscibility temperature phase coexistence should disappear (Veatch and Keller 2003, 2005). To explore a physiological temperature, erythro-GUVs and GUVs of 2:1:2 POPC/SM/chol were subjected to injection of 5 mM TX-100 at 37 °C. The same chain of events was observed, namely macroscopic Lo/Ld phase separation and selective solubilization of the Ld phase. However, the relative Lo/Ld ratio decreased, and consequently the insoluble fraction detected at 37 °C also decreased, as expected, and roughly half of the initial vesicle surface area, of both compositions, was insoluble to TX-100 (see Table 2).
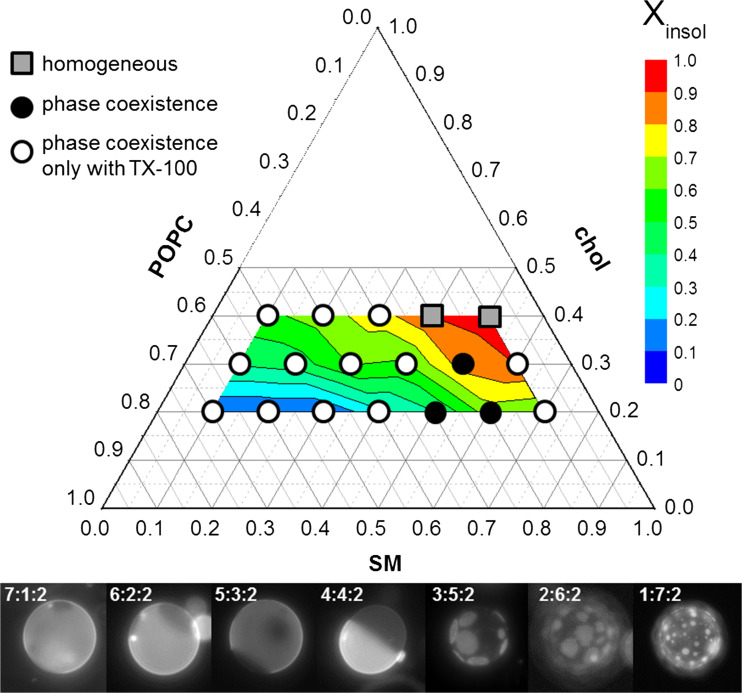
The relative Lo/Ld fraction and the extent of solubilization induced by TX-100 were shown to be dependent on the relative proportion of the constituent lipids (Casadei et al. 2014a). To explore the raft-like ternary mixture POPC/SM/chol in detail, a wide range of compositions was explored in respect to Lo/Ld phase separation and extension of solubilization (Caritá et al. 2017). To that purpose, three fixed concentrations of cholesterol were chosen (20, 30 and 40 mol%) and the proportions of POPC and SM in the mixture were varied at intervals of 10 mol%. First, GUVs of those compositions were exposed to TX-100 below its cmc to determine which compositions exhibited macroscopic Lo/Ld phase separation in the absence and in the presence of TX-100, which enabled the construction of phase diagrams of the POPC/SM/chol mixtures, at room temperature. Then, the GUVs were exposed to a flux of TX-100 with a micropipette to quantify the extent of the insoluble fraction, Xinsol, in the same way as described above. The results obtained are summarized in Fig. 9 in a combined phase-solubility diagram. The symbols indicate the phase diagram. Filled circles show the compositions that exhibited Lo/Ld phase separation, even in the absence of TX-100. The region is very limited, as reported by other groups (Veatch and Keller 2005), and only some of the GUVs were found with domains. Open circles indicate that the compositions for which Lo/Ld phase separation was promoted by TX-100. These represent the majority of the compositions explored. The images below the diagram show representative images of GUVs with 20 mol% cholesterol and with varying proportions of POPC/SM. At high POPC fraction, the Ld fraction dominates and (dark) Lo domains are detected. As SM replaces POPC the relative Lo/Ld proportion increases, and eventually the Lo phase becomes the matrix and (bright) domains are seen. Only two compositions explored (shown as squares) did not exhibit phase separation irrespective of the presence of TX-100. In fact, such compositions, rich in SM and cholesterol, are probably in the Lo phase.
Fig. 9.
Phase-solubility diagram of the POPC/SM/chol mixture. The symbols indicate the phase diagram with and without 0.1 mM TX-100 (see text), whereas the color contour map showa the Xinsol values measured from at least 20 GUVs for each composition exposed to micropipette injection of TX-100. The fluorescence images show representative GUVs along the 20 mol% cholesterol line in the presence of 0.1 mM TX-100. Figure adapted from Caritá et al. (2017)
If the phase diagrams in the presence and absence of TX-100 are compared with phase diagrams obtained with similar compositions, it becomes clear that the diagram obtained without TX-100, which shows a very limited region with detectable phase separation, coincides with a diagram obtained by optical microscopy. On the other hand, the phase diagram exhibiting an extensive region of macroscopic phase separation in the presence of TX-100 is similar to diagrams obtained with techniques that can detect nanoscopic domains (Almeida et al. 2003, 2005; Halling et al. 2008). It is therefore realistic to propose that the main effect of TX-100 is to induce the coalescence of sub-microscopic domains, which can then be detected. This has in fact been previously proposed by others (Giocondi et al. 2000; Pathak and London 2011).
The solubilization extent was quantified for all compositions and Xinsol is shown as a color contour map in Fig. 9. The compositions in the POPC-rich corner are almost completely solubilized by TX-100, whereas they become increasingly resistant as the fraction of SM and cholesterol in the mixture increases, until virtually insoluble mixtures are detected in the opposite corner.
Concluding remarks
In this review, complementary aspects of the solubilization process of membranes with different complexity were investigated and discussed using a wide range of experimental approaches. On the one hand, human erythrocyte membrane, in its intrinsic complexity, was totally or partially solubilized by different series of detergents, which were classified by the strength of their hemolytic action. EPR experiments allowed characterization of the prevalent structures (pure and mixed membranes/micelles) formed in the different stages of membrane solubilization process by TX-100 and detergent-resistant membranes obtained from erythrocytes at 4 and 37 °C were characterized. On the other hand, biomimetic models of tuned lipid composition in the presence of the ubiquitous detergent TX-100 were investigated in detail. It was shown that the lipid composition and phase state are crucial to the solubilization process. Pure phospholipid membranes were completely solubilized by TX-100, whereas the presence of cholesterol rendered the mixture partially or completely insoluble (when the Lo phase was reached). Of great biological relevance, TX-100 induced macroscopic Ld/Lo phase separation in raft-like mixtures and was able to solubilize the Ld fraction only. Therefore, the membrane domains can be induced by TX-100 since lipids are reassembled during the action of the detergent upon the bilayer.
The results discussed here represent the main outcomes of the studies performed in the groups of Dr. de Paula and Dr. Riske, who worked in complementary aspects of the same problem and, more recently, combined their skills to study the physical chemical aspects underlying the solubilization process of complex biomembranes.
Acknowledgements
We are grateful to Elsevier for the permission to use Fig. 4, originally published in Biochim. Biophys. Acta 1808:164-170, 2011; 10.1016/j.bbamem.2010.10.016. The authors are grateful to Dr. M. Teresa Lamy for the use of the EPR equipment, and Dr. Shirley Schreier for inspiring discussions.
Abbreviations
- ASB
aminosulfobetaines
- C
Cholate
- CB
Cascade blue – Dextran
- CHAPS
Cholamido propyl dimethylammonio propanesulfonate
- chol
Cholesterol
- cmc
Critical micelle concentration
- Cprot
Detergent concentration for maximum protection against hemolysis, at hyposmotic condition
- Csat (Csol)
Detergent concentration for the onset (complete) membrane solubilization
- DC
Desoxycholate
- DRM
Detergent resistant membrane fractions
- GC
Glycocholate
- GD
Glycochenodeoxycholate
- GUVs
Giant unilamellar vesicles
- HLB
Hydrophilic-lipophilic balance
- Ht
Hematocrit
- LC
Lithocholate
- Ld
Liquid disordered phase
- Lo
Liquid ordered phase
- LUV
Large unilamellar vesicles
- POPC
Palmitoyl oleoyl phosphatidylcholine
- PTS
Pyrenetetrasulphonic acid
- Resat (Resol)
Effective detergent/lipid molar ratio for the onset (complete) solubilization
- SASL
Doxyl stearic acid spin label
- SM
Sphingomyelin
- SRB
Sulforhodamine B
- TC
Taurocholate
- TD
Taurochenodeoxycholate
- TX-100
Triton X-100
- UD
Ursodeoxycholate
- VL
Viologen
Funding
This study was funded by FAPESP (09/901–1 – EP; 11/22171–6 and 13/20499–0 – KAR; 10/18516–5 - CCD), CNPq (308,621/2013–1 – EP; 472,054/2011–2, 158,413/2013–0 – KAR; 479,993/2011–4 – CCD) and INCT-FCx (KAR).
Compliance with ethical standards
Conflicts of interest
Karin A. Riske declares that she has no conflicts of interest. Cleyton C. Domingues declares that he has no conflicts of interest. Bruna R. Casadei declares that she has no conflicts of interest. Bruno Mattei declares that he has no conflicts of interest. Amanda C. Caritá declares that she has no conflicts of interest. Rafael B. Lira declares that he has no conflicts of interest. Paulo S. C Preté declares that he has no conflicts of interest. Eneida de Paula declares that she has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri.
References
- Ahyayauch H, Collado MI, Goñi FM, Lichtenberg D. Cholesterol reverts TX-100 preferential solubilization of sphingomyelin over phosphatidylcholine: A 31P-NMR study. FEBS Lett. 2009;583:2859–2864. doi: 10.1016/j.febslet.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Ahyayauch H, Bennouna M, Alonso A, Goñi FM. Detergent effects on membranes at subsolubilizing concentrations: Transmembrane lipid motion, bilayer permeabilization, and vesicle lysis/reassembly are independent phenomena. Langmuir. 2010;26:7307–7313. doi: 10.1021/la904194a. [DOI] [PubMed] [Google Scholar]
- Ahyayauch H, Collado M, Alonso A, Goñi FM. Lipid bilayers in the gel phase become saturated by triton X-100 at lower surfactant concentrations than those in the fluid phase. Biophys J. 2012;102:2510–2516. doi: 10.1016/j.bpj.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RFM, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/ cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RFM, Loura LMS, Fedorov A, Prieto M. Lipid rafts have different sizes depending on membrane composition: A time-resolved fluorescence resonance transfer study. J Mol Biol. 2005;346:1109–1120. doi: 10.1016/j.jmb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Aranda S, Riske KA, Lipowsky R, Dimova R. Morphological transitions of vesicles induced by alternating electric fields. Biophys J. 2008;95:L19–L21. doi: 10.1529/biophysj.108.132548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnulphi C, Sot J, García-Pacios M, Arrondo JL, Alonso A, Goñi FM. Triton X-100 partitioning into sphingomyelin bilayers at subsolubilizing detergent concentrations: Effect of lipid phase and a comparison with dipalmitoylphosphatidylcholine. Biophys J. 2007;93:3504–3514. doi: 10.1529/biophysj.107.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatolli LA. Direct observation of lipid domains in free standing bilayers: From simple to complex lipid mixtures. Chem Phys Lipids. 2003;122:137–145. doi: 10.1016/s0009-3084(02)00184-6. [DOI] [PubMed] [Google Scholar]
- Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Campos DDP, Cassu SN, Garcia RBR, Queiroz AHAS, RFB G, Kawachi EY. SAXS and DSC evaluation of interactions between H2O and Renex-100. Quim Nova. 2012;35:355–359. [Google Scholar]
- Caritá AC, Mattei B, Domingues CC, de Paula E, Riske KA (2017) Effect of triton X-100 on raft-like lipid mixtures: Phase separation and selective Solubilization. Langmuir. 10.1021/acs.langmuir.7b01134 [DOI] [PubMed]
- Casadei BR, Domingues CC, de Paula E, Riske KA. Direct visualization of the action of triton X-100 on giant vesicles of erythrocyte membrane lipids. Biophys J. 2014;106:2417–2425. doi: 10.1016/j.bpj.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei BR, Carvalho PO, Riske K, Barbosa RM, de Paula E, Domingues CC. Brij detergents reveal new aspects of membrane microdomain in erythrocytes. Mol Membr Biol. 2014;31:195–205. doi: 10.3109/09687688.2014.949319. [DOI] [PubMed] [Google Scholar]
- Ciana A, Balduini C, Minetti G. Detergent-resistant membranes in human erythrocytes and their connection to the membrane-skeleton. J Biosci. 2005;30:317–328. doi: 10.1007/BF02703669. [DOI] [PubMed] [Google Scholar]
- Ciana A, Achilli C, Balduini C, Minetti G. On the association of lipid rafts to the spectrin skeleton in human erythrocytes. Biochim Biophys Acta. 2011;1808:183–190. doi: 10.1016/j.bbamem.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Ciana A, Achilli C, Minetti G. Membrane rafts of the human red blood cell. Mol Membr Biol. 2014;31:47–57. doi: 10.3109/09687688.2014.896485. [DOI] [PubMed] [Google Scholar]
- Crepaldi Domingues C, Ciana A, Buttafava A, Balduini C, de Paula E, Minetti G. Resistance of human erythrocyte membranes to triton X-100 and C12E8. J Membr Biol. 2009;227:39–48. doi: 10.1007/s00232-008-9142-4. [DOI] [PubMed] [Google Scholar]
- de la Maza A, Parra JL. Vesicle-micelle structural transition of phosphatidylcholine bilayers and triton X-100. Biochem J. 1994;303:907–914. doi: 10.1042/bj3030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues CC, Malheiros SVP, de Paula E. Solubilization of human erythrocyte membranes by ASB detergents. Braz J Med Biol Res. 2008;41:758–764. doi: 10.1590/s0100-879x2008000900003. [DOI] [PubMed] [Google Scholar]
- Domingues CC, Ciana A, Buttafava A, Casadei B, Balduini C, de Paula E, Minetti G. Effect of cholesterol depletion and temperature on the isolation of detergent-resistant membranes from human erythrocytes. J Membr Biol. 2010;234:195–205. doi: 10.1007/s00232-010-9246-5. [DOI] [PubMed] [Google Scholar]
- Duwe HP, Sackmann E (1990) Bending elasticity and thermalexcitations of lipid bilayer vesicles: modulation by solutes. Phys Acta 163:410–428
- El Kirat K, Morandat S. Cholesterol modulation of membrane resistance to triton X-100 explored by atomic force microscopy. Biochim Biophys Acta. 2007;1768:2300–2309. doi: 10.1016/j.bbamem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Galembeck E, Alonso A, Meirelles NC. Effects of polyoxyethylene chain length on erythrocyte hemolysis induced by poly[oxyethylene (n) nonylphenol] non-ionic surfactants. Chem Biol Interact. 1998;113:91–103. doi: 10.1016/s0009-2797(98)00006-4. [DOI] [PubMed] [Google Scholar]
- Gandhi VM, Cherian KM. Red cell haemolysis test as an in vitro approach for the assessment of toxicity of karanja oil. Toxicol in Vitro. 2000;14:513–516. doi: 10.1016/s0887-2333(00)00046-1. [DOI] [PubMed] [Google Scholar]
- Giocondi MC, Vié V, Lesniewska E, Goudonnet JP, Le Grimellec C. In situ imaging of detergent-resistant membranes by atomic force microscopy. J Struct Biol. 2000;131:38–43. doi: 10.1006/jsbi.2000.4266. [DOI] [PubMed] [Google Scholar]
- Goñi FM. The basic structure and dynamics of cell membranes: An update of the singer–Nicolson model. Biochim Biophys Acta. 2014;1838:1467–1476. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Griffin WC. Classification of surface-active agents by HLB. J Soc Cosmet Chem. 1949;1:311–326. [Google Scholar]
- Hägerstrand H, Isomaa B. Amphiphile-induced antihaemolysis is not causally related to shape changes and vesiculation. Chem Biol Interact. 1991;79:335–347. doi: 10.1016/0009-2797(91)90113-l. [DOI] [PubMed] [Google Scholar]
- Hait SK, Moulik SP. Determination of critical micelle concentration (cmc) of nonionic surfactants by donor–acceptor interaction with iodine and correlation of cmc with hydrophile–lipophile balance and other parameters of the surfactants. J Surfactant Deterg. 2001;4:303–309. [Google Scholar]
- Halling KK, Ramstedt B, Nystrom JH, Slotte P, Nyholm TKM. Cholesterol interactions with fluid-phase phospholipids: Effect on the lateral organization of the bilayer. Biophys J. 2008;95:3861–3871. doi: 10.1529/biophysj.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: Contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle FA, Petruzielo RS, Pan J, Drazba P, Kučerka N, Standaert RF, Feigenson GW, Katsaras J. Bilayer thickness mismatch controls domain size in model membranes. J Am Chem Soc. 2013;135:6853–6859. doi: 10.1021/ja3113615. [DOI] [PubMed] [Google Scholar]
- Heerklotz H. Triton promotes domain formation in lipid raft mixture. Biophys J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H. Interactions of surfactants with lipid membranes. Q Rev Biophys. 2008;41:205–264. doi: 10.1017/S0033583508004721. [DOI] [PubMed] [Google Scholar]
- Heerklotz H, Seelig J. Correlation of membrane/water partition coefficients of detergents with the critical micelle concentration. Biophys J. 2000;78:2435–2440. doi: 10.1016/S0006-3495(00)76787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H, Szadkowska H, Anderson T, Seelig J. The sensitivity of lipid domains to small perturbations demonstrated by the effect of triton. J Mol Biol. 2003;329:793–799. doi: 10.1016/s0022-2836(03)00504-7. [DOI] [PubMed] [Google Scholar]
- Helenius A, Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hjelmeland LM. Solubilization of native membrane proteins. Methods Enzymol. 1990;182:253–264. doi: 10.1016/0076-6879(90)82021-s. [DOI] [PubMed] [Google Scholar]
- Ingelmo-Torres M, Gaus K, Herms A, González-Moreno E, Kassan A, Bosch M, Grewal T, Tebar F, Enrich C, Pol A. Triton X-100 promotes a cholesterol-dependent condensation of the plasma membrane. Biochem J. 2009;420:373–381. doi: 10.1042/BJ20090051. [DOI] [PubMed] [Google Scholar]
- Ipsen JH, Karlstrom G, Mouritsen OG, Wennerstrom H, Zuckermann MJ. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Hägerstrand H. Effects of nonionic amphiphiles at sublytic concentrations on the erythrocyte membrane. Cell Biochem Funct. 1988;6:183–190. doi: 10.1002/cbf.290060306. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Hägerstrand H, Paatero G, Engblom AC. Permeability alterations and antihaemolysis induced by amphiphiles in human erythrocytes. Biochim Biophys Acta. 1986;860:510–524. doi: 10.1016/0005-2736(86)90548-1. [DOI] [PubMed] [Google Scholar]
- Jones MN. Surfactants in membrane solubilisation. Int J Pharm. 1999;177:137–159. doi: 10.1016/s0378-5173(98)00345-7. [DOI] [PubMed] [Google Scholar]
- Kamata K, Manno S, Ozaki M, Takakuwa Y. Functional evidence for presence of lipid rafts in erythrocyte membranes: Gsalpha in rafts is essential for signal transduction. Am J Hematol. 2008;83:371–375. doi: 10.1002/ajh.21126. [DOI] [PubMed] [Google Scholar]
- Koumanov KS, Tessier C, Momchilova AB, Rainteau D, Wolf C, Quinn PJ. Comparative lipid analysis and structure of detergentresistant membrane raft fractions isolated from human and ruminant erythrocytes. Arch Biochem Biophys. 2005;434:150–158. doi: 10.1016/j.abb.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U, le Maire M, Møller JV. The mechanism of detergent solubilization of liposomes and protein-containing membranes. Biophys J. 1998;75:2932–2946. doi: 10.1016/S0006-3495(98)77735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Suzuki KG, Kasai RS, Ritchie K, Fujiwara T K (2011) Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem Sci 36:604–615 [DOI] [PubMed]
- Lasch J. Interaction of detergents with lipid vesicles. Biochim Biophys Acta. 1995;1241:269–292. doi: 10.1016/0304-4157(95)00010-o. [DOI] [PubMed] [Google Scholar]
- Le Maire M, Champeil P, Moller JV. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta. 2000;1508:86–111. doi: 10.1016/s0304-4157(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Leidl K, Liebisch G, Richter D, Schmitz G (2008) Mass spectrometric analysis of lipid species of human circulating blood cells. Biochim Biophys Acta 1781:655–664 [DOI] [PubMed]
- Levental I, Veatch SL. The continuing mystery of lipid rafts. J Mol Biol. 2016;428:4749–4764. doi: 10.1016/j.jmb.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Momsen MM, Smaby JM, Brockman HL, Brown RE. Cholesterol decreases the interfacial elasticity and detergent solubility of sphingomyelins. Biochemistry. 2001;40:5954–5963. doi: 10.1021/bi002791n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim Biophys Acta. 1985;821:470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D, Opatowski E, Kozlov MM. Phase boundaries in mixtures of membrane-forming amphiphiles and micelle-forming amphiphiles. Biochim Biophys Acta. 2000;1508:1–19. doi: 10.1016/s0304-4157(00)00004-6. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D, Goni F, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D, Ahyaauch H, Goñi FM. The mechanism of detergent solubilization of lipid bilayers. Biophys J. 2013;105:289–299. doi: 10.1016/j.bpj.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke D. Detergents: an overview. Methods Enzymol. 2009;463:603–617. doi: 10.1016/S0076-6879(09)63034-2. [DOI] [PubMed] [Google Scholar]
- Maggio B, Fanani ML, Rosetti CM, Wilke N. Biophysics of sphingolipids II. Glycosphingolipids: An assortment of multiple structural information transducers at the membrane surface. Biochim Biophys Acta. 2006;1758:1922–1944. doi: 10.1016/j.bbamem.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Malheiros SVP, Meirelles NC, de Paula E. Pathways involved in trifluoperazine-, dibucaine-and praziquantel-induced hemolysis. Biophys Chem. 2000;83:89–100. doi: 10.1016/s0301-4622(99)00125-8. [DOI] [PubMed] [Google Scholar]
- Mattei B, França ADC, Riske KA. Solubilization of binary lipid mixtures by the detergent triton X-100: The role of cholesterol. Langmuir. 2015;31:378–386. doi: 10.1021/la504004r. [DOI] [PubMed] [Google Scholar]
- Mattei B, Lira RB, Perez KR, Riske KA. Membrane permeabilization induced by triton X-100: The role of membrane phase state and edge tension. Chem Phys Lipids. 2017;202:28–37. doi: 10.1016/j.chemphyslip.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Miseta A, Bogner P, Szark A, Kellermayer M, Galambos C, Wheatley DN, Cameronil IL. Permeability effect of non-lytic concentrations of Brij series detergents on the metabolism-independent ion properties of erythrocytes. Biophys J. 1995;69:2563–2568. doi: 10.1016/S0006-3495(95)80127-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: Elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Nomura F, Nagata M, Inaba T, Hiramatsu H, Hotani H, Takiguchi K. Capabilities of liposomes for topological transformation. Proc Natl Acad Sci U S A. 2001;98:2340–2345. doi: 10.1073/pnas.041419098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partearroyo MA, Urbaneja MA, Goñi FM. Effective detergent lipid ratios in the solubilization of phosphatidylcholine vesicles by tTriton X-100. FEBS Lett. 1992;302:138–140. doi: 10.1016/0014-5793(92)80424-f. [DOI] [PubMed] [Google Scholar]
- Partearroyo MA, Alonso A, Goñi FM, Tribout M, Paredes SJ. Solubilization of phospholipid bilayers by surfactants belonging to the triton X series: Effect of polar group size. J Colloid Interface Sci. 1996;178:156–159. [Google Scholar]
- Passot S, Fonseca F, Alarcon-Lorca M, Rolland D, Marin M. Physical characterisation of formulations for the development of two stable freeze-dried proteins during both dried and liquid storage. Eur J Pharm Biopharm. 2005;60:335–348. doi: 10.1016/j.ejpb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Pathak P, London E. Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation. Biophys J. 2011;101:2417–2425. doi: 10.1016/j.bpj.2011.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra SK, Alonso A, Goñi FM. Detergent solubilisation of phospholipid bilayers in the gel state: The role of polar and hydrophobic forces. Biochim Biophys Acta. 1998;1373:112–118. doi: 10.1016/s0005-2736(98)00095-9. [DOI] [PubMed] [Google Scholar]
- Patra SK, Alonso A, Arrondo JLR, Goñi FM. Liposomes containing sphingomyelin and cholesterol: Detergent solubilisation and infrared spectroscopic studies. J Liposome Res. 1999;9:247–260. [Google Scholar]
- Preté PSC, Malheiros SVP, Meirelles NC, de Paula E. Quantitative assessment of human erythrocyte membrane solubilization by triton X-100. Biophys Chem. 2002;97:1–5. doi: 10.1016/s0301-4622(02)00043-1. [DOI] [PubMed] [Google Scholar]
- Preté PSC, Gomes K, Malheiros SVP, Meirelles NC, de Paula E. Solubilization of human erythrocyte membranes by non-ionic surfactants of the polyoxyethylene alkyl ethers series. Biophys Chem. 2002;97:45–54. doi: 10.1016/s0301-4622(02)00042-x. [DOI] [PubMed] [Google Scholar]
- Preté PSC, de Paula E, Meirelles NC, Malheiros SVP, Goñi FM, Schreier S. Multiple stages of detergent-erythrocyte membrane interaction – A spin label study. Biochim Biophys Acta. 2011;1808:164–170. doi: 10.1016/j.bbamem.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Regen S. Lipid–lipid recognition in fluid bilayers: Solving the cholesterol mystery. Curr Opin Chem Biol. 2002;6:729–735. doi: 10.1016/s1367-5931(02)00398-8. [DOI] [PubMed] [Google Scholar]
- Requero MA, Goñi FM, Alonso A. The membrane-perturbing properties of palmitoyl-coenzyme-a and palmitoylcarnitine - a comparative study. Biochemistry. 1995;34:10400–10405. doi: 10.1021/bi00033a011. [DOI] [PubMed] [Google Scholar]
- Riske KA, Sudbrack TP, Archilha NL, Uchoa AF, Schroder AP, Marques C, Baptista MS, Itri R. Giant vesicles under oxidative stress induced by a membrane-anchored photosensitizer. Biophys J. 2009;97:1362–1370. doi: 10.1016/j.bpj.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas MG, Gennaro AM. Detergent resistant domains in erythrocyte membranes survive after cell cholesterol depletion: An EPR spin label study. Chem Phys Lipids. 2003;122:165–169. doi: 10.1016/s0009-3084(02)00189-5. [DOI] [PubMed] [Google Scholar]
- Roda A, Hoffman AF, Mysels KJ. The influence of bile salt structure on self-association in aqueous solutions. J Biol Chem. 1983;258:6362–6370. [PubMed] [Google Scholar]
- Rodi PM, Cabeza MS, Gennaro AM. Detergent solubilization of bovine erythrocytes: Comparison between the insoluble material and the intact membrane. Biophys Chem. 2006;122:114–122. doi: 10.1016/j.bpc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Rodi PM, Bocco Gianello MD, Corregido MC, Gennaro AM. Comparative study of the interaction of CHAPS and triton X-100 with the erythrocyte membrane. Biochim Biophys Acta. 2014;1838:859–866. doi: 10.1016/j.bbamem.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Goñi FM, Alonso A. Surfactant-induced release of liposomal contents. A survey of methods and results. Biochim Biophys Acta. 1988;937:127–134. doi: 10.1016/0005-2736(88)90234-9. [DOI] [PubMed] [Google Scholar]
- Salzer U, Prohaska R. Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood. 2001;97:1141–1143. doi: 10.1182/blood.v97.4.1141. [DOI] [PubMed] [Google Scholar]
- Samuel BU, Mohandas N, Harrison T, McManus H, Rosse W, Reid M, Haldar K. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J Biol Chem. 2001;276:29319–29329. doi: 10.1074/jbc.M101268200. [DOI] [PubMed] [Google Scholar]
- Schnitzer E, Kozlov MM, Lichtenberg D. The effect of cholesterol on the solubilization of phosphatidylcholine bilayers by the non-ionic surfactant triton X-100. Chem Phys Lipids. 2005;135:69–82. doi: 10.1016/j.chemphyslip.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schönfeldt N. Surface active ethylene oxide adducts. London: Pergamon; 1969. [Google Scholar]
- Schreier S, Malheiros SVP, de Paula E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim Biophys Acta. 2000;1508:210–234. doi: 10.1016/s0304-4157(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci U S A. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim Biophys Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Seeman P. Erythrocyte membrane stabilization by local anesthetics and tranquilizers. Biochem Pharmacol. 1966;15:1753–1766. doi: 10.1016/0006-2952(66)90214-0. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharm Rev. 1972;24:58–55. [PubMed] [Google Scholar]
- Sheetz MP. Integral membrane protein interaction with triton cytoskeletons of erythrocytes. Biochim Biophys Acta. 1979;557:122–134. doi: 10.1016/0005-2736(79)90095-6. [DOI] [PubMed] [Google Scholar]
- Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sonino S, Prinetti A. Membrane domains and the ‘lipid raft’ concept. Curr Med Chem. 2013;20:4–21. [PubMed] [Google Scholar]
- Sot J, Collado MI, Arrondo JLR, Alonso A, Goñi FM. Triton X-100-resistant bilayers: Effect of lipid composition and relevance to the raft phenomenon. Langmuir. 2002;18:2828–2835. [Google Scholar]
- Steck TL. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974;62:1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbrack TP, Archilha NL, Itri R, Riske KA. Observing the solubilization of lipid bilayers by detergents with optical microscopy of GUVs. J Phys Chem B. 2011;115:269–277. doi: 10.1021/jp108653e. [DOI] [PubMed] [Google Scholar]
- Tragner D, Csordas A. Biphasic interaction of triton detergents with the erythrocyte membrane. Biochem J. 1987;244:605–609. doi: 10.1042/bj2440605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamaloukas A, Szadkowska H, Heerklotz H. Nonideal mixing in multicomponent lipid/detergent systems. J Phys Condens Matter. 2006;18:1125–1138. doi: 10.1088/0953-8984/18/28/S02. [DOI] [PubMed] [Google Scholar]
- Uragami M, Tokutake N, Yan X, Regen SL. Is the linkage region of sphingolipids responsible for lipid raft formation? J Am Chem Soc. 2001;123:5124–5125. doi: 10.1021/ja015715w. [DOI] [PubMed] [Google Scholar]
- Veatch SL, Keller SL. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch SL, Keller SL. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys Rev Lett. 2005;94:148101–148104. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- Yawata Y. Cell membrane: The red blood cell as a model. Weinheim: Wiley-VCH; 2003. [Google Scholar]
- Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ. Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods. 2004;56:339–347. doi: 10.1016/j.mimet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Yu J, Fischman DA, Steck TL. Selective solubilization of proteins and phospholipids from red blood cells membranes by nonionic detergents. J Supramol Struct. 1973;1:233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]