Atherosclerotic lesions form preferentially in arterial regions characterized by slow and irregular patterns of blood flow such as those found on the inner curvature of bifurcation branch points. Due to this non-random distribution, extensive research has focused on the role of shear stress, or the mechanical drag force exerted on the endothelial lining of blood vessels. Blood flow that follows a high shear-stress, unimpeded laminar pattern encourages homeostatic mechanisms in the endothelium and protects against atherosclerosis. The transition from laminar to disturbed flow elicits changes in endothelial cell behavior that include increased inflammatory signaling through the activation of NF-κB, increased expression of leukocyte adhesion receptors and the recruitment of immune cells. Focal areas exposed to detrimental shear stress, together with the synergistic effects of dyslipidemia, age and hyperglycemia, initiate and promote the growth of atherosclerotic lesions. The mechanisms by which endothelial cells sense and respond to these changes in flow have been intensively studied but gaps in our knowledge remain.
In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Feng et al report on the ability of low shear stress to upregulate HIF1α and glycolytic programming in endothelial cells. HIF1α is a master regulator of the cellular response to hypoxia and its expression is associated with changes in metabolism, inflammation and angiogenesis1, 2. The ability of low oxygen tensions to increase atherosclerosis in ApoE mice3 and the genetic deletion of HIF1α, selectively in endothelial cells4 or macrophages5, to protect against atherosclerosis collectively suggest that hypoxia plays a pathogenic role. Arterial blood carries abundant levels of oxygen and therefore hypoxia has been hypothesized to occur deep within the core of large atherosclerotic lesions and this is supported by the detection of low oxygen concentrations and HIF1α expression within plaques in both animal models and humans6, 7. An interesting observation by Feng et al was that HIF1α expression was selectively increased in the low-flow inner curvature of non-atherosclerotic porcine aorta as well as in cultured endothelial cells exposed to low shear stress in the presence of atmospheric oxygen (findings recently confirmed by others8). This data suggests that the upregulation of HIF1α may also play a role in the initiation of atherosclerosis. How mechanical stress on the endothelium regulates HIF1α expression was an important next question. Feng et al, found that low and oscillatory shear stress on endothelial cells increased the activation of NF-κB which drives expression of HIF1α mRNA, as well as increased expression of Cezanne or OTUD7B, an editor of ubiquitin chains that preserves HIF1α protein expression9. While the ability of oscillatory shear to activate NF-κB is well described10, others have found that the ability of disturbed flow to induce HIF1α expression is mediated instead by Nox4-derived reactive oxygen species in manner that is independent of NF-κB8. Important differences between these studies include the type of cell used (HUVEC versus HAEC8), strategies to inhibit NFκB (Rel siRNA, IκBα overexpression versus a NEMO binding domain peptide) and the approaches used to model disturbed flow (orbital shaking or an Ibidi parallel-plate versus a cone and plane).
HIF1α is a key transcription factor that orchestrates metabolic reprogramming in hypoxic cells towards glycolysis. Enhanced glycolysis can also occur in normoxic cells, a phenomena first described by Warburg11. Glycolysis not only supports enhanced rates of proliferation and migration, but has emerged as a powerful regulator of angiogenesis and inflammation12–14. Feng et al and others found increased expression of glycolytic enzymes in normoxic endothelial cells exposed to low or disturbed flow in culture, as well as in partially ligated carotid arteries and atheroprone regions of porcine aorta8. HIF1α and its family member, HIF2α (EPAS1), were both upregulated by disturbed flow but increased expression of glycolytic enzymes was dependent only on HIF1α8. HIF1α and NF-κB have a complicated interrelationship which is also observed in endothelial cells exposed to changes in flow. Hypoxia, HIF1α and increased expression of glycolytic enzymes are connected with increased inflammation 15, 16 and NF-κB can drive increased HIF1α expression 17. In endothelial cells exposed to disturbed flow, silencing both HIF1α and select glycolytic enzymes decreases NF-κB activation as well as the expression of pro-inflammatory genes8. HIF1α is not the only shear stress sensitive transcription factor and previous studies have identified KLF2 as a gene that is strongly upregulated by laminar flow18 and suppressed by disturbed flow 8. In effects opposite to HIF1α, KLF2 has been shown to repress inflammatory signaling19 and glycolytic metabolism 20. Whether KFL2 impacts disturbed flow-induced upregulation of HIF1α is not yet known. This is an important question as KLF2 has been shown to potently inhibit HIF1α expression and function21. In contrast, silencing HIF1α in endothelial cells exposed to disturbed flow resulted in increased expression of KLF2 suggesting that the mechanism by which disturbed flow decreases KLF2 expression is via increased HIF1α 8.
The posttranslational modification of proteins by the addition of ubiquitin, a small 8.5kDa “ubiquitous” protein, to select lysine residues is an important regulator of protein function and cell signaling. Protein degradation is one of the best known consequence of ubiquitin modification, but ubiquitin can also alter protein conformation and function and subcellular targeting 22. In normoxic conditions HIF1α typically undergoes VHL-dependent ubiquitination and degradation23, 24. An underappreciated aspect of ubiquitin modification is its reversibility and proteins targeted for elimination can earn a reprieve through the actions of a group of enzymes known as DUBs (DeUBiquitinating enzymes). The role of DUBs in shear stress and atherosclerosis is poorly understood. Otud7b (Cezanne) is a DUB that belongs to A20 like ovarian tumor domain subfamily. In addition to targeting protein substrates with Lys48- and Lys63- ubiquitin chains, Cezanne specifically breaks ubiquitin chains linked to Lys11, endowing it with potentially important roles in regulating protein stability and signaling25. Cezanne has emerged as an important regulator of both NF-κB signaling and HIF1α expression 9, 26–28. In the study by Feng et al, Cezanne was upregulated by low flow and its expression was necessary to stabilize NF-κB-induced HIF1α protein expression. The inability of Cezanne to impact disturbed-flow induced-upregulation of NF-κB is an apparent contradiction of previous findings27, 29. The authors address this conundrum by suggesting shear stress may activate NF-kB in a manner that is immune to Cezanne mediated de-ubiquitination.
In summary, Feng et al have expanded our knowledge of the role of HIF1α in the development of atherosclerosis. In specific they show that in addition to a role in regulating intraplaque angiogenesis in advanced lesions, HIF1α also functions in the early stages of atherosclerosis to initiate lesion formation by promoting inflammatory signaling in arterial regions exposed to low or non-laminar shear stress. Endothelial cells in culture and in regions of blood vessels exposed to low and turbulent flow have increased HIF1α expression along with the upregulation of numerous glycolytic enzymes and increased inflammatory signaling through enhanced activation of NF-κB (see outline in Figure 1). While these results are in excellent agreement with a recent publication 8, important gaps in our knowledge remain including a better understanding of the shear stress-dependent signaling events leading to expression of HIF1α. A role for Nox4 in shear-stress mediated induction and stabilization of HIF1α as proposed by others8 is complicated by numerous studies showing that loss of Nox4 exacerbates atheroclerosis30 although there may be confounding temporal considerations. How shear-stress impacts Cezanne expression is also ambiguous with some publications showing little to no effect compared to proinflammatory cytokine such as TNFα and upregulation by laminar flow 26, 31. The impact of KLF2 on HIF1α expression and how Cezanne and other DUBs affect shear-dependent changes in NF-κB await further clarification. VEGF is robustly upregulated by HIF-1α and has been shown to be proatherogenic 32, but whether shear-dependent changes in VEGF have an important role is not known. The effect of low ambient oxygen concentrations on atherosclerosis is also complex and while 3 week exposure to hypoxia in ApoE null mice 3 and chronic intermittent hypoxia increase lesion burden 33, the long term adaptation to hypoxia is protective in both mice and humans at altitude 34.
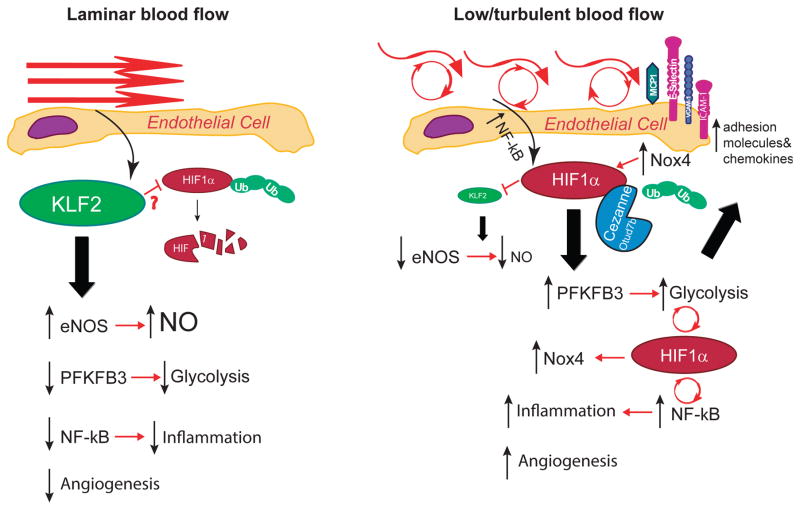
Figure 1. Mechanosensitive pathways in endothelial cells subject to (A) laminar flow or (B) disturbed flow.
Laminar shear stress upregulates KLF2 which has been shown to inhibit HIF1α by promoting its degradation and collectively these events lead to increased expression of homeostatic enzymes such as eNOS, inhibition of NF-κB and inflammation, decreased angiogenesis and suppression of key glycolytic enzymes such as PFKFB3. In contrast, turbulent flow and oscillatory shear stimulate NF-κB which induces HIF1α resulting in the loss of KLF2. HIF1α protein expression is stabilized by the upregulation of Cezanne which removes ubiquitin modifications and also by Nox4. HIF1α drives increased glycolysis, inflammatory signaling via NF-κB and expression of adhesion molecules as well as increased angiogenesis.
Acknowledgments
Sources of Funding
This study was supported by grants from National Institutes of Health, 1R01HL124773-01A1, 1R01HL125926-01A1
References
- 1.Carmeliet P, Dor Y, Herbert JM, et al. Role of hif-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakano D, Hayashi T, Tazawa N, Yamashita C, Inamoto S, Okuda N, Mori T, Sohmiya K, Kitaura Y, Okada Y, Matsumura Y. Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein e-knockout mice. Hypertens Res. 2005;28:837–845. doi: 10.1291/hypres.28.837. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar S, Hartmann P, Karshovska E, Rinderknecht FA, Subramanian P, Gremse F, Grommes J, Jacobs M, Kiessling F, Weber C, Steffens S, Schober A. Endothelial hypoxia-inducible factor-1alpha promotes atherosclerosis and monocyte recruitment by upregulating microrna-19a. Hypertension (Dallas, Tex: 1979) 2015;66:1220–1226. doi: 10.1161/HYPERTENSIONAHA.115.05886. [DOI] [PubMed] [Google Scholar]
- 5.Aarup A, Pedersen TX, Junker N, Christoffersen C, Bartels ED, Madsen M, Nielsen CH, Nielsen LB. Hypoxia-inducible factor-1alpha expression in macrophages promotes development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1782–1790. doi: 10.1161/ATVBAHA.116.307830. [DOI] [PubMed] [Google Scholar]
- 6.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, Gassmann M, Gardner LB, Fisher EA. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circulation research. 2011;109:1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu D, Huang RT, Hamanaka RB, Krause M, Oh MJ, Kuo CH, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, Mutlu GM. Hif-1alpha is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife. 2017:6. doi: 10.7554/eLife.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bremm A, Moniz S, Mader J, Rocha S, Komander D. Cezanne (otud7b) regulates hif-1alpha homeostasis in a proteasome-independent manner. EMBO reports. 2014;15:1268–1277. doi: 10.15252/embr.201438850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 11.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 12.Cheng SC, Quintin J, Cramer RA, et al. Mtor- and hif-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (New York, NY) 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoors S, De Bock K, Cantelmo AR, et al. Partial and transient reduction of glycolysis by pfkfb3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, Kandala S, Li Q, Li H, Zhang C, Caldwell RB, Fulton DJ, Su Y, Hoda MN, Zhou G, Wu C, Huo Y. Endothelial pfkfb3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34:1231–1239. doi: 10.1161/ATVBAHA.113.303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa b through the phosphorylation of i kappa b alpha on tyrosine residues. Cancer research. 1994;54:1425–1430. [PubMed] [Google Scholar]
- 16.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. Hif-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. Nf-kappab links innate immunity to the hypoxic response through transcriptional regulation of hif-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung kruppel-like factor (klf2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 19.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. Klf2 is a novel transcriptional regulator of endothelial proinflammatory activation. The Journal of experimental medicine. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doddaballapur A, Michalik KM, Manavski Y, Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M, Dimmeler S, Boon RA. Laminar shear stress inhibits endothelial cell metabolism via klf2-mediated repression of pfkfb3. Arterioscler Thromb Vasc Biol. 2015;35:137–145. doi: 10.1161/ATVBAHA.114.304277. [DOI] [PubMed] [Google Scholar]
- 21.Kawanami D, Mahabeleshwar GH, Lin Z, Atkins GB, Hamik A, Haldar SM, Maemura K, Lamanna JC, Jain MK. Kruppel-like factor 2 inhibits hypoxia-inducible factor 1alpha expression and function in the endothelium. The Journal of biological chemistry. 2009;284:20522–20530. doi: 10.1074/jbc.M109.025346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: Implications for human pathobiology. Annual review of pharmacology and toxicology. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 23.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of hif-alpha to the von hippel-lindau ubiquitylation complex by o2-regulated prolyl hydroxylation. Science (New York, NY) 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 24.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of hif1alpha ubiquitination by a reconstituted von hippel-lindau (vhl) tumor suppressor complex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mevissen TET, Kulathu Y, Mulder MPC, Geurink PP, Maslen SL, Gersch M, Elliott PR, Burke JE, van Tol BDM, Akutsu M, Oualid FE, Kawasaki M, Freund SMV, Ovaa H, Komander D. Molecular basis of lys11-polyubiquitin specificity in the deubiquitinase cezanne. Nature. 2016;538:402–405. doi: 10.1038/nature19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enesa K, Zakkar M, Chaudhury H, Luong le A, Rawlinson L, Mason JC, Haskard DO, Dean JL, Evans PC. Nf-kappab suppression by the deubiquitinating enzyme cezanne: A novel negative feedback loop in pro-inflammatory signaling. The Journal of biological chemistry. 2008;283:7036–7045. doi: 10.1074/jbc.M708690200. [DOI] [PubMed] [Google Scholar]
- 27.Luong le A, Fragiadaki M, Smith J, Boyle J, Lutz J, Dean JL, Harten S, Ashcroft M, Walmsley SR, Haskard DO, Maxwell PH, Walczak H, Pusey C, Evans PC. Cezanne regulates inflammatory responses to hypoxia in endothelial cells by targeting traf6 for deubiquitination. Circulation research. 2013;112:1583–1591. doi: 10.1161/CIRCRESAHA.111.300119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe J, Berk BC. Cezanne paints inflammation by regulating ubiquitination. Circulation research. 2013;112:1526–1528. doi: 10.1161/CIRCRESAHA.113.301518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enesa K, Evans P. The biology of a20-like molecules. Advances in experimental medicine and biology. 2014;809:33–48. doi: 10.1007/978-1-4939-0398-6_3. [DOI] [PubMed] [Google Scholar]
- 30.Fulton DJ, Barman SA. Clarity on the isoform-specific roles of nadph oxidases and nadph oxidase-4 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:579–581. doi: 10.1161/ATVBAHA.116.307096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nature medicine. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 33.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JG, Sung HJ, Amar MJ, Pryor M, Remaley AT, Allen MD, Noguchi AC, Springer DA, Kwon J, Chen J, Park JH, Wang PY, Hwang PM. Low ambient oxygen prevents atherosclerosis. J Mol Med (Berl) 2016;94:277–286. doi: 10.1007/s00109-016-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]