Abstract
Although borderline personality disorder (BPD) traits decline from adolescence to adulthood, comorbid psychopathology such as symptoms of major depressive disorder (MDD), alcohol use disorder (AUD), and drug use disorders (DUDs) likely disrupt this normative decline. Using a longitudinal sample of female twins (N = 1,763), we examined if levels of BPD traits were correlated with changes in MDD, AUD, and DUD symptoms from ages 14–24. A parallel process biometric latent growth model examined the contributions of genetic and environmental factors to the relationships between developmental components of these phenotypes. Higher BPD trait-levels predicted a greater rate of increase in AUD and DUD symptoms, and higher AUD and DUD symptoms predicted a slower rate of decline of BPD traits from ages 14–24. Common genetic influences accounted for the associations between BPD traits and each disorder, as well as the interrelationships of AUD and DUD symptoms. Both genetic and nonshared environmental influences accounted for the correlated levels between BPD traits and MDD symptoms, but solely environmental influences accounted for the correlated changes between the two over time. Results indicate that higher levels of BPD traits may contribute to an earlier onset and faster escalation of AUD and DUD symptoms, and substance use problems slow the normative decline in BPD traits. Overall, our data suggests that primarily genetic influences contribute to the comorbidity between BPD features and substance use disorder symptoms. We discuss our data in the context of two major theories of developmental psychopathology and comorbidity.
Keywords: Borderline Personality Disorder, Alcohol Use Disorder, Drug Use Disorder, Major Depressive Disorder, Comorbidity, longitudinal stability and change, adolescence
Borderline personality disorder (BPD) is characterized by affective instability, impulsivity, impaired social functioning, and identity disturbance (American Psychiatric Association, 2013). BPD is related to a number of negative outcomes including interpersonal and occupational impairment, and suicide (Skodol et al., 2002). Particularly concerning are the high rates of comorbidity between BPD and other psychiatric disorders, including major depressive disorder (MDD), alcohol use disorder (AUD), and illicit drug use disorders (DUD) (McGlashan et al., 2000; Zanarini et al., 2004; Zimmerman & Mattia, 1999). For those who meet diagnostic criteria for BPD, lifetime comorbidity rates with MDD and AUD or DUD have been reported as high as 32.1% and 30.9%, respectively (Grant et al., 2008). Moreover, the association between BPD and several areas of functional impairment can be attributed to a general impairment shared between BPD and MDD, AUD, and DUDs (Lenzenweger, Lane, Loranger, & Kessler, 2007), although there is evidence that BPD predicts functional impairment over-and-above co-morbid psychopathology (Skodol et al., 2002). In order to better inform treatment frameworks for BPD, it is especially important to investigate the developmental origins and etiological influences that contribute to this comorbidity.
Normative Developmental Change
In epidemiological samples, BPD prevalence and trait levels decline from mid-adolescence into young adulthood (Bernstein et al., 1993; Bornovalova, Hicks, Iacono, & McGue, 2009; Cohen, Crawford, Johnson, & Kasen, 2005; Johnson et al., 2000; Lenzenweger, 1999). There is also evidence for similar declines in adolescents and adults with clinically elevated levels of BPD symptoms (Cohen et al., 2005; Grilo et al., 2004; Johnson et al., 2000; Lenzenweger, 1999; Meijer, Goedhart, & Treffers, 1998; Paris, Brown, & Nowlis, 1987; Shea et al., 2002; Zanarini, Frankenburg, Hennen, & Silk, 2003). The normative decline in BPD levels is likely due to developmental changes associated with personality maturation. Indeed, declines in the normal range personality traits of negative emotionality and behavioral disinhibition – traits that are core to the definition of BPD – show most pronounced changes from late adolescence to young adulthood (Durbin et al., 2016; Johnson et al., 2007; Roberts, Caspi, & Moffitt, 2001). In contrast, MDD prevalence rates spike in mid-adolescence and increase into young adulthood, after which they remain relatively stable. Risk for AUD and DUD increases in late adolescence, peaks in young adulthood, and then shows a substantial decline by age 30 (Chassin, Flora, & King, 2004; Compton, Thomas, Stinson, & Grant, 2007; Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Merikangas et al., 2010; Wittchen, Nelson, & Lachner, 1998). The rise and subsequent decline in AUD and DUD symptoms likely represents extreme versions of maturational changes in behavioral disinhibition. Likewise, the rise in MDD symptoms during adolescence likely represents maladaptive variation in neuroticism and social problem-solving (Keller & Nesse, 2005; Moffitt, 1993; Nettle, 2006; Watson & Andrews, 2002).
Evidence for Common Cause, Predisposition, and Pathoplasty Models
Several models have been proposed to account for personality-psychopathology associations, most notably the predisposition, pathoplasty, and common cause models (Durbin & Hicks, 2014). The predisposition model posits that BPD traits increase risk for the onset of a new disorder. The pathoplasty model predicts that BPD traits affect the course of disorders, such that higher BPD traits contribute to greater severity and/or chronicity of a disorder. The common cause model posits that some third variable, such as emotion dysregulation or disinhibition, accounts for the comorbidity between BPD traits and other disorders. While each of these models could independently account for BPD-psychopathology associations, it is important to note that they can operate simultaneously. Also, these models ignore normative developmental shifts in the mean-levels of traits and the prevalence of disorders, and thus do not account for how these age-related changes might contribute to the comorbidity between BPD traits and other disorders.
The common cause model has received the majority of empirical attention and suggests that BPD traits and symptoms of comorbid disorders are derived partly from shared neurobiological influences. Seminal theories posit that BPD traits result partly from the interplay between the same environmental risk (e.g., traumatic life events) and pre-existing, biologically influenced tendencies that exacerbate comorbid disorders, such as negative emotionality, sensitivity to emotional cues, and behavioral disinhibition (Linehan, 1993). Given that impulsivity is related to BPD traits, AUD, and DUD symptoms throughout development, it has received special attention in efforts to identify a common diathesis (Berlin, Rolls, & Iversen, 2005; Brodsky et al., 2001; De Wit, 2009; Dick et al., 2010). Recent models of BPD further emphasize developmental processes that contribute to heterotypic continuity (Kaufman, Crowell, & Stepp, 2015) and include evidence from behavior genetic methodology and neuroimaging to support the notion that impulsivity is a common feature of BPD traits and AUD and DUD symptoms (Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, 2009; Crowell, Beauchaine, & Linehan, 2009). These models posit that the expression of an underlying biological system for a phenotype, such as trait impulsivity (Coccaro et al., 1989; Friedel, 2004), can gives rise to multiple maladaptive phenotypic outcomes in the context of other biological and environmental influences (Beauchaine et al., 2009). Similarly, neuroticism has been considered as one possible source in BPD-MDD comorbidity (Distel et al., 2009; Hettema, Neale, Myers, Prescott, & Kendler, 2006; Soldz, Budman, Demby, & Merry, 1993; Trull, 1992; Clarkin, Hull, Cantor, Sanderson, 1993; Soldz, Budman, Demby, & Merry, 1993).
Building on well-established evidence that differences in neural responses to rewarding stimuli covary with impulsivity (Martin & Potts, 2004; Wilbertz et al., 2012), recent neuroimaging evidence suggests that reduced activation in neural regions that process rewards are associated with higher levels of BPD traits, such as self-injurious behavior (Sauder, Derbidge, & Beauchaine, 2016). Similar findings have been observed in participants with MDD symptoms and elevated alcohol or substance use (Beck et al., 2009; Jentsch & Taylor, 1999; Must et al., 2006). Further evidence suggests that BPD patients and participants with lesions in brain regions associated with reward processing, such as the orbitofrontal cortex, exhibit elevated impulsivity, inappropriate behavior, and other common BPD characteristics compared with a control group (Berlin, Rolls, & Iversen, 2005; Forbes et al., 2009). With respect to the BPD-MDD comorbidity, recent neuroimaging work suggests that variability in structure and function of the prefrontal cortex, anterior cingulate, hippocampus, and amygdala may represent a neurobehavioral risk factor for development of neuroticism that has downstream effects on BPD and MDD comorbidity (Cremers et al., 2010; Davidson, Pizzagalli, Nitschke, & Putnam, 2002; DeYoung et al., 2010; Driessen et al., 2000; Haas, Omura, Constable, & Canli, 2007; Hazlett et al., 2005; Soloff et al., 2003; Tzschoppe et al., 2014). These data emphasize the importance of neurodevelopmental influences on BPD and commonly comorbid psychopathology, and provide suggestive evidence that the common cause model may partially explain co-development and change in BPD traits and symptoms of comorbid psychopathology. However, a longitudinal test of the common cause model is needed.
In a prior study with a subset of the present sample, we provided a test of the predisposition model for BPD traits and a composite of substance use (tobacco, alcohol, and marijuana quantity and frequency) in 14 and 18 year old female twins (Bornovalova, Hicks, Iacono, & McGue, 2013). BPD traits and substance use were correlated at each age, but BPD traits at age 14 did not predict substance use at age 18 after accounting for the stability of BPD traits and substance use (and vice versa). These results were consistent with a common cause but not predisposition model; however, they failed to rule out the pathoplasty model. Additionally, while this study focused on quantity and frequency of substance use, it did not examine the association with substance use disorder symptoms.
Finally, there is accumulating evidence for the pathoplasty model. For example, recurrent or chronic MDD is associated with a failure to exhibit normative declines in the personality trait of stress reaction, while persistent AUD is associated with a lack of normative decline in aggression and behavioral disinhibition (Durbin & Hicks, 2014). Given the strong connection between BPD traits and these normal-range personality traits, it is also likely that BPD traits will fail to decline when accompanied by chronic MDD and substance use disorder symptoms; conversely, the remission of comorbid disorders may facilitate a reduction in BPD traits. For instance, a large community sample of adolescent females reported that increasing BPD symptoms were associated with worsening social and mental health outcomes (Wright, Zalewski, Hallquist, Hipwell, & Stepp, 2015). Likewise, adult clinical samples report that the rate of decline in BPD traits is associated with the rate of decline for the personality trait of neuroticism (Wright, Hopwood, & Zanarini, 2015), as well as decline in co-occurring MDD, AUD, and DUD symptoms (De Panfilis et al., 2011; Gunderson et al., 2008; Zanarini et al., 2011; Zanarini Frankenburg, Hennen, Reich, & Silk, 2004). Data from the Collaborative Longitudinal Personality Disorder Study, for instance, indicated that patients with BPD and MDD negatively impacted each other’s time to remission and accelerated time to relapse (Grilo et al., 2005; Gunderson et al, 2014); similar results have been reported for substance use disorders (Walter et al., 2009). Likewise, results from the McLean Study of Adult Development have repeatedly documented that initial levels and remission status in BPD predict change in major depressive, drug, and alcohol use disorders (and vice versa; Frankenburg, Fitzmaurice, & Zanarini, 2014; Zanarini et al., 2004; Zanarini et al, 2011; Zanarini, Frankenburg, Hennen, Reich, & Silk, 2005).
Current Study
We sought to advance our understanding of the association between BPD traits and symptoms of comorbid disorders in a number of ways. First, we focused on examining both the pathoplasty and common cause models of BPD traits and AUD, DUD, and MDD symptoms (due to the lack of support in our prior research with this sample, we did not examine the predisposition model). By including symptoms of multiple disorders we were able to examine convergent and discriminant processes contributing to BPD trait-levels and symptoms of commonly co-occurring disorders. Second, we extended the developmental period of interest from age 14 to 24, a period when there are declines in BPD traits and large increases in MDD, AUD, and DUD symptoms. Notably, most studies that have examined the comorbidity between BPD traits and other disorders have used adult samples (Gunderson et al., 2008; Zanarini et al., 2004) with less developmental change in these traits and disorders. Third, we used our twin sample to estimate genetic and environmental contributions to BPD traits and their overlap with symptoms of the disorders. Several large cross-sectional studies have investigated both the heritability of BPD traits (Distel et al., 2011; Distel et al., 2009; Distel et al., 2008; Kendler et al., 2008; Kendler et al., 2011; Reichborn-Kjennerud et al., 2015; Reichborn-Kjennerud et al., 2010; Torgersen et al., 2008; Torgersen et al., 2000) as well as the genetic and environmental overlap between BPD traits and MDD, AUD, and DUD symptoms in adults (Distel et al., 2012; Kendler et al., 2011). The current study extends this work to modeling the etiological influences on longitudinal relationships as well. Finally, we modeled the cross-sectional and longitudinal relationships between BPD features and MDD, AUD, and DUD symptoms simultaneously. This allowed us to test the common cause and pathoplasty models for multiple disorders, thereby connecting the current study to the broader developmental psychopathology literature.
A small but growing literature indicates that BPD tendencies are influenced roughly equally by additive and non-additive genetic (35–50%) and non-shared environmental (50–60%) sources (Distel et al., 2009; Distel et al., 2008; Kendler et al., 2008; Kendler et al., 2011), Genetic factors account for much of the association between BPD traits with AUD and DUD symptoms in adults (Bornovalova et al., 2013; Distel et al., 2012; Kendler et al., 2011), whereas the association with MDD symptoms is largely accounted by environmental factors (Kendler et al., 2011). These previous studies have been almost exclusively cross-sectional and focused on adults. We hoped to extend this work to the co-development between BPD traits and symptoms of multiple disorders from mid-adolescence through young adulthood.
We also capitalized on the longitudinal nature of the data to fit biometric latent growth models to better understand the co-development between BPD traits and symptoms of MDD, AUD, and DUD (as well as the interrelationships within MDD, AUD, and DUD symptoms). Evidence consistent with the common cause model exists if there are significant correlations between the intercepts (average levels) and slopes (linear rate of change) for BPD traits and symptoms of MDD, AUD, and DUD. Common cause would also be inferred if common genetic or environmental influences accounted for any of the cross-sectional or longitudinal relationships between BPD traits and MDD, AUD, and DUD symptoms. Evidence consistent with the pathoplasty model exists if the intercept for BPD traits are correlated with the slopes of MDD, AUD, and DUD symptoms (and vice versa); that is, if average levels of BPD traits predicted a more severe course (i.e., greater increase) in symptoms of the disorder. Likewise, pathoplasty would be inferred if high average levels of MDD, AUD, and DUD symptoms are associated with a lack of normative declines in BPD trait levels (i.e., shallower slopes).
Method
Sample
Participants were adolescent female twins taking part in the Minnesota Twin Family Study (MTFS), an ongoing population-based, longitudinal study of twins and their families (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Keyes et al., 2009). Birth records and public databases were used to locate more than 90% of families that included a twin birth in the state of Minnesota from 1975 to 1984 and 1988 to 1994. Eligible twins and their families were a) living within a one-day drive of Minneapolis with at least one biological parent, and b) had no mental or physical handicap precluding participation. All protocols were approved by the institutional review board. Parents and children gave informed consent or assent as appropriate.
The MTFS intake sample includes an 11-year-old and a 17-year-old cohort consisting of same-sex male and female monozygotic (MZ) and dizygotic (DZ) twins. The current study focused on the female twins, as the male twins only had BPD data at two assessment time points. Intake and follow-up assessments are scheduled to coincide with major transitions in the lives of adolescents and young adults. BPD traits were first assessed at age 14 for the younger cohort, and at age 17 for the older cohort. Follow-up assessments of BPD traits were conducted at age 17 and 24 in the younger cohort and at ages 20 and 24 in the older cohort. In the current study, the cohorts were combined and matched by age of assessment. The actual ages at each assessment (corrected for in all analyses) were 14.88 (SD = .57), 17.89 (SD = .69), 20.83 (SD = .61), and 25.09 (SD = .73). AUD, DUD, and MDD symptoms were assessed using the same time points and procedures, then combined and corrected for age variability in the same manner as BPD traits. Retention rates were excellent with approximately 90% participation at each wave.
The final sample included 1121 MZ (or identical) twins and 642 DZ (or fraternal) twins. The sample sizes for each assessment time point were as follows: age 14 (N = 1080; 674 twins from MZ pairs, 406 twins from DZ pairs); age 17 (N = 1602; 1026 twins from MZ pairs, 576 twins from DZ pairs); age 20 (N = 1389; 879 twins from MZ pairs, 510 twins from DZ pairs); age 24 (N = 1260; 797 twins from MZ pairs, 463 twins from DZ pairs). Zygosity was determined by agreement among 3 estimates: MTFS staff evaluations of the twins’ physical similarity; parents’ completion of a standard zygosity questionnaire; and twin similarity on an algorithm of ponderal and cephalic indices and fingerprint ridge count. A serological analysis was performed if the 3 estimates did not agree. Consistent with the racial/ethnic makeup of the recruitment area, 95.3% of the sample was white. Additionally, the mean maternal and paternal years of education among the families of origin were 13.57 (2.09) and 13.67 (2.61), respectively.
Measures
BPD Traits
The Minnesota Borderline Personality Disorder Scale (MBPD; Bornovalova, Hicks, Patrick, Iacono, & McGue, 2011) is a self-report inventory of BPD traits that provides a dimensional measure of BPD trait severity. The MBPD was developed using items from the Multidimensional Personality Questionnaire (MPQ; Tellegen, 1982; Patrick, Curtin, & Tellegen, 2002) and asks participants to respond to 19-items on a 4-point scale (agree, somewhat agree, somewhat disagree, disagree). Items for the MBPD were selected due to overlap with BPD symptomatology and were drawn from the following MPQ scales: stress reaction (e.g., Mood often fluctuates), alienation (e.g., Often betrayed by friends), control (e.g., Often act impulsively), aggression (e.g., Sometimes enjoy saying mean things), well-being (e.g., Rarely feel happy), and absorption (e.g. Sometimes feel presence of people not actually there). While the MPQ was designed to measure normal personality functioning, the MBPD scale is correlated with established self-report measures of BPD traits (rs= 0.80–0.89) and BPD diagnosis and symptom counts (rs = 0.60–0.66, respectively) from structured interviews in community and clinical samples (Bornovalova et al., 2011; Rojas et al., 2014; Rojas, Hicks, Stark, Hopwood, & Bornovalova, 2015). MBPD scores also show high reliability and temporal stability (Rojas et al., 2015) as well as theoretically expected associations with criterion variables in the BPD trait nomological network, such as impulsivity, antisocial behaviors, interpersonal problems, MDD symptoms, and alcohol and drug use (Bornovalova et al., 2011; Bornovalova et al., 2013; Rojas et al., 2013; Rojas et al., 2015). In this sample, internal consistency reliability was > .80 for all follow-ups, and the score distributions exhibited minimal skew and kurtosis (< 1 for all time points). Therefore, MBPD scores can be used to make reasonable inferences about BPD traits. To adjust for any variability in age at each assessment, the BPD trait data were conditioned on target age (ages 14, 17, 20, or 24 depending on the assessment wave; i.e., age variability around each assessment point regressed out; Bornovalova et al., 2009; Johnson et al., 2007). Raw scores are plotted for descriptive purposes in Figure 1. To aid with model fitting in all non-descriptive analyses, MBPD scores were recoded to range between 1 (maximum possible score) and 0 (minimum possible score) using the following equation: [(MBPD individual score-min)/(max-min) = (MBPD individual score-19)/57]. This rescaling minimized the differences in variance between the variables in the models while maintaining all the original properties of the MBPD scores.
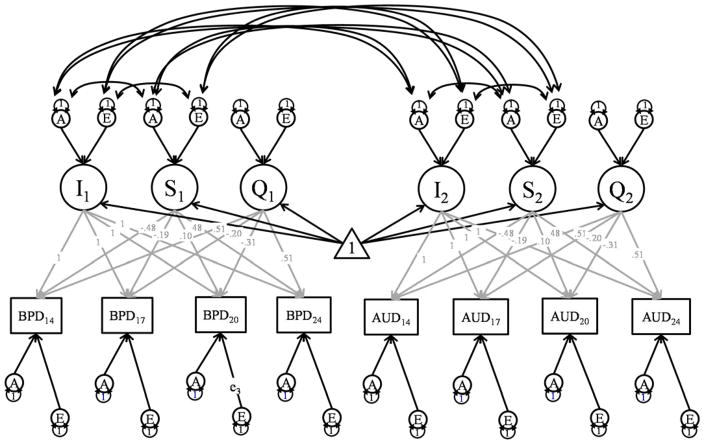
Figure 1.
Bivariate Growth Model for One Twin
Note: BPD refers to Borderline Personality Disorder traits, while AUD refers to Alcohol Use Disorder symptoms. In the full model, BPD traits, AUD, MDD, and DUD were included. An abbreviated figure is presented to increase clarity. Each assessment point is assumed to reflect an effect of general level of the phenotype/intercept (I), linear slope (S), and quadratic slope (Q), and assessment-specific additive genetic (A), shared environmental (C), and nonshared environmental effects (E). The intercept and slope effects are also decomposed into correlated additive genetic, shared environmental, and nonshared environmental effects. Subscripted numbers refer to the age of measurement, and numbers above the grey paths coefficients indicate fixed factor loadings for the growth parameters. Common Environmental effects were estimated in the fully saturated model but they were subsequently removed, as they did not improve the fit of the model.
MDD, AUD and DUD Symptoms
Symptoms of MDD, AUD, and DUD were assessed using the Diagnostic Interview for Children and Adolescents – Revised (DICA-R) (Reich & Welner, 1988), the Structured Clinical Interview for DSM-III-R (Spitzer, Williams, Gibbon, & First, 1987), and the Substance Abuse Module of the Composite International Diagnostic Interview (Robbins, Cottler, & Babor, 1990). Clinical interviews were conducted by trained staff members at the University of Minnesota. Maternal reports of these symptoms were also collected at ages 14 and 17. Consistent with evidence that each informant provides unique information in the assessment of psychopathology, we utilized a best estimate approach and considered a symptom present if reported by either the twin or parent (Burt, Krueger, McGue, & Iacono, 2003; Burt, Krueger, McGue, & Iacono, 2001). An established diagnostic consensus procedure was used to maximize accuracy of symptom assignment. For each participant, a clinical case conference consisting of at least two advanced clinical psychology graduate students was conducted in which all interview data was reviewed, referring to audiotapes when necessary. The two diagnosticians were required to reach consensus regarding symptom presence prior to assigning any symptoms. Once consensus was reached regarding the presence/absence of symptoms, a computer algorithm was used to determine diagnosis. The consensus process yielded diagnostic kappa reliabilities that ranged from 0.71 to 0.92. All symptom counts were log transformed to reduce skew and kurtosis (see Table 1 for means and SDs, as well as the range of the raw and log-transformed scales). As with MBPD scores, the MDD, AUD, and DUD data were conditioned on target age to account for any variability in age at each assessment.
Table 1.
Descriptive statistics and twin Correlations and 95% CIs for Borderline Personality Disorder (BPD) traits, Major Depressive Disorder (MDD) symptoms, Alcohol Use Disorder (AUD) symptoms, and Drug Use Disorder (DUD) symptoms across ages 14 to 24
| Raw Mean (SD) | Raw Range | Transformed Mean (SD) | Transformed Range | Twin Correlations | ||
|---|---|---|---|---|---|---|
| rMZ | rDZ | |||||
| BPD14 | 41.14 (9.53) | 18.45, 70.78 | .39 (0.17) | −.01,.91 | .49 (.40, .57) | .38 (.25, .50) |
| BPD17 | 40.71 (8.72) | 19.39, 70.59 | .38 (0.15) | .01,.91 | .50 (.43, .57) | .27 (.16, .38) |
| BPD20 | 37.78 (8.52) | 18.16, 64.82 | .33 (0.15) | −.01,.80 | .37 (.24 .49) | .23 (.03, .41) |
| BPD24 | 35.53 (7.84) | 18.14, 62.39 | .29 (0.14) | −.02,.76 | .48 (.40, .56) | .23 (.10, .35) |
| MDD14 | .72 (1.89) | 0, 9 | .71 (0.61) | 0, 2.84 | .30 (.20, .40) | .30 (.16, .42) |
| MDD17 | 1.28 (2.49) | 0, 9 | 1.27 (0.78) | 0, 3.17 | .35 (.27, .42 | .29 (.18, .39) |
| MDD20 | .96 (2.22) | 0, 9 | .96 (0.7) | 0, 2.95 | .27 (.18 .36) | .21 (.09, .33) |
| MDD24 | 1.25 (2.53) | 0, 9 | 1.25 (0.78) | 0, 3.22 | .19 (.09, .29) | .17 (.04, .30) |
| AUD14 | .07 (0.48) | 0, 9 | .04 (0.2) | −.18, 2.32 | .52 (.44, .59) | .30 (.16, .43) |
| AUD17 | .34 (1.06) | 0, 9 | .15 (0.42) | −.32, 2.34 | .55 (.49, .61 | .17 (.05, .29) |
| AUD20 | .45 (1.12) | 0, 9 | .25 (0.47) | −.28, 2.39 | .29 (.20 .38) | .25 (.13, .37) |
| AUD24 | .52 (1.23) | 0, 9 | .27 (0.5) | −.24, 2.34 | .37 (.28, .45) | .13 (.00, .26) |
| DUD14 | .08 (0.57) | 0, 8 | .04 (0.23) | −.1, 2.21 | .35 (.25, .44) | .43 (.30, .54) |
| DUD17 | .27 (1.09) | 0, 9 | .11 (0.39) | −.43, 2.34 | .47 (.40 .54 | .36 (.25, .46) |
| DUD20 | .34 (1.19) | 0, 9 | .16 (0.44) | −.13, 2.39 | .35 (.27 .43) | .36 (.25, .46) |
| DUD24 | .29 (1.03) | 0, 9 | .13 (0.4) | −.02, 2.31 | .55 (.48, .62) | .12 (−.01, .24) |
Note: MDD, AUD, and DUD symptom variables underwent a log+1 transformation for all analyses, after which they were corrected for age variability around each age of assessment (accounting for negative values under transformed range); MZ and DZ correlations are presented for the log-transformed values.
Analytic Approach
We fit a multivariate biometric latent growth model to BPD traits and MDD, AUD, and DUD symptoms between the ages of 14 and 24. This model allows us to simultaneously model the developmental trajectories of each phenotype using a set of latent factors that estimate the average level (intercept) and rate of change (both linear and quadratic) in each phenotype, as well as the covariance between the latent growth parameters (Molenaar & Rovine, 1998; Neale & McArdle, 2000; Singer & Willett, 2003).1,2 Multivariate growth models allowed us to examine the covariance between the latent growth parameters across phenotypes, such as a) the correlation between the intercept of BPD traits and the intercept of the other phenotypes (e.g., do people with higher levels of BPD traits have more symptoms of other psychopathology?); b) the correlation between the linear slope of BPD traits and the linear slope of the other phenotypes (e.g., do people who decrease in BPD traits more slowly also have a faster rate of increase in other symptoms of psychopathology?), c) the correlation between the intercept of BPD traits and the linear slope of the other phenotypes (e.g., do people with higher BPD traits increase faster in AUD symptoms?); d) the correlation between the linear slope of BPD traits and the intercept of the other phenotype (e.g., do BPD traits decrease more slowly among people with higher average levels of AUD symptoms?). (a) and (b) provide evidence for common cause, whereas (c) and (d) provide evidence of pathoplasty.
We used the twin data to decompose the variance of the latent growth parameters into additive genetic (A - additive effect of individual genes summed over loci on trait variance), shared environmental (C - environmental influences that increase similarity between members of a twin pair), and unshared environmental (E - environmental factors that contribute to differences between members of a twin pair, including measurement error) variance components. This allowed us to examine genetic and environmental influences on the co-development between BPD traits and MDD, AUD, and DUD symptoms. To determine the best fitting model for each phenotype, we progressively dropped parameters for the variance components and time specific residuals and compared model fit of nested models to identify a parsimonious model that was consistent across all four phenotypes (see supplemental materials for model-fitting results). Because the common environmental factors did not significantly improve the fit of any models, we present the results with these parameters fixed at zero. Finally, we estimated the genetic and environmental contributions to covariance between growth parameters for BPD traits and the other phenotypes. A path depiction of the bivariate growth model for one twin (which generalizes to the multivariate case) is presented in Figure 1. All models were fit with Full Information Maximum Likelihood (which is robust to most forms of missingness) in OpenMx 2.0 (Little & Rubin, 1983; Neale et al., 2016).
Results
Within Trait Biometric Latent Growth Parameters
Descriptive statistics for all of the variables, such as means, standard deviations, and twin correlations by zygosity for all phenotypes and ages are reported in Table 1. We present the means, variances, and proportion of additive genetic (A) and unique environmental (E) variance of the latent growth factors for each phenotype in Table 2, and a spaghetti plot of the trajectories of each phenotype in Figure 2. Consistent with our previous report, the negative slope for BPD traits indicated that trait BPD significantly decreased from ages 14 to 24 (Bornovalova et al., 2009). In contrast, MDD, AUD, and DUD symptoms all had positive linear slopes, indicating that on average there was an increase in symptoms for each disorder from ages 14 to 24. Biometric model fitting (Table 3) indicated that the best fitting biometric models included only genetic and nonshared environmental components for the latent growth factors and residual variances of each phenotype at each assessment. Results from the biometric variance decompositions (Table 2) revealed that the intercept of each phenotype was highly heritable (0.64 to 0.78) with small to medium nonshared environmental influences (0.36 to 0.22). The linear slope for BPD traits and AUD and DUD symptoms exhibited significant genetic influences (0.33 to 0.65), while the linear slope for MDD symptoms was mostly determined by non-shared environment influences (0.82). Finally, although the quadratic variance component did not have sufficient variation to reliably decompose into genetic and environmental influences, it improved model fit and was subsequently retained in all models3 .
Table 2.
Model Predicted Means and Total Variances and Standardized Genetic and Unique Environmental Variance Components of Borderline Personality Disorder (BPD) traits, Major Depressive Disorder (MDD) symptoms, Alcohol Use Disorder (AUD) symptoms, and Drug Use Disorder (DUD) symptoms for the univariate phenotypes.
| BPD Traits | MDD Symptoms | AUD Symptoms | DUD Symptoms | ||
|---|---|---|---|---|---|
| Intercept | Mean | .41 (.40, .42) | 1.08 (1.05, 1.12) | .20 (.18, .22) | .13 (.11, .15) |
| Total Variance | .02 (.01, .02) | .14 (.12, .17) | .06 (.05, .06) | .06 (.05, .07) | |
| Proportion A Variance | .64 (.57, .71) | .64 (.50, .77) | .74 (.64, .83) | .78 (.71, .83) | |
| Proportion E Variance | .36 (.30, .43) | .36 (.23, .50) | .26 (.17, .36) | .23 (.17, .29) | |
|
| |||||
| Linear Slope | Mean | −.12 (−.14, −.11) | .49 (.42, .55) | .24 (.21, .27) | .10 (.07, .14) |
| Total Variance | .02 (.01, .02) | .34 (.17, .53) | .17 (.14, .20) | .18 (.14, .21) | |
| Proportion A Variance | .33 (.14, .62) | .18 (.00, .62) | .50 (.31, .68) | .65 (.53, .77) | |
| Proportion E Variance | .67 (.40, .86) | .82 (.38, 1.00) | .50 (.32, .69) | .36 (.23, .47) | |
|
| |||||
| Quadratic Slope | Mean | −.01 (−.02, .00) | −.15 (−.21, −.09) | −.08 (−.10, −.05) | −.07 (−.10, −.05) |
| Total Variance | .01 (.00, .01) | .03 (.00, .13) | .03 (.02, .04) | .02 (.01, .04) | |
| Proportion A Variance | .00 (.00, .30) | .00 (.00, 1.00) | .00 (.00, .36) | .76 (.14, 1.00) | |
| Proportion E Variance | 1.00 (.70, 1.00) | 1.00 (.00, 1.00) | 1.00 (.64, 1.00) | .24 (.00, .86) | |
Note: All symptom counts were subjected to a log transformation [log(x+1)] to reduce skew and kurtosis. To aid with model fitting, MBPD was recoded to range between 1 (maximum possible score) and 0 (minimum possible score) [(MBPD individual score-min) (max-min) = (MBPD individual score-19)/57); accordingly, the intercept can be interpreted as the level (form 0 to 1), and the slope can be interpreted as percent change over the 10 year interval.
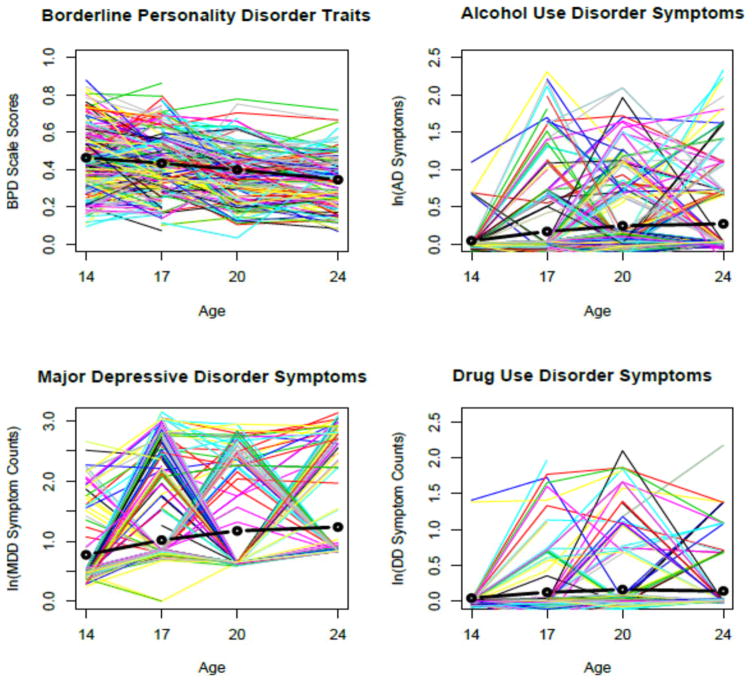
Figure 2.
Predicted Growth trajectories for Borderline Personality Disorder traits, Major Depressive disorder symptoms, Alcohol Use Disorder symptoms, and Drug Use Disorder symptoms.
Note: Each point in the graphs represent expected means for the respective phenotype, while the black lines depict mean trajectories. The surrounding lines are 250 randomly sampled individuals from the dataset that illustrate variability around the mean trajectory. MDD, AUD, and DUD symptom variables underwent a log+1 transformation for all analyses, and log-transformed data are plotted above. MBPD was recoded to range between 1 (maximum possible score) and 0 (minimum possible score) [(MBPD individual score-min) (max-min) = (MBPD individual score-19)/57).
Table 3.
Biometric model fitting results for the Univariate Latent Growth Models for Borderline Personality Disorder (BPD) traits, Major Depressive Disorder (MDD) symptoms, Alcohol Use Disorder (AUD) symptoms, and Drug Use Disorder (DUD) symptoms.
| Model | ep | −2LL | df | AIC | −2LL | df | p |
|---|---|---|---|---|---|---|---|
| Borderline Personality Disorder Traits | |||||||
| Full Model | 27 | −5083.33 | 4313 | −13709.33 | |||
| No Common Environment* | 19 | −5079.28 | 4321 | −13721.28 | - | - | - |
| No Correlation between Latent Growth Factors | 17 | −5074.85 | 4323 | −13720.85 | 4.43 | 2 | .109 |
| No A Component for the Latent Growth Factors | 15 | −4876.55 | 4325 | −13526.55 | 202.73 | 4 | .000 |
| No E Component for the Latent Growth Factors | 15 | −4875.07 | 4325 | −13525.07 | 204.21 | 4 | .000 |
| No Specific A Component for the time points | 15 | −5061.39 | 4325 | −13711.39 | 17.89 | 4 | .000 |
| Major Depressive Disorder Symptoms | |||||||
| Full Model | 27 | 11362.79 | 5277 | 808.7853 | |||
| No Common Environment* | 19 | 11369.94 | 5285 | 799.94 | |||
| No Correlation between Latent Growth Factors | 17 | 11382.73 | 5287 | 808.73 | 12.79 | 2 | .002 |
| No A Component for the Latent Growth Factors | 15 | 11425.13 | 5289 | 847.13 | 55.19 | 4 | .000 |
| No E Component for the Latent Growth Factors | 15 | 11408.97 | 5289 | 830.97 | 39.02 | 4 | .000 |
| No Specific A Component for the time points | 15 | 11492.39 | 5289 | 914.39 | 122.45 | 4 | .000 |
| Alcohol Use Disorder Symptoms | |||||||
| Full Model | 27 | 4102.93 | 5279 | −6455.067 | |||
| No Common Environment* | 19 | 4104.01 | 5287 | −6469.99 | |||
| No Correlation between Latent Growth Factors | 17 | 4214.28 | 5289 | −6363.72 | 110.27 | 2 | .000 |
| No A Component for the Latent Growth Factors | 15 | 4260.9 | 5291 | −6321.1 | 156.9 | 4 | .000 |
| No E Component for the Latent Growth Factors | 15 | 4158.97 | 5291 | −6423.03 | 54.97 | 4 | .000 |
| No Specific A Component for the time points | 15 | 4191.17 | 5291 | −6390.83 | 87.17 | 4 | .000 |
| Drug Use Disorder Symptoms | |||||||
| Full Model | 27 | 2979.85 | 5277 | −7574.153 | |||
| No Common Environment* | 19 | 2982 .00 | 5285 | −7588 | |||
| No Correlation between Latent Growth Factors | 17 | 3130.05 | 5287 | −7443.95 | 148.05 | 2 | .000 |
| No A Component for the Latent Growth Factors | 15 | 3200.70 | 5289 | −7377.3 | 218.7 | 4 | .000 |
| No E Component for the Latent Growth Factors | 15 | 3050.72 | 5289 | −7527.28 | 68.72 | 4 | .000 |
| No Specific A Component for the time points | 15 | 3037.44 | 5289 | −7540.56 | 55.44 | 4 | .000 |
Note: ep, estimated parameters; −2LL, −2 log likelihood; AIC, Akaike Information Criterion.
As the common environment did not significantly contribute to variation in any of the phenotypes, it was dropped from the analysis. Accordingly, the No Common Environment model is the model against which subsequent reduced models were tested, and it was always the best fitting model.
Cross-Phenotype Biometric Latent Growth Parameters
Table 4 presents the phenotypic correlations, Table 5 presents the additive genetic correlations, and Table 6 presents the unique environmental correlations for the growth parameters across the four phenotypes. Because we focused on the relationship between BPD traits and the three other phenotypes, the first two columns of Tables 4–6 are of primary interest. The other columns speak to broader co-development of MDD, AUD, and DUD symptoms.
Table 4.
Total correlations between the latent growth parameters for Borderline Personality Disorder (BPD) traits, Major Depressive Disorder (MDD) symptoms, Alcohol Use Disorder (AUD) symptoms, and Drug Use Disorder (DUD) symptoms.
| BPD intercept | BPD slope | AUD intercept | AUD slope | MDD intercept | MDD slope | DUD intercept | DUD slope | |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| BPD intercept | 1.00 | |||||||
| BPD slope | −.02 (−.15,.07) | 1.00 | ||||||
| AUD intercept | .44 (.37, .50) | −.12 (−0.22, −0.02) | 1.00 | |||||
| AUD slope | .26 (.18, .35) | .17 (.06, .29) | .57 (.49, .63) | 1.00 | ||||
| MDD intercept | .56 (.49, .63) | .02 (−.09, .13) | .39 (.30, .48) | .26 (.16, .37) | 1.00 | |||
| MDD slope | .02 (−.09, .14) | .49 (.33, .71) | .11 (−.02, .25) | .23 (.08, .38) | .35 (.18, .53) | 1.00 | ||
| DUD intercept | .32 (.26, .39) | −.15 (−.26, −.06) | .71 (.65, .76) | .38 (.29, .47) | .46 (.38, .53) | −.11 (−.23, .02) | 1.000 | |
| DUD slope | .11 (.02, .19) | .15 (.05, .27) | .24 (.14, .34) | .59 (.49, .68) | .24 (.14, .35) | .05 (−.09, .19) | .56 (.48, .64) | 1.00 |
Note: Key relationships of interest between BPD intercept and slope and the intercept and slope of MDD, AUD, and DUD are separated by vertical lines. All significant relationships are bolded.
Table 5.
Genetic correlations between the latent growth parameters for Borderline Personality Disorder (BPD) traits, Major Depressive Disorder (MDD) symptoms, Alcohol Use Disorder (AUD) symptoms, and Drug Use Disorder (DUD) symptoms.
| BPD intercept | BPD slope | AUD intercept | AUD slope | MDD intercept | MDD slope | DUD intercept | DUD slope | |
|---|---|---|---|---|---|---|---|---|
| BPD intercept | 1.00 | |||||||
| BPD slope | −.22 (−.42, −.02) | 1.00 | ||||||
| AUD intercept | .46 (.35, .56) | −.24 (−.49, −.02) | 1.00 | |||||
| AUD slope | .18 (.03, .33) | .45 (.18, .78) | .41 (.25, .53) | 1.00 | ||||
| MDD intercept | .62 (.51, .74) | .00 (−.27, .28) | .41 (.26, .56) | .36 (.16,.56) | 1.00 | |||
| MDD slope | .22 (−.12, .56) | .55 (−.05, .95) | −.02 (−.48, .32) | .59 (.12, .94) | .46 (−.08, .78) | 1.00 | ||
| DUD intercept | .37 (.27, .47) | −.20 (−.42, −.01) | .72 (.63, .80) | .31 (.17, .46) | .50 (.37, .63) | −.25 (−.59, .84) | 1.00 | |
| DUD slope | .06 (−.08, .19) | .36 (.12, .64) | .11 (−.04, .26) | .60 (.44, .77) | .35 (.17, .53) | .30 (−.12, .70) | .56 (.44, .68) | 1.00 |
Note: Key relationships of interest between BPD intercept and slope and the intercept and slope of MDD, AUD, and DUD are separated by vertical lines. All significant relationships are bolded.
Table 6.
Nonshared environmental correlations between the latent growth parameters for Borderline Personality Disorder (BPD) traits, Major Depressive Disorder (MDD) symptoms, Alcohol Use Disorder (AUD) symptoms, and Drug Use Disorder (DUD) symptoms.
| BPD intercept | BPD slope | AUD intercept | AUD slope | MDD intercept | MDD slope | DUD intercept | DUD slope | |
|---|---|---|---|---|---|---|---|---|
| BPD intercept | 1.00 | |||||||
| BPD slope | .16 (−.07, .35) | 1.00 | ||||||
| AUD intercept | .41 (.24, .58) | −.001 (−.22, .22) | 1.00 | |||||
| AUD slope | .40 (.23, .58) | −.06 (−.28, .14) | .93 (.81, .98) | 1.00 | ||||
| MDD intercept | .45 (.28, .62) | .03 (−.19, .26) | .36 (.12, .60) | .11 (−.14, .36) | 1.00 | |||
| MDD slope | −.12 (−.35, .09) | .47 (.22, .81) | .28 (−.02, .56) | .01 (−.30, .31) | .34 (.01, .66) | 1.00 | ||
| DUD intercept | .21 (.06, .35) | −.13 (−.34, .05) | .68 (.48, .87) | .58 (.37, .80) | .36 (.15, .58) | .01 (−.24, .28) | 1.00 | |
| DUD slope | .20 (.05,.37) | −.04 (−.25, .15) | .56 (.35, .79) | .57 (.36, .82) | .04 (−.19, .27) | −.14 (−.42, .12) | .57 (.41 .71) | 1.00 |
Note: Key relationships of interest between BPD intercept and slope and the intercept and slope of MDD, AUD, and DUD are separated by vertical lines. All significant relationships are bolded.
Evidence for common cause
Consistent with the common cause model, the BPD trait intercept was significantly correlated with the intercepts for MDD, AUD, and DUD symptoms, indicating that those with higher BPD trait levels tended to experience more symptoms of MDD, AUD, and DUD. The intercept for BPD traits showed significant genetic and moderate nonshared environmental correlations with the intercepts of MDD, AUD, and DUD symptoms (Tables 4 and 5), indicating greater genetic relative to environmental overlap between BPD traits and the other phenotypes. A similar pattern was evident for MDD, AUD, and DUD symptoms: the intercepts were significantly correlated with each other, and evidenced similar pattern of genetic and environmental covariation.
Next, the linear slope for BPD traits was correlated with the linear slopes for MDD, AUD, and DUD symptoms. This indicates that after accounting for the average level, greater increases in symptoms of MDD, AUD, and DUD were associated with a slower decline in BPD traits. Alternatively, this could be stated as the smaller the increase in symptoms of MDD, AUD, and DUD, the greater the decline in BPD traits. The genetic correlation between the slopes was significant and of moderate effect, while the nonshared environmental correlations were not significant, with one notable exception. The nonshared environmental correlation between BPD trait slope and MDD symptom slope was moderate (.47) and significant. No other nonshared environmental correlations approached significance. Therefore, genetic influences primarily accounted for the associations between the linear slopes of BPD traits and AUD and DUD symptoms. In contrast, nonshared environmental influences primarily accounted for the association between the linear slopes of BPD traits and MDD symptoms. With respect to other phenotypes, MDD symptom slope predicted AUD but not DUD symptom slope. This relationship was accounted for by overlapping genetic factors, with no significant evidence for nonshared environmental overlap. AUD and DUD symptom slopes were significantly correlated, showing strong and roughly equal influences of common genetic and nonshared environmental factors.
Evidence for pathoplasty
The intercept for BPD traits was correlated with the linear slope of AUD and DUD symptoms, but not MDD symptoms. Consistent with the pathoplasty hypothesis, individuals who had higher levels of BPD traits increased in AUD and DUD symptoms more rapidly. Furthermore, the nonshared environmental correlations between BPD trait intercept and the linear slopes of AUD and DUD symptoms were significant and contributed more to the covariance than genetic factors. The linear slope for BPD traits was negatively correlated with the intercepts of AUD and DUD symptoms, and unrelated to the MDD symptoms intercept. This indicates that individuals with more AUD and DUD symptoms were associated with a slower rate of decline in BPD traits, even after adjusting for the average trait level. The associations between the linear slope of BPD traits and the intercepts of AUD and DUD symptoms were almost entirely due to genetic influences. As for the other phenotypes, MDD symptom intercept was positively correlated with AUD and DUD symptom slope (but not vice versa), with this relationship accounted for solely by genetic overlap. Unsurprisingly, AUD symptom intercept was correlated with DUD symptom slope (and vice versa), with somewhat more robust nonshared environmental than genetic overlap. Taken together, the slope-intercept correlations across all four phenotypes suggest that an individual’s comorbid psychopathology is associated with the trajectory of other maladaptive traits.
Discussion
Using a large, longitudinal twin sample, we conducted the first study to investigate the genetic and environmental influences on the co-development between BPD traits and MDD, AUD, and DUD symptoms from middle adolescence to young adulthood. Previous studies have reported that BPD traits tend to decline over time, and that declines in BPD traits are associated with declines in the symptoms of comorbid disorders (Bornovalova et al., 2009; Gunderson et al., 2008; Lenzenweger, 1999). Few studies, however, have considered the developmental context of the associations between BPD traits and symptoms of other disorders—specifically, during a period when there are dramatic changes in the mean-levels of BPD traits, MDD, AUD, and DUD symptoms—allowing for hypotheses about developmental processes that might contribute to these associations. Next, no study has examined the genetic and environmental influences on the co-development between BPD traits and symptoms of other disorders. Finally, the current paper simultaneously modeled the total, genetic, and nonshared environmental correlations between BPD traits, MDD, AUD, and DUD symptoms. Results from this model align the current paper with the broader models within developmental psychopathology that suggest that disorders rarely develop discretely and independently. Rather, there is an interdependence and interaction among multiple forms of psychopathology that necessitate a whole-organism approach.
In terms of developmental change, we replicated previous epidemiological findings that BPD traits decreased, while symptoms of MDD, AUD, and DUD increased from ages 14 to 24 (Chen & Jacobson, 2012; Dussault, Brendgen, Vitaro, Wanner, & Tremblay, 2011). The decline in BPD traits was consistent with longitudinal research on normal personality development that finds large declines in negative emotionality and behavioral disinhibition during the transition from adolescence into young adulthood (Johnson et al., 2000; Roberts, Walton, & Viechtbauer, 2006). Our findings suggest similar maturational processes may contribute to age-related declines in BPD traits.
The current results support two models of comorbidity: the common cause model and the pathoplasty models. For common cause, we detected medium to large associations between a) the average level of BPD traits and the average levels of the other disorders and b) changes in BPD traits and changes in other disorders associations. Consistent with previous work (Distel et al., 2012; James & Taylor, 2008; Kendler et al., 2011), these associations were primarily due to genetic influences. Beyond BPD, there were large genetic correlations between MDD, AUD, and DUD as well. The data suggest that there are transdiagnostic, heritable influences (e.g., temperament traits) that contribute to the average level as well as the covariation in change of BPD traits, MDD, AUD, and DUD symptoms from ages 14 to 24.
Importantly, correlated rates of change provide especially strong evidence of a common cause model, suggesting that a similar process is affecting fluctuations in both BPD traits and symptoms of other psychopathology. Also consistent with a common cause model, there was substantial genetic and environmental overlap between the intercepts and slopes among BPD traits, AUD, DUD, and MDD symptoms. Interestingly, genetic influences primarily accounted for the correlated changes between BPD traits and AUD and DUD symptoms, while environmental influences primarily accounted for the correlated changes between BPD traits and MDD symptoms. Also, the correlations between the slope for BPD traits and the changes in AUD and DUD symptoms were small, while the correlation between the slope of BPD traits and MDD symptoms was large. Consistent with previous cross-sectional reports (Kendler et al., 2011), common nonshared environmental factors also accounted for the correlated slopes between BPD traits and MDD symptoms. The large, nonshared environmental correlation stands in contrast to the moderate to large genetic correlations between the growth parameters for BPD traits and AUD and DUD, and suggests that a markedly different process is driving the correlated rates of change between BPD traits and MDD symptoms. While complex processes involving both genetic and environmental factors likely contribute to the co-development of BPD traits and symptoms of commonly comorbid psychopathology, it is possible that environmental experiences (e.g., stressful life events, changes in relationships) have more pronounced effects on correlated changes in both BPD traits and MDD symptoms, with more gradual maturational changes in heritable characteristics (e.g., behavioral disinhibition) accounting for the majority of correlated changes between BPD traits and AUD and DUD symptoms.
On a broader level, the genetic correlations among the multiple forms of psychopathology are consistent with recent findings from molecular genetic research (Lichtenstein, Carlstrom, Rastam, Gillberg, & Anckarsater, 2010; Maier et al., 2015). The cross-disorder group from the Psychiatric Genetics Consortium, for instance, reported that some genetic risk factors seem to be shared across multiple disorders (attention deficit hyperactivity disorder, autism spectrum disorder, bipolar disorder, MDD, and schizophrenia; Smoller et al., 2013). Like previous work, our results provide support for the common cause and pathoplasty models across multiple forms of psychopathology. However, unlike existing molecular genetic work, we were able to model longitudinal relationships. This allowed us to begin disentangling common cause and pathoplasty at a finer level.
Given our results, an obvious question concerns identification of the common causes underlying the co-development between BPD traits and symptoms of other disorders. One possibility is common genetic variants influence both disorders – a possibility that should be explored in the cross-disorder group of the Psychiatric Genetics Consortium. As briefly alluded to above, a likely answer is that broad neurobehavioral processes such as negative emotionality, rejection sensitivity, emotional dysregulation, or behavioral disinhibition have non-specific effects and contribute to a diverse set of phenotypes including internalizing and externalizing disorders (Beauchaine, Gatzke-Kopp, & Mead, 2007; Iacono et al., 1999; Lenzenweger, 2010; Lilienfeld, 2003; Silk, Steinberg, & Morris, 2003; Young et al., 2009). With regard to behavioral disinhibition, the literature clearly documents elevated impulsivity in patients with BPD, AUD, and DUDs (Lee, Bagge, Schumacher, & Coffey, 2010; Links, Heslegrave, Mitton, Vanreekum, & Patrick, 1995; Wilson, Fertuck, Kwitel, Stanley, & Stanley, 2006) – suggesting that the latter serves as a process common to the two disorders (Bornovalova, Lejuez, Daughters, Rosenthal, & Lynch, 2005; Trull, Sher, Minks-Brown, Durbin, & Burr, 2000).
Common neurobiological changes throughout development may account for the correlated slopes between BPD traits and AUD and DUD symptoms. Developmental imaging studies suggest that the brain systems governing the socioemotional, cognitive, and decision-making processes that contribute to disinhibited behavior exhibit dramatic remodeling during middle and late adolescence (Spear, 2010; Wahlstrom, White, & Luciana, 2010). Distinct changes in adolescent neural processing of social and emotional information are thought to impact the functioning of the prefrontal cortex and subsequent self-regulation of motivational states and impulsive behavior (Ernst & Fudge, 2009; Spear, 2010; Steinberg, 2007). Notably, development of these networks involve changes in the amygdala, nucleus accumbens, orbitofrontal cortex, medial prefrontal cortex, and superior temporal sulcus (Nelson, Leibenluft, McClure, & Pine, 2005) that occur at the same time as a dramatic remodeling of the dopaminergic neural reward system (Spear, 2000; Wahlstrom et al., 2010). The reward system also shows enhanced responsivity to anticipating and receiving rewards of disinhibited behavior in adolescence relative to adulthood (Bjork et al., 2004; Ernst & Fudge, 2009; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Joseph, Liu, Jiang, Lynam, & Kelly, 2009). Cross-sectionally, structural and functional variability in these systems has been documented in BPD, AUDs, and DUDs (Bowirrat & Oscar-Berman, 2005; Everitt & Robbins, 2005; Friedel, 2004; Hyman, Malenka, & Nestler, 2006; Kelley & Berridge, 2002; Koob, 1992; Makris et al., 2008; Wrase et al., 2007). Competition between the socioemotional and cognitive control system (Drevets & Raichle, 1998), altered timing in the maturation of these systems (Steinberg, 2007), or incomplete functional integration of neural signals implicated in the socioemotional and cognitive control systems (Stevens, 2009) may be driving the correlated changes in BPD, AUD, and DUD as well. While the latter notion is speculative, data from large, epidemiological projects that include imaging (e.g., Adolescent Brain and Cognitive Development; National Consortium on Alcohol and Neurodevelopment in Adolescence) will be able to test it empirically.
Several findings were also consistent with the pathoplasty model. First, average levels of BPD traits were associated with greater increases in AUD and DUD symptoms, suggesting that BPD traits increase the likelihood of a transition from use to problem use and the escalation of AUD and DUD symptoms. Likewise, AUD and DUD symptoms were associated with a slower decline in BPD traits, suggesting substance use disorder symptoms disrupt maturational processes and contribute to the persistence of BPD traits. This result is consistent with other studies of BPD comorbidity in adolescence and adulthood (Burke & Stepp, 2012; Rohde, Lewinsohn, Kahler, Seeley, & Brown, 2001; Zanarini et al., 2004). For instance, Zanarini et al. (2004) reported that the absence of substance use disorders at a 6-year follow-up was the strongest predictor of the remission of BPD in patients diagnosed with BPD.
Additionally, pathoplasty was evident for symptoms of MDD on AUD and DUD symptoms. That is, higher mean-levels of MDD symptoms were associated with greater increases in symptoms of AUD and DUD. Notably, this association was not reversed; that is, greater mean-levels of AUD and DUD symptoms were not associated with changes in MDD symptoms. Prior work on has provided mixed evidence on the temporal ordering of MDD and substance use problems, with some studies reporting that drug and alcohol use predicts MDD levels (Bovasso, 2001; Rohde et al., 2001; Stice, Burton, & Shaw, 2004), others that MDD symptoms predict substance use symptoms (Abraham & Fava, 1999), and still others reporting reciprocal relationships (Hettema, Prescott, & Kendler, 2003). Differences in sample composition and measurement approaches may account for many of the disparities. Our results suggest that unipolar mood symptoms are indicative of a more severe and persistent course of AUDs and DUDs.
When examining the evidence for the common cause and the pathoplasty hypotheses, two important observations should be underscored. First, it appears that the evidence for pathoplasty is more associated with unique environmental factors, while the evidence for common cause is more associated with common/shared genetic factors, with the notable exception of the MDD intercept correlations with AUD and DUD linear slopes. Second, the magnitudes of the relationships that support the common cause hypothesis are slightly stronger than those that support the pathoplasty hypothesis.
Although unique, these findings are qualified by several limitations. First, nearly all participants were females of European American ancestry, limiting generalizability. BPD has traditionally been investigated in samples of middle to upper class, educated Caucasian females in psychiatric inpatient facilities (Silk, Lee, Hill, & Lohr, 1995; Zanarini, Frankenburg, Khera, & Bleichmar, 2001; Zanarini et al., 2002). However, it is possible that BPD-psychopathology relationships may differ across gender and ethnic groups, particularly considering evidence that rates of psychopathology, including personality disorders, differ across racial/ethnic groups (McGilloway, Hall, Lee, & Bhui, 2010; Turner & Gil, 2002). Second, the rate of BPD diagnosis is unknown in the current sample. Nevertheless, rates of similar disorders (e.g., antisocial personality disorder) are consistent with large, representative samples (Hamdi & Iacono, 2014). Third, the MBPD scale is a self-report questionnaire (albeit, well-validated) that provides a dimensional/trait rather than symptom measure of BPD. In contrast, MDD, AUD, and DUD symptoms were clinician-determined symptom counts. The differences in measurement introduce the potential concern of method effect that is not accounted for in the data, and likely reduces the effect sizes between BPD traits and the disorders given the different method of measurement. While interviews are often assumed to be superior to questionnaire measures, there is limited evidence for the incremental validity of one method over the other, though each approach has particular strengths and weaknesses (Hopwood et al., 2008). Ideally, the current study should be replicated using a multi-method (i.e., trait- and interview-based measures) approach. Finally, the current study was not set up to arbitrate between common cause and pathoplasty hypotheses (although the two may operate simultaneously).
Together, our results are indicative of meaningful co-developmental processes between BPD traits and MDD, AUD, and DUD symptoms. Moreover, our results further highlight the construct validity of the MBPD. The availability of a quantitative and dimensional measure of BPD traits that can be applied in a general population sample will further bridge the gap between psychopathology and normal behavior – a perspective consistent with the National Institute of Mental Health’s Research Domain Criteria framework (Insel et al., 2010). Our results set the stage for several future studies. The most apparent follow-up study would investigate temperamental and environmental processes that account for the genetic and environmental correlations between BPD traits and MDD, AUD, and DUD symptoms. One approach is to test if there is a corresponding reduction in the genetic correlation between BPD traits and AUD symptoms after accounting for a preexisting vulnerability (e.g., behavioral disinhibition). Next, this work should be replicated in another sample – preferably using a multi-method approach to the measurement of psychopathology. Likewise, this study should be replicated using males and more diverse samples. Finally, there is a clear need to extend these models of comorbidity into middle and late adulthood, as there is a clear drop-off in our understanding of BPD comorbidity past middle adulthood. Exploration of these questions will provide further insight into etiological influences on BPD and its comorbidity with other psychopathology throughout the lifespan.
Acknowledgments
Data for this project were collected at the University of Minnesota. This work was supported by National Institute of Drug Abuse Grant DA05147, DA034606, DA 036216, and P30DA028807 and National Institute on Alcohol Abuse and Alcoholism grants AA09367 and AA015621. Marina A. Bornovalova was supported by National Institute of Drug Abuse Grant DA032582. Brad Verhulst was supported by National Institute of Drug Abuse Grants DA026119 and DA018637. Brian M. Hicks was supported by DA025868. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. No conflict of interest exists for any of the authors.
Footnotes
For these models, factor loadings on the latent growth factors varied to account for differing measurement intervals. Also, orthogonal polynomial contrast weights were used for the linear and quadratic loadings to remove non-essential multicollinearity between the latent factors. The intercept was anchored at the age midpoint (~age 19).
Negligible variance for higher-order growth factors – such as quadratic growth factors – is common and correlations with parameters with virtually zero variance are generally uninformative and can lead to empirical under-identification. Accordingly, the models estimated the variance of the quadratic parameter but not the covariance of the quadratic parameter with the intercept or linear factors.
The confidence intervals for the quadratic term were large and included both 0 and 1. In our models, its correlation with other growth parameters was fixed to zero. The intercept-slope correlations of the individual phenotypes was included in the models and is reported elsewhere (Bornovalova et al, 2009).
References
- Tellegen A. Brief manual for the Multidimensional Personality Questionnaire. University of Minnesota; Minneapolis: 1982. [Google Scholar]
- Abraham HD, Fava M. Order of onset of substance abuse and depression in a sample of depressed outpatients. Comprehensive Psychiatry. 1999;40(1):44–50. doi: 10.1016/s0010-440x(99)90076-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74(2):174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: A Biology× Sex× Environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21(03):735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, … Heinz A. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66(8):734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity, and the orbitofrontal cortex. American Journal of Psychiatry. 2005;162(12):2360–2373. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Cohen P, Velez CN, Schwabstone M, Siever LJ, Shinsato L. Prevalence and stability of the DSM-III-R personality-disorders in a community-based survey of adolescents. American Journal of Psychiatry. 1993;150(8):1237–1243. doi: 10.1176/ajp.150.8.1237. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/jneurosci.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, McGue M. Stability, change, and heritability of borderline personality disorder traits from adolescence to adulthood: A longitudinal twin study. Development and Psychopathology. 2009;21(4):1335–1353. doi: 10.1017/S0954579409990186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, McGue M. Longitudinal Twin Study of Borderline Personality Disorder Traits and Substance Use in Adolescence: Developmental Change, Reciprocal Effects, and Genetic and Environmental Influences. Personality Disorders-Theory Research and Treatment. 2013;4(1):23–32. doi: 10.1037/a0027178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Patrick CJ, Iacono WG, McGue M. Development and validation of the Minnesota Borderline Personality Disorder scale. Assessment. 2011;18(2):234–252. doi: 10.1177/1073191111398320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Huibregtse BM, Hicks BM, Keyes M, McGue M, Iacono W. Tests of a direct effect of childhood abuse on adult borderline personality disorder traits: a longitudinal discordant twin design. Journal of Abnormal Psychology. 2013;122(1):180. doi: 10.1037/a0028328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Lejuez CW, Daughters SB, Rosenthal MZ, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clinical Psychology Review. 2005;25(6):790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bovasso GB. Cannabis abuse as a risk factor for depressive symptoms. American Journal of Psychiatry. 2001;158(12):2033–2037. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2005;132B(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Oquendo M, Ellis SP, Haas GL, Malone KM, Mann JJ. The relationship of childhood abuse to impulsivity and suicidal behavior in adults with major depression. American Journal of Psychiatry. 2001;158(11):1871–1877. doi: 10.1176/appi.ajp.158.11.1871. [DOI] [PubMed] [Google Scholar]
- Burke JD, Stepp SD. Adolescent Disruptive Behavior and Borderline Personality Disorder Symptoms in Young Adult Men. Journal of Abnormal Child Psychology. 2012;40(1):35–44. doi: 10.1007/s10802-011-9558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono W. Parent-child conflict and the comorbidity among childhood externalizing disorders. Archives of General Psychiatry. 2003;60(5):505–513. doi: 10.1001/archpsyc.60.5.505. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono WG. Sources of covariation among attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: The importance of shared environment. Journal of Abnormal Psychology. 2001;110(4):516–525. doi: 10.1037/0021-843x.110.4.516. [DOI] [PubMed] [Google Scholar]
- Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113(4):483–498. doi: 10.1037/0021-843x.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chen P, Jacobson KC. Developmental trajectories of substance use from early adolescence to young adulthood: Gender and racial/ethnic differences. Journal of Adolescent Health. 2012;50(2):154–163. doi: 10.1016/j.jadohealth.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkin JF, Hull JW, Cantor J, Sanderson C. Borderline personality disorder and personality traits: A comparison of SCID-II BPD and NEO-PI. Psychological Assessment. 1993;5:472. [Google Scholar]
- Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB, … Davis KL. Serotonergic studies in patients with affective and personality disorders: correlates with suicidal and impulsive aggressive behavior. Archives of General Psychiatry. 1989;46(7):587–599. doi: 10.1001/archpsyc.1989.01810070013002. [DOI] [PubMed] [Google Scholar]
- Cohen P, Crawford TN, Johnson JG, Kasen S. The Children in the Community Study of developmental course of personality disorder. Journal of Personality Disorders. 2005;19(5):466–486. doi: 10.1521/pedi.2005.19.5.466. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States - Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJA, … Roelofs K. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49(1):963–970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality: Elaborating and extending linehan’s theory. Psychological Bulletin. 2009;135(3):495. doi: 10.1037/a0015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- De Panfilis C, Politi V, Fortunati R, Cazzolla R, Scaramuzzino M, Marchesi C, Maggini C. Two-year follow-up of borderline personality disorder patients in Italy: A preliminary report on prognosis and prediction of outcome. International Journal of Social Psychiatry. 2011;57(5):528–537. doi: 10.1177/0020764010368619. [DOI] [PubMed] [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing Predictions From Personality Neuroscience: Brain Structure and the Big Five. Psychological Science. 2010;21(6):820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Review: understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15(2):217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Middeldorp CM, Trul TJ, Derom CA, Willemsen G, Boomsma DI. Life events and borderline personality features: The influence of gene-environment interaction and gene-environment correlation. Psychological Medicine. 2011;41(4):849–860. doi: 10.1017/S0033291710001297. [DOI] [PubMed] [Google Scholar]
- Distel MA, Rebollo-Mesa I, Willemsen G, Derom CA, Trull TJ, Martin NG, Boomsma DI. Familial Resemblance of Borderline Personality Disorder Features: Genetic or Cultural Transmission? Plos One. 2009;4(4) doi: 10.1371/journal.pone.0005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, de Moor MMH, Vink JM, Geels LM, van Beek JHDA, … Boomsma DI. Borderline personality traits and substance use: Genetic factors underlie the association with smoking and ever use of cannabis, but not with high alcohol consumption. Journal of Personality Disorders. 2012;26(6):867–879. doi: 10.1521/pedi.2012.26.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, … Boomsma DI. Heritability of borderline personality disorder features is similar across three countries. Psychological Medicine. 2008;38(9):1219–1229. doi: 10.1017/s0033291707002024. [DOI] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Willemsen G, Vink JM, Derom CA, Lynskey M, … Boomsma DI. The Five-Factor Model of Personality and Borderline Personality Disorder: A Genetic Analysis of Comorbidity. Biological Psychiatry. 2009;66(12):1131–1138. doi: 10.1016/j.biopsych.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12(3):353–385. [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, … Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Hicks BM. Personality and Psychopathology: A Stagnant Field in Need of Development. European Journal of Personality. 2014;28(4):362–386. doi: 10.1002/per.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin CE, Hicks BM, Blonigen DM, Johnson W, Iacono WG, McGue M. Personality Trait Change Across Late Childhood to Young Adulthood: Evidence for Nonlinearity and Sex Differences in Change. European Journal of Personality. 2016;30(1):31–44. doi: 10.1002/per.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussault F, Brendgen M, Vitaro F, Wanner B, Tremblay RE. Longitudinal links between impulsivity, gambling problems and depressive symptoms: a transactional model from adolescence to early adulthood. Journal of Child Psychology and Psychiatry. 2011;52(2):130–138. doi: 10.1111/j.1469-7610.2010.02313.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Forbes E, Brown S, Kimak M, Ferrell R, Manuck S, Hariri A. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14(1):60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenburg FR, Fitzmaurice GM, Zanarini MC. The Use of Prescription Opioid Medication by Patients With Borderline Personality Disorder and Axis II Comparison Subjects: A 10-Year Follow-Up Study. Journal of Clinical Psychiatry. 2014;75(4):357–361. doi: 10.4088/JCP.13m08557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel RO. Dopamine dysfunction in borderline personality disorder: a hypothesis. Neuropsychopharmacology. 2004;29(6):1029–1039. doi: 10.1038/sj.npp.1300424. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in Reward Processing and Its Influence on Inhibitory Control in Adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, … Ruan WJ. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: Results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2008;69(4):533–545. doi: 10.4088/JCP.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Sanislow CA, Gunderson JG, Pagano ME, Yen S, Zanarini MC, … McGlashan TH. Two-Year Stability and Change of Schizotypal, Borderline, Avoidant, and Obsessive-Compulsive Personality Disorders. Journal of Consulting and Clinical Psychology. 2004;72(5):767–775. doi: 10.1037/0022-006X.72.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Sanislow CA, Shea MT, Skodol AE, Stout RL, Gunderson JG, … McGlashan TH. Two-year prospective naturalistic study of remission from major depressive disorder as a function of personality disorder comorbidity. Journal of Consulting and Clinical Psychology. 2005;73(1):78–85. doi: 10.1037/0022-006x.73.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson JG, Stout RL, Sanislow CA, Shea MT, McGlashan TH, Zanarini MC, … Skodol AE. New episodes and new onsets of major depression in borderline and other personality disorders. Journal of Affective Disorders. 2008;111(1):40–45. doi: 10.1016/j.jad.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson JG, Stout RL, Shea MT, Grilo CM, Markowitz JC, Morey LC, … McGlashan TH. Interactions of borderline personality disorder and mood disorders over ten years. Journal of Clinical Psychiatry. 2014;75(8) doi: 10.4088/JCP.13m08972. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: Personality-dependent activation in the amygdala and subgenual anterior cingulate (vol 121, pg 249, 2007) Behavioral Neuroscience. 2007;121(6):1173–1173. doi: 10.1037/0735-7044.121.6.1173. [DOI] [PubMed] [Google Scholar]
- Hamdi NR, Iacono WG. Lifetime prevalence and co-morbidity of externalizing disorders and depression in prospective assessment. Psychological Medicine. 2014;44(2):315–324. doi: 10.1017/s0033291713000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, … Buchsbaum MS. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biological Psychiatry. 2005;58(8):614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. American Journal of Psychiatry. 2006;163(5):857–864. doi: 10.1176/appi.ajp.163.5.857. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS. The effects of anxiety, substance use and conduct disorders on risk of major depressive disorder. Psychological Medicine. 2003;33(8):1423–1432. doi: 10.1017/s0033291703008365. [DOI] [PubMed] [Google Scholar]
- Hopwood CJ, Morey LC, Edelen MO, Shea MT, Grilo CM, Sanislow CA, … Skodol AE. A comparison of interview and self-report methods for the assessment of borderline personality disorder criteria. Psychological Assessment. 2008;20(1):81–85. doi: 10.1037/1040-3590.20.1.81. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family. Study. Development and Psychopathology. 1999;11(4):869–900. doi: 10.1017/S0954579499002369. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- James LM, Taylor J. Revisiting the structure of mental disorders: Borderline personality disorder and the internalizing/externalizing spectra. British Journal of Clinical Psychology. 2008;47:361–380. doi: 10.1348/014466508x299691. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Skodol AE, Hamagami F, Brook JS. Age-related change in personality disorder trait levels between early adolescence and adulthood: A community-based longitudinal investigation. Acta Psychiatrica Scandinavica. 2000;102(4):265–275. doi: 10.1034/j.1600-0447.2000.102004265.x. [DOI] [PubMed] [Google Scholar]
- Johnson W, Hicks BM, McGue M, Iacono WG. Most of the girls are alright, but some aren’t: Personality trajectory groups from ages 14 to 24 and some associations with outcomes. Journal of Personality and Social Psychology. 2007;93(2):266–284. doi: 10.1037/0022-3514.93.2.266. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Liu X, Jiang Y, Lynam D, Kelly TH. Neural Correlates of Emotional Reactivity in Sensation Seeking. Psychological Science. 2009;20(2):215–223. doi: 10.1111/j.1467-9280.2009.02283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman EA, Crowell SE, Stepp SD. Self-injury, borderline personality development, and the externalizing spectrum. Oxford Handbook of Externalizing Spectrum Disorders. 2015:61–78. [Google Scholar]
- Keller MC, Nesse RM. Is low mood an adaptation? Evidence for subtypes with symptoms that match precipitants. Journal of Affective Disorders. 2005;86(1):27–35. doi: 10.1016/j.jad.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Roysamb E, Tambs K, Torgersen S, … Reichborn-Kjennerud T. The Structure of Genetic and Environmental Risk Factors for DSM-IV Personality Disorders A Multivariate Twin Study. Archives of General Psychiatry. 2008;65(12):1438–1446. doi: 10.1001/archpsyc.65.12.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. The American Journal of Psychiatry. 2011;168(1):29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes MA, Malone SM, Elkins IJ, Legrand LN, McGue M, Iacono WG. The Enrichment Study of the Minnesota Twin Family Study: Increasing the yield of twin families at high risk for externalizing psychopathology. Twin Research and Human Genetics. 2009;12(5):489–501. doi: 10.1375/twin.12.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends in Pharmacological Sciences. 1992;13(5):177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Bagge CL, Schumacher JA, Coffey SF. Does Comorbid Substance Use Disorder Exacerbate Borderline Personality Features? A Comparison of Borderline Personality Disorder Individuals With vs. Without Current Substance Dependence. Personality Disorders-Theory Research and Treatment. 2010;1(4):239–249. doi: 10.1037/a0017647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF. Stability and change in personality disorder features: The Longitudinal Study of Personality Disorders. Archives of General Psychiatry. 1999;56(11):1009–1015. doi: 10.1001/archpsyc.56.11.1009. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF. Current status of the scientific study of the personality disorders: an overview of epidemiological, longitudinal, experimental psychopathology, and neurobehavioral perspectives. Journal of the American Psychoanalytic Association. 2010;58(4):741–778. doi: 10.1177/0003065110386111. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the national comorbidity survey replication. Biological Psychiatry. 2007;62(6):553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The Genetics of Autism Spectrum Disorders and Related Neuropsychiatric Disorders in Childhood. American Journal of Psychiatry. 2010;167(11):1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO. Comorbidity between and within childhood externalizing and internalizing disorders: Reflections and directions. Journal of Abnormal Child Psychology. 2003;31(3):285–291. doi: 10.1023/a:1023229529866. [DOI] [PubMed] [Google Scholar]
- Linehan M. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press; 1993. [Google Scholar]
- Links PS, Heslegrave RJ, Mitton JE, Vanreekum R, Patrick J. Borderline personality disorder and substance abuse: consequences of comorbidity. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie. 1995;40(1):9–14. doi: 10.1177/070674379504000105. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. On jointly estimating parameters and missing data by maximizing the complete-data likelihood. American Statistician. 1983;37(3):218–220. doi: 10.2307/2683374. [DOI] [Google Scholar]
- Maier R, Moser G, Chen GB, Ripke S, Coryell W, Potash JB … Psychiat Genomics, C. Joint Analysis of Psychiatric Disorders Increases Accuracy of Risk Prediction for Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. American Journal of Human Genetics. 2015;96(2):283–294. doi: 10.1016/j.ajhg.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, … Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Reward sensitivity in impulsivity. Neuroreport. 2004;15(9):1519–1522. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- McGilloway A, Hall RE, Lee T, Bhui KS. A systematic review of personality disorder, race and ethnicity: prevalence, aetiology and treatment. BMC Psychiatry. 2010;10 doi: 10.1186/1471-244x-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Grilo CM, Skodol AE, Gunderson JG, Shea MT, Morey LC, … Stout RL. The Collaborative Longitudinal Personality Disorders Study: Baseline Axis I/II and II/II diagnostic co-occurrence. Acta Psychiatrica Scandinavica. 2000;102(4):256–264. doi: 10.1034/j.1600-0447.2000.102004256.x. [DOI] [PubMed] [Google Scholar]
- Meijer M, Goedhart AW, Treffers PDA. The persistence of borderline personality disorder in adolescence. Journal of Personality Disorders. 1998;12(1):13–22. doi: 10.1521/pedi.1998.12.1.13. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui LH, … Swendsen J. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100(4):674–701. doi: 10.1037/0033-295x.100.4.674. [DOI] [PubMed] [Google Scholar]
- Molenaar P, Rovine M. The covariance between level and shape in the latent growth curve model with estimated basis vector coefficients. Methods of Psychological Research Online. 1998;3(2):95–108. [Google Scholar]
- Must A, Szabó Z, Bódi N, Szász A, Janka Z, Kéri S. Sensitivity to reward and punishment and the prefrontal cortex in major depression. Journal of Affective Disorders. 2006;90(2):209–215. doi: 10.1016/j.jad.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, … Boker SM. OpenMx 2.0: Extended structural equation and statistical modeling. Psychometrika. 2016;81(2):535–549. doi: 10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, McArdle JJ. Structured latent growth curves for twin data. Twin Research. 2000;3(3):165–177. doi: 10.1375/136905200320565454. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nettle D. The evolution of personality variation in humans and other animals. American Psychologist. 2006;61(6):622–631. doi: 10.1037/0003-066x.61.6.622. [DOI] [PubMed] [Google Scholar]
- Paris J, Brown R, Nowlis D. Long-term follow-up of borderline patients in a general hospital. Comprehensive Psychiatry. 1987;28(6):530–535. doi: 10.1016/0010-440X(87)90019-8. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the multidimensional personality questionnaire. Psychological Assessment. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z. Revised version of the Diagnostic Interview for Children and Adolescents (DICA-R) St. Louis, MO: Department of Psychiatry, Washington University School of Medicine; 1988. [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Ystrom E, Orstavik R, Aggen SH, Tambs K, … Kendler KS. A longitudinal twin study of borderline and antisocial personality disorder traits in early to middle adulthood. Psychological Medicine. 2015;45(14):3121–3131. doi: 10.1017/s0033291715001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Røysamb E, Ørstavik RE, Neale MC, Torgersen S, Kendler KS. Major depression and dimensional representations of DSM-IV personality disorders: a population-based twin study. Psychological Medicine. 2010;40(09):1475–1484. doi: 10.1017/S0033291709991954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Caspi A, Moffitt TE. The kids are alright: Growth and stability in personality development from adolescence to adulthood. Journal of Personality and Social Psychology. 2001;81(4):670–683. doi: 10.1037/0022-3514.81.4.670. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132(1):1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Robbins LN, Cottler LB, Babor T. Composite international diagnostic interview–expanded substance abuse module (CIDI-SAM) St. Louis, MO: Washington University School of Medicine; 1990. [Google Scholar]
- Rohde P, Lewinsohn PM, Kahler CW, Seeley JR, Brown RA. Natural course of alcohol use disorders from adolescence to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(1):83–90. doi: 10.1097/00004583-200101000-00020. [DOI] [PubMed] [Google Scholar]
- Rojas EC, Cummings JR, Bornovalova MA, Hopwood CJ, Racine SE, Keel PK, … Klump KL. A Further Validation of the Minnesota Borderline Personality Disorder Scale. Personality Disorders-Theory Research and Treatment. 2014;5(2):146–153. doi: 10.1037/per0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas EC, Hicks BM, Stark S, Hopwood CJ, Bornovalova MA. Elaborating on the construct validity of the Minnesota Borderline Personality Disorder Scale (MBPD): A multi-sample, longitudinal examination. Psychological Assessment. 2015;27(1):332. doi: 10.1037/pas0000061. [DOI] [PubMed] [Google Scholar]
- Sauder CL, Derbidge CM, Beauchaine TP. Neural responses to monetary incentives among self-injuring adolescent girls. Development and Psychopathology. 2016;28(01):277–291. doi: 10.1017/S0954579415000449. [DOI] [PubMed] [Google Scholar]
- Shea MT, Stout R, Gunderson J, Morey LC, Grilo CM, McGlashan T, … Keller MB. Short-term diagnostic stability of schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders. The American Journal of Psychiatry. 2002;159(12):2036–2041. doi: 10.1176/appi.ajp.159.12.2036. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silk KR, Lee S, Hill EM, Lohr NE. Borderline personality disorder symptoms and severity of sexual abuse. American Journal of Psychiatry. 1995;152(7):1059–1064. doi: 10.1176/ajp.152.7.1059. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Skodol AE, Gunderson JG, McGlashan TH, Dyck IR, Stout RL, Bender DS, … Sanislow CA. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. American Journal of Psychiatry. 2002;159(2):276–283. doi: 10.1176/appi.ajp.159.2.276. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI … Psychiatric Genomics, C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/s0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldz S, Budman S, Demby A, Merry J. Representation of personality disorders in circumplex and five-factor space: Explorations with a clinical sample. Psychological Assessment. 1993;5:41. [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D. Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Research-Neuroimaging. 2003;123(3):153–163. doi: 10.1016/s0925-4927(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. New York, NY: WW Norton & Company; 2010. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute. Biometrics Research. 1987;11 [Google Scholar]
- Steinberg L. Risk taking in adolescence - New perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16(2):55–59. doi: 10.1111/j.1467-8721.2007.00475.x. [DOI] [Google Scholar]
- Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain and Cognition. 2009;70(1):1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Stice E, Burton EM, Shaw H. Prospective relations between bulimic pathology, depression, and substance abuse: Unpacking Comorbidity in adolescent girls (vol 72, pg 62, 2004) Journal of Consulting and Clinical Psychology. 2004;72(4):587–587. doi: 10.1037/0022-006X.72.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Roysamb E, Neale MC, Kendler KS. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychological Medicine. 2008;38(11):1617–1625. doi: 10.1017/s0033291708002924. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Lygren S, Oien PA, Skre I, Onstad S, Edvardsen J, … Kringlen E. A twin study of personality disorders. Comprehensive Psychiatry. 2000;41(6):416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- Trull TJ. DSM-III-R personality disorders and the five-factor model of personality: An empirical comparison. Journal of Abnormal Psychology. 1992;101:553. doi: 10.1037//0021-843x.101.3.553. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clinical Psychology Review. 2000;20(2):235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Gil AG. Psychiatric and substance use disorders in South Florida - Racial/ethnic and gender contrasts in a young adult cohort. Archives of General Psychiatry. 2002;59(1):43–50. doi: 10.1001/archpsyc.59.1.43. [DOI] [PubMed] [Google Scholar]
- Tzschoppe J, Nees F, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, … Consortium I. Aversive Learning in Adolescents: Modulation by Amygdala-Prefrontal and Amygdala-Hippocampal Connectivity and Neuroticism. Neuropsychopharmacology. 2014;39(4):875–884. doi: 10.1038/npp.2013.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews. 2010;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Gunderson JG, Zanarini MC, Sanislow CA, Grilo CM, McGlashan TH, … Skodol AE. New onsets of substance use disorders in borderline personality disorder over 7 years of follow-ups: findings from the Collaborative Longitudinal Personality Disorders Study. Addiction. 2009;104(1):97–103. doi: 10.1111/j.1360-0443.2008.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PJ, Andrews PW. Toward a revised evolutionary adaptationist analysis of depression: the social navigation hypothesis. Journal of Affective Disorders. 2002;72(1):1–14. doi: 10.1016/s0165-0327(01)00459-1. [DOI] [PubMed] [Google Scholar]
- Wilbertz G, van Elst LT, Delgado MR, Maier S, Feige B, Philipsen A, Blechert J. Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. Neuroimage. 2012;60(1):353–361. doi: 10.1016/j.neuroimage.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Wilson ST, Fertuck EA, Kwitel A, Stanley MC, Stanley B. Impulsivity, suicidality and alcohol use disorders in adolescents and young adults with borderline personality disorder. International Journal of Adolescent Medicine and Health. 2006;18(1):189–196. doi: 10.1515/ijamh.2006.18.1.189. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychological Medicine. 1998;28(1):109–126. doi: 10.1017/s0033291797005928. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, … Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wright A, Zalewski M, Hallquist M, Hipwell A, Stepp S. Developmental trajectories of borderline personality disorder symptoms and psychosocial functioning in adolescence. Journal of Personality Disorders. 2015;29:200. doi: 10.1521/pedi_2015_29_200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AGC, Hopwood CJ, Zanarini MC. Associations Between Changes in Normal Personality Traits and Borderline Personality Disorder Symptoms Over 16 Years. Personality Disorders-Theory Research and Treatment. 2015;6(1):1–11. doi: 10.1037/per0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral Disinhibition: Liability for Externalizing Spectrum Disorders and Its Genetic and Environmental Relation to Response Inhibition Across Adolescence. Journal of Abnormal Psychology. 2009;118(1):117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenbur FR, Weingeroff JL, Reich DB, Fitzmaurice GM, Weiss RD. The course of substance use disorders in patients with borderline personality disorder and Axis II comparison subjects: a 10-year follow-up study. Addiction. 2011;106(2):342–348. doi: 10.1111/j.1360-0443.2010.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Hennen J, Reich DB, Silk KR. Axis I comorbidity in patients with borderline personality disorder: 6-year follow-up and prediction of time to remission. American Journal of Psychiatry. 2004;161(11):2108–2114. doi: 10.1176/appi.ajp.161.11.2108. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Hennen J, Reich DB, Silk KR. The McLean Study of Adult Development (MSAD): Overview and implications of the first six years of prospective follow-up. Journal of Personality Disorders. 2005;19(5):505–523. doi: 10.1521/pedi.2005.19.5.505. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Hennen J, Silk KR. The longitudinal course of borderline psychopathology: 6-year prospective follow-up of the phenomenology of borderline personality disorder. The American Journal of Psychiatry. 2003;160(2):274–283. doi: 10.1176/appi.ajp.160.2.274. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Khera GS, Bleichmar J. Treatment histories of borderline inpatients. Comprehensive Psychiatry. 2001;42(2):144–150. doi: 10.1053/comp.2001.19749. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Vujanovic AA, Hennen J, Reich DB, Silk KR. Axis II comorbidity of borderline personality disorder: Description of 6-year course and prediction to time-to-remission. Acta Psychiatrica Scandinavica. 2004;110(6):416–420. doi: 10.1111/j.1600-0447.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Yong L, Frankenburg FR, Hennen J, Reich DB, Marino MF, Vujanovic AA. Severity of reported childhood sexual abuse and its relationship to severity of borderline psychopathology and psychosocial impairment among borderline inpatients. Journal of Nervous and Mental Disease. 2002;190(6):381–387. doi: 10.1097/01.nmd.0000018963.57744.7e. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Comprehensive Psychiatry. 1999;40(4):245–252. doi: 10.1016/S0010-440X(99)90123-2. [DOI] [PubMed] [Google Scholar]