Abstract
TRPM7 is a member of the melastatin-related subfamily of TRP channels and represents a protein that contains both an ion channel and a kinase domain. The protein is ubiquitously expressed and represents the only ion channel known that is essential for cellular viability. TRPM7 is a divalent cation-selective ion channel that is permeable to Ca2+ and Mg2+, but also conducts essential metals such as Zn2+, Mn2+, and Co2+, as well as nonphysiologic or toxic metals such as Ni2+, Cd2+, Ba2+, and Sr2+. The channel is constitutively open but strongly downregulated by intracellular levels of Mg2+ and MgATP and other Mg-nucleotides. Reducing the cellular levels of these regulators leads to activation of TRPM7-mediated currents that exhibit a characteristic nonlinear current–voltage relationship with pronounced outward rectification due to divalent influx at physiologically negative voltages and monovalent outward fluxes at positive voltages. TRPM7 channel activity is also actively regulated following receptor-mediated changes in cyclic AMP (cAMP) and protein kinase A activity. This regulation as well as that by Mg-nucleotides requires a functional endogenous kinase domain. The function of the kinase domain is not completely understood, but may involve autophosphorylation of TRPM7 as well as phosphorylation of other target proteins such as annexin and myosin IIA heavy chain. Based on these properties, TRPM7 is currently believed to represent a ubiquitous homeostatic mechanism that regulates Ca2+ and Mg2+ fluxes based on the metabolic state of the cell. Physiologically, the channel may serve as a regulated transport mechanism for these ions that could affect cell adhesion, cell growth and proliferation, and even cell death under pathological stress such as anoxia.
Keywords: TRPM7, MagNuM, Magnesium, Mg-nucleotide, Divalent cation channel
1 Introduction
TRPM7 belongs to the melastatin-related subfamily of TRP-related ion channels, which comprises eight members with homologous architecture, but distinctive biophysical properties and activation mechanisms. TRPM7 is a bi-functional protein that contains both ion channel and protein kinase domains (Nadler et al. 2001; Runnels et al. 2001; Ryazanova et al. 2001) and provides a pathway for the transport of Ca2+ and Mg2+ as well trace metal ions (Nadler et al. 2001; Monteilh-Zoller et al. 2003). The protein was originally discovered in three independent studies that used very different approaches to clone it: Ryazanova et al. identified TRPM7 by screening data bases for homologs of human eukaryotic elongation factor 2 kinase (Ryazanova et al. 2001), Runnels et al. found TRPM7 in a yeast two-hybrid screen using the C2 domain of phospholipase C as a bait (Runnels et al. 2001), and Nadler et al. obtained TRPM7 using a bioinformatics approach aimed at identifying novel ion channels expressed in immune cells (Nadler et al. 2001). The latter two studies overexpressed TRPM7 in a heterologous expression system and provided the first electrophysiological characterization of the channel; however, they arrived at very different conclusions about the selectivity, activation mechanism, and the role of the channel’s endogenous kinase domain. Since then, TRPM7 has been the subject of numerous studies and at the center of lively and controversial discussions.
2 Channel Properties
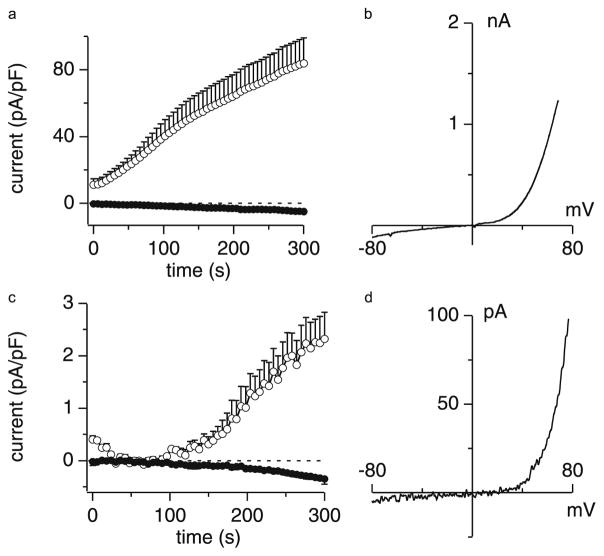
The full-length TRPM7 protein is composed of 1,863 amino acid residues (Nadler et al. 2001; Runnels et al. 2001; Ryazanova et al. 2001). Its currents are characterized by a reversal potential of approximately 0 mV and very strong outward rectification, with little inward current in the physiological rage of −70 mV to 0 mV and large outward currents above +50 mV (Fig. 1a, b). Endogenous TRPM7 currents in various cell types are small and rarely exceed 20 pA/pF (Fig. 1c, d; Nadler et al. 2001; Hermosura et al. 2002; Kozak et al. 2002; Prakriya and Lewis 2002).
Fig. 1.
a–d Development of heterologous and endogenous TRPM7 currents. a Representative development of heterologous TRPM7 currents in overexpressing HEK-293 cells where free Mg2+]i was kept at 800 μM and MgATP was omitted (n = 6). Data points correspond to average and normalized current amplitudes measured at −80 mV (closed circles) and +80 mV (open circles) and plotted as a function of time. Note that TRPM7 develops immediately. b Current–voltage (I–V) relationship of heterologous TRPM7 extracted from an example cell at 200 s. c Representative development of endogenous TRPM7-like MagNuM currents measured in RBL-2H3 cells. Average inward (closed circles) and outward (open circles) currents at −80 mV and +80 mV, respectively (n = 6), recorded under conditions that suppress ICRAC and support MagNuM activation (omission of MgATP, free [Mg2+]i = 760 μM, free [Ca2+]i = 100 nM). Note that MagNuM starts to develop around 80 s into the experiment. d Representative I–V relationship of endogenous TRPM7-like MagNuM extracted at 300 s after whole-cell establishment
At physiological, negative membrane potentials TRPM7 conducts very little inward current and exclusively transports divalent cations such as Ca2+ and Mg2+ from the extracellular space into the cytosol down their concentration gradient (Nadler et al. 2001). However, at positive membrane potentials, where divalent ions do not experience sufficient driving force to enter the cell, the outward transport rates of intracellular cations such as K+ or Cs+ increase, and at potentials beyond +50 mV the monovalent cation fluxes become quite prominent and shape the characteristic outwardly rectifying current–voltage relationship of TRPM7. Only when completely removing extracellular divalent cations, TRPM7 will transport monovalent cations inwardly and this will linearize the current–voltage relationship, revealing both TRPM7’s exquisite specificity for divalent cation transport and the lack of any significant voltage dependence (Nadler et al. 2001).
Since the outwardly rectifying current–voltage relationship of TRPM7 macroscopic currents is based on the channel’s permeation properties, the microscopic behavior of TRPM7 in single-channel recordings also exhibits nonlinear outward rectification when divalent cations are present. Under these conditions, single-channel events are not detectable at negative voltages, and single-channel outward currents gradually increase in a nonlinear fashion at positive voltages (Runnels et al. 2001). It is therefore not possible to determine an accurate slope conductance estimate. Because of this, the slope conductance of 105 pS obtained by linear regression by Runnels et al. (2001) is likely an overestimate. Under similar experimental conditions, Nadler et al. (2001) arrived at a single-channel conductance of approximately 40 pS at +60 mV, and this is in good agreement with the single-channel conductance of approximately 40 pS obtained by Kerschbaum et al. (1999) under symmetric divalent-free conditions at negative membrane voltages (Kerschbaum and Cahalan 1999). The latter study attributed these 40-pS single channels to store-operated calcium release-activated calcium (CRAC) channels; however, in retrospect, these channels have been identified as TRPM7 (Nadler et al. 2001; Hermosura et al. 2002; Kozak et al. 2002; Prakriya and Lewis 2002). The asymmetrical permeation properties of TRPM7, with exclusive divalent ion influx at negative voltages and exclusive efflux of monovalent cations at high positive voltages, as well as the pronounced block of monovalent cation permeation by divalent cations, renders classical analyses of selectivity based on constant field equations inaccurate, since these rely on the assumption that the permeating ion species move through the channel independently of each other. Therefore, TRPM7 cannot be classified as a nonselective cation channel, and the proposed permeability of cations relative to that of Cs+ of 1.1, 0.97, and 0.34 for K+, Na+, and Ca2+, respectively (Runnels et al. 2001), does not reflect the channel’s true selectivity (Monteilh-Zoller et al. 2003).
The unusual permeation profile of TRPM7 is further evident from ion substitution experiments in which other divalent cations were tested for permeation relative to Ca2+ with the sequence Zn2+ ≈ Ni2+≫Ba2+>Co2+>Mg2+≥Mn2+≥≥Sr2+≥Cd2+≥Ca2+ (Monteilh-Zoller et al. 2003). Interestingly, it has recently been proposed that the selectivity of TRPM7 may be regulated by extracellular pH (Jiang et al. 2005).
3 Channel Activation and Regulation
TRPM7 channels appear to be constitutively active in resting cells, albeit at very low levels of less than 10% of maximal activity (Nadler et al. 2001), and this is true for both heterologously expressed and native channels. TRPM7 channel activity can be either up- or downregulated, and a number of positive and negative modulators have been identified, although some of the proposed mechanisms are controversial.
3.1 Regulation by Free Mg2+ and Mg·ATP
The two most significant factors that control TRPM7 channel activity are free Mg2+ ions and Mg-complexed nucleotides, which tonically inhibit the channels and represent the primary reason for the low activity observed in resting cells (Nadler et al. 2001). Nadler et al. (2001) observed that perfusing cells with intracellular solutions in which MgATP is omitted but free Mg2+ is present at physiological levels of approximate 700–900 μM can elicit large TRPM7-mediated currents in whole-cell patch-clamp experiments. Increasing MgATP concentrations in the pipette solution while keeping free Mg2+ levels constant gradually decreased the TRPM7 activation in a dose-dependent manner so that at physiological levels of 3–4 mM MgATP there was no increase in current, and higher concentrations even inhibited the resting TRPM7 current. The Mg-nucleotide-mediated regulation of TRPM7 was observed both in human embryonic kidney (HEK)-293 cells overexpressing TRPM7 as well as in cells such as Jurkat T cells and RBL-2H3, where MgATP-free pipette solutions activate native currents with identical properties as TRPM7 that are completely suppressed by millimolar concentrations of MgATP. A similar nucleotide-mediated regulation of TRPM7 has also been observed in human retinoblastoma cells, where MgATP appears to be very effective in inhibiting TRPM7 whole-cell currents (Hanano et al. 2004). The MgATP-dependent inhibition of TRPM7 is not due to ATP hydrolysis, since MgATPγS is also effective in suppressing the current (Nadler et al. 2001). Interestingly, the suppression of TRPM7 is not limited to adenine nucleotides, since GTP was found to be similarly effective (Nadler et al. 2001; Demeuse et al. 2006).
The nucleotide-dependent regulation of TRPM7 is not mediated by the free nucleotide, since perfusing cells with NaATP fails to suppress TRPM7 currents and in fact causes a massive activation of TRPM7 (Nadler et al. 2001; Demeuse et al. 2006). The strong activation of TRPM7 by Na-ATP was also observed by Clapham and colleagues and was interpreted in favor of a kinase-mediated activation mechanism (Runnels et al. 2001). However, this interpretation turned out to be incorrect, since Nadler et al. (2001) demonstrated that free Mg2+ is another important factor in regulating TRPM2 activity. Since the phosphate groups of nucleotides can coordinate Mg2+ ions, they represent very strong Mg2+ chelators, so that NaATP will chelate free cytosolic Mg2+ to very low levels and thereby cause a strong activation of TRPM7. Therefore, the physiological, Mg-complexed form of ATP inhibits the channel, whereas the free nucleotide activates the channel by chelating free Mg2+. Since both Mg-nucleotides and free Mg2+ can regulate TRPM7 activity, omitting both and additionally chelating residual Mg2+ by strong buffers such as N-hydroxyethylenediaminetriacetic acid (HEDTA) or NaATP yields maximal activation of TRPM7 (Demeuse et al. 2006).
It should be noted that Kozak and Cahalan have questioned the regulation by Mg-nucleotides (Kozak and Cahalan 2003). While they confirmed the original observation that MgATP in the presence of ethyleneglycoltetraacetic acid (EGTA) can inhibit TRPM7, they also reported that this inhibition is lost when using a strong Mg2+ chelator, HEDTA, leading them to conclude that free Mg2+ alone can explain the effect. The study compared experiments in which free Mg2+ was buffered to 270 μM with either 12 mM EGTA or by 3 mM EGTA+2.5 mM HEDTA+5 mM MgATP, and its conclusion regarding the absence of MgATP-dependent regulation of TRPM7 rests entirely on the accuracy of these two data sets. Both solutions reportedly caused a very similar inhibition of TRPM7 by approximate 75%. The strong suppression of TRPM7 by the reference solution containing 270 μM certainly is surprising, as it would suggest an IC50 of free Mg2+ that is well below the typical IC50 values of approximately 700 μM observed by several laboratories (Nadler et al. 2001; Kozak et al. 2002; Prakriya and Lewis 2002; Schmitz et al. 2003; Demeuse et al. 2006). A recent study by Demeuse et al. (2006) has reevaluated the experimental conditions employed by Kozak and Cahalan and confirmed that the MgATP-containing solution indeed causes approximately 80% inhibition of TRPM7 currents (Demeuse et al. 2006). However, the reference solution containing only EGTA and 270 μM free Mg2+ did not produce any significant inhibition compared to completely Mg2+-free conditions (Demeuse et al. 2006). The study further demonstrated that MgATP can inhibit TRPM7 in the complete absence of any exogenous chelator, suggesting that MgATP can also regulate TRPM7 channel activity in physiologically more relevant circumstances.
Demeuse et al. (2006) also provided a comprehensive assessment of the dual regulation of TRPM7 by free Mg2+ and Mg-nucleotides and found that the two factors act in synergy to regulate TRPM7 (Demeuse et al. 2006). They established dose-response curves of TRPM7 current inhibition by increasing the MgATP concentration at fixed low (~200 μM), physiological (~800 μM), or high (~1600 μM) free Mg2+ concentrations. There was only a modest shift in the IC50 for MgATP-mediated inhibition of TRPM7 from 3.3 mM (at 200 μM free Mg2+) to 2 mM (at 800 μM free Mg2+) and 1.6 mM (at 1.6 mM free Mg2+). Although this amounts to a twofold shift in the IC50 for MgATP, this shift occurs at relatively low concentrations of free Mg2+ and is essentially complete at 800 μM free Mg2+, suggesting that slight variations in free Mg2+ around physiological levels may not have a major impact on the nucleotide sensitivity of TRPM7. Conversely, the efficacy of free Mg2+ is shifted by about sixfold when increasing MgATP from 0 to 6 mM. The IC50 of free Mg2+ in the absence of any added ATP is very close to physiological levels in resting cells (720 μM), whereas the IC50 for free Mg2+ in the presence of 6 mM MgATP is 130 μM. For physiologically relevant MgATP concentrations between 1 and 4 mM, the shift in IC50 for Mg2+ is fourfold. Therefore, it appears that free Mg2+ and MgATP are acting in synergy (or cooperatively) to inhibit TRPM7. However, free Mg2+ is not the only divalent cation that can inhibit TRPM7 intracellularly, since most divalent cations will inhibit TRPM7 with various degrees of potency from the cytosol (Kozak and Cahalan 2003) and this effect is also seen with various polyamines and even protons (Kerschbaum et al. 2003; Kozak et al. 2005). Kozak and Cahalan (2005) have proposed that this inhibitory effect of charged cations is due to screening the head group phosphates on membrane phospholipids that would otherwise promote TRPM7 activity (Kozak et al. 2005). If this were the case, then the Mg2+-dependent inhibition of TRPM7 would be extraneous to the protein itself and would be difficult to reconcile with the significant shifts of Mg2+ sensitivity of point mutations and kinase deletion mutants of TRPM7 (Schmitz et al. 2003; Demeuse et al. 2006). These studies presented an alternative model that takes this into account and favors a separate Mg2+ binding site within the TRPM7 protein itself (Schmitz et al. 2003; Demeuse et al. 2006).
In addition to MgATP, Demeuse et al. (2006) also found other purine and pyrimidine nucleotide triphosphates to inhibit TRPM7 currents. Except for 5′ triphosphate (ITP), all nucleotides synergize with free Mg2+, with ATP being the most potent. The sequence of potency was determined as follows: ATP>TTP>CTP≥GTP≥UTP. Not only did the triphosphate nucleotide prove effective in suppressing TRPM7, but to a lesser degree also the dinucleotides, whereas nucleotide monophosphates, which cannot coordinate Mg2+ into a complex, were completely ineffective in modulating TRPM7 currents.
Since free Mg2+ and MgATP differentially affect each other’s inhibitory efficacy, the nucleotide-dependent regulation of TRPM7 appears to be mediated by two distinct binding sites. Demeuse et al. (2006) proposed that a binding site within the endogenous kinase domain mediates the nucleotide effect, since two point mutations that affect nucleotide binding and phosphotransferase activity of the kinase domain reduce both free Mg2+ and Mg-nucleotide-mediated inhibition of channel activity (Schmitz et al. 2003; Demeuse et al. 2006). Additional characterizations of these mutants demonstrate the loss of MgATP and MgGTP block in the phosphotransferase-deficient mutant K1648R. The lysine residue mutated is implicated in nucleotide binding and catalysis and is conserved in classical kinases (Yamaguchi et al. 2001). In the second mutant, G1799D, a site that might coordinate the Mg-phosphate complex, some regulation remains, even though phosphotransferase activity is lost. Interestingly, a truncation mutant of TRPM7 that lacks the entire kinase domain exhibits supersensitivity to both Mg2+ and MgATP (Schmitz et al. 2003), but exhibits no specificity toward nucleotide species (Demeuse et al. 2006). A hypothesis that explains this seemingly paradoxical behavior proposes that the truncation of the C-terminal kinase domain exposes the Mg2+ binding site normally masked by the kinase domain and converts it into a high-affinity site that can be blocked by very low levels of free Mg2+. In addition, this site now becomes available for binding Mg-nucleotides. However, since the kinase deletion mutant does not discriminate between nucleotide species, it might simply accept the Mg-phosphate moiety as a binding partner.
3.2 Regulation by the Endogenous Kinase Domain
Since TRPM7 contains both an ion channel and a kinase domain, an important question is whether these two functional domains interact and regulate each other. Conflicting accounts of the kinase’s importance for ion channel activity have been presented, ranging from one extreme that the kinase domain is essential for gating the channel (Runnels et al. 2001)—others support a modulatory role of the kinase in shaping Mg2+ and Mg-nucleotide sensitivity (Schmitz et al. 2003; Demeuse et al. 2006)—to the opposite extreme of complete dissociation of kinase and channel activities (Matsushita et al. 2005).
Clapham and colleagues (Runnels et al. 2001) suggested that the kinase domain is absolutely required for channel activation based on the effects of ATP and the absence of TRPM7 currents in two mouse TRPM7 mutants that were designed to inactivate phosphotransferase activity [G1796D and the double mutant C1809A/C1812A; although based on structural considerations, the latter mutant may not represent a phosphotransferase-deficient construct (Yamaguchi et al. 2001)]. This interpretation is probably incorrect, since the ATP effect is attributable to Mg2+ chelation and the lack of TRPM7 currents when expressing the mutant channels was likely due to failed expression of TRPM7 mutant proteins, since studies from two other laboratories have demonstrated that mutated human TRPM7 channels without phosphotransferase activity still exhibit channel activation comparable to wildtype TRPM7 (Schmitz et al. 2003; Matsushita et al. 2005). Both studies are in general agreement that kinase activity is not essential to activate TRPM7. They mutated a lysine residue to aspartate (K1648R), which is implicated in catalysis and is conserved in classical kinases, and found that this mutant is activated normally when reducing cytosolic Mg2+ levels. Matsushita et al. (2005) observed that this mutant channel exhibited the same Mg2+ sensitivity as wildtype TRPM7 (Matsushita et al. 2005), leading them to conclude that the kinase and channel activity are completely dissociated. However, this interpretation may be overstated, as it is based on just two relatively high concentrations of Mg2+ (4 and 6 mM) and the data set for 4 mM actually hints at a reduced efficacy of Mg2+-induced inhibition in the mutant compared to wildtype channels. Schmitz et al. (2003) had previously provided a comprehensive dose-response analysis of phosphotransferase-deficient mutants of human TRPM7 (K1648R and G1799D). Based on their analysis, the differences in the inhibition by Mg2+ at high concentrations are indeed relatively small; however, at intermediate concentrations around the IC50, there is in fact a reduced sensitivity of these mutants to inhibition by Mg2+. In a more recent study, this reduced sensitivity to Mg2+ has been confirmed and extended, suggesting that the kinase domain provides the site for Mg-nucleotide binding, which interacts with a separate Mg2+ binding site to regulate TRPM7 channel activity (Demeuse et al. 2006).
An important question relates to whether the nucleotide binding alone is sufficient to regulate TRPM7 channel activity or whether kinase activity per se also plays a role, since this domain can autophosphorylate the channel. The primary sites of autophosphorylation are a matter of controversy, since some groups mainly observe phosphorylation of serine (Ryazanova et al. 2004; Matsushita et al. 2005), while another study proposes threonine as a primary target (Hermosura et al. 2005). Matsushita et al. (2005) mutated two serine residues at positions 1511 and 1567 to alanine, and this resulted in a large decrease in autophosphorylation compared with wildtype proteins; however, this mutant did not show any obvious changes in its regulation of Mg2+. However, as discussed above, this analysis was performed at relatively high concentrations of Mg2+, so that subtle changes at intermediate or low Mg2+ concentrations or possible effects on MgATP-mediated regulation may have gone unnoticed. At variance with this report, Hermosura et al. (2005) identified threonine 1428 as a significant locus for TRPM7 autophosphorylation, since a T1428I point mutation not only reduced the phosphate incorporation by 30%, but also changed the sensitivity of the channel toward inhibition by free Mg2+. The authors found that 1 mM of added free Mg2+ reduced the currents of the T1428I mutant by approximately 50% compared to Mg2+-free solutions. This value on its own would not suggest an overly sensitive channel, since it is in fact very similar to the degree of Mg2+-induced inhibition observed in numerous studies on heterologous or native TRPM7 (Nadler et al. 2001; Kozak et al. 2002; Prakriya and Lewis 2002; Schmitz et al. 2003; Demeuse et al. 2006). However, in the context of that study, the same concentration of Mg2+ produced a surprisingly small inhibition of wildtype TRPM7 (less than 20%), which is at variance with values obtained by others. Therefore it remains doubtful that the phosphorylation state of that particular threonine residue is important for shaping Mg2+ and/or Mg-nucleotide sensitivity. It should be noted that a sensitization would be rather unexpected and in apparent conflict with the reduced Mg2+ and MgATP sensitivity reported for phosphotransferase-deficient mutants (Schmitz et al. 2003), which would likely also result in a dephosphorylated protein.
In summary, it would appear that the kinase domain, while not essential for gating TRPM7 channels, might modulate channel activity by shaping its sensitivity toward free Mg2+ and Mg-nucleotides. Whether or not the autophosphorylated channel contributes to this modulation awaits additional experimental efforts.
3.3 Regulation by Receptor Stimulation
While concentrations of cellular free Mg2+ and Mg-nucleotide levels provide an important “passive” regulatory mechanism that tonically reduces TRPM7 activity to approximately 10% of maximum, the channels can also be “actively” modulated following receptor stimulation. Two conflicting reports have proposed regulatory mechanisms of TRPM7 by phosphatidylinositol bisphosphate (PIP2) (Runnels et al. 2002) and by cyclic AMP (cAMP) signaling (Takezawa et al. 2004). Clapham and colleagues (2002) suggested that receptor-mediated PIP2 is required to maintain TRPM7 activity, and its breakdown by receptor-mediated stimulation of phospholipase C (PLC) is responsible for the inhibition TRPM7 they observed when stimulating cells with agonists that couple to PLC. Although several ion channels, including members of the TRPM family [TRPM4 (Zhang et al. 2005; Nilius et al. 2006), TRPM5 (Liu and Liman 2003), and TRPM8 (Liu and Qin 2005; Rohacs et al. 2005)] are subject to PIP2-mediated regulation, the case for TRPM7 has not been made without ambiguity. Most of the experiments presented in support of PIP2 regulation were performed in cells that overexpress TRPM7 or with receptor agonists that can couple to PLC (or both). Such overexpression is prone to significant pitfalls. Thus, the overexpression of TRPM7 leads to a complete suppression of PLC-mediated signaling and likely abolishes any PIP2 breakdown, without affecting the receptor-mediated regulation of TRPM7 by muscarinic agonists (Takezawa et al. 2004). This is presumably due to the fact that TRPM7 binds to and inhibits PLC (Runnels et al. 2001). The overexpression of Gq-coupled receptors faces the problem of G protein promiscuity (Xiao 2000; Milligan and Kostenis 2006), where receptors may couple to other G proteins and activate signaling pathways unrelated to PLC. Another potential problem is the fact that the cells under investigation can have endogenous receptors that are activated in parallel with the overexpressed receptors, which is the case for muscarinic receptors, as some of them couple to PLC (M1, M3, M5) whereas others couple to Gi (M2, M4).
Takezawa et al. (2004) demonstrated that the muscarinic receptor-mediated inhibition of TRPM7 in HEK-293 cells by endogenous muscarinic receptors likely occurs via Gi-coupled pathways, since the effect was suppressed by pertussis toxin. They further demonstrated that TRPM7 currents are upregulated by isoproterenol (a beta receptor agonist that couples to Gs) or by perfusing cells with elevated cAMP. Furthermore, these modulations were inhibited by protein kinase A inhibitors but not by the PLC antagonist U73122, suggesting that the receptor signaling cascade that regulates TRPM7 indeed involves Gi and/or Gs-coupled receptors that elevate or decrease cAMP levels, which in turn regulate the activity of PKA. Interestingly, this PKA-dependent regulation additionally requires TRPM7’s functional endogenous kinase, since phosphotransferase-deficient mutants lose their ability to be regulated via this mechanism (Takezawa et al. 2004). It remains to be established whether the cAMP-dependent and the MgATP-mediated regulation of TRPM7 is caused by two separate and unrelated mechanisms or the two are somehow linked. For example, it is conceivable that the cAMP-induced activation of PKA could induce a shift in MgATP sensitivity of TRPM7 or conversely, changes in MgATP could determine the activity of PKA and TRPM7’s endogenous kinase activity.
4 Physiological Functions
TRPM7 is found in virtually every mammalian cell, although it appears to be expressed in variable amounts in different cells. Nadler et al. (2001) and Hermosura et al. (2002) characterized native TRPM7-like currents in Jurkat T, RBL-2H3, and HEK-293 cells and named these endogenous currents MagNuM (for magnesium-nucleotide-regulated metal ion currents), because they were inhibited by high concentrations of MgATP and transport divalent metal ions. Unfortunately, two later publications (Kozak et al. 2002; Prakriya and Lewis 2002) did not follow the original nomenclature and introduced the new term MIC (for Mg2+-inhibited cation current), which is based on the observation that Mg2+ also inhibits TRPM7 as described by the same report that originally designated the current as MagNuM (Nadler et al. 2001). Because MagNuM activates under the same experimental conditions typically used to study the store-operated Ca2+ current ICRAC (Hermosura et al. 2002; Kozak et al. 2002; Prakriya and Lewis 2002), and both ICRAC and MagNuM will carry large monovalent cation currents when using divalent-free extracellular solutions (Hermosura et al. 2002), endogenous TRPM7 channels had in fact been recorded before (Kerschbaum and Cahalan 1998, 1999; Fomina et al. 2000), but were erroneously interpreted to represent CRAC channels (Kerschbaum and Cahalan 1999). Monovalent currents that are unmasked by removal of divalent cations from the extracellular solution and are possibly due to TRPM7 have also been observed previously in myocytes (Mubagwa et al. 1997), aortic smooth muscle cells (Zakharov et al. 1999), and hippocampal neurons (Xiong et al. 1997). Based on the whole-cell current densities of approximately 2–10 pA/pF at +80 mV that one typically sees in a range of different cell types, and assuming a single-channel conductance of approximately 40 pS and open probabilities of approximately 90%, one would estimate about 10–100 channels per cell. Fomina et al. (2000) estimated the number of TRPM7 channels in resting lymphocytes to be approximately 15, but this number is upregulated to approximately 140 channels in activated cells. In smooth muscle cells, the number of TRPM7 channels can be doubled by quick recruitment from a cytosolic pool in response to laminar flow (Oancea et al. 2006).
So far, most of the information available on the physiological functions of TRPM7 in mammalian systems has been derived from cellular assays. Unfortunately, a conventional TRPM7 knockout in the mouse has proved to be lethal at early embryonic stages (A. Ryazanov, personal communication) and characterization of TRPM7 at the rodent animal level will have to await an inducible knockout. TRPM7 orthologs have been identified in Danio rerio (zebrafish) and Caenorhabditis elegans, although it remains to be determined whether the physiological context in these organisms translates into mammalian systems. The zebrafish mutant touchtone/nutria is caused by mutations in TRPM7 and exhibits severe growth retardation and gross alterations in skeletal development in addition to embryonic melanophore and touch-response defects (Elizondo et al. 2005). In C. elegans a Mg2+-sensitive current was found in intestinal cells with electrophysiological properties that are very similar to TRPM7/MagNuM (Estevez et al. 2003; Estevez and Strange 2005), and Teramoto et al. (2005) have recently identified GTL-1 and GON-2 as possible candidates underlying this current (Teramoto et al. 2005).
The divalent cation transport function of TRPM7 appears to be vital to cell survival, since cellular knockout of TRPM7 in avian B cells induces death of the cells over a 48–72 h time frame (Schmitz et al. 2003). The crucial role of Mg2+ transport, at least in the case of avian B cells, is evident from ion substitution experiments, which demonstrate that growing TRPM7-deficient cells in medium with high Mg2+ concentrations, but not high Ca2+, can rescue the lethal phenotype and restore normal cell growth and proliferation (Schmitz et al. 2003). On the other hand, in retinoblastoma cells, the role of Ca2+ influx through TRPM7 may be more significant, since molecular suppression of TRPM7 in these cells by small interfering RNA (siRNA) silencing markedly reduces TRPM7 currents and the magnitude of resting Ca2+ influx, which is paralleled by decreased TRPM7 immunoreactivity, decelerated cell proliferation, and retarded G1/S cell cycle progression (Hanano et al. 2004). In addition to Ca2+ and Mg2+, TRPM7 is also capable of transporting other divalent cations, exhibiting a strong preference for the essential trace metals Zn2+, Mn2+, and Co2+ (Monteilh-Zoller et al. 2003). While undeniably crucial for cellular function, if they accumulate above trace levels, these ions are highly toxic. This is particularly true for zinc, which is one of the most abundant transition metals in the brain. Excessive vesicular release of Zn2+ is thought to play a key role in neuronal cell death following ischemia (Lee et al. 2000). In addition to its acute neurotoxicity, zinc may contribute to the pathogenesis of chronic neurodegenerative conditions such as Alzheimer’s disease, where it is found to accumulate in amyloid plaques (Maynard et al. 2005). Furthermore, TRPM7 also allows significant entry of toxic metals such as Ni2+ and Cd2+ (Monteilh-Zoller et al. 2003). This permeation profile coupled with the constitutive activity of TRPM7 indicates that it may represent a ubiquitous basal influx pathway for metal ions with the potential of significant physiological and toxicological repercussions that remain to be studied in more detail.
While the knock down or complete knock out of TRPM7 compromises cell growth and proliferation, overexpression of TRPM7 in heterologous cell systems such as HEK-293 cells also has harmful consequences for cell viability. Nadler et al. (2001) observed that these normally adherent cells detach after about 24–48 h and eventually die (Nadler et al. 2001), presumably because of excessive TRPM7 channel activity. Two recent studies have investigated this phenomenon in more detail, and although they arrive at different conclusions regarding the underlying mechanisms that may be responsible for it, both studies implicate TRPM7 in affecting cell adhesion. Su et al. (2006) found that overexpression of TRPM7 in cells causes loss of cell adhesion. This was determined to be due to the Ca2+-dependent protease m-calpain requiring TRPM7 activity by presumably creating a high Ca2+ environment that is localized to adhesion points.
Clark et al. (2006), studying NIE-E115 neuroblastoma cells, also found TRPM7-mediated changes in cell adhesion. This study suggested that both Ca2+- and kinase-dependent mechanisms were involved. It was found that mild overexpression of TRPM7 in NIE-E115 cells causes increases intracellular Ca2+ levels accompanied by kinase-independent cell spreading and the formation of focal adhesions. Kinase-dependent effects of TRPM7 were seen after bradykinin stimulation, which caused increased Ca2+ entry into these cells that the authors attribute to TRPM7 activation. This was accompanied by the formation of podosomes in TRPM7 overexpressing cells, but not in wildtype cells, and the effect involves the TRPM7 kinase-mediated phosphorylation of myosin IIA heavy chain (Clark et al. 2006). The interaction between TRPM7 and myosin IIA is strictly Ca2+-dependent, but additionally requires an active kinase domain, as the kinase-dead mutant does not support this interaction. It remains to be determined whether the discrepancies between these two studies reflect differences in TRPM7 expression levels or cell type-dependent mechanisms.
A further role of TRPM7 in neuronal cells has been suggested by Aarts et al. (2003), who subjected cultured cortical neurons to prolonged oxygen and glucose deprivation (OGD), an experimental model for ischemia that causes anoxic cell death. OGD caused significant influx of Ca2+ that correlated with the amount of cell death. The underlying membrane currents were attributed to TRPM7, since gene silencing by siRNA targeted against TRPM7 inhibits the currents and protects neurons from cell death. One complicating factor in these studies was that molecular silencing of TRPM7 was accompanied by a parallel reduction in TRPM2, which represents another candidate channel for mediating neuronal cell death, as it is activated by reactive oxygen species (Hara et al. 2002). It is therefore possible that both channels may have contributed to some of the effects on cell death.
Acknowledgments
This work was supported by NIH grants R01-GM065360 to A.F. and R01-NS040927 to R.P.
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeuse P, Penner R, Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006;127:421–434. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005;15:667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Estevez AY, Strange K. Calcium feedback mechanisms regulate oscillatory activity of a TRP-like Ca2+ conductance in C. elegans intestinal cells. J Physiol. 2005;567:239–251. doi: 10.1113/jphysiol.2005.091900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AY, Roberts RK, Strange K. Identification of store-independent and store-operated Ca2+ conductances in Caenorhabditis elegans intestinal epithelial cells. J Gen Physiol. 2003;122:207–223. doi: 10.1085/jgp.200308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomina AF, Fanger CM, Kozak JA, Cahalan MD. Single channel properties and regulated expression of Ca2+ release-activated Ca2+ (CRAC) channels in human T cells. J Cell Biol. 2000;150:1435–1444. doi: 10.1083/jcb.150.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–419. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci U S A. 2005;102:11510–11515. doi: 10.1073/pnas.0505149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaum HH, Cahalan MD. Monovalent permeability, rectification, and ionic block of store-operated calcium channels in Jurkat T lymphocytes. J Gen Physiol. 1998;111:521–537. doi: 10.1085/jgp.111.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 1999;283:836–839. doi: 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- Kerschbaum HH, Kozak JA, Cahalan MD. Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J. 2003;84:2293–2305. doi: 10.1016/S0006-3495(03)75035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Cahalan MD. MIC channels are inhibited by internal divalent cations but not ATP. Biophys J. 2003;84:922–927. doi: 10.1016/S0006-3495(03)74909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Matsushita M, Nairn AC, Cahalan MD. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J Gen Physiol. 2005;126:499–514. doi: 10.1085/jgp.200509324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD, Nairn AC. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J Biol Chem. 2005;280:20793–20803. doi: 10.1074/jbc.M413671200. [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX. Metals and amyloid-beta in Alzheimer’s disease. Int J Exp Pathol. 2005;86:147–159. doi: 10.1111/j.0959-9673.2005.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006;147(Suppl 1):S46–55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubagwa K, Stengl M, Flameng W. Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle. J Physiol. 1997;502:235–247. doi: 10.1111/j.1469-7793.1997.235bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a MgATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca(2+)-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Ryazanova LV, Pavur KS, Petrov AN, Dorovkov MV, Ryazanov AG. Novel type of signaling molecules: protein kinases covalently linked with ion channels. Mol Biol. 2001;35:271–283. [PubMed] [Google Scholar]
- Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG. Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem. 2004;279:3708–3716. doi: 10.1074/jbc.M308820200. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Su LT, Agapito MA, Li M, WTNS, Huttenlocher A, Habas R, Yue L, Runnels LW. Trpm7 regulates cell adhesion by controlling the calcium dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto T, Lambie EJ, Iwasaki K. Differential regulation of TRPM channels governs electrolyte homeostasis in the C. elegans intestine. Cell Metab. 2005;1:343–354. doi: 10.1016/j.cmet.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP. Cell logic for dual coupling of a single class of receptors to G(s) and G(i) proteins. Circ Res. 2000;87:635–637. doi: 10.1161/01.res.87.8.635. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Lu W, MacDonald JF. Extracellular calcium sensed by a novel cation channel in hippocampal neurons. Proc Natl Acad Sci U S A. 1997;94:7012–7017. doi: 10.1073/pnas.94.13.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell. 2001;7:1047–1057. doi: 10.1016/s1097-2765(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Zakharov SI, Mongayt DA, Cohen RA, Bolotina VM. Monovalent cation and L-type Ca2+ channels participate in calcium paradox-like phenomenon in rabbit aortic smooth muscle cells. J Physiol. 1999;514:71–81. doi: 10.1111/j.1469-7793.1999.071af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 2005;280:39185–39192. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]