The tone of precapillary blood vessels is important for haemodynamic resistance. However, the precise localization of the resistance and how this is best assessed is controversial. In a letter to the editor, Furness (2017) raises this important discussion in relation to responses of rat mesenteric arteries of approximately 150–300 μm internal diameter – termed ‘principal’ (Furness & Marshall, 1974; Hebert & Marshall, 1985) or ‘feeding’ (Christensen & Mulvany, 1993; Fenger‐Gron et al. 1995; Fenger‐Gron et al. 1997) arteries. We are grateful to Professor Furness for his comments on the physiological relevance of vascular function tests using mesenteric small arteries.
In our recent paper (Nyvad et al. 2017), we addressed the question whether the substantial information on rat mesenteric artery physiology and pharmacology collected from in vitro experiments is useful for understanding the role of these arteries in regulation of tissue perfusion and blood pressure in vivo. We investigated this question using intravital microscopy; and in order to restrict drug exposure to the studied vascular segment and reduce effects on upstream and downstream blood vessels and intestinal wall metabolism, we isolated the arterial segment in a small chamber. This is a significant modification of previously described intravital microscopy of the mesenteric circulation (Chambers & Zweifach, 1944; Furness & Marshall, 1974; Hebert & Marshall, 1985; Le Noble et al. 1987; Westcott & Segal, 2013) and allows for a more direct comparison with previous in vitro work. We found that the mesenteric arteries showed qualitatively similar functional responses in vitro and in vivo and suggested that myograph data provide a good indication of vascular behaviour in vivo.
Relative contribution of principal arteries and terminal arterioles to vascular resistance
In his letter, Furness (2017) stresses that it is the terminal and precapillary arterioles with diameter < 30 μm that contribute most to the increased resistance in response to circulating noradrenaline. This conclusion is mainly based on elegant experiments by Hebert and Marshall (1985) who showed that intravenous injections resulting in plasma noradrenaline concentration of approximately 15–30 nm (comparable to plasma noradrenaline concentration during stress, haemorrhage or exhaustive exercise; Hebert & Marshall, 1985) led to increased blood pressure, dilatation of principal arteries and constriction of terminal arterioles. However, other authors have shown that injection of larger doses of noradrenaline to give plasma concentrations of approximately 200 nm increased resistance of both principal arteries and terminal arterioles of conscious rats (Christensen & Mulvany, 1993; Fenger‐Gron et al. 1995, 1997). Whether high and low noradrenaline concentrations were applied, the largest resistance increase was in the terminal arterioles.
Intravenous injection of noradrenaline is clearly not physiological in rats. Noradrenaline in plasma originates from adrenal glands and from sympathetic varicosities (Goldstein et al. 1983). Vascular sympathetic varicosities are in close proximity to vascular smooth muscle cells and elevate the noradrenaline concentration in their immediate surroundings (Bevan et al. 1985). As pointed out by Furness (2017) in his letter, the principal mesenteric arteries are innervated with high density, while the terminal arterioles have little or no innervation (Furness, 1973). This leads to relatively high noradrenaline content in the wall of innervated mesenteric arteries even under resting conditions (Berkowitz et al. 1971; Pfeil et al. 2014). Under physiological conditions where plasma noradrenaline concentration is high, the sympathetic tone in principal arteries is also high and both principal arteries and terminal arterioles may therefore contribute to the increased resistance. Support for an important role of principal arteries came from experiments where acute stress increased blood pressure in conscious rats by ∼30 mmHg (Fenger‐Gron et al. 1997). The acute stress was associated with increased resistance of principal arteries by almost 200% while total intestinal resistance increased similarly. This suggests an important contribution to the increased resistance from both principal arteries and terminal arterioles. The same authors (Fenger‐Gron et al. 1997) demonstrated that while angiotensin II infusion almost exclusively increased the resistance in the smaller arterioles, serotonin predominantly increased the resistance of the principal arteries. The evidence therefore suggests that the relative contribution of the terminal arterioles and the principal arteries to regulation of resistance depends on the physiological conditions.
Effects of luminally vs. topically applied noradrenaline
A second point stressed in the letter by Furness (2017) is the observation that responses of mesenteric arteries to circulating noradrenaline differ from those observed with topical application. In our set‐up, we directly compared the two ways of applying noradrenaline. We confirmed that intravenous injection of noradrenaline (in a dose that gives a plasma noradrenaline concentration of about 15 nm, which was sufficient to raise blood pressure) caused dilatation of the principal arteries, whereas 15 nm noradrenaline applied topically (i.e. in the bath) had no effect (Fig. 1). Furthermore, we showed that even a high dose of noradrenaline, which causes a steady state plasma concentration of about 200 nm caused an initial dilatation followed by a sustained vasoconstriction, whereas 200 nm noradrenaline added to the bath only caused vasoconstriction. Lew and Duling (Lew et al. 1989; Lew & Duling, 1990, 1992) discussed the reason for this apparent difference in the sensitivities and demonstrated that for hydrophilic vasoconstrictors the potency was two orders of magnitude lower when applied luminally than from the adventitial side. On the other hand, lipophilic vasoconstrictors had similar potency whether applied luminally or from the adventitial side (Lew & Duling 1992). Based on these findings, they suggested that the endothelium constitutes a barrier that reduces the concentration of hydrophilic vasoconstrictors at the smooth muscle membrane relative to the blood. This would nicely explain the result and could potentially also be important for a third point raised by Furness (2017) – the apparent higher sensitivity of the constrictor response of the terminal arterioles to noradrenaline compared to the principal arteries.
Figure 1. Low plasma noradrenaline concentration (∼15 nm) relaxed the principle arteries while the topical application of the same concentration of noradrenaline did not.
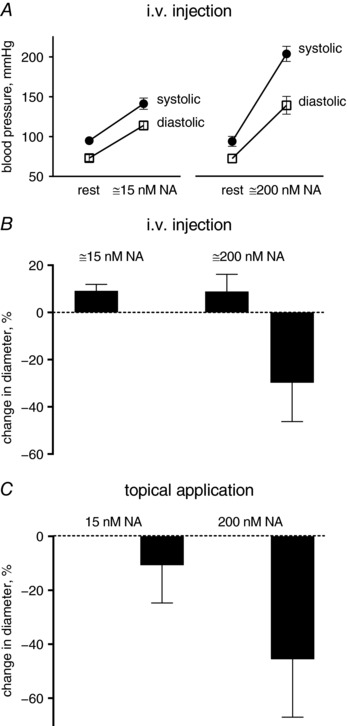
Higher noradrenaline concentration (∼200 nm) constricted the principle arteries when applied either systemically or topically. The experimental set‐up was as described previously (Nyvad et al. 2017). (A) Blood pressure was measured by carotid artery catheter in rats anaesthetized with ketamine and xylazine. Noradrenaline was injected intravenously to achieve plasma concentrations of approximately 15 and 200 nm. (B) Vascular responses to intraluminal noradrenaline were measured as the principle artery diameter changes in the experimental chamber. (C) Topical applications of noradrenaline were made to the chamber to achieve final concentrations of 15 and 200 nm, as indicated. n = 3.
Noradrenaline sensitivity of principal arteries and terminal arterioles
It is possible that the barrier function of the endothelium may vary between principal arteries and terminal arterioles, but as far as we know this has not been addressed. Other possibilities for the apparent difference in potency of noradrenaline are that the vasodilator effect of low plasma noradrenaline concentration in the principal arteries masks a vasoconstrictor effect or that presynaptic uptake of noradrenaline or activation of presynaptic α2‐adrenoceptors reduce the noradrenaline concentration near the smooth muscle α‐adrenoceptors in the densely innervated principal arteries. Finally, it may be possible that the density or subtype of adrenoceptors or their downstream signalling differ in smooth muscle cells of principal arteries compared to terminal arterioles. As far as we know, these possibilities have not been addressed.
In conclusion, mesenteric arteries of approximately 150–300 μm inner diameter can be considered as resistance arteries relevant for physiological control of peripheral resistance. The resistance of these arteries is modulated by sympathetic innervation and regulated in a bimodal fashion by blood‐borne noradrenaline.
As highlighted above, we agree with the final conclusion in Furness's letter that ‘responses to circulating noradrenaline differ from those observed when vasomotor nerves are stimulated’. In our view, this difference in responses to blood‐borne noradrenaline and noradrenaline released in the vascular wall underscores that both pathways should be investigated as they may very well both represent physiological or pathophysiological conditions. As illustrated in Fig. 1 and in our recent paper (Nyvad et al. 2017), the intravital technique provides a valuable platform for evaluating both circulating and topical administration of noradrenaline and other vasoactive substances.
Additional information
Competing interests
The authors declare no conflict of interest.
Funding
The study was supported by Carlsberg Foundation (case no CF14‐0226).
Acknowledgements
We thank Ebbe Boedtkjer for critical comments and Jørgen Andresen for excellent technical assistance.
Linked articles This is a reply to a Letter to the Editor by Furness. To read the Letter to the Editor, visit https://doi.org/10.1113/JP275097.
References
- Berkowitz BA, Tarver JH & Spector S (1971). Norepinephrine in blood vessels: concentration, binding, uptake and depletion. J Pharmacol Exp Ther 177, 119–126. [PubMed] [Google Scholar]
- Bevan JA, Bevan RD & Laher I (1985). Role of alpha‐adrenoceptors in vascular control. Clin Sci (Lond) 68(Suppl 10), 83s–88s. [DOI] [PubMed] [Google Scholar]
- Chambers R & Zweifach BW (1944). The topography and function of the mesenteric capillary circulation. Am J Anat 75, 173–205. [Google Scholar]
- Christensen KL & Mulvany MJ (1993). Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vasc Res 30, 73–79. [DOI] [PubMed] [Google Scholar]
- Fenger‐Gron J, Mulvany MJ & Christensen KL (1995). Mesenteric blood pressure profile of conscious, freely moving rats. J Physiol 488, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger‐Gron J, Mulvany MJ & Christensen KL (1997). Intestinal blood flow is controlled by both feed arteries and microcirculatory resistance vessels in freely moving rats. J Physiol 498, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB (1973). Arrangement of blood vessels and their relation with adrenergic nerves in the rat mesentery. J Anat 115, 347–364. [PMC free article] [PubMed] [Google Scholar]
- Furness JB (2017). The physiological relevance of constriction of mesenteric arteries by topically applied noradrenaline. J Physiol 595, 6783–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB & Marshall JM (1974). Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J Physiol 239, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, McCarty R, Polinsky RJ & Kopin IJ (1983). Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension 5, 552–559. [DOI] [PubMed] [Google Scholar]
- Hebert MT & Marshall JM (1985). Direct observations of responses of mesenteric microcirculation of the rat to circulating noradrenaline. J Physiol 368, 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Noble LM, Tangelder GJ, Slaaf DW, Smits JF & Struyker‐Boudier HA (1987). Adrenergic stimulation of the rat mesenteric vascular bed: a combined micro‐ and macrocirculatory study. Pflugers Arch 410, 250–256. [DOI] [PubMed] [Google Scholar]
- Lew MJ & Duling BR (1990). Arteriolar reactivity in vivo is influenced by an intramural diffusion barrier. Am J Physiol Heart Circ Physiol 259, H574–H581. [DOI] [PubMed] [Google Scholar]
- Lew MJ & Duling BR (1992). Access of blood‐borne vasoconstrictors to the arteriolar smooth muscle. J Vasc Res 29, 341–346. [DOI] [PubMed] [Google Scholar]
- Lew MJ, Rivers RJ & Duling BR (1989). Arteriolar smooth muscle responses are modulated by an intramural diffusion barrier. Am J Physiol Heart Circ Physiol 257, H10–H16. [DOI] [PubMed] [Google Scholar]
- Nyvad J, Mazur A, Postnov DD, Straarup MS, Soendergaard AM, Staehr C, Brondum E, Aalkjaer C & Matchkov VV (2017). Intravital investigation of rat mesenteric small artery tone and blood flow. J Physiol 595, 5037–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeil U, Kuncova J, Bruggmann D, Paddenberg R, Rafiq A, Henrich M, Weigand MA, Schluter KD, Mewe M, Middendorff R, Slavikova J & Kummer W (2014). Intrinsic vascular dopamine – a key modulator of hypoxia‐induced vasodilatation in splanchnic vessels. J Physiol 592, 1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott EB & Segal SS (2013). Ageing alters perivascular nerve function of mouse mesenteric arteries in vivo . J Physiol 591, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]


