Abstract
Type I (α and β) and type III (λ) IFNs are induced upon viral infection through host sensory pathways that activate IFN regulatory factors (IRFs) and nuclear factor κB. Secreted IFNs induce autocrine and paracrine signaling through the JAK-STAT pathway, leading to the transcriptional induction of hundreds of IFN-stimulated genes, among them sensory pathway components such as cGAS, STING, RIG-I, MDA5, and the transcription factor IRF7, which enhance the induction of IFN-αs and IFN-λs. This positive feedback loop enables a very rapid and strong host response that, at some point, has to be controlled by negative regulators to maintain tissue homeostasis. Type I IFN signaling is controlled by the inducible negative regulators suppressor of cytokine signaling 1 (SOCS1), SOCS3, and ubiquitin-specific peptidase 18 (USP18). The physiological role of these proteins in IFN-γ signaling has not been clarified. Here we used knockout cell lines and mice to show that IFN-λ signaling is regulated by SOCS1 but not by SOCS3 or USP18. These differences were the basis for the distinct kinetic properties of type I and III IFNs. We found that IFN-α signaling is transient and becomes refractory after hours, whereas IFN-λ provides a long-lasting IFN-stimulated gene induction.
Keywords: Hepatitis C virus (HCV), interferon, JAK, STAT transcription factor, suppressor of cytokine signaling 3 (SOCS3), IFN λ
Introduction
Type I and type III IFNs are induced in virus-infected cells and provide an important first line of defense through the rapid induction of hundreds of IFN-stimulated genes (ISGs)3 that collectively establish an antiviral state. Type I IFNs (IFN-αs and IFN-β) bind to the ubiquitously expressed IFN-α receptor (IFNAR), which consists of two major subunits: IFNAR1 and IFNAR2c. Each receptor subunit constitutively binds to a single specific member of the JAK family: IFNAR1 to tyrosine kinase 2 (TYK2) and IFNAR2c to JAK1. Upon binding of the two chains by type I IFNs, TYK2 and JAK1 transactivate each other by mutual tyrosine phosphorylation and then initiate a cascade of tyrosine phosphorylation events on the intracellular domains of the receptors and on STAT1 and STAT2. STAT1 and STAT2 combine with a third transcription factor, IFN regulatory factor 9 (IRF9), to form interferon-stimulated gene factor 3 (ISGF3). ISGF3 binds to interferon-stimulated response elements (ISREs) in the promoters of ISGs. Alternatively, IFN-activated STAT1 can form homodimers. These STAT dimers bind a different class of response elements, the γ-activated sequence elements (1). Activation of the JAK–STAT pathway is tightly controlled by several negative regulatory mechanisms. SOCS1 and 3 are rapidly induced and inhibit STAT activation (2). Mechanistically, SOCS proteins simultaneously bind the receptors and the JAKs and prevent STATs from access to the receptor kinase complex (3). Ubiquitin-specific peptidase 18 (USP18) is induced later but remains highly expressed for days (4). USP18 is responsible for the long-lasting refractoriness of IFN-α signaling in the liver (5). The physiological role of these inducible negative feedback loops is to prevent tissue damage caused by the potent pro-inflammatory effects of type I IFNs (6).
In humans, the type III IFN family consists of four members, IFN-λ1–4. They all bind to the IFN-λ receptor (IFNLR), consisting of the ubiquitously expressed IL-10R2 chain (shared with the IL-10 receptor) and a unique IFN-λ receptor chain (IFNλR1) whose expression is mainly restricted to epithelial cells (7–9). Activation of the IFNLR by ligand binding results in the activation of ISGF3 and STAT1 homodimers, the same transcription factor complexes that are induced by type I IFNs. As a consequence, despite using different receptors, IFN-λs induce highly similar sets of ISGs as IFN-α/βs (10–14). However, at least in cell culture, ISG induction followed distinct kinetic profiles for IFN-α and IFN-λ. Although gene induction was rapid and transient with IFN-α, IFN-λ induced a slower but more sustained increase in ISG expression (15, 16). There is indirect evidence that this difference could result from the lack of USP18 binding to the IFNLR. In cell culture and in mice, USP18 was induced by both IFN-α and IFN-λ, but only IFN-α signaling was inhibited by USP18 (17, 18). Contradictory to these findings, a more recent report identified USP18 as a novel inhibitor of IFN-λ signaling (19). The role of SOCS1 and SOCS3 in regulating IFN-λ signaling remains to be clarified as well. Overexpression of SOCS1 in Huh7 human hepatoma cells inhibited the antiviral activity of both IFN-α and IFN-λ (20). However, whether physiological expression levels of SOCS1 and/or SOCS3 can inhibit IFN-λ signaling remains to be shown.
The IFN-λ system has been intensely studied in the context of hepatitis C virus (HCV) infections because genetic variants of the IFN-λ gene locus are strongly associated with the ability of the host response to clear the viral infection in the acute phase and with response to treatment with pegylated IFN-α and ribavirin in chronic hepatitis C (21). Genetic evidence points to IFN-λ4 as the key regulator of the host response to HCV. A variant allele (TT at position rs368234815) with an inactivating frameshift mutation in exon 1 of the IFN-λ4 gene is found with a frequency that increases from Africa (0.29–0.44) to the New World (0.51–0.65) and Europe (0.58–0.77) and reaches 0.94–0.97 in East Asia (22). Homozygosity for the functionally inactive IFN-λ4 gene (TT/TT) is associated with spontaneous clearance of HCV in the acute phase and a high cure rate of therapies with pegylated IFN-α and ribavirin (23–25). It is not entirely clear why the host benefits from lack of a functional IFN-λ4. A pertinent observation relates to the activation status of the endogenous IFN system in the liver. Patients with wild-type IFN-λ4 (encoded by the rs368234815 ΔG allele) mount a strong innate immune response and have a permanent and strong expression of hundreds of ISGs in the liver (26). Among these ISGs are also inducible negative regulators of IFN signaling such as USP18. The continuous high expression of USP18 makes it highly unlikely that type I IFNs are drivers of the innate immune response in chronic hepatitis C. Indeed, there is genetic evidence that IFN-λ4 is the driver of ISG expression in chronic hepatitis C (25). To better understand the mechanisms of ISG induction in chronic hepatitis C, differences in negative regulation of type I and type III interferons have to be elucidated.
In this work, we investigated the role of SOCS1, SOCS3, and USP18 in IFN-λ signaling in cells with physiological IFNLR expression and deletion or overexpression of all three inhibitors and in mice deficient for SOCS1 or USP18. We identify SOCS1 as a physiologically relevant inducible inhibitor of IFN-λ–induced JAK–STAT signaling. SOCS3 and USP18 are important for regulating IFN-α but not IFN-λ signaling.
Results
IFN-λ induces sustained gene expression in cells despite strong induction of USP18
The selective and restricted expression of IFNLR1 limits the biological activity range of IFN-λ primarily to mucosal epithelial tissues (27). We have previously shown very low expression of IFNLR1 in the human liver that can, however, be induced in patients with chronic hepatitis C, particularly with the IFN-λ4 wild-type genotype (TT/ΔG and ΔG/ΔG), to levels that make hepatocytes responsive to IFN-λ (28). To analyze IFN-λ signaling in a cell line with similar IFNLR1 expression, we used a clone of Huh7 cells (a widely used human hepatoma cell line) that has been stably transfected with IFNLR1, Huh7 LR clone 3 (designated Huh7 LR in this manuscript) (28). Huh7 LR cells express around 10,000 copies of IFNLR1 per 40 ng of total RNA (Fig. 1A). Huh7 LR cells were then stimulated with saturating concentrations of human IFN-α and human IFN-λ1 for up to 48 h. The kinetics of the activation of JAK–STAT signaling were assessed with antibodies specific for tyrosine-phosphorylated STAT1 (p-STAT1), STAT2 (p-STAT2), and STAT3 (p-STAT3). IFN-α induced strong but transient STAT1 and STAT2 activation (Fig. 1B). Induction of the two early ISGs, RSAD2 and GBP5 (4), was also transient, with an expression peak 8 h after IFN-α stimulation (Fig. 1C). IFN-λ1 showed prolonged activation of STAT1 and STAT2 despite an even stronger induction of USP18 at the 24 h time point (Fig. 1B). Consistently, ISG induction was sustained and increased compared with IFN-α (Fig. 1C). The induction of pSTAT3 was not significantly different between IFN-α and IFN-λ1. These results are in accordance with published data obtained with other cell lines and primary human hepatocytes (11, 15, 16).
Figure 1.
IFN-α– but not IFN-λ–induced STAT1 phosphorylation becomes refractory to continuous stimulation. A, liver biopsies from chronic hepatitis C patients (n = 16) were divided into three groups based on their IFNL4 genotype (rs368234815; TT/TT, TT/dG, and dG/dG). Total RNA from biopsies and Huh7 and Huh7 LR cells were prepared. Expression of the IFNLR1 transcript was analyzed by quantitative PCR. Results (mean ± S.D.) are shown as copy numbers per 40 ng of total RNA. B and C, Huh7 LR cells were stimulated with 1000 IU/ml IFN-α or 100 ng/ml IFN-λ1 for the indicated times. B, p-STAT1, STAT1, p-STAT2, STAT2, p-STAT3, STAT3, USP18, SOCS1, SOCS3, and actin were visualized using specific antibodies. Shown are representative blots from two independent experiments. C, transcripts of interferon-stimulated genes (RSAD2, IFI27, and GBP5) were quantified by PCR. Results (mean ± S.D., n = 3) are shown as relative expression to GAPDH. ut, untreated.
IFN-λ signaling is inhibited in cells overexpressing SOCS1, SOCS3, or USP18
Huh7 LR cells were then transiently transfected with expression plasmids for SOCS1, SOCS3, and USP18 and stimulated with saturating concentrations of IFN-α and IFN-λ1 for 30 min. All three proteins were strongly expressed and inhibited both IFN-α– and IFN-λ1–induced STAT1 phosphorylation (Fig. 2A). The inhibitory effect of USP18 on IFN-λ signaling was unexpected, given the sustained p-STAT1 and ISG induction shown in Fig. 1. We therefore systematically compared the expression levels of the negative regulators obtained by physiological stimulation with IFNs versus those obtained in cells transfected with expression plasmid. As shown in Fig. 2B, transfection resulted in expression levels that were 2–3 orders of magnitude higher than those induced by maximal physiological stimulation with IFNs. The mRNA results were also confirmed at the protein level (Fig. 2B). We conclude that all three inhibitors have the potential to inhibit both IFN-α and IFN-λ signaling at supraphysiological expression levels.
Figure 2.
Overexpression of SOCS1, SOCS3, and USP18 leads to a reduction of IFN-α– and IFN-λ–mediated STAT1 phosphorylation. A, Huh7 LR cells were transiently transfected with control, SOCS1, SOCS3, or USP18 expression plasmids. 24 h later, cells were stimulated with 1000 IU/ml IFN-α or 100 ng/ml IFN-λ1 for 30 min, and p-STAT1, STAT1, SOCS1, SOCS3, USP18, and actin were visualized by immunoblotting. Shown are representative blots from three independent experiments. B, Huh7 LR cells were transfected with SOCS1, SOCS3, or USP18 expression plasmids for 24 h. The mRNA expression levels of SOCS1, SOCS3, and USP18 were analyzed by quantitative PCR and compared with the endogenously induced SOCS1, SOCS3, or USP18 upon IFN-α or IFN-λ1 stimulation at the indicated time points. The results (mean ± S.D., n = 3) are shown as relative expression to GAPDH. Protein levels of SOCS1, SOCS3, and USP18 and actin were visualized using specific antibodies. ox, overexpression; ut, untreated.
SOCS1-deficient cells are hyperresponsive to IFN-λ
To assess the physiological relevance of SOCS1, SOCS3, and USP18 for IFN-λ signaling, we generated knockout cell lines from Huh7 LR cells using the CRISPR-Cas9 technology (supplemental Fig. 1). The rationale for this loss-of-function experiment was the expectation that loss of a physiologically relevant inhibitor of IFN signaling must result in hyperactivation of the signal transduction pathway with increased ISG induction. By definition, any inducible inhibitor of IFN signaling has to restrict ISG induction at some point and to a relevant degree. We first tested this hypothesis using a luciferase reporter gene under the control of an ISRE promoter (i.e. the Mx1 promoter, ISRE-Mx1-Luc). To do so, we transfected control and knockout cells with the pGL3-ISRE-Mx1-Luc plasmid together with a constitutively expressed Renilla luciferase construct for normalization. 20 h after transfection, they were stimulated with IFN-λ1, IFN-λ3, IFN-λ4, IFN-α, and IFN-β or left untreated, and luciferase activity was quantified 4, 8, and 24 h later. The luciferase activity in untreated cells did not differ significantly between cell lines but increased at least 15-fold within 4 h after IFN addition. At 4 h and 8 h, but not at 24 h, SOCS1 knockout cells showed significantly higher expression of the reporter constructs when stimulated with IFN-λ1, IFN-λ3, or IFN-λ4 (Fig. 3 and supplemental Fig. 2). IFN-α signaling was enhanced in USP18 knockout cells at all time points (Fig. 3), whereas IFN-β signaling was enhanced at the 8 h time point only (supplemental Fig. 2). Type I IFN–induced expression of the reporter plasmid was also significantly enhanced at some time points in SOCS1 and SOCS3 knockout cells (Fig. 3 and supplemental Fig. 2). We confirmed these findings by quantifying the expression levels of ISGs, including RSAD2, GBP5, and IFI27, as well as the three inducible regulators, SOCS1, SOCS3, and USP18, in the knockout cell lines after stimulation with IFN-λ1 and IFN-α (Fig. 4 and supplemental Fig. 3). Expression of RSAD2, GBP5, and IFI27 in untreated control and knockout cells was detectable and did not differ significantly between the cell lines. The same ISGs were up-regulated 10- to 100-fold 4 h after IFN-α or IFN-λ1 stimulation (Fig. 4). Importantly however, IFN-λ1–induced ISG expression was significantly enhanced in SOCS1 knockout cells at 8 and 24 h, but this increase was not observed in SOCS3 or USP18 knockout cells. For GBP5, this enhancement could already be observed 4 h after IFN-λ1 stimulation, although it did not reach statistical significance. Furthermore, we confirmed the known negative regulatory effect of USP18 on IFN-α signaling. We conclude that, in Huh7 LR cells, SOCS1 is an inducible and physiologically relevant negative regulator of IFN-λ1, IFN-λ3, and IFN-λ4.
Figure 3.

SOCS1 is a modulator of IFN-λ-signaling. Control, SOCS1−/−, SOCS3−/−, and USP18−/− cells were transfected with pGL3-ISRE-Mx1-Luc and pGL4-CMV-Renilla-Luc plasmids and, 20 h later, stimulated with 100 ng/ml IFN-λ1, 50 ng/ml IFN-λ4, or 1000 IU/ml IFN-α for 4 h, 8 h, and 24 h or left untreated. The firefly luciferase values were normalized to Renilla luciferase, and the results (mean ± S.D., n = 2) are expressed as firefly/Renilla ratio. Unpaired t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Figure 4.
SOCS1 is a modulator of IFN-λ–induced ISGs expression in vitro. Control, SOCS1−/−, SOCS3−/−, and USP18−/− cells were stimulated with 1000 IU/ml IFN-α or 100 ng/ml IFN-λ1 for 4 h, 8 h, and 24 h or left untreated, and the expression levels of RSAD2, GBP5, and IFI27 were analyzed by quantitative PCR. The results (mean ± S.D., n = 2) are shown as relative expression to GAPDH. Unpaired t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
SOCS1 is a physiological inhibitor of IFN-λ signaling in vivo
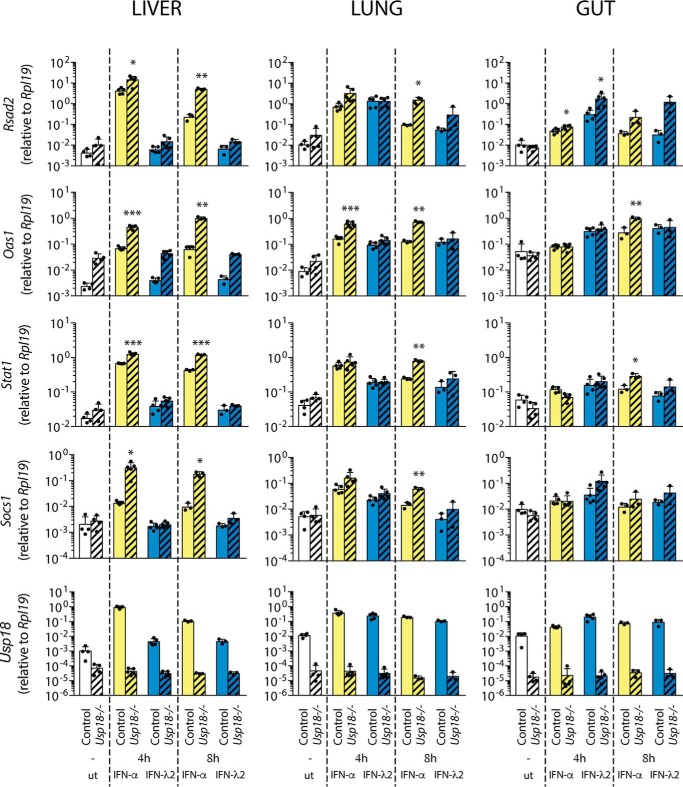
To test whether the results obtained in Huh7 LR cells are valid in vivo, we analyzed IFN-λ and IFN-α signaling in mice deficient for Socs1 or Usp18 and their corresponding control mice. Because Socs1 knockout mice suffer from IFN-γ–mediated lethal toxicity, they were bread as Ifng/Socs1 double knockout mice, with Ifng knockout mice serving as controls, as described previously (5, 29). The mice were sacrificed 4 and 8 h after IFN injections, and the liver, lung, and gut were analyzed for induction of seven different ISGs (Rsad2, Oas1, Stat1, Usp18, Gbp5, and Ifi27). Socs1 knockout resulted in significantly increased expression of all IFN-λ2–induced ISGs at 8 h in the lung and at 4 h in the gut, the two IFN-λ responsive organs, but not in the liver, which is devoid of IFNLR1 in mice (Fig. 5). Although Socs1 knockout also resulted in enhanced expression of three of six ISGs in the lung and gut upon IFN-α injection (Fig. 5), it did not affect IFN-α–mediated ISG induction levels in the liver (Fig. 5 and supplemental Fig. 4A). In contrast, IFN-α induced all tested ISGs to significantly higher levels in the livers of Usp18 knockout mice compared with control mice at all time points (Fig. 6 and supplemental Fig. 4B). The effect of Usp18 knockout on IFN-α signaling in the lung occurred mainly at 8 h, and it was much less pronounced in the gut. The effect of Usp18 knockout on IFN-λ signaling was minimal, with only Rsad2 and Ifi27 being significantly increased at one time point in the gut and lung, respectively (Fig. 6 and supplemental Fig. 4B). From these results, we conclude that IFN-α signaling in these mice is predominantly inhibited by Usp18, whereas IFN-λ signaling is regulated by Socs1.
Figure 5.
Depletion of Socs-1 increased IFN-λ–induced ISGs expression in vivo. Control and Socs1−/− mice were subcutaneously injected with PBS, 1000 units/g mouse IFN-α, or 50 ng/g mouse IFN-λ2. The liver, the lung, and the gut were collected 4 h and 8 h after injection, and total RNA was prepared. The expression of Rsad2, Oas1, Stat1, Usp18, and Socs1 was measured by quantitative PCR. The results (mean ± S.D.) are shown as relative expression to Rpl19. Three to four animals were used per time point and condition. Unpaired t test with Welch's correction; *, p < 0.05; **, p < 0.01; ***, p < 0.001. ut, untreated.
Figure 6.
Depletion of Usp18 increased IFN-α–induced ISGs expression in vivo. Control and Usp18−/− mice were subcutaneously injected with PBS, 1000 units/g mouse IFN-α, or 50 ng/g mouse IFN-λ2. The liver, the lung, and the gut were collected 4 h and 8 h after injection, and total RNA was prepared. The expression of Rsad2, Oas1, Stat1, Socs1, and Usp18 was measured by quantitative PCR. The results (mean ± S.D.) are shown as relative expression to Rpl19. Three to five animals were used per time point and condition. Unpaired t test with Welch's correction; *, p < 0.05; **, p < 0.01; ***, p < 0.001. ut, untreated.
Discussion
Type I IFNs (IFN-α/β) are potent and critically important cytokines that control innate and adaptive immune responses to infection, cancer, and other inflammatory stimuli. Positive feedback amplification through autocrine and paracrine induction of IFN-α gene transcription allows a very rapid and strong host response to infections (30). The extent and duration of this response is tightly controlled by several mechanisms to avoid adverse effects on tissue homeostasis. The fact that several clinically important autoimmune diseases, such as systemic lupus erythematosus and Aicardi-Goutières syndrome, are associated with uncontrolled type I IFN activities demonstrates the important role of negative regulators in keeping the system in balance (6, 31). SOCS1 is an inducible negative regulator of IFN-α signaling that transiently restricts phosphorylation of STATs in the first hours. However, its role as a negative regulator of IFN-α signaling is limited. The fact that the lethal phenotype of Socs1 knockout mice is rescued by additional knockout of Ifng demonstrates that the main role of SOCS1 in the IFN system is to control IFN-γ (29). Furthermore, deletion of Socs1 does not prevent the induction of refractoriness to IFN-α in the mouse liver (5). The main negative regulator of IFN-α seems to be USP18. This is demonstrated by this work, by previous work that showed that refractoriness to IFN-α stimulation is abrogated in Usp18 knockout mice (5), and by a rare genetic interferonopathy, pseudo-TORCH syndrome, which is caused by human USP18 deficiency (32).
Negative regulation of type III IFNs is fundamentally different from type I IFNs. The inducible up-regulation of USP18 does not induce refractoriness of IFN-λ signaling (this work and Refs. 11, 15–18).). Of note, as shown in Fig. 2, USP18 indeed has the potential to inhibit IFN-λ signaling, but only at expression levels that are not achieved by physiological induction stimuli. We conclude that USP18 is not a physiological inhibitor of IFN-λ signaling. IFN-λ signaling is rather controlled by SOCS1, and contrary to IFN-α, not only in the first hours of stimulation but also at later time points (Fig. 4). Of note, a long-lasting negative regulatory effect of SOCS1 on IFN-λ1 signaling has been found previously in shRNA knockdown experiments in A549 human alveolar epithelial cells (33). SOCS1 not only controls the closely related IFN-λ1 and IFN-λ3 but also IFN-λ4. We postulate that the prolonged inhibitory effects of SOCS1 on IFN-λ signaling result from its sustained up-regulation for at least 48 h, which contrasts the transient induction of SOCS1 by IFN-α (Fig. 2B).
In conclusion, we show that the IFN-α and the IFN-λ systems not only differ in terms of tissue distribution of their cognate receptors (27) but are also controlled by distinct negative regulatory mechanisms of their signal transduction through the JAK–STAT pathway. IFN-α signaling is transient and shut down after 6–8 h by USP18, a very strong inhibitor of STAT tyrosine phosphorylation at the IFNAR–kinase complex. IFN-λ signaling is not affected by USP18. It is controlled by SOCS1, but SOCS1 does not shut down STAT phosphorylation completely, allowing long-lasting stimulation of ISG transcription by IFN-λ. We postulate that these differences between IFN-α and IFN-λ in the negative regulation of JAK–STAT signaling are responsible for the previously described differences in the kinetics of ISG induction. Long-lasting activation of JAK–STAT signaling allows the IFN-λ system to mount a sustained antiviral state in mucosal epithelial cells that are constantly exposed to pathogens. IFN-α is a more powerful defense system that is activated when pathogenic viruses have breached the mucosal surfaces and invaded the systemic circulation and other organs. In most instances, it is only transiently activated because prolonged activation of IFN-α signaling is detrimental for tissue homeostasis and can also negatively impact the cellular immune response.
Experimental procedures
Cell culture
A human hepatoma cell line (Huh7) was cultured in DMEM (Gibco) supplemented with 10% heat-inactivated FBS (Gibco) and 1% penicillin-streptomycin (Gibco). Huh7 LR cells constitutively overexpressing IFNLR1 (28) and SOCS1−/−, SOCS3−/−, and USP18−/− cells were cultured in DMEM-10% FBS, 1% penicillin-streptomycin (Gibco) supplemented with 1 mg/ml G418 (Calbiochem).
Animals
Socs1−/− Ifng−/− (Socs1−/−), Ifng−/− (control), Ubp43−/− (Usp18−/−), and FVB (control) mice were described previously (29, 34–37). The animals were bred and maintained in the animal facility of the Department of Biomedicine of the University Hospital Basel under specific-pathogen-free conditions on a 12-h day and 12-h night schedule with ad libitum access to food and drinking water. Experiments were conducted with the approval of the Animal Care Committee of the Canton Basel-Stadt, Switzerland. Six- to eight-week-old males were used, and the animals were euthanized by CO2 narcosis. Resected organs were immediately frozen in liquid nitrogen and kept at −70 °C until further processing. Subcutaneous injections with PBS, mouse IFN-α, or mouse IFN-λ2 were performed between 8 a.m. and 5 p.m.
Human liver biopsies
Liver biopsies from chronic HCV-infected patients (n = 16, patient characteristics are shown in supplemental Table 1) were obtained in the outpatient clinic of the Division of Gastroenterology and Hepatology, University Hospital Basel, Switzerland. Biopsy material that was not needed for routine histopathology was used for research purposes after obtaining written informed consent. The use of biopsy material for this project was approved by the Ethikkommission Nordwest- und Zentralschweiz, Basel, Switzerland, protocol number M989/99. Total DNA was isolated from liver biopsies using the DNeasy Blood & Tissue Kit (Qiagen, Hombrechtikon, Switzerland) according to the instructions of the manufacturer. IFN-λ4 genotype was determined as described previously (25).
Plasmids, antibodies, and reagents
Human IFN-α (Roferon-A) was purchased from Roche Pharma SA (Reinach, Switzerland). Human IFN-β (Betaferon) was obtained from Bayer HealthCare Pharmaceuticals (Bayer Consumer Care AG, Basel, Switzerland). Human IFN-λ1 and mouse IFN-λ2 were from PeproTech (LuBioScience GmbH, Luzern, Switzerland), and human IFN-γ was from Biolegend (Lucerna-Chem AG, Luzern, Switzerland). Human IFN-λ3 and human IFN-λ4 were generated as described previously (9) by Prof. Rune Hartmann (Aarhus University, Aarhus, Denmark), and mouse IFN-α was a gift from Prof. Radek Skoda (University Hospital Basel). Phospho-STAT1 (Tyr-701, 58D6), phospho-STAT3 (Tyr-705, D3A7 XP), STAT3 (124H6), and USP18 (D4E7) antibodies were from Cell Signaling Technology (BioConcept, Allschwil, Switzerland), and STAT1 (C-term) and STAT2 antibodies were from BD Biosciences (Allschwil, Switzerland). Phospho-STAT2 (Tyr689) and SOCS1 (4H1) antibodies were from EMD Millipore (Merck & Co., Schaffhausen, Switzerland). SOCS3 (H-103) and β-actin were from Santa Cruz Biotechnology (LabForce AG, Muttenz, Switzerland) and Sigma-Aldrich Chemie GmbH (Buchs, Switzerland), respectively.
Whole-cell lysates and Western blotting analysis
Whole-cell lysates and immunoblots were prepared and used as described previously (38).
Total RNA extraction and quantitative PCR
Total RNA from cell lines was isolated using NucleoSpin RNA (Macherey-Nagel AG, Oensingen, Switzerland) according to the instructions of the manufacturer. cDNA was generated from 1 μg of total RNA using Moloney murine leukemia virus reverse transcriptase (Promega AG, Dübendorf, Switzerland). Total RNA from human biopsies and mouse samples was isolated using TRIzol according to the instructions of the manufacturer. 1 μg of total RNA was incubated with rDNaseI using the DNA-free kit (Ambion). cDNA was generated using the TaqMan reverse transcription reagent kit (Applied Biosystems) according to the recommendations of the manufacturer. Real-time quantitative PCR was performed using FastStart Universal SYBR Green Master Mix (Roche Diagnostics AG, Rotkreuz, Switzerland) and the ABI 7500 detection system (Applied Biosystems, Thermo Fisher Scientific, Zug, Switzerland). Primers are listed in supplemental Table 2. The specificity of the PCR primers was assessed by sequencing the PCR product. Gene transcript expression levels were calculated using the ΔCt method. To quantitate IFNLR1 transcript levels, dilutions of plasmids containing the IFNLR1 ORF (28) were used as standard curves (dilutions ranged from 108 to 100 copies of plasmid).
SOCS1, SOCS3, and USP18 overexpression
The pCMV6 plasmid containing the SOCS1 gene (RC220847) was purchased from Origene Technologies Inc. (Rockville, MD) and used to overexpress SOCS1. Human USP18 and human SOCS3 coding sequences were cloned into the pCMV6-entry and pCMV-MIR vectors, respectively, using XhoI and BamHI restriction sites for USP18 and XhoI and KpnI restriction sites for SOCS3. Huh7 LR cells (2 × 105/well) were seeded onto a 6-well plate, and 2 μg of expression plasmid was transfected using JetPrimeTM (Polyplus Transfection, VWR International GmbH, Dietikon, Switzerland) according to the instructions of the manufacturer.
Generation of SOCS1, SOCS3, and USP18 knockout cell lines
Briefly, the CRISPR design tool from the Zhang laboratory (http://crispr.mit.edu)4 was used to design single-guide RNA constructs (supplemental Table 3). Phosphorylated and annealed single-guide RNAs were cloned into pSpCas9(BB)-2A-GFP (PX458) (Addgene, 48138, deposited by Feng Zhang) using BbsI restriction sites. Plasmids were verified by sequencing. 48 h post-transfection, GFP-positive cells were single cell–sorted by FACS. To confirm successful gene targeting in sorted clones, genomic DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen AG) and subjected to PCR amplification using specific primers for the region of interest (supplemental Table 2). PCR fragments were then cloned into a pGEMT-easy expression vector (Promega AG) and transformed into Escherichia coli TOP-10 chemically competent cells (Invitrogen). Colonies were analyzed by sequencing using the T7 primer. Finally, the absence of SOCS1, SOCS3, or USP18 protein was confirmed by immunoblotting.
IFN activity reporter assay
The ISRE-Mx1 firefly luciferase reporter construct (pGL3-Mx1P-FF-Luc, a gift from Rune Hartmann) and pGL4-CMV-Renilla-Luc (a gift from Jacek Krol) were electroporated using Cytomix (39, 40) into SOCS1−/−, SOCS3−/−, USP18−/−, or control cells. 20 h after electroporation, cells were treated with IFN-α (1000 UI/ml), IFN-β (1000 UI/ml), IFN-λ1 (100 ng/ml), IFN-λ3 (200 ng/ml), or IFN-λ4 (50 ng/ml) for 4, 8, and 24 h. Cells were then lysed with passive lysis buffer (Promega AG), and firefly luciferase levels were measured, followed by Renilla luciferase levels, using a multimode microplate reader (Centro XS3 LB960, Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany). The firefly luciferase was normalized in each well to Renilla luciferase.
Statistical analysis
Prism4 (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis.
Author contributions
T. B., M. C.L., and F. H. T. D. conducted the experiments, analyzed the results, and prepared the figures. M. H. H. recruited patients and obtained the liver biopsies. All authors conceived the idea for the project and wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Hans Henrik Gad and Rune Hartmann for providing recombinant human IFN-λ3 and human IFN-λ4 and the ISRE luciferase reporter construct. We thank Petr Broz and Roland Dreier for excellent technical support with CRISPR/Cas9 technology. We thank Stefan Wieland for critical reading of the manuscript.
This work was supported by Swiss National Science Foundation Grants 310030B_147089 and 310030_166202 (to M. H. H.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. 1–4 and Tables 1–3.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party–hosted site.
- ISG
- IFN-stimulated gene
- IFNAR
- IFN-α receptor
- ISRE
- IFN-stimulated response element
- SOCS
- suppressor of cytokine signaling
- IFNLR
- IFN-λ receptor
- HCV
- hepatitis C virus.
References
- 1. Darnell J. E., Jr. (1997) STATs and gene regulation. Science 277, 1630–1635 [DOI] [PubMed] [Google Scholar]
- 2. Krebs D. L., and Hilton D. J. (2001) SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19, 378–387 [DOI] [PubMed] [Google Scholar]
- 3. Kershaw N. J., Murphy J. M., Liau N. P., Varghese L. N., Laktyushin A., Whitlock E. L., Lucet I. S., Nicola N. A., and Babon J. J. (2013) SOCS3 binds specific receptor-JAK complexes to control cytokine signaling by direct kinase inhibition. Nat. Struct. Mol. Biol. 20, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dill M. T., Makowska Z., Trincucci G., Gruber A. J., Vogt J. E., Filipowicz M., Calabrese D., Krol I., Lau D. T., Terracciano L., van Nimwegen E., Roth V., and Heim M. H. (2014) Pegylated IFN-alpha regulates hepatic gene expression through transient Jak/STAT activation. J. Clin. Invest. 124, 1568–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarasin-Filipowicz M., Wang X., Yan M., Duong F. H., Poli V., Hilton D. J., Zhang D.-E., and Heim M. H. (2009) α Interferon induces long-lasting refractoriness of JAK-STAT signaling in the mouse liver through induction of USP18/UBP43. Mol. Cell. Biol. 29, 4841–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Porritt R. A., and Hertzog P. J. (2015) Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 36, 150–160 [DOI] [PubMed] [Google Scholar]
- 7. Kotenko S. V., Gallagher G., Baurin V. V., Lewis-Antes A., Shen M., Shah N. K., Langer J. A., Sheikh F., Dickensheets H., and Donnelly R. P. (2003) IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4, 69–77 [DOI] [PubMed] [Google Scholar]
- 8. Donnelly R. P., Sheikh F., Kotenko S. V., and Dickensheets H. (2004) The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukocyte Biol. 76, 314–321 [DOI] [PubMed] [Google Scholar]
- 9. Hamming O. J., Terczyńska-Dyla E., Vieyres G., Dijkman R., Jørgensen S. E., Akhtar H., Siupka P., Pietschmann T., Thiel V., and Hartmann R. (2013) Interferon λ 4 signals via the IFNλ receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J. 32, 3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doyle S. E., Schreckhise H., Khuu-Duong K., Henderson K., Rosler R., Storey H., Yao L., Liu H., Barahmand-pour F., Sivakumar P., Chan C., Birks C., Foster D., Clegg C. H., Wietzke-Braun P., et al. (2006) Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 44, 896–906 [DOI] [PubMed] [Google Scholar]
- 11. Marcello T., Grakoui A., Barba-Spaeth G., Machlin E. S., Kotenko S. V., MacDonald M. R., and Rice C. M. (2006) Interferons α and λ inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131, 1887–1898 [DOI] [PubMed] [Google Scholar]
- 12. Lauber C., Vieyres G., Terczyńska-Dyla E., Anggakusuma Dijkman R., Gad H. H., Akhtar H., Geffers R., Vondran F. W., Thiel V., Kaderali L., Pietschmann T., and Hartmann R. (2015) Transcriptome analysis reveals a classical interferon signature induced by IFNλ4 in human primary cells. Genes Immun. 16, 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou Z., Hamming O. J., Ank N., Paludan S. R., Nielsen A. L., and Hartmann R. (2007) Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 81, 7749–7758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crotta S., Davidson S., Mahlakoiv T., Desmet C. J., Buckwalter M. R., Albert M. L., Staeheli P., and Wack A. (2013) Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 9, e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jilg N., Lin W., Hong J., Schaefer E. A., Wolski D., Meixong J., Goto K., Brisac C., Chusri P., Fusco D. N., Chevaliez S., Luther J., Kumthip K., Urban T. J., Peng L. F., et al. (2014) Kinetic differences in the induction of interferon stimulated genes by interferon-α and interleukin 28B are altered by infection with hepatitis C virus. Hepatology 59, 1250–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolen C. R., Ding S., Robek M. D., and Kleinstein S. H. (2014) Dynamic expression profiling of type I and type III interferon-stimulated hepatocytes reveals a stable hierarchy of gene expression. Hepatology 59, 1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. François-Newton V., Magno de Freitas Almeida G., Payelle-Brogard B., Monneron D., Pichard-Garcia L., Piehler J., Pellegrini S., and Uzé G. (2011) USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon α response. PLoS ONE 6, e22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makowska Z., Duong F. H., Trincucci G., Tough D. F., and Heim M. H. (2011) Interferon-β and interferon-λ signaling is not affected by interferon-induced refractoriness to interferon-α in vivo. Hepatology 53, 1154–1163 [DOI] [PubMed] [Google Scholar]
- 19. Burkart C., Arimoto K., Tang T., Cong X., Xiao N., Liu Y. C., Kotenko S. V., Ellies L. G., and Zhang D. E. (2013) Usp18 deficient mammary epithelial cells create an antitumour environment driven by hypersensitivity to IFN-λ and elevated secretion of Cxcl10. EMBO Mol. Med. 5, 1035–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu B., Chen S., Guan Y., and Chen L. (2015) Type III interferon induces distinct SOCS1 expression pattern that contributes to delayed but prolonged activation of Jak/STAT signaling pathway: implications for treatment non-response in HCV patients. PLoS ONE 10, e0133800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heim M. H., Bochud P. Y., and George J. (2016) Host-hepatitis C viral interactions: the role of genetics. J. Hepatol. 65, S22–32 [DOI] [PubMed] [Google Scholar]
- 22. Key F. M., Peter B., Dennis M. Y., Huerta-Sánchez E., Tang W., Prokunina-Olsson L., Nielsen R., and Andrés A. M. (2014) Selection on a variant associated with improved viral clearance drives local, adaptive pseudogenization of interferon λ 4 (IFNL4). PLoS Genet. 10, e1004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prokunina-Olsson L., Muchmore B., Tang W., Pfeiffer R. M., Park H., Dickensheets H., Hergott D., Porter-Gill P., Mumy A., Kohaar I., Chen S., Brand N., Tarway M., Liu L., Sheikh F., et al. (2013) A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 45, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bibert S., Roger T., Calandra T., Bochud M., Cerny A., Semmo N., Duong F. H., Gerlach T., Malinverni R., Moradpour D., Negro F., Müllhaupt B., Bochud P. Y., and Swiss Hepatitis C Cohort Study (2013) IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J. Exp. Med. 210, 1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terczyńska-Dyla E., Bibert S., Duong F. H., Krol I., Jørgensen S., Collinet E., Kutalik Z., Aubert V., Cerny A., Kaiser L., Malinverni R., Mangia A., Moradpour D., Müllhaupt B., Negro F., et al. (2014) Reduced IFNlambda4 activity is associated with improved HCV clearance and reduced expression of interferon-stimulated genes. Nat. Commun. 5, 5699. [DOI] [PubMed] [Google Scholar]
- 26. Heim M. H. (2013) Innate immunity and HCV. J. Hepatol. 58, 564–574 [DOI] [PubMed] [Google Scholar]
- 27. Sommereyns C., Paul S., Staeheli P., and Michiels T. (2008) IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4, e1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duong F. H., Trincucci G., Boldanova T., Calabrese D., Campana B., Krol I., Durand S. C., Heydmann L., Zeisel M. B., Baumert T. F., and Heim M. H. (2014) IFN-λ receptor 1 expression is induced in chronic hepatitis C and correlates with the IFN-λ3 genotype and with nonresponsiveness to IFN-α therapies. J. Exp. Med. 211, 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alexander W. S., Starr R., Fenner J. E., Scott C. L., Handman E., Sprigg N. S., Corbin J. E., Cornish A. L., Darwiche R., Owczarek C. M., Kay T. W., Nicola N. A., Hertzog P. J., Metcalf D., and Hilton D. J. (1999) SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98, 597–608 [DOI] [PubMed] [Google Scholar]
- 30. Marié I., Durbin J. E., and Levy D. E. (1998) Differential viral induction of distinct interferon-α genes by positive feedback through interferon regulatory factor-7. EMBO J. 17, 6660–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee-Kirsch M. A. (2017) The type I Interferonopathies. Annu. Rev. Med. 68, 297–315 [DOI] [PubMed] [Google Scholar]
- 32. Meuwissen M. E., Schot R., Buta S., Oudesluijs G., Tinschert S., Speer S. D., Li Z., van Unen L., Heijsman D., Goldmann T., Lequin M. H., Kros J. M., Stam W., Hermann M., Willemsen R., et al. (2016) Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 213, 1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei H., Wang S., Chen Q., Chen Y., Chi X., Zhang L., Huang S., Gao G. F., and Chen J. L. (2014) Suppression of interferon λ signaling by SOCS-1 results in their excessive production during influenza virus infection. PLoS Pathog. 10, e1003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Starr R., Metcalf D., Elefanty A. G., Brysha M., Willson T. A., Nicola N. A., Hilton D. J., and Alexander W. S. (1998) Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. U.S.A. 95, 14395–14399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., and Stewart T. A. (1993) Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 [DOI] [PubMed] [Google Scholar]
- 36. Kim K. I., Yan M., Malakhova O., Luo J.-K., Shen M.-F., Zou W., de la Torre J. C., and Zhang D.-E. (2006) Ube1L and protein ISGylation are not essential for α/β interferon signaling. Mol. Cell. Biol. 26, 472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ritchie K. J., Malakhov M. P., Hetherington C. J., Zhou L., Little M.-T., Malakhova O. A., Sipe J. C., Orkin S. H., and Zhang D.-E. (2002) Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev. 16, 2207–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duong F. H., Filipowicz M., Tripodi M., La Monica N., and Heim M. H. (2004) Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology 126, 263–277 [DOI] [PubMed] [Google Scholar]
- 39. Koutsoudakis G., Perez-del-Pulgar S., Coto-Llerena M., Gonzalez P., Dragun J., Mensa L., Crespo G., Navasa M., and Forns X. (2011) Cell culture replication of a genotype 1b hepatitis C virus isolate cloned from a patient who underwent liver transplantation. PLoS ONE 6, e23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van den Hoff M. J., Christoffels V. M., Labruyère W. T., Moorman A. F., and Lamers W. H. (1995) Electrotransfection with “intracellular” buffer. Methods Mol. Biol. 48, 185–197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.