Abstract
Objectives
Sexual minority women are at risk for infection with human papillomavirus (HPV); yet, relatively little is known about the prevalence of HPV infection among this population.
Methods
We analysed data from the 2003–2012 National Health and Nutrition Examination Survey among women aged 20–59 (n=7132). We examined two dimensions of sexual orientation (sexual identity and sexual behaviour) and used weighted logistic regression to determine how HPV infection outcomes (any HPV type, high-risk HPV type and vaccine HPV type) vary by dimension.
Results
Similar patterns emerged for sexual identity and sexual behaviour. In bivariate analyses, HPV infection outcomes were more common among non-heterosexual women compared with heterosexual women (any type: 49.7% vs 41.1%; high-risk type: 37.0% vs 27.9%), as well as among women who reported any same-sex partners compared with women who reported only opposite-sex partners (any type: 55.9% vs 41.0%; high-risk type: 37.7% vs 28.2%; vaccine type: 19.1% vs 14.0%) (p<0.05). When we disaggregated measures of sexual orientation into subgroups, bisexual women and women who reported partners of both sexes had greater odds of HPV infection outcomes (p<0.05 in bivariate analyses). Multivariate models attenuated several of these differences, though lesbian women and women who reported only same-sex partners had lower odds of most HPV infection outcomes in multivariate analyses (p<0.05).
Conclusions
HPV infection is common among sexual minority women, though estimates vary depending on how sexual orientation is operationalised. Results can help inform targeted HPV and cervical cancer prevention efforts for sexual minority women.
INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted infection in the USA,1 and infections can cause several adverse health outcomes. Oncogenic HPV types are associated with several cancer types, including cervical, vaginal, vulvar and anal cancers.2 In fact, persistent infection with oncogenic HPV types causes almost all cervical cancers.2 Non-oncogenic HPV types can cause anogenital warts.3
A large body of research has examined the burden of HPV infection among sexual minority men,4 but much less is known about infection among sexual minority women (ie, women who identify as non-heterosexual or have a history of same-sex sexual partners). Data suggest there are over four million sexual minority women in the USA.5 Many sexual minority women lack knowledge about HPV and perceive themselves to be at very low risk for HPV infection,6–9 even though they are in fact at risk. HPV can be transmitted between female sexual partners,10 and many sexual minority women have current or past male sexual partners from whom they could have acquired HPV.11 The lack of knowledge and low perceived risk for HPV infection may be due in part to sexual minority women often not being mentioned specifically in sexual health education programmes and materials, sexual health policies or cervical cancer screening guidelines.9,12,13
Only a few studies have examined the prevalence of genital HPV infection among sexual minority women, with estimates ranging from 13% to 51% for infection with any type of HPV.10,14–16 There may be several reasons for the heterogeneity of these estimates, including some studies relying on convenience samples of women from a limited geographic area, modest sample sizes and differences in HPV testing methodology.
Research examining HPV infection among sexual minority women is also affected by the complexities of assessing and operationalising sexual orientation, which is a multidimensional construct. Two dimensions of sexual orientation are sexual identity (ie, considering oneself to be lesbian, bisexual, heterosexual, etc.) and sexual behaviour (ie, having sex with opposite-sex partners, same-sex partners, etc.), and there is discordance between these dimensions (eg, not all women with a history of same-sex partners identify as lesbian or bisexual).17 Further, each dimension can be operationally defined in various ways, including aggregating sexual minority women into a single analytical group (eg, combining lesbian and bisexual women) and disaggregating women into analytical subgroups due to potential heterogeneity between subgroups.17 Past studies have shown health outcomes vary by which dimension of sexual orientation is examined and by the way each dimension is operationally defined.17,18 Thus, to effectively intervene and communicate information about HPV infection among sexual minority women, it is important to examine multiple dimensions of sexual orientation and multiple operational definitions within each dimension.
The current study examined genital HPV infection among a population-based national sample of sexual minority women from the National Health and Nutrition Examination Survey (NHANES). In doing so, we examined the prevalence of HPV infection for two dimensions of sexual orientation (sexual identity and sexual behaviour) and multiple operational definitions within each dimension. Results will provide valuable data on HPV infection among sexual minority women, which will be highly useful in informing future HPV and cervical cancer prevention efforts.
METHODS
Study design
We conducted secondary analyses of public-use data from the NHANES, which is described extensively elsewhere.19 Briefly, the NHANES is conducted by the Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of adults and children in the USA. To obtain a nationally representative sample of the USA non-institutionalised civilian population, the NHANES uses a complex, stratified, multistage probability sample design with unequal probabilities of selection. The NHANES collects data from consented individuals through in-person questionnaires and physical examinations. Questionnaires are completed in participants’ homes, with audio computer-assisted self-interviewing (ACASI) software used to ensure privacy for sensitive questions (eg, sexual identity and behaviour). Following questionnaires, physical examinations are administered by trained personnel in mobile examination centres. Starting with the 2003–2004 survey cycle, the NHANES asked female participants aged 14–59 who underwent physical examinations to provide self-collected cervicovaginal swabs for HPV testing.
We analysed data from five NHANES survey cycles: 2003–2004, 2005–2006, 2007–2008, 2009–2010 and 2011–2012. These represent all cycles that collected cervicovaginal samples for HPV testing and had public-use datasets available at the time of our study. The NHANES is a series of cross-sectional surveys, meaning that each cycle is independent from other cycles. Data from multiple cycles can, however, be combined and analysed.19
We analysed data on a total of 7132 women from the 2003–2012 NHANES that were aged 20–59, provided cervicovaginal samples that were adequate for interpretation of HPV testing results and answered survey items regarding sexual orientation (sexual identity and/or sexual behaviour). We could not include female participants aged 14–19 who provided cervicovaginal samples, because sexual orientation data for these ages are not included in public-use datasets. Response rates for women aged 20–59 were over 70% for all NHANES survey cycles examined.19 Data collection for the NHANES received approval from the National Center for Health Statistics Research Ethics Review Board. The Institutional Review Board at the Ohio State University deemed our secondary analyses did not meet the definition of human subjects research.
Specimen collection and HPV testing
Women self-collected a cervicovaginal specimen using a swab (Catch-All Sample Collection Swab; Epicentre). HPV DNA testing was performed on the collected specimens using the Research Use Only Linear Array (LA) genotyping test (Roche Diagnostics). The test included probes to detect 37 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, XR(52), 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89 and IS39). Additional details about specimen collection and HPV testing have been provided previously.15
We examined three HPV infection outcomes (yes or no for each): (a) infection with any HPV type; (b) infection with a high-risk (ie, oncogenic) HPV type (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 64, 66, 67, 68, 69, 70, 73, 82 or IS39) and (c) infection with a vaccine HPV type (6, 11, 16, 18, 31, 33, 45, 52 or 58). We used the same high-risk HPV classification scheme as past analyses of NHANES data.15 The vaccine HPV types are those types protected against by at least one of the currently available HPV vaccines.20
Questionnaire data
Sexual identity
The NHANES assessed sexual identity with the item, “Do you think of yourself as…”. Responses included heterosexual or straight (hereafter, ‘heterosexual’); bisexual; homosexual or lesbian (hereafter, ‘lesbian’) or other. We used the resulting data to create two operational definitions of sexual identity for analyses. The first definition aggregated women with a non-heterosexual identity, resulting in two analytical categories: heterosexual or non-heterosexual (ie, bisexual, lesbian and other). In accordance with recent recommendations,17 the second definition disaggregated the ‘non-heterosexual’ group, resulting in four analytical categories: heterosexual; bisexual; lesbian or other.
Sexual behavior
The NHANES collected information on respondents’ lifetime reports of sex (vaginal, anal or oral) with males and females separately. We used these data to create two operational definitions of sexual behaviour for analyses. The first definition aggregated women who reported same-sex partners, resulting in three analytical categories: opposite-sex only; any same-sex or no sex. The second definition disaggregated the ‘any same-sex’ group, resulting in four analytical categories: opposite-sex only; both sexes; same-sex only or no sex.
Demographic and health-related characteristics
The NHANES assessed various demographic and health-related characteristics (table 1).
Table 1.
Characteristics of women from the 2003–2012 National Health and Nutrition Examination Survey (n=7132)
| n (weighted %) | |
|---|---|
| Survey cycle | |
| 2003–2004 | 1184 (18.9) |
| 2005–2006 | 1369 (20.5) |
| 2007–2008 | 1559 (20.9) |
| 2009–2010 | 1616 (19.5) |
| 2011–2012 | 1404 (20.2) |
| Sexual orientation | |
| Sexual identity | |
| Heterosexual | 6571 (93.6) |
| Bisexual | 243 (3.4) |
| Lesbian | 87 (1.2) |
| Other | 203 (1.8) |
| Sexual behaviour | |
| Opposite-sex only | 6139 (88.0) |
| Both sexes | 577 (8.4) |
| Same-sex only | 23 (0.3) |
| No sex | 293 (3.4) |
| Demographic characteristics | |
| Age (years) | |
| 20–29 | 1914 (23.9) |
| 30–39 | 1755 (23.6) |
| 40–49 | 1866 (27.6) |
| 50–59 | 1597 (24.9) |
| Race/ethnicity | |
| Non-Hispanic white | 3148 (67.9) |
| Non-Hispanic black | 1608 (12.7) |
| Hispanic | 1899 (13.4) |
| Other | 477 (5.9) |
| Relationship/marital status | |
| Not married | 2905 (36.3) |
| Married/living with a partner | 4224 (63.7) |
| Education | |
| Less than a high school degree | 1543 (14.4) |
| High school degree | 1517 (21.1) |
| More than a high school degree | 4068 (64.5) |
| Poverty level | |
| Below | 1556 (15.4) |
| At or above | 5158 (84.6) |
| Health-related characteristics | |
| Healthcare visit in the last year | |
| No | 907 (11.5) |
| Yes | 6224 (88.5) |
| Smoking status | |
| Never smoker | 4388 (58.5) |
| Former smoker | 1127 (18.4) |
| Current smoker | 1615 (23.1) |
| Age at first sexual intercourse | |
| 15 or younger | 1807 (24.0) |
| 16 or older | 5298 (76.0) |
| Total number of sexual partners during lifetime | |
| 4 or fewer | 3480 (46.8) |
| 5 or more | 3563 (53.2) |
Table reports raw frequencies and weighted percentages. Totals may not sum to stated sample size due to missing data. Per cents may not sum to 100% due to rounding.
Data analysis
We conducted parallel analyses for each dimension of sexual orientation (sexual identity and behaviour). Applying the aggregated and disaggregated operational definitions of each dimension, we first determined the prevalence of each HPV infection outcome. We then constructed logistic regression models for each outcome separately, producing ORs and 95% CIs. Bivariate models contained only measures of sexual identity or behaviour (either the aggregated or disaggregated operational definitions for a given dimension) and controlled for NHANES survey cycle (to account for potential temporal effects). Multivariate models controlled for NHANES survey cycle and demographic and health-related characteristics. Analyses applied procedures for analysing complex survey data in SAS V.9.3 (Cary, North Carolina, USA) and appropriate sampling weights in determining percentages and effect estimates. Frequencies were not weighted. Statistical tests were two-tailed, with a critical α of 0.05.
RESULTS
Participant characteristics
Most women (93.6%) were identified as heterosexual, with 3.4% identified as bisexual, 1.2% as lesbian and 1.8% as other (table 1). Sexual behaviour followed a similar pattern, as most women reported only opposite-sex partners (88.0%), with fewer reporting partners of both sexes (8.4%), only same-sex partners (0.3%) or no sex (3.4%). Most women were <50 years old (75.1%), non-Hispanic white (67.9%), married or living with a partner (63.7%), had at least a high school degree (85.6%) and were at or above poverty level (84.6%). Sexual minority women, whether defined by sexual identity or behaviour, differed from other women on almost all demographic and health-related characteristics examined (all p<0.05; see online supplementary appendix 1).
HPV infection
Among all women, the prevalence of any HPV type was 41.6%. About 28.5% of all women were infected with a high-risk HPV type, and about 14.1% were infected with a vaccine HPV type.
Sexual identity
Overall, about 49.7% of non-heterosexual women were infected with HPV, 37.0% were infected with a high-risk HPV type and 16.7% were infected with a vaccine HPV type (figure 1A). In bivariate analyses, non-heterosexual women had greater odds of infection with any HPV type (OR=1.44, 95% CI 1.16 to 1.78) and with a high-risk HPV type (OR=1.52, 95% CI 1.20 to 1.93), compared with heterosexual women (table 2). These differences were attenuated in multivariate analyses (all p>0.05).
Figure 1.
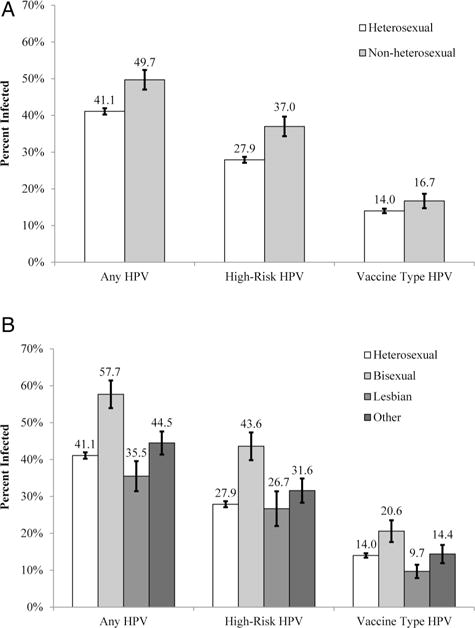
Human papillomavirus (HPV) infection among women by two operational definitions of sexual identity: (A) applies the aggregated definition; (B) applies the disaggregated definition. Bars indicate the SEs.
Table 2.
HPV infection among women by sexual identity and sexual behaviour, 2003–2012 NHANES
| Any HPV infection
|
High-risk HPV infection*
|
Vaccine type HPV infection†
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bivariate
|
Multivariate
|
Bivariate
|
Multivariate
|
Bivariate
|
Multivariate
|
|||||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sexual identity | ||||||||||||
| Aggregated | ||||||||||||
| Heterosexual | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Non-heterosexual | 1.44 (1.16 to 1.78) | 0.001 | 0.83 (0.64 to 1.08) | 0.159 | 1.52 (1.20 to 1.93) | <0.001 | 0.91 (0.70 to 1.18) | 0.455 | 1.26 (0.92 to 1.72) | 0.152 | 0.75 (0.53 to 1.06) | 0.099 |
| Disaggregated | ||||||||||||
| Heterosexual | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Bisexual | 2.00 (1.47 to 2.73) | <0.001 | 0.96 (0.68 to 1.36) | 0.834 | 2.01 (1.47 to 2.75) | <0.001 | 0.99 (0.70 to 1.39) | 0.941 | 1.63 (1.10 to 2.43) | 0.015 | 0.79 (0.51 to 1.24) | 0.309 |
| Lesbian | 0.79 (0.49 to 1.27) | 0.326 | 0.43 (0.25 to 0.72) | 0.002 | 0.95 (0.52 to 1.71) | 0.853 | 0.56 (0.30 to 1.05) | 0.069 | 0.67 (0.32 to 1.41) | 0.287 | 0.41 (0.20 to 0.84) | 0.014 |
| Other | 1.15 (0.83 to 1.61) | 0.401 | 1.00 (0.69 to 1.44) | 0.996 | 1.21 (0.83 to 1.75) | 0.329 | 1.06 (0.71 to 1.58) | 0.768 | 1.05 (0.61 to 1.79) | 0.866 | 0.98 (0.55 to 1.73) | 0.934 |
| Sexual behaviour | ||||||||||||
| Aggregated | ||||||||||||
| Opposite-sex only | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Any same-sex | 1.87 (1.49 to 2.35) | <0.001 | 1.01 (0.78 to 1.32) | 0.916 | 1.55 (1.22 to 1.96) | <0.001 | 0.88 (0.68 to 1.15) | 0.345 | 1.47 (1.11 to 1.95) | 0.007 | 0.87 (0.64 to 1.18) | 0.374 |
| No sex | 0.36 (0.25 to 0.52) | <0.001 | 0.37 (0.25 to 0.55) | <0.001 | 0.35 (0.23 to 0.52) | <0.001 | 0.33 (0.22 to 0.51) | <0.001 | 0.47 (0.28 to 0.81) | 0.006 | 0.47 (0.28 to 0.79) | 0.004 |
| Disaggregated | ||||||||||||
| Opposite-sex only | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Both sexes | 1.92 (1.53 to 2.41) | <0.001 | 1.06 (0.81 to 1.37) | 0.684 | 1.57 (1.24 to 1.98) | <0.001 | 0.90 (0.69 to 1.17) | 0.436 | 1.50 (1.14 to 1.99) | 0.005 | 0.90 (0.66 to 1.23) | 0.500 |
| Same-sex only | 0.75 (0.29 to 1.95) | 0.554 | 0.30 (0.11 to 0.80) | 0.016 | 1.07 (0.39 to 2.93) | 0.900 | 0.45 (0.15 to 1.31) | 0.144 | 0.56 (0.15 to 2.09) | 0.392 | 0.25 (0.07 to 0.96) | 0.044 |
| No sex | 0.36 (0.25 to 0.52) | <0.001 | 0.37 (0.25 to 0.54) | <0.001 | 0.35 (0.23 to 0.52) | <0.001 | 0.33 (0.22 to 0.51) | <0.001 | 0.47 (0.28 to 0.81) | 0.006 | 0.47 (0.28 to 0.78) | 0.004 |
Bivariate models adjusted for NHANES survey cycle. Multivariate models adjusted for NHANES survey cycle and all demographic and health-related characteristics (except poverty status due to a large amount of missing data for this variable). Multivariate models included n=7000 women for sexual identity and n=7017 women for sexual behaviour.
High-risk (ie, oncogenic) HPV types included types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 64, 66, 67, 68, 69, 70, 73, 82 and IS39.
Vaccine HPV types included types 6, 11, 16, 18, 31, 33, 45, 52 and 58.
HPV, human papillomavirus; NHANES, National Health and Nutrition Examination Survey; Ref., referent group.
When disaggregated, HPV infection outcomes varied greatly among non-heterosexual women (figure 1B). Outcomes were most common among bisexual women and lowest among lesbian women, and this was seen for infection with any HPV type (bisexual=57.7%, lesbian=35.5%), a high-risk HPV type (bisexual=43.6%, lesbian=26.7%) and a vaccine HPV type (bisexual=20.6%, lesbian=9.7%). In bivariate analyses, bisexual women had greater odds of all HPV infection outcomes compared with heterosexual women (all p<0.05), but these differences were no longer statistically significant in multivariate analyses (all p>0.05) (table 2). In multivariate analyses, lesbian women had lower odds of infection with any HPV type (OR=0.43, 95% CI 0.25 to 0.72) and with a vaccine HPV type (OR=0.41, 95% CI 0.20 to 0.84) compared with heterosexual women.
Sexual behavior
About 55.9% of women who reported any same-sex partners, when aggregated, were infected with HPV, 37.7% were infected with a high-risk HPV type and 19.1% were infected with a vaccine HPV type (figure 2A). In bivariate analyses, women who reported any same-sex partners had greater odds of infection with any HPV type (OR=1.87, 95% CI 1.49 to 2.35), a high-risk HPV type (OR=1.55, 95% CI 1.22 to 1.96) and a vaccine HPV type (OR=1.47, 95% CI 1.11 to 1.95) compared with women who reported only opposite-sex partners (table 2). Most of the differences were attenuated in multivariate analyses, though women who reported no sex continued to have lower odds of all HPV infection outcomes compared with women who reported only opposite-sex partners (all p<0.05).
Figure 2.
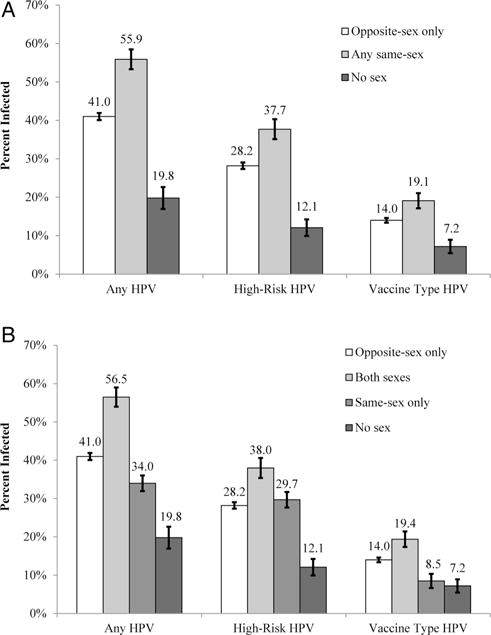
Human papillomavirus (HPV) infection among women by two operational definitions of sexual behaviour: (A) applies the aggregated definition; (B) applies the disaggregated definition. Bars indicate the SEs.
When disaggregated, HPV infection outcomes were highest among women who reported partners of both sexes (any HPV type=56.5%; high-risk HPV type=38.0%; vaccine HPV type=19.4%) (figure 2B). Prevalence estimates were lower among all other analytical groups, including women who reported only same-sex partners (any HPV type=34.0%; high-risk HPV type=29.7%; vaccine HPV type=8.5%). In bivariate analyses, women who reported a history of partners of both sexes had greater odds of all HPV infection outcomes compared with women who reported only opposite-sex partners (all p<0.05) (table 2). These differences were again no longer statistically significant in multivariate analyses (all p>0.05). In multivariate analyses, women who reported only same-sex partners had lower odds of infection with any HPV type (OR=0.30, 95% CI 0.11 to 0.80) and with a vaccine HPV type (OR=0.25, 95% CI 0.07 to 0.96) compared with women who reported only opposite-sex partners. Women who reported no sex also had lower odds of all HPV infection outcomes (all p<0.05).
DISCUSSION
Sexual minority women are at risk for HPV infection; yet, past studies examining the prevalence of infection among this population have often been limited by convenience samples and modest sample sizes.10,14–16 Among a population-based national sample, we found that, whether defined by sexual identity or behaviour, about half of sexual minority women had some type of HPV infection, over one-third were infected with a high-risk HPV type and nearly 20% were infected with a vaccine HPV type. Previous studies reported estimates ranging from 13% up to 51% for infection with any HPV type among sexual minority women.10,14–16 Our results provide the most comprehensive data to date on HPV infection among sexual minority women and suggest the prevalence is likely towards the higher end of this range.
The current study is the first, to our knowledge, to examine HPV infection across multiple dimensions of sexual orientation and apply multiple operational definitions within each dimension. Although the overall pattern of results between sexual identity and behaviour was qualitatively similar, prevalence estimates between dimensions differed quantitatively by up to 6.2%. For example, when examining sexual identity, we found that 49.7% of non-heterosexual women were infected with any HPV type, but when examining sexual behaviour, 55.9% of women who reported any same-sex partners were infected with any HPV type. Importantly, much larger within-dimension variations existed when examining disaggregated subgroups of sexual minority women (eg, HPV infection outcomes were much more common among bisexual women compared with lesbian women). These results provide further support that treating sexual minority women as a homogenous group obscures important differences that exist among the disaggregated subgroups.17 Thus, to fully capture the complex relationship between sexual orientation and health outcomes, it is necessary to assess multiple dimensions of sexual orientation and apply different operational definitions within each dimension.
We believe our results have additional implications because they can be used to help inform future HPV and cervical cancer prevention efforts targeting sexual minority women. Research suggests that fewer than half of young sexual minority women have received any doses of HPV vaccine,21 and sexual minority women, particularly those who identify as lesbian or report only same-sex partners, are less likely to have had a recent Pap test compared with heterosexual women.22,23 There are several reasons why sexual minority women are not receiving these health services, including lower access to and use of healthcare, lack of health insurance, past experiences of discrimination in receiving healthcare, concerns about disclosure of sexual orientation and knowledge deficits about HPV and cervical cancer (including misperceptions that they are at low risk for these health outcomes).9,12,13 Future efforts promoting cervical cancer screening and HPV vaccine are therefore needed for this population, particularly since sexual minority women are often not mentioned in sexual health education programmes and materials, sexual health policies or cervical cancer screening guidelines.9,12,13 Our results offer novel insight on the prevalence of HPV infection among sexual minority women, which can be further tailored to specific audiences by sexual identity or sexual behaviour subgroup. Such information is needed to effectively communicate with sexual minority women about their risk for HPV infection, which in turn may help motivate cervical cancer screening and HPV vaccination behaviours. It will also be important for future efforts to educate sexual minority women about modes of HPV transmission. Specifically, women should know that HPV can be transmitted between female partners10 and through various forms of contact (eg, genital–genital, anal–genital, oral–genital and digital–genital) and the sharing of sex toys.24,25
Our results can also be used to help educate healthcare providers about HPV infection among sexual minority women and the importance of Pap testing and HPV vaccine for this population. Healthcare providers and their recommendations play a key role in whether or not women receive these health services;21,26 yet, many providers may not receive adequate training and support in understanding the health needs of sexual minority women. Indeed, medical schools report a median of only 5 h of content related to sexual minority health in their curriculum.27 It is critical that healthcare providers and medical students are better educated about the health needs of this population and trained in effective, culturally sensitive communication.12 Related healthcare settings should be a safe environment where patients are comfortable discussing sensitive topics, such as sexual identity and sexual behaviour. This is important since disclosure of sexual orientation is correlated with receipt of preventive services, including Pap testing, among sexual minority women.26,28
Study strengths include analysing data from a population-based national sample of sexual minority women and infection data based on HPV DNA testing. We were able to examine two dimensions of sexual orientation and apply multiple operational definitions within each dimension. The distribution of sexual identity in our study mirrors patterns from other large studies,5 giving us further confidence in sample validity. Our study also has several limitations. Although HPV DNA testing is a strong indicator of current HPV infection, it does not measure lifetime exposure since some HPV infections clear from the body.15 Data on sexual identity and behaviour were self-reported by participants and may be subject to social desirability bias, but the ACASI software used can increase response accuracy.29 Our measure of sexual behaviour was based on participants’ lifetime sexual behaviour, and few women reported only same-sex partners. We were not able to account for HPV vaccination status because this was not assessed for all survey cycles. However, HPV vaccine was not available until 2006 in the USA, and uptake was very low among adult women during the years included in analyses.30
Our study suggests HPV infection is common among sexual minority women, and many are infected with a high-risk HPV type or a type that could be protected against by HPV vaccine. Prevalence estimates varied slightly between sexual orientation dimensions and greatly depending on how a dimension was operationally defined, underscoring the importance of examining sexual minority status using various approaches. Our results can inform future HPV and cervical cancer prevention efforts that target sexual minority women and healthcare providers.
Supplementary Material
Key messages.
-
▸
Genital human papillomavirus (HPV) infection is common among sexual minority women.
-
▸
HPV prevalence estimates vary slightly between sexual orientation dimensions and greatly depending on how a dimension is operationally defined.
-
▸
Results can inform future HPV and cervical cancer prevention efforts that target sexual minority women.
Acknowledgments
Funding Supported by the National Cancer Institute of the National Institutes of Health under Award Number R03CA198115.
Competing interests PLR has received research grants from Merck Sharp & Dohme and Cervical Cancer-Free America, via an unrestricted educational grant from GlaxoSmithKline. These funds were not used to support this research study.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/sextrans-2016-052536).
This work was presented at the 2016 annual meetings for the American Society of Preventive Oncology and Society of Behavioural Medicine.
Handling editor: Jackie A Cassell
Contributors: PLR conceived the study, performed data analysis and wrote the initial manuscript draft. A-LM conceived of the study and provided direction for data analysis and manuscript writing. Both authors helped to conceptualise ideas, interpret findings and review drafts of the manuscript.
Ethics approval: The Ohio State University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data analysed for this study were public-use data from the National Health and Nutrition Examination Survey (NHANES).
References
- 1.Centers for Disease Control and Prevention (CDC) Genital HPV infection—fact sheet. 2012 http://www.cdc.gov/std/HPV/STDFact-HPV.htm.
- 2.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(Suppl 10):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(Suppl 3):S3/35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 5.Gates GJ. How many people are lesbian, gay, bisexual, and transgender? 2011 http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf.
- 6.Eaton L, Kalichman S, Cain D, et al. Perceived prevalence and risks for human papillomavirus (HPV) infection among women who have sex with women. J Womens Health (Larchmt) 2008;17:75–83. doi: 10.1089/jwh.2006.0256. [DOI] [PubMed] [Google Scholar]
- 7.McNair R, Power J, Carr S. Comparing knowledge and perceived risk related to the human papilloma virus among Australian women of diverse sexual orientations. Aust N Z J Public Health. 2009;33:87–93. doi: 10.1111/j.1753-6405.2009.00345.x. [DOI] [PubMed] [Google Scholar]
- 8.Pelullo CP, Di Giuseppe G, Angelillo IF. Human papillomavirus infection: knowledge, attitudes, and behaviors among lesbian, gay men, and bisexual in Italy. PLoS ONE. 2012;7:e42856. doi: 10.1371/journal.pone.0042856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power J, McNair R, Carr S. Absent sexual scripts: lesbian and bisexual women’s knowledge, attitudes and action regarding safer sex and sexual health information. Cult Health Sex. 2009;11:67–81. doi: 10.1080/13691050802541674. [DOI] [PubMed] [Google Scholar]
- 10.Marrazzo JM, Koutsky LA, Stine KL, et al. Genital human papillomavirus infection in women who have sex with women. J Infect Dis. 1998;178:1604–9. doi: 10.1086/314494. [DOI] [PubMed] [Google Scholar]
- 11.Marrazzo JM, Gorgos LM. Emerging sexual health issues among women who have sex with women. Curr Infect Dis Rep. 2012;14:204–11. doi: 10.1007/s11908-012-0244-x. [DOI] [PubMed] [Google Scholar]
- 12.Peitzmeier SM. Promoting cervical cancer screening among lesbians and bisexual women. 2013 http://fenwayhealth.org/documents/the-fenway-institute/policy-briefs/PolicyFocus_cervicalcancer_web.pdf.
- 13.Fish J. Cervical screening in lesbian and bisexual women: a review of the worldwide literature using systematic methods. 2009 http://www.glhv.org.au/files/screening-lesbians-bisexual-women.pdf.
- 14.Marrazzo JM, Koutsky LA, Kiviat NB, et al. Papanicolaou test screening and prevalence of genital human papillomavirus among women who have sex with women. Am J Public Health. 2001;91:947–52. doi: 10.2105/ajph.91.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204:566–73. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 16.Massad LS, Xie X, Minkoff H, et al. Abnormal pap tests and human papillomavirus infections among HIV-infected and uninfected women who have sex with women. J Low Genit Tract Dis. 2014;18:50–6. doi: 10.1097/LGT.0b013e3182942733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DD, Blosnich JR, Farmer GW, et al. Operational definitions of sexual orientation and estimates of adolescent health risk behaviors. LGBT Health. 2014;1:42–9. doi: 10.1089/lgbt.2013.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostwick WB, Boyd CJ, Hughes TL, et al. Dimensions of sexual orientation and the prevalence of mood and anxiety disorders in the United States. Am J Public Health. 2010;100:468–75. doi: 10.2105/AJPH.2008.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2015. http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 20.Petrosky E, Bocchini JA, Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 21.McRee AL, Katz ML, Paskett ED, et al. HPV vaccination among lesbian and bisexual women: findings from a national survey of young adults. Vaccine. 2014;32:4736–42. doi: 10.1016/j.vaccine.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agenor M, Kriéger N, Austin SB, et al. Sexual orientation disparities in Papanicolaou test use among US women: the role of sexual and reproductive health services. Am J Public Health. 2014;104:e68–73. doi: 10.2105/AJPH.2013.301548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews AK, Brandenburg DL, Johnson TP, et al. Correlates of underutilization of gynecological cancer screening among lesbian and heterosexual women. Prev Med. 2004;38:105–13. doi: 10.1016/j.ypmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano AR, Nyitray AG, Kreimer AR, et al. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer. 2015;136:2752–60. doi: 10.1002/ijc.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson TA, Schick V, Herbenick D, et al. A study of human papillomavirus on vaginally inserted sex toys, before and after cleaning, among women who have sex with women and men. Sex Transm Infect. 2014;90:529–31. doi: 10.1136/sextrans-2014-051558. [DOI] [PubMed] [Google Scholar]
- 26.Tracy JK, Schluterman NH, Greenberg DR. Understanding cervical cancer screening among lesbians: a national survey. BMC Public Health. 2013;13:442. doi: 10.1186/1471-2458-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obedin-Maliver J, Goldsmith ES, Stewart L, et al. Lesbian, gay, bisexual, and transgender-related content in undergraduate medical education. JAMA. 2011;306:971–7. doi: 10.1001/jama.2011.1255. [DOI] [PubMed] [Google Scholar]
- 28.Reiter PL, McRee AL. Cervical cancer screening (Pap testing) behaviours and acceptability of human papillomavirus self-testing among lesbian and bisexual women aged 21–26 years in the USA. J Fam Plann Reprod Health Care. 2015;41:259–64. doi: 10.1136/jfprhc-2014-101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: II. Accuracy of self-reports. Ann Behav Med. 2003;26:104–23. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor LD, Hariri S, Sternberg M, et al. Human papillomavirus vaccine coverage in the United States, National Health and Nutrition Examination Survey, 2007–2008. Prev Med. 2011;52:398–400. doi: 10.1016/j.ypmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


