SECTION 1
An 8-year-old girl with mild dysmorphic features presented for evaluation of developmental delay and staring spells. She had been born late preterm and spent 1 month in the neonatal intensive care unit. She was generally healthy other than her developmental delay, which improved somewhat with physical, occupational, and speech therapy. At the first clinic visit, her mother reported loss of previously mastered vocabulary and struggles with fine motor skills such as buttoning. She also noted repetitive movements, obsessive behaviors, and hand flapping.
Question for consideration:
What is the initial evaluation for a child with developmental delay?
SECTION 2
A total of 1%–3% of children younger than 5 are diagnosed with global developmental delay. The differential diagnosis is broad and includes genetic, structural, and metabolic causes.1 Initial evaluation should include neuroimaging, with MRI being preferable to CT. MRI brain will identify an abnormality such as a malformation, atrophy, white matter disease, demyelination, or injury in 48%–92% of children with developmental delay.1 Evaluation should also include genetic testing such as karyotype, array comparative genomic hybridization (aCGH), gene-specific testing, and whole exome sequencing (WES).2 aCGH is now often considered the first-line genetic test because it will identify an etiology in up to 20% of individuals with developmental delay through detection of genomic deletions and duplications. In contrast, the diagnostic yield for karyotype is less than 10%. Therefore, while karyotype will identify chromosomal abnormalities such as Down syndrome, Turner syndrome, or large genomic deletions, it is now frequently omitted in the evaluation of the developmentally delayed child. Single gene, gene panel, or WES is subsequently ordered if clinically indicated. WES is ordered to evaluate for de novo mutations in previously identified developmental genes when there are no clinical features pointing towards a specific syndrome. Notably, some complex genomic abnormalities such as ring chromosomes are not detected by aCGH, gene testing, or WES. Therefore, children with terminal chromosome deletions should be evaluated for a ring chromosome by karyotype.
EEG is not indicated for routine evaluation of developmental delay unless the child exhibits regression or seizures.2
Routine screening for metabolic causes of global developmental delay has a yield of only 1%, although it increases to 5% in specific populations with other key clinical features. Therefore, routine screening is not recommended.1
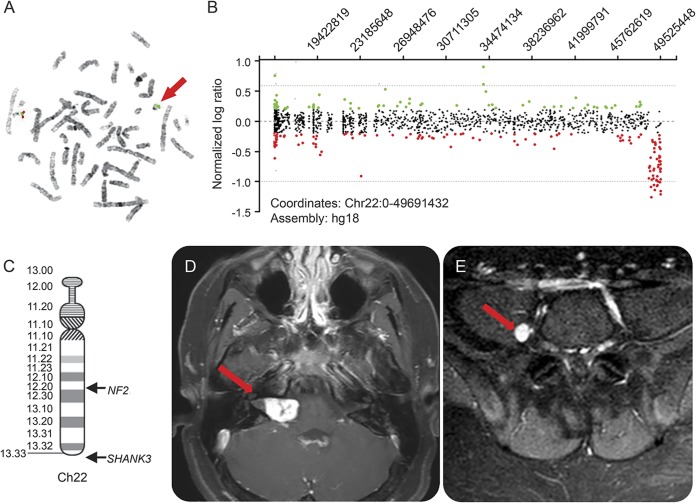
Our patient's initial evaluation included an MRI brain that was unremarkable and a karyotype that revealed a ring chromosome 22 including a terminal deletion of the long arm. A ring chromosome is when the p and q arms of the chromosome fuse together and often occurs due to deletion of one arm. Subsequent high-resolution karyotype with subtelomeric fluorescent in situ hybridization analysis confirmed the diagnosis (figure, A) and demonstrated a double-sized ring chromosome 22 in 15% of cells. In order to better define the size of the deletion, a high-resolution aCGH was performed, which identified a 1.036-Mb deletion in the subtelomeric region of the long arm of chromosome 22 involving 20 genes including SHANK3, consistent with the diagnosis of Phelan-McDermid syndrome (PMS) (figure, B and C). To complete the evaluation, the patient was assessed with an Autism Diagnostic Observation Schedule and met criteria for autism.
Figure. Genetic and neuroimaging evaluation.
(A) Fluorescent in situ hybridization analysis depicts a normal chromosome 22 with Cosmid probe n85a3 of the SHANK3 gene in red and the control probe in green, and a ring chromosome with only green signal indicating the deletion of the SHANK3 gene at band 22q13.3 (red arrow). (B) High-resolution array comparative genomic hybridization shows all the oligo probes for chromosome 22 with a copy number loss of chromosome band 22q13.3 of 1.036 Mb (depicted as red circles). (C) Chromosome 22 ideogram shows the relative locations of SHANK3 and NF2. (D) Axial T1 postcontrast MRI brain. Red arrow points to bulky lobular, enhancing mass extending from the right cerebellopontine angle to the right internal auditory canal, consistent with a schwannoma. (E) Axial T1 postcontrast MRI spine with right lumbar paraspinal enhancing focus just medial to the right psoas muscle concerning for a small schwannoma.
After receiving the diagnosis, the patient was lost to follow-up for several years. At age 16, the family noted a change in the patient's personality. She previously related well with other children and was described as sweet and kind. Her parents expressed concern for new episodes of unprovoked anger. Her family also noted the development of painless growths on both arms, most predominant on the left arm and wrist.
Questions for consideration:
What is the evaluation for behavioral regression in a child with developmental delay?
How do the growths on the patient’s arm relate to her other signs and symptoms?
SECTION 3
In a child with developmental delay, frustration related to communication difficulties from impaired hearing or speech delay can manifest as aggression. New-onset seizure activity can also manifest as behavior changes such as postictal agitation or irritability related to poor sleep following nocturnal seizures. Therefore, MRI brain, EEG, and audiology testing were ordered for this patient.
Brain MRI revealed a large, enhancing right cerebellopontine angle to internal auditory canal vestibular schwannoma (figure, D) and a punctate contralateral vestibular schwannoma that were not present on the prior brain MRI brain at 8 years of age. Based on these findings, MRI spine was also ordered and identified enhancing foci in multiple paraspinal areas, which were concerning for schwannomas (figure, E). EEG revealed a focus of sharp transients over the left temporal region suggestive of an epileptogenic focus. The patient subsequently had an event concerning for seizure in which she was staring and nonresponsive and then fell to the ground. Audiology testing was not able to establish hearing sensitivity due to the patient's inability to complete the task. However, crossed acoustic reflexes were absent, suggesting impairment of the auditory nerve.
Given the presence of bilateral vestibular as well as spinal schwannomas, the growths on her arms were likely peripheral schwannomas. The patient was referred to oncology for further evaluation. She was determined to meet criteria for neurofibromatosis type 2 (NF2). Comprehensive auditory evaluation was difficult to obtain secondary to the patient's intellectual disability. She was evaluated by neurosurgery, who did not recommend surgical intervention as hearing was intact to voice. She was treated with bevacizumab with significant improvement in her mood and monitored with serial brain imaging every 3 months and serial spine imaging every 6 months. An ophthalmologic consultation was obtained to evaluate for ocular findings of NF2, such as cataracts, orbital meningiomas, and retinal hamartomas, which were absent.
DISCUSSION
PMS, or 22q13.3 deletion syndrome, is a rare neurologic syndrome characterized by global developmental delay leading to moderate to severe intellectual disability with severe language impairment.3 Additional features include poor eye contact, anxiety, and reduced social interactions consistent with autism spectrum disorder.3 Dysmorphic features on physical examination can include large fleshy hand, bulbous nose, long eyelashes, hypoplastic nails, dysplastic ears, and dolichocephaly as well as neonatal hypotonia.4 Baseline renal ultrasound is recommended as more than one-quarter of children will have renal abnormalities ranging from reflux to absent kidneys. Individuals with PMS may also have developmental structural brain abnormalities, including delayed myelination, thin corpus callosum, and arachnoid cysts. Finally, children with PMS can have sleep abnormalities, including bedtime resistance, delayed onset, sleep anxiety, parasomnias, sleep-disordered breathing, and daytime sleepiness.3,4
Patients with PMS can have variable phenotype possibly related to size of their deletion on the long arm of chromosome 22.5 The majority of neurologic features, however, are believed to be due to haploinsufficiency of the SHANK3 gene.6 The SHANK3 protein is enriched in the postsynaptic density of excitatory synapses. Along with other members of the SHANK protein family, SHANK3 is believed to play an important role in synaptogenesis, synapse maintenance, and synapse plasticity.6
NF2 is diagnosed using the Manchester criteria7:
Bilateral vestibular schwannomas or
Unilateral vestibular schwannoma and at least 2 other features including meningioma, glioma, schwannoma, or juvenile posterior lenticular opacities
The hallmark feature is bilateral vestibular schwannomas. However, additional criteria allow for the diagnosis in a patient with at least 2 meningiomas and unilateral vestibular schwannoma or at least 2 meningiomas and 2 of the other features including glioma, neurofibroma, schwannoma, and cataract.7 Although schwannomas are benign, they can cause symptoms ranging from hearing loss to brainstem compression and are therefore usually monitored with serial imaging.8 These symptoms are caused by disruption of the NF2 gene on chromosome 22 (figure, C), which codes for merlin protein.
Ring chromosomes were first described by Lilian Morgan in 1926 and have been reported for all chromosomes. Terminal deletions can result in a ring chromosome when the truncated arm fuses with another end and forms a ring. Ring chromosomes are mitotically unstable, leading to somatic loss and resultant mosaicism. When the ring is lost during mitotic cell division, cells become monosomic for the affected chromosome. There are multiple reports of patients with ring chromosome 22, all of whom shared features of PMS, including speech delay, seizures, and autism.9 Rearrangements of chromosome 22, including ring 22, have also been reported to cause NF2.10 In the case of ring chromosome 22 due to a large, terminal deletion like in our patient, cells become monosomic for chromosome 22, and then have only 1 copy of NF2. In patients with NF2 secondary to ring chromosome 22, it is possible that aspects of their phenotype are related to mosaicism of SHANK3 haploinsufficiency. We therefore recommend that children diagnosed with PMS through aCGH should be evaluated for ring chromosome by karyotype. If ring 22 is present, they should be evaluated as recommended for children of affected parents, who have a 50% risk of developing NF2. This includes baseline and then annual ocular, skin, and neurologic examinations for children 2–10 years of age. After age 10, children should also undergo annual audiology screening and MRI brain every 2 years.8
Patients with global developmental delay should undergo evaluation with neuroimaging and genetic testing selected based on phenotype specific features. PMS is a rare cause of developmental delay, but given the association with NF2, it is important to include karyotype if terminal deletion of the long arm of chromosome 22 is identified. Ring chromosome can occur with any chromosomal terminal deletion and will put the patient at risk for mosaic monosomy. This case also highlights the growing recognition of dual genetic diagnoses in a subset of patients with complex phenotypic presentations.
AUTHOR CONTRIBUTIONS
Dr. Lyons-Warren saw the patient at a clinic visit, completed a history and physical examination, and discussed the patient with Dr. Holder. Dr. Lyons-Warren wrote the first draft of the manuscript including the initial literature review search. Dr. Cheung performed and analyzed the chromosome 22q13 FISH and aCGH and reviewed and edited the manuscript for content. Dr. Holder is the primary physician who manages the patient presented in this manuscript, ordered the test that identified the ring 22 mutation, and formulated the idea for the manuscript. Dr. Holder provided critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Dr. Holder is supported by a K08 award from the National Institute of Neurological Disorders and Stroke (NS091381) as well as the Robbins Foundation.
DISCLOSURE
A. Lyons-Warren and S. Cheung report no disclosures relevant to the manuscript. J. Holder is supported by a K08 award from the National Institute of Neurological Disorders and Stroke (NS091381) as well as the Robbins Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Shevell M, Ashwal S, Donley D, et al. Practice parameter: evaluation of the child with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2003;60:367–380. [DOI] [PubMed] [Google Scholar]
- 2.Silove N, Collins F, Ellaway C. Update on the investigation of children with delayed development. J Paediatr Child Health 2013;49:519–525. [DOI] [PubMed] [Google Scholar]
- 3.Phelan K, McDermid HE. The 22q13.3 deletion syndrome (Phelan-McDermid syndrome). Mol Syndromol 2012;2:186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolevzon A, Angarita B, Bush L, et al. Phelan-McDermid syndrome: a review of the literature and practice parameters for medical assessment and monitoring. J Neurodev Disord 2014;6:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwanenburg RJ, Ruiter SA, van den Heuvel ER, Flapper BC, Van Ravenswaaij-Arts CM. Developmental phenotype in Phelan-McDermid (22q13.3 deletion) syndrome: a systematic and prospective study in 34 children. J Neurodev Disord 2016;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonaglia MC, Giorda R, Beri S, et al. Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet 2011;7:e1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kresak JL, Walsh M. Neurofibromatosis: a review of NF1, NF2, and schwannomatosis. J Pediatr Genet 2016;5:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardern-Holmes S, Fisher G, North K. Neurofibromatosis type 2. J Child Neurol 2017;32:9–22. [DOI] [PubMed] [Google Scholar]
- 9.Guilherme RS, Soares KC, Simioni M, et al. Clinical, cytogenetic, and molecular characterization of six patients with ring chromosomes 22, including one with concomitant 22q11.2 deletion. Am J Med Genet A 2014;164A:1659–1665. [DOI] [PubMed] [Google Scholar]
- 10.Tsilchorozidou T, Menko FH, Lalloo F, et al. Constitutional rearrangements of chromosome 22 as a cause of neurofibromatosis 2. J Med Genet 2004;41:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]