Abstract
Although small molecule inhibitors of B-cell receptor-associated kinases have revolutionized therapy in chronic lymphocytic leukemia (CLL), responses are incomplete. Pro-survival signaling emanating from the microenvironment may foster therapeutic resistance of the malignant B cells resident in the protective lymphoid niches. B-cell activating factor (BAFF) is critical to the survival of both healthy and neoplastic B cells. However, the pro-survival pathways triggered by BAFF have not been fully characterized. Here we show that BAFF elicited resistance to spontaneous and drug-induced apoptosis in stromal co-cultures, induced activation of both canonical and non-canonical NFκB signaling pathways, and triggered B-cell receptor signaling in CLL cells, independently of IGHV mutational status. SYK, a proximal kinase in the B-cell receptor signaling cascade, acted via STAT3 to bolster transcription of the anti-apoptotic protein Mcl-1, thereby contributing to apoptosis resistance in BAFF-stimulated cells. SYK inhibitor entospletinib downregulated Mcl-1, abrogating BAFF-mediated cell survival. BAFF-B-cell receptor crosstalk in neoplastic B cells was mediated by SYK interaction with TRAF2/TRAF3 complex. Thus, SYK inhibition is a promising therapeutic strategy uniquely poised to antagonize crosstalk between BAFF and B-cell receptor, thereby disrupting the pro-survival microenvironment signaling in chronic lymphocytic leukemia.
Introduction
Soluble mediators derived from mesenchymal stromal cells, nurse-like cells, dendritic cells and T cells present in the protective niches (lymph nodes and bone marrow) prolong survival of neoplastic B cells in chronic lymphocytic leukemia (CLL).1–3 Lymph node-resident CLL cells exhibit gene signatures indicating activation of the B-cell receptor (BCR) and nuclear factor-κB (NFκB) pathways.4 Novel inhibitors of the BCR-associated kinases (BCRi) have made a significant clinical impact in CLL in part via induction of B-cell egress from niches wherein stromal support is lost. Ibrutinib and idelalisib, small molecule inhibitors of Bruton’s tyrosine kinase (BTK) and phosphoinositide 3-kinase-δ (PI3K-δ), respectively, have improved outcomes in CLL.5 However, patients who progress on, or who are intolerant of BCRi therapy have poor outcomes.6,7 Improved understanding of microenvironment signaling will foster development of novel effective therapeutic approaches in CLL.
Tumor necrosis factor receptor (TNFR) superfamily ligands, CD40L and BAFF/APRIL (B-cell activating factor/A proliferation-inducing ligand), are ubiquitously secreted in the stromal niches and promote fitness of the neoplastic clone.2 BAFF/APRIL ligands and their receptors are indispensable in B-cell survival.8–11 BAFF/APRIL share homology and are able to bind two TNFR - BCMA (B-cell maturation antigen) and TACI (transmembrane activator of the calcium modulator and cyclophilin ligand-interactor), whereas BAFF alone can bind BAFF receptor (BAFF-R, BR3).12 Like other TNFR ligands, BAFF/APRIL activate NFκB signaling, a major common pathway which mediates anti-apoptotic responses in CLL cells through induction of Bcl-2 family proteins and chemokine networks.12–16 Both signal through BCMA/TACI to activate the canonical NFκB in CLL, where the IκB kinase complex phosphorylates IκB, triggering its ubiquitination and leading to nuclear translocation of the NFκB dimers, predominantly p50/RelA and p50/c-Rel.8,13 Meanwhile, BAFF-R/BR3 signals through an intermediary complex, which involves adaptor proteins TRAF2/TRAF3, NFκB-inducing kinase (NIK), and inhibitor of apoptosis (IAP) family proteins cIAP1/2.12 While the exact mechanism remains elusive, it is believed that, in unstimulated B cells, NIK is constitutively bound to TRAF3 and degraded. When BAFF engages BR3, the NIK/TRAF/cIAP complex is recruited to the receptor, followed by TRAF3 repression, thus allowing NIK to persist and activate IκB kinase-1 (IKK1). IKK1 catalyzes proteasome-assisted processing of NFκB2 (p100) precursor, thereby inducing the non-canonical (alternative) NFκB pathway.12
Despite significant progress in understanding the role of BAFF/APRIL signaling in healthy and neoplastic B cells, the role of BAFF-mediated NFκB activation in CLL has not been thoroughly studied. Furthermore, the mechanistic implications of targeting BCR signaling using novel BCRi have not been elucidated in this context. Here we explored the mechanistic underpinnings of CLL cell survival in response to BAFF signaling, uncovering the functional significance of the BCR-associated kinases and the pro-survival Bcl-2 family proteins in this setting.
Methods
Patients’ samples and cell culture
Peripheral blood and bone marrow (where applicable) were obtained from patients with CLL at the Center for Hematologic Malignancies at the Oregon Health and Science University (Portland, OR, USA) after informed consent following approval by the Institutional Review Board (IRB#4422). Mononuclear cells were isolated using standard Ficoll-Hypaque techniques (Amersham, Piscataway, NJ, USA), rendering more than 90% CD5+/CD19+ cells, as determined by flow cytometry (FACSCanto). CLL cells were cultured in RPMI-1640 supplemented with 15% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 25 mM HEPES, 100 μM nonessential amino acids and 1 mM sodium pyruvate (Life Technologies, Grand Island, NY, USA). For stimulation with soluble factors, CLL cells were seeded at 1×106/mL in the presence of 5 μg/μL soluble goat F(ab’)2 anti-human IgM antibody (sol-IgM; Southern Biotech, Birmingham, AL, USA) or 25 ng/mL soluble human BAFF (sol-BAFF; Cell Signaling Technology, Danvers, MA, USA). CLL samples were analyzed for IGHV mutations using the IGH Somatic Hypermutation Assay v.2.0 (Invivoscribe, San Diego, CA, USA), as previously described.16
BAFF-expressing Chinese hamster ovary cells (BAFF-CHO) were obtained from Dr. Robert Woodland (University of Massachusetts, Worcester, MA, USA).17 Those cells were maintained in MEM-α supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 μM nonessential amino acids. CHO-K1 cells not expressing BAFF were used as control [American Type Culture Collection (ATCC), Manassas, VA, USA].
Chronic lymphocytic leukemia cells were cultured on BAFF-expressing (or control) cells under the stromal conditions previously described.16 Briefly, stromal cells were seeded to achieve 80–100% confluence; on the following day, CLL cells were plated at a 50:1 ratio and incubated at 37°C in 5% CO2. Cultures were then treated with drugs as indicated. At harvest, CLL cells were gently washed off the stromal layer. When harvested for protein and mRNA analysis, CLL cells were transferred to a new plate and incubated for an additional 60 minutes (min) to allow re-attachment of stromal cells, thus minimizing contamination of CLL cells.
Chronic lymphocytic leukemia cell apoptosis was quantified using the ApoScreen Annexin-V Apoptosis Staining Kit (Southern Biotech) in the CD19+ population, as previously described.16 Expression of BAFF ligand and receptors in paired peripheral blood-bone marrow samples and in BAFF-expressing stromal cells was quantified by flow cytometry using the following antibodies: CD257(BAFF)-PE (Clone 1D6, eBioScience, San Diego, CA, USA), CD256(APRIL)-PE, CD267(TACI)-PE, CD269(BCMA)-PE, CD268(BR3)-VioBlue (Miltenyi Biotech, San Diego, CA, USA).
Chemotaxis assays across polycarbonate Transwell inserts were performed as described.18 Briefly, CLL cells (107/mL) were incubated at 37°C in 5% CO2 with 5 μg/mL of anti-IgM or 25 ng/mL BAFF with or without drugs. After 1 hour (h), CLL cells were washed and 100 μL of cell suspension (106 cells) was added to the top chamber of a Transwell culture insert (Corning) with a diameter of 6.5 mm and a pore size of 5 μm. Filters were then transferred to wells containing serum-free medium with or without 200 ng/mL CXCL12 (Cell Signaling). After a 3-h incubation, the cells in the lower chamber were aliquoted for counting by flow cytometry for 20 seconds in duplicates. A 1:20 dilution of input cells was counted under the same conditions.
Statistical analysis
Paired or unpaired Student t-tests were performed in GraphPad Prism software (La Jolla, CA, USA). P<0.05 was considered statistically significant. Microarray data were analyzed for functional significance using Pathway Studio software (Ariadne Genomics/Elsevier, Rockville, MD, USA). Data are presented as mean±Standard Error (SE) throughout the manuscript.
Results
BAFF predominantly activates non-canonical NFκB and up-regulates the pro-survival Bcl-2 proteins in CLL cells
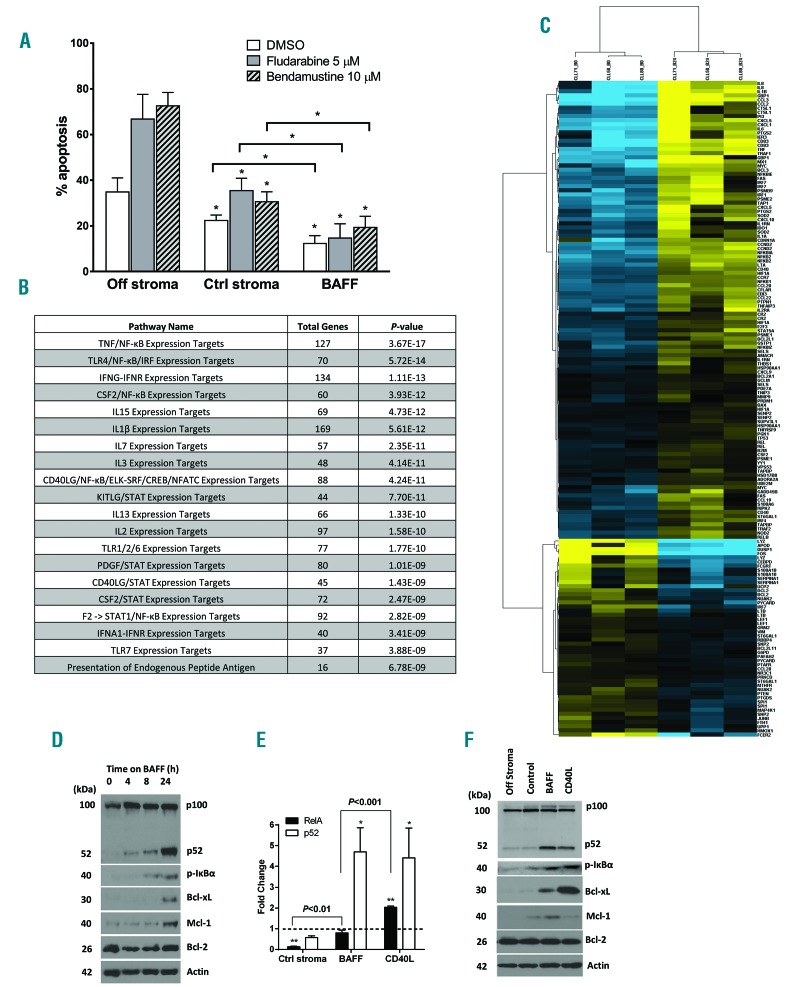
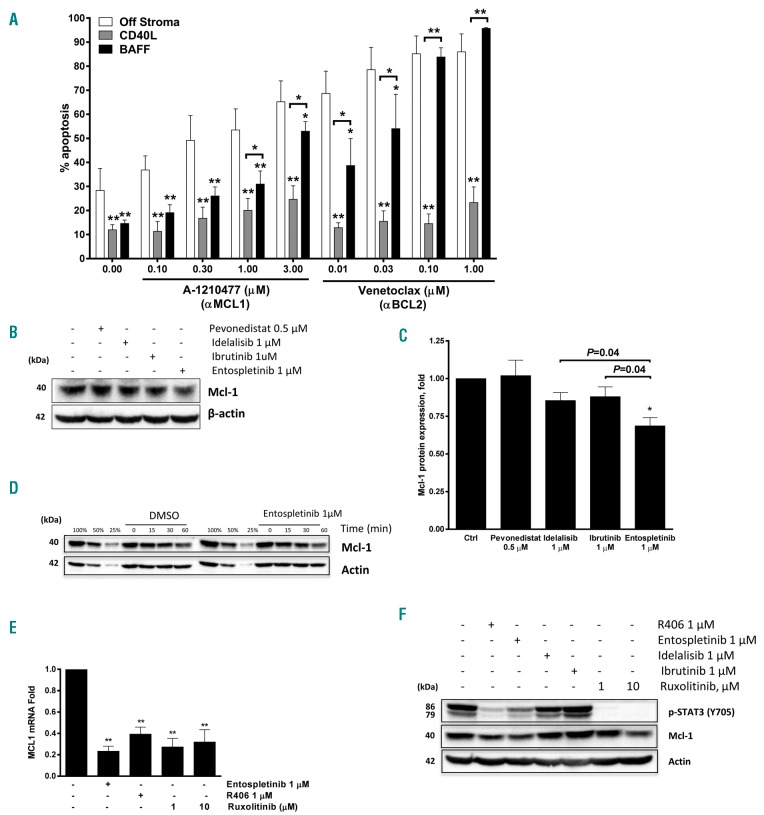
First, we analyzed expression of BAFF ligands and receptors in CLL cells sourced from peripheral blood and the bone marrow. BAFF receptors BR3, BCMA and TACI, and their ligands BAFF and APRIL were expressed in both compartments (Online Supplementary Figure S1). In our previous work, we had partially reconstituted the lymph node microenvironment by employing co-cultures of the primary CLL cells with CD40L-expressing fibroblasts. In this model, neoplastic B cells exhibited induction of NFκB pathways and Bcl-2 proteins, accompanied by protection from both spontaneous and drug-induced apoptosis, thereby partially replicating the resistant stromal niche.16 Here, to determine the effect of BAFF ligand on NFκB activation in primary CLL cells, we established an in vitro model where primary CLL cells were cultured in the presence of engineered BAFF-expressing CHO cells, as described in the Methods section (Online Supplementary Figure S2). In this model, cells were subjected to continuous stimulation by BAFF cytokine. BAFF-stimulated CLL cells were rescued from spontaneous apoptosis (12.3±3.2% cell apoptosis after 24-h incubation), compared to cells cultured off stroma (34.8±6.2%) or on control stroma (22.4±2.4%), and were resistant to chemotherapy-induced apoptosis (Figure 1A).
Figure 1.
B-cell activating factor (BAFF) promotes activation of pro-survival pathways in chronic lymphocytic leukemia (CLL) cells. (A) CLL cells (n=10) were cultured on BAFF-expressing or parental cells for 24 hours (h), followed by incubation with the indicated drugs, or vehicle control (ctrl), for an additional 24 h. As a reference, cells were treated off stroma. Apoptosis within CD19+ subset of cells was determined by Annexin V and 7-AAD staining. Data are presented as mean±Standard Error (SE). *P<0.05 compared to ‘off stroma’. (B and C) CLL cells from 3 individual samples were co-cultured with BAFF-expressing stroma for 24 h. RNA was isolated from the purified CLL B cells and microarray analysis was performed as described in the Methods section. Heatmap shows hierarchical clustering of expression profiles of the 122 differentially expressed NFκB target genes (yellow: upregulation; blue: downregulation). (D) CLL cells from 3 individual patients were co-cultured with BAFF-expressing stroma for 4–24 h. Whole-cell protein lysates were subjected to immunoblotting. (E) CLL cells (n=4) were co-cultured with control, BAFF- or CD40L-expressing stroma for 24 h. p52 and p65/RelA activity was determined in whole-cell protein lysates using the TransAM NFκB activity assay. The dotted line represents activity measured in freshly isolated cells (at 0 h), which has been set at 1 (*P<0.05, **P<0.01 compared against that). (F) CLL cells (n=4) were co-cultured under the indicated conditions for 24 h. Whole-cell protein lysates were subjected to immunoblotting.
We then employed gene expression profiling to determine the pathways induced by BAFF in CLL. Of the genes incorporated in the probe set, 7254 were expressed in CLL. Of these, 4844 were differentially expressed in response to BAFF stimulation (P<0.01). Using a cut-off value of at least 1.5-fold change, we identified 2251 genes whose expression was significantly modulated by BAFF stimulation (P<0.01) (Figure 1B). We determined that receptor signaling and expression target pathways involving NFκB were most significantly associated with the upregulated genes (P<0.0001). Importantly, of the more than 400 known NFκB transcriptional targets, 181 genes were expressed in CLL and 122 were modulated by BAFF (P<0.01), among which 70 showed at least 50% induction, including anti-apoptotic genes and chemokines (Figure 1C). We confirmed up-regulated transcription of several NFκB target genes (Online Supplementary Figure S3A).
We studied BAFF-mediated NFκB activation in additional detail. BAFF led to strong upregulation of the non-canonical NFκB pathway in CLL (Figure 1D–F). Processing of the non-canonical precursor protein NFκB2 (p100) occurred as early as 4 h after co-culture with BAFF-expressing stroma, and further increased by 24 h (Figure 1D). In agreement with this finding, BAFF led to a 4.5-fold increase in non-canonical (p52) activity in a DNA-binding ELISA assay, comparable with the effects of CD40L (Figure 1E). By contrast, the canonical NFκB pathway was less prominently induced by BAFF. Phosphorylation of IκBα, a negative pathway regulator, was detectable after 8 h (Figure 1D). RelA/p65 DNA binding was enhanced compared with cells cultured on control stroma, but was less pronounced compared with CD40L stimulation (Figure 1E).
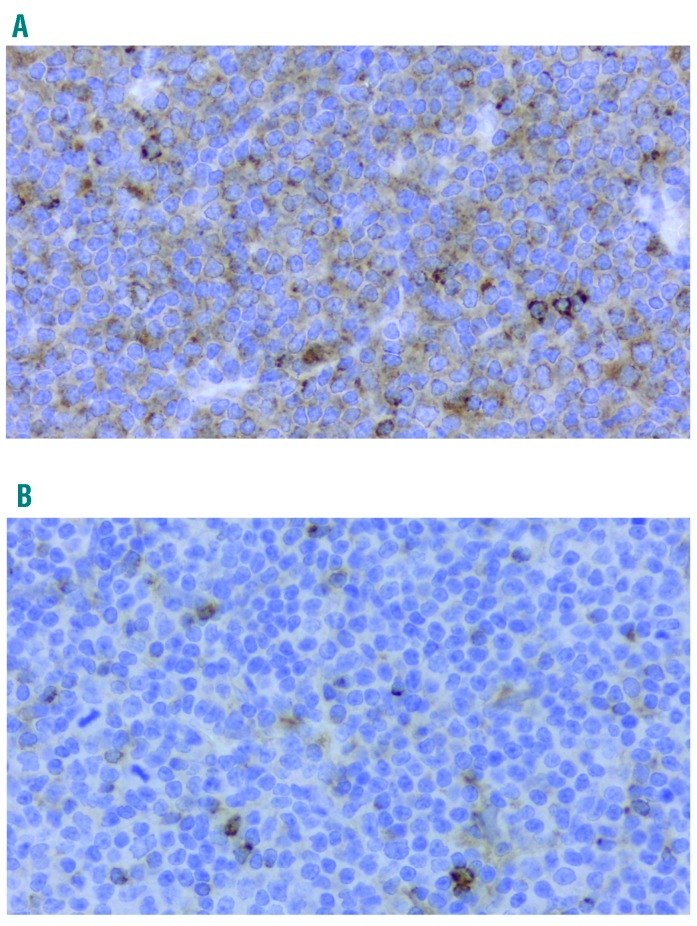
Deregulation of balance between the pro- and anti-apoptotic Bcl-2 family members determines cell fate in response to TNFR signaling.15,16,19 We found that the anti-apoptotic proteins Bcl-xL and Mcl-1 were induced in CLL cells in response to BAFF stimulation, while Bcl-2 expression was unchanged (Figure 1D). While CD40L predominantly up-regulated Bcl-xL (Figure 1F),20 BAFF mostly induced Mcl-1 (Figure 1D and Online Supplementary Figure S3B). It has been previously reported that focal CD40L expression by T cells may be restricted to lymphoid proliferation centers where it contributes to strong NFκB activation,21 while BAFF-R is ubiquitously expressed across many B-cell malignancies including CLL.22,23 Consistent with this, and together with our in vitro results, we found that, whereas Mcl-1 was diffusely expressed in CLL lymphatic tissue, Bcl-xL staining was scattered (Figure 2).
Figure 2.
Expression of Mcl-1 and Bcl-xL in chronic lymphocytic leukemia (CLL) lymph nodes. Lymphatic tissue from patients with CLL (n=10) were subjected to immunocytochemistry for Mcl-1 (A) and Bcl-xL (B), as described in the Methods section (40×).
Thus, in BAFF-expressing CLL-stromal cell co-cultures, non-canonical NFκB and Mcl-1 are induced to a greater extent than the canonical NFκB and Bcl-xL, a bona fide NFκB target.
BAFF induces activation of BCR-associated kinases independent of IGHV mutational status in CLL
The mechanisms underlying canonical NFκB activation in BAFF-stimulated B cells are not well understood. In CLL and other B-cell neoplasia, BCR ligation has previously been implicated as a trigger of NFκB signaling.24 Therefore, and also because Mcl-1 is a recognized target of the BCR signaling cascade,25 we aimed to determine if BAFF co-opts BCR to activate NFκB and induce Mcl-1 in CLL. In the short-term experiments which follow, we eliminated the effects of CLL-stromal contact and stromal-conditioned media by using soluble BAFF, and compared our findings against IgM crosslinking.
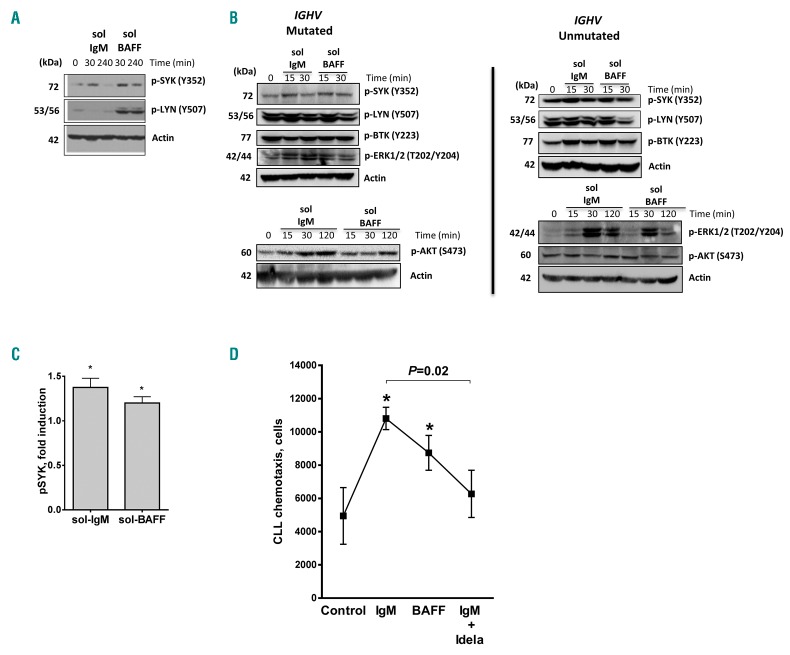
Consistent with previous reports, we found that SYK was constitutively phosphorylated in a subset of CLL samples (data not shown).26 BCR engagement is known to lead to a rapid increase in phosphorylation of the BCR-associated kinases in CLL cells.26,27 While stimulation with soluble IgM leads to transient signaling, immobilized IgM leads to sustained activation of BCR kinases (>1 h), accompanied by enhanced CLL-cell survival.26,28 We found that BAFF induced rapid activation of the proximal kinases SYK and LYN at the phosphorylation sites relevant to BCR signaling, which was sustained for 4 h in some samples (Figure 3A).
Figure 3.
B-cell activating factor (BAFF) activates BCR signaling in chronic lymphocytic leukemia (CLL) cells. (A–C) CLL cells were stimulated with 5 μg/μL sol-IgM or 25 ng/μL sol-BAFF. Cells were lysed at the indicated time points and subjected to immunoblotting. A representative result of 10 independent experiments is shown (includes 4 unmut-IGHV and 6 mut-IGHV). Densitometry (C) was performed on immunoblots from 10 individual CLL samples after 15 minutes (min) stimulation with IgM or BAFF. (D) CLL cells were incubated or not with idelalisib (5 μM) for 1 hour (h) and stimulated with sol-IgM or sol-BAFF for 30 min. Cell migration using 200 ng/mL CXCL12 was evaluated as described in the Methods section. P<0.05 versus control.
IGHV mutational status is a strong determinant of response to BCR stimulation in CLL.27,29 We found that phosphorylation of the proximal BCR-associated kinases SYK, LYN, and BTK in response to BAFF ligation was variable between samples. However, their activation did not correlate with IGHV mutational status, with 3 of 4 unmutated and 4 of 6 mutated samples exhibiting SYK activation (Figure 3B and C). When present, ERK activation in response to BAFF occurred after 15 min, and waned by 120 min, mirroring IgM-mediated effects. By contrast, AKT phosphorylation occurred later (>2 h following stimulation), suggesting that BAFF activates AKT independent of BCR.
BCR activation modulates cytokine synthesis, and CLL cell adhesion and migration.30 Consistent with earlier reports,18 we found that IgM crosslinking enhanced CLL migration toward CXCL12 2-fold, and this was partially inhibited in the presence of idelalisib, a PI3K-δ inhibitor (Figure 3D). Interestingly, we found that BAFF also induced CLL cell chemotaxis, suggesting that BAFF signaling may play a role in CLL cell homing. Finally, in our gene profiling experiments reported above, we found that of the 157 genes involved in the BCR pathway, 63 were significantly modulated by BAFF (Online Supplementary Figure S4).
In summary, BAFF stimulation co-opts BCR signaling in CLL cells, independently of their IGHV mutational status, thereby contributing to CLL cell survival.
SYK inhibition thwarts BAFF-mediated survival in CLL by targeting Mcl-1
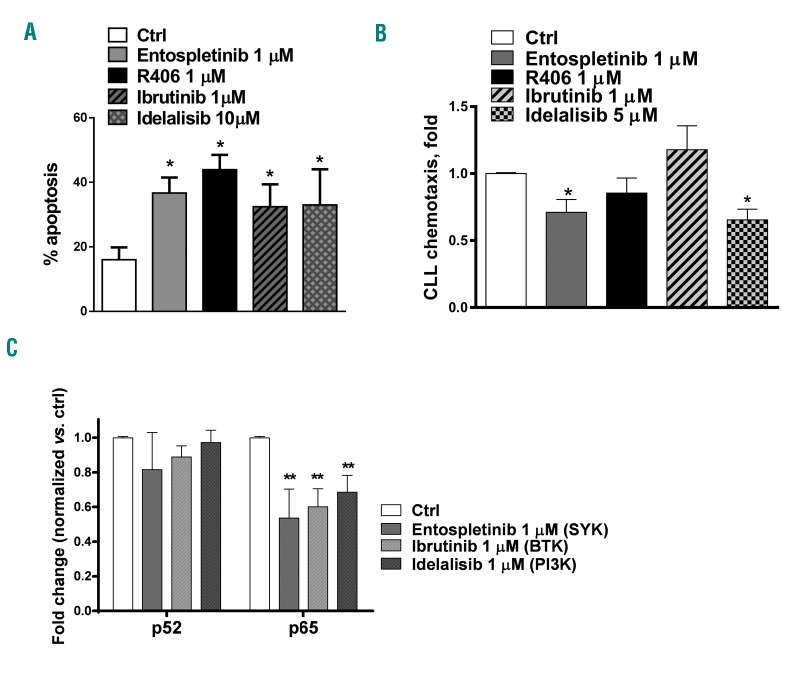
Given the co-operation between BAFF and BCR signaling, we explored the effects of targeting BCR signaling on BAFF-mediated events. CLL cells were cultured on BAFF-expressing stroma for 24 h and then exposed to BCRi. SYK inhibitors entospletinib (GS-9973) and R406 effectively antagonized survival of BAFF-stimulated CLL cells, while pharmacological inhibitors of BTK and PI3K were slightly less active (Figure 4A and Online Supplementary Figure S5). Entospletinib and idelalisib partially abrogated chemotaxis of BAFF-stimulated CLL cells (Figure 4B), echoing observations made in a setting of IgM crosslinking.31,32 As with IgM stimulation, the anti-chemotactic effect of BCRi was likely dependent on their ability to abrogate the autocrine secretion of chemokines, many of which are NFκB transcriptional targets.18,33
Figure 4.
Inhibitors of BCR-associated kinases abrogate B-cell activating factor (BAFF)-mediated canonical NFκB activation in chronic lymphocytic leukemia (CLL). (A) CLL cells (n=6) were cultured on BAFF-expressing stroma for 24 hours (h), followed by incubation with the indicated drugs, or vehicle control (ctrl), for an additional 24 h. Apoptosis within the CD19+ subset of cells was determined by Annexin V and 7-AAD staining. Data are presented as mean±Standard Error (SE). *P<0.05 compared to vehicle control. (B) CLL cells (n=4) were incubated with the indicated drugs or vehicle control for 1 h, followed by stimulation with 25 ng/mL sol-BAFF for 30 minutes (min). Cell migration using 200 ng/mL CXCL12 was evaluated as described in the Methods section. (C) CLL cells (n=4) were co-cultured with BAFF-expressing stroma for 24 h, and incubated with the indicated drugs for an additional 24 h. p52/RelA activity was determined in nuclear protein lysates using the TransAM NFκB activity assay (ActivMotif). **P<0.01 compared to untreated control.
Next, we studied the mechanism of SYK inhibition-induced apoptosis in this setting. It has been previously shown that ibrutinib down-modulates NFκB signaling in CLL in vivo.34 Thus, we hypothesized that targeting kinases within the BCR signaling cascade would antagonize NFκB activation in BAFF-stimulated CLL cells. We found that all tested BCRi tested abrogated the canonical, but not the non-canonical NFκB (Figure 4C and Online Supplementary Figure S6A). By contrast, pevonedistat, an inhibitor of NEDD8-activating enzyme previously shown by us to disrupt NFκB activity in CD40-stimulated CLL cells,16 abrogated both NFκB pathways and induced apoptosis in BAFF-stimulated CLL cells (Online Supplementary Figure S6A–C).
Since SYK is the key molecule in BCR activation, we further explored how SYK inhibition could counter BAFF-mediated CLL cell survival. SYK is involved in regulation of Mcl-1 in healthy and neoplastic B cells.25 Mcl-1 is a protein with a short half-life (approx. 30 min), whose expression is influenced by many regulatory networks.35 While CLL cells cultured off stroma quickly lose Mcl-1 (data not shown), BAFF-expressing stroma up-regulated Mcl-1 after 8–24 h (Figure 1D). The pro-survival effects of BAFF-expressing stroma were reversed by A-1210477, an Mcl-1 specific BH3-mimetic (Figure 5A).36 While less efficacious than off stroma, BH3-mimetic venetoclax abrogated survival of BAFF-stimulated CLL cells, indicating their continued dependence on Bcl-2 (Figure 5A). While BAFF signaling resulted in partial rescue from low concentrations of venetoclax, CD40L-expressing stroma exhibited a high degree of protection from both BH3-mimetics (i.e. αMcl-1 and αBcl-2), suggesting that Bcl-xL may play a particularly important role in resistance to apoptosis in CLL. Treatment with pevonedistat did not down-regulate Mcl-1 in CLL cells, confirming that BAFF-mediated induction of Mcl-1 is NFκB-independent. By contrast, inhibition of SYK reduced Mcl-1 protein levels more effectively than targeting other molecules within the BCR signaling cascade (Figure 5B and C).
Figure 5.
SYK inhibition down-regulates Mcl-1 via STAT3. (A) Chronic lymphocytic leukemia (CLL) cells were co-cultured with B-cell activating factor (BAFF)-expressing stroma for 24 hours (h), followed by incubation with the indicated drugs for 24 h. Cells were also treated off stroma for 24 h. Apoptosis within CD19+ subset of cells was determined by Annexin V and 7-AAD staining (n=6). Data are presented as mean±Standard Error (SE). *P<0.05, **P<0.01, compared to ‘off stroma’ control, or as shown. (B and C) CLL cells were co-cultured with BAFF-expressing stroma for 24 h, followed by incubation with the indicated drugs for 24 h in the presence of caspase inhibitor QVD-OPh (1 μM). Cells were lysed and subjected to immunoblotting. Densitometry chart (C) represents data from 6 individual CLL samples. Data are presented as mean±Standard Error (SE). *P<0.05 compared to control. (D) CLL cells (4 individual samples) were co-cultured with BAFF-expressing stroma for 24 h, followed by addition of 100 μg/mL cycloheximide and 1 μM entospletinib or vehicle control. Cells were lysed at the indicated time points and subjected to immunoblotting. (E and F) CLL cells (n=4) were co-cultured with BAFF-expressing stroma for 24 h, treated with SYK inhibitors (entospletinib, R406), JAK1/2 inhibitor (ruxolitinib), BTK inhibitor (ibrutinib) or PI-3Kδ inhibitor (idelalisib) for 24 h in the presence of caspase inhibitor QVD-OPh (1 μM), followed by collection of mRNA and protein.
We further explored how SYK inhibition deregulated Mcl-1. Entospletinib did not enhance Mcl-1 degradation in BAFF-stimulated CLL cells (Figure 5D). Similarly, pharmacological targeting of MEK/ERK, which antagonize Mcl-1 degradation,37 did not modulate Mcl-1 levels in BAFF-stimulated CLL cells (Online Supplementary Figure S7A). By contrast, targeting SYK significantly repressed Mcl-1 mRNA (Figure 5E). SYK activation induces phosphorylation and nuclear translocation of signal transducer and activator of transcription 3 (STAT3),38 a transcriptional regulator of Mcl-1.39 STAT3 phosphorylation was lost in CLL cells cultured off stroma (Online Supplementary Figure S7B). By contrast, STAT3 remained activated in BAFF-expressing stromal co-cultures (Figure 5F). Inhibition of SYK, but not BTK or PI3K, abrogated STAT3 phosphorylation in this setting. Similarly, ruxolitinib, a JAK/STAT inhibitor, abrogated STAT3 activation, accompanied by downregulation of Mcl-1 transcript and protein (Figure 5E and F).
Thus, BAFF-mediated induction of Mcl-1 contributes to CLL cell survival, and may be abrogated via disrupting the SYK-STAT3 axis.
BAFF induces SYK interaction with TRAF2/TRAF3 signaling complex
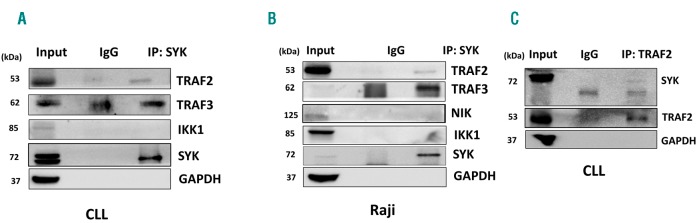
Since BAFF promoted CLL cell survival via SYK-mediated upregulation of the canonical NFκB and Mcl-1, we asked how BAFF activates SYK. We supposed that SYK complexing with TRAF2/TRAF3 may be responsible for BAFF-induced SYK activation in neoplastic B cells. CLL cells and Raji B-cell lymphoma cells were used to address this question. Immunoprecipitation with SYK monoclonal antibodies showed association of SYK with TRAF3 and TRAF2 in both BAFF-stimulated CLL and Raji cells (Figure 6A and B). SYK binding was subsequently confirmed in the reverse experiments with TRAF2 monoclonal antibodies (Figure 6C).
Figure 6.
SYK interacts with TRAF2/TRAF3 in neoplastic B cells. Cells were stimulated with 25 ng/mL sol-B-cell activating factor (BAFF) for 30 minutes (min). Proteins lysates were subjected to immunoprecipitation experiments using indicated antibodies as described in the Methods section. A representative blot of 3 independent experiments is shown. CLL: chronic lymphocytic leukemia.
Since NIK participates in TRAF2/3 complex, it could be responsible for SYK phosphorylation. NIK expression was low in CLL and Raji lymphoma cells, complicating interpretation of experimental results involving NIK genetic knockdown (data not shown). While BAFF stimulation induced SYK phosphorylation in Raji cells, engineered expression of NIK40 did not modulate SYK activation either in the absence or in the presence of BAFF (Online Supplementary Figure S8A). At the same time, pharmacological targeting of IKK failed to prevent BAFF-mediated SYK activation and SYK did not complex with either NIK or IKK1 in immunoprecipitation experiments (Figure 6A and B, and Online Supplementary Figure S8B and C).
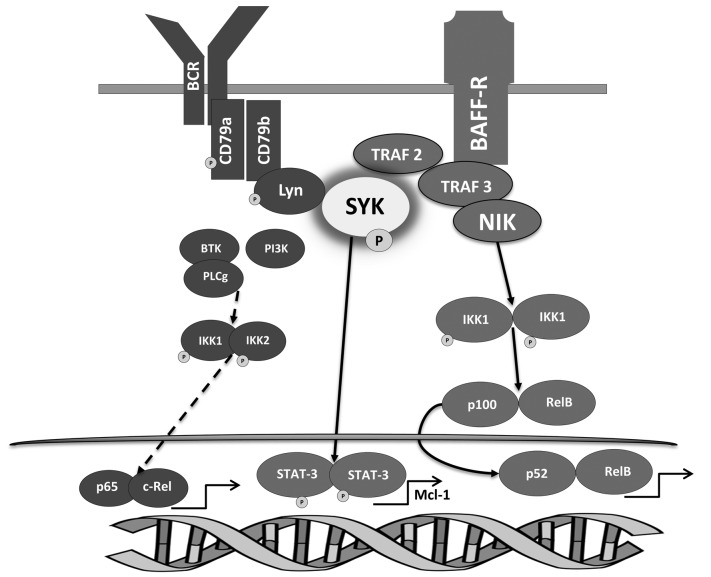
Thus, BAFF-BCR crosstalk in neoplastic B cells is at least in part mediated by SYK interaction with TRAF2/TRAF3 signaling complex (Figure 7).
Figure 7.
B-cell activating factor (BAFF)-BCR crosstalk in chronic lymphocytic leukemia (CLL) cells. BAFF-R engagement stabilizes NIK within the NIK/TRAF2/TRAF3/cIAP1/2 complex, promoting the non-canonical NFκB pathway activity. SYK recruitment to TRAF2/TRAF3 assists BAFF-mediated activation of BCR signaling, which contributes to activation of the canonical NFκB. Concurrently, SYK induces STAT3 transcription factor, thereby up-regulating Mcl-1, a pro-survival Bcl-2 family member.
Discussion
Along with the BCR, many concurrently active pathways ensure survival of the neoplastic B cells in the protective niche. We and others previously demonstrated that primary CLL cells co-cultured with CD40L-expressing stroma activate the canonical and non-canonical NFκB pathways, accompanied by upregulation of the pro-survival Bcl-2 family proteins (Bcl-xL), and acquire therapeutic resistance, including to BCRi.14–16 Here we demonstrate that BAFF signaling had a distinct effect on CLL cell survival. CLL cells co-cultured with BAFF-expressing stroma were resistant to both spontaneous and drug-induced apoptosis. BAFF triggered robust activation of the non-canonical and, to a lesser extent, canonical NFκB, leading to distinctive expression of Bcl-2 family proteins, predominantly Mcl-1.
BAFF was previously shown to co-opt BCR signaling in mouse splenic B lymphocytes, manifested by phosphorylation of the BCR-associated CD79A subunit and SYK.41 Here, we present, for the first time, evidence that such crosstalk exists in primary human neoplastic B cells. We detected activation of the BCR-associated kinases, including SYK, BTK, and ERK in CLL cells stimulated with BAFF. Importantly, while BCR response to BAFF was transient in mouse B cells,41 it was sustained in CLL. Although CLL samples with unmutated IGHV have an enhanced capacity to respond to BCR engagement,28 both CLL subsets responded to BAFF. The ability of the mutated CLL to respond to BAFF suggests that surface IgM expression is an unlikely pre-requisite for successful transduction of BAFF signal to the BCR.24
In a mouse, engineered BCR loss prevented SYK activation by BAFF, suggesting that intracellular tyrosine activation motif (ITAM)-related sequences on CD79A/B may be involved in BAFF signal transmission.41 We observed direct interaction between TRAF2/TRAF3 adaptor protein complex and SYK in CLL cells, potentially implicating this interaction in BAFF-BCR crosstalk in neoplastic B cells (Figure 6D). It is possible that SYK induction in CLL by CD40L also occurs through TRAF2/3 interaction.42
When BAFF-R is engaged, the NIK/TRAF/cIAP complex is recruited to the receptor, thus allowing NIK to persist and activate IKK1. Therefore either NIK or IKK1 may be involved in BAFF-mediated SYK activation. Furthermore, the role of the BCR structures (CD79A/B) or LYN, a BCR-associated kinase constitutively active in CLL cells,43 as well as BCR-associated protein phosphatases, needs to be investigated in this setting. Additional experiments will be required to decipher the exact contributions of the individual kinases and BCR structural components in BAFF-BCR crosstalk in CLL, and will be hampered by the technical challenges of eliminating those individual players in primary B cells. It is conceivable that many different conditions need to be fulfilled where co-operative action involving an intact BCR structure and the SRC family protein kinases is required for BAFF-mediated activation of SYK and BCR signal propagation.
It remains unclear whether activation of both canonical and non-canonical NFκB is necessary to ensure BAFF-mediated CLL cell survival. Experiments in murine splenic B cells suggest that the two NFκB pathways may be redundant, since the double Nfkb1−/−Nfkb2−/− mouse, but not single mutations, recapitulated a BAFF/BAFF-R-deficiency phenotype.44 However, recent data suggest complementary roles for both pathways, which promote BAFF-induced B-cell survival and maturation via distinct gene expression programs.45 Earlier studies demonstrated that selectively blocking canonical NFκB abrogated survival advantage inferred by CLL cell exposure to soluble BAFF.8 Furthermore, although BCR signaling does not promote processing of p100, the latter is a positively regulated target of BCR-mediated gene transcription, where ultimately BCR signaling may enhance non-canonical NFκB signaling.46,47 We show that BCRi predominantly inactivated canonical NFκB and did not completely reverse protection in BAFF-expressing stromal co-cultures. Meanwhile, pevonedistat is an investigational small molecule that forms a covalent adduct with NEDD8, a ubiquitin-like modifier, thus disrupting the functionality of Cullin-RING ubiquitin ligases and leading to accumulation of their substrates, including inhibitor of NFκB (IκB) and NFκB2/p100, with a net outcome of inactivation of the canonical and non-canonical NFκB, respectively.16 While pevonedistat abrogated NFκB signaling in BAFF-stimulated CLL cells, apoptosis was not complete, suggesting that NFκB-independent signaling pathways, including BCR, contribute to BAFF-mediated survival in CLL. Additional studies are needed to elucidate the role of NFκB in BAFF-mediated CLL cell survival. Ultimately, a combination of strategies aimed at delivering a multi-pronged attack will be required to fully neutralize the pro-survival pathways induced by BAFF.
Subsequent studies should help elucidate the exact ligand-receptor interactions leading to BCR activation in CLL. BAFF and APRIL bind the two TNFR superfamily members, BCMA and TACI, with high affinity. It would be important to confirm whether APRIL stimulation could replicate some of the BAFF ligand effects. While our data suggest that BAFF-BR3 interaction may be necessary for BCR activation via TRAF2/TRAF3 complex, isolated and concurrent blockade of the individual signaling receptors9 will be necessary to elucidate their importance in regulation of NFκB and BCR signaling pathways, as well as response to BCRi in BAFF-simulated CLL cells.
SYK, a key initiating kinase in the BCR signaling cascade, is an attractive therapeutic target in lymphoid malignancies. Entospletinib is a novel orally available kinase inhibitor which has greater selectivity for SYK compared with R406.48 Entospletinib showed a favorable toxicity profile and induced responses in 61% of patients with relapsed/refractory CLL.48 It has been previously demonstrated that SYK inhibitors, such as R406, block BCR engagement-mediated induction of Mcl-1 in CLL cells.25 While this manuscript was in preparation, Bojarczuk et al. reported that other BCRi (ibrutinib, idelalisib) were also able to inhibit Mcl-1; however, they were not as effective as SYK inhibitors.49 Interestingly, we found that neither BTK or PI3Kδ inhibition neutralized Mcl-1 to the same degree as entospletinib in BAFF-expressing stromal co-culture model. In a setting of BCR engagement, BCRi prevented inactivation of glycogen synthase kinase-3 (GSK-3), thereby presumably leading to Mcl-1 degradation.35 By contrast, we did not observe enhanced Mcl-1 degradation following SYK inhibition in BAFF-stimulated CLL cells. It is possible that BAFF signaling incapacitates GSK-3 in a BCR-independent manner, e.g. through PI3K/AKT, assisted by the co-receptor CD19,10,50,51 such that concurrent inhibition of multiple pathways, resulting in abrogation of both AKT-dependent and AKT-independent (Protein kinase C-dependent) inactivation of GSK-3, may be necessary to enhance Mcl-1 turnover. Importantly, SYK inhibition resulted in decreased phosphorylation of STAT3, a known SYK target and a positive regulator of Mcl-1 transcription.38,39 Thus, transcriptional downmodulation rather than enhanced turnover underlies loss of Mcl-1 following SYK inhibition in BAFF-stimulated CLL cells. This finding also suggests that BTK and PI3Kδ inhibitors may less effectively target Mcl-1 in the tumor microenvironment than SYK inhibitors, informing future therapeutic development of BCRi.
High expression of Mcl-1 predicts adverse outcomes following chemo-immunotherapy in CLL.52 Given our findings, a relevant question would be whether responses in B-cell malignancies may be bolstered by concurrent inhibition of SYK and other BCR-associated kinases. While a clinical trial of a combination of entospletinib and idelalisib, a PI3Kδ inhibitor, in patients with CLL was halted early due to treatment-emergent pneumonitis,53 clinical trials evaluating safety and efficacy of entospletinib and BTK inhibitor combinations are ongoing.
Thus, our study throws light on the crosstalk between BAFF and BCR signaling pathways in neoplastic B cells, and provides insights into the mechanistic effects of SYK inhibitors in CLL.
Supplementary Material
Acknowledgments
We would like to thank Allison Berger, Ph.D., for the helpful review of this manuscript.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/11/1890
References
- 1.Pascutti MF, Jak M, Tromp JM, et al. IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood. 2013;122(17):3010–3019. [DOI] [PubMed] [Google Scholar]
- 2.Caligaris-Cappio F, Bertilaccio MT, Scielzo C. How the microenvironment wires the natural history of chronic lymphocytic leukemia. Semin Cancer Biol. 2014;24:43–48. [DOI] [PubMed] [Google Scholar]
- 3.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. [PubMed] [Google Scholar]
- 4.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danilov AV. Targeted therapy in chronic lymphocytic leukemia: past, present, and future. Clin Ther. 2013;35(9):1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015;1(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo T, Nishio M, Enzler T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood. 2007;109(2):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kern C, Cornuel JF, Billard C, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103(2):679–688. [DOI] [PubMed] [Google Scholar]
- 10.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237(1):205–225. [DOI] [PubMed] [Google Scholar]
- 11.Nishio M, Endo T, Tsukada N, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106(3):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244(1):115–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. [DOI] [PubMed] [Google Scholar]
- 14.Tromp JM, Tonino SH, Elias JA, et al. Dichotomy in NF-kappaB signaling and chemoresistance in immunoglobulin variable heavy-chain-mutated versus unmutated CLL cells upon CD40/TLR9 triggering. Oncogene. 2010;29(36):5071–5082. [DOI] [PubMed] [Google Scholar]
- 15.Vogler M, Butterworth M, Majid A, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–4413. [DOI] [PubMed] [Google Scholar]
- 16.Godbersen JC, Humphries LA, Danilova OV, et al. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-kappaB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res. 2014;20(6):1576–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One. 2008;3(9):e3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danilov AV, Soderquist RS, Bates DJ, Eastman A. Toward a cure for chronic lymphocytic leukemia: an attack on multiple fronts. Expert Rev Anticancer Ther. 2013;13(9):1009–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soderquist R, Bates DJ, Danilov AV, Eastman A. Gossypol overcomes stroma-mediated resistance to the BCL2 inhibitor ABT-737 in chronic lymphocytic leukemia cells ex vivo. Leukemia. 2013;27(11):2262–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herreros B, Rodriguez-Pinilla SM, Pajares R, et al. Proliferation centers in chronic lymphocytic leukemia: the niche where NF-kappaB activation takes place. Leukemia. 2010;24(4):872–876. [DOI] [PubMed] [Google Scholar]
- 22.Rodig SJ, Shahsafaei A, Li B, Mackay CR, Dorfman DM. BAFF-R, the major B cell-activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum Pathol. 2005;36(10):1113–1119. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura N, Hase H, Sakurai D, et al. Expression of BAFF-R (BR 3) in normal and neoplastic lymphoid tissues characterized with a newly developed monoclonal antibody. Virchows Arch. 2005;447(1):53–60. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313–4320. [DOI] [PubMed] [Google Scholar]
- 25.Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23(4):686–697. [DOI] [PubMed] [Google Scholar]
- 26.Petlickovski A, Laurenti L, Li X, Marietti S, Chiusolo P, Sica S, Leone G, Efremov DG. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105(12):7. [DOI] [PubMed] [Google Scholar]
- 27.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101(3):1087–1093. [DOI] [PubMed] [Google Scholar]
- 28.Deglesne PA, Chevallier N, Letestu R, et al. Survival response to B-cell receptor ligation is restricted to progressive chronic lymphocytic leukemia cells irrespective of Zap70 expression. Cancer Res. 2006;66(14):7158–7166. [DOI] [PubMed] [Google Scholar]
- 29.Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood. 2007;109(10):4424–4431. [DOI] [PubMed] [Google Scholar]
- 30.Packham G, Stevenson F. The role of the B-cell receptor in the pathogenesis of chronic lymphocytic leukaemia. Semin Cancer Biol. 2010;20(6):391–399. [DOI] [PubMed] [Google Scholar]
- 31.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. [DOI] [PubMed] [Google Scholar]
- 32.Thijssen R, ter Burg J, van Bochover GGW, et al. The pan phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor SAR245409 (voxtalisib/XL765) blocks survival, adhesion and proliferation of primary chronic lymphocytic leukemia cells. Leukemia. 2015;30(2):337–345. [DOI] [PubMed] [Google Scholar]
- 33.Bernard S, Danglade D, Gardano L, et al. Inhibitors of BCR signalling interrupt the survival signal mediated by the micro-environment in mantle cell lymphoma. Int J Cancer. 2015;136(12):2761–2774. [DOI] [PubMed] [Google Scholar]
- 34.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123(21):3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ertel F, Nguyen M, Roulston A, Shore GC. Programming cancer cells for high expression levels of Mcl1. EMBO Rep. 2013;14(4):328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leverson JD, Zhang H, Chen J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015;6:e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23(31):5301–5315. [DOI] [PubMed] [Google Scholar]
- 38.Uckun FM, Qazi S, Ma H, Tuel-Ahlgren L, Ozer Z. STAT3 is a substrate of SYK tyrosine kinase in B-lineage leukemia/lymphoma cells exposed to oxidative stress. Proc Natl Acad Sci. 2010;107(7):2902–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epling-Burnette PK, Liu JH, Catlett-Falcone R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107(3):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao G, Sun SC. Negative regulation of the nuclear factor kappa B-inducing kinase by a cis-acting domain. J Biol Chem. 2000;275(28):21081–21085. [DOI] [PubMed] [Google Scholar]
- 41.Schweighoffer E, Vanes L, Nys J, et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013;38(3):475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parente-Ribes A, Skanland SS, Burgler S, et al. Spleen tyrosine kinase inhibitors reduce CD40L-induced proliferation of chronic lymphocytic leukemia cells but not normal B cells. Haematologica. 2016;101(2):e59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contri A, Brunati AM, Trentin L, et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest. 2005;115(2):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3(10):958–965. [DOI] [PubMed] [Google Scholar]
- 45.Almaden JV, Liu YC, Yang E, et al. B-cell survival and development controlled by the coordination of NF-kappaB family members RelB and cRel. Blood. 2016;127(10):1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cancro MP. Signalling crosstalk in B cells: managing worth and need. Nat Rev Immunol. 2009;9(9):657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro I, Wright JA, Damdinsuren B, et al. B cell receptor-mediated sustained c-Rel activation facilitates late transitional B cell survival through control of B cell activating factor receptor and NF-kappaB2. J Immunol. 2009;182(12):7729–7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015;125(15):2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bojarczuk K, Sasi BK, Gobessi S, et al. BCR signaling inhibitors differ in their ability to overcome Mcl-1-mediated resistance of CLL B cells to ABT-199. Blood. 2016;127(25):3192–3201. [DOI] [PubMed] [Google Scholar]
- 50.Jellusova J, Miletic AV, Cato MH, et al. Context-specific BAFF-R signaling by the NF-kappaB and PI3K pathways. Cell Rep. 2013;5(4):1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henley T, Kovesdi D, Turner M. B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. Eur J Immunol. 2008;38(12):3543–3548. [DOI] [PubMed] [Google Scholar]
- 52.Awan FT, Kay NE, Davis ME, et al. Mcl-1 expression predicts progression-free survival in chronic lymphocytic leukemia patients treated with pentostatin, cyclophosphamide, and rituximab. Blood. 2009;113(3):535–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127(20):2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.