Abstract
Intravenous rituximab plus chemotherapy is standard treatment for diffuse large B-cell lymphoma. A subcutaneous formulation of rituximab is expected to simplify and shorten drug preparation and administration, and to reduce treatment burden. MabEase (clinicaltrials.gov Identifier: 01649856) examined efficacy, safety and patient satisfaction with subcutaneous rituximab plus chemotherapy in treatment-naïve patients with diffuse large B-cell lymphoma. Patients were randomized 2:1 to subcutaneous rituximab (intravenous 375 mg/m2 cycle 1; subcutaneous 1,400 mg cycles 2–8) or intravenous rituximab (375 mg/m2 cycles 1–8) plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 14 or 21 days. The primary endpoint was investigator-assessed complete response/unconfirmed complete response. Secondary endpoints included safety, treatment satisfaction (Cancer Treatment Satisfaction Questionnaire and Rituximab Administration Satisfaction Questionnaire), time savings, and survival. Of 576 randomized patients, 572 (378 subcutaneous; 194 intravenous) received treatment. End of induction complete response/unconfirmed complete response rates were 50.6% (subcutaneous) and 42.4% (intravenous). After a median 35 months, median overall, event-free and progression-free survivals were not reached. Grade ≥3 adverse events (subcutaneous 58.3%; intravenous 54.3%) and administration-related adverse events (both groups 21%) were similar between arms. Injection-site reactions were more common with subcutaneous injections (5.7% versus 0%, respectively). Rituximab Administration Satisfaction Questionnaire scores for ‘impact on activities of daily living’, ‘convenience’, and ‘satisfaction’ were improved with subcutaneous versus intravenous injections; Cancer Therapy Satisfaction Questionnaire scores were similar between arms. Median administration time (6 minutes vs. 2.6 to 3.0 hours), chair/bed and overall hospital times were shorter with subcutaneous versus intravenous rituximab. Overall, subcutaneous and intravenous rituximab had similar efficacy and safety, with improved patient satisfaction and time savings.
Introduction
Intravenous (IV) rituximab plus chemotherapy is standard treatment for diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL)1,2 on the basis of improved efficacy versus chemotherapy alone.3–8 In addition to the primary treatment goal of optimizing patient outcomes, such as response rate, progression-free and overall survival, simplifying treatment and reducing treatment burden are also important aims for patients and healthcare providers. Subcutaneous (SC) dosing has the potential to simplify administration, reduce the treatment burden for patients, and reduce resource utilization at the treatment facility.9–13
To address these needs in patients with non-Hodgkin lymphoma (NHL), a SC formulation of rituximab has been developed. Two studies using a pharmacokinetic and clinical bridging approach, SABRINA and SparkThera, have demonstrated pharmacokinetic non-inferiority for rituximab SC compared with the IV formulation in patients with FL.14–16 In SABRINA, when given in a three-weekly dosing schedule as first-line treatment, geometric mean rituximab trough concentrations at cycle 7 were 83.13 mg/mL for rituximab IV and 134.58 mg/mL for rituximab SC, with comparable efficacy and safety between formulations.14,15 In addition, the phase Ib SAWYER study demonstrated pharmacokinetic non-inferiority and similar safety profiles for rituximab SC and IV (both with fludarabine and cyclophosphamide) in first-line chronic lymphocytic leukemia patients.17,18 The accumulating clinical data, along with the established efficacy and safety of rituximab IV,19,20 supported the authorization of rituximab SC for patients with NHL, and rituximab SC has subsequently been approved in Europe and elsewhere for the treatment of DLBCL and FL.21–23 The SC formulation takes approximately 5 minutes to administer versus 1.5 to 6 hours for rituximab IV,19,21 and studies have confirmed that this new presentation offers improved patient convenience and healthcare resource savings over the IV form.13,24
Herein we report the final analysis of the MabEase study in patients with previously untreated CD20+ DLBCL, an aggressive form of NHL that is treated with curative intent. The objectives of MabEase were to examine the efficacy and safety of rituximab SC versus the IV formulation as part of a rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen. Patient satisfaction with treatment was also assessed.
Methods
Study design
MabEase (clinicaltrials.gov Identifier: 01649856) is a phase IIIb, multicenter, randomized, open-label study. The study was conducted in line with International Conference on Harmonisation E6 guidelines for Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by independent ethics committees at each center. The first patient was enrolled on 22 August 2012, and the data cut-off for the current analysis was 18 September 2016. All patients provided written informed consent. Additional methodological details are provided in the Online Supplementary Appendix.
Patients
Eligible patients were aged 18–80 years with untreated histologically confirmed CD20+ DLBCL, International Prognostic Index (IPI) 1–5 or IPI 0 with bulky disease (one lesion ≥7.5 cm), had at least one bidimensionally measurable lesion ≥1.5 cm at its largest dimension by computed tomography (CT), positron emission tomography-CT (PET-CT), or magnetic resonance imaging (MRI), had adequate hematologic function, and Eastern Cooperative Oncology Group (ECOG) performance status ≤2 (detailed inclusion/exclusion criteria are supplied in the Online Supplementary Appendix).
Randomization
Patients were randomized 2:1 via a centralized interactive voice/web response system to receive rituximab SC or IV, and were stratified according to age (<60 or ≥60 years), IPI risk category (low, low-intermediate, high-intermediate, high), and chemotherapy regimen (CHOP-14, CHOP-21).
Procedures
All patients were scheduled to receive eight cycles of rituximab in accordance with the prescribing information for rituximab in DLBCL.19–21 In addition, patients received six to eight cycles of CHOP chemotherapy every 14 (CHOP-14) or 21 (CHOP-21) days (see the Online Supplementary Appendix). The planned CHOP regimen for each patient was chosen by the center prior to randomization. However, patients scheduled to receive eight cycles of CHOP who achieved a complete response (CR)/unconfirmed complete response (CRu) after cycle 4 could be reduced to two additional rituximab plus CHOP cycles (for a total of six CHOP cycles), followed by two rituximab monotherapy cycles (for a total of eight rituximab cycles). Patients randomized to rituximab SC received rituximab IV 375 mg/m2 on day one of cycle 1, then rituximab SC 1,400 mg on day one of the subsequent seven cycles (eight rituximab doses in total). Patients randomized to the IV arm received rituximab 375 mg/m2 on day one of each cycle. All patients received rituximab IV during cycle 1 in order to allow appropriate intervention in the event of an administration-related reaction (ARR).
Outcomes
The primary endpoint was investigator-assessed CR/CRu rate according to Cheson 1999 criteria25 at the end of induction (EOI) in the intent-to-treat (ITT) population.
Secondary endpoints included patient satisfaction measured by the Cancer Therapy Satisfaction Questionnaire (CTSQ) and Rituximab Administration Satisfaction Questionnaire (RASQ) and time savings; namely rituximab administration time, chair/bed time, and hospital time. Other secondary endpoints were progression-free survival (PFS), event-free survival (EFS), disease-free survival (DFS), and overall survival (OS). The end of the study was defined as the last patient visit in the follow-up period when all patients had been followed for at least 24 months after their last dose of study treatment.
Statistical analyses
This was a descriptive study designed to exclude major differences in efficacy between treatment arms as measured by CR/CRu rates. There was no formal statistical hypothesis for treatment comparison. The calculated sample size (600 patients) was based on the primary endpoint, and no power calculation was performed for PFS.
The final analysis was performed in the ITT population when the last patient had completed at least 24 months of follow up after the EOI, or when one of the following had been documented for all randomized patients: disease recurrence, withdrawal from study, loss to follow up or death, or whichever occurred first.
An exploratory analysis was conducted to investigate potential correlations between the incidence of adverse events (AEs) and serious adverse advents (SAEs) in different body surface area (BSA) categories and age, sex, or type of AE (MedDRA v17.1 System Organ Class preferred terms).
Results
Study population
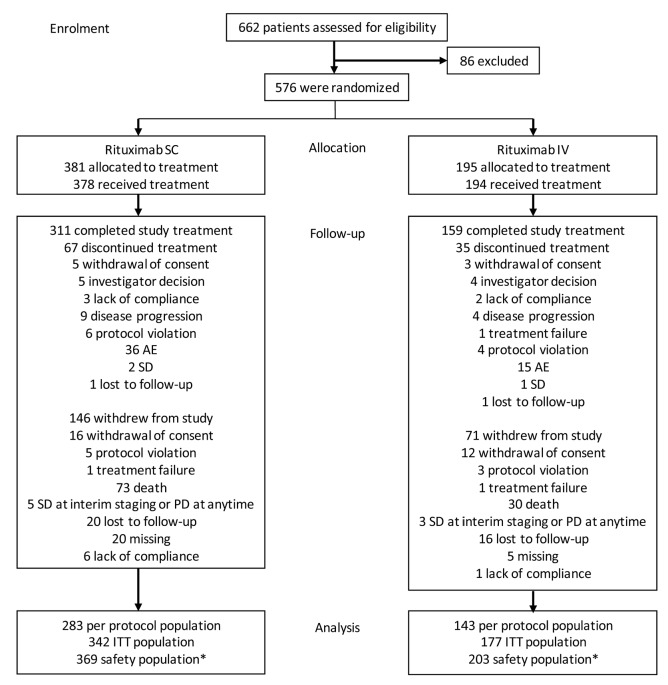
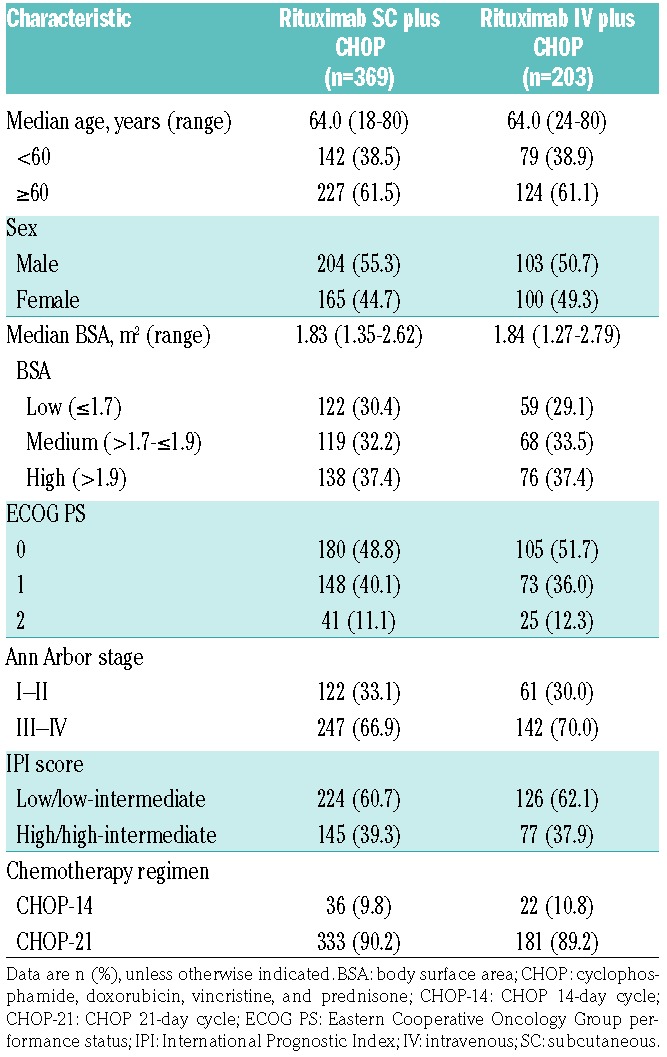
Overall, 576 patients were enrolled and randomized (SC, 381; IV, 195) from 151 in- or outpatient treatment centers in 25 countries. Of these, 572 received at least one dose of rituximab; however, nine patients randomized to receive rituximab SC only received the first rituximab IV infusion. The safety population therefore comprised 369 patients in the SC arm and 203 in the IV arm (369 and 188, respectively, from cycle 2 onwards). The ITT population comprised 519 patients (SC, 342; IV, 177; Figure 1). Baseline demographics and disease characteristics were balanced between treatment groups (Table 1).
Figure 1.
Patient disposition. *Nine patients randomized to SC only received the first IV infusion and were analyzed as IV in the safety population. AE: adverse event; ITT: intent-to-treat; IV: intravenous: SC: subcutaneous; PD: progressive disease; SD: stable disease.
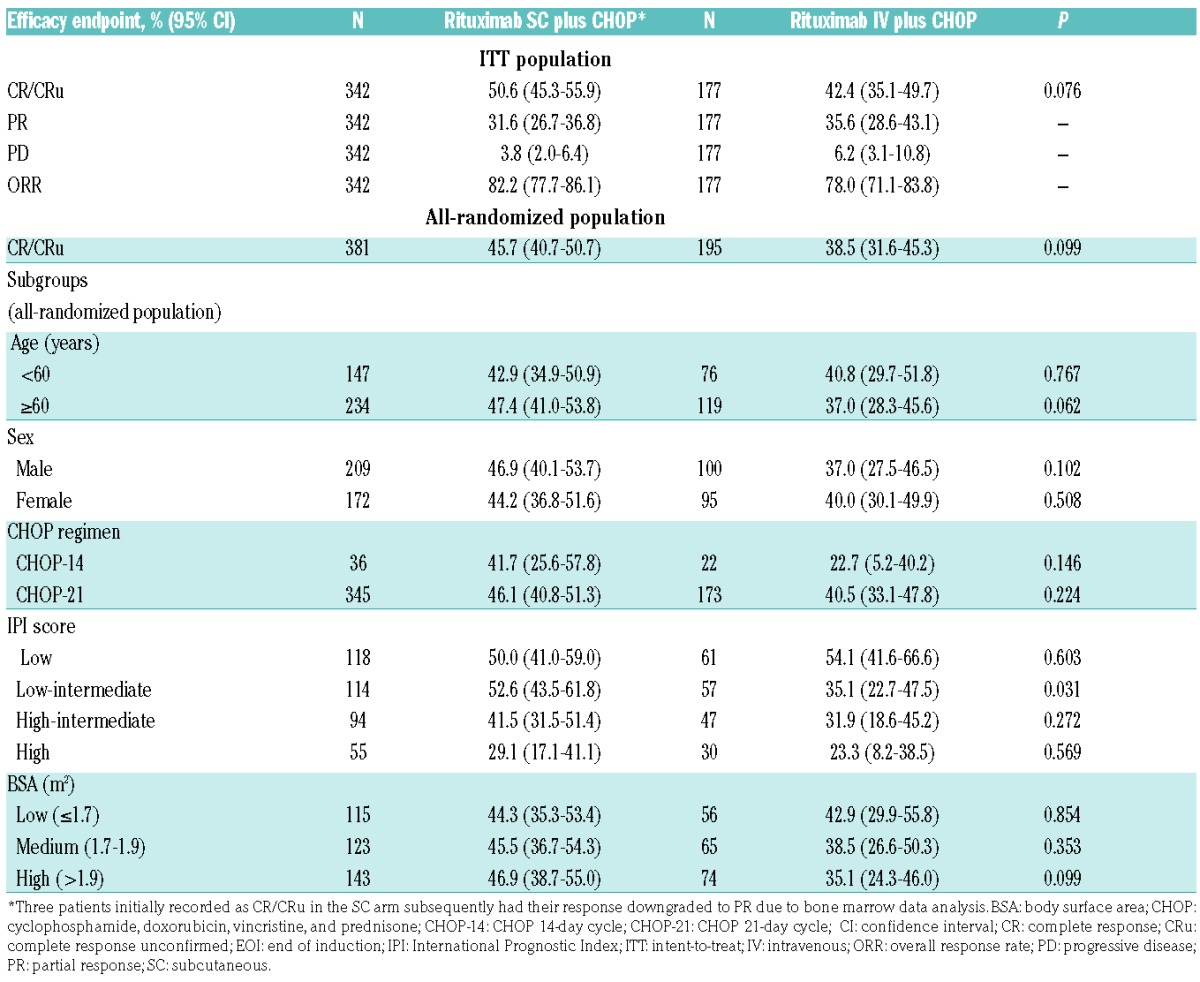
Table 1.
Patient baseline demographics and disease characteristics (safety population).
Of 576 randomized patients, 102 (SC, 67 [17.6%]; IV, 35 [17.9%]) discontinued study treatment before the end of cycle 8, predominantly because of AEs (SC, 36 [9.4%]; IV, 15 [7.7%]), and 217 patients (SC, 146 [38.3%]; IV, 71 [36.4%]) withdrew from the study altogether, predominantly because of death (SC, 73 [19.2%]; IV, 30 [15.4%]; Figure 1). In the randomized patient population, 311 of 381 SC patients and 159 of 195 IV patients completed the treatment period. There were 217 (69.8%) SC patients and 117 (73.6%) IV patients still in follow up at the end of the study.
The median duration of rituximab exposure was 4.9 months in both groups (safety population). Overall, 82.2% of patients received eight rituximab cycles. Median durations of CHOP exposure were 4.6 and 4.4 months in the rituximab SC and IV arms, respectively. Overall, 85.7%, 47.4% and 46.7% of patients received six, seven, and eight CHOP cycles, respectively.
Efficacy
In the ITT population at EOI, rates of investigator-assessed CR/CRu (95% CI) were 50.6% (45.3%–55.9%) and 42.4% (35.1%–49.7%), P=0.076, in the SC and IV groups, respectively. Partial response (PR) and progressive disease (PD) rates were similar between treatment arms. CR/CRu rates (95% CI) for all randomized patients were 45.7% (40.7%–50.7%) for rituximab SC and 38.5% (31.6%–45.3%) for IV, P=0.099. When stratified by age, sex, BSA, CHOP regimen, and IPI score, statistically significantly higher CR/CRu rates with SC treatment versus IV were seen in patients with low-intermediate IPI scores along with a trend towards higher rates in patients aged ≥60 years (Table 2). Overall, CR/CRu rates were higher in patients receiving CHOP-21 than CHOP-14 (Table 2), although the latter regimen was used in only small numbers of patients.
Table 2.
Efficacy endpoints at EOI treatment.
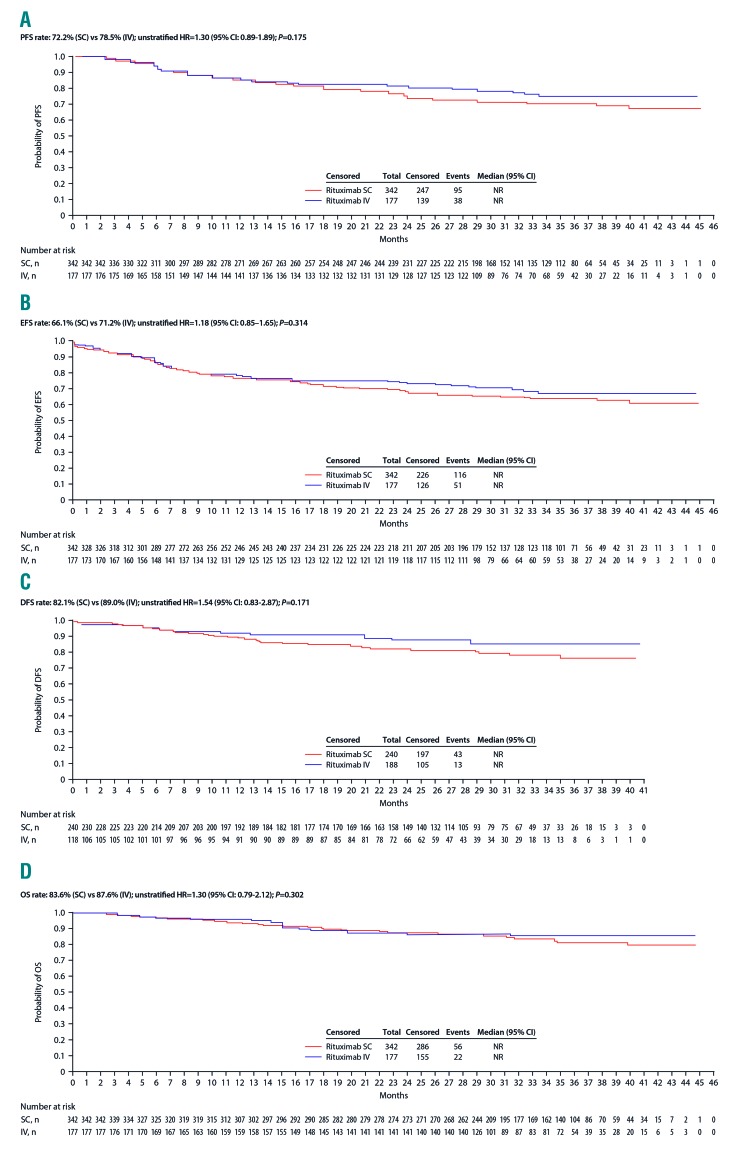
After a median follow-up of 35 months, median survival in the ITT population was not reached for PFS, EFS, DFS or OS. Statistical analyses showed no significant differences between treatment groups (Figure 2). At the time of the final data lock, 56 of 342 rituximab SC patients (16.4%) had died, while 22 of 177 (12.4%) rituximab IV patients had died. Analysis of PFS and EFS showed that 72.2% of SC and 78.5% of IV patients had not progressed, relapsed or died, while 66.1% of SC and 71.2% of IV patients had not experienced an event (Figure 2). PFS was also generally similar between treatments for the subgroups with high SC CR/CRu rates (aged ≥60 years and with low-intermediate IPI scores; Table 2; Online Supplementary Appendix), although a higher proportion of SC patients receiving CHOP-14 had progressed, relapsed or died (14/36; 38.9% vs. 2/22; 9.1%; P=0.041). In addition, significantly higher proportions of SC patients with low BSA had progressed, relapsed or died (43/115; 37.4% vs. 9/56; 16.1%; P=0.01) or experienced an event (51/115; 44.3% vs. 13/56; 23.2%; P=0.02).
Figure 2.
Secondary time-to-event endpoints for rituximab SC and rituximab IV (intent-to-treat population). Analyses presented are (A) progression-free survival, (B) event-free survival, (C) disease-free survival, and (D) overall survival. CI: confidence interval; DFS: disease-free survival; EFS: event-free survival; HR: hazard ratio; IV: intravenous; OS: overall survival; PFS, progression-free survival; SC: subcutaneous.
At 24 months of follow up, PFS (95% CI) was 75.0% (69.9%–79.4%) in the SC group and 81.5% (74.7%–86.6%) in the IV group (P=0.175), and EFS (95% CI) was 68.6% (63.3%–73.4%) and 73.4% (66.0%–79.4%), respectively (P=0.456).
Safety
Safety profiles were similar between arms, with no unexpected safety signals (Online Supplementary Appendix). Most AEs were grade 2 or 3 (339 [60.9%] of 557 patients in the safety population with cycle 2 dosing or beyond completed). In cycle 2 or later (all patients received rituximab IV in cycle 1), 58.3% of SC and 54.3% of IV patients experienced at least one AE of grade ≥3. ARRs were reported in 20.9% of SC patients and 21.3% of IV patients on or after cycle 2. Ten patients (2.7%) receiving rituximab SC experienced an ARR of grade ≥3 on or after cycle 2, compared with nine patients (4.8%) receiving IV. Injection site reactions were reported by 5.7% of patients receiving SC therapy; there were no such reactions with IV administration (P=0.0002). One injection site reaction (an episode of injection site pain in the SC group) was grade ≥3; the remainder were grade <3.
In cycle 2 or later, 141 SC (38.2%) and 62 IV patients (33.0%) reported at least one SAE, most commonly febrile neutropenia (FN), neutropenia, and pneumonia. A higher proportion of patients experienced FN as an SAE in the SC versus the IV arm (11.7% vs. 6.4%, P=0.0515), consistent with the higher incidence of grade 3/4 FN in the SC arm (12.5% vs. 6.9%, P=0.0575).
A similar proportion of patients in each group in the safety population discontinued rituximab treatment because of AEs (SC, 30 [8.1%]; IV, 19 [9.4%]), the most common of which were infections and infestations (2.4% and 2.5% of patients in the SC and IV groups, respectively; all treatment cycles). When cycle 1 was excluded, discontinuation rates were 7.9% for SC and 5.3% for IV rituximab; infection and infestation rates were 2.4% (SC) and 1.1% (IV). More SC patients (138 [37.4%]) had an interruption/delay in their rituximab treatment due to AEs when compared with the IV arm (56 [27.6%]; safety population, all cycles). The most common reasons (>2% of patients) were neutropenia (SC, 34 [9.2%]; IV, 14 [6.9%]), FN (SC, 13 [3.5%]; IV, 1 [0.5%]), pneumonia (SC, 8 [2.2%]; IV, 6 [3.0%]), neutrophil count decreased (SC, 20 [5.4%]; IV, 8 [3.9%]), and white blood cell count decreased (SC, 10 [2.7%]; IV, 3 [1.5%]).
Death due to treatment-emergent AEs (i.e., grade 5 AE) was reported in 32 patients in the SC group (8.7%) and 14 in the IV group (6.9%). The main causes were infections and infestations (SC, 14 [3.8%]; IV, 4 [2.0%]), cardiac disorders (SC, 4 [1.4%]; IV, 4 [2.0%]), and respiratory, thoracic, and mediastinal disorders (SC, 2 [0.5%]; IV, 3 [1.5%]).
In the exploratory analysis, treatment effect on grade ≥3 AEs and SAEs (i.e., rituximab SC vs. IV) was not modified by BSA, age group or sex (Online Supplementary Appendix).
Treatment satisfaction
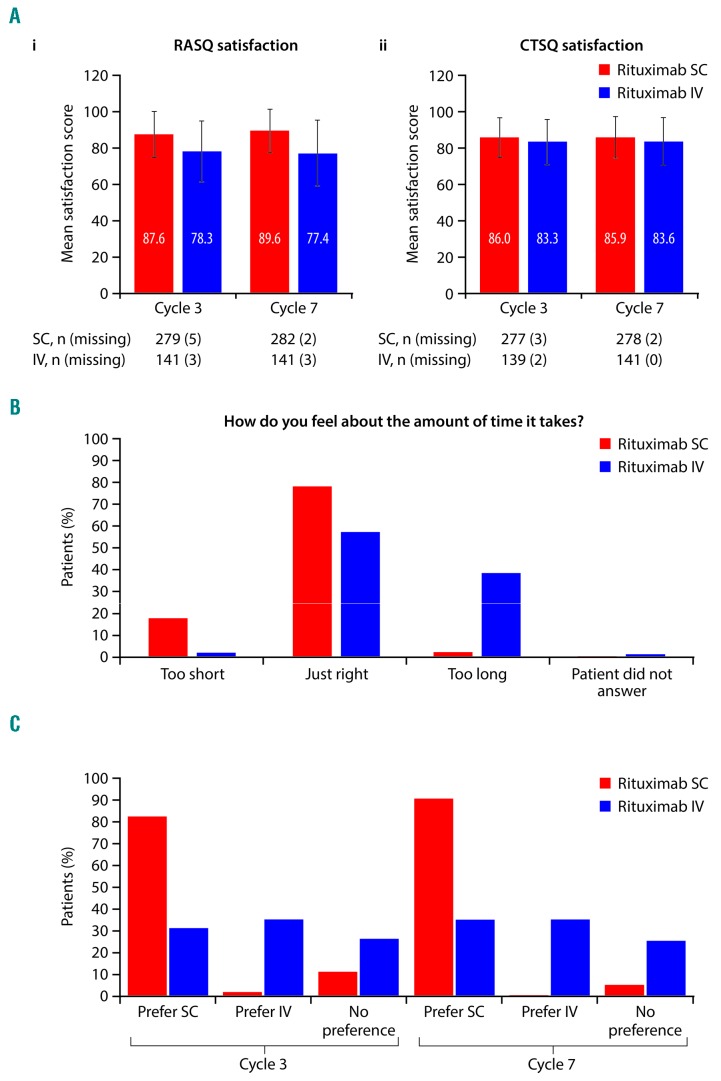
Mean RASQ scores for ‘impact on activities of daily living’, ‘convenience’, and ‘satisfaction’ were improved with SC versus IV rituximab. Overall, 428 patients (SC, 284; IV, 144) completed the RASQ at cycle 7 and were included in the RASQ analysis. The mean RASQ scores were higher across all domains for rituximab SC versus IV (Table 3), with mean satisfaction scores of 89.6 and 77.4 for the SC and IV groups, respectively (Figure 3). More patients in the rituximab SC group versus the IV group thought that the length of time taken for the SC injection/IV infusion was ‘just right’ (78.9% SC vs. 57.6% IV). When patients in the SC group were asked which treatment they would prefer, if given the option, 90.8% stated a preference for SC over IV.
Table 3.
Mean (SD) RASQ and CTSQ scores at cycles 3 and 7 (ITT RASQ and CTSQ populations).
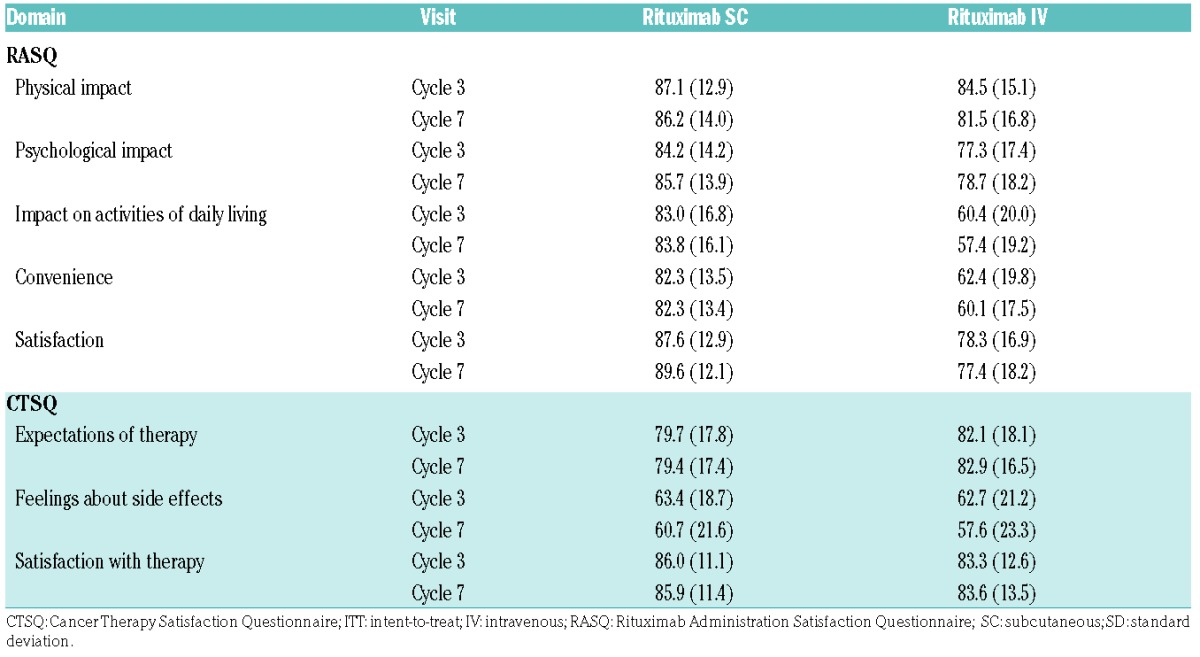
Figure 3.
Patient satisfaction and preference. (A) Patient satisfaction assessed by (i) RASQ and (ii) CTSQ at cycles 3 and 7, (B) Time taken to receive SC injection/IV infusion (RASQ individual question) at cycle 7, (C) Treatment preferences (RASQ individual question) at cycles 3 and 7. CTSQ: Cancer Therapy Satisfaction Questionnaire; IV: intravenous; RASQ: Rituximab Administration Satisfaction Questionnaire; SC: subcutaneous.
CTSQ scores were similar between arms (Figure 3 and Table 3). A total of 421 patients were included in the CTSQ analysis, with 421 (280 SC and 141 IV) completing the questionnaire at cycle 7. The mean CTSQ satisfaction score and scores for individual domains were similar between the treatment arms (Table 3). RASQ and CTSQ results for cycle 3 were similar to those for cycle 7.
Time savings
The median administration time (cycles 2–8) was substantially shorter for SC (6 minutes) than IV rituximab (range: 2.6 to 3.0 hours). Chair/bed and overall hospital times were also shorter with SC treatment. In cycle 2, 82.9% of patients in the SC arm had a chair/bed time ≤4 hours, whereas 61.2% in the IV arm had a chair/bed time ≥4 hours. For each cycle from cycle 2 onwards, a higher proportion of SC than IV patients spent <2 hours in a chair/bed receiving rituximab (ranges: 27%–56% SC vs. <1%–5% IV). In cycle 2, 64.8% of SC patients required ≤6 hours of hospital time overall, whereas 51.6% of those receiving rituximab IV required ≥6 hours.
Discussion
MabEase was a randomized, phase IIIb study designed to exclude major differences in efficacy and safety between rituximab SC and IV treatment arms in newly diagnosed DLBCL patients treated with R-CHOP. The primary endpoint results indicated similar efficacy of the rituximab SC and IV formulations in our overall study population.
We note that patient demographics in MabEase differed from those in many key trials of R-CHOP in DLBCL patients. For example, the patient population in the MabThera International Trial group (MInT) study was younger (18–60 years), with low IPI risk scores,4 whereas the populations in the LNH98.53 LNH03-6B,26 and rituximab with CHOP over age 60 years (RICOVER-60)8 studies were older (60–80 years). However, we suggest that comparisons of outcomes with these studies, although indirect, are valid and informative once demographic differences are taken into consideration.
Overall, previous trials of R-CHOP regimens including rituximab IV reported CR/CRu rates ranging from 58% to 86% in patients with previously untreated DLBCL.3,4,8,26,27 The CR/CRu rates in our study (SC, 50.6%; IV, 42.4%) were lower, which may relate to the number of patients in the ITT population who did not complete the planned course of treatment. The MabEase ITT population included all patients who completed a baseline assessment and at least one on-treatment efficacy assessment, with the first efficacy assessment conducted at interim staging at the end of cycle 4. However, approximately 18% of patients discontinued study treatment before the end of cycle 8, predominantly because of AEs. For SC and IV patients who completed all eight cycles of induction, CR/CRu rates were 57% and 47%, respectively. In addition, distinguishing PR from CRu using a CT scan alone is difficult. Current criteria recommend using PET scans where possible in order to better delineate disease extent and treatment response in DLBCL.28 However, due to limited PET availability, the MabEase study protocol prespecified the use of CT scans only for all tumor assessments. This may also have contributed to the apparently low CR rate. Another limitation was the lack of centralized radiologic review. For these aforementioned reasons the CR rates in MabEase should be interpreted with caution. Despite the lower CR rate, the overall response rate in our study (CR/CRu plus PR; approximately 80%) was similar to observations in previous studies.3,4,8,26
Some trends towards higher CR/CRu rates were seen with SC treatment in some subgroups, but these did not translate into improvements in PFS or EFS, with a small number of subgroups showing increased rates of progression, relapse or death, or increased event rates. We note that these analyses were exploratory only, however, and that patient numbers in the subgroups were too small to permit any conclusions to be drawn. In particular, very few patients (approximately 10%) received CHOP-14 in MabEase, and it is therefore not possible to draw meaningful comparisons with response rates in the CHOP-21 or overall populations. Survival results overall were similar for both formulations in the ITT population.
In MabEase, similar safety profiles were observed in the SC and IV arms. There were no new safety signals, and the rate of treatment-related deaths was comparable with rates reported in other studies.3,8,26 ARRs with rituximab have been well characterized in previous studies, particularly during cycle 1.29 To minimize ARR risk, rituximab was infused at a low initial rate, which was then increased incrementally. All patients received rituximab IV during cycle 1. Consistent with the reported SparkThera (phase Ib) and SABRINA (phase III) studies,14–16 we observed a higher rate of ARRs with rituximab SC versus IV. As expected, injection site reactions were more common with SC than with IV treatment, but these were mostly mild/moderate (< grade 3) and manageable.
The most frequent SAE in this study was FN (SC, 11.7%; IV 6.4%), although there was no difference between groups in rates of treatment discontinuation due to AEs or infections. FN and grade 3 neutropenia were more frequently reported in the rituximab SC arm, and we do not have a comprehensive explanation for this observation. However, in DLBCL studies with rituximab dose intensification, and thus higher rituximab serum levels, more neutropenia and/or FN were also reported.30,31 Compared with other studies in DLBCL, the overall incidence of FN in our study (9.9%) was similar to that reported by Cunningham et al. among patients receiving the CHOP-21 regimen (11%),27 and to specific analysis of FN among the DLBCL cohort of the PrefMab crossover phase IIIb study in which patients with DLBCL or FL received rituximab SC and IV in different sequences (9.4%; data not reported by Rummel et al.).24 Analysis of patient subgroups showed a trend towards higher incidence of AEs and SAEs in patients with low BSA. These effects were not significant, however, and no significant interaction effect was found for AEs of grade ≥3 or SAEs for any of the covariates (BSA, age, or sex).
CTSQ results showed that patients had similar levels of satisfaction with the R-CHOP treatment when used with either SC or IV rituximab, which is consistent with CTSQ data obtained in the PrefMab study.24 However, RASQ data suggested that most rituximab SC patients, given the option, preferred to have the SC injection over the IV infusion; again this finding reiterates the preference for rituximab SC expressed by 80.7% of patients in PrefMab.24 Consistent with our findings, in PrefMab, rituximab SC scored more highly for satisfaction with therapy (87.5% vs. 75.0% for IV), impact on activities of daily living and convenience of therapy (both 83.3% vs. 58.3%).24 Of note, the CTSQ was designed for use in a wide range of cancer types and stages.32 In contrast, the RASQ was developed specifically for the assessment of patients’ perceptions of the impact of treatment administration route.33
The use of rituximab SC resulted in substantial savings in clinic time. These findings concur with a time and motion analysis based on data collected within the MabCute study34 in patients with indolent NHL, which evaluated aspects of SC and IV administration of rituximab in real-world clinical practice.13 Reductions in chair time could potentially reduce waiting lists, increase the efficiency of oncology units, and increase the availability of appointments. In addition, the healthcare practitioner time gained could be deployed in other activities. Cost minimization data from The Netherlands also indicate the potential for cost savings with rituximab SC when compared with IV dosing.35 Although reductions in clinic time could also reduce the opportunity for healthcare professionals to provide patient support, the RASQ findings indicated that patients felt that this factor did not compromise their treatment, and that they had sufficient time to discuss their treatment with their healthcare providers.
In conclusion, the MabEase study showed no major differences between the efficacy of rituximab SC and IV therapy in treatment-naïve patients with DLBCL. Overall, safety was similar between arms but with a higher incidence of FN and injection site reactions in the rituximab SC arm. The higher RASQ scores in the rituximab SC arm suggest that patient satisfaction, convenience, and effect on daily living were improved with rituximab SC compared with IV. Attempts to improve care for patients with DLBCL, including short treatment intervals, consolidation with high-dose chemotherapy and autologous stem cell transplantation and replacement of rituximab with the type II CD20 antibody obinutuzumab, have so far been unsuccessful.29,36–38 Combined with previous evidence, the results of this study provide support for the use of rituximab SC in this setting.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/11/1913
Funding
This study was sponsored by F. Hoffmann-La Roche Ltd. Editorial support under the direction of the lead author was provided by Cheryl Wright, PhD and Susan Browne, PhD, of Gardiner-Caldwell Communications (Macclesfield, UK) and funded by F. Hoffmann-La Roche Ltd.
References
- 1.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–125. [DOI] [PubMed] [Google Scholar]
- 2.Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii76–82. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. [DOI] [PubMed] [Google Scholar]
- 5.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417–1423. [DOI] [PubMed] [Google Scholar]
- 6.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. [DOI] [PubMed] [Google Scholar]
- 7.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. [DOI] [PubMed] [Google Scholar]
- 8.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–116. [DOI] [PubMed] [Google Scholar]
- 9.Lundin J, Kimby E, Bjorkholm M, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood. 2002;100(3):768–773. [DOI] [PubMed] [Google Scholar]
- 10.Pivot X, Gligorov J, Muller V, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962–970. [DOI] [PubMed] [Google Scholar]
- 11.Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013;109(6): 1556–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stilgenbauer S, Zenz T, Winkler D, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27(24):3994–4001. [DOI] [PubMed] [Google Scholar]
- 13.De Cock E, Kritikou P, Sandoval M, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One. 2016;11(6):e0157957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies A, Merli F, Mihaljevic B, et al. Pharmacokinetics and safety of subcutaneous rituximab in follicular lymphoma (SABRINA): stage 1 analysis of a randomised phase 3 study. Lancet Oncol. 2014;15(3):343–352. [DOI] [PubMed] [Google Scholar]
- 15.Davies AJ, Merli F, Mihaljevic B, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol. 2017;4(6):e272–e282. [DOI] [PubMed] [Google Scholar]
- 16.Salar A, Avivi I, Bittner B, et al. Comparison of subcutaneous versus intravenous administration of rituximab as maintenance treatment for follicular lymphoma: results from a two-stage, phase IB study. J Clin Oncol. 2014;32(17):1782–1791. [DOI] [PubMed] [Google Scholar]
- 17.Assouline S, Buccheri V, Delmer A, et al. Pharmacokinetics and safety of subcutaneous rituximab plus fludarabine and cyclophosphamide for patients with chronic lymphocytic leukaemia. Br J Clin Pharmacol. 2015;80(5):1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assouline S, Buccheri V, Delmer A, et al. Pharmacokinetics, safety, and efficacy of subcutaneous versus intravenous rituximab plus chemotherapy as treatment for chronic lymphocytic leukaemia (SAWYER): a phase 1b, open-label, randomised controlled non-inferiority trial. Lancet Haematol. 2016;3(3):e128–e138. [DOI] [PubMed] [Google Scholar]
- 19.MabThera Summary of Product Characteristics. European Medicines Agency; 2017. [updated 6 July 2017; cited 25 September 2017]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdf Last accessed 25 September 2017. [Google Scholar]
- 20.Rituxan Highlights of Prescribing Information. US Food and Drug Administration; 2012. [updated October 2012; cited 25 September 2017]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103705s5367s5388lbl.pdf Last accessed 25 September 2017. [Google Scholar]
- 21.MabThera 1400 mg Solution for Subcutaneous Injection. European Medicines Agency; 2017. [updated 15 August 2017; cited 25 September 2017]. Available from: <http://www.medicines.org.uk/emc/medicine/28732/SPC/MabThera+1400+mg+Solution+for+Subcutaneous+Injection> Last accessed 25 September 2017. [Google Scholar]
- 22.Rituximab (MabThera SC) Australian Public Assessment Report. Therapeutic Goods Administration; 2014. [updated 13 October 2014; cited 25 September 2017]. Available from: https://www.tga.gov.au/auspar/auspar-rituximab-3 Last accessed 25 September 2017. [Google Scholar]
- 23.Rituxan SC Product Monograph. Hoffmann-La Roche Ltd.; 2016. [updated 9 September 2016; cited 25 September 2017]. Available from: http://www.rochecana-da.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/RituxanSC/RituxanSC_PM_E.pdf Last accessed 25 September 2017. [Google Scholar]
- 24.Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28(4):836–842. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 26.Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14(6):525–533. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817–1826. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs. 2010;14(2):E10–21. [DOI] [PubMed] [Google Scholar]
- 30.Murawski N, Pfreundschuh M, Zeynalova S, et al. Optimization of rituximab for the treatment of DLBCL (I): dose-dense rituximab in the DENSE-R-CHOP-14 trial of the DSHNHL. Ann Oncol. 2014;25(9):1800–1806. [DOI] [PubMed] [Google Scholar]
- 31.Lugtenburg PJ, de Nully Brown P, van der Holt B, et al. Randomized phase III study on the effect of early intensification of rituximab in combination with 2-weekly CHOP chemotherapy followed by rituximab or no maintenance in patients with diffuse large B-cell lymphoma: Results from a HOVON-Nordic Lymphoma Group study. J Clin Oncol. 2016;34(suppl; abstr 7504). [Google Scholar]
- 32.Trask PC, Tellefsen C, Espindle D, Getter C, Hsu MA. Psychometric validation of the cancer therapy satisfaction questionnaire. Value Health. 2008;11(4):669–679. [DOI] [PubMed] [Google Scholar]
- 33.Rule S, Briones J, Smith R, et al. Preference for rituximab subcutaneous (SC) and intravenous (IV) among patients with CD20+ Non-Hodgkin’s Lymphoma (NHL) completing the RASQ measure in randomized phase III studies Prefmab and Mabcute. Value Health. 2014;17(7):A537. [DOI] [PubMed] [Google Scholar]
- 34.Rule S, Briones J, Carella AM, et al. A randomized comparison of maintenance therapy with subcutaneous rituximab for 2 years versus until progression in patients with indolent non-Hodgkin’s lymphoma: interim safety data from the Mabcute Study. Blood. 2013;122(21):3052. [Google Scholar]
- 35.Bax P, Postma MJ. Cost-minimization of MabThera intravenous versus subcutaneous administration. Value Health. 2013;16:A390–A391. [Google Scholar]
- 36.Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369(18):1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz N, Nickelsen M, Ziepert M, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13(12):1250–1259. [DOI] [PubMed] [Google Scholar]
- 38.Vitolo U, Trnêný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017. August 10.1200/JCO.2017733402 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.