SIPP mediates self-incompatibility in Nicotiana and interacts with StEP in mitochondria of pollen tubes.
Abstract
In Solanaceae, the S-specific interaction between the pistil S-RNase and the pollen S-Locus F-box protein controls self-incompatibility (SI). Although this interaction defines the specificity of the pollen rejection response, the identification of three pistil essential modifier genes unlinked to the S-locus (HT-B, 120K, and NaStEP) unveils a higher degree of complexity in the pollen rejection pathway. We showed previously that NaStEP, a stigma protein with homology with Kunitz-type protease inhibitors, is essential to SI in Nicotiana spp. During pollination, NaStEP is taken up by pollen tubes, where potential interactions with pollen tube proteins might underlie its function. Here, we identified NaSIPP, a mitochondrial protein with phosphate transporter activity, as a novel NaStEP-interacting protein. Coexpression of NaStEP and NaSIPP in pollen tubes showed interaction in the mitochondria, although when expressed alone, NaStEP remains mostly cytosolic, implicating NaSIPP-mediated translocation of NaStEP into the organelle. The NaSIPP transcript is detected specifically in mature pollen of Nicotiana spp.; however, in self-compatible plants, this gene has accumulated mutations, so its coding region is unlikely to produce a functional protein. RNA interference suppression of NaSIPP in Nicotiana spp. pollen grains disrupts the SI by preventing pollen tube inhibition. Taken together, our results are consistent with a model whereby the NaStEP and NaSIPP interaction, in incompatible pollen tubes, might destabilize the mitochondria and contribute to arrest pollen tube growth.
Cross-pollination has decisively contributed to the widespread distribution of angiosperms, and in many species the pistil has played an active role in the rejection of self-pollen and the acceptance of pollen coming from genetically unrelated plants. Thus, the pistil has evolved to some extent to safeguard the species identity as well as to produce a vigorous progeny with new allelic combinations.
Several species avoid self-fertilization through self-incompatibility (SI), a genetically controlled system by the polymorphic S-locus (de Nettancourt, 2001). In Solanaceae, Plantaginaceae, and Rosaceae, the S-locus includes two tightly linked genes: the male and female determinants. The product of the female determinant is a pistil extracellular glycoprotein known as S-RNase (Anderson et al., 1986; McClure et al., 1989). S-RNases are secreted to the stylar extracellular matrix and incorporated into both compatible and incompatible pollen tubes (Luu et al., 2000; Goldraij et al., 2006), apparently using an MdABCF transporter localized at the pollen tube membrane as described in apple (Malus domestica; Meng et al., 2014).
Once the S-RNases are inside the pollen tubes, large amounts of these enzyme molecules are compartmentalized inside the vacuoles. If the cross is incompatible, vacuoles break down, releasing S-RNases into the cytoplasm, RNA is hydrolyzed, and the pollen tube stops growing. In contrast, if a compatible cross takes place, the S-RNases remain confined in intact vacuoles, and the pollen tubes can grow toward the ovary (Goldraij et al., 2006).
The male S-determinant encodes the cytosolic protein called S-locus F-box (SLF; Lai et al., 2002; Entani et al., 2003; Ushijima et al., 2003; Sijacic et al., 2004; Wang et al., 2004). An important characteristic of SLF is an F-box domain at the N terminus. F-box proteins are a component of the SCF (Skp1, Cullin-1, and F-box protein) E3 ligase complex, which is involved in ubiquitin-mediated protein degradation by the 26S proteasome (Qiao et al., 2004; Hua and Kao, 2008; Williams et al., 2015). Within the SCF complex, Cullin-1 is a scaffold and Skp1 connects the scaffold to an F-box protein, which, in turn, recruits the target protein (Vierstra, 2003; Xu et al., 2009).
Several SLF genes have been identified at the S-locus in Solanaceae and Rosaceae, subfamily Maloidea (Wang et al., 2004; Wheeler and Newbigin, 2007; Ashkani and Rees, 2016). In particular, in S2- and S3-haplotypes of Petunia inflata, 17 SLF genes have been found to aid the recognition of several S-RNase variants (Sijacic et al., 2004; Kubo et al., 2010; Williams et al., 2015). Based on the specificity of these interactions, multiple SLF proteins expressed in a specific pollen S-haplotype have been proposed to collaboratively recognize and detoxify nonself S-RNases, allowing only self S-RNases to exert their cytotoxic effect on self pollen (Kubo et al., 2010; Williams et al., 2015).
Although the interaction between SLF and S-RNase defines the S-specific pollen rejection, there are modifier genes unlinked to the S-locus that also are essential to the SI response (de Nettancourt, 2001; Zhang and Xue, 2008; McClure et al., 2011). To date, three pistil modifier genes have been identified: 120K (Hancock et al., 2005), HT-B (McClure et al., 1999), and NaStEP (Jiménez-Durán et al., 2013).
The HT-B protein presents a C-terminal domain rich in Asn and Asp (McClure et al., 1999; Kondo and McClure, 2008). HT-B is expressed only in mature pistils and has been described in Solanum, Nicotiana, and Petunia spp. (McClure et al., 1999; Kondo et al., 2002; O’Brien et al., 2002; Sassa and Hirano, 2006; Puerta et al., 2009). In the particular case of Solanum habrochaites, there is no HT-B gene, but there is a related HT-A gene, which may act as a substitute for the HT-B function in this species (Covey et al., 2010). Immunolocalization assays show that HT-B, like S-RNases, is taken up by compatible and incompatible pollen tubes during pollination (Goldraij et al., 2006). In incompatible crosses, HT-B levels decrease slightly in pollen tubes; however, in compatible crosses, HT-B levels inside pollen tubes decrease by 75% to 97% (Goldraij et al., 2006; Jiménez-Durán et al., 2013). Apparently, HT-B is needed to halt pollen tube growth, and in agreement, the down-regulation of HT genes results in the breakdown of SI in Nicotiana (McClure et al., 1999), Petunia (Puerta et al., 2009), and Solanum (Kondo et al., 2002; O’Brien et al., 2002) spp.
The arabinogalactan glycosylated protein 120K accumulates abundantly in the extracellular matrix in mature styles of Nicotiana alata (Schultz et al., 1997); like S-RNases, 120K is taken up by pollen tubes and targeted to vacuoles (Lind et al., 1996; Goldraij et al., 2006). Loss-of-function assays show that 120K is essential to SI, because its suppression by RNA interference (RNAi) disrupts self-pollen rejection (Hancock et al., 2005). Protein-protein interaction experiments gave evidence of 120K complexes with style proteins, including S-RNases, NaPELP III, Nap11 (Cruz-García et al., 2005), and the pollen C2 domain-containing protein NaPCCP. This last protein also associates with the endomembrane system via phosphatidylinositol 3-phosphate (Lee et al., 2008, 2009).
NaStEP (N. alata Stigma-Expressed Protein) is an abundant stigma-specific protein of SI Nicotiana spp. (Busot et al., 2008). In mature papillary stigmatic cells, NaStEP remains stored in the vacuoles, but upon pollination, the cell wall of these papillary cells becomes punctured and NaStEP relocalizes to the stigmatic exudate (Busot et al., 2008), and from there it can be taken up by compatible and incompatible pollen tubes (Jiménez-Durán et al., 2013). NaStEP is homologous to Kunitz-type protease inhibitors (Busot et al., 2008) and inhibits subtilisin in vitro, in a specific manner (Jiménez-Durán et al., 2013). RNAi-mediated suppression of NaStEP prevents S-specific pollen rejection (Jiménez-Durán et al., 2013). Likewise, NaStEP protects HT-B stability in pollen tubes by a yet unidentified mechanism, because when NaStEP is absent, HT-B is degraded inside pollen tubes in both compatible and incompatible crosses (Jiménez-Durán et al., 2013). This last evidence, suggests an interaction of these two modifier genes at some point of the pollen rejection pathway in Nicotiana spp., which currently is vaguely known. Consequently, it becomes important to determine if additional pollen proteins are required by NaStEP to exert its function in pollen rejection.
Here, a mitochondrial NaStEP-interacting protein was identified and designated as NaSIPP (N. alata Self-Incompatibility Pollen Protein), and convincing evidence of the ability of NaSIPP to recruit NaStEP to the mitochondria in pollen tubes is provided. In addition, NaSIPP transcript was detected specifically in mature pollen of SI and SC (self-compatible) Nicotiana spp. Notably, the NaSIPP orthologs in SC species have accumulated extensive mutations in the coding region, so that the encoded product is unlikely to produce a functional protein. According to these data and further evidence given below, NaSIPP represents a novel mitochondrial protein with phosphate carrier activity that is essential to SI.
RESULTS
Identifying NaStEP Pollen Protein Partners
To identify pollen and pollen tube proteins possibly interacting with NaStEP, we performed a yeast two-hybrid assay using NaStEP as bait to screen a Nicotiana rastroensis pollen/pollen tube cDNA library fused to a transcription factor activation domain (AD), according to Fields and Song (1989). Positive clones were selected under stringent conditions for further analysis.
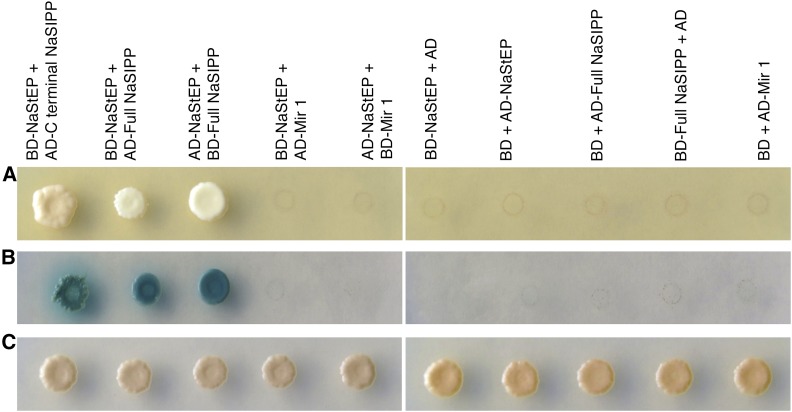
From the above experiment, we recovered a cDNA encoding the C-terminal end of an MPC (Mitochondrial Phosphate Carrier)-like protein. To confirm that the interaction was mediated by the bait (NaStEP) and prey (MPC sequence) pair, we cotransformed yeast with an empty vector (Binding Domain or BD) or the bait (BD-NaStEP) and the candidate prey protein and selected positive transformants under stringent medium. From this assay, we only recovered clones coexpressing the BD-NaStEP fusion and the AD-C-terminal end of MPC-like cDNA (Fig. 1A). In addition, in a similar assay, NaStEP was not found to interact with Mir1, an MPC from Saccharomyces cerevisiae.
Figure 1.
NaStEP interacts with the pollen protein NaSIPP. Interaction between NaStEP and NaSIPP was detected by a yeast two-hybrid assay. A, Cotransformed S. cerevisiae growing on the quadruple dropout, or QDO, medium (synthetic dextrose [SD]/Trp-Leu-Ade-His). B, Yeast growth on QDO medium supplemented with 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (X-α-Gal) and aureobasidin; positive interactions support growth and turn blue. C, Yeast growth on double dropout, or DDO, medium (SD/Trp-Leu). Full NaSIPP, The whole NaSIPP protein; Mir1, MPC of S. cerevisiae. BD and AD empty vectors were used as negative controls.
The cDNA coding the C-terminal end of MPC-like was 431 bp long. Northern-blot analysis using this cDNA as probe showed the accumulation of this MPC-like transcript specifically in mature N. rastroensis pollen (Supplemental Fig. S1). Therefore, we cloned its full-length cDNA by 5′ RACE from N. rastroensis and N. alata. The resulting sequence was named NaSIPP.
When tested by yeast two-hybrid assay, the full-length NaSIPP cDNA remained positive for interaction with NaStEP. Therefore, the NaSIPP C terminus has enough exposure in the complete protein to account for its interaction with NaStEP.
NaSIPP Belongs to the Phosphate Carrier Family
According to a multiple sequence alignment of NaSIPP and other MPC sequences from both functionally characterized and putative MPCs (Supplemental Fig. S2A), NaSIPP belongs to the MPC subfamily within the mitochondrial carrier family, which includes a number of membrane proteins known to transport solutes across the mitochondrial membranes (Palmieri et al., 2011). Three tandem repeats of a domain, known as mitochondrial carrier domain (PROSITE PS50920, PFAM PF00153, and IPR00193; Palmieri, 2004) are conserved in the primary sequences of all mitochondrial carrier family members. Each domain is about 100 amino acids long and contains two hydrophobic transmembrane segments connected through a hydrophilic loop and is characterized by the sequence motif PX[D/E]XX[K/R]X[K/R] (20–30 residues) [D/E]GXXXX[W/Y/F][K/R]G (Palmieri, 1994, 2004). This signature has been used to identify mitochondrial carriers in eukaryotic sequenced genomes (Palmieri, 1994, 2004).
A phylogenetic analysis of 21 MPC sequences from yeast, plants, and animals based on amino acid sequences (Supplemental Fig. S2B) clearly defined a plant and animal clade. Notably, this sequence comparison shows S. cerevisiae Pic2 as a closer relative to the animal and plant MPC than to Mir1, a second homolog encoded in the S. cerevisiae genome.
The MPC plant sequences could be further separated into three subgroups: (1) legume MPC (Glycine max and Medicago truncatula), sharing 95.3% sequence identity; (2) Solanaceae MPC (five sequences sharing 84.8% sequence identity); and (3) a diverse set of plant sequences from different plant families that were clustered together, although they do not form a well-defined group. The Solanaceae subgroup is represented by five MPCs, and the higher identity is between the two carriers from Solanum spp., with 99.2% identity, follow by the Nicotiana spp. carriers, which share 98.8% identity. NaSIPP formed part of this subgroup and presents a high identity with a Nicotiana tomentosiformis MPC, followed by the Solanum spp. carriers (95.3%) and the Ipomoea spp. MPC (88.3%).
Expression of NaSIPP Rescues the Mitochondrial Defect of the Yeast Mutant Δmir1
Because the NaSIPP sequence has MPC protein family features, we evaluated its ability to complement the Δmir1 mutant of S. cerevisiae. The amino acids known to be required for phosphate transport in Mir1 are His-32, Lys-42, Thr-43, Thr-79, Lys-90, Glu-126, Arg-140, Arg-142, Lys-179, Lys-187, Asp-236, and Arg-276. When these residues are mutated, the ability to transport phosphate is suppressed (Briggs et al., 1999; Phelps et al., 2001; Wohlrab et al., 2002). Sequence alignment of NaSIPP with multiple functional and putative MPCs showed high conservation in these 12 residues for NaSIPP (Supplemental Fig. S2A), in agreement with its possible phosphate transport function.
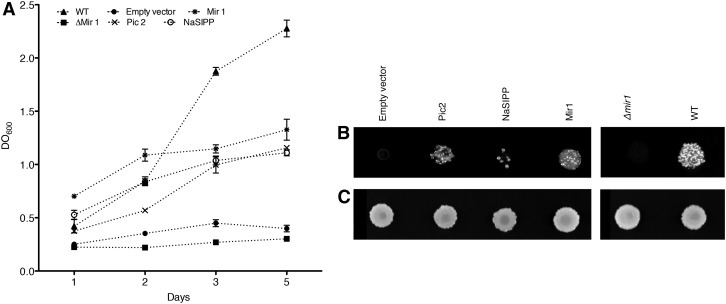
In S. cerevisiae, there are two functionally redundant MPCs, Mir1 and Pic2. Mir1 is more abundant than Pic2 under normal conditions (Murakami et al., 1990), whereas the expression of Pic2 is induced by high temperature (Hamel et al., 2004). NaSIPP shares 50% identity with Pic2 and 40.3% with Mir1, which offered an opportunity to evaluate whether NaSIPP rescues the Δmir1 mutant. To test this, we transformed the Δmir1 S. cerevisiae strain with NaSIPP using the yeast expression vector pYES-DEST52. As shown in Figure 2, NaSIPP partially rescued the Δmir1 mutant, although transformants grew slower in glycerol (a nonfermentable substrate) compared with those transformed by Mir1. According to this result, NaSIPP can provide phosphate transport to the yeast mutant, but with reduced efficiency. Differences between NaSIPP and Mir1 in kinetic or regulatory properties, or the absence of some unidentified factor, may limit NaSIPP function in yeast, but that issue was beyond the scope of this study.
Figure 2.
NaSIPP is a phosphate transporter and partially complements the absence of Mir1 in S. cerevisiae. A, Growth curve of the yeast mutant Δmir1, the wild-type (WT) strain, and the Δmir1 yeast transformed with the plasmid pYES-DEST52 (empty vector) and with construct Pic2::pYES-DEST52, NaSIPP::pYES-DEST52, and Mir1::pYES-DEST52. Yeast were grown on liquid glycerol medium at 30°C. B, Yeast were grown on solid glycerol medium at 30°C for 10 d. C, Yeast replica plated on solid Glc medium were incubated for 3 d at 30°C.
NaSIPP Three-Dimensional Model
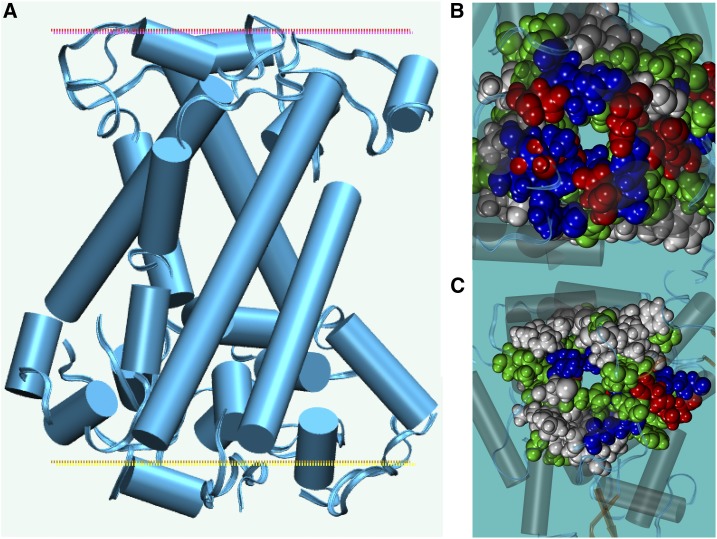
Mitochondrial carrier members have divergent sequences (15%–20% identity) but share predicted membrane topologies with six transmembrane helices, as do NaSIPP and ATP/ADP translocators. The yeast mitochondrial ATP/ADP translocator was used as a template to model NaSIPP, and extensive molecular dynamics simulations in an explicit mixed-lipid membrane, with explicit water and ions, were used to improve the model (see “Materials and Methods”). The final model had an Rd.HMM score considered as highly reliable (Martínez-Castilla and Rodríguez-Sotres, 2010), similar to those found for NMR experimental solutions of protein three-dimensional structures, and the scoring method has a very low false positive rate. The predicted structure of NaSIPP had an all-α structure, forming a core of six transmembrane regions (Fig. 3A). The upper soluble domain had a discoidal shape (Fig. 3A, red dotted line), while the bottom domain was quasi-globular (Fig. 3A, yellow dotted line). Both N and C termini were on the bottom domain.
Figure 3.
Schematic representation of the predicted three-dimensional structure of NaSIPP. A, The model is shown as a cartoon from the membrane side. B and C, Translucent cartoon representations shown from the top (B), showing the region indicated by the red dotted line in A, and from the bottom (C), showing the region indicated by the yellow dotted line in A. Amino acid atoms are represented as Van der Waals spheres and colored by amino acid type: red, acidic; blue, basic; green, polar neutral; light gray, hydrophobic. These images were prepared using Visual Molecular Dynamics (Humphrey et al., 1996).
The three-dimensional model of this protein has a central channel dominated by positive (at neutral pH) and neutral polar side chains (Fig. 3B), but the entrance at the top had several negatively charged chains (Fig. 3C). In this model, Asp-298 forms a salt bridge with Arg-316, which obstructs the pore, but a pH change could allow protonation of the acidic side chains to open the gate and allow phosphate transport. Thus, the model’s structural features are consistent with the partial complementation found of the Δmir1 mutant by NaSIPP.
Subcellular Localization of NaSIPP
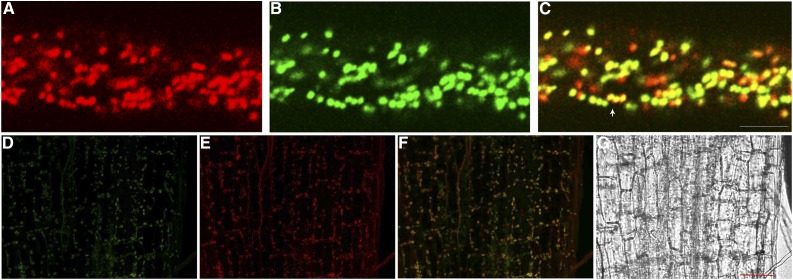
Several members of the mitochondrial carrier family localize to mitochondria, although some have been localized in the plasma membrane, specifically in the caveolae microdomain (Lisanti et al., 1994; Bàthori et al., 1999), or in small vesicles (Wandrey et al., 2004), peroxisomes, glyoxisomes, or plastids (Fukao et al., 2001; Palmieri et al., 2001; Bedhomme et al., 2005; Leroch et al., 2005). Thus, to determine the precise subcellular localization of NaSIPP, we expressed NaSIPP fused with the tomato (Solanum lycopersicum) fluorescent protein and under the control of the pollen-specific promoter Lat52 (Lat52::NaSIPP-Tomato) in transiently transformed Nicotiana tabacum pollen tubes. Furthermore, NaSIPP-Tomato signal was analyzed in these pollen tubes in the presence of the mitochondrial marker Mit-GFP (mitochondrial targeting sequence fused to GFP; Logan and Leaver, 2000). The results indicated that the NaSIPP-Tomato fluorescence signal was associated with motile organelles throughout the pollen tube cytoplasm, which displayed a movement similar to a reverse-fountain pattern (Fig. 4A; Supplemental Movie S1), characteristic of elongating pollen tubes (Cheung and Wu, 2008). Cotransformation of the mitochondrial marker Mit-GFP (Fig. 4B) showed clear overlap of NaSIPP-Tomato and Mit-GFP on the same motile organelles observed previously (Fig. 4C, yellow signal; Supplemental Movie S2), providing evidence of NaSIPP mitochondrial localization in N. tabacum pollen tubes.
Figure 4.
Subcellular localization of NaSIPP. A to C, Coexpression of NaSIPP-Tomato with the mitochondrial marker Mit-GFP. Labeled compartments in N. tabacum pollen tubes expressing NaSIPP-Tomato (red; A), Mit-GFP (green; B), and merge (yellow; C) are shown. The white arrow shows the colocalization of NaSIPP-Tomato and Mit-GFP. The pollen grains were transformed by microprojectile bombardment. Bar = 5 μm. D, Transiently transformed Arabidopsis seedlings expressing the 35S::NaSIPP-GFP construct (green) in hypocotyl cells. E, MitoTracker Red FM signal is observed in red. F, Merge of D and E (yellow). G, Bright field. Bar = 50 μm.
To obtain further support for the mitochondrial localization of NaSIPP, we transiently expressed the construct NaSIPP-GFP in Arabidopsis (Arabidopsis thaliana) seedlings via Agrobacterium tumefaciens, along with staining with the mitochondrial marker MitoTracker Red FM. We found NaSIPP-GFP fluorescence signal colocalizing with MitoTracker Red FM (Fig. 4, D–G), indicating that the necessary information to target NaSIPP to the plant mitochondria is contained within its amino acid sequence.
Interaction between NaStEP and NaSIPP in Plant Cells
To establish the interaction between NaStEP and NaSIPP in plant cells, we performed a bimolecular fluorescence complementation (BiFC) assay. We used vectors containing the Ubiquitin10 promoter that drives a moderate expression in plant cells and mitigates potential problems, such as false positive (Grefen et al., 2010).
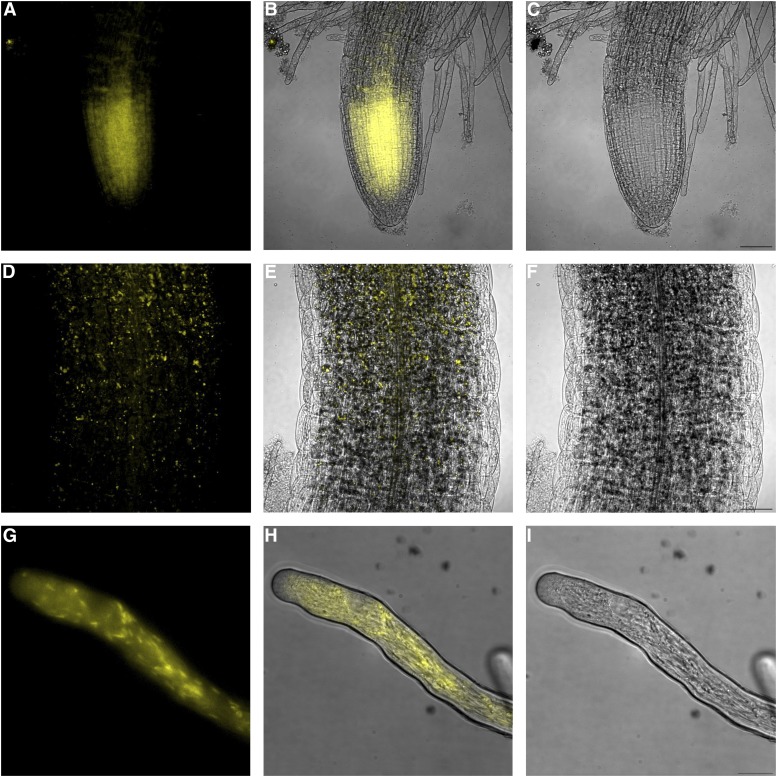
We fused each gene to the N- and C-terminal halves of yellow fluorescent protein (YFP) and used them to transform A. tumefaciens. Transient coexpression of NaStEP and NaSIPP constructs in roots and hypocotyl epidermis of Arabidopsis seedlings led to the restoration of YFP fluorescence, prominently in the meristematic and elongation zones of the roots (Fig. 5, A–C) and lower hypocotyl (Fig. 5, D–F). Fluorescence was not observed in seedlings cotransformed with either half of the split YFP vector in combination with an empty vector (Supplemental Fig. S3, A–H).
Figure 5.
NaStEP interacts with NaSIPP in plant cells. Interaction between NaStEP and NaSIPP was detected by BiFC assay. A, Coexpression of NaStEP-nYFP and NaSIPP-cYFP constructs in roots transiently transformed in Arabidopsis seedlings. B, Merge of A and C. C, Bright field. Bar = 50 μm. D, Coexpression of NaStEP-nYFP and NaSIPP-cYFP constructs in hypocotyls transiently transformed in Arabidopsis seedlings. E, Merge of D and F. F, Bright field. The YFP fluorescence is shown in yellow. Bar = 50 μm. G, Coexpression of NaStEP-NVenus and NaSIPP-CVenus constructs of N. tabacum pollen tubes transformed by microprojectile bombardment. H, Merge of G and I. I, Bright field. The Venus fluorescence is shown in yellow. Bar = 40 μm.
Additionally, we performed a BiFC assay in N. tabacum pollen tubes. We fused each gene to the N- and C-terminal halves of the Venus protein and under the control of the pollen-specific promoter Lat52. Even though the frequencies of transient expression were low, ranging from 0.001% to 0.002%, the transient coexpression of NaStEP and NaSIPP constructs in transformed N. tabacum pollen tubes led to the restoration of Venus fluorescence, mainly in small sausage-shaped organelles resembling mitochondria that were distributed throughout the pollen tube (Fig. 5, G–I). Fluorescence was not observed in pollen tubes cotransformed with either half of the split Venus vector in combination with an empty vector (Supplemental Fig. S3, I–F). These data demonstrate the NaStEP-NaSIPP interaction in vivo, both in pollen tubes and in other heterologous plant tissues.
The Interaction of NaSIPP and NaStEP Is Associated with Mitochondria
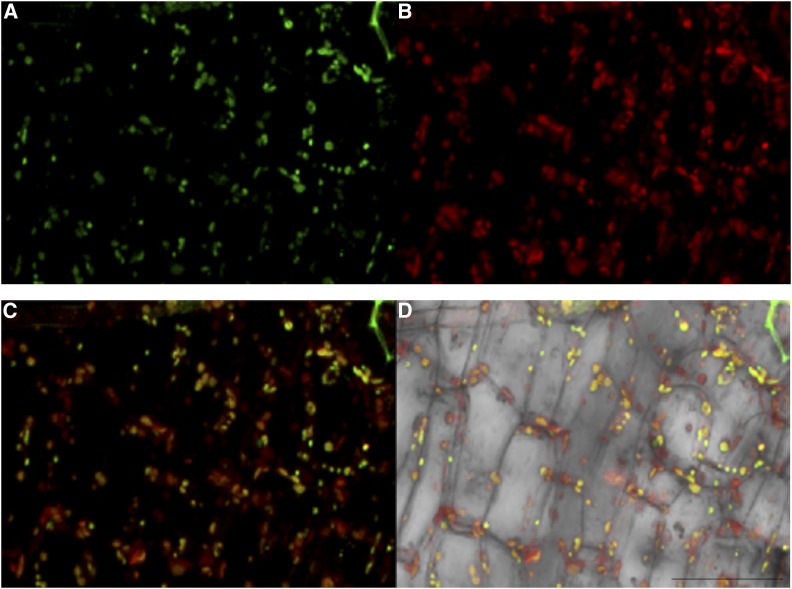
To examine if the NaSIPP-NaStEP complex associates with the mitochondria, we performed a BiFC assay in Arabidopsis seedlings, which were also treated with MitoTracker Red FM, in order to determine whether the reconstituted YFP signal could be detected in mitochondria. Figure 6A shows NaStEP-nYFP and NaSIPP-cYFP constructs coexpressed in Arabidopsis seedlings, where the YFP fluorescence was reestablished, as shown before, and how NaStEP and NaSIPP (Fig. 6A, green signal) colocalize with the MitoTracker Red FM signal coming from mitochondria (Fig. 6, B–D). Altogether, these data give evidence of an in vivo complex between NaStEP and NaSIPP located in mitochondria. The time resolution of the events is not enough to indicate whether the complex forms in the mitochondria or a preformed complex is targeted to this organelle, and this could be one of the questions to answer in the future.
Figure 6.
Physical interaction between NaStEP and NaSIPP occurs in mitochondria. Colocalization of the BiFC signal with the mitochondrial marker MitoTracker Red FM is shown in Arabidopsis seedlings. A, Localization of the interaction between NaStEP-nYFP and NaSIPP-cYFP (green) expressed in hypocotyl cells. B, MitoTracker Red FM signal is observed in red. C and D Merged signals (yellow). Arabidopsis seedlings were transiently transformed. Bar = 50 μm.
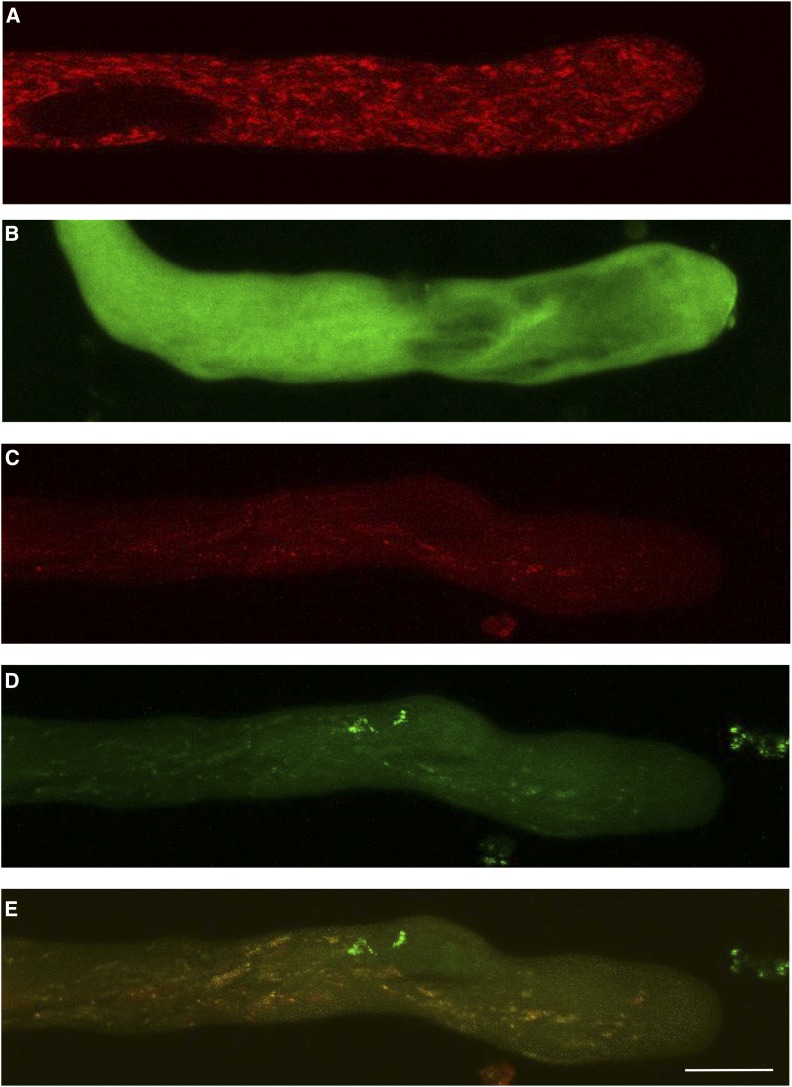
BiFC complementation provides evidence of the interaction between two proteins in vivo, but since it involves a covalent bond, the complex has an extended long life. To provide further evidence of the interaction between NaStEP and NaSIPP using a more dynamic probe, N. tabacum pollen grains were transformed by microprojectile bombardment using both constructs, Lat52::NaStEP-GFP and Lat52::NaSIPP-Tomato, and the GFP and/or Tomato fluorescence was monitored by confocal microscopy. When NaSIPP was expressed alone, the red Tomato fluorescence was distributed to discrete structures in pollen tubes (Fig. 7A). By contrast, NaStEP-GFP signal was distributed randomly in the pollen tube cytoplasm (Fig. 7B). When both proteins were coexpressed, the localization pattern of NaStEP changed from a cytoplasm distribution to a punctate pattern (Fig. 7, C–E), suggesting again a physical interaction between NaStEP and NaSIPP, which apparently mediates the translocation of the cytoplasmic NaStEP to the mitochondria.
Figure 7.
Interaction between NaStEP and NaSIPP occurs in the mitochondria of pollen tubes. A, Localization pattern of NaSIPP-Tomato (red). B, NaStEP-GFP (green). C, Coexpression of NaSIPP-Tomato with NaStEP-GFP on the red channel. D, The green channel. E, Merge (yellow). The pollen tubes were transformed by microprojectile bombardment. Bar = 10 μm.
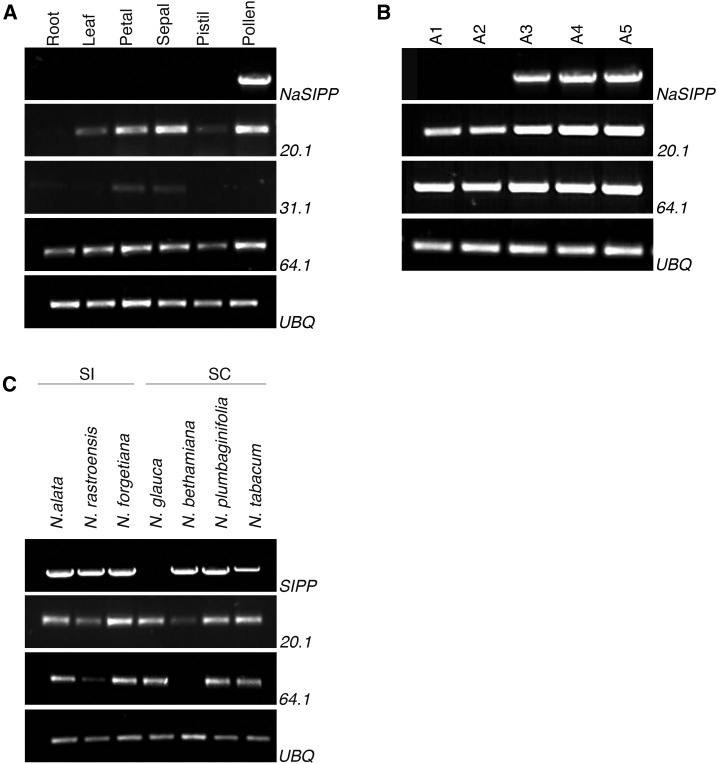
The NaSIPP Transcript Accumulates Highly in Mature Pollen of Nicotiana spp.
A BLAST analysis on the National Center for Biotechnology Information site (http://www.ncbi.nlm.nih.gov) with NaSIPP cDNA as probe found three MPC-like cDNAs of N. tabacum sharing high identity to the NaSIPP coding sequence: XM_016632920.1 (20.1), XM_016617131.1 (31.1), and XM_016600064.1 (64.1). The expression patterns of these MPC-like mRNAs were compared with those of NaSIPP transcript by reverse transcription-PCR analysis, using specific primers for each MPC-like transcript, and in several N. alata organs (Fig. 8A), developing anthers at various stages (Fig. 8B), and mature pollen from Nicotiana spp. (Fig. 8C). The results show the MPC-like 20.1 and 64.1 transcripts expressed on reproductive and no reproductive tested organs and MPC-like 31.1 mRNA detected on all organs, with the exception of pollen grains. Besides, the 20.1 and 64.1 transcripts were amplified in all the anther development stages evaluated, whereas NaSIPP mRNA was detected only in mature pollen (Fig. 8, A and B). In addition, when we tested the presence of MPC-like transcripts in mature pollen of different Nicotiana spp. (Fig. 8C), the 20.1 and 64.1 mRNAs were present in most of the Nicotiana spp. In the case of SIPP, a cDNA was amplified with high similarity to the NaSIPP cDNA in all the Nicotiana spp., with the only exception being Nicotiana glauca. Nevertheless, when all of these cDNAs were sequenced, we found insertions/deletions in the sequences from SC Nicotiana spp. (Nicotiana plumbaginifolia, N. tabacum, and Nicotiana benthamiana), and the nucleotide sequences are predicted to encode for proteins with significant differences from NaSIPP, including frame-shift mutations and/or premature stop codons. If expressed, the putative corresponding protein products are unlikely to be functional (Supplemental Fig. S4, A and B). By contrast, all the SIPP cDNAs from SI Nicotiana spp. (N. alata, N. rastroensis, and Nicotiana forgetiana) show better conservation in their amino acid sequences (Supplemental Fig. S3, A and B) and appear to encode proteins sharing all the features associated with their predicted functions, in agreement with their possible participation in the SI response.
Figure 8.
The NaSIPP transcript is accumulated specifically in mature pollen of Nicotiana spp. A, mRNA levels of different MPCs in different organs of N. alata. B, Developmental anther stages: A1, 0.5 to 1 cm; A2, 1.1 to 2 cm; A3, 2.1 to 3.5 cm; A4, 3.5 to 6 cm; A5, 6 cm, mature flower. C, Detection of transcripts in different genetic Nicotiana spp. backgrounds. Primers used were specific for the genes shown at the right: NaSIPP, XM_0166322920.1 (20.1), XM_016649631.1 (31.1), and XM_016600064.1 (64.1). Ubiquitin (UBQ) was used as a loading control.
NaSIPP Suppression in Pollen Tubes
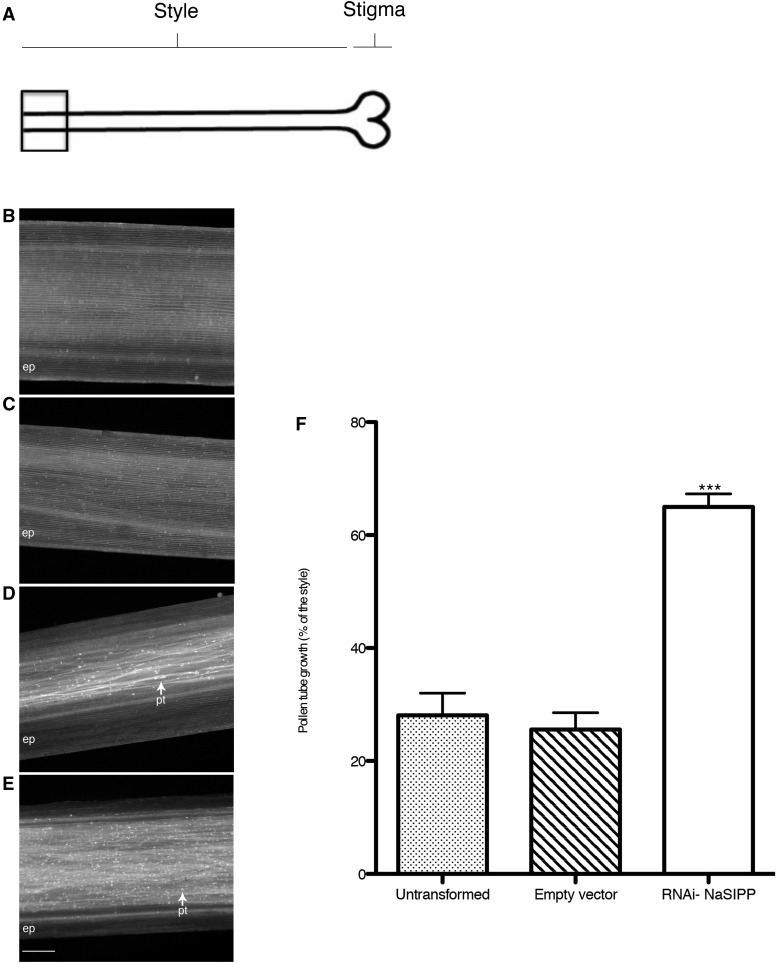
To test if NaSIPP plays a role in SI, we transformed N. alata SA2 pollen by microprojectile bombardment with the construct RNAi-NaSIPP under the control of the NTP303 promoter (NTP303p). The transformed pollen grains were used to pollinate SI N. alata SA2SA2 pistils (incompatible cross), and we evaluated the effect on pollination, observing pollen tube growth through the style after 72 h of pollination (Fig. 9A). If NaSIPP is playing a role in pollen rejection, its suppression would allow pollen tubes to reach the base of the style in an incompatible cross (when the S-allele in pollen matches with one of the pistil S-alleles); otherwise, pollen tube growth should be inhibited in the upper one-third of the style, as happens in the SI N. alata.
Figure 9.
Suppression of NaSIPP in pollen tubes disrupts SI in N. alata. Pistils from SI N. alata were pollinated with SA2 pollen and prepared for imaging after 72 h of pollination. A, As shown in the diagram, the field of view is at or very near the base of the style. B, SI N. alata SA2SA2 pistils pollinated with untransformed SA2 pollen. Epidermal tissue (ep) also is visible. The untransformed control pollen shows normal S-allele-specific pollen rejection because no SA2 pollen tubes are evident. C, SI N. alata SA2SA2 pistils pollinated with SA2 pollen bombarded with the empty vector. D, Pollen tubes reaching the base of a style of SI N. alata SA2SA2, pollinated with SA2 pollen bombarded with the construct RNAi-NaSIPP. Pollen tubes (pt) appear as fiber with brightly stained callose plugs (arrow). E, SI N. alata SC10SC10 pistils with SA2 pollen bombarded with the construct RNAi-NaSIPP. F, Histogram of pollen tube lengths after SI N. alata SA2SA2 pistils were pollinated with untransformed SA2 pollen, with pollen grains bombarded with empty vector, and with the construct RNAi-NaSIPP. Results are averages and se of 28 pollinations with untransformed pollen grains, 67 pollinations with pollen grains transformed with the empty vector, and 89 pollinations with pollen grains transformed with the RNAi-NaSIPP construct. The error bars represent se; asterisks represent statistical significance (P < 0.05, one-way ANOVA with Dunnett’s posttest).
When SI N. alata SA2SA2 pistils were pollinated with untransformed SA2 pollen (incompatible cross), the pollen tubes did not reach the base of the style (Fig. 9B), as expected, because the pollen tube growth was inhibited in the upper segment of the style. Here, the average length of the pollen tube was equivalent to 28% of the style length (se = 3.9; n = 28 pistils analyzed; Fig. 9F; Supplemental Table S1).
Pollen transformed with the empty vector displayed a similar behavior to the wild-type pollen, and the average pollen tube growth was equivalent to 26% of the style (se = 2.9; n = 67 pistils analyzed; Fig. 9, C and F; Supplemental Tables S1 and S2).
Transformation of pollen grains with RNAi-NaSIPP resulted in a notable increase in the number of pollen tubes reaching the base of the style over those pollen tubes coming from untransformed pollen or pollen transformed with the empty vector (Fig. 9, D and F), and the average length of the pollen tube was equivalent to 64.9% of the style (se = 2.3; n = 89 pistils analyzed). Moreover, the difference was statistically highly significant (P < 0.0001; Supplemental Tables S1 and S2) between the pollen transformed with RNAi-NaSIPP and the pollen transformed with the empty vector. As predicted, RNAi made normally incompatible pollen tubes grow significantly longer, and many did reach the base of the style, suggesting that the RNAi-mediated suppression of NaSIPP expression impairs SI and giving further support to the proposed role of NaSIPP as a novel pollen modifier gene, essential to the SI response in Nicotiana spp.
On the other hand, when we pollinated SI N. alata SC10SC10 pistils with SA2 pollen bombarded with the construct RNAi-NaSIPP, most of the pollen tubes reached the base of the style (Fig. 9E), as expected for a compatible cross.
DISCUSSION
In this study, we identified a novel pollen MPC, NaSIPP, that interacts with NaStEP, and the resulting complex was found associated to the mitochondria. Likewise, evidence is provided of NaSIPP being an essential gene in the pollen rejection response in Nicotiana spp.
The interaction of NaStEP with a mitochondrial protein like NaSIPP was initially unexpected, because we previously demonstrated the role of the stigma-located NaStEP protein as a proteinase inhibitor and its ability to enhance HT-B stability inside the vacuoles of pollen tubes (Jiménez-Durán et al., 2013). However, the interaction of NaStEP with the mitochondrial membrane protein NaSIPP agrees with some reports describing Kunitz-type inhibitors interacting with membrane ion channels, which are able to induce membrane permeability changes (Lancelin et al., 1994; Harvey, 2001; Peigneur et al., 2011; García-Fernández et al., 2016).
The specific expression of NaSIPP in mature pollen and its ability to complement a phosphate transport-deficient mutant could relate this protein to energy requirements during pollen tube growth. The NaSIPP transcript was found expressed in mature pollen of both SI and SC Nicotiana spp.; however, their orthologs in SC Nicotiana spp. have accumulated frame-shifting mutations that generate premature stop codons, and even if those proteins are translated, they are unlikely to be functional (Supplemental Fig. S4B). Therefore, the expression pattern of the functional NaSIPP in SI Nicotiana spp. backgrounds and the SI disruption when NaSIPP was suppressed are strongly consistent with a key role for this protein in the SI response in Nicotiana spp., probably after its interaction with NaStEP.
Recent evidence points to some MPCs as structural components of the permeability transition pore (PTP; Leung et al., 2008; Gutiérrez-Aguilar et al., 2010; Varanyuwatana and Halestrap, 2012). The PTP is a nonspecific channel in the mitochondrial membrane (Haworth and Hunter, 1979; Crompton et al., 1987), and it is usually closed. The opening of the PTP may result from a number of stimuli, including a calcium overload of the mitochondrial matrix (Hunter and Haworth, 1979; Al-Nasser and Crompton, 1986), which triggers an increase in the mitochondrial inorganic phosphate pools (Crompton and Costi, 1988; Kushnareva et al., 1999; Arpagaus et al., 2002).
An open PTP allows an unrestricted movement of solutes, increasing the mitochondrial permeability and producing the collapse of mitochondrial membrane potential (Bhosale et al., 2015). As a result, ATP synthesis stops and a cellular energy crisis take place (Halestrap et al., 1998, 2004). If NaSIPP is a component of PTP-like, its interaction with NaStEP might be part of the mechanism of PTP opening and cause the pollen tube to stop growing as part of the S-specific pollen rejection response. Many aspects of this proposal are still hypothetical and should be challenged by further studies, some of which are in progress in our group.
The opened PTP has been shown to mediate the cytochrome c release, along with other cell death factors (Petronilli et al., 1994; Doran and Halestrap, 2000), which are early crucial events in both the animal and plant intrinsic pathway of programmed cell death (PCD) activation (Beers, 1997; Balk et al., 1999; Stein and Hansen, 1999; Sun et al., 1999; Lam and del Pozo, 2000). However, we do not know if this happens in SI in Nicotiana spp., and it would be interesting to explore in the future, since PCD has been implicated in the gametophytic pollen rejection response (Thomas and Franklin-Tong, 2004) and different hallmarks of PCD also have been reported in other species, such as Pyrus pyrifolia (Wang et al., 2009; Wang and Zhang, 2011), Olea europaea (Serrano et al., 2010), and N. alata (Roldán et al., 2012).
Although whether the SI response in Nicotiana spp. involves a PCD program has yet to be tested, the features of NaSIPP and the possibility that PCD might be part of the pollen rejection response in Nicotiana spp. make NaSIPP a good candidate for an active role if such an SI-specific cascade takes place in these species.
It is well know that interaction between S-RNase and SLF determines either the compatibility or incompatibility phenotype. But further evidence indicated the participation of HT-B, NaStEP, and NaSIPP somewhere downstream of this interaction. NaStEP enters both compatible and incompatible pollen tubes early in pollination (Jiménez-Durán et al., 2013), and considering its properties, NaStEP might play roles at the cytoplasm and at the mitochondria in the pollen rejection response. Thus, in an incompatible cross, NaStEP might function as a proteinase inhibitor protecting HT-B from degradation. On the other hand, there is evidence that NaStEP in the pollen tube interacts with NaSIPP in the mitochondria, and this interaction could create an energy crisis and contribute to pollen tube growth inhibition. Somehow in a compatible cross, the interaction between NaSIPP and NaStEP might be impaired or altered, through an unknown mechanism, related to the unspecific S-allele interaction between SLF and S-RNase. Under this last condition, we postulate that the S-RNases remain confined to intact vacuoles, HT-B would be degraded, and the mitochondria would stay healthy to provide ATP and support the growth of the pollen tubes toward the ovary.
Our future goal is to propose a comprehensive model of the SI mechanism by identifying all the factors required in the S-RNase-dependent pollen rejection pathway and their participation in the SI response-related mechanism. Here, we give evidence that NaSIPP is an essential gene for SI, and our findings suggest a possible pivotal participation of mitochondria in the SI Nicotiana spp. response.
MATERIALS AND METHODS
Plant Materials
SI Nicotiana alata (SA2SA2, SC10SC10 genotype), SC Nicotiana glauca, and SC Nicotiana tabacum ‘Praecox’ have been described previously (Murfett et al., 1994, 1996; Beecher and McClure, 2001). SC Nicotiana plumbaginifolia (inventory no. TW107) and SI Nicotiana forgetiana (inventory no. TW50) were gifts from Bruce McClure’s laboratory. SI Nicotiana rastroensis and Nicotiana benthamiana have been described previously (Jiménez-Durán et al., 2013). N. tabacum variety Petit Havana SR1 was used for pollen transformation. All the plants were grown in soil under greenhouse conditions.
Yeast Two-Hybrid Assay, Clone Identification, and Sequencing
NaStEP Bait
The cDNA of NaStEP (accession no. EU253563) was amplified using the following primers: forward, 5′-CCGGAATTCTCATCTTTCACTTCCACCAATCCCATTGTC-3′; and reverse, 5′-GCGCTGCAGTTATGCATCAGTCTTCTGGAATTTCTCGAAGAC-3′. The PCR product was cloned into a pGBKT7 vector (Clontech). Transformation of the NaStEP construct was performed in Saccharomyces cerevisiae Y2HGold cells, according to the manufacturer’s instructions (Yeastmaker Yeast Transformation System 2, Clontech).
Pollen-Pollen Tube cDNA Library
mRNA was purified from total RNA from N. rastroensis pollen and pollen tubes (germinated during 16 h at 30°C) using the PolyATtract mRNA Isolation System (Promega). Total RNA was isolated with TRIzol (Invitrogen). For cDNA library construction, a 1:1 mix of mRNA from pollen and pollen tubes was used for cDNA synthesis using the CDS III and CDS III/6 primers, according to the manufacturer’s instructions (Make Your Own Mate & Plate Library System; Clontech). The S. cerevisiae strain employed was Y187.
The cDNA library was screened using BD-NaStEP as bait. The screening was performed using yeast mating. Transformed cells were plated on QDO/X-α-gal medium (SD/-Trp-Leu-His-Ade) supplemented with 40 μg mL−1 X-α-gal, according to the manufacturer’s instructions (Matchmaker Gold Yeast Two-Hybrid System; Clontech).
Positive interactions were confirmed by yeast mating between the BD-NaStEP and AC-prey proteins and then growing on DDO and QDO/X-α-gal/aureobasidin A medium; the plates contained 125 ng mL−1 aureobasidin A, according to the manufacturer’s instructions (Matchmaker Gold Yeast Two-Hybrid System; Clontech).
Full NaSIPP cDNA Cloning
RNA was isolated from N. alata and N. rastroensis mature pollen with TRIzol (Invitrogen), and cDNA was prepared using the SMARTer RACE cDNA Amplification kit (Clontech). A full-length clone was recovered, cloned into pGEM-T-Easy, and sequenced.
Confirmation of NaStEP-Full Length NaSIPP Interaction by Yeast-Two Hybrid Analysis
The cDNAs of NaStEP and NaSIPP were amplified using the following primers: forward, 5′-CACCATGTCATCTTTCACTTCCA-3′; reverse, 5′-TGCATCAGTCTTCTGGAATTTCTC-3′; and forward, 5′-CACCATGGCCTACACACACAACT-3′; reverse, 5′-CTTGGCAGGGGCAGGTG-3′, respectively. As a negative control, Mir1 was used; the cDNA of Mir1 was amplified using the following primers: forward, 5′-CACCATGTCTGTGTCTGCT-3′; and reverse, 5′-ATGACCACCACCACCAATTTC-3′. PCR products were cloned into pENTR-D-TOPO vector (Invitrogen), according to the manufacturer’s instructions. A subsequent LR reaction was performed in pDEST32 and pDEST22 (Invitrogen) for NaSIPP, NaStEP, and Mir1. Yeast transformation of those constructs was performed in Y2HGold and Y187 cells, according to the manufacturer’s instructions (Yeastmaker Yeast Transformation System 2; Clontech).
Positive interactions were confirmed by yeast mating and then growing on DDO and QDO/X-α-gal/aureobasidin A medium; the plates contained 125 ng mL−1 aureobasidin A, according to the manufacturer’s instructions (Matchmaker Gold Yeast Two-Hybrid System; Clontech).
Transformation of Arabidopsis Seedlings
Agrobacterium tumefaciens strain GV3101 cells carrying the clones of interest were grown during a first cycle (20 h, 200 rpm, and 28°C) in 5 mL of Luria-Bertani medium with 50 μg mL−1 rifampicin and 100 μg mL−1 spectinomycin. A second cycle of growth was started by inoculation of an aliquot from the first culture at a 1:1,000 dilution in fresh medium and then culturing until late exponential growth phase (OD600 of 1.5–2). The bacteria were harvested and resuspended in 10 mm MgCl2 with 100 μm acetosyringone (Sigma-Aldrich) and incubated for 1 h. For cocultivation with Arabidopsis (Arabidopsis thaliana), the bacteria were resuspended in 0.5× Murashige and Skoog basal salt medium (Sigma-Aldrich), pH 7.2, with 0.003% Silwet-77 to a final OD600 of 0.5 (Campanoni et al., 2007).
Transformation of Pollen Grains
Pollen Tubes
Five milligrams of mature pollen grains of N. tabacum (8 × 105 cells, as counted with TC20; Bio-Rad) was used in each bombardment trial and suspended in 500 μL of pollen germination medium (Cheung et al., 2002). The pollen suspension was dispersed in 35-mm petri dishes with germination medium and solidified with 0.7% agarose. The slides were maintained in the dark in a humid chamber at 26°C to 28°C. After 8 to 12 h, the pollen tubes were observed.
Transient transformation of pollen grains was accomplished with microprojectile bombardment equipment from Bio-Rad (Biolistic PDS-1000/He) according to Chen et al. (2002) and with the manufacturer’s recommended protocol. The pollen grain samples were bombarded twice to increase the yield of transformed pollen tubes. The pollen tubes were observed directly on glass slides. On average, 25 pollen tubes were counted for each sample, unless indicated otherwise. The average transformation efficiency was roughly 0.003%.
Subcellular Localization
For subcellular localization in pollen tubes, the constructs were under the control of the pollen-specific promoter Lat52 (Twell et al., 1989): 5 μg of Lat52::NaSIPP-Tomato and 3 μg of Lat52::Mit-GFP (Logan and Leaver, 2000) were mixed with 10 μL of spermidine (0.1 m), 25 μL of CaCl2 (2.5 m), and 25 μL of tungsten particles (60 mg mL−1). The pollen grains were transformed and germinated as described above. A Nikon E800 confocal microscope was used to observe the fluorescence.
The construct 35S::NaSIPP-GFP was used to transform A. tumefaciens strain GV3101 cells, which were used for Arabidopsis seedling transformation (described above). A solution of 20 nm MitoTracker Red FM (Invitrogen) was used as a mitochondrial marker.
Complementation of the Δmir1 Yeast Mutant
Strains, Media, and Plasmids
The yeast strains BY4741 MATα; his3 Δ1; leu2 Δ0; met15 Δ0; ura3 Δ (WT) and BY4741 MATα; his3 Δ1; leu2 Δ0; met15 Δ0; ura3 Δ0; YJR077c: kanMX4 (Δmir1) were used for the complementation test. The yeast strains were a gift from Salvador Uribe-Carvajal’s laboratory.
The cDNA of Pic2 was amplified using the following primers: forward, 5′-CACCATGGAGTCCAATAAACAACC-3′; and reverse, 5′-ATAAGAATGCGGCCGCCTAACCGGTGGTTGGTAA-3′. The PCR product were cloned into pENTR-D-TOPO vector (Invitrogen). The constructs Pic2:pENTR NaSIPP:pENTR and Mir1:pENTR (described above) was used for LR recombination in pYES-DEST52 vector (Invitrogen).
Yeast transformation with the NaSIPP, Pic2, Mir1, and empty vector (pYES-DEST52) constructs was performed in Δmir1 BY4741 cells, according to the manufacturer’s instructions (Yeastmaker Yeast Transformation System 2; Clontech). The transformed yeasts were grown on selective medium (-Ura; Clontech).
Growth Conditions
The strains WT and Δmir BY4741 were incubated in yeast peptone dextrose preculture medium for 24 h at 30°C under agitation at 200 rpm, subsequently cultured in YPGal (carbon source, Gal) medium for 24 h at 30°C under agitation at 200 rpm, and finally cultured in YPGly (carbon source, glycerol) medium for 5 d at 30°C under agitation at 200 rpm.
Transformed yeasts were incubated in SD-Ura (carbon source, Glc) preculture medium for 24 h at 30°C, subsequently cultured in SGal-Ura (carbon source, Gal) medium for 24 h at 30°C under agitation at 200 rpm, and then cultured in SGly-Ura (carbon source, glycerol) medium for 5 d at 30°C under agitation at 200 rpm. The evaluation of growth was realized every day according to the OD600.
For the evaluation of growth in solid medium, an aliquot of 100 μL of the YPGal and SGal-Ura cultures was taken and spotted on plates that contained glycerol medium (YPGly and SGly-Ura) and 2% agar (w/v).
RNA Transcript Analysis
RNA was isolated with TRIzol (Invitrogen) from N. alata pistil, pollen, sepal, leaf, root, and petal materials as well as from anthers at different developmental stages (A1, 0.5–1 cm; A2, 1.1–2 cm; A3, 2.1–3.5 cm; A4, 3.5–6 cm; and A5, 6 cm, mature flower). Mature pollen RNA (stage A5) was isolated from the following species: N. alata, N. forgetiana, N. rastroensis, N. glauca, N. plumbaginifolia, N. tabacum, and N. benthamiana. cDNA was made from all RNA samples using Moloney murine leukemia virus reverse transcriptase (Sigma-Aldrich), according to the manufacturer’s instructions.
The cDNA of NaSIPP was amplified using the following specific primers: forward, 5′-GACACGGCTTCTTCTTCACCATTCTC-3′; and reverse, 5′-TCTTCACTTGGCAGGGGCA-3′. The cDNA of MPC-like from N. tabacum XM_016632920.1 (LOC107808402) was amplified using the following specific primers: forward, 5′-ATGGAGTATATTGATCCTGCAAAGTACA-3′; and reverse, 5′-TCGTGTATGGTATCTGTCGTCC-3′. The cDNA of MPC-like from N. tabacum XM_016617131.1 (LOC107823043) was amplified using the following specific primers: forward, 5′-ATGGCGTTTCCAGATAGCTCGACT-3′; and reverse, 5′-GGTGTCGGAATGACATGTTTATAGAGTTG-3′. The cDNA of MPC-like from N. tabacum XM_016600064.1 (LOC107779610) was amplified using the following specific primers: forward, 5′-ATGGAGAACTCACGCCGTCA-3′; and reverse, 5′-TTGGTAATCCATCTGACAATCCCCT-3′.
RNAi Construction, NaSIPP Suppression, and Pollination Phenotype
The NTP303 promoter was amplified using the following primers: forward, 5′-CCGGAGGTCCTGATACACTCGCAAC-3′; and reverse, 5′-CCGCTCGAGCATGACGTTGTTTTT-3′. The PCR product contains the XhoI and PpuMI restriction sites and was inserted at the RNAi vector pBADC.
The construct NaSIPP:pENTR (described above) was used for LR recombination into the RNAi vector to generate sense and antisense NaSIPP (RNAi-NaSIPP).
Freshly collected SA2 pollen grains of SI N. alata were transient transformed with 5 μg of RNAi-NaSIPP construct by microprojectile bombardment, as described above. The pollen grains were used to pollinate SA2SA2 pistils (incompatible cross) and SC10SC10 pistils (compatible cross). Effects on pollination behavior were evaluated by staining style squashes with decolorized Aniline Blue (Kho and Baer, 1968). The pollen tubes were counted, and their growth along the style was evaluated at 72 h after pollination, using an AmScope FM320T microscope.
Development of a Reliable Model for the Three-Dimensional Structure of NaSIPP
Taking advantage of the distant relationship to ATP/ADP translocators, NaSIPP models were obtained from SAMT-T08 (Karplus, 2009), I-TASSER (Zhang, 2008), and HHpred (Karplus et al., 1998)/modeler (Eswar et al., 2007) and scored for biological appropriateness using Rd.HMM (Martínez-Castilla and Rodríguez-Sotres, 2010). The most appropriate prediction (Rd.HMM) was placed in a mixed-lipid membrane (Martínez et al., 2009) and subjected to molecular dynamics simulations (NPT cubic box, TIP3P water, 0.15 m NaCl PME electrostatics, SHAKE for C-H bonds, ∆t 2 fs, AMBER 99SB-ildn force-field, 9), with the following temperature and time scheme: (1) 313 K, 100 ns; 328 K, 100 ns; (2) five rounds of 298 to 413 K heating; 413 K, 3 ns; 413 to 320 K cooling, 10 ns; 320 to 298 K cooling, 6 ns; (3) five rounds of 298 to 413 K heating, 3 ns; 398 K, 10 ns; 398 to 320 K cooling, 6 ns; 320 to 298 K cooling, 6 ns. Conformers were recovered by clustering the 320 to 298 K trajectory sections, and their energy was minimized. The conformer with the highest Rd.HMM had departed significantly from the starting template but was comparable in quality to NMR structural solutions (score of ∼0.4 times the length of the NaSIPP amino acid sequence; Martínez-Castilla and Rodríguez-Sotres, 2010).
BiFC Assay
Arabidopsis Seedlings
The NaSIPP:pENTR and NaStEP:pENTR (described above) constructs was used for LR recombination in pUBC-nYFP and pUBC-cYFP vectors (Grefen et al., 2010), yielding the NaStEP-nYFP and NaSIPP-cYFP fusion proteins. The BiFC analyses were performed by transient transformation of Arabidopsis seedlings (described above). A solution of 20 nm MitoTracker Red FM (Invitrogen) was used as a mitochondrial marker.
N. tabacum Pollen Tubes
Seven micrograms of Lat52::NaSIPP-CVenus and Lat52::NaStEP-NVenus constructs were mixed with 10 μL of spermidine (0.1 m), 25 μL of CaCl2 (2.5 m), and 25 μL of tungsten particles (60 mg mL−1). The pollen grains were transformed and germinated as described above. A fluorescence microscope was used to observe the reestablishment of Venus fluorescence.
In addition, the pollen grains were cobombarded with NaStEP-NV and empty vector (CV), or with the NaSIPP-CV and empty vector (NV), as described above. A rapid screening was performed looking for fluorescence on all the glass slides where the pollen grains were deposited. Then, a screening was carried out with selected preparations, performing detailed observations of the pollen tubes. An average of 200 pollen tubes were analyzed.
For the coexpression assays of NaStEP-GFP and NaSIPP-Tomato in pollen tubes, 5 μg of the Lat52::NaSIPP-Tomato construct and 7 μg of the Lat52::NaStEP-GFP construct were used. The transient transformation of pollen grains was done as described above.
Microscopic Observations
Confocal images were obtained on Olympus FV1000 and Nikon E800 microscopes. GFP fluorescence was excited with the 458- or 488-nm argon laser lines; YFP and Venus fluorescence was excited with the 514-nm laser line; Aniline Blue fluorescence was excited with the 390-nm laser line; MitoTracker Red FM (Invitrogen) and Tomato fluorescence was excited with the 581-nm laser line. Emitted light was collected through an NFT515 dichroic and 505- to 530-nm (GFP), 535- to 590-nm (YFP), 380- to 390-nm (Aniline Blue), and 600- to 650-nm (MitoTracker Red FM; Invitrogen) band-pass filters.
Accession Number
Sequence data from this article can be found in the GenBank database under nucleotide sequence BankIt1982585 Seq KY471417.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The NaSIPP transcript is detected specifically in mature pollen in N. rastroensis.
Supplemental Figure S2. Sequence alignment of NaSIPP, functional and putative MPCs.
Supplemental Figure S3. BiFC assay controls.
Supplemental Figure S4. Sequence alignment of different Nicotiana spp. SIPP sequences.
Supplemental Table S1. Evaluation of pollen tube length after RNAi-NaSIPP pollen transformation.
Supplemental Table S2. NaSIPP suppression disrupts S-specific pollen rejection.
Supplemental Movie S1. Lat52::NaSIPP-Tomato localization.
Supplemental Movie S2. Coexpression of Lat52::NaSIPP-Tomato and Lat52::Mit-Green.
Acknowledgments
We thank Yanjiao Zou for assistance in microprojectile bombardment, Jorge Herrera Díaz for assistance in yeast complementation assays, Javier Andres Juárez-Díaz for assistance in BiFC assays, Karina Jiménez-Durán for confocal microscopy support, Yuridia Cruz-González Zamora for technical assistance, María Teresa Olivera-Flores for greenhouse support, and Janai M. García-Valencia for assistance with imaging. We thank the anonymous reviewers for the thoughtful comments scientifically and for improving the presentation of our data. We also thank the RCN on Integrative Pollen Biology for facilitating collaboration. We thank the UNAM-DGTIC staff for help in the compilation and maintenance of the required software.
Glossary
- SI
self-incompatibility
- RNAi
RNA interference
- SC
self-compatible
- BiFC
bimolecular fluorescence complementation
- PTP
permeability transition pore
- PCD
programmed cell death
- SD
synthetic dextrose
Footnotes
This work was supported by grants IN217816 and RN217816 (PAPIIT-UNAM), 329718/234690 (to L.E.G.-V.), and 236602 from Consejo Nacional de Ciencia y Tecnología and by grants 0955910 (RCN on Integrative Pollen Biology) and 1147165 from the National Science Foundation (to A.Y.C). Computing resources were provided by the LANCAD-UNAM-DGTIC-215 supercomputing project.
Articles can be viewed without a subscription.
References
- Al-Nasser I, Crompton M (1986) The entrapment of the Ca2+ indicator arsenazo III in the matrix space of rat liver mitochondria by permeabilization and resealing: Na+-dependent and -independent effluxes of Ca2+ in arsenazo III-loaded mitochondria. Biochem J 239: 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Cornish EC, Mav SL, Williams EG, Hoggart R, Atkinson A, Boning I, Greg B, Simpson RJ, Roche PJ, et al. (1986) Cloning of cDNA for a stylar glycoprotein associated with expression of self-incompatibility in Nicotiana alata. Nature 321: 38–44 [Google Scholar]
- Arpagaus S, Rawyler A, Braendle R (2002) Occurrence and characteristics of the mitochondrial permeability transition in plants. J Biol Chem 277: 1780–1787 [DOI] [PubMed] [Google Scholar]
- Ashkani J, Rees DJ (2016) A comprehensive study of molecular evolution at the self-incompatibility locus of Rosaceae. J Mol Evol 82: 128–145 [DOI] [PubMed] [Google Scholar]
- Balk J, Leaver CJ, McCabe PF (1999) Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett 463: 151–154 [DOI] [PubMed] [Google Scholar]
- Bàthori G, Parolini I, Tombola F, Szabò I, Messina A, Oliva M, De Pinto V, Lisanti M, Sargiacomo M, Zoratti M (1999) Porin is present in the plasma membrane where it is concentrated in caveolae and caveolae-related domains. J Biol Chem 274: 29607–29612 [DOI] [PubMed] [Google Scholar]
- Bedhomme M, Hoffmann M, McCarthy EA, Gambonnet B, Moran RG, Rébeillé F, Ravanel S (2005) Folate metabolism in plants: an Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J Biol Chem 280: 34823–34831 [DOI] [PubMed] [Google Scholar]
- Beecher B, McClure BA (2001) Expressing self-incompatibility RNases (S-RNases) in transgenic plants. Methods Mol Biol 160: 65–85 [DOI] [PubMed] [Google Scholar]
- Beers EP. (1997) Programmed cell death during plant growth and development. Cell Death Differ 4: 649–661 [DOI] [PubMed] [Google Scholar]
- Bhosale G, Sharpe JA, Sundier SY, Duchen MR (2015) Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann N Y Acad Sci 1350: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C, Mincone L, Wohlrab H (1999) Replacements of basic and hydroxyl amino acids identify structurally and functionally sensitive regions of the mitochondrial phosphate transport protein. Biochemistry 38: 5096–5102 [DOI] [PubMed] [Google Scholar]
- Busot GY, McClure B, Ibarra-Sánchez CP, Jiménez-Durán K, Vázquez-Santana S, Cruz-García F (2008) Pollination in Nicotiana alata stimulates synthesis and transfer to the stigmatic surface of NaStEP, a vacuolar Kunitz proteinase inhibitor homologue. J Exp Bot 59: 3187–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanoni P, Sutter JU, Davis CS, Littlejohn GR, Blatt MR (2007) A generalized method for transfecting root epidermis uncovers endosomal dynamics in Arabidopsis root hairs. Plant J 51: 322–330 [DOI] [PubMed] [Google Scholar]
- Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu HM, Cheung AY (2002) The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 14: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Chen CY, Glaven RH, de Graaf BH, Vidali L, Hepler PK, Wu HM (2002) Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14: 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Covey PA, Kondo K, Welch L, Frank E, Sianta S, Kumar A, Nuñez R, Lopez-Casado G, van der Knaap E, Rose JK, et al. (2010) Multiple features that distinguish unilateral incongruity and self-incompatibility in the tomato clade. Plant J 64: 367–378 [DOI] [PubMed] [Google Scholar]
- Crompton M, Costi A (1988) Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress: a potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur J Biochem 178: 489–501 [DOI] [PubMed] [Google Scholar]
- Crompton M, Costi A, Hayat L (1987) Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J 245: 915–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-García F, Hancock CN, Kim D, McClure B (2005) Stylar glycoproteins bind to S-RNase in vitro. Plant J 42: 295–304 [DOI] [PubMed] [Google Scholar]
- de Nettancourt D. (2001) Incompatibility and Incongruity in Wild and Cultivated Plants. Springer-Verlag, New York [Google Scholar]
- Doran E, Halestrap AP (2000) Cytochrome c release from isolated rat liver mitochondria can occur independently of outer-membrane rupture: possible role of contact sites. Biochem J 348: 343–350 [PMC free article] [PubMed] [Google Scholar]
- Entani T, Iwano M, Shiba H, Che FS, Isogai A, Takayama S (2003) Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 8: 203–213 [DOI] [PubMed] [Google Scholar]
- Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen M, Pieper U, Sali A (2007) Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci Chapter 2: Unit 2.9 [DOI] [PubMed] [Google Scholar]
- Fields S, Song O (1989) A novel genetic system to detect protein-protein interactions. Nature 340: 245–246 [DOI] [PubMed] [Google Scholar]
- Fukao Y, Hayashi Y, Mano S, Hayashi M, Nishimura M (2001) Developmental analysis of a putative ATP/ADP carrier protein localized on glyoxysomal membranes during the peroxisome transition in pumpkin cotyledons. Plant Cell Physiol 42: 835–841 [DOI] [PubMed] [Google Scholar]
- García-Fernández R, Peigneur S, Pons T, Alvarez C, González L, Chávez MA, Tytgat J (2016) The Kunitz-type protein ShPI-1 inhibits serine proteases and voltage-gated potassium channels. Toxins (Basel) 8: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldraij A, Kondo K, Lee CB, Hancock CN, Sivaguru M, Vazquez-Santana S, Kim S, Phillips TE, Cruz-García F, McClure B (2006) Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature 439: 805–810 [DOI] [PubMed] [Google Scholar]
- Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR (2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64: 355–365 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Aguilar M, Pérez-Martínez X, Chávez E, Uribe-Carvajal S (2010) In Saccharomyces cerevisiae, the phosphate carrier is a component of the mitochondrial unselective channel. Arch Biochem Biophys 494: 184–191 [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion: a target for cardioprotection. Cardiovasc Res 61: 372–385 [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Kerr PM, Javadov S, Woodfield KY (1998) Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta 1366: 79–94 [DOI] [PubMed] [Google Scholar]
- Hamel P, Saint-Georges Y, de Pinto B, Lachacinski N, Altamura N, Dujardin G (2004) Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol Microbiol 51: 307–317 [DOI] [PubMed] [Google Scholar]
- Hancock CN, Kent L, McClure BA (2005) The stylar 120 kDa glycoprotein is required for S-specific pollen rejection in Nicotiana. Plant J 43: 716–723 [DOI] [PubMed] [Google Scholar]
- Harvey AL. (2001) Twenty years of dendrotoxins. Toxicon 39: 15–26 [DOI] [PubMed] [Google Scholar]
- Haworth RA, Hunter DR (1979) The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467 [DOI] [PubMed] [Google Scholar]
- Hua Z, Kao TH (2008) Identification of major lysine residues of S(3)-RNase of Petunia inflata involved in ubiquitin-26S proteasome-mediated degradation in vitro. Plant J 54: 1094–1104 [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14: 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA (1979) The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys 195: 453–459 [DOI] [PubMed] [Google Scholar]
- Jiménez-Durán K, McClure B, García-Campusano F, Rodríguez-Sotres R, Cisneros J, Busot G, Cruz-García F (2013) NaStEP: a proteinase inhibitor essential to self-incompatibility and a positive regulator of HT-B stability in Nicotiana alata pollen tubes. Plant Physiol 161: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus K. (2009) SAM-T08, HMM-based protein structure prediction. Nucleic Acids Res 37: W492–W497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus K, Barrett C, Hughey R (1998) Hidden Markov models for detecting remote protein homologies. Bioinformatics 14: 846–856 [DOI] [PubMed] [Google Scholar]
- Kho YO, Baer J (1968) Observing pollen tubes by means of fluorescence. Euphytica 17: 298–302 [Google Scholar]
- Kondo K, McClure B (2008) New microsome-associated HT-family proteins from Nicotiana respond to pollination and define an HT/NOD-24 protein family. Mol Plant 1: 634–644 [DOI] [PubMed] [Google Scholar]
- Kondo K, Yamamoto M, Matton DP, Sato T, Hirai M, Norioka S, Hattori T, Kowyama Y (2002) Cultivated tomato has defects in both S-RNase and HT genes required for stylar function of self-incompatibility. Plant J 29: 627–636 [DOI] [PubMed] [Google Scholar]
- Kubo K, Entani T, Takara A, Wang N, Fields AM, Hua Z, Toyoda M, Kawashima S, Ando T, Isogai A, et al. (2010) Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 330: 796–799 [DOI] [PubMed] [Google Scholar]
- Kushnareva YE, Haley LM, Sokolove PM (1999) The role of low (< or = 1 mM) phosphate concentrations in regulation of mitochondrial permeability: modulation of matrix free Ca2+ concentration. Arch Biochem Biophys 363: 155–162 [DOI] [PubMed] [Google Scholar]
- Lai Z, Ma W, Han B, Liang L, Zhang Y, Hong G, Xue Y (2002) An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol Biol 50: 29–42 [DOI] [PubMed] [Google Scholar]
- Lam E, del Pozo O (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44: 417–428 [DOI] [PubMed] [Google Scholar]
- Lancelin JM, Foray MF, Poncin M, Hollecker M, Marion D (1994) Proteinase inhibitor homologues as potassium channel blockers. Nat Struct Biol 1: 246–250 [DOI] [PubMed] [Google Scholar]
- Lee CB, Kim S, McClure B (2009) A pollen protein, NaPCCP, that binds pistil arabinogalactan proteins also binds phosphatidylinositol 3-phosphate and associates with the pollen tube endomembrane system. Plant Physiol 149: 791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CB, Swatek KN, McClure B (2008) Pollen proteins bind to the C-terminal domain of Nicotiana alata pistil arabinogalactan proteins. J Biol Chem 283: 26965–26973 [DOI] [PubMed] [Google Scholar]
- Leroch M, Kirchberger S, Haferkamp I, Wahl M, Neuhaus HE, Tjaden J (2005) Identification and characterization of a novel plastidic adenine nucleotide uniporter from Solanum tuberosum. J Biol Chem 280: 17992–18000 [DOI] [PubMed] [Google Scholar]
- Leung AW, Varanyuwatana P, Halestrap AP (2008) The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem 283: 26312–26323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind JL, Bönig I, Clarke AE, Anderson MA (1996) A style-specific 120 kDa glycoprotein enters pollen tubes of Nicotiana alata in vivo. Sex Plant Reprod 9: 75–86 [Google Scholar]
- Lisanti MP, Scherer PE, Tang Z, Sargiacomo M (1994) Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol 4: 231–235 [DOI] [PubMed] [Google Scholar]
- Logan DC, Leaver CJ (2000) Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J Exp Bot 51: 865–871 [PubMed] [Google Scholar]
- Luu DT, Qin X, Morse D, Cappadocia M (2000) S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 407: 649–651 [DOI] [PubMed] [Google Scholar]
- Martínez L, Andrade R, Birgin EG, Martínez JM (2009) PACKMOL: a package for building initial configurations for molecular dynamics simulations. J Comput Chem 30: 2157–2164 [DOI] [PubMed] [Google Scholar]
- Martínez-Castilla LP, Rodríguez-Sotres R (2010) A score of the ability of a three-dimensional protein model to retrieve its own sequence as a quantitative measure of its quality and appropriateness. PLoS ONE 5: e12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure B, Cruz-García F, Romero C (2011) Compatibility and incompatibility in S-RNase-based systems. Ann Bot (Lond) 108: 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure B, Mou B, Canevascini S, Bernatzky R (1999) A small asparagine-rich protein required for S-allele-specific pollen rejection in Nicotiana. Proc Natl Acad Sci USA 96: 13548–13553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA, Haring V, Ebert PR, Anderson MA, Simpson RJ, Sakiyama F, Clarke AE (1989) Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature 342: 955–957 [DOI] [PubMed] [Google Scholar]
- Meng D, Gu Z, Li W, Wang A, Yuan H, Yang Q, Li T (2014) Apple MdABCF assists in the transportation of S-RNase into pollen tubes. Plant J 78: 990–1002 [DOI] [PubMed] [Google Scholar]
- Murakami H, Blobel G, Pain D (1990) Isolation and characterization of the gene for a yeast mitochondrial import receptor. Nature 347: 488–491 [DOI] [PubMed] [Google Scholar]
- Murfett J, Atherton TL, Mou B, Gasser CS, McClure BA (1994) S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 367: 563–566 [DOI] [PubMed] [Google Scholar]
- Murfett J, Strabala TJ, Zurek DM, Mou B, Beecher B, McClure BA (1996) S-RNase and interspecific pollen rejection in the genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8: 943–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien M, Kapfer C, Major G, Laurin M, Bertrand C, Kondo K, Kowyama Y, Matton DP (2002) Molecular analysis of the stylar-expressed Solanum chacoense small asparagine-rich protein family related to the HT modifier of gametophytic self-incompatibility in Nicotiana. Plant J 32: 985–996 [DOI] [PubMed] [Google Scholar]
- Palmieri F. (1994) Mitochondrial carrier proteins. FEBS Lett 346: 48–54 [DOI] [PubMed] [Google Scholar]
- Palmieri F. (2004) The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch 447: 689–709 [DOI] [PubMed] [Google Scholar]
- Palmieri F, Pierri CL, De Grassi A, Nunes-Nesi A, Fernie AR (2011) Evolution, structure and function of mitochondrial carriers: a review with new insights. Plant J 66: 161–181 [DOI] [PubMed] [Google Scholar]
- Palmieri L, Rottensteiner H, Girzalsky W, Scarcia P, Palmieri F, Erdmann R (2001) Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J 20: 5049–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneur S, Billen B, Derua R, Waelkens E, Debaveye S, Béress L, Tytgat J (2011) A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem Pharmacol 82: 81–90 [DOI] [PubMed] [Google Scholar]
- Petronilli V, Nicolli A, Costantini P, Colonna R, Bernardi P (1994) Regulation of the permeability transition pore, a voltage-dependent mitochondrial channel inhibited by cyclosporin A. Biochim Biophys Acta 1187: 255–259 [DOI] [PubMed] [Google Scholar]
- Phelps A, Briggs C, Haefele A, Mincone L, Ligeti E, Wohlrab H (2001) Mitochondrial phosphate transport protein: reversions of inhibitory conservative mutations identify four helices and a nonhelix protein segment with transmembrane interactions and Asp39, Glu137, and Ser158 as nonessential for transport. Biochemistry 40: 2080–2086 [DOI] [PubMed] [Google Scholar]
- Puerta AR, Ushijima K, Koba T, Sassa H (2009) Identification and functional analysis of pistil self-incompatibility factor HT-B of Petunia. J Exp Bot 60: 1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Wang H, Zhao L, Zhou J, Huang J, Zhang Y, Xue Y (2004) The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell 16: 582–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán JA, Rojas HJ, Goldraij A (2012) Disorganization of F-actin cytoskeleton precedes vacuolar disruption in pollen tubes during the in vivo self-incompatibility response in Nicotiana alata. Ann Bot (Lond) 110: 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa H, Hirano H (2006) Identification of a new class of pistil-specific proteins of Petunia inflata that is structurally similar to, but functionally distinct from, the self-incompatibility factor HT. Mol Genet Genomics 275: 97–104 [DOI] [PubMed] [Google Scholar]
- Schultz CJ, Hauser K, Lind JL, Atkinson AH, Pu ZY, Anderson MA, Clarke AE (1997) Molecular characterisation of a cDNA sequence encoding the backbone of a style-specific 120 kDa glycoprotein which has features of both extensins and arabinogalactan proteins. Plant Mol Biol 35: 833–845 [DOI] [PubMed] [Google Scholar]
- Serrano I, Pelliccione S, Olmedilla A (2010) Programmed-cell-death hallmarks in incompatible pollen and papillar stigma cells of Olea europaea L. under free pollination. Plant Cell Rep 29: 561–572 [DOI] [PubMed] [Google Scholar]
- Sijacic P, Wang X, Skirpan AL, Wang Y, Dowd PE, McCubbin AG, Huang S, Kao TH (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429: 302–305 [DOI] [PubMed] [Google Scholar]
- Stein JC, Hansen G (1999) Mannose induces an endonuclease responsible for DNA laddering in plant cells. Plant Physiol 121: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YL, Zhao Y, Hong X, Zhai ZH (1999) Cytochrome c release and caspase activation during menadione-induced apoptosis in plants. FEBS Lett 462: 317–321 [DOI] [PubMed] [Google Scholar]
- Thomas SG, Franklin-Tong VE (2004) Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 429: 305–309 [DOI] [PubMed] [Google Scholar]
- Twell D, Wing R, Yamaguchi J, McCormick S (1989) Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet 217: 240–245 [DOI] [PubMed] [Google Scholar]
- Ushijima K, Sassa H, Dandekar AM, Gradziel TM, Tao R, Hirano H (2003) Structural and transcriptional analysis of the self-incompatibility locus of almond: identification of a pollen-expressed F-box gene with haplotype-specific polymorphism. Plant Cell 15: 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanyuwatana P, Halestrap AP (2012) The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion 12: 120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. (2003) The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci 8: 135–142 [DOI] [PubMed] [Google Scholar]
- Wandrey M, Trevaskis B, Brewin N, Udvardi MK (2004) Molecular and cell biology of a family of voltage-dependent anion channel porins in Lotus japonicus. Plant Physiol 134: 182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, Xu GH, Jiang XT, Chen G, Wu J, Wu HQ, Zhang SL (2009) S-RNase triggers mitochondrial alteration and DNA degradation in the incompatible pollen tube of Pyrus pyrifolia in vitro. Plant J 57: 220–229 [DOI] [PubMed] [Google Scholar]
- Wang CL, Zhang SL (2011) A cascade signal pathway occurs in self-incompatibility of Pyrus pyrifolia. Plant Signal Behav 6: 420–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tsukamoto T, Yi KW, Wang X, Huang S, McCubbin AG, Kao TH (2004) Chromosome walking in the Petunia inflata self-incompatibility (S-) locus and gene identification in an 881-kb contig containing S2-RNase. Plant Mol Biol 54: 727–742 [DOI] [PubMed] [Google Scholar]
- Wheeler D, Newbigin E (2007) Expression of 10 S-class SLF-like genes in Nicotiana alata pollen and its implications for understanding the pollen factor of the S locus. Genetics 177: 2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Wu L, Li S, Sun P, Kao TH (2015) Insight into S-RNase-based self-incompatibility in Petunia: recent findings and future directions. Front Plant Sci 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlrab H, Annese V, Haefele A (2002) Single replacement constructs of all hydroxyl, basic, and acidic amino acids identify new function and structure-sensitive regions of the mitochondrial phosphate transport protein. Biochemistry 41: 3254–3261 [DOI] [PubMed] [Google Scholar]
- Xu G, Ma H, Nei M, Kong H (2009) Evolution of F-box genes in plants: different modes of sequence divergence and their relationships with functional diversification. Proc Natl Acad Sci USA 106: 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xue Y (2008) Molecular biology of S-RNase-based self-incompatibility. In Franklin-Tong VE, ed, Self-Incompatibility in Flowering Plants: Evolution, Diversity, and Mechanisms. Springer-Verlag, Berlin, pp 193–215 [Google Scholar]