Abstract
The ATP-binding cassette transporter ABCC4 (Multidrug resistance protein 4, MRP4) mRNA level is a strong predictor of poor clinical outcome in neuroblastoma which may relate to its export of endogenous signaling molecules and chemotherapeutic agents. We sought to determine whether ABCC4 contributes to development, growth, and drug response in neuroblastoma in vivo. In neuroblastoma patients, high ABCC4 protein levels were associated with reduced overall survival. Inducible knockdown of ABCC4 strongly inhibited the growth of human neuroblastoma cells in vitro and impaired the growth of neuroblastoma xenografts. Loss of Abcc4 in the Th-MYCN transgenic neuroblastoma mouse model did not impact tumor formation, however Abcc4-null neuroblastomas were strongly sensitized to the ABCC4 substrate drug irinotecan. Our findings demonstrate a role for ABCC4 in neuroblastoma cell proliferation and chemoresistance and provide rationale for a strategy where inhibition of ABCC4 should both attenuate the growth of neuroblastoma and sensitize tumors to ABCC4 chemotherapeutic substrates.
Keywords: neuroblastoma, ABCC4, drug resistance, chemotherapy, xenograft
Introduction
Neuroblastoma is a extracranial solid tumor of infancy and early childhood arising from the sympatico-adrenal lineage of the neural crest (1). It is a heterogenous disease with few recurrent somatic mutations (2), and treatment for high-risk disease still relies heavily on conventional cytotoxic agents. ABCC4, encoding for the ATP-binding cassette transporter protein ABCC4 (Multidrug resistance protein 4, MRP4), is transcriptionally regulated by MYCN (3), which is a driver of neuroblastoma tumorigenesis (4) and an established poor prognostic factor (5, 6) and high ABCC4 mRNA expression strongly predicts poor clinical outcome across multiple patient cohorts (7, 8). In cultured cells, ABCC4 confers resistance to several anti-cancer drugs, including the camptothecin irinotecan, a drug used in the treatment of neuroblastoma (9), however it is unknown whether ABCC4 protein expression has prognostic value or affects chemotherapy responsein tumors. ABCC4 also exports endogenous signaling molecules that may influence tumor survival and proliferation, including cyclic nucleotides and eicosanoids (10, 11) and we previously reported that transient RNAi-mediated ABCC4 knockdown reduced proliferation and colony-forming ability in two neuroblastoma cell lines (7) in the absence of chemotherapy. More rigorous assessment of ABCC4 as a therapeutic target in neuroblastoma is clearly warranted.
Here we demonstrate that suppression of ABCC4 inhibits the growth of multiple neuroblastoma cell lines in vitro and established human neuroblastoma xenografts in immune-deficient mice, suggesting that blocking ABCC4 function might be beneficial in established tumors, even without chemotherapy. In a neuroblastoma-prone transgenic mouse model, constitutive absence Abcc4 did not affect neuroblastoma formation suggesting that ABCC4 function does not contribute to the genesis of this tumor. Nonetheless, the murine neuroblastomas derived from the Abcc4-deficient animals were sensitized to irinotecan in an allograft model. Our findings demonstrate ABCC4 inhibition as an approach to chemosensitization of neuroblastomas.
Materials and methods
Tissue microarray
Tissue microarray (TMA) sections with clinical annotation, from the Children’s Hospital at Westmead Tumor Bank, were stained with hematoxylin and eosin (H&E) or for ABCC4 (rat monoclonal anti-MRP4 M4I-10, Abcam ab15602, 1:50 dilution, 3 μg/mL). Cores from 98 patients diagnosed between 1979 and 2013 were scored for ABCC4 staining by a pediatric pathologist blinded to clinical parameters (12). Staining was scored for intensity (0, absent; 1, weak; 1.5 weak-moderate; 2, moderate, 2.5 moderate-strong; and 3, strong) and percentage of positive staining (0, 0%; 1, 1–10%; 2, 11–50% and 3, 51–100%) and overall score (0–9) determined by multiplying staining intensity and percentage scores, with duplicate cores averaged. Photos were from an Olympus BX53 light microscope and DP-73 camera with cellSens software.
Cell culture
Cells lines verified by short tandem repeat profiling (CellBank Australia, Westmead Australia) were cultured at 37°C, 5% CO2 in Dulbecco’s Modified Eagles Medium with 10% fetal bovine serum (FBS; ThermoTrace, Nobel Park, Australia) for BE(2)-C, or Roswell Park Memorial Institute medium with 10% FBS (CHP-134 and NB69). Transfections used Lipofectamine RNAiMAX (Life Technologies, Mulgrave Australia) and 20 nM siRNA (Supplementary Table 1). Stable cell lines expressing doxycycline-inducible ABCC4 shRNA were generated by lentiviral transduction with the pFH1UTG vector (13) (Supplementary Table 1) packaged using the psPAX2 and pMD2.G plasmids (gift from Didier Trono, Lausanne, Switzerland). Doxycycline treatment was 1 μg/mL (72 h pre-treatment, media change to replenish doxycycline every 48 h).
Western blot
Western blotting antibodies were: ABCC4 (rat monoclonal M4I-10; Enzo Life Sciences, Waterloo, NSW; 1:1000), α-tubulin (mouse monoclonal DM1A; Abcam; 1:3000), total actin (rabbit polyclonal A2066; Sigma-Aldrich; 1:2000), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; mouse monoclonal G-9, sc365062; Santa Cruz Biotechnology, Dallas, TX; 1:5000), Na+/K+-ATPase α (rabbit polyclonal H-300, sc-28800; Santa Cruz Biotechnology; 1:1000), peroxidase-conjugated goat anti-rat IgG (VWR International, Murarrie, QLD; 1:10000) and sheep anti-mouse horseradish peroxidase (GE Healthcare, Rydalmere, NSW; 1:50000).
Proliferation and colony assays
Cell viability assays were in 6-well plates. Transfected cells were plated 24h after siRNA treatment, and stably transduced cells were plated in 1 μg/mL doxycycline. Viable cell number was determined using trypan blue exclusion. For colony assays, untreated or doxycycline-treated cells were plated at 200–500 cells/well, stained with 0.5% crystal violet in 50% methanol after 9–14 days, scanned, and counted using Quantity One Software (Bio-Rad) or ImageJ.
Animal studies
All animal studies were approved by the University of New South Wales Animal Care and Ethics Committee and conducted according to the Animal Research Act, 1985 (New South Wales, Australia) and the Australian Code of Practice for Care and Use of Animals for Scientific Purposes (2013).
For xenografts, 1×106 BE(2)-C cells stably transduced with inducible shRNAs were subcutaneously engrafted into one flank of Balb/c nude mice. After 7 days, mice received either control diet or diet supplemented with 600 mg/kg doxycycline (Specialty Feeds, Glen Forrest, WA, Australia). Tumors were measured every second day using a vernier caliper. Th-MYCN transgenic mice (4),(14) (C57BL/6, Balb/c, 129/Sv-ter background) were crossed with Abcc4−/− mice (C57BL/6, 129/SvJ background) (15) and Th-MYCNTg/−Abcc4+/− offspring interbred for 10 generations. Th-MYCNTg/Tg mice of each Abcc4 genotype were palpated twice weekly and humanely killed by cervical dislocation or carbon dioxide–mediated asphyxiation when a ~1000 mm3 tumor was detected.
For allograft experiments, tumors were harvested from Th-MYCNTg/Tg/Abcc4+/+ and Th-MYCNTg/Tg/Abcc4−/− mice, dissociated (5×106 cells/mL), resuspended (1:1) with ice-cold Matrigel, and injected (0.2 mL) subcutaneously into one flank of Balb/c nude mice. Tumors were measured every second day using a vernier caliper. Drug treatment (25 mg/kg intraperitoneal (IP) irinotecan or 2 mg/kg IP cisplatin for 5 consecutive days) commenced when tumors reached 100–125 mm3. Time to reach 1000 mm3 was calculated and the saline control was subtracted to account for variability between growth rates of individual tumors.
Representative tumor sections were examined tumor morphology and viability following H&E staining. Percentage of viable tumor was calculated as 100% – (% of hemorrhage, necrosis and apoptosis).
Statistical analyses
Statistical analyses were conducted using Prism version 6 (GraphPad Software, La Jolla, CA). Survival analyses were computed by the Kaplan Meier method and compared between groups using the log-rank test. Patient overall survival (OS) was defined as the time to death within 5 years from initial diagnosis or until last contact if no event occurred. Other statistical methods are as described in the Results and figure legends.
Results
ABCC4 protein levels are associated with poor outcome in neuroblastoma
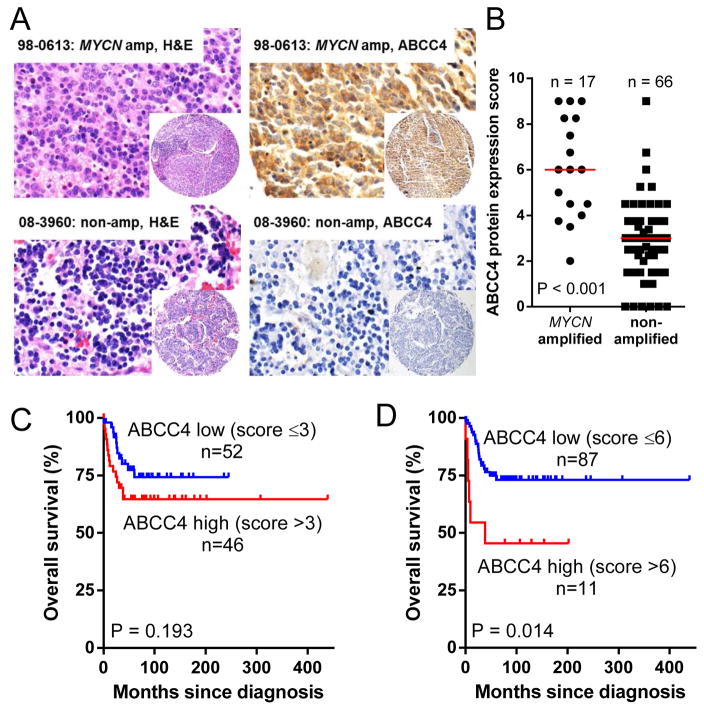
To investigate the relationship between ABCC4 protein expression and MYCN amplification and neuroblastoma outcome, tissue microarray (TMA) cores from 98 patients with primary untreated neuroblastoma (n=81), ganglioneuroma (n=12) or ganglioneuroblastoma (n=5) were immunohistochemically stained for ABCC4 (Figure 1A) and scored (0–9) for staining intensity and percentage of staining within each core. Staining of cytoplasm, neuropil, and Schwannian stroma were observed. Clinical characteristics for the cohort are shown in Table 1 and are broadly representative of the disease in terms of age at diagnosis, frequency of MYCN amplification and INSS stage (16). Poorer overall survival was associated with the established prognostic indicators of INSS Stage 3 or 4 (Supplementary Figure 1A; P < 0.001), age >18 months at diagnosis (Supplementary Figure 1B; P = 0.047) and MYCN amplification (Supplementary Figure 1C; P < 0.001). ABCC4 protein expression was higher in tumors with MYCN amplification (Figure 1B), with a median expression score of 6 for MYCN-amplified tumors and 3 for non-amplified tumors (< 0.001, Mann Whitney test). When dichotomized at the median ABCC4 staining score (≤3 vs >3), the 5-year survival rates were 74% ± 7% and 65% ± 7% for patients with low or high ABCC4 expression, respectively (Figure 1C; P = 0.193). When dichotomized based on ABCC4 staining score ≤6 vs >6 (87 vs 11 tumors, approximately upper decile as previously described for ABCC4 mRNA (7)), higher ABCC4 was strongly associated with reduced overall survival (P = 0.014), with a 5-year survival rate of 73% ± 5% versus 46% ± 15% in patients with low versus high ABCC4 expression, respectively (Figure 1D).
Figure 1. ABCC4 protein levels are associated with poor outcome in neuroblastoma.
(A) Immunohistochemical staining of sample cores from a neuroblastoma TMA showing tumor morphology by H&E staining (left panels) and ABCC4 staining (right panels). A representative MYCN-amplified sample with high ABCC4 staining (score = 9) is shown in the upper right panel and a representative MYCN non-amplified, ABCC4-negative sample (score = 0) in the bottom right panel. 600x magnification for larger panels; 100x for insets. (B) Distribution of ABCC4 protein expression scores across the TMA. Bars represent median expression score, P value from Mann Whitney test. (C, D) Kaplan-Meier curves showing the probability of overall survival for patients with tumors dichotomized into high or low ABCC4 protein expression at different cut-points.
Table 1.
Clinical characteristics for the 98 patient tissue microarray cohort
| Clinical characteristic | Total | % |
|---|---|---|
| Age at diagnosis | ||
| ≤ 18 months | 52 | 53.1 |
| > 18 months | 46 | 46.9 |
|
| ||
| MYCN status | ||
| Single copy | 66 | 67.4 |
| Amplified | 17 | 17.3 |
| Unknown | 15 | 15.3 |
|
| ||
| INSS stage | ||
| Stage 1,2,4S | 47 | 48.0 |
| Stage 3,4 | 50 | 51.0 |
| Unknown | 1 | 1.0 |
|
| ||
| Death from disease1 | 26 | 26.5 |
Deaths attributed to neuroblastoma within 5 years of diagnosis.
Down-regulation of ABCC4 inhibits proliferation of human neuroblastoma cell lines
We previously showed that transient ABCC4 knockdown (siRNAs ABCC4.1 and ABCC4.5) decreased proliferation and induced neurite formation in the BE(2)-C cell line (7). To confirm that these phenotypes were attributable to ABCC4 knockdown, we designed two additional ABCC4 siRNA duplexes (ABCC4.6 and ABCC4.7; Supplementary Table 1) and transiently transfected BE(2)-C cells. ABCC4 protein knockdown was comparable for each duplex (Supplementary Figure 2A), as was suppression of cell proliferation in short-term assays (P < 0.05; Supplementary Figure 2B), confirming that reduced proliferation is a consequence of ABCC4 suppression. The percentage of neurite-positive cells was increased with ABCC4 siRNA duplex ABCC4.5 (P < 0.001, Supplementary Figure 2C), but not with duplexes ABCC4.6 (P = 0.967) or ABCC4.7 (P = 0.557), indicating that neurite formation is not consistently related to ABCC4 suppression. Duplexes ABCC4.6 and ABCC4.7 were subsequently selected for in vivo studies.
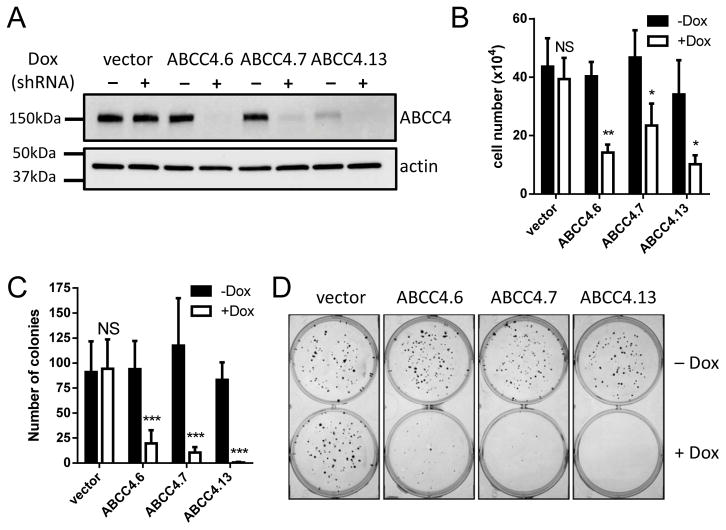
To enable sustained ABCC4 suppression, shRNA sequences equivalent to ABCC4.6 and ABCC4.7 and an additional shRNA targeting the ABCC4 3′UTR (ABCC4.13) were cloned into a doxycycline-inducible expression system (Supplementary Table 1). Efficient knockdown of ABCC4 protein in transduced BE(2)-C cells was confirmed following induction with doxycycline (Figure 2A) and slowed cell proliferation in short term assays (>50% reduction in cell number for each duplex compared to untreated cells; P ≤ 0.05 for each; Figure 2B, Supplementary Figure 3A–D) and in colony assays (>80% reduction in colony numbers for each duplex; P ≤ 0.001; Figure 2C–D).
Figure 2. Sustained suppression of ABCC4 inhibits neuroblastoma cell growth and abolishes colony formation.
(A) Efficient inducible knockdown of ABCC4 is achieved in BE(2)-C cells following doxycycline (Dox) treatment, with each of three independent shRNA duplexes (72h timepoint). Actin is shown as a loading control. (B) Inducible ABCC4 knockdown inhibits BE(2)-C cell proliferation in short-term assays at 72h post-induction for each duplex compared its untreated control (t test). (C) Inducible ABCC4 knockdown abolishes the formation of BE(2)-C cell colonies for each duplex compared to its untreated control (t test). (D) Representative images for colony assays quantitated in panel C. Data in B and C show means and SD derived from three independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001.
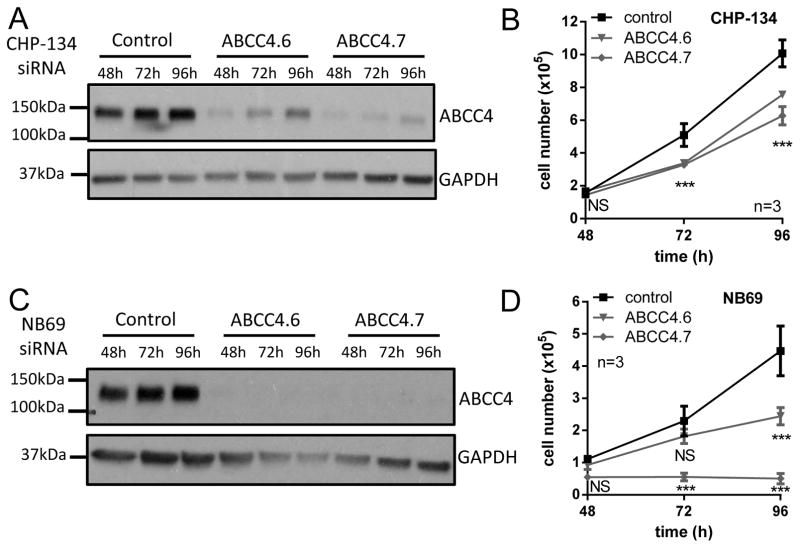
These findings were confirmed in the MYCN-amplified CHP-134 cell line (17), with cell numbers at 96h post-transfection 75% (P ≤ 0.001) and 62% of control (P ≤ 0.001), respectively for ABCC4.6 and ABCC4.7 siRNAs (Figure 3A–B) and in the non-amplified NB69 (17) cell line, with cell numbers of 55% of control (P ≤ 0.001) for ABCC4.6 and abolition of proliferation for ABCC4.7 (Figure 3C–D).
Figure 3. Downregulation of ABCC4 inhibits proliferation of neuroblastoma cell lines.
Knockdown of ABCC4 with two independent siRNA duplexes inhibits proliferation of the MYCN-amplified CHP-134 cell line (A, B) and the MYCN single copy NB69 (C, D) cell line compared to non-silencing control siRNA at 72h. GAPDH is shown as a loading control in A and C. Data in B and D show means and SD derived from three independent experiments. *** P < 0.001, Dunnett’s multiple comparisons test following 2-way ANOVA.
ABCC4 knockdown slows the growth of xenografted human neuroblastoma cells
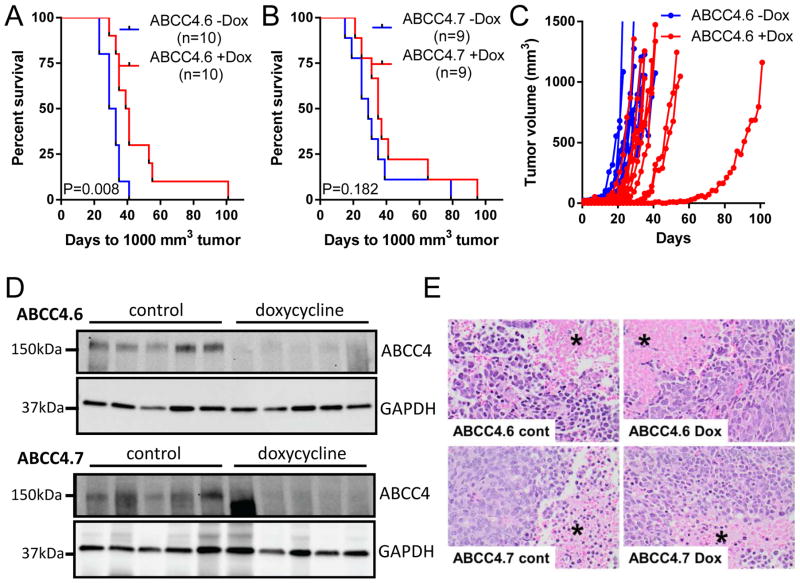
To test the effects of ABCC4 knockdown in vivo, BE(2)-C cells with stable, doxycycline-inducible ABCC4 knockdown (ABCC4.6 or ABCC4.7) were xenografted subcutaneously into Balb/c nude mice, with randomization to doxycycline or control diet 7 days later. ABCC4 knockdown increased the median time to 1000 mm3 tumor from 31 to 40 days (Figure 4A) in the ABCC4.6 xenografts, and from 29 to 35 days (Figure 4B) in the ABCC4.7 xenografts. Growth curves for individual ABCC4.6 tumors are shown in Figure 4C. ABCC4 suppression was maintained in tumors harvested at ~1000 mm3 (Figure 4D) albeit with a lower differential than achieved in vitro. On histological examination (Figure 4E), no difference in percentage viable tumor was observed between the control and doxycycline-treated tumors for either shRNA construct (64.4±13.7% and 63.5±11.0%, P = 0.961 for ABCC4.6; 79.9±7.0% and 81.1±5.7%, P = 0.892 for ABCC4.7).
Figure 4. ABCC4 knockdown slows the growth of xenografted human neuroblastoma cells.
(A, B) Induction of ABCC4 knockdown in established BE(2)-C tumors (doxycycline treatment commenced 7 days post-engraftment) extends survival (P = 0.008 for ABCC4.6, P = 0.182 for ABCC4.7, n = 9–10/group). (C) Tumor growth in individual mice for ABCC4.6 with and without doxycycline. (D) Doxycycline-induced knockdown of ABCC4 is maintained in xenografted tumors to the experimental end-point for both ABCC4.6 and ABCC4.7. GAPDH is shown as a loading control and a different tumor sample is shown in each lane. (E) Histological appearance of representative xenografts from each cohort (H&E staining). Coagulative necrosis, where cell outlines are preserved (*), is seen in all treatment groups. Magnification: 400x.
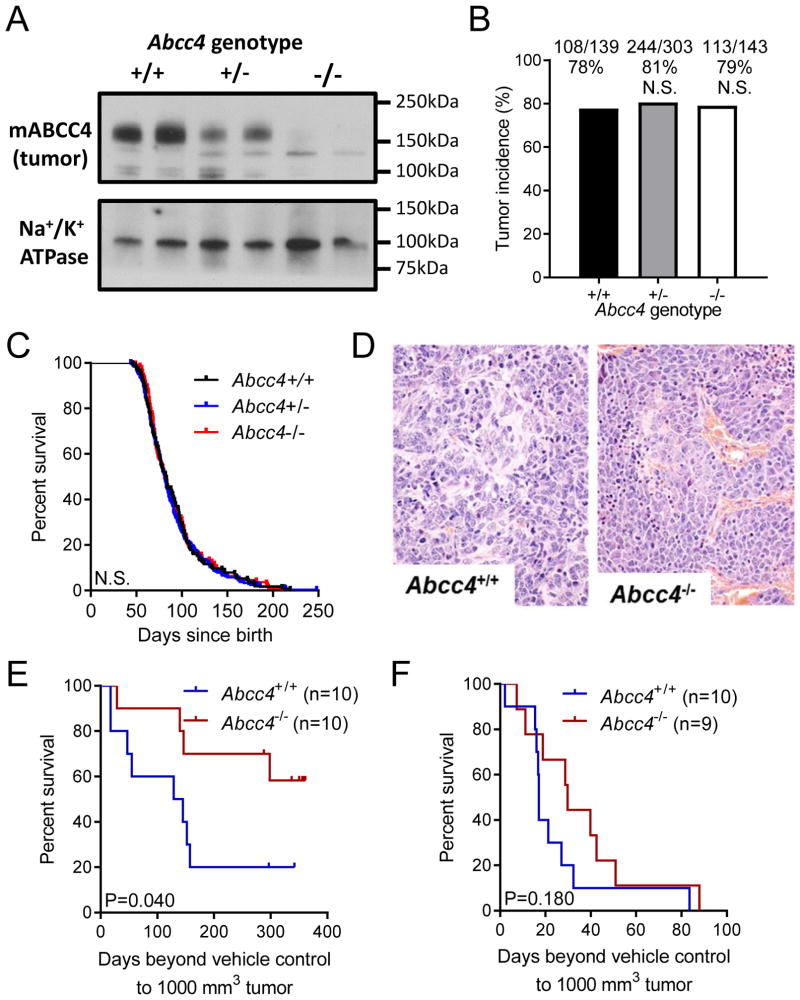
Constitutive absence of ABCC4 does not alter tumorigenesis in a murine model of neuroblastoma
To investigate whether loss of ABCC4 impacts on tumor initiation, we crossed neuroblastoma-prone Th-MYCN mice and mice with targeted disruption of Abcc4. Tumors arising in Abcc4+/− mice had reduced ABCC4 protein levels and those arising in Abcc4−/− mice had no detectable ABCC4 protein (Figure 5A). Tumor incidence was not altered by loss of either one (OR = 0.842, 95% CI = 0.516–1.38, P = 0.525, Fisher’s exact test) or both Abcc4 alleles (OR = 0.925, 95% CI = 0.525–1.63, P = 0.885, Fisher’s exact test) (Figure 5B). Tumor latency was unchanged between Abcc4 genotypes, with a median age to ~1000 mm3 tumor of 81 days for Abcc4+/+ mice, 82 days for Abcc4+/− mice (HR = 0.944, 95% CI = 0.753–1.18; P = 0.611) and 83 days for Abcc4−/− mice (HR = 0.983, 95% CI = 0.754–1.28; P = 0.895) (Figure 5C). All tumours examined were Schwannian-stroma poor neuroblastic tumours with similar degree of haemorrhage and necrosis, with no histological differences observed between genotypes (Figure 5D).
Figure 5. Loss of ABCC4 does not alter tumorigenesis or tumor progression in a transgenic mouse model of neuroblastoma, but sensitizes tumors to irinotecan.
(A) ABCC4 protein expression in Th-MYCN mouse tumors derived from animals with Abcc4+/+, Abcc4+/−, and Abcc4−/− genotypes. Samples are membrane preparations and Na+/K+ ATPase is shown as a loading control. (B) Loss of Abcc4 does not alter tumor incidence in Th-MYCN mice. Number of tumor-bearing mice and total mice for each genotype are indicated above each bar (P=1.0 and P=0.725 for loss of one and both Abcc4 alleles respectively, compared to wild-type control). (C) Loss of Abcc4 does not alter tumor latency in Th-MYCN mice, as measured by median age to occurrence of a medium palpable tumor (P = 0.689 and P = 0.7071 for loss of one and both Abcc4 alleles respectively, compared to wild-type control). (D) Histological appearance of representative Abcc4+/+ and Abcc4−/− Th-MYCN mouse tumors (H&E staining). Magnification: 400x. (E,F) Loss of ABCC4 significantly sensitizes allografted transgenic mouse neuroblastomas to the ABCC4 substrate irinotecan (E; P = 0.040, log-rank test), but not to cisplatin (F; P = 0.1797, log-rank test).
Loss of ABCC4 alters tumor sensitivity to chemotherapeutic drugs
Next, we investigated whether ABCC4 contributes to tumor drug sensitivity in the Th-MYCN mouse model treated with the ABCC4 substrate irinotecan and with cisplatin, which is not an ABCC4 substrate. To avoid pharmacokinetic differences due to systemic ABCC4 loss, we harvested and dissociated tumors from Th-MYCNTg/Tg/Abcc4+/+ and Th-MYCNTg/Tg/Abcc4−/− mice and established allografts in Balb/c nude mice, as described previously (18). Mice engrafted with Abcc4+/+ tumors and treated with irinotecan had a median survival time of 137 days, with 2 of 10 mice surviving long-term. Survival was extended in mice engrafted with Abcc4−/− tumors, with 6 of 10 mice treated with irinotecan surviving long term (HR = 3.209, 95% CI = 1.081–11.07) (Figure 5E). In contrast, loss of Abcc4−/− did not significantly alter sensitivity to cisplatin (HR = 1.789, 95% CI = 0.769–4.887) (Figure 5F).
Discussion
Previous studies suggest ABCC4 as an attractive therapeutic target, as its knockdown suppresses proliferation of a range of cancer cell lines in vitro and in xenograft models (19–21). ABCC4 may be particularly relevant in neuroblastoma, given the association between high ABCC4 expression and poor clinical outcome (7, 8). In contrast to previous studies which used constitutive ABCC4 knockdown, the present study mimics pharmacological inhibition by inducing knockdown in established tumours. The striking impact on cells in cell culture and the reduced growth of xenografted tumors support a role for this transporter in neuroblastoma biology.
The observation that loss of Abcc4 has no impact on tumor formation in the Th-MYCN transgenic neuroblastoma mouse model has several possible non-mutually exclusive explanations. First, ABCC4 may not contribute to the genesis of neuroblastoma, but may play a role in sustaining established tumors, as indicated by the xenograft model. Second, tumors arising in Abcc4-null mice might harbor adaptive changes that allow their development in the absence of Abcc4. Third, functional differences might exist between the human and mouse protein, as suggested by differences in the effects of ABCC4 inhibition or down-regulation on human and mouse skin dendritic cell migration (22),(23) and by differences in the affinity of human and mouse ABCC4 for the substrate cGMP (24).
Notably, we observed that loss of Abcc4 sensitizes allografted Th-MYCN mouse tumors to irinotecan, which along with its active metabolite SN-38 is an ABCC4 substrate (8, 25). As these experiments were conducted as allografts, this chemosensitzation can be attributed to loss of tumor ABCC4 rather than to altered drug clearance as has been previously reported for another camptothecin, topotecan, in Abcc4-deficient mice (15). As irinotecan is used to treat relapsed neuroblastoma (9), pharmacological inhibition of ABCC4 may be of clinical benefit in this disease. Currently, no selective inhibitors of ABCC4 with proven in vivo efficacy are available and the most widely used small molecule inhibitor of ABCC4, the cysteinyl leukotriene receptor antagonist MK-571, also has inhibitory activity against a range of ABC transporters, including ABCC1, ABCC2, ABCC3 and ABCC5 (26, 27) as well as apparent phosphodiesterase inhibitory activity (28). While more selective ABCC4 inhibitors have been described (29), their suitability for in vivo use is unknown. This study provides support for the continued development of ABCC4 inhibitors, and illustrates a model in which these inhibitors could be assessed.
In conclusion, we have shown that downregulation of ABCC4 slows the progression of neuroblastoma xenografts and that loss of ABCC4 sensitizes neuroblastoma tumors to irinotecan. Inhibition of ABCC4 is therefore a potential strategy for chemosensitization in this disease, providing rationale for the further development of small molecule ABCC4 inhibitors.
Supplementary Material
Suppression of ABCC4 slows progression of neuroblastoma xenografts
Loss of ABCC4 sensitizes tumors to the camptothecin irinotecan
Inhibition of ABCC4 may be a chemosensitization strategy to pediatric neuroblastoma
Acknowledgments
Grant support: National Health and Medical Research Council Program Grant APP1016699 (MDN, MH) and Project Grant APP1029543 (MJH, JIF), Cancer Institute New South Wales Translational Program Grant 10/TPG/1-03 (MDN, MH), R01CA194206, NCI P30 CA021765-35 and CA21865 and ALSAC (JDS).
The authors acknowledge the Children’s Hospital at Westmead Tumour Bank for provision of the human neuroblastoma tissue microarray sections. Children’s Cancer Institute Australia is affiliated with UNSW Australia and the Sydney Children’s Hospitals Network.
Funding: This work was supported by: the National Health and Medical Research Council (Program Grant APP1016699, Project Grant APP1029543), Cancer Institute New South Wales (Translational Program Grant 10/TPG/1-03), the National Institutes of Health (R01CA194206, NCI P30 CA021765-35 and CA21865) and The American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45(3):279–84. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porro A, Haber M, Diolaiti D, Iraci N, Henderson M, Gherardi S, et al. Direct and coordinate regulation of ATP-binding cassette transporter genes by Myc factors generates specific transcription signatures that significantly affect the chemoresistance phenotype of cancer cells. J Biol Chem. 2010;285(25):19532–43. doi: 10.1074/jbc.M109.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss W, Aldape K, Mohapatra G, Feuerstein B, Bishop J. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224(4653):1121–4. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 6.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313(18):1111–6. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 7.Henderson MJ, Haber M, Porro A, Munoz MA, Iraci N, Xue C, et al. ABCC multidrug transporters in childhood neuroblastoma: clinical and biological effects independent of cytotoxic drug efflux. J Natl Cancer Inst. 2011;103(16):1236–51. doi: 10.1093/jnci/djr256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris MD, Smith J, Tanabe K, Tobin P, Flemming C, Scheffer GL, et al. Expression of multidrug transporter MRP4/ABCC4 is a marker of poor prognosis in neuroblastoma and confers resistance to irinotecan in vitro. Mol Cancer Ther. 2005;4(4):547–53. doi: 10.1158/1535-7163.MCT-04-0161. [DOI] [PubMed] [Google Scholar]
- 9.Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29(2):208–13. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10(2):147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher JI, Williams RT, Henderson MJ, Norris MD, Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist Updat. 2016;26:1–9. doi: 10.1016/j.drup.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Garcia H, Miecznikowski JC, Safina A, Commane M, Ruusulehto A, Kilpinen S, et al. Facilitates chromatin transcription complex is an “accelerator” of tumor transformation and potential marker and target of aggressive cancers. Cell Rep. 2013;4(1):159–73. doi: 10.1016/j.celrep.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herold MJ, van den Brandt J, Seibler J, Reichardt HM. Inducible and reversible gene silencing by stable integration of an shRNA-encoding lentivirus in transgenic rats. Proc Natl Acad Sci U S A. 2008;105(47):18507–12. doi: 10.1073/pnas.0806213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkhart CA, Cheng AJ, Madafiglio J, Kavallaris M, Mili M, Marshall GM, et al. Effects of MYCN antisense oligonucleotide administration on tumorigenesis in a murine model of neuroblastoma. J Natl Cancer Inst. 2003;95(18):1394–403. doi: 10.1093/jnci/djg045. [DOI] [PubMed] [Google Scholar]
- 15.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–21. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–97. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiele CJ. Neuroblastoma Cell Lines. In: Masters J, editor. Human Cell Culture. Lancaster, UK: Kluwer Academic Publishers; 1998. pp. 21–53. [Google Scholar]
- 18.Burkhart CA, Watt F, Murray J, Pajic M, Prokvolit A, Xue C, et al. Small-molecule multidrug resistance-associated protein 1 inhibitor reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma. Cancer Res. 2009;69(16):6573–80. doi: 10.1158/0008-5472.CAN-09-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Gu J, Xu L, Qu C, Zhang Y, Zhang W. RNAi-mediated silencing of ATP-binding cassette C4 protein inhibits cell growth in MGC80-3 gastric cancer cell lines. Cell Mol Biol (Noisy-le-grand) 2014;60(1):1–5. [PubMed] [Google Scholar]
- 20.Zhang Z, Wang J, Shen B, Peng C, Zheng M. The ABCC4 gene is a promising target for pancreatic cancer therapy. Gene. 2012;491(2):194–9. doi: 10.1016/j.gene.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Guo Y, Yue W, Zhang L, Gu M, Wang Y. ABCC4 is required for cell proliferation and tumorigenesis in non-small cell lung cancer. Onco Targets Ther. 2014;7:343–51. doi: 10.2147/OTT.S56029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Ven R, Scheffer GL, Reurs AW, Lindenberg JJ, Oerlemans R, Jansen G, et al. A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood. 2008;112(6):2353–9. doi: 10.1182/blood-2008-03-147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Ven R, de Groot J, Reurs AW, Wijnands PG, van de Wetering K, Schuetz JD, et al. Unimpaired immune functions in the absence of Mrp4 (Abcc4) Immunol Lett. 2009;124(2):81–7. doi: 10.1016/j.imlet.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 24.de Wolf CJ, Yamaguchi H, van der Heijden I, Wielinga PR, Hundscheid SL, Ono N, et al. cGMP transport by vesicles from human and mouse erythrocytes. FEBS J. 2007;274(2):439–50. doi: 10.1111/j.1742-4658.2006.05591.x. [DOI] [PubMed] [Google Scholar]
- 25.Tian Q, Zhang J, Tan TM, Chan E, Duan W, Chan SY, et al. Human multidrug resistance associated protein 4 confers resistance to camptothecins. Pharm Res. 2005;22(11):1837–53. doi: 10.1007/s11095-005-7595-z. [DOI] [PubMed] [Google Scholar]
- 26.Keppler D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. In: Fromm MF, Kim RB, editors. Handb Exp Pharmacol. Berlin: Springer-Varlag; 2011. pp. 299–323. 2010/11/26 ed. [DOI] [PubMed] [Google Scholar]
- 27.Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994;269(45):27807–10. [PubMed] [Google Scholar]
- 28.Xie M, Rich TC, Scheitrum C, Conti M, Richter W. Inactivation of multidrug resistance proteins disrupts both cellular extrusion and intracellular degradation of cAMP. Mol Pharmacol. 2011;80(2):281–93. doi: 10.1124/mol.111.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung L, Flemming CL, Watt F, Masada N, Yu DM, Huynh T, et al. High-throughput screening identifies Ceefourin 1 and Ceefourin 2 as highly selective inhibitors of multidrug resistance protein 4 (MRP4) Biochem Pharmacol. 2014;91(1):97–108. doi: 10.1016/j.bcp.2014.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.